Abstract:
Autologous platelet-gel (APG) is the process of harvesting ones own cells (platelets), concentrating them most often through centrifugation, exposing them to an agonist which induces activation which releases intrinsic substances, and applying them to a target area to accelerate wound healing. APG is attractive because it concentrates a large number of biologically active substances, which are primarily proteins that participate in complex series of mechanisms involved in inflammation and wound healing. It has been used in numerous applications including sports medicine, dermatology, and surgery. However, there are few prospective randomized trials that have compared it in a rigorous manner to other techniques or to placebo. The following report is a review of APG, which includes a description of its perceived benefit, identification of the various modalities where it has been used, and criticisms concerning its use.
Keywords: autologous platelet gel, growth factors, wound healing
“No idea is so outlandish that it should not be considered with a searching but at the same time a steady eye.”
Winston Churchill
“A person with a new idea is a crank until the idea succeeds.”
Mark Twain
Few areas of blood management have been simultaneously so widely revered yet broadly questioned as the process of autologous platelet gel (APG). An equal balance of skepticism tempers the degree of enthusiasm where a believer’s passion is only surpassed by a critic’s admonitions. APG is described as the process of harvesting ones own cells (platelets), concentrating them, exposing them to an agonist, activating them to release their intrinsic substances, and applying them to an area of the body in need of healing.
It’s growth over the past decade can best be described as geographically variant where certain locations have seen explosive growth while others have seen little to no acceptance. It is clearly a technology in search of a science, which suffers from the conventional wisdom of a lack of evidence-base. Its origins can be traced to the techniques of intra-operative plasmapheresis.
Although the process of plasmapheresis has been in clinical use for over 50 years, its application as a perioperative clinical tool was not appreciated until the mid 1970s. Initial research focused on the separation of autologous whole blood into fractions that could be administered throughout the surgical procedure. In cardiac surgery this technique became popular in the late 1980s, but controversy surrounding its reproducible efficacy led to its decline in the late 1990s (1–5). Although a number of studies promoted the benefits of plasmapheresis as a blood conservation measure, an equal number failed to show a reduction in allogeneic blood product utilization.
During the past two decades, however, this technique has reemerged not based upon its original potential to improve hemostasis, but instead related to the contribution of the platelet and its biological affect on wound healing (6). A secondary benefit of this process, and one that has promoted its popularity, is the derivation of the product from an autologous source. It is harvested from the same individual to whom it will be applied. The growth of APG has occurred most rapidly during the past decade both in the United States and across Europe and is beginning to expand in Asia. What makes APG so attractive is the ease of which the platelets can be harvested using point-of-care devices that are used both in clinics and physician offices as well as throughout operating theaters. The technique for making APG begins with a modified plasmapheresis procedure that yields a platelet-rich plasma (PRP) fraction either by a centrifugation procedure or through the process of ultrafiltration (the former being the most used technique). Once the platelets are harvested they are activated and degranulate releasing various intrinsic proteins primarily by exocytosis from alpha granules (7). The PRP fraction usually has a platelet count of 4–6 times that found in the unfractionated sample with a normal concentration of fibrinogen. Hence, APG can also be termed fibrin-platelet gel as a result of combining coagulation with cellular activation. Since white cells are retained as well, some have coined the term platelet-rich-leukocyte plasma. The following review is not intended to be comprehensive or inclusive and readers are directed elsewhere for a more in-depth evaluation (8,9), but instead to address several major clinical applications and controversies surrounding the use of APG in surgery.
BIOLOGICAL ACTIVITY
The mechanism of activity whereby platelets participate in wound healing and tissue regeneration is complex and poorly understood. However, its foundation is based upon the biological systems that are involved in wound healing (10,11). Platelets participate in wound healing in a number of ways but are critically important especially during injury where there is a loss of vascular integrity and hemorrhage. Once platelets are activated they degranulate and secrete intracellular substances, some of which are called chemokines or growth factors, which attract and activate macrophages and fibroblasts (10). Complement is activated which modulates various protein driven amplification systems that recruit leukocytes and other chemotactic substances to the area of injury. The platelet-released chemokines, cytokines that have chemotactic activity, accelerate the rate of activation of monocytes and macrophages, which in turn serve an important roll in the propagation of tissue regeneration (12). Chemokines also modulate cellular actions that include autocrine, paracrine, and endocrine systems (13). The process of making PRP concentrates platelets which similarly increases the concentration of chemokines, which is thought to accelerate the healing process. Since the PRP is collected from the same patient to whom the growth factors will be applied, the entire process is termed autologous growth factors (AGF).
