Abstract
SCCRO (squamous cell carcinoma-related oncogene; also known as DCUN1D1) is a highly conserved gene that functions as an E3 in neddylation. Although inactivation of SCCRO in yeast results in lethality, SCCRO−/− mice are viable. The exclusive presence of highly conserved paralogues in higher organisms led us to assess whether compensation by SCCRO paralogues rescues lethality in SCCRO−/− mice. Using murine and Drosophila models, we assessed the in vivo activities of SCCRO and its paralogues in cullin neddylation. We found that SCCRO family members have overlapping and antagonistic activity that regulates neddylation and cell proliferation activities in vivo. In flies, both dSCCRO and dSCCRO3 promote neddylation and cell proliferation, whereas dSCCRO4 negatively regulates these processes. Analysis of somatic clones showed that the effects that these paralogues have on proliferation serve to promote cell competition, leading to apoptosis in clones with a net decrease in neddylation activity. We found that dSCCRO and, to a lesser extent, dSCCRO3 rescue the neddylation and proliferation defects promoted by expression of SCCRO4. dSCCRO and dSCCRO3 functioned cooperatively, with their coexpression resulting in an increase in both the neddylated cullin fraction and proliferation activity. In contrast, human SCCRO and SCCRO4 promote, and human SCCRO3 inhibits, neddylation and proliferation when expressed in flies. Our findings provide the first insights into the mechanisms through which SCCRO family members cooperatively regulate neddylation and cell proliferation.
Keywords: head and neck cancer, lung cancer, oncogene, tumor suppressor gene, ubiquitylation (ubiquitination)
Introduction
The highly conserved tripartite enzymatic cascade that results in posttranslational modification by ubiquitin regulates the activity of proteins involved in diverse cellular processes (1–3). Given its essential role, ubiquitination itself is subject to considerable regulatory control, primarily by modulation of E3 activity, the specificity- and rate-limiting step in the reaction (4). For cullin-RING ligase-type ubiquitination E3s, the largest class of E3s in mammals, assembly of the enzymatic complex serves as a key regulator of function. Neddylation, a process analogous to ubiquitination that results in conjugation of the ubiquitin-like protein NEDD8 to cullins, serves as a signal for assembly and activation of cullin-RING ligase complexes (5–15). We and others have shown that SCCRO6/DCUN1D1 is an essential component of the E3 ligase for neddylation (16–21). Conservation of SCCRO from yeast to humans strongly suggests that it is required for fundamental processes in eukaryotic cells. Lending support to this idea, knocking out SCCRO orthologues DCN1 and Dcn1p in Caenorhabditis elegans and Saccharomyces cerevisiae results in lethality (19). Our finding that SCCRO knock-out mice are viable raises questions about the requirement of SCCRO for normal cellular function and development in higher organisms (17).
The presence of SCCRO paralogues in higher organisms suggests that the viability of SCCRO−/− mice might be attributable to compensation by its paralogues (20). Analysis of model organisms with completely sequenced genomes revealed that a single SCCRO gene in plants, fungi, and metazoans evolved into five genes in higher organisms. Sequence analysis of the five SCCRO family members showed that they all contain a highly conserved central and C-terminal PONY domain but possess divergent N-terminal regions (16, 18). On the basis of N-terminal sequence homology and phylogeny, the SCCRO family of genes can be classified into three subgroups. SCCRO/DCUN1D1 and SCCRO2/DCUN1D2 appear to have resulted from a gene duplication event early in the deuterostome/chordate lineage, as they are both present in Danio rerio but are co-orthologues to a single gene (DCUN1D1) found in the invertebrates Drosophila melanogaster and Anopheles gambiae. SCCRO and SCCRO2 possess a UBA-like domain in their N terminus that binds to ubiquitin and ubiquitinated proteins (20). The amino acid sequences of SCCRO and SCCRO2 in mice and humans share >80% sequence identity. Mammalian SCCRO4/DCUN1D4 and SCCRO5/DCUN1D5 contain a canonical nuclear localization sequence in their N terminus and share 65% sequence identity, with orthology to a single insect gene. SCCRO4 protein has an extended N terminus before the nuclear localization sequence-like sequence, which is attributable to a conserved upstream translation initiation sequence that is not present in SCCRO5. The last subgroup includes a single gene (SCCRO3) that is conserved in the purple sea urchin Strongyloides purpuratus and is more divergent than its mammalian orthologue but is recognizable as an orthologue in Drosophila and Anopheles. SCCRO3 is distinguished by a long N-terminal region consisting of a NORS (nonordered secondary structure) domain and an N-myristoylation sequence (MGXXXT), and it has a less-conserved PONY domain compared with other SCCRO family members. The finding of these properties suggests the possibility that the N terminus of SCCRO3 could serve as a tether, linking the protein to plasma or an organelle membrane via a fatty acid anchor (23).
Results of in vivo studies suggest that, in addition to assembling the neddylation E3 complex, SCCRO/DCUN1D1 facilitates nuclear localization of neddylation components, which is required for cullin neddylation (20). The high degree of conservation of the PONY domain, which is required for neddylation-promoting activity, supports the proposition that all SCCRO paralogues play a role in neddylation activity. The presence of variable N-terminal domains that are all directly or indirectly involved in subcellular compartmentalization suggests that the SCCRO paralogues may have divergent activities in vivo, which remain to be defined (20, 23–27). Using murine and Drosophila models, we provide evidence that SCCRO and its paralogues have overlapping and independent activity that regulates neddylation and cell proliferation in vivo. Moreover, we show that cell proliferation has an effect on accompanying up- or down-regulation of SCCRO and its paralogues, which results in competition between cells.
Experimental Procedures
Antibodies
The following antibodies were used in this study: rat anti-Elav and mouse anti-Dlg (Developmental Studies Hybridoma Bank, Iowa City, IA); rabbit anti-Pax2 (gift from Dr. Markus Noll, University of Zurich, Switzerland); rabbit anti-Cul1 (Invitrogen); mouse anti-Cul3 and anti-CAND1 (BD Biosciences); anti-α-tubulin (Calbiochem); rabbit anti-ROC1 (Abcam, Cambridge, MA); mouse anti-HA (Santa Cruz Biotechnology, Dallas, TX); rabbit anti-Myc (Novus, Littleton, CO); chicken anti-GFP and rabbit anti-Casp3 (Abcam). The anti-SCCRO monoclonal antibody was produced and used as described previously (17). The mouse polyclonal antibodies against the N-terminal portions of dSCCRO, dSCCRO3, and dSCCRO4 were generated by a commercial source (Antibody Research, St. Charles, MO). The secondary antibodies used were Alexa Fluor 488-conjugated goat anti-chicken IgY and Alexa Fluor 568-conjugated goat anti-rabbit IgG (Invitrogen).
Drosophila Stocks and Crosses
The flies used in this study were cultured on standard yeast-cornmeal-agar medium at 25 °C in a humidified room. The Gal4/UAS system was used for ectopically expressing genes of interest. The Gal4 lines include Ey-Gal4 (Bloomington #5535), GMR-Gal4 (Bloomington #8440), Ey-GMR-Gal4, and Ptc-Gal4 (gifts from Dr. Lai), Ubi-Gal4 (Bloomington #32551), and Act5c-Gal4 (Bloomington #3954). UAS-dSCCRO RNAi lines were obtained from the Vienna Drosophila Stock Center (Vienna, Austria), including dSCCRO RNAi (28006 and 104190), dSCCRO3 RNAi (101492 and 32179), and dSCCRO4 RNAi (28237, 104418, and 104778). Knockdown of dSCCRO and its paralogues was achieved by crossing these lines with the Gal4 drivers listed above. UAS-dSCCRO and UAS-hSCCRO constructs were generated by cloning the full-length coding sequences plus N-terminal-attached HA (for dSCCRO and hSCCRO) and Myc tag (for dSCCRO3 and dSCCRO4) into the EcoRI and NotI sites of pUAST vector. At least three independent transgenic lines were generated for each construct by use of a commercial source (BestGene, Chino Hills, CA) and were crossed with different Gal4 drivers.
Generation of Null Mutants of Drosophila SCCRO and Its Paralogues
A series of deletions uncovering the loci of dSCCROand its paralogues was generated using the jump-out technique, as described previously (28). The break points of these deletions were mapped by amplifying and sequencing the corresponding genomic regions by use of primers: 1) 5′-CGCGTCGCATAGTAATCGATAAG-3′ and 5′-GCATTTACGCTAAAGCAGACACAC-3′ for CG7427 (dSCCRO); 2) 5′-ACATACCAACGACCAACTACC-3′ and 5′-GCTCTTCATTTCTCGCTTCCT-3′ for CG13322 (dSCCRO3); and 3) 5′-CTGAAGAATAGACCTAGACAGTCAG-3′ and 5′-GTAACATAATGACGACGCAAAGGC-3′, 5′-TCTTCTTGAACTCCAATCCCAGATAG-3′ and CATATAGCCAAGCTGTATCTGCG-3′, AGCAGCGTCTTCGAAAATCCT and TTGCCGTGCTACTGACCATAT-3′, and TCTGTCTGGCATCGTGTTTAC-3′ and CTCGCTGTATCGTTTCAATGC-3′ for CG6597 (dSCCRO4). Western blot analysis was performed to check protein expression of dSCCRO and its paralogues. The enhancer-trap line 12091 (Bloomington Drosophila Stock Center, Bloomington, IN), which carries a P-element with a lacZ expression cassette inserted 126 bp upstream of the first ATG codon of dSCCRO (CG7427) locus, was crossed with the transposase line (Δ2–3) to mobilize the P-element. Two deletions (J34 and J156) were confirmed to have lost the entire coding sequence, and Western blot analysis with the anti-dSCCRO antibody detected no dSCCRO protein in the mutant embryonic lysates. The enhancer-trap line 112238 (Drosophila Genetic Research Center, Kyoto, Japan) carries a Gal4 expression cassette inserted in the second intron of dSCCRO3 (CG13322). Jump-out of this P-element generated one deletion, J155, that lost the dSCCRO3 promoter region and coding sequences, including the first N-terminal 204 amino acids. dSCCRO3 protein was not detected by Western blot analysis in J155 embryonic lysate. The line 22470 (Bloomington Drosophila Stock Center), which has a P-element inserted in the 5′-UTR region of dSCCRO4 (CG6597), was used to generate deletions uncovering dSCCRO4 (CG6597); eight deletions were recovered. However, using several pairs of primers located in the neighboring genes, we were not able to determine the break points, which suggests that these deletions may also uncover the genes, including CG32225, CG32226, and CG12306. Despite repeated efforts to do so, a mutant uncovering the dSCCRO4 locus could not be generated. Moreover, the presence of CG32225 in the fourth intron of SCCRO4 limited attempts to rescue jump-out mutants by use of genomic SCCRO4 clones.
