Introduction
Natural health products (NHPs), such as herbal medicines, probiotics, vitamins and minerals, are used regularly by 73% of Canadians.1 Many consumers believe that NHPs derived from plants (such as herbal medicines) are safe because they are “natural.”1 However, some plant-derived NHPs can interact with pharmaceutical medications, potentially resulting in serious harm for patients.2 A recent active surveillance study found that approximately 45% of consumers presenting to a Canadian pharmacy report using NHPs and prescription drugs concomitantly, with 7.4% describing an adverse event.3
It is important for clinicians to initiate patient communication about NHP use and be knowledgeable about the potential risks to ensure patient safety. Unless clinicians ask, they may be unaware of patient NHP use. Most NHPs can be purchased from many sources without involvement of a health professional, and many patients do not disclose NHP use to clinicians.4 However, education about NHP interactions is not always included within medical and pharmacy curricula,5 and new evidence on NHP–drug interactions is constantly emerging. In addition, a quick search of the medical literature might identify contradictory information or poor-quality reports that are challenging to interpret. Clinicians could benefit from effective knowledge translation tools to identify and prevent potential NHP–drug interactions in their patients. As pharmacists have confirmed the utility of a NHP–drug interaction grid6 as an effective knowledge translation tool,7 we undertook a scoping review to identify and incorporate new NHP–drug interactions.
Data sources
We started with our team’s prior work in this area,6 which includes 29 plant-derived NHPs cross-referenced with 27 drug classes, and identified NHPs that have documented nephrotoxic and/or hepatotoxic effects. The process for selecting plant-derived NHPs included on the tool has been described previously.6
Medical databases (MEDLINE, EMBASE and International Pharmaceutical Abstracts) were searched from January 2007 to December 2012. Search terms included medical subject headings/key words for each of the NHPs, combined with the terms clinical trials, case studies and case reports.
Study selection
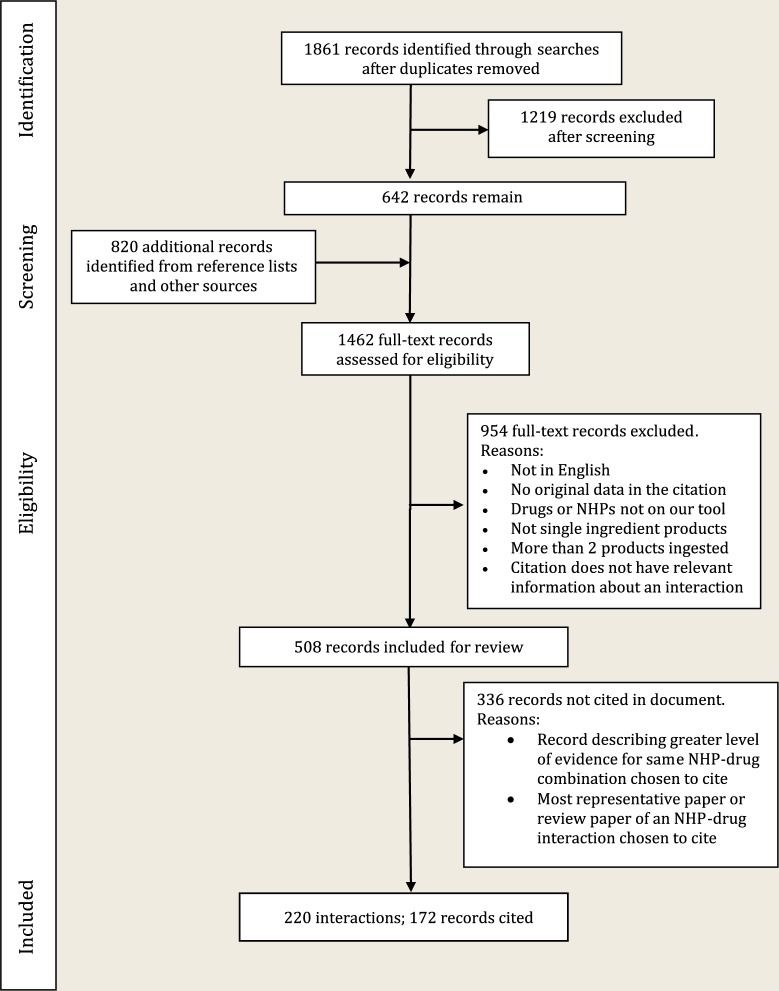
One reviewer screened titles and abstracts of identified publications for select NHPs and pharmaceutical drugs. Inclusion criteria included 1) English language; 2) studies containing original data; 3) clinical, animal studies or in vitro studies; 4) studies that could potentially describe an interaction (theoretical or actual) of a single ingredient NHP with a pharmaceutical drug or a hepatotoxic or nephrotoxic reaction (Figure 1).