There are a large number of these AGF that have been studied. Some of these include transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), transforming growth factor-β (TGF-β), and vascular endothelial growth factor (8,9). These proteins regulate various processes involved in wound healing and tissue regeneration by regulating cell proliferation, cell differentiation, angiogenesis, matrix deposition, and tissue remodeling (14,15). In toto, these systems potentiate and accelerate the rate of wound healing above that possible under normal biological regulation without supplementation via concentration and activation of platelets.
CLINICAL APPLICATIONS
One of the most attractive facets of APG is its broad applicability for improving wound healing across a diverse population of patients. Estimates of its yearly potential use range into the hundreds of thousands of patients in the United States alone (Table 1). However, the majority of cases reported thus far in the literature have been focused on three main areas: dermatology, orthopedics, and maxofacillary surgery. Other developing services that have reported on its use include cardiac surgery and plastic surgery.
Table 1. Estimated number of platelet gel by specialty in United States.*.
| Specialty | Yearly Number |
|---|---|
| Cardiovascular | 350,000 |
| Orthopedic | |
| Spine | 350,000 |
| Joints (knees, hips, shoulders) | 500,000 |
| Foot and ankle | 1,000,000 |
| Regenerative injection therapy | |
| Sports medicine | 1,000,000 |
| Oral maxofacillary/periodontal | 500,000 |
| ENT | 50,000 |
| Plastic and reconstructive/cosmetic | 200,000 |
| Obstetrics/gynecology | 100,000 |
| Wound care/soft tissue | ? |
| Veterinary | ? |
Estimates obtained from personal communications with Harvest Technologies, Plymouth, MA, July 27, 2009.
Dermatology and Soft Tissue Healing
The use of APG in dermatology is predicated upon the intrinsic benefits of various growth factors to accelerate tissue regeneration and reepithelialization. It has been shown that PDGF stimulates cell division more efficiently in normal skin fibroblasts than in scar fibroblasts and decreases glycosaminoglycan synthesis in skin and scar fibroblasts (16). Using a hamster model, Dvorak and associates demonstrated that angiogenesis was enhanced when PDGF was added to a fibrin gel preparation and postulated that this growth factor served as an additional mitogen for cell proliferation (17). Soheil et al. have shown that excessive scar contracture can result from excessive fibroblast formation leading to hypertrophic scar formation, but PDGF levels were higher in normal and keloid fibroblasts (18).
Chronic non-healing wounds such as venous ulcers has long challenged the medical industry and plagued patients, especially those with diabetes. Most of the advances in this area have involved wound dressings and compression therapies, so applications of APG are extremely attractive in this setting. Hom and associates from Minnesota recently reported a prospective, single-blind study comprising 80 full-thickness skin-punch wounds (4 mm diameter) where APG was applied (19). They used digital planimetry to show that both the size of the wound and the velocity of wound closure were significantly improved in the APG group. However, when the platelet count exceeded 6-times baseline levels, epithelialization and granulation formation appeared 3 days earlier when compared to the control group. They hypothesized that the faster velocity of wound healing was a function of the higher AGF concentration of various chemokines in the treatment group, which ranged from 4–12 times higher in the PRP sample as opposed to baseline. One retrospective study of over 26,000 diabetic patients presenting with chronic wounds demonstrated that the use of an autologous platelet releasate resulted in improved rates of healings as compared with standard therapies (20). Saldalamacchia et al. randomized the use of APG to standard therapies in 14 diabetic patients who underwent the treatment for 5 weeks (21). Patients treated with APG had a significant reduction in wound size (71.9 ± 22.5–9.2 ± 67.8%, p < .39) and smaller wound areas, although significance was not achieved.