Somatic Clone Analysis
The flies with chromosomes carrying dSCCRO deletions of J34 and J156 were recombined with FRT80B and then crossed with flies carrying w HspFLP; Ubi-GFP FRT80B. The somatic recombination was induced by 1 h of heat shock at 37 °C during the second and third instar larval stages to generate two types of tissue in the eyes: dSCCRO−/− (marked as the white color in the adult eyes because of loss of the white gene) and dSCCRO+/+ (marked as dark red in the adult eyes because of two copies of the white gene). The sizes of wild-type and mutant clones were then determined. The whole-eye clone of the dSCCRO mutant was generated by crossing J34 FRT80B or J156 FRT80B males with virgins of w; eyFLP/CyO; GMR-Hid FRT80B/TM6B, with 1 h of heat shock at 37 °C during the first and second instar larval stages. Overexpression clones of dSCCRO and its paralogues were generated by crossing UAS-dSCCRO lines with yw hFLP; Act-Gal4≫UAS-LacZ, +/SM with 20 min of heat shock at 37 °C during the second instar larval stage.
Overexpression of the dSCCRO and dSCCRO4 Subset of Cells in the Eye Imaginal Disc
The flip-out technique was used to ectopically express dSCCRO and dSCCRO4 in a subset of clones marked by GFP in the eye imaginal disk. Virgins of yw hFLP; Act>CD2>Gal4; UAS-GFP (gift from Dr. Lai) were crossed with UAS-dSCCRO or UAS-dSCCRO4 and allowed to lay eggs overnight. To induce Gal4 expression and activate both UAS-GFP and UAS-dSCCRO or UAS-dSCCRO4, larvae were subjected to 1 h of heat shock at 37 °C during the first to second instar larval stages. The imaginal eye discs of late-third-instar larvae were dissected in cold PBS and then stained with anti-GFP (green) and anti-Casp3 (red) antibodies.
Wing Measurement
The 1-week-old flies were collected and fixed in ethanol:glycerol mix (3:1) overnight. The wings were then dissected and mounted in 80% glycerol on glass slides covered with coverslips. A 5× objective lens was used to photograph the whole wing; 40× was used for the middle wing patches. The distance between veins L3 and L4 was measured as total points by use of Photoshop (Adobe, San Jose, CA), and the cell number was determined by counting the total bristles in the same area. The relative cell size was calculated as the area size divided by the total number of bristles.
Touch Sensitivity Analysis
The larvae were staged 80 h after eggs were laid, at 25 °C (early third instar larvae). Single larvae were transferred onto agarose plates for scoring, as follows: 0, no response; 1, stop or hesitate; 2, retract and continue forward; 3, retract and turn <90°; 4, retract and turn >90°. Each larva was tested four times, and scores were summed. The mean scores were calculated for comparison.
Fertility Analysis
Virgin flies were collected among wild-type (w118) and mutant (J34, J155, and J155/J34 double mutant) flies on the same day. Ten breeding pairs of each genotype were set up and raised at 25 °C. The flies were flipped every 3 days into new tubes with food and yeast. The F1 flies were counted and averaged for a single breeding pair.
Survival Analysis
Twenty female flies of each genotype that had hatched on the same day were collected and raised at 25 °C. The flies were flipped every day into fresh tubes with food and yeast. The number of living flies was assessed daily.
Generation of SCCRO−/− Mice
All animal experiments were approved by the Institutional Animal Care and Use Committee of Memorial Sloan Kettering Cancer Center and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. SCCRO−/− mice were generated by use of exon trapping in ES cells with the retroviral gene trap vector pGT01xf (BayGenomics, San Francisco, CA). The generation, mapping, and PCR genotyping protocols were as described previously (17, 20).
Neddylation Assay
For in vivo neddylation, cell lysates were directly subjected to immunoblotting for cullin(s). In vitro neddylation was performed as described previously (17). To determine the ratio of neddylated to nonneddylated Cul1 or Cul3, Western blots were scanned and analyzed using ImageJ software (National Institutes of Health, Bethesda, MD).
Real-time PCR
RNA was extracted from tissues by mechanical homogenization in Trizol reagent (Invitrogen) and was further purified using the RNeasy kit with on-column DNase digestion (Qiagen, Germantown, MD) in accordance with the manufacturer's instructions. The iScript cDNA Synthesis kit (Bio-Rad) was used for first-strand cDNA synthesis after pretreatment with DNase I (Invitrogen) in accordance with the manufacturers' protocols. Subsequent individual PCR reactions used Platinum PCR Supermix (Invitrogen). Primer pairs used for quantitative RT-PCR on murine tissues were: mDcun1D1_RT2: GAAGGGCACACTCACTCACA; RT 2R: GAGACCTGACCCACACGAAG, mDcun1D1_RT3_F: GGAATCTTCTGTTAGACTTCAGTTC; RT3_R: TGTTGTACTTTTTGTCCCAGCA; mDcun1D1ex1_RT1_F: GGAGGAGGAGGGGAGAGG; RT1_R: TTGTGTGAAGATCATAAACTGACG; mDcun1D1ex1AB_RT1_F: TGACAGACTGGCTCTGAACAA; _RT1_R: CATTTTGAGAAAGACAACTTACTGC; mDcun1D1ex1C_RT1_F: GCAGTCCTTCCTTCAGCTTC; RT1_R: TGCAGTTTTCTCACTAGATTGTGT; mDcun1D2_RT1_F: CTAAGAACCCTGGGCAGAAG; RT1_R:TGATGCTCCAGCAGAAATGT; mDcun1D3_RT1_F: GAATTTCGAGTGCTGCTCTT; _RT1_R: ACAGATCCCATCAATGCTGT; mDcun1D4_RT2_F: CAACACTGGCAAGCATTCAT;RT2_R: AGTCCGAAGAACTGCAAGAG; mDcun1D5_RT1_F: AGAACAGTTCATGCCGATCTT; RT1_R: AGGTTTGCATTGTCTTCACG; m18s_F: GTAACCCGTTGAACCCCATT;_R: CCATCCAATCGGTAGTAGCG; mGAPDH F: TGCACCACCAACTGCTTAGC; R: GGCATGGACTGTGGTCATGAG.
For quantitative RT-PCR, reactions were prepared using iQ SYBER green Supermix (Bio-Rad) in accordance with the manufacturer's instructions and were run on a MyQ single-color real-time PCR detection system (Bio-Rad). PCR settings were the same for all primer sets: after a 5-min denaturation step at 95 °C, each PCR cycle consisted of 30 s of denaturation at 95 °C, 30 s of annealing at 58 °C, and 30 s of elongation at 72 °C for 40 cycles. Two primer sets were used for each experimental run in triplicate on a 96-well plate along with controls. The details of real-time PCR data acquisition and data analysis have been described previously (29).
GST Pulldown Assays and Immunoblotting
Protein was extracted from mouse tissue using Tissue-PE LB buffer (Geno-Tech) in accordance with the manufacturer's directions and separated by SDS/PAGE, transferred to PVDF membranes (Whatman), and probed with selected antibodies. Fly protein lysates were prepared from overnight-collected imaginal discs and brains from third-instar larvae by use of 3× SDS sample buffer, in accordance with the manufacturer's protocols (New England BioLabs, Ipswich, MA). All proteins were resolved on SDS-PAGE gels and subjected to Western blot analysis using the indicated antibodies.
Subcellular Localization
HeLa cells were transfected with the appropriate florescent-tagged gene-bearing or empty vectors (pEGFP-N2, pEGFP-C2 (Clontech), pGex-4T3, and phCMV3 [Genlantis]) and imaged 24 h after transfection using a Nikon IX51 fluorescent microscope with standard excitation and filter settings for enhanced GFP fluorescence.
Statistical Analysis
Quantitative comparisons were made using Student's t test. SPSS 19 was used for all statistical analyses (IBM, Armonk, NY). Qualitative comparisons were made using the Mann-Whitney U test. A two-tailed p ≤ 0.05 was considered to indicate statistical significance.