Figure 1.
PRISMA flow diagram
Additional records were identified from reference lists of review articles, the Natural Medicines Database8 (formerly known as Natural Medicines Comprehensive Database), Stockley’s Herbal Medicines Interactions9 and a textbook titled Herb, Nutrient and Drug Interactions.10 Full-text articles of included publications were retrieved.
Data were collected in a standardized fashion, including (if appropriate) reason for exclusion, and if included: 1) NHP involved, 2) drug class/drug involved, 3) colour assignment as per the level of evidence and 4) brief description of the documented interaction.
We defined 6 levels of evidence:
Purple square: Clinical evidence describes an NHP–drug interaction in humans, based on randomized clinical trials or case reports.
- Purple dot: Clinical evidence describes an NHP–drug interaction, but we had reservations about the available data, for reasons such as
- insufficient details provided in reporting,
- instances of product misuse (for example, patient overdose),
- adulteration or contamination of the NHP and/or poor manufacturing processes provide alternative explanations for the adverse event,
- the intervention was an isolated constituent(s) of the NHP rather than the plant extract and used in a way that was inconsistent with clinical use, and
- conflicting (contradictory) clinical evidence.
Orange square: Evidence describes an interaction postulated in theory but not yet reported in practice.
Yellow square: Interactions based on preclinical studies (animal studies or in vitro) for which the clinical significance is unknown.
Blue square: No reported interactions or theoretical interactions identified from the literature.
White dot: Clinical evidence documenting the lack of interaction after concurrent use.
Representative papers describing the strongest level of evidence of interaction for each NHP‒drug combination and supporting documentation underwent an independent second review to confirm the classification and to finalize interaction details. Disagreements were decided by group discussion to reach consensus. All remaining interactions coded red and orange on our earlier tool6 were confirmed from reference lists and secondary sources. Interactions previously coded yellow6 for which there were no new literature reports were transferred without review. Interaction details were developed for each clinical interaction, with relevant citations identified. For interactions documented in multiple papers, a review paper was cited.
The results were synthesized into a colour-coded NHP‒drug interaction grid for clinicians.
Results
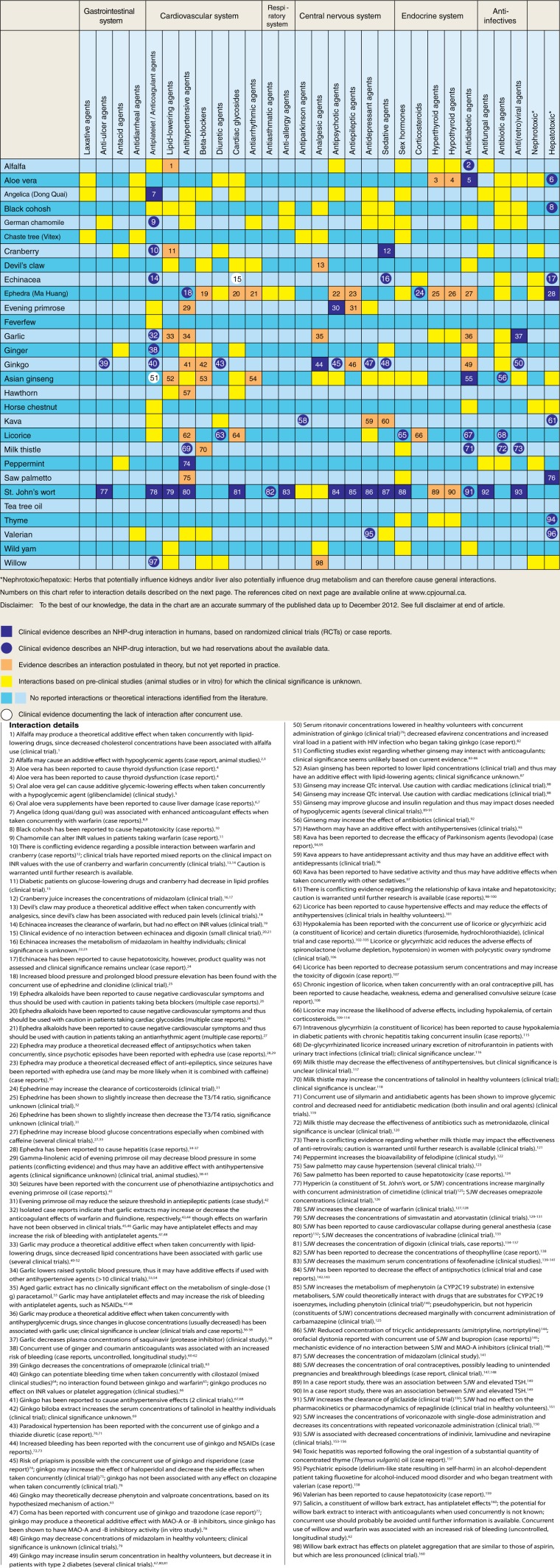