Stacey et al. examined APG in a randomized double-blind study on chronic venous ulcers and found no benefit of this technique when compared with placebo improvement (22). However, these authors pointed out that the autologous platelet lysate that was created contained concentrations of PDGF and EGF that were a thousand fold less than had been seen to show benefit in animal models. Similarly a small controlled randomized trial on APG demonstrated no benefit of this technique when applied to a variety of patients presenting with non-healing wounds (23). The authors did note that the patients were not stratified according to wound size and that the control patients had larger wounds at the start of the trial. This trial used a technique for preparing APG that required sending a collected sample to an outside laboratory for processing and is no longer commercially available.
Orthopedics and Maxofacillary Surgery
The healing of bones is a complex interaction of growth factors, cytokines and the various cells (chondrocytes, osteoblasts, osteoprogenitor) associated with bone growth (13). Growth factors play a critical and pivotal role in bone repair. It is thought that the supplementation of intrinsic growth factors with those either from recombinant sources (recombinant bone morphogenetic proteins) or from those concentrated from PRP may enhance the potential for improving the rate of bone healing. Much of the early work in the area of APG occurred in dental and craniofacial surgical areas and has been summarized in the 2005 monograph by Marx and Garg (24). In dental surgery, PDGF stimulates cementogenesis and osteogenesis increasing bone growth.
The use of growth factors in spinal surgery has appeal because of the potential for acceleration of consolidation of graft materials into a fusion mass and secondarily to increase stabilization (25). Initial enthusiasm for using APG in spinal fusion surgery (26,27) has been tempered by the appearance of studies suggesting questionable benefit. Feiz-Erfan et al. used APG combined with allograft bone in 50 patients who underwent anterior cervical fusion with allograft bone for patients with soft herniated cervical disc or degenerative disc disease (28). Patients were assessed radiographically for up to 2 years post surgery and those patients presenting with degenerative disc had improved fusion rates compared with control; those with soft disc herniation showed no benefit.
Weiner and Walker completed a retrospective non-randomized trial of AGF use in 59 patients who underwent lumbar fusion with (n = 32) and without (n = 27) AGF (29). The use of AGF resulted in inferior rates of arthrodesis compared with autogenous bone graft alone, which caused the authors to reject the use of AGF for lumbar spinal surgery. Some believe it a timing issue in so much that absence of an adequate carrier for PG reduces its retention at the wound site due to the action of fluid movement (30). Carreon et al. performed a retrospective review of patients undergoing posterolateral spinal fusions and found no benefit of its application compared with matched patients who did not receive APG (31).
The use APG is increasing in other areas of orthopedic surgery. Rodeo has described the application of APG in rotator cuff tendon repair where healing occurs between tendon and bone (32). This application uses the physical properties of the platelet coagulum to directly suture the “plug” to the tendon repair site where osteoinductive growth factors are directly released. Zavadil and associates conducted a randomized clinical study on APG in patients undergoing total shoulder arthroplasty (33). What these authors found was that patients receiving APG had a significant increase in internal rotation measurement and lower postoperative pain, which required less analgesia. Galasso et al. reported on the use of APG in 22 patients undergoing reoperative surgery for long bone non-unions and found improved results with few complications, although these were poorly defined (34). Recently the use of APG has been expanded to include sports medicine with varying degrees of success seen in repair of Achilles tendon (35), hamstring tendons (36), and anterior cruciate ligament surgery (37).