Results
Mammalian SCCRO Paralogues Promote Cullin Neddylation
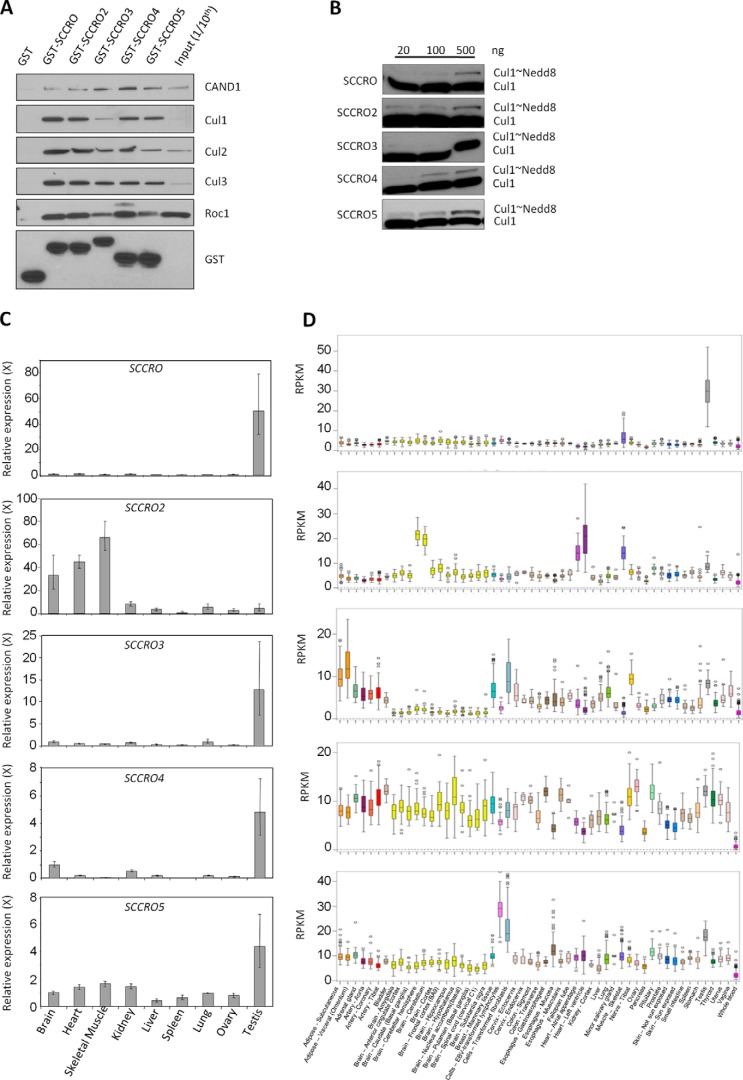
Given that the SCCRO orthologue DCN1 is essential for development in yeast and C. elegans, it was somewhat unexpected that SCCRO−/− mice were found to be viable, albeit with runting and male-specific infertility (19, 20). The unique presence of SCCRO paralogues in higher organisms raises the possibility that they may compensate for the loss of SCCRO in these mice. To address this hypothesis, we first determined whether, similar to SCCRO, the paralogues promote cullin neddylation; we tested this by assessing their interactions with neddylation components and their neddylation activity in vitro. Using GST pulldown in lysates from HeLa cells transfected with GST-tagged SCCRO or its paralogues (Fig. 1A), we found that all paralogues bound to cullins, ROC1, and CAND1. Only four of the five paralogues (SCCRO, SCCRO2, SCCRO4, and SCCRO5), which were found to bind to UBC12 (27), promoted neddylation of cullins in vitro (Fig. 1B). In all SCCRO paralogues, mutations in the DAD patch or the PONY domain resulted in loss of binding to the neddylation components and loss of neddylation-promoting activities (data not shown). This finding suggests that four of the five SCCRO paralogues promote cullin neddylation and, therefore, are able to putatively compensate for loss of SCCRO in SCCRO−/− mice.
FIGURE 1.
A, Western blot on GST pulldown products using the indicated constructs. SCCRO and its paralogues were probed for components of the neddylation pathway. All SCCRO paralogues bind to neddylation components. B, Western blot for Cul1 neddylation on products from in vitro neddylation assays supplemented with SCCRO or its paralogues. All paralogues promote neddylation except SCCRO3. C, results from quantitative real-time PCR showing tissue-specific expression of SCCRO and its paralogues. SCCRO expression is disproportionately higher in the testis. D, results from analysis of data from the Genotype-Tissue Expression project showing tissue-specific mRNA expression of SCCRO and its paralogues. SCCRO expression is disproportionally higher in testis compared with other paralogues in other tissues. RPKM, fragments per kilobase of exon per million reads mapped.
Tissue-specific Expression Patterns Explain the Isolated Defect in Spermatogenesis of SCCRO−/− Mice
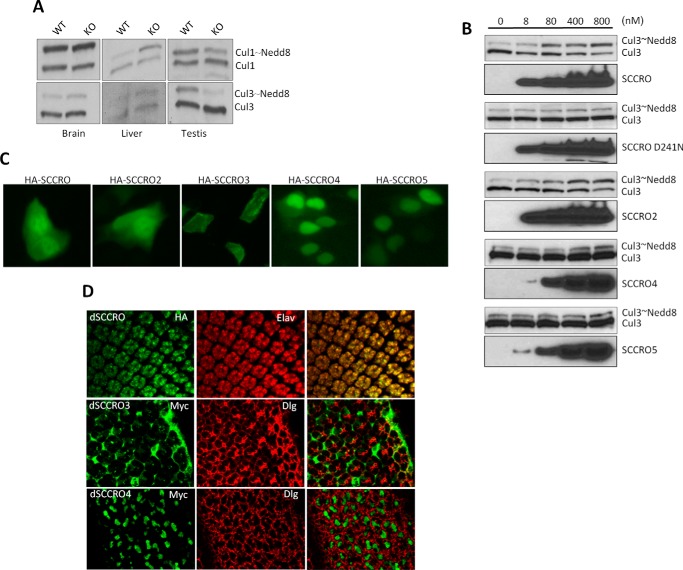
The only detectable physiological phenotype in SCCRO−/− mice was male-specific infertility. To begin to determine the cause of the infertility, we first assessed expression of SCCRO and its paralogues in normal mouse tissues by use of quantitative real-time PCR. One or more SCCRO paralogues was expressed at levels similar to those of SCCRO in all organs tested except the testis. Normalized expression of all SCCRO paralogues other than SCCRO2 was highest in testis, but SCCRO expression was disproportionately higher (SCCRO expression was 50-fold higher than brain, whereas SCCRO2–5 were 4–13-fold higher) (Fig. 1C). Analysis of human data from the Genotype-Tissue Expression project (GTExPORTAL) showed expression patterns similar to ours, confirming that SCCRO was the dominant paralogue expressed in testis (Fig. 1D). Consistent with this, we found that between SCCRO−/− and SCCRO+/+ mice levels of neddylated Cul3 were significantly reduced in testis but not in other organs (Fig. 2A). To confirm that the paralogues can compensate for loss of SCCRO, we performed activated neddylation assays on testis lysates from SCCRO−/− mice, with recombinant SCCRO or SCCRO paralogues added to the reactions. We found that the addition of SCCRO2, but not other SCCRO paralogues, could rescue neddylation of Cul3 to levels similar to those seen in the same lysates supplemented with SCCRO and in testis lysates from SCCRO+/+ mice (Fig. 2B). Combined, these findings suggest that phenotypic defects and decreased Cul3 neddylation in the testis of SCCRO−/− mice may be attributable to the absence of compensation by SCCRO2. These results may provide a basis for the defective spermatogenesis and viability observed in SCCRO−/− mice.
FIGURE 2.
A, results from Western blot for Cul1 and Cul3 in the lysates from the indicated organs from 6-week-old SCCRO−/− and littermate control wild-type mice showing decreased neddylation in testis from SCCRO−/− mice. B, Western blots showing results from in vitro neddylation assay on lysates from testis from SCCRO−/− mice supplemented with SCCRO or SCCRO paralogues. SCCRO and SCCRO2 can rescue neddylation defects in SCCRO−/− mice. The addition of SCCRO-D241N, a neddylation-dead mutant of SCCRO, to the lysates served as a control for these experiments. C, live cell fluorescence of HeLa cells transfected with GFP-tagged SCCRO or its paralogues showing pan-cellular localization of SCCRO and SCCRO2, membrane localization of SCCRO3, and nuclear localization of SCCRO4 and SCCRO5. D, photomicrographs of eye imaginal discs from flies engineered to express HA- or Myc-tagged dSCCRO or its paralogues. Shown are the results of immunostaining using antibodies against HA- and Myc (first column), cellular markers (Elav (nuclear) and Dlg (membrane; middle column), and merged images (last column). Subcellular localization of SCCRO orthologues is preserved in flies.
SCCRO and Its Paralogues Are Conserved in Flies
The presence of multiple paralogues in mice combined with the complexity of manipulating the genome makes assessment of the in vivo activities of individual SCCRO family members difficult in mice. In contrast, D. melanogaster have only three SCCRO paralogues, each belonging to a separate subgroup on the basis of their N-terminal domain: dSCCRO, which encodes a 288-amino acid protein with an N-terminal UBA-like domain (5–50 amino acids), similar to its mammalian orthologue SCCRO; dSCCRO3, which encodes a 334-amino acid protein with a less conserved but recognizable myristoylation site after the first methionine, similar to its mammalian orthologue SCCRO3; dSCCRO4, which encodes a 248-amino acid protein with a nuclear localization sequence near the N terminus, similar to its mammalian orthologue SCCRO4. We confirmed that all N-terminal loci were functional by assessing localization of HA-tagged human SCCRO and its paralogues in HeLa cells by use of immunofluorescence (Fig. 2C). Our previous studies showed that the major function of the N-terminal domains of SCCRO and its paralogues is compartmentalization, as mutation of the critical residues in the N-terminal domains abrogated subcellular localization for all family members (20, 24, 27). To assess the function of the three paralogues in flies, HA-tagged dSCCRO and Myc-tagged dSCCRO3 and dSCCRO4 were expressed in the eye imaginal discs by use of GMR-Gal4 driver, and their subcellular localization was assessed by immunofluorescence. We found that, similar to their mammalian orthologues, dSCCRO was predominantly located in the nucleus, dSCCRO3 was predominantly located at the membrane, and dSCCRO4 was exclusively located in the nucleus (Fig. 2D). These findings confirm that the function of N-terminal domains is conserved in flies.