The updated NHP‒drug interaction tool is shown in Figure 2. It includes 23 interactions coded purple, 37 interactions coded purple dot, 36 interactions coded orange, 123 interactions coded yellow and 2 interactions coded white dot. NHP‒drug interactions documented in in vitro or animal studies that are new to this tool from the previous tool are summarized in Table 1.
Figure 2.
Natural health product–drug interaction tool
Table 1.
Plant-derived natural health product (NHP)–drug interactions documented in in vitro or animal studies*
| NHP | Drug categories |
|---|---|
| Black cohosh | Antihypertensive agents11 |
| Chaste tree (Vitex) | Analgesic agents12 |
| Devil’s claw | Beta-blockers,13 cardiac glycosides13 |
| Garlic | Antibiotic agents14 |
| Ginger | Antibiotic agents15 |
| Ginkgo biloba | Antibiotic agents16 |
| Asian ginseng | Cardiac glycosides,17 antihypertensive agents,18 antidepressant agents19,20 |
| Licorice | Antidepressant agents21 |
Coded yellow in Figure 2
Of the 29 NHPs, 27 had interactions with multiple drug categories cited in the medical literature—22 had interactions documented in clinical studies, 6 had only theoretical interactions, and 1 had no interactions.
Interpretation
Our NHP–drug interaction tool is comprehensive for 29 plant-derived NHPs. However, caution must be applied, as evidence in this field is constantly emerging (for example, we identified 97 clinically documented interactions compared with 59 in the first version).
There are many published studies regarding NHP‒drug interactions; however, our study’s strengths are 1) it captures a large amount of clinically relevant information, including 29 popular plant-derived NHPs and 27 drug classes, including nephrotoxicity and hepatotoxicity; 2) it is categorized and colour coded for ease of use; 3) it has succinct details for rapid clinical interpretation; and 4) it cites original data documented in medical literature.
This tool has many differences from our first version. First, the classification system was updated, to allow for more nuanced interpretation. We avoided the colours red and green, as these can be interpreted as “bad (unsafe)” or “good (safe),” when reality is more complex. A known interaction can be used to promote favourable outcomes. For example, extract of milk thistle (silymarin) in combination with antidiabetic agents can improve glycemic control and reduce the need for antidiabetic medication (both insulin and oral agents).22 However, safe concurrent use requires clinical knowledge and close monitoring of the patient to confirm there are no adverse reactions and that the desired clinical effect is achieved. Whether or not concurrent use of products is desirable depends on the patient’s clinical condition and the goals of treatment. As such, we chose a neutral colour (purple), so that the inference of safe/unsafe is removed, prompting clinicians to use the interaction details and their clinical judgment to determine if concurrent use is advisable or acceptable for their patients.
