Abstract
Inhibitors that target the retroviral enzyme reverse transcriptase (RT) have played an indispensable role in the treatment and prevention of HIV-1 infection. They can be grouped into two distinct therapeutic groups; namely the nucleoside and nucleotide RT inhibitors (NRTIs), and the nonnucleoside RT inhibitors (NNRTIs). NRTIs form the backbones of most first- and second-line antiretroviral therapy (ART) regimens formulated for the treatment of HIV-1 infection. They are also used to prevent mother-to-child transmission, and as pre-exposure prophylaxis in individuals at risk of HIV-1 infection. The NNRTIs nevirapine (NVP), efavirenz and rilpivirine also used to form part of first-line ART regimens, although this is no longer recommended, while etravirine can be used in salvage ART regimens. A single-dose of NVP administered to both mother and child has routinely been used in resource-limited settings to reduce the rate of HIV-1 transmission. Unfortunately, the development of HIV-1 resistance to RT inhibitors can compromise the efficacy of these antiviral drugs in both the treatment and prevention arenas. Here, we provide an up-to-date review on drug-resistance mutations in HIV-1 RT, and discuss their cross-resistance profiles, molecular mechanisms and clinical significance.
Keywords: HIV, reverse transcriptase, resistance, mutations, nucleoside, nonnucleoside
Introduction
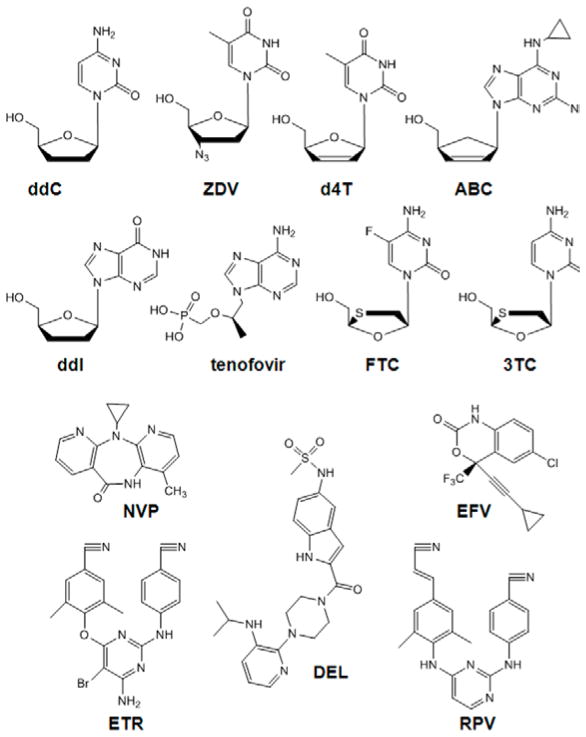
HIV-1 reverse transcriptase (RT) catalyzes the conversion of the viral single-stranded RNA into double-stranded DNA, which then serves as a substrate for integration into the human genome. RT is a multifunctional enzyme and exhibits both DNA polymerase and ribonuclease H (RNase H) activity. Due to its indispensable role in virus replication, 18 different antiviral drugs and drug combinations have been approved by the United States Food and Drug Administration that target HIV-1 RT (Table 1; https://aidsinfo.nih.gov/education-materials/fact-sheets/21/58/fda-approved-hiv-medicines). These RT inhibitors (RTIs) can be classified into two distinct therapeutic groups; namely the nucleoside and nucleotide RTIs (NRTIs), and the nonnucleoside RTIs (NNRTIs) (Figure 1). Once metabolized in the cell to their active diphosphate or triphosphate forms, NRTIs inhibit reverse transcription by competing with the analogous dNTP substrate for binding and incorporation into the newly synthesized DNA chain. Incorporation of an NRTI into the nascent viral DNA chain results in termination of any further nucleic acid synthesis. The NNRTIs are chemically distinct from the NRTIs and do not require intracellular metabolism for activity. Instead they bind to HIV-1 RT at a site, which is located approximately 10 Å from the DNA polymerase active site, termed the NNRTI-binding pocket, and inhibit reverse transcription by an allosteric mechanism of action.
Table 1.
FDA-approved antiretroviral drugs or drug combinations that target RT
| Brand Name | Generic Name | Approval Date NRTIs | Notes |
|---|---|---|---|
| Retrovir | zidovudine, azidothymidine, ZDV | March 1987 | Generic form available |
| Videx | didanosine, dideoxyinosine, ddI | October 1991 | Generic form available |
| Hivid | zalcitabine, dideoxycytidine, ddC | June 1992 | No longer marketed due to serious adverse events |
| Zerit | stavudine, d4T | June 1994 | Use is being phased out due to long-term, irreversible side effects |
| Epivir | lamivudine, 3TC | November 1995 | Generic form available |
| Ziagen | abacavir sulfate, ABC | December 1998 | |
| Viread | tenofovir disoproxil fumarate, TDF | October 2001 | |
| Emtriva | emtricitabine, (−)-FTC | July 2003 | |
| NNRTIs | |||
| Viramune | nevirapine, NVP | June 1996 | |
| Rescriptor | delavirdine, DLV | April 1997 | Efficacy < NVP and EFV and is not recommended as part of initial therapy. Cross-resistance among the NNRTI class limits DLV use in second-line therapy. |
| Sustiva | efavirenz, EFV | September 1998 | |
| Intelence | etravirine, ETR | January 2008 | |
| Edurant | rilpivirine, RPV | May 2011 | |
| NRTI combinations | |||
| Combivir | 3TC+ZDV | September 1997 | |
| Trizivir | ABC+3TC+ZDV | November 2000 | |
| Epzicom | ABC+3TC | August 2004 | |
| Truvada | (−)-FTC+TDF | August 2004 | |
| NRTI/NNRTI combinations | |||
| Atripla | (−)-FTC+TDF+EFV | July 2006 | |
| Complera | (−)-FTC+TDF+RPV | August 2011 | |
Figure 1.