dSCCRO and dSCCRO3 Knock-out Flies Are Viable
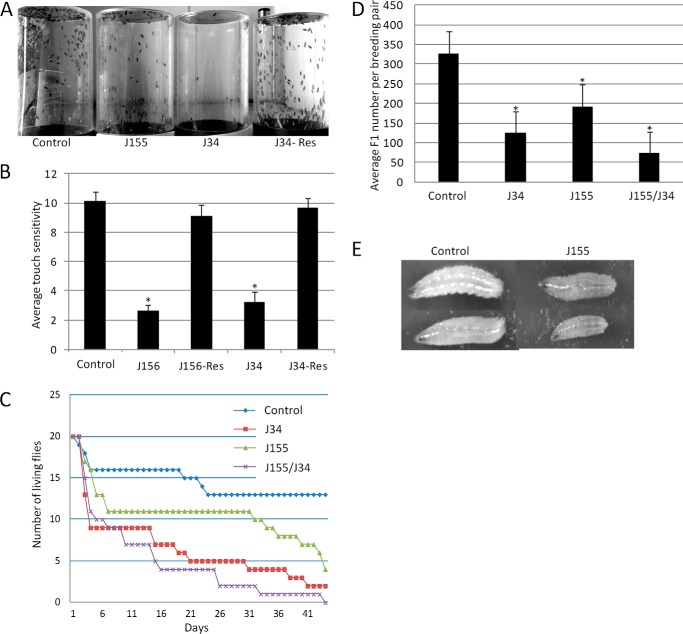
To begin to assess the in vivo functions of dSCCRO and its paralogues, we generated null mutants for all three family members in flies by mobilizing the P-elements inserted in or close to the transcription units; we then mapped the deletions by genomic sequencing. Two deletions (J34 and J156) were generated that lost the entire dSCCRO coding sequence without affecting its neighboring genes. A dSCCRO3 deletion (J155), which was generated using a similar approach, uncovered 204 amino acids, including the entire N-terminal and one-third of the PONY domain. Western blot analysis confirmed the absence of dSCCRO or dSCCRO3 expression in these deletions, compared with wild-type controls, confirming that they were null mutants (data not shown). Phenotypic analysis of the dSCCRO and dSCCRO3 mutants showed that they were fully viable, with no gross morphologic changes. Further analysis revealed that the dSCCRO mutants (J34 and J156) had behavioral changes (Fig. 3, A and B), significantly reduced life-span (Fig. 3C), and partial female infertility (Fig. 3D). The presence of infertility in female fly SCCRO mutants, in contrast to infertility in male mice, was not unexpected. Sex determination in mammals differs significantly from that in flies, with the default pathway for germ line sex determination changing from male in flies to female in mice. Consistent with this concept, previous studies have shown that genes that affect female germ cell development in flies affect male germ cell development in mice (30). In addition, dSCCRO3 mutants (J155) had significantly slower growth during embryonic and larval stages, although they caught up in size by the time of hatch (Fig. 3E). As the viability of the genomic deletion mutants was unexpected, to begin to determine whether viability resulted from compensation, we generated a double mutant by combining dSCCRO deletions on the third chromosome with dSCCRO3 deletions on the second chromosome. The dSCCRO/dSCCRO3 double mutant had a further reduction in life-span and increased infertility in females, suggesting that dSCCRO and dSCCRO3 may have overlapping activities in vivo (data not shown). Eight dSCCRO4 mutants were generated that contained large deletions whose break points could not be precisely mapped by genomic sequencing. All of the dSCCRO4 mutants had embryonic lethality. A complementation test among these mutants revealed that none of them could complement any others for lethality, suggesting that they uncover the same genomic region. However, because neighboring genes were also deleted, we cannot ascribe the lethality solely to the loss of dSCCRO4. Thus, the effects of the loss of all dSCCRO family members on development could not be assessed.
FIGURE 3.
A, images of glass jars showing upward migration of flies 30 min after flies were shaken to the bottom of the jar. Note the decrease in the migration of dSCCRO mutants (J34) but not dSCCRO3 mutants (J155). The migration defect in dSCCRO mutants could be rescued by re-expression of dSCCRO (j34-Res). B, graph showing results form touch sensitivity test (means ± S.E.). A significant decrease (asterisks indicate p < 0.01) in touch sensitivity was seen in two different dSCCRO mutants (J156 and J34) compared with control wild-type flies; this was rescued by re-expression of dSCCRO (J156-Res and J34-Res). C, graph showing actuarial survival in wild-type (Control) and mutant female flies (n = 20 for each). Survival was decreased in dSCCRO (J34) and dSCCRO3 (J155) mutant flies. Survival was further reduced in dSCCRO and SCCRO3 double mutants (J155/J34). D, graph showing results from breeding female mutants with wild-type males. A significant decrease (the asterisks indicates p < 0.05) in the number of F1 offspring was seen in both dSCCRO (J34) and dSCCRO3 (J155); double mutants (J155/J34) were increased. E, images showing representative same-age third instar larvae from control (wild-type) and dSCCRO3 mutant (J155) flies. Note the smaller size of the dSCCRO3 mutants.
Loss of dSCCRO Negatively Affects Cell Proliferation
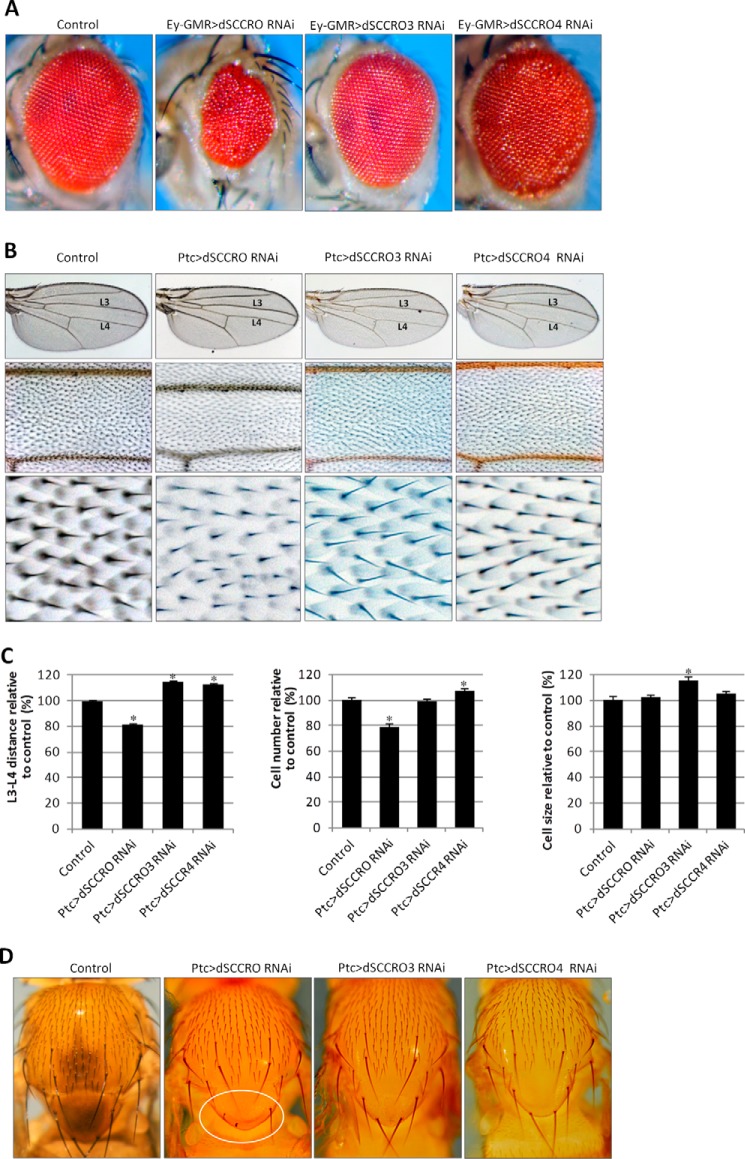
We have previously shown that loss of SCCRO in mice results in runting, likely due to defects in cell proliferation (20). However, body size was normal in dSCCRO mutants, raising questions about the role of SCCRO in regulating cell proliferation in flies. To determine whether the SCCRO role in cell proliferation is conserved, we assessed the effects of conditional SCCRO knockdown by RNAi using the Gal4/UAS system. As with the genomic deletions, expression of dSCCRO RNAi by Ubi-Gal4 had no effect on development or viability in two independent RNAi lines tested (28006 and 104190). In contrast, expression of the same RNAi lines under the control of the Act5c-Gal4 promoter resulted in lethality. Interestingly, we found that GFP is ubiquitously expressed when driven by Ubi-Gal4, but it was variably expressed under the control of the Act5c-Gal4 promoter, with the highest expression levels observed in the central and peripheral nervous systems (data not shown). These findings suggest that imbalanced, but not balanced, SCCRO expression causes detrimental effects during development. To determine the effects of differential SCCRO expression, we used Ey-GMR-Gal4 to drive expression of UAS-RNAi in eye primordial cells during the embryonic stages and in all the cells behind the morphogenic furrow during the larval stages. Knockdown of dSCCRO resulted in significantly smaller, rough eyes, with the absence of the entire eye in extreme cases (Fig. 4A). The absence of cell identity changes in eye discs, on the basis of immunostaining for the photoreceptor marker (Elav), and in cone and pigment cells (Pax2) suggests that dSCCRO regulates cell proliferation without affecting cell differentiation (data not shown). Furthermore, to confirm and quantify the effects of SCCRO on cell proliferation, we used Ptc-Gal4 to express RNAi against dSCCRO in the middle wing patch between veins L3 and L4. In this model, the presence of bristles for each cell allowed reliable quantification of total cell numbers. Determining the distance and total number of cells between veins L3 and L4 can be used to screen for changes in cell size and/or number. Knockdown of dSCCRO in the middle wing patch led to a significant reduction in the L3-L4 distance, due to a significant reduction in cell number, but there was no change in cell size or morphology (Fig. 4, B and C). In addition, macrochaetae were smaller or absent when dSCCRO was knocked down using the Ptc-Gal4 promoter (Fig. 4D).