AUTOLOGOUS PLATELET GEL USE AT GEISINGER HEALTH SYSTEM
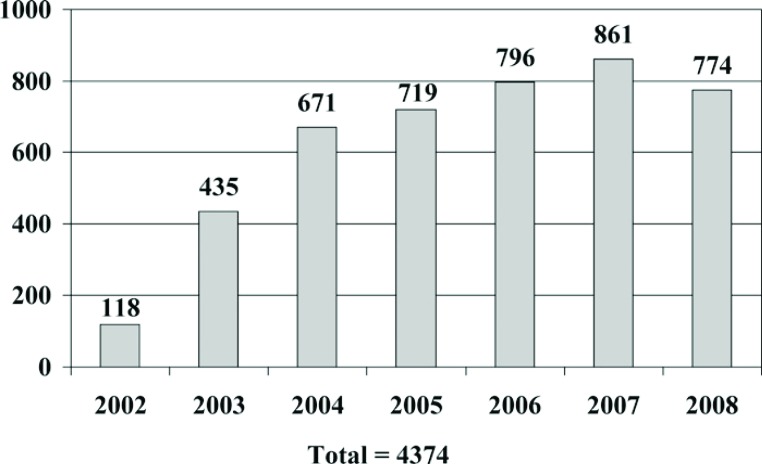
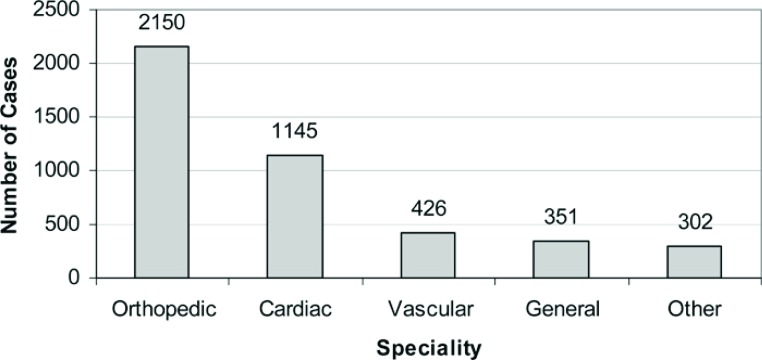
We began using the techniques of APG in 2002 with total figures for one of our hospitals shown in Figure 1 . From 2002 through 2008 we performed a total of 4374 procedures where AGF were applied. This represents various specialties with the preponderance clustered in orthopedic and cardiac surgery (Figure 2). Within orthopedics we began our experience with APG spine surgery but have since shifted to the use of autologous stem cells for spinal fusions. Presently the majority of our orthopedic cases involve regenerative injection therapy for rotator cuff and shoulder surgeries (52.1% of non-cardiac cases performed in 2008) followed by its use in anterior cruciate ligament surgery (18.3% of non-cardiac cases performed in 2008). APG is also applied for non-union and tendon surgeries although to a lesser degree (5.3% of non-cardiac cases performed in 2008). Seventy percent of these procedures are performed on an outpatient basis with patients receiving this therapy for same-day surgery. More recently we have expanded our application of this technique in the area of wound management for non-healing wounds (38).
Figure 1.
Geisinger Health System platelet gel use from 2002 through 2009 (2009 total estimated).
Figure 2.
Geisinger Health System autologous platelet gel case by specialty (2002–2008).
We examined the effects of APG on infection rate by retrospectively reviewing data on all patients undergoing cardiac surgery at our institution (39). Of the 2259 patients reviewed, 382 had received APG applied to all surgical incisions combined with a mixture of 10% calcium chloride (5 mL) and bovine thrombin (5000 units). The incidence of superficial infection was significantly lower in the APG group (n = .3%) vs. either an historical group of 929 patients (1.5%) and a concurrent group of patients (n = 948) who did not receive APG (1.8%), p < .05. Similarly the occurrence of sternal infections was lower as well (APG, 0% vs. No APG, 1.5%; p < .029 and APG vs. historical control, 1.7%; p < .01). Although this was not a prospective randomized trial and APG was applied to all patients operated upon by two of four surgeons, no other selection criteria was observed to confound the outcomes. Englert and associates conducted a prospective randomized trial on cardiac patients receiving APG and found lower rates of postoperative incision pain, and in a follow-up retrospective study, found reduced rates of sternal infections, and shortened intensive care unit and hospital length of stay (40,41). A group of researchers from the University of Bielefeld in Bielefeld Germany recently reported that APG had no effect on improving wound healing post-saphenectomy in coronary artery bypass graft patients (42). These researchers found a reduction in the number of large-area hematomas but otherwise found no benefit to APG application.