Structures of RTIs approved for clinical use
Acquired drug resistance in HIV-infected individuals failing first-line therapy containing RTIs
Until recently, a first-line regimen for antiretroviral therapy (ART)-naïve individuals consisted of two NRTIs plus an NNRTI, a ritonavir-boosted protease inhibitor (PI/r), or an integrase inhibitor (INI). The most widely used NRTI combinations include ABC/3TC, TDF/FTC and ZDV/3TC and NNRTI include one of the following: EFV, RPV or NVP. However, EFV was typically preferred on the basis of its potency, although RPV may be used as an alternative NNRTI option in subjects with pre-treatment HIV RNA < 100,000 copies/mL. NVP used to be an option in women with pretreatment CD4+ counts ≤250 cells/mm3 or in men with pretreatment CD4+ counts ≤400 cells/mm3.
Virologic failure (i.e., the inability to achieve or maintain suppression of viral replication) in an HIV-infected individual on first-line ART occurs for multiple reasons, including sub-optimal adherence, drug intolerance/toxicity or drug resistance. HIV-1 drug resistance arises from the genetic variability of the virus population and selection of subpopulations of resistant variants with therapy [1]. HIV-1 genetic variability is due to the high rate of HIV-1 replication (1010 rounds of replication per day), the error-prone nature of the RT enzyme, which lacks proofreading activity (3 x 10−5 mutation per base pair per cycle), and genetic recombination when viruses of different sequence infect the same cell. Consequently, multiple genetically distinct variants (termed quasi-species) evolve within an individual in the years following infection. The selection of a drug resistant variant depends on the extent to which virus replication continues during therapy, the ease of acquisition of a particular mutation, and the effect of drug resistance mutations on drug susceptibility and viral fitness [1].
HIV-1 resistance to RTIs typically correlates with mutations in RT (Table 2). NRTI resistance mutations can be classified into two groups depending on their phenotypic mechanism of resistance. The thymidine analog mutations (TAMs) M41L, D67N, K70R, L210W, T215F/Y and K219Q/E are typically selected by ZDV (or d4T) and increase the ability of RT to excise a chain-terminating NRTI-monophosphate from a chain-terminated DNA primer [2]. In contrast, the mutations K65R, K70E, L74I/V and M184I/V increase the selectivity of RT for incorporation of the natural dNTP substrate versus the NRTI-diphosphate/triphosphate [3–6]. Rarely, virologic failure can also be associated with multi-NRTI resistance mutations including the Q151M complex [7], and the β3-β4 insertions and deletions [8]. HIV-1 resistance to NNRTIs is associated with the acquisition of one or more mutations in the NNRTI-binding pocket of RT [9]. NNRTI resistance mutations can impact inhibitor binding by: (i) removing one or more favorable interactions between the inhibitor and NNRTI-binding pocket; (ii) by introducing steric barriers to NNRTI binding; or (iii) by introducing or eliminating inter-residue contacts in the NNRTI-binding pocket, which interfere with the ability of other residues in the pocket to fold down over the NNRTI.
Table 2.
RT mutations associated with resistance to NRTIs and NNRTIs used in first-line ART
| Drug | Discrimination Mutations | NRTI Resistance Mutations Excision Enhancing Mutations | Multi-NRTI Resistance Mutations | Notes |
|---|---|---|---|---|
| ABC | K65R1, K70E, L74I/V, Y115F | Q151M2
T69 Insertions3 |
|
|
| FTC | K65R, M184I/V | Q151M T69 Insertions |
||
| 3TC | K65R, M184I/V | Q151M T69 Insertions |
||
| TDF | K65R, K70E | Q151M T69 Insertions |
||
| ZDV | M41L, D67N, K70R, L210W, T215F/Y, K219Q/E4 | Q151M T69 Insertions |
||
| NNRTI Resistance Mutations | ||||
| Drug | Resistance Mutations | Notes | ||
| EFV | L100I, K101P, K103N/S, V106M, V108I, Y181C/I, Y188L, G190A/S, P225H, M230L | There are several other mutations and polymorphisms in the HIV-1 NNRTI binding pocket that can reduce drug susceptibility, including Y318F, K238T/N, P236L, L234I, P225H, V179D/E/F/I/T, I132M/L, V106A/I, K103H/T/R/Q/E, K101H/Q/R/N/A/T, A98G and V90I. | ||
| NVP | L100I, K101P, K103N/S, V106M, V108I, Y181C/I, Y188C/L/H, G190A/S, M230L | |||
| RPV | K101E/P, E138A/G/K/Q/R, V179L, Y181C/I/V, Y188l, H221Y, F227C, M230I/L | |||
First-line therapy that included both NRTIs and NNRTIs has also been associated with the selection of mutations in the connection domain of HIV-1 RT. For example, the N348I mutation can appear early in therapy and is found to be highly associated with TAMs, M184V/I and the NNRTI resistance mutations K103N, Y181C/I, and G190A/S [10,11]. N348I appears to be significantly associated with therapies that contain ZDV (or d4T) and NVP, and decreases HIV-1 susceptibility to both drugs [10,11].