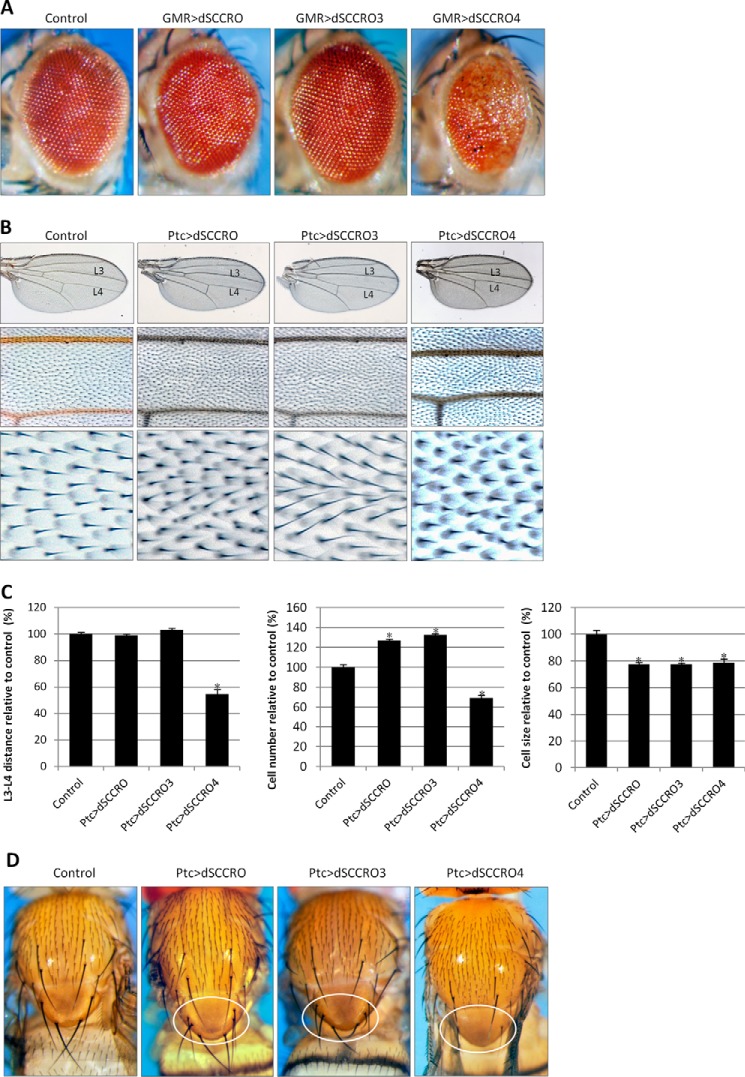
FIGURE 4.
A, representative images showing a magnified view of the eyes of control flies and flies expressing RNAi against the indicated dSCCRO paralogues. There is a decrease in size in flies expressing RNAi under the control of an eye-specific promoter (Ey-GMR-Gal4) against dSCCRO and dSCCRO3 and an increase in those expressing RNAi against dSCCRO4. Eyes from Ey-GMR-Gal4/− flies are shown as a control for these experiments. B, representative images of the whole wing (top row) and a magnified view of the middle wing patch (middle row) and cells from the middle wing patch (bottom row) from flies expressing Ptc>dSCCRO RNAi, Ptc>dSCCRO3 RNAi, or Ptc>dSCCRO4 RNAi, and controls (Ptc-Gal4/−). There is a decrease in the width of the middle wing patch in flies expressing RNAi against dSCCRO and an increase in those expressing RNAi against dSCCRO3 or dSCCRO4. C, bar graph showing the mean L3-L4 distance (left plot), cell number (middle plot), and cell size (right plot) in control vector and RNAi-expressing flies (lines indicate ± S.E.; asterisks indicate p ≤ 0.05). Changes in the middle wing patch are related to an increase in cell number but not cell size in flies expressing RNAi against dSCCRO, an increase in cell size but not cell number in flies expressing RNAi against dSCCRO3, and an increase in cell number but not cell size in flies expressing RNAi against dSCCRO4. D, representative magnified images showing macrochaetae on the notum from control and RNAi-expressing flies as indicated. The circled area in the second panel shows an area shortened and missing macrochaetae in flies expressing RNAi against dSCCRO.
Loss of dSCCRO3 Inhibits Proliferation, Whereas Loss of dSCCRO4 Promotes Proliferation
To determine whether the other dSCCRO paralogues also affect cell proliferation, we performed comparable analyses of tissue-specific RNAi knockdowns of dSCCRO3 and dSCCRO4 in the eye and the wing (Fig. 4). In contrast to knockdown of dSCCRO, knockdown of dSCCRO3 by Act5c-Gal4 or Ubi-Gal4 did not cause lethality (data not shown). This may be explained by the fact that dSCCRO3 is expressed in significantly fewer tissues during development compared with dSCCRO.7 However, Ey-GMR-Gal4-driven expression of dSCCRO3 RNAi resulted in a reduction in eye size, suggesting that, similar to dSCCRO, dSCCRO3 affects cell proliferation. Interestingly, in contrast to the role that dSCCRO3 plays in the eye, knocking it down led to an increase in the L3-L4 distance. Further analysis suggested that the increased L3-L4 distance was attributable to the significantly increased cell size (total cell number was not increased) (Fig. 4, B and C). However, we observed that, as with flies that expressed Ptc>dSCCRO RNAi, flies expressing Ptc>dSCCRO3 RNAi had smaller whole-wing sizes compared with wild-type controls. Similarly, Ptc>dSCCRO3RNAi knockdown of dSCCRO3 caused a slight decrease in the size of macrochaetae on nota (Fig. 4D). Combined, these data suggest that dSCCRO3 may regulate both cell size and cell proliferation.
General expression of dSCCRO4 RNAi by either Act5c-Gal4 or Ubi-Gal4 resulted in lethality during the late pupal stages, suggesting that dSCCRO4 may be essential for development. Interestingly, knockdown of dSCCRO4 by Ey-GMR-Gal4 resulted in larger, rough eyes (Fig. 4A). Similar to the findings in the eye, knockdown of dSCCRO4 in the wing caused a significant increase in the L3-L4 distance. Quantitative analyses revealed that the changes in the L3-L4 distance accompanying the conditional expression of dSCCRO4 RNAi were attributable to a change in cell number but not in cell size (Fig. 4C). Ptc>dSCCRO4 RNAi knockdown of dSCCRO4 caused an increase in the size of macrochaetae on nota (Fig. 4D). These findings suggest that dSCCRO4 negatively regulates cell proliferation.
Overexpression of dSCCRO or Its Paralogues Affects Proliferation
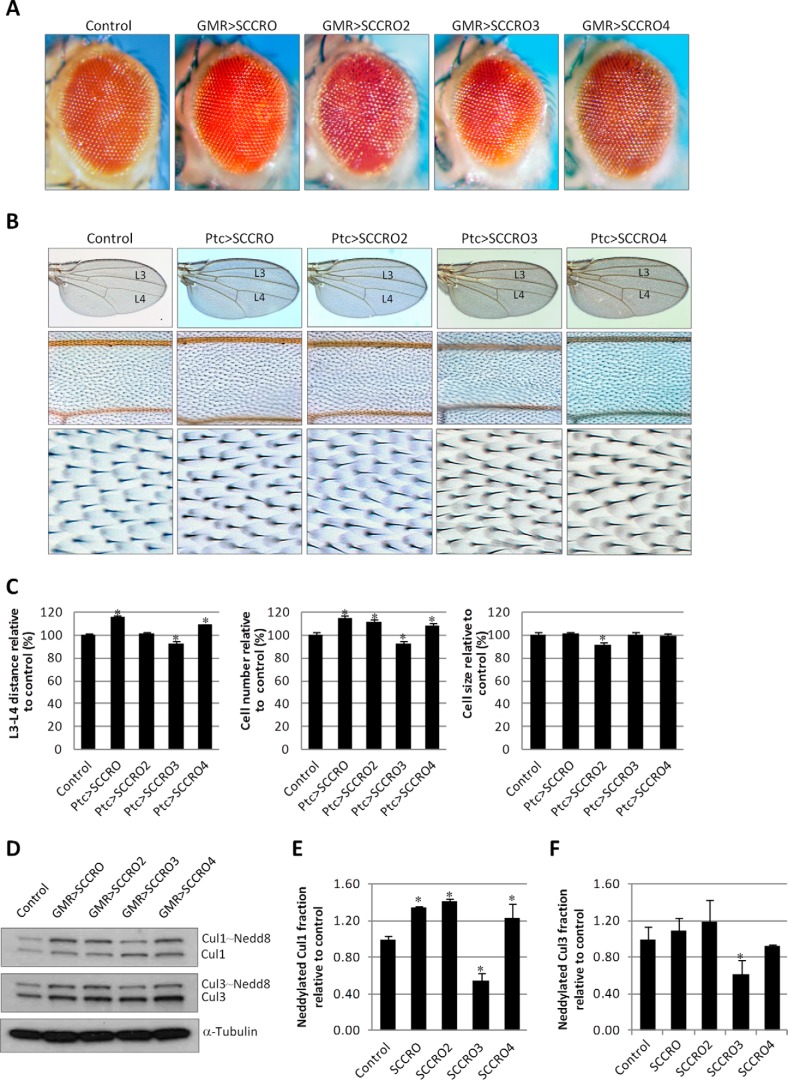
To confirm the role that dSCCRO and its paralogues play in cell proliferation, we overexpressed them in the eye and wing by use of Gal4 drivers (Fig. 5). Expression of a single copy of UAS-dSCCRO by use of the GMR-Gal4 promoter had no effect on eye development at room temperature and caused only slight roughness of the eye at 29 °C (data not shown). However, in flies with four copies of UAS-dSCCRO, eyes became markedly larger, bulging, and rougher (Fig. 5A). As with dSCCRO, overexpression of dSCCRO3 caused an increase in eye size in a dosage-dependent manner, with the most severe eye phenotype observed in flies with four copies of UAS-dSCCRO3 (Fig. 5A). In contrast, overexpression of one copy of UAS-dSCCRO4 driven by GMR-Gal4 resulted in smaller, rough eyes at room temperature (Fig. 5A). Increasing UAS-dSCCRO4 to four copies led to smaller eyes, with severe roughness and dark pigmentation on the surface of some ommatidia (Fig. 5A).
FIGURE 5.
A, representative images showing a magnified view of eyes from flies expressing four copies of the indicated constructs under the control of an eye-specific promoter (Ey-GMR-Gal4). Expression of dSCCRO and dSCCRO3 caused larger and rough eyes, whereas expression of dSCCRO4 caused smaller eyes. Eyes from Ey-GMR-Gal4/− flies are shown as a control for these experiments. B, representative images of the whole wing (top row) and a magnified view of the middle wing patch (middle row), and cells from the middle wing patch (bottom row) from flies expressing Ptc>2x UAS-dSCCRO, Ptc>2x UAS-dSCCRO3, Ptc>1x SCCRO4, or wild-type controls (Ptc-gal4/−). C, bar graph showing mean L3-L4 distance (left plot), cell number (middle plot), and cell size (right plot) in control vector and flies expressing dSCCRO and its paralogues (lines indicate ± S.E.; asterisks indicate p ≤ 0.05). Note that the increase in cell number associated with expression of dSCCRO and dSCCRO3 did not affect the distance between L3-L4, as it also resulted in a decrease in cell size. The decrease in the distance between L3 and L4 in flies expressing dSCCRO4 was due to a decrease in both the total number and size of cells. D, representative magnified images showing macrochaetae on the notum from flies expressing the indicated dSCCRO paralogues or controls. The circled areas show extra macrochaetae in dSCCRO- and dSCCRO3-expressing flies; there were missing macrochaetae in dSCCRO4-expressing flies.