CONTROVERSIES
The acceptance of APG therapy is geographical and is often related to the number of champions at individual centers. Although we perform a large number of APG procedures at Geisinger Health System, the majority of these are conducted by a handful of surgeons. Even within specialties amongst the same surgical groups, we have both believers and non-believers. Caution was raised by Snyder and Calhoun in an early editorial on AGF appropriately titled “Topical Platelet Growth Factor Therapy: Of Lotions and Potions” (43). These authors correctly identified that one of the major problems of AGF preparations is assessing the quality of the biological preparation. The American Association of Blood Banks has begun the process of regulating this heretofore not regulated practice. Quality controls are all but absent and perhaps the most foreboding problem is assessing the efficacy of AGF therapy (44). The paucity of randomized clinical trials makes assessing benefit challenging and what studies do exist are often retrospective with very few actually containing cohort matched groups. The paucity in clinical outcomes data makes interpreting results difficult, as it should be. With the estimates of use of AGF therapy growing to the millions, it is a technique in dire need of well-designed prospective evaluations. Two additional problems exist for most centers contemplating developing an AGF service: Reimbursement from third-party payers and meeting personnel challenges. The disposable costs for providing APG vary from center to center. The range in costs for the disposable kits at our facility ranges from 175 to 450 United States dollars. This does not include the additional pharmacy costs of commercial thrombin, calcium chloride, or the use of autologous thrombin.
Production of APG involves the mechanical process of phlebotomization, exposure of the collected whole blood to synthetic surfaces, centrifugation, and activation with a platelet agonist. All of these begin the process of platelet activation regardless of the level of anticoagulation and the concentration of AGF becomes dependent upon the viability of the platelet (7). If growth factors are released prematurely their efficacy may be reduced or lost. Some individuals, including these authors, therefore collect and use the platelet-poor plasma fraction, which may retain some level of AGF, especially applying this on the cutaneous surfaces. The type of anticoagulant used to collect the whole blood has been shown to influence AGF yield with acid citrate dextrose and citrate theophylline adenosine dipyridamole shown to be superior to heparin and sodium citrate (7).
There are at least nine commercial manufactures of platelet gel devices in the United States alone and numerous “home-made” systems as well. Differences may exist in both the yield of platelet concentrate and the quality of end-product when using different devices and techniques for the production of AGF (44). Mazzucco has demonstrated this variability in comparing three commercially available devices and one manual procedure (45). They found a variation range of 5–27 fold amongst the tested devices in AGF production, which may or may not influence the degree of wound healing achieved. Varying the concentration of AGF has been shown to influence the rate of angiogenesis in endothelial cells, which may explain the discrepancies in the literature when attempting to interpret interstudy comparisons (46).
The rate of release of AGF from the platelet may have an effect with target tissue benefiting from a gradual and sustained uptake of chemokines. In an animal study of platelet-clot stability, Rademakers and associates demonstrated a rapid liquefication, within 60 minutes when plate-let-clot was exposed to pericardial surfaces (47).
The use of commercial bovine based thrombin preparations has come under intense scrutiny because of the potential for disease transmission and immune-mediated coagulopathies (48). The fact that severe coagulopathies can result in association with the use of bovine thrombin preparations has prompted the FDA to require pharmaceutical companies to insert a black-box warning in product information of these agents despite recent developments at increasing purification techniques (49). It remains to be seen whether the appearance of recombinant thrombin preparations or autologous thrombin processes will supplant bovine thrombin in the Unites States. Whitlow and associates also reported that the impact of cost and a lack of training in the techniques to the personnel administering it have also hindered its spread (50).
And perhaps most importantly, the healing process is a complex interaction of numerous cellular and acellular mechanisms that are based in the complexities of molecular biology. The influences of disease state, nutritive health, and patient medication history are all but a few of the confounding factors that make evaluation of the efficacy of APG difficult, if not impossible.
CONCLUSION
The use of APG has had a gradual and subdued penetration as a clinical modality to enhance wound healing. This tempered acceptance has its foundation linked to the paucity of strong evidence for its expansion, and although used in centers throughout the world, is most often linked to individual perceived notion of benefit. Its growth, or decline, will most assuredly be linked to the appearance of well-designed randomized trials as they appear in the literature. Until then it will remain a modality used passionately by those convinced of its perceived benefit to enhance healing.