We decided to include an option for when evidence was emerging but not yet conclusive (i.e., a dot vs a fully shaded square). Typically, emerging harms are reported as case reports; these can vary considerably in terms of quality of reporting.23 The same issues were described in Fugh-Berman and Ernst24—that it was not always possible to ascribe causality in case reports to a particular NHP‒drug interaction, often because of a lack of full reporting. Often, NHPs are not well described in documentations in terms of species name, manufacturer or quality, leading to questions about the reproducibility of an interaction. In addition, if information such as the specific plant part used (e.g., leaves, roots) and type of preparation is lacking, the product’s chemical profile is unknown, since the profile of chemical constituents is not uniform throughout a plant. In addition, our team was not comfortable attributing the effect of a highly concentrated component of a plant-derived NHP (such as glycyrrhizin isolated from licorice) to that of the whole plant–derived extract, recognizing that plant-derived NHPs are often large and complex substances with many active bioingredients, some of which may work synergistically to provide a clinical effect. Their absorption, distribution, metabolism and excretion is often not well understood. We differentiate between adverse events that have been documented in humans rather than postulated in theory or seen in animal models for this reason. A recent literature review revealed that in some cases, preclinical studies have been shown to predict clinical reactions and in some cases they do not; for example, CYP3A4-mediated interactions documented in vitro were predictive for clinical interactions seen in St. John’s wort but not for milk thistle or Panax ginseng.25 However, these interactions can alert clinicians to the possibility of a clinical interaction and promote clinical vigilance.
Limitations
Our study has several limitations. Given the scope of the work undertaken, we took a scoping review approach, rather than a full systematic review for all NHPs and drugs of interest. As our primary interest was to inform clinicians of clinically relevant interactions, our search strategy underrepresents preclinical data. Many studies of cytochrome P450 (CYP) enzyme inhibition/induction are conducted in in vitro/animal settings and thus are also underrepresented based on the search strategy. The absence of interactions (coded white dot) is also underreported, as this was not the focus of our searches.
Finally, our study was also limited by the quality of available data. As the current risk-of-bias tool used by the Cochrane Collaboration26 is more appropriate when assessing studies of effectiveness, rather than adverse events,27 we did not formally assess risk of bias. Instead, we assessed reports for causality and used expert judgment to identify which were less consistent with how products are typically used in clinical practice. Since we classified drugs by their clinical effect rather than their pharmacologic class, we added as much clarity as possible in the interaction details.
Future directions for NHP‒drug interaction research are many and varied. More primary research is needed. For several reasons, adverse reactions associated with NHP‒drug interactions may not be reported.3,28-32 Most national pharmacovigilance strategies are based on a passive surveillance system for reporting of suspected adverse drug reactions; this approach is well known to suffer from underreporting (both the quantity and quality of reported adverse reactions are often insufficient for meaningful interpretation).31,33 Active surveillance is a strategy to identify NHP‒drug interactions at a faster rate than passive reporting of adverse events or by developing case studies, as described in Necyk.3 All new evidence must be weighed against previous evidence to determine the clinical significance of the interaction in question. Future updates of our knowledge translation tool would also benefit from a larger scope, for example, the inclusion of more instances in which products have been taken concurrently with no documented interactions, adding interaction details to preclinical interactions and adding literature search terms relating to cytochrome P450. Knowledge translation tools are most helpful when they are up to date, and given the volume of relevant publications in this field, we are working to develop an app that can be more easily updated.
Conclusion
The NHP‒drug interaction tool that has been developed can help clinicians determine if their patient is at risk. We urge clinicians to inquire about NHP use at every patient encounter and to report all suspected NHP‒drug interactions to their respective national pharmacovigilance center (e.g., Marketed Health Products Directorate, Health Canada).
Disclaimer
To the best of our knowledge, the data in the chart are an accurate summary of the published data up to December 2012. The data have been reviewed by a panel of researchers and clinicians. Rare or currently undocumented interactions are always possible. Remember that the likelihood that a drug interaction may occur will also vary depending on a patient’s individual characteristics, as well as on the quality of the plant-derived NHP, the part of the plant used, how a specific product is manufactured and what NHPs are combined together. Some of the common names listed here (e.g., Asian ginseng) refer to several different species or subspecies. We caution that all interactions are underreported and evidence is still emerging.