To quantify the effects of overexpression of dSCCRO and its paralogues, we ectopically expressed them in the middle wing patch by use of Ptc-Gal4 (Fig. 5, B and C). We found that both dSCCRO and dSCCRO3 caused a significant increase in the cell number, with no change in the L3-L4 distance, due to significantly reduced cell size compared with the wild-type controls. In contrast, dSCCRO4 caused a decrease in the L3-L4 distance due to a reduction in cell number and cell size. Moreover, overexpression of both dSCCRO and dSCCRO3 by use of Ptc-Gal4 led to extra macrochaetae on nota, whereas overexpression of dSCCRO4 caused smaller and missing macrochaetae on nota (Fig. 5D). These results suggest that dSCCRO and dSCCRO3 function as positive regulators of cell proliferation, whereas dSCCRO4 functions as a negative regulator of both cell proliferation and cell growth in vivo.
dSCCRO and Its Paralogues Affect Cell Competition
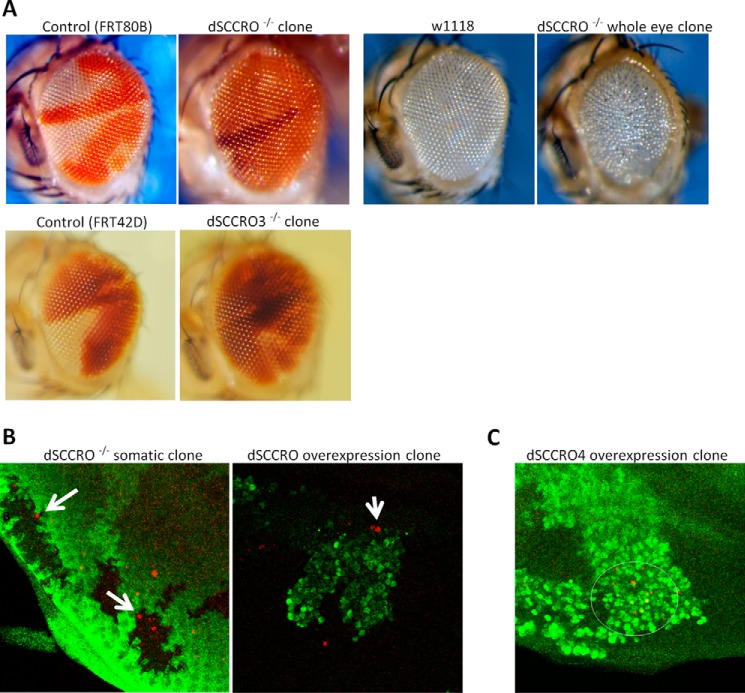
The finding that dSCCRO and its paralogues affect cell proliferation only when they are differentially expressed in vivo suggests that these genes may contribute to cell competition. To determine the presence of cell competition, we generated somatic clones in the adult eye by use of FRT chromosome recombination. This model allows direct comparison of growth differences between dSCCRO mutant cells and wild-type cells in a single organ in vivo. When dSCCRO function was intact in both clones in control flies, the number of the red (w+/+) and white (w−/−) clones were approximately equal (Fig. 6A). However, when dSCCRO mutant clones (w−/− dSCCRO−/−) were generated in the eye imaginal discs, their numbers were significantly reduced compared with wild-type twin spots (w+/+ dSCCRO+/+) (Fig. 6A). In fact, the majority of the SCCRO mutant clones were eventually eliminated from the adult eyes, and these eyes remained normal in size and morphologic appearance. Interestingly, when all of the wild-type clones were eliminated in the eye by GMR-Hid-promoted apoptosis, eyes in flies containing only dSCCRO mutant clones were smaller than those in wild-type flies, and they resembled the phenotype seen in flies with conditional knockdown of SCCRO in the eye by use of RNAi (Fig. 6A).
FIGURE 6.
A, representative images showing a magnified view of eyes with clones from flies of the FRT80B control, dSCCRO−/− (J34), wild-type control (w1118), and dSCCRO−/− (J34) with the whole eye clone, FRT42 control, and dSCCRO3−/− (J155). Control flies maintain an equal proportion of both clones (red and white). In contrast, both dSCCRO−/− and dSCCRO3−/− clones (white patch) are smaller than wild-type twin spots (dark red), suggesting that the mutant cells have a defect in proliferation (second panel). Although eye size is normal in dSCCRO−/− somatic clones, it is markedly reduced in dSCCRO−/− whole eye clones (compare with w1118 eye). B, representative fluorescent microscopic images showing Caspase 3 (red) and GFP (green) expression in dSCCRO−/− somatic clones (left) and dSCCRO overexpressing flip out clones (right). Note the presence of apoptotic cells (red) in dSCCRO−/− somatic GFP-negative clones that border SCCRO wild-type GFP-positive (green) clones. In the dSCCRO overexpression clones (right panel); however, apoptosis (red) is primarily present in GFP-negative cells, SCCRO wild-type cells that border SCCRO-overexpressing GFP-positive cells (green). C, fluorescent microscopic images showing Caspase 3 (red) and GFP (green) expression in dSCCRO4 flip out clones. Apoptotic (red) cells were primarily observed in cells overexpressing dSCCRO4 (GFP-positive cells).
Previous studies have shown that slowly dividing cells are promoted to undergo apoptosis by normally proliferating neighboring cells as a mechanism to preserve organ size (31–33). To assess for apoptosis in the dSCCRO mutant clones, we stained the eye discs carrying somatic mutant clones with anti-Casp3 antibody to allow identification of apoptotic cells. We found increased apoptosis in dSCCRO−/− clones, especially in cells at or close to clone boundaries (Fig. 6B). To confirm that dSCCRO and its paralogues contribute to cell competition, we generated dSCCRO and dSCCRO4 overexpression clones in the eye imaginal discs by use of the flip-out technique. This confers overexpression of the target gene in only a subset of cells from the same lineage in the eye along with GFP. When dSCCRO was overexpressed in the eye imaginal disc by use of the flip-out technique, staining for Caspase3 showed increased apoptosis in GFP-negative cells (with lower levels of dSCCRO) adjacent to GFP-positive cells (with higher levels of dSCCRO) (Fig. 6B). In contrast, when dSCCRO4 was overexpressed by use of the flip-out technique, staining for Caspase3 showed increased apoptosis in GFP-positive, dSCCRO4-overexpressing cells (Fig. 6C). Combined, these findings confirm that the effects that dSCCRO and its paralogues have on proliferation serve to promote cell competition in vivo.
SCCRO Family Members Have Overlapping, Antagonistic, and Independent Activity
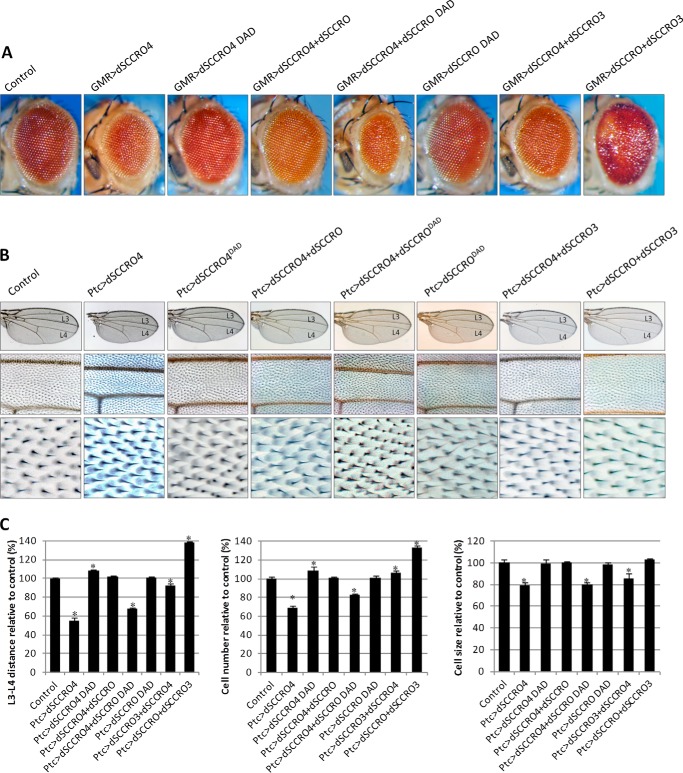
The findings on loss-of-function and gain-of-function mutant flies indicate that both dSCCRO and dSCCRO3 work as positive regulators, whereas dSCCRO4 functions as a negative regulator of cell proliferation in flies. To determine whether the family members work independently, cooperatively, and/or antagonistically in vivo, we performed a series of epistasis experiments in which dSCCRO and its paralogues were coexpressed in varying combinations in the eye by use of the GMR-Gal4 promoter (Fig. 7A). The small, rough eye accompanying dSCCRO4 overexpression was fully rescued by coexpression of dSCCRO, but not by dSCCRODAD, a mutant deficient in neddylation activity; this finding suggests that dSCCRO4 and dSCCRO work antagonistically in vivo. Interestingly, the eyes in which both dSCCRO4 and dSCCRODAD were overexpressed were even smaller than those in which only dSCCRO4 was overexpressed. This finding is consistent with the dominant negative activity of dSCCRODAD in vivo, which was observed in our previous work using mammalian models (20). Coexpression of dSCCRO3 only partially rescued the small-eye phenotype caused by overexpression of dSCCRO4, which suggests that dSCCRO3 may not be as potent as dSCCRO in vivo or that dSCCRO3 has nonoverlapping activities. Coexpression of dSCCRO and dSCCRO3 led to a more severe eye phenotype than that caused by expression of either dSCCRO or dSCCRO3 alone (Fig. 7A), suggesting that these proteins have cooperative and/or overlapping functions in vivo.