REFERENCE
- 1.Stammers AH, Kratz J, Johnson T, Crumbley J, Merrill J.. Hematological assessment of patients undergoing plasmapheresis during cardiac surgery. J Extra Corpor Technol. 1993;25:6–14. [PubMed] [Google Scholar]
- 2.Rubens FD, Fergusson D, Wells PS, Huang M, McGowan JL, Laupacis A.. Platelet-rich plasmapheresis in cardiac surgery:A meta-analysis of the effect on transfusion requirements. J Thorac Cardiovasc Surg. 1998;116:641–7. [DOI] [PubMed] [Google Scholar]
- 3.Menges T, Welters I, Wagner RM, Boldt J, Dapper F, Hempelmann G.. The influence of acute preoperative plasmapheresis on coagulation tests, fibrinolysis, blood loss and transfusion requirements in cardiac surgery. Eur J Cardiothorac Surg. 1997;11:557–63. [DOI] [PubMed] [Google Scholar]
- 4.Armellin G, Sorbara C, Bonato R, Pittarello D, Dal Cero P, Giron G.. Intraoperative plasmapheresis in cardiac surgery. J Cardiothorac Vasc Anesth. 1997;11:13–7. [DOI] [PubMed] [Google Scholar]
- 5.Pivalizza EG, Abramson DC.. Autologous platelet-rich plasmapheresis in repeat cardiac surgery. Anesth Analg. 1996;82:885–6. [DOI] [PubMed] [Google Scholar]
- 6.Rhee JS, Black M, Schubert U, et al. . The functional role of blood platelet components in angiogenesis. Thromb Haemost. 2004;92:394–402. [DOI] [PubMed] [Google Scholar]
- 7.Lei H, Gui L, Xiao R.. The effects of anticoagulants on the quality and biological efficacy of platelet-rich plasma. Clin Biochem. 2009;26 (epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 8.Everts PA, Knape JT, Weibrich G, et al. . Platelet-rich plasma and platelet gel: A review. J Extra Corpor Technol. 2006;38:174–87. [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta S, Watson JT.. Platelet rich concentrate: Basic science and current clinical applications. J Orthop Trauma. 2008;22:432–8. [DOI] [PubMed] [Google Scholar]
- 10.Singer AJ, Clark RA.. Cutaneous wound healing. N Engl J Med. 1999;341:738–46. [DOI] [PubMed] [Google Scholar]
- 11.Szpaderska AM, DiPietro LA.. Inflammation in surgical wound healing: Friend or foe? Surgery. 2005;137:571–3. [DOI] [PubMed] [Google Scholar]
- 12.Charo IF, Ransohoff RM.. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354: 610–2. [DOI] [PubMed] [Google Scholar]
- 13.Devescovi V, Leonardi E, Ciapetti G, Cenni E.. Growth factors in bone repai. Chir Organi Mov. 2008;92:161–8. [DOI] [PubMed] [Google Scholar]
- 14.Giusti I, Rughetti A, D’Ascenzo S, et al. . Identification of an optimal concentration of platelet gel for promoting angiogenesis in human endothelial cells. Transfusion. 2009;49:771–8. [DOI] [PubMed] [Google Scholar]
- 15.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT.. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. [DOI] [PubMed] [Google Scholar]
- 16.Savage K, Siebert E, Swann D.. The effect of platelet-derived growth factor on cell division and glycosaminoglycan synthesis by human skin and scar fibroblasts. J Invest Dermatol. 1987;89:93–9. [DOI] [PubMed] [Google Scholar]
- 17.Dvorak HF, Harvey VS, Estrella P, Brown LF, McDonagh J, Dvorak AM.. Fibrin containing gels induce angiogenesis. Implications for tumor stroma generation and wound healing. Lab Invest. 1987;57: 673–86. [PubMed] [Google Scholar]
- 18.Soheil Y, Venters G, Vu S, et al. . Role of growth factors in scar contraction: An in vitro analysis. Ann Plast Surg. 1995;34:495–501. [DOI] [PubMed] [Google Scholar]