In some instances, interactions between different RTI resistance mutations can be antagonistic or complementary. For example, the NRTI discrimination mutations K65R, K70E, L74V and M184V and the NNRTI mutation Y181C reverse HIV-1 resistance to ZDV when added to a genetic background containing TAMs [6,12–14]. In contrast, the RPV resistance mutation E138K compensates for the poor replicative capacity of HIV-1 containing M184I [15]. As a consequence of this interaction, the combination of these two mutations (i.e., E138K + M184I) was the most frequent mutation combination in therapy-naive individuals who failed a first-line ART regimen containing TDF/FTC/RPV in the phase III ECHO and THRIVE clinical trials [16].
Subtype differences in HIV-1 resistance to RTIs
The diversity of HIV-1 has given rise to a large number of variants, including nine subtypes (A–D, F–H, J–K), six sub-subtypes (A1–A4, F1–F2), multiple (> 48) circulating recombinants forms and thousands of unique recombinant forms. Despite the fact that non-subtype B strains are responsible for 90% of global infections, the majority of research on HIV-1 drug resistance has focused on subtype B viruses. Importantly, there is growing increasing evidence of subtype differences in RTI drug resistance. For example, subtype C viruses harbor GTG (valine) at codon 106 in RT whereas subtype B harbors the GTA (valine) polymorphism. This genetic variation facilitates the emergence of subtype C virus with the V106M mutation (GTG to ATG) that confers high-level resistance to EFV and NVP [17]. Furthermore, subtype C viruses harbor AAA (lysine), AAG (lysine) and AAG (lysine) at codons 64, 65 and 66 of RT, respectively. In contrast, all other HIV-1 subtypes harbor AAG (lysine) and AAA (lysine) at the same codons. There is clinical evidence demonstrating frequent and early emergence of K65R on TDF-based first-line ART regimens in South Africa [18] where subtype C is predominant. In this regard, the difference in selection of K65R between subtypes B and C may be related to the template nucleotide sequence and preferential pausing of reverse transcription at the homopolymeric stretch of adenine bases at codons 64, 65 and 66 of RT [19].
Acquired drug resistance in individuals failing second- and third-line ART containing RTIs
For HIV-1-infected individuals failing a first line NNRTI-containing regimen, second-line ART typically consists of two NRTIs plus a PI/r. For individuals failing PI/r or INI-containing first-line regimens, NNRTIs can also be used in second-line ART. The NRTI backbone used in second-line ART is dependent on several considerations, including availability as a fixed-dose combination, tolerability and resistance mutation risk. The following sequence of second-line NRTI options is recommended: (i) after failure on a TDF + FTC (or 3TC)-based first-line regimen, AZT + 3TC should be used; (ii) after failure of an AZT + 3TC–based first-line regimen, TDF + FTC (or 3TC) should be used. Other NRTI drugs such as ABC and ddI are acceptable as potential back-up options in special situations, but are not recommended as preferred alternatives, since they have no specific advantage and add complexity and cost. Typically, third-line regimes should include new drugs with minimal risk of cross-resistance to previously used regimens, such as INIs and the next-generation NNRTIs (i.e., ETR) and PIs.
The mutations associated with NRTI resistance in individuals failing second-line ART are similar to those described for first-line ART. Resistance to the NNRTI ETR is conferred by combinations of the following mutations: L100I, K101E/P, E138A/G/K/Q, Y181C/I/V, Y188L, G190A/S/E and M230L (see http://hivdb.stanford.edu/DR/NNRTIResiNote.html).
Multi-RTI Drug Resistance
There is a significant degree of cross-resistance between the NRTIs and, independently, the NNRTIs. While resistance to an NRTI may develop by more than one route (as described above), there are some common pathways of cross-resistance (Table 2). TAMs confer some degree of cross-resistance to all NRTIs [20], whereas K65R decreases HIV-1 susceptibility to all NRTIs, except ZDV [12]. M184V, typically associated with FTC and 3TC resistance, may also cause resistance to ABC and ddI [20]. The Q151M complex [7] and the β3–β4 insertions and deletions [8] in HIV-1 RT can lead to resistance to all existing NRTIs as a class. Of concern, NRTI cross-resistance can lead to difficulties in finding effective antiretroviral therapies for infected-individuals who have already received extensive NRTI treatment. In the NNRTI class, cross-resistance is extensive (Table 2). In a recent analysis [21], 16 mutations at ten positions in HIV-1 RT were significantly associated with the greatest contribution to reduced phenotypic susceptibility to one or more NNRTI, including 14 mutations at six positions for NVP (K101P, K103N/S, V106A/M, Y181C/I/V, Y188C/L and G190A/E/Q/S); 10 mutations at six positions for EFV (L100I, K101P, K103N, V106M, Y188C/L and G190A/E/Q/S); 5 mutations at four positions for ETR (K101P, Y181I/V, G190E and F227C); and 6 mutations at five positions for RPV (L100I, K101P, Y181I/V, G190E and F227C). Of note, K101P and G190E were found to markedly reduce susceptibility to all NNRTIs.
HIV drug resistance in mothers and infants following use of ART to prevent mother-to-child transmission
The transmission of HIV-1 from an HIV-1-positive mother to her child can occur during pregnancy, labor, delivery or breastfeeding. In 1994, ZDV monotherapy was shown to reduce mother-to-child-transmission (MTCT) by 67.5% in a randomized placebo-controlled trial conducted in infants [22]. However, monotherapy does not suppress viral replication to undetectable levels, and consequently ZDV-resistance has been detected during pregnancy, and has been transmitted to infants [23]. Because combination ART regimens containing at least three drugs were more potent than monotherapy in women taking ART to treat their HIV infection, a lower rate of MTCT was observed compared to ZDV-monotherapy [24].
For many reasons, including insufficient infrastructure and lack of funds, women in resource-limited settings can have limited access to antiviral drugs to prevent MTCT. Consequently, shortened, simplified and inexpensive regimens were developed to prevent MTCT. In this regard, the HIVNET 012 Trial demonstrated that single-dose of NVP (sdNVP) given to the mother during labor and to mothers of breastfeeding infants after birth, reduced MTCT by 50% at 6 weeks of age and by 38% at 18 months of age [25]. Unfortunately, sdNVP selects resistant viruses at high rates in both mothers and infected infants; 10 to 75% of mothers and 4 to 87% of children [26] and is no longer recommended. Prevalence of NVP resistance after sdNVP varies across HIV subtypes, with higher rates in subtypes C and D compared to subtype A [27]. NVP resistance also confers cross-resistance to EFV. As described above, since both of these NNRTIs form part of first-line ART regimens used globally to treat HIV-infected individuals, it is particularly concerning. In response to these finding, the World Health Organization recommended that in addition to sdNVP, ZDV monotherapy (or ART) should be administered to the mother during late gestation and that short course combination ART “tails” - such as ZDV/3TC, ZDV/ddI or TDF/FTC - be administered to the mother and infant to further suppress viral replication and increase the genetic barrier to resistance.
Drug resistance and pre-exposure prophylaxis
Pre-exposure prophylaxis (PrEP) involves the use of antiretroviral drugs, specifically TDF and FTC, to prevent HIV-1 infection in high-risk populations. TDF and FTC fixed dose combination regimen (coformulated as a single pill called Truvada) are favored as PrEP agents because: (i) they exhibit potent activity against all HIV-1 subtypes; (ii) they act early in the viral replication cycle; (iii) they possess long intracellular half-lives; (iv) they are able to achieve high concentrations in the genital tract; (v) they have a convenient daily dosing with few drug interactions; and (vi) they have established safety profiles. Six efficacy trials of orally administered TDF and/or FTC/TDF as PrEP for HIV prevention have been completed (Table 3). Four of these trials demonstrated that PrEP reduced the risk of HIV acquisition, with intention-to-treat comparisons against placebo showing HIV protection efficacies between 44% and 75% [28–31]. A dose–response relationship between adherence and HIV protection was demonstrated across PrEP efficacy trials. In general, HIV-1 protection correlated with adherence. Specifically, no protection was found in two trials (FEM-PrEP and VOICE) in which adherence to PrEP was very low [32,33].
Table 3.
Clinical trials that used TDF and/or FTC/TDF as PrEP for HIV prevention
| Clinical Trial | Population | Design | Relative reduction in HIV incidence in intention-to-treat analysis |
|---|---|---|---|
| Partners PrEP | 4,747 heterosexual men and women with known HIV- infected partners (serodiscordant couples) | 1:1:1 randomization to daily TDF, FTC/TDF or placebo | TDF: 67% (95% CI: 44% to 81%; p < 0.0001) FTC/TDF: 75% (95% CI: 5% to 87%; p < 0.0001) |
| TDF2 | 1,219 heterosexual men and women | 1:1 randomization to daily oral FTC/TDF or placebo |
FTC/TDF: 63% (95 % CI: 22% to 83%; p = 0.01) |
| iPrEx | 2,499 MSM and transgender women | 1:1 randomization to daily oral FTC/TDF or placebo |
FTC/TDF: 44% (95% I: 15% to 63%; p = 0.005) |
| Fem-PrEP | 2120 women | 1:1 randomization to daily oral FTC/TDF or placebo |
FTC/TDF: 6% (p = 0.8). No statistically significant reduction in HIV incidence |
| VOICE | 3,019 women (plus 2010 women receiving tenofovir or placebo gel) | 1:1:1 randomization to daily oral TDF, FTC/TDF or placebo |
TDF: -49% (p = 0.07) FTC/TDF: -4% (p > 0.2) No statistically significant reduction in HIV incidence |
| Bagkok Tenofovir Study | 2,413 injection drug users | 1:1 randomization to daily oral TDF or placebo | TDF: 48.9% (95% CI 9.6% to 72.2 %; p = 0.01) |
PrEP could, in principle, lead to increased levels of drug resistance. For example, PrEP may be ineffective against resistant HIV strains, which could lead to a relative increase in transmitted drug-resistance. Furthermore, if an individual becomes infected while using PrEP, the drugs could select for drug-resistant HIV-1. However, in the early PrEP studies, none of the infections that occurred during the trials were with drug resistant strains. Another risk occurs when individuals start using PrEP while already infected with HIV-1. In the iPrEx and Partners PrEP studies, there were at least 3 examples of acquired drug-resistance due to the use of PrEP by previously infected individuals [28,29]. As described for sdNVP for the prevention of MTCT, the development of TDF- or FTC-resistance from exposure to PrEP agents could significantly influence first-line ART. However, as described above, the overall rates of acquired resistance due to PrEP vs MTCT are much lower, and additional research is required to comprehensively assess the frequency of PrEP acquired resistance.
Minority (Low-Frequency) Variants
Standard genotyping assays can only detect variants that have a frequency of around 20 % or higher in an HIV-1 infected individual. In recent years, however, novel technologies have allowed investigators to detect variants at much lower frequencies, depending on the assay. In general, these studies have demonstrated a higher frequency of drug resistant variants in both ART-naïve and -experienced individuals, and that pre-existing minority variants can compromise subsequent therapy, as described below.
The OCTANE Trial 1 showed that women who had received sdNVP are more likely to fail a first-line ART regimen containing NVP compared to one containing ritonavir-boosted lopinavir, even in participants in whom resistance due to sdNVP use was not detectable by standard genotyping [34]. Follow-up analyses revealed that participants who harbored low-frequencies of K103N or Y181C (as detected by allele specific PCR assay), had > 3 times the risk of virologic failure compared to women without these mutations [34]. The association between NNRTI-resistant minority variants and treatment failure has also been documented in several other studies of women and children exposed to sdNVP [35–38].
The effect of drug-resistant minority variants in ART-naive subjects has been studied in individuals on an NNRTI-based first-line regimen. In a pooled analysis of ten studies involving 985 participants, none of whom had detectable NNRTI and NRTI resistance by standard genotyping, ~ 14% were found to harbor either an NNRTI or NRTI minority variant [39]. Importantly, this study showed that the presence of a minority resistance mutation at baseline was associated with more than twice the risk of virologic failure. Studies in treatment-naive African patients have also shown an association between NNRTI minority variant detection and an increased risk of treatment failure [35]. However, a recently reported analysis of OCTANE Trial 2 of treatment-naive African women did not detect a significant association between NNRTI minority variant detection and risk of treatment failure [40]. In NNRTI-experienced patients, the presence of minority variants also increases the risk of treatment failure [39]. Even though minority variants can now be detected, its clinical application is unclear. However, it may be beneficial in choosing the appropriate regimen in treatment-experienced individuals.
New RTIs in clinical development
There is an ongoing effort to develop new RTIs, both for the treatment and prevention of HIV-1 infection. Currently, there are two NRTIs [EFdA, and tenofovir alafenamide (TAF)] and an NNRTI (doravirine) in clinical development (Figure 2). Additionally, a long-acting injectable formulation of RPV (RPV-LA) has been developed, and is currently being assessed with the integrase inhibitor GSK1265744 in an ongoing phase II study to determine whether they can maintain virologic suppression in HIV-infected individuals (ClinicalTrials.gov Identifier: NCT01641809). RPV-LA is also under evaluation as a PrEP agent. Finally, the ASPIRE study is currently assessing whether dapivirine (TMC120), an analog of ETR and RPV, can safely prevent HIV-1 infection when continuously released in the vagina from a silicone ring replaced once a month. While all of these RTIs will help diversify the therapeutic options available for the treatment and prevention of HIV-1 infection, it is unclear whether any of them will be active against existing RTI-resistant variants. In this regard, an ongoing challenge in the field continues to be the development of inhibitors active against drug-resistant HIV-1.
Figure 2.
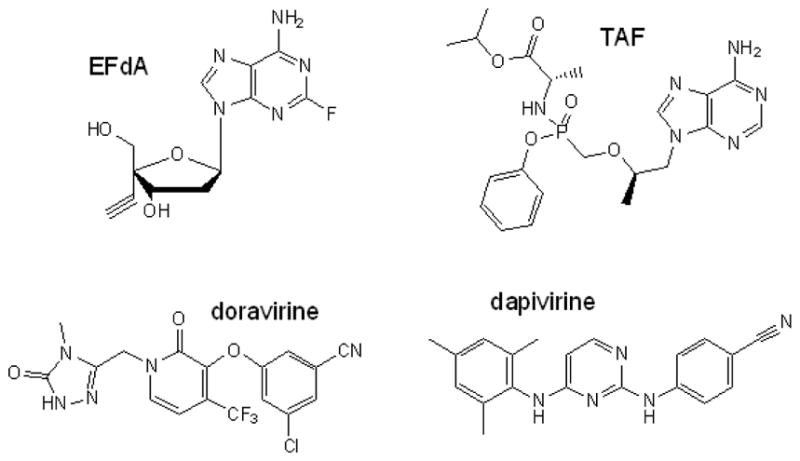
Structures of RTIs in clinical development
Overcoming drug resistance: RTIs with distinct mechanisms of action
One approach to overcoming RTI resistance involves the development of RTIs that bind to different sites in HIV-1 RT and exert mechanisms of action distinct from the NRTIs and NNRTIs. The RNase H activity of HIV-1 RT is essential for virus infectivity and therefore, represents a viable target for drug discovery. However, very few inhibitors of this activity have been identified, none of these showed reproducible antiviral activity, and consequently none have progressed to clinical trials. Another novel class of RTI is termed the nucleotide-competing RTIs (NcRTIs) [41]. NcRTIs inhibit HIV-1 RT by binding to the DNA polymerase active site and act as competitive inhibitors of dNTPs. The first NcRTI described, INDOPY-1, was largely unaffected by NRTI and NNRTI-associated resistance, although M184V and Y115F decreased susceptibility to this inhibitor [41]. A more potent novel benzo[4,5]furo[3,2,d]pyrimidin-2-one (BFPY) chemical series of NcRTIs, represented by Compound A, was recently described. These NcRTIs retain potency against RT containing M184V, but select for a W153L mutation [42]. The W153L mutation decreases HIV-1 replication capacity, confers high-level resistance to Compound A, hyper-susceptibility to ABC, TDF, 3TC and FTC, and has no effect on NVP. Residue W153 is highly conserved in RT, but is not located at the dNTP binding site. . Recent studies suggest that W153L, alone or in a background of clinically relevant mutations for NRTIs and NNRTIs, may severely diminish viral replicative fitness by impairing RT enzyme processivity and polymerization efficiency. W153L can also reverse resistance to TDF in HIV-1 containing K65R; indeed the K65R/W153L double mutant HIV-1 is hypersusceptibility to TDF [85]. In view of the fact that K65R confers hypersusceptibility to Compound A while W153L alone or together with K65R can hypersensitize TDF, it may be advantageous to co-administer a NcRTI with a resistance profile similar to Compound A together with TDF to supress viral replication in general as well as that of viruses that are resistant to either of these drugs. Hopefully, a modified version of compound A that possesses improved pharmacokinetics will be developed that will make possible the simultaneous administration of such a compound together with TDF, or with the new generation of TDF-like prodrugs such as TAF or CMX157.
Executive Summary.
Reverse transcriptase inhibitors (RTIs) are routinely used to treat HIV-1 infection.
RTIs can also to reduce mother-to-child-transmission of HIV-1 and have been shown to prevent infection in high-risk populations.
There are two distinct classes of RT inhibitors, namely the nucleoside RTIs (NRTIs) and the nonnucleoside RTIs (NNRTIs).
RTI therapy selects for viruses that have mutations in HIV-1 reverse transcriptase.
NRTI-associated resistance mutations can be broadly categorized into two groups depending on whether they confer resistance by a discrimination or excision phenotype.
HIV-1 resistance to NNRTIs correlates directly with mutations of one or more RT residues in the NNRTI-binding pocket.
There is growing increasing evidence of subtype differences in RTI drug resistance.
Pre-existing RTI-resistance, even present as minor species, can compromise the efficacy of combination antiretroviral therapies that contain RTIs.
New RTIs are in clinical development that will help diversify the therapeutic options available for the treatment and prevention of HIV-1 infection.
Acknowledgments
Research in the Sluis-Cremer laboratory was supported by grants AI081571, GM068406 and AI071846 from the National Institutes of Health (NIH), United States of America. Research in the Schinazi laboratory was supported by NIH CFAR grant 2P30-AI-050409 and the Department of Veterans Affairs. Research in the Wainberg lab is supported by the Canadian Institutes for Health Research
References
- 1.Chen R, Quinones-Mateu ME, Mansky LM. Drug resistance, virus fitness and HIV-1 mutagenesis. Curr Pharm Des. 2004;10:4065–4070. doi: 10.2174/1381612043382404. [DOI] [PubMed] [Google Scholar]
- 2.Meyer PR, Matsuura SE, Mian AM, et al. A mechanism of AZT resistance: an increase in nucleotide-dependent primer unblocking by mutant HIV-1 reverse transcriptase. Mol Cell. 1998;4:35–43. doi: 10.1016/s1097-2765(00)80185-9. [DOI] [PubMed] [Google Scholar]
- 3.Feng JY, Anderson KS. Mechanistic studies examining the efficiency and fidelity of DNA synthesis by the 3TC-resistant mutant (184V) of HIV-1 reverse transcriptase. Biochemistry. 1998;38:9440–9448. doi: 10.1021/bi990709m. [DOI] [PubMed] [Google Scholar]
- 4.Selmi B, Boretto J, Sarfati SR, et al. Mechanism-based suppression of dideoxynucleotide resistance by K65R human immunodeficiency virus reverse transcriptase using an alpha-boranophosphate nucleoside analogue. J Biol Chem. 2001;276:48466–48472. doi: 10.1074/jbc.M107003200. [DOI] [PubMed] [Google Scholar]
- 5.Deval J, Navarro JM, Selmi B, et al. A loss of viral replicative capacity correlates with altered DNA polymerization kinetics by the human immunodeficiency virus reverse transcriptase bearing the K65R and L74V dideoxynucleoside resistance substitutions. J Biol Chem. 2004;279:25489–25496. doi: 10.1074/jbc.M313534200. [DOI] [PubMed] [Google Scholar]
- 6.Sluis-Cremer N, Sheen CW, Zelina S, et al. Molecular Mechanism by Which the K70E Mutation in Human Immunodeficiency Virus Type 1 Reverse Transcriptase Confers Resistance to Nucleoside Reverse Transcriptase Inhibitors. Antimicrob Agents Chemother. 2007;51:48–53. doi: 10.1128/AAC.00683-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumi S, Kosalaraksa P, Tsang H, et al. Pathways for the emergence of multi-dideoxynucleoside-resistant HIV-1 variants. AIDS. 2003;17:1127–1137. doi: 10.1097/00002030-200305230-00003. [DOI] [PubMed] [Google Scholar]
- 8.Menéndez-Arias L, Matamoros T, Cases-González CE. Insertions and deletions in HIV-1 reverse transcriptase: consequences for drug resistance and viral fitness. Curr Pharm Des. 2006;12:1811–1825. doi: 10.2174/138161206776873608. [DOI] [PubMed] [Google Scholar]
- 9.Ren J, Stammers DK. Structural basis for drug resistance mechanisms for non-nucleoside inhibitors of HIV reverse transcriptase. Virus Res. 2008;134:157–170. doi: 10.1016/j.virusres.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Brehm JH, Koontz DL, Wallis CL, et al. Frequent emergence of N348I in HIV-1 subtype C reverse transcriptase with failure of initial therapy reduces susceptibility to reverse-transcriptase inhibitors. Clin Infect Dis. 2012;55:737–745. doi: 10.1093/cid/cis501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yap SH, Sheen CW, Fahey J, et al. N348I in the connection domain of HIV-1 reverse transcriptase confers zidovudine and nevirapine resistance. PLoS Med. 2007;4:e335. doi: 10.1371/journal.pmed.0040335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parikh UM, Zelina S, Sluis-Cremer N, Mellors JW. Molecular mechanisms of bi-directional antagonism between K65R and thymidine analog mutations in HIV-1 reverse transcriptase. AIDS. 2007;21:1405–1414. doi: 10.1097/QAD.0b013e3281ac229b. [DOI] [PubMed] [Google Scholar]
- 13.Miranda LR, Gotte M, Liang F, Kuritzkes DR. The L74V mutation in human immunodeficiency virus type 1 reverse transcriptase counteracts enhanced excision of zidovudine monophosphate associated with thymidine analog resistance mutations. Antimicrob Agents Chemother. 2005;49:2648–2656. doi: 10.1128/AAC.49.7.2648-2656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gotte M, Arion D, Parniak MA, Wainberg MA. The M184V mutation in the reverse transcriptase of human immunodeficiency virus type 1 impairs rescue of chain-terminated DNA synthesis. J Virol. 2000;74:3579–3585. doi: 10.1128/jvi.74.8.3579-3585.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Z, Kuritzkes DR. Interaction of reverse transcriptase (RT) mutations conferring resistance to lamivudine and etravirine: effects on fitness and RT activity of human immunodeficiency virus type 1. J Virol. 2011;85:11309–11314. doi: 10.1128/JVI.05578-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rimsky L, Van Eygen V, Hoogstoel A, et al. 96-week resistance analyses of rilpivirine in treatment-naive, HIV-1-infected adults from the ECHO and THRIVE Phase III trials. Antivir Ther. 2013;18:967–977. doi: 10.3851/IMP2636. [DOI] [PubMed] [Google Scholar]
- 17.Brenner B, Turner D, Oliveira M, et al. A V106M mutation in HIV-1 clade C viruses exposed to efavirenz confers cross-resistance to non-nucleoside reverse transcriptase inhibitors. AIDS. 2003;17:F1–5. doi: 10.1097/00002030-200301030-00001. [DOI] [PubMed] [Google Scholar]
- 18.Sunpath H, Wu B, Gordon M, et al. High rate of K65R for antiretroviral therapy-naive patients with subtype C HIV infection failing a tenofovir-containing first-line regimen. AIDS. 2012;26:1679–1684. doi: 10.1097/QAD.0b013e328356886d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Invernizzi CF, Coutsinos D, Oliveira M, et al. Signature nucleotide polymorphisms at positions 64 and 65 in reverse transcriptase favor the selection of the K65R resistance mutation in HIV-1 subtype C. J Infect Dis. 2009;200:1202–1206. doi: 10.1086/605894. [DOI] [PubMed] [Google Scholar]
- 20.Whitcomb JM, Parkin NT, Chappey C, Hellmann NS, Petropoulos CJ. Broad nucleoside reverse-transcriptase inhibitor cross-resistance in human immunodeficiency virus type 1 clinical isolates. J Infect Dis. 2003;188:992–1000. doi: 10.1086/378281. [DOI] [PubMed] [Google Scholar]
- 21.Melikian GL, Rhee SY, Varghese V, et al. Non-nucleoside reverse transcriptase inhibitor (NNRTI) cross-resistance: implications for preclinical evaluation of novel NNRTIs and clinical genotypic resistance testing. J Antimicrob Chemother. 2014;69:12–20. doi: 10.1093/jac/dkt316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connor EM, Mofenson LM. Zidovudine for the reduction of perinatal human immunodeficiency virus transmission: pediatric AIDS Clinical Trials Group Protocol 076-results and treatment recommendations. Pediatr Infect Dis J. 1995;14:536–541. doi: 10.1097/00006454-199506000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Frenkel LM, Wagner LE, 2nd, Demeter LM, et al. Effects of zidovudine use during pregnancy on resistance and vertical transmission of human immunodeficiency virus type 1. Clin Infect Dis. 1995;20:1321–1326. doi: 10.1093/clinids/20.5.1321. [DOI] [PubMed] [Google Scholar]
- 24.Cooper ER, Charurat M, Mofenson L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484–494. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 25.Jackson JB, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362:859–868. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- 26.Arrive E, Newell ML, Ekouevi DK, et al. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int J Epidemiol. 2007;36:1009–1021. doi: 10.1093/ije/dym104. [DOI] [PubMed] [Google Scholar]
- 27.Eshleman SH, Guay LA, Mwatha A, et al. Comparison of mother to-child transmission rates in Ugandan women with subtype A versus D HIV-1 who received single-dose nevirapine prophylaxis: HIV Network For Prevention Trials 012. J Acquir Immune Defic Syndr. 2005;39:593–597. [PubMed] [Google Scholar]
- 28.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 31.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 32.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marrazzo J, Ramjee G, Nair G, et al. Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE (MTN 003). Presented at: 20th Conference on Retroviruses and Opportunistic Infections; March 3–6, 2013; Atlanta, GA. p. Abstract 26LB. [Google Scholar]
- 34.Boltz VF, Zheng Y, Lockman S, et al. Role of low-frequency HIV-1 variants in failure of nevirapine-containing antiviral therapy in women previously exposed to single-dose nevirapine. Proc Natl Acad Sci USA. 2011;108:9202–9207. doi: 10.1073/pnas.1105688108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coovadia A, Hunt G, Abrams EJ, et al. Persistent minority K103N mutations among women exposed to single-dose nevirapine and virologic response to nonnucleoside reverse-transcriptase inhibitor-based therapy. Clin Infect Dis. 2009;48:462–472. doi: 10.1086/596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rowley CF, Boutwell CL, Lee EJ, et al. Ultrasensitive detection of minor drug-resistant variants for HIV after nevirapine exposure using allele-specific PCR: clinical significance. AIDS Res Hum Retroviruses. 2010;26:293–300. doi: 10.1089/aid.2009.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacLeod IJ, Rowley CF, Thior I, et al. Minor resistant variants in nevirapine- exposed infants may predict virologic failure on nevirapine containing ART. J Clin Virol. 2010;48:162–167. doi: 10.1016/j.jcv.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehman DA, Wamalwa DC, McCoy CO, et al. Low-frequency nevirapine resistance at multiple sites may predict treatment failure in infants on nevirapine-based treatment. J Acquir Immune Defic Syndr. 2012;60:225–233. doi: 10.1097/QAI.0b013e3182515730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li JZ, Paredes R, Ribaudo HJ, et al. Low-frequency HIV-1 drug resistance mutations and risk of NNRTI-based antiretroviral treatment failure: a systematic review and pooled analysis. JAMA. 2011;305:1327–1335. doi: 10.1001/jama.2011.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boltz VF, Bao Y, Lockman S, et al. Low-Frequency Nevirapine (NVP)-Resistant HIV-1 Variants Are Not Associated With Failure of Antiretroviral Therapy in Women Without Prior Exposure to Single-Dose NVP. J Infect Dis. 2014 doi: 10.1093/infdis/jit635. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jochmans D, Deval J, Kesteleyn B, et al. Indolopyridones inhibit human immunodeficiency virus reverse transcriptase with a novel mechanism of action. J Virol. 2006;80:12283–12292. doi: 10.1128/JVI.00889-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajotte D, Tremblay S, Pelletier A, et al. Identification and characterization of a novel HIV-1 nucleotide-competing reverse transcriptase inhibitor series. Antimicrobial Agents Chemother. 2013;57:2712–2718. doi: 10.1128/AAC.00113-13. [DOI] [PMC free article] [PubMed] [Google Scholar]