FIGURE 7.
A, representative images showing a magnified view of eyes from flies expressing the indicated SCCRO paralogues and selected neddylation-dead mutants alone or in combination under the control of an eye-specific promoter (Ey-GMR-Gal4). GMR-Gal4/− served as control flies for these experiments. Note the smaller eyes in dSCCRO4-overexpressing mutant clones but not dSCCRO-DAD mutant clones. Coexpression of dSCCRO, but not dSCCRO-DAD, rescued the size defect seen with dSCCRO4 expression. Coexpression of dSCCRO3 only partially rescued the size defect seen with dSCCRO4 expression. Coexpression of dSCCRO and dSCCRO3 caused larger, rough eyes. B, representative images of the whole wing (top row) and a magnified view of the middle wing patch (middle row), and cells from the middle wing patch (bottom row) from flies expressing dSCCRO paralogues or mutants and controls. C, bar graph showing mean L3-L4 distance (left plot), cell number (middle plot), and cell size (right plot) in control vector and RNAi-expressing flies (lines indicate ± S.E.; asterisks indicate p ≤ 0.05).
To validate our findings, we repeated these experiments using the Ptc-GAL4 wing system (Fig. 7B). Similar to the findings in the eye, narrowing of the L3-L4 distance caused by dSCCRO4 overexpression was fully rescued by coexpression of dSCCRO and dSCCRO3 but not the dSCCRODAD mutant. Interestingly, the decrease in cell size in the middle wing patch resulting from dSCCRO4 overexpression was rescued by coexpression of dSCCRO, but not dSCCRO3, which was associated with an increase in cell size. When dSCCRO and dSCCRO3 were coexpressed under the control of the Ptc-Gal4 promoter, the total number of cells was significantly increased compared with either dSCCRO or dSCCRO3 overexpression alone (Fig. 7C). These results suggest that in flies dSCCRO and its paralogues have cooperative, antagonistic, and independent activities that regulate cell proliferation and cell size.
SCCRO and Its Paralogues Regulate Cullin Neddylation
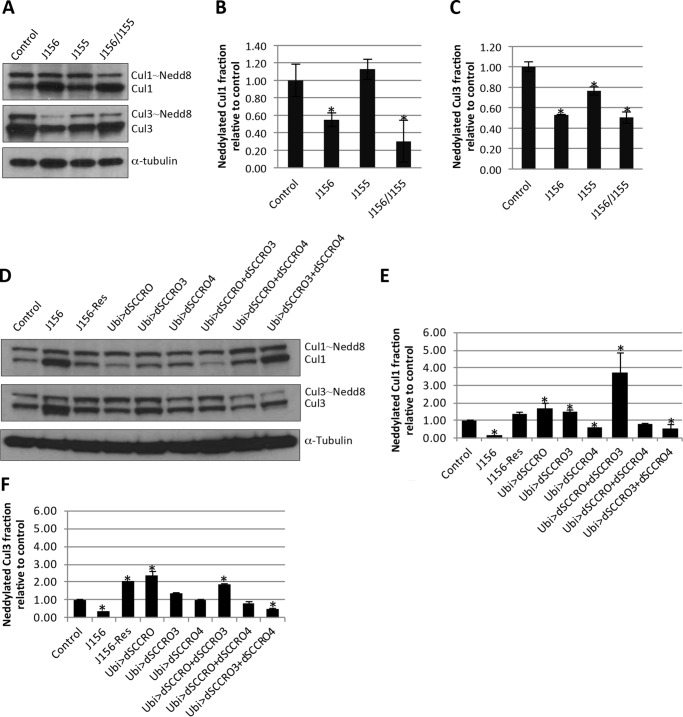
To assess the effects of SCCRO paralogues on neddylation activity, total protein lysates were prepared from third-instar larval eye discs and from brains from wild-type flies, genomic knock-out flies, or flies expressing extra copies of dSCCRO and/or its paralogues. Western blot analysis showed that the neddylated Cul1 and Cul3 fractions were smaller in lysates from dSCCRO (J156) loss-of-function genomic mutants than in lysates from wild-type controls (Fig. 8, A–C). The defect in cullin neddylation in dSCCRO mutants was rescued by re-expression of dSCCRO confirming that the observed decrease in cullin neddylation was attributable to loss of dSCCRO (J156-Res; Fig. 8, D–F). Although the neddylated Cul1 fraction was not reduced in the dSCCRO3 (J155) mutant, it was further reduced in lysates from flies in which both dSCCRO and dSCCRO3 were lost, suggesting that dSCCRO3 plays a minor or possibly a cooperative role in cullin neddylation in vivo (Fig. 8, A–C). Western blot analysis of lysates from the larval eye and brain tissues in which dSCCRO was overexpressed under the control of an ubiquitin promoter (Ubi>SCCRO) showed an increase in the neddylated Cul1 and Cul3 fractions. An increase in the neddylated Cul1 and Cul3 fractions was also observed in tissues with overexpression of dSCCRO3 (Ubi>SCCRO3), but the magnitude of the increase was less than that observed in tissues with overexpression of dSCCRO. Coexpression of SCCRO and SCCRO3 had an additive effect on the increase in the neddylated Cul1 and Cul3 fractions. In sharp contrast, overexpression of dSCCRO4 resulted in a decrease in the neddylated Cul1 fraction but not the neddylated Cul3 fraction (Fig. 8, D–F). Moreover, coexpression of dSCCRO and dSCCRO4 partially rescued the neddylation defect that resulted from dSCCRO4 overexpression alone (Fig. 8, D–F). To validate the role of SCCRO and its paralogues in neddylation, we generated mutations in the DAD patch of dSCCRO and its paralogues and expressed them under the GMR-Gal4 promoter. In contrast to expression of wild-type genes, the eyes of flies expressing DAD patch mutants of UAS-dSCCRO and its paralogues were phenotypically normal and had no change in the neddylated Cul1 or Cul3 fraction even when multiple copies of the gene were expressed (data not shown). Combined, these findings suggest that in flies dSCCRO family members regulate cullin neddylation, with dSCCRO and dSCCRO3 promoting, and dSCCRO4 inhibiting, neddylation and that the functional PONY domain is necessary for the in vivo activities of dSCCRO and its paralogues.
FIGURE 8.
A, Westerns blots for Cul1 and Cul3 on lysates from third instar larval eye discs and brains from genomic mutants of dSCCRO3 (J156), dSCCRO (J155), or both. B, bar graphs of the results from densitometry analyses using ImageJ software showing the ratio of neddylated:nonneddylated Cul1 relative to controls (lines indicate ± S.E.; the asterisk indicates p ≤ 0.05). C, bar graphs of results from densitometry analyses using ImageJ software showing the ratio of neddylated:nonneddylated Cul3 relative to controls (lines indicate ± S.E.; the asterisk indicates p ≤ 0.05). D, Western blots for Cul1 or Cul3 on lysates from third instar larval eye discs and brains from wild-type control, SCCRO deletion mutant (J156), SCCRO deletion mutant expressing genomic rescue construct (J156-Res), and indicated overexpression clones. E and F, bar graphs of the results from densitometry analyses using ImageJ software showing the ratio of neddylated:nonneddylated Cul1 or Cul3 relative to controls (lines indicate ± S.E.; the asterisk indicates p ≤ 0.05).
Mammalian SCCRO3 and SCCRO4 Paralogues Have Divergent Activities
In contrast to our findings in flies, we have previously shown that SCCRO3 antagonizes and SCCRO4 promotes neddylation activity in mammalian models (27). To assess their function in flies, we expressed human SCCRO, SCCRO2, SCCRO3, and SCCRO4 under the GMR-GAL4 and PTC-GAL4 promoters. Interestingly, we found that in both the eye and the wing middle patch, expression of SCCRO and SCCRO4 promoted cell proliferation, whereas expression of SCCRO3 resulted in a decrease in cell number and organ size (Fig. 9, A–C). These findings are identical to those observed in mammalian models (17, 20). To determine whether the effects on proliferative activity correlate with the effects on neddylation, we assessed the effects of expression of human SCCRO paralogues on the levels of neddylated Cul1 and Cul3 in flies. We found that overexpression of SCCRO, SCCRO2, and SCCRO4 increased and overexpression of SCCRO3 decreased the levels of neddylated Cul3 and, to a lesser extent, neddylated Cul1 (Fig. 9, D–F). Combined, these findings suggest that the function and the neddylation activity of SCCRO3 and SCCRO4 are reversed in flies and humans.
FIGURE 9.
A, representative images showing a magnified view of eyes from flies expressing the indicated human SCCRO paralogues under the control of an eye-specific promoter (Ey-GMR-Gal4) and control flies (Ey-GMR-Gal4/−). B, representative images of the whole wing (top row) and a magnified view of the middle wing patch (middle row) and cells from the middle wing patch (bottom row) from flies expressing one copy of human SCCRO paralogues and control (Ptc-Gal4/−). C, bar graph showing mean L3-L4 distance (left plot), cell number (middle plot), and cell size (right plot) in control vector and RNAi-expressing flies (lines indicate ± S.E.; asterisks indicate p ≤ 0.05). D, Western blot for Cul1 and Cul3 on lysates from eye imaginal discs and brains from flies with overexpression of human SCCRO and its paralogues, as indicated. E and F, bar graphs showing the ratio of neddylated to nonneddylated Cul1 or Cul3 relative to controls (lines indicate ± S.E.; asterisks indicate p ≤ 0.05).