- 19.Hom DB, Linzie BM, Huang TC.. The healing effects of autologous platelet gel on acute human skin wounds. Arch Facial Plast Surg. 2007;9:174–83. [DOI] [PubMed] [Google Scholar]
- 20.Margolis DJ, Kantor J, Santana J, Strom BL, Berlin JA.. Effectiveness of platelet releasate for the treatment of diabetic neuropathic foot ulcers. Diabetes Care. 2001;24:483–8. [DOI] [PubMed] [Google Scholar]
- 21.Saldalamacchia G, Lapice E, Cuomo V, et al. . A controlled study of the use of autologous platelet gel for the treatment of diabetic foot ulcers. Nutr Metab Cardiovasc Dis. 2004;14:395–6. [DOI] [PubMed] [Google Scholar]
- 22.Stacey MC, Mata SD, Trengove NJ, Mather CA.. Randomised double-blind placebo controlled trial of topical autologous platelet lysate in venous ulcer healing. Eur J Vasc Endovasc Surg. 2000;20:296–301. [DOI] [PubMed] [Google Scholar]
- 23.Krupski WC, Reilly LM, Perez S, Moss KM, Crombleholme PA, Rapp JH.. A prospective randomized trial of autologous platelet-derived wound healing factors for treatment of chronic nonhealing wounds: A preliminary report. J Vasc Surg. 1991;14:526–32. [PubMed] [Google Scholar]
- 24.Marx RE, Garg AK.. Dental and Craniofacial Applications of Platelet-Rich Plasma. Chicago: Quintessence Publishing Co, Inc.; 2005:154. [Google Scholar]
- 25.Castro FP.. Role of activated growth factors in lumbar spinal fusions. J Spinal Disord Tech. 2004;17:3804. [DOI] [PubMed] [Google Scholar]
- 26.Lowery GL, Kulkarni S, Pennisi AE.. Use of autologous growth factors in lumbar spinal fusion. Bone. 1999;25:47S–50S. [DOI] [PubMed] [Google Scholar]
- 27.Walsh WR, Loefler A, Nicklin S, et al. . Spinal fusion using an autologous growth factor gel and a porous resorbable ceramic. Eur Spine J. 2004;13:359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feiz-Erfan I, Harrigan M, Sonntag VK, Harrington TR.. Effect of autologous platelet gel on early and late graft fusion in anterior cervical spine surgery. J Neurosurg Spine. 2007;7:496–502. [DOI] [PubMed] [Google Scholar]
- 29.Weiner BK, Walker M.. Efficacy of autologous growth factors in lumbar intertransverse fusions. Spine. 2003;28:1968–70. [DOI] [PubMed] [Google Scholar]
- 30.Tsai CH, Hsu HC, Chen YG, Lin MJ, Chen HT.. Using the growth factors-enriched platelet glue in spinal fusion and its efficiency. J Spinal Disord Tech. 2009;22:246–50. [DOI] [PubMed] [Google Scholar]
- 31.Carreon LY, Glassman SD, Anekstein Y, Puno RM.. Platelet gel (AGF) fails to increase fusion rates in instrumented posterolateral fusions. Spine. 2005;30:E243–6. [DOI] [PubMed] [Google Scholar]
- 32.Rodeo SA.. Biologic augmentation of rotator cuff tendon repair. J Shoulder Elbow Surg. 2007;16:191S–7S. [DOI] [PubMed] [Google Scholar]
- 33.Zavadil DP, Satterlee CC, Costigan JN, Holt DW, Shostrom VK.. Autologous platelet gel and platelet-poor plasma reduce pain with total shoulder arthroplasty. J Extra Corpor Technol. 2007;39: 177–82. [PMC free article] [PubMed] [Google Scholar]
- 34.Galasso O, Mariconda M, Romano G, et al. . Expandable intramedullary nailing and platelet rich plasma to treat long bone non-unions. J Orthop Traumatol. 2008;9:129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sánchez M, Anitua E, Azofra J, Andía I, Padilla S, Mujika I.. Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35:245–51. [DOI] [PubMed] [Google Scholar]