Acknowledgments
We would like to thank Soleil Surette, Alison Henry and Susanne King-Jones for conducting the literature searches, as well as Amanda Filippelli and Simran Jassar for reviewing papers and secondary sources to develop preliminary results.
Footnotes
Author Contributions:A. Kutt coordinated the project and contributed to the overview, methods, results determination and writing the manuscript. L. Girard initiated the project, reviewed papers for inclusion, developed preliminary results, drafted the initial manuscript and reviewed the manuscript. C. Necyk reviewed papers, developed preliminary results and reviewed the manuscript. H. Boon, J. Barnes and P. Gardiner provided expert opinion, developed classification system and process, confirmed results and interaction details and reviewed the manuscript. S. Vohra conceived the idea, initiated the project, provided overall direction and supervision and provided final approval of the manuscript.
Declaration of Conflicting Interests:The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding:The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Canadian Institutes of Health Research 10.13039/501100000024 CIHR PSI 85001 Vohra/Project G118160705.
References
- 1. Health Canada. Natural Health Product Tracking Survey–2010. Ipsos-Reid. Available: http://epe.lac-bac.gc.ca/100/200/301/pwgsc-tpsgc/por-ef/health/2011/135-09/report.pdf (accessed Dec. 2, 2015).
- 2. Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: an updated systematic review. Drugs 2009;69:1777-98. [DOI] [PubMed] [Google Scholar]
- 3. Necyk C, Tsuyuki RT, Boon H, et al. Pharmacy study of natural health product reactions (SONAR): a cross-sectional study using active surveillance in community pharmacies to detect adverse events associated with natural health products and assess causality. BMJ Open 2014;4:e003431. doi: 10.1136/bmjopen-2013-003431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Esmail N. Complementary and alternative medicine in Canada: trends in use and public attitudes 1997-2006. Public Policy Sources 2007;87:1-53. [Google Scholar]
- 5. Konefal J. The challenge of educating physicians about complementary and alternative medicine. Acad Med 2002;77:847-50. [DOI] [PubMed] [Google Scholar]
- 6. Cvijovic K, Boon H, Barnes J, et al. A tool for rapid identification of potential herbal medicine–drug interactions. Can Pharm J (Ott) 2009;142:224-7. [Google Scholar]
- 7. Vohra S, Cvijovic K, Boon H., et al. Study of natural health product adverse reactions (SONAR): active surveillance of adverse events following concurrent natural health product and prescription drug use in community pharmacies. PLoS ONE 2012;7:e45196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Natural Medicines Database. Available: https://naturalmedicines.therapeuticresearch.com/databases.aspx (accessed Jun. 24, 2015).
- 9. Williamson E, Driver S, Baxter K, Preston CL, editors. Stockley’s herbal medicines interactions. London (UK): Pharmaceutical Press; Available: http://www.medicinescomplete.com/mc/shmi/current/ (accessed Dec. 18, 2014). [Google Scholar]
- 10. Stargrove B, Treasure J, McKee D. Herb, nutrient, and drug interactions: clinical implications and therapeutic strategies. St. Louis (MO): Mosby/Elsevier; 2008. [Google Scholar]
- 11. Genzzanni E, Sorrentino L. Vascular action of acteina: active constituent of Actaea racemosa L. Nature 1962(194):544-5. [DOI] [PubMed] [Google Scholar]
- 12. Gupta RK, Tandon VR. Antinociceptive activity of Vitex negundo Linn leaf extract. Indian J Physiol Pharmacol 2005;49:163-70. [PubMed] [Google Scholar]
- 13. Circosta C, Occhiuto F, Ragusa S, et al. A drug used in traditional medicine: Harpagophytum procumbens DC. II. Cardiovascular activity. J Ethnopharmacol 1984;11:259-74. [DOI] [PubMed] [Google Scholar]
- 14. Tsao S, Yin M. In vitro activity of garlic oil and four diallyl sulphides against antibiotic-resistant Pseudomonas aeruginosa and Klebsiella pneumoniae. J Antimicrob Chemother 2001;47:665-70. [DOI] [PubMed] [Google Scholar]