Discussion
SCCRO functions as a component of the E3 for neddylation and is highly conserved from yeast to humans (19). The role of SCCRO in the neddylation E3 is to promote neddylation of cullins, which signals for assembly and activation of ubiquitination cullin-RING ligase-type E3 complexes (17, 18, 20). Thus, SCCRO putatively regulates the ubiquitination of multiple essential cellular proteins. The functional importance of SCCRO was established by the lethality resulting from its inactivation in C. elegans and yeast (19). It was, therefore, somewhat unexpected that targeted inactivation of SCCRO in mice did not result in lethality (17, 20). Although it is possible that SCCRO is not required for development in mice, the exclusive presence of closely related paralogues in higher organisms raises the prospect that they may compensate for the loss of SCCRO. In support of this hypothesis, severe defects in spermatogenesis were observed in SCCRO−/− mice; the testis was the only organ in which expression of SCCRO was disproportionately higher than expression of its paralogues. A decrease in cullin neddylation was consistently and exclusively present in the testis of SCCRO−/− mice, which could be rescued by the addition of SCCRO or SCCRO2, its most closely related paralogues. Combined, these findings suggest that SCCRO paralogues may have overlapping activities in higher organisms. However, the essential role that compartmentalization plays in the function of SCCRO and its paralogues confounds the significance of in vitro assays, making in vivo analyses irreplaceable for the assessment of functional interaction in vivo (34). The complexity of the genome and its manipulation in mice convolutes in vivo analyses of gene function in mice. The relative simplicity of genome manipulation in Drosophila flies combined with their established role in assessing the activity of neddylation components makes Drosophila flies the ideal model for assessing functional redundancy among SCCRO and its paralogues (35–38). In addition, the presence of a single homologue in each of the three SCCRO family subgroups facilitates genetic dissection of the pathway in flies.
A high degree of sequence conservation and the finding that paralogues of dSCCRO interact with cullins (by use of large scale yeast two-hybrid screens) suggest that their function is conserved in flies (39). Interestingly, genomic deletion of dSCCRO was not lethal in flies. Similar results were obtained with knockdown of dSCCRO under the control of a ubiquitously expressed promoter, which approximates the homogenic background seen in genomic deletion clones. Conversely, developmental defects or lethality was observed in the heterogenic environment created by RNAi knockdown of dSCCRO by use of conditional promoters. These findings suggest that dSCCRO may contribute to cell competition, a process in which unfit cells are eliminated during development, allowing normal cells to populate the organism (34). The role of dSCCRO in cell competition was validated in experiments using somatic clones, which showed near complete elimination of SCCRO-deficient cells caused by apoptosis during the development of targeted tissues. These findings suggest that cells deficient in SCCRO fail to compete with neighboring wild-type cells. Moreover, consistent with principles of cell competition, “loser” cells survive when surrounded by cells with the same genotype as seen with genomic deletion or global RNAi knockdown of dSCCRO. Furthermore, we observed that a competitive advantage was conferred to cells in which SCCRO was overexpressed. The findings in gain-of-function mutants suggest that dSCCRO may promote the generation of supercompetitors, as originally described for dmyc (40, 41). These initial findings would suggest a mechanism through which SCCRO promotes oncogenesis, with its overexpression putatively providing a survival advantage to neoplastic clones.
How does SCCRO promote cell competition? Cell competition was first described in cells heterozygous for minute mutations in Drosophila that have impairments in ribosome biogenesis and, consequently, protein synthesis (34, 42). After that discovery, multiple genes and pathways have been shown to promote cell competition, including several factors that regulate cell proliferation (31–33, 43, 44). Although the precise mechanisms remain to be defined, our findings in flies and mice suggest that SCCRO plays a role in the regulation of cell proliferation. Deregulation of dSCCRO did not cause changes in structure or organization, but it did cause significant changes in cell number in targeted organs and tissues. Conditional knockdown of SCCRO caused a decrease in cell number; overexpression caused an increase. Similar findings were observed in mice in which runting was related to decreased cell proliferation (20). Interestingly, defects in cell proliferation in both SCCRO-deficient flies and MEFs from SCCRO−/− mice could be rescued by expression of SCCRO, but not in neddylation-deficient mutants, suggesting that neddylation activity is required.
The results of our experiments in flies suggest that all SCCRO paralogues have an effect on proliferative activity. Interestingly, we found that the effects conferred by loss of dSCCRO3 and gain-of-function mutants on cell proliferation and competition overlap with but are less potent than those conferred by loss of dSCCRO. In contrast, dSCCRO4 had the opposite effect on proliferation; loss of dSCCRO4 led to increased proliferation, and its overexpression led to decreased proliferation. Moreover, expression of dSCCRO can salvage the phenotype in dSCCRO3 loss-of-function mutants, and their coinactivation or overexpression has additive effects on the phenotype. The phenotype in dSCCRO4-deficient flies was rescued by overexpression of dSCCRO or dSCCRO3, suggesting that dSCCRO4 may antagonize the function of dSCCRO and dSCCRO3. Furthermore, it appears that dSCCRO3 and dSCCRO4 affect not only cell proliferation but also cell size. Combined, these findings led us to propose that SCCRO paralogues have overlapping and/or antagonistic activities in vivo.
The central phenotypic effects of dSCCRO and its paralogues involve their roles in regulating cullin neddylation. The lack of phenotypic changes in flies expressing neddylation-dead mutants confirmed that neddylation-promoting activity is required for the in vivo activity of dSCCRO and its paralogues. In flies, dSCCRO4 functions as an antagonist of neddylation and proliferation in vivo. In contrast, it appears that both SCCRO4 and SCCRO5 promote neddylation and proliferation in mice and humans (24). Similarly, whereas dSCCRO3 promotes neddylation in flies, SCCRO3 appears to function as an antagonist of SCCRO neddylation activity in mice and humans (27). We found that in flies expression of human SCCRO3 results in a decrease in neddylated Cul1 and Cul3 and proliferative activity, whereas expression of human SCCRO4 increases these factors. Although the precise reasons for the difference in functions in flies and higher organisms are not clear, there are several possibilities. Although the PONY domain is highly conserved between the two, there is a significant difference in the N-terminal regions of dSCCRO4 and mammalian SCCRO4. Similarly, sequence analysis suggests that dSCCRO3 is only distantly related to its mammalian orthologue, which has a significantly shorter NORS (nonordered secondary structure) domain. Moreover, we previously reported that mammalian SCCRO3 does not conform to reaction processivity paradigms in its interactions with Ubc12 (27). In addition, SCCRO3 bound to an alternate E2 (UBE2F) with higher affinity than to UBC12 to promote neddylation (22). Interestingly, there is no orthologue for UBE2F in flies, which suggests that dSCCRO3 may have evolved to lose its interaction with Ubc12 in higher organisms. Moreover, even though their effects on neddylation are divergent, subcellular localization by the N-terminal domains of dSCCRO3 and dSCCRO4 is conserved in flies. Although the conservation of subcellular domain activity validates their functional importance, their precise role in regulating neddylation activity is not clear.
Combined, our findings establish the functional importance of SCCRO in development and demonstrate that SCCRO family members cooperatively regulate neddylation, which in turn affects cell proliferation and cell competition. Moreover,dSCCRO3 may be better classified as SCCRO4, whereas dSCCRO4 may be better classified as SCCRO3 on the basis of their effects on cellular and biochemical function. In addition, recent findings from our group and others suggest that dysregulation of this gene family plays an important role in cancer pathogenesis, especially in squamous cell carcinomas of the lung and head and neck. These findings suggest that SCCRO family members promote oncogenesis through the development of supercompetitors. Our analyses suggest that their effects on cullin neddylation are central to the activity of all SCCRO paralogues. However, the precise cullin-RING ligases and their ubiquitination targets through which SCCRO and its family members impart their cellular effects remain to be identified.
Author Contributions
B. S. supervised the entire project including conception and design of experiments, analysis and interpretation of data, and writing and final approval of the manuscript. T. V. and S. Y. R. helped with project conception and design of experiments and interpretation of data. G. H., A. K., and R. J. H. R. designed, performed, and analyzed the experiments shown in Figs. 1 and 2. W. F., J. S., and J. C. L. designed, performed, and analyzed the experiments shown in Figs. 3–9. All authors reviewed the results and approved the final version of the manuscript.
This work was supported by NCI, National Institutes of Health Grant P30 CA008748, NCI, National Institutes of Health Grant U54CA137788/U54CA132378 (to B. S. and T. V.), and a grant from Hackers for Hope (to B. S.). The authors declare they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
W. Fu, J. Sun, G. Huang, J. C. Liu, A. Kaufman, R. J. H. Ryan, S. Y. Ramanathan, T. Venkatesh, and B. Singh, unpublished data.
- SCCRO (Dcun1D1)
- squamous cell carcinoma-related oncogene
- dSCCRO
- Drosophila SCCRO
- PONY
- potentiation of neddylation.
References
- 1. Hershko A., and Ciechanover A. (1998) The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 [DOI] [PubMed] [Google Scholar]
- 2. Finley D., and Chau V. (1991) Ubiquitination. Annu. Rev. Cell Biol. 7, 25–69 [DOI] [PubMed] [Google Scholar]
- 3. Pickart C. M. (2001) Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70, 503–533 [DOI] [PubMed] [Google Scholar]
- 4. Berndsen C. E., and Wolberger C. (2014) New insights into ubiquitin E3 ligase mechanism. Nat. Struct. Mol. Biol. 21, 301–307 [DOI] [PubMed] [Google Scholar]
- 5. Osaka F., Saeki M., Katayama S., Aida N., Toh-E A., Kominami K., Toda T., Suzuki T., Chiba T., Tanaka K., and Kato S. (2000) Covalent modifier NEDD8 is essential for SCF ubiquitin-ligase in fission yeast. EMBO J. 19, 3475–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duda D. M., Borg L. A., Scott D. C., Hunt H. W., Hammel M., and Schulman B. A. (2008) Structural insights into NEDD8 activation of cullin-RING ligases: conformational control of conjugation. Cell 134, 995–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hori T., Osaka F., Chiba T., Miyamoto C., Okabayashi K., Shimbara N., Kato S., and Tanaka K. (1999) Covalent modification of all members of human cullin family proteins by NEDD8. Oncogene 18, 6829–6834 [DOI] [PubMed] [Google Scholar]
- 8. Kawakami T., Chiba T., Suzuki T., Iwai K., Yamanaka K., Minato N., Suzuki H., Shimbara N., Hidaka Y., Osaka F., Omata M., and Tanaka K. (2001) NEDD8 recruits E2-ubiquitin to SCF E3 ligase. EMBO J. 20, 4003–4012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morimoto M., Nishida T., Honda R., and Yasuda H. (2000) Modification of cullin-1 by ubiquitin-like