- 36.Orrego M, Larrain C, Rosales J, et al. . Effects of platelet concentrate and a bone plug on the healing of hamstring tendons in a bone tunnel. J Arthroscop Rel Surg. 2008;24:1373–80. [DOI] [PubMed] [Google Scholar]
- 37.Ventura A, Terzaghi C, Borgo E, Verdoia C, Gallazzi M, Failoni S.. Use of growth factors in ACL surgery. Preliminary results. J Orthop Traumatol. 2005;6:76–9. [Google Scholar]
- 38.Klayman MH, Trowbridge CC, Stammers AH, Wolfgang GL, Zijerdi DA, Bitterly TJ.. Autologous platelet concentrate and vacuum-assisted closure device use in a non-healing total knee replacement. J Extra Corpor Technol. 2006;38:44–7. [PMC free article] [PubMed] [Google Scholar]
- 39.Trowbridge CC, Stammers AH, Woods E, Yen B, Klayman M, Gilbert C.. Use of platelet gel and its effects on infection in cardiac surgery. J Extra Corpor Technol. 2005;37:381–6. [PMC free article] [PubMed] [Google Scholar]
- 40.Englert SJ, Estep TH, Ellis-Stoll CC.. Autologous platelet gel applications during cardiovascular surgery: Effect on wound healing. J Extra Corpor Technol. 2005;37:148–52. [PMC free article] [PubMed] [Google Scholar]
- 41.Englert SJ, Estep TH, Ellis-Stoll CC.. Postoperative surgical chest and leg incision sites using platelet gel: A retrospective study. J Extra Corpor Technol. 2008;40:225–8. [PMC free article] [PubMed] [Google Scholar]
- 42.Buchwald K, Kaltschmidt C, Haardt H, Laczkovics A, Reber D.. Autologous platelet gel fails to show beneficial effects on wound healing after saphenectomy in CABG patients. J Extra Corpor Technol. 2008;40:196–202. [PMC free article] [PubMed] [Google Scholar]
- 43.Snyder EL, Calhoun BC.. Topical platelet growth factor therapy: Of lotions and potions. Transfusion. 2001;41:1186–9. [DOI] [PubMed] [Google Scholar]
- 44.Waters JH, Roberts KC.. Database review of possible factors influencing point-of-care platelet gel manufacture. J Extra Corpor Technol. 2004;36:250–4. [PubMed] [Google Scholar]
- 45.Mazzucco L, Balbo V, Cattana E, Guaschino R, Borzini P.. Not every PRP-gel is born equal. Evaluation of growth factor availability for tissues through four PRP-gel preparations: Fibrinet, RegenPRP-Kit, Plateltex and one manual procedure. Vox Sang. 2009;97:110–8. [DOI] [PubMed] [Google Scholar]
- 46.Giusti I, Rughetti A, D’Ascenzo S, et al. . Identification of an optimal concentration of platelet gel for promoting angiogenesis in human endothelial cells. Transfusion. 2009;49:771–8. [DOI] [PubMed] [Google Scholar]
- 47.Rademakers LM, Gründeman PF, Bolderman RW, van der Veen FH, Maessen JG.. Stability of an autologous platelet clot in the pericardial sac: An experimental and clinical study. J Thorac Cardiovasc Surg. 2009;137:1190–4. [DOI] [PubMed] [Google Scholar]
- 48.Ness P, Creer M, Rodgers GM, et al. . Recognition, Evaluation and Treatment of Acquired Coagulopathy Consensus (RETACC) Panel. Building an immunemediated coagulopathy consensus: Early recognition and evaluation to enhance post-surgical patient safety. Patient Saf Surg. 2009;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kessler CM, Ortel TL.. Recent developments in topical thrombins. Thromb Haemost. 2009;102:15–24. [DOI] [PubMed] [Google Scholar]
- 50.Whitlow J, Shackelford AG, Sievert AN, Sistino JJ.. Barriers to the acceptance and use of autologous platelet gel. Perfusion. 2008;23:283–9. [DOI] [PubMed] [Google Scholar]