- 15. Nagoshi C, Shiota S, Kuroda T, et al. Synergistic effect of [10]-gingerol and aminoglycosides against vancomycin-resistant enterococci (VRE). Biol Pharm Bull 2006;29:443-7. [DOI] [PubMed] [Google Scholar]
- 16. Jung HW, Chang SO, Kim CS, et al. Effects of ginkgo biloba extract on the cochlear damage induced by local gentamicin installation in guinea pigs. J Korean Med Sci 1998;13:525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dasgupta A, Wu S, Actor J, et al. Effect of Asian and Siberian ginseng on serum digoxin measurement by five digoxin immunoassays: significant variation in digoxin-like immunoreactivity among commercial ginsengs. Am J Clin Pathol 2003;119:298-303. [DOI] [PubMed] [Google Scholar]
- 18. Jeon BH, Kim CS, Kim HS, et al. Effect of Korean red ginseng on blood pressure and nitric oxide production. Acta Pharmacologica Sin 2000;21:1095-100. [PubMed] [Google Scholar]
- 19. Kimura T, Saunders PA, Kim HS, et al. Interactions of ginsenosides with ligand-bindings of GABA(A) and GABA(B) receptors. Gen Pharmacol 1994;25:193-9. [DOI] [PubMed] [Google Scholar]
- 20. Tsang D, Yeung HW, Tso WW, et al. Ginseng saponins: influence on neurotransmitter uptake in rat brain synaptosomes. Planta Medica 1985;51:221-4. [DOI] [PubMed] [Google Scholar]
- 21. Ofir R, Tamir S, Khatib S, et al. Inhibition of serotonin re-uptake by licorice constituents. J Mol Neurosci 2003;20:135-40. [DOI] [PubMed] [Google Scholar]
- 22. Hussain SA. Silymarin as an adjunct to glibenclamide therapy improves long-term and postprandial glycemic control and body mass index in type 2 diabetes. J Med Food 2007;10:543-7. [DOI] [PubMed] [Google Scholar]
- 23. Gardiner P, Adams D, Filippelli A, et al. A systematic review of the reporting of adverse events associated with medical herb use among children. Glob Adv Health Med 2013;2:46-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fugh-Berman A, Ernst E. Herb–drug interactions: review and assessment of report reliability. Br J Clin Pharmacol 2001;52:587-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goey AK, Mooiman KD, Beijnen JH, et al. Relevance of in vitro and clinical data for predicting CYP3A4-mediated herb–drug interactions in cancer patients. Cancer Treat Rev 2013;39:773-83. [DOI] [PubMed] [Google Scholar]
- 26. Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Cochrane Collaboration; 2011. Available: http://handbook.cochrane.org (accessed Dec. 2, 2015).
- 27. Zorzela L, Golder S, Liu Y, et al. Quality of reporting in systematic reviews of adverse events: a systematic review. BMJ 2014;8:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Charrois TL, Hill RL, Vu D, et al. Community identification of natural health product-drug interactions. Ann Pharmacother 2007;41:1124-29. [DOI] [PubMed] [Google Scholar]
- 29. Barnes J. Quality, efficacy, and safety of complementary medicines: fashions, facts, and the future. Part II: efficacy and safety. Br J Clin Pharmacol 2003;55:331-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Grootheest K, Olsson S, Couper M, de Jong-van den Berg L. Pharmacists’ role in reporting adverse drug reactions in an international perspective. Pharmacoepidemiol Drug Saf 2004;13:457-64. [DOI] [PubMed] [Google Scholar]
- 31. Barnes J, Mills SY, Abbot NC, et al. Different standards for reporting ADRs to herbal remedies and conventional OTC medicines: face-to-face interviews with 515 users of herbal remedies. Br J Clin Pharmacol 1998;45:496-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barnes J. Pharmacovigilance of herbal medicines. Drug Safety 2003;26:829-51. [DOI] [PubMed] [Google Scholar]
- 33. Wiktorowicz ME, Lexchin J, Moscou K, et al. Keeping an eye on prescription drugs, keeping Canadians safe: a commissioned discussion paper. Health Council of Canada. 2010. Available: http://publications.gc.ca/collections/collection_2011/ccs-hcc/H174-21-2010-eng.pdf (accessed Dec. 2, 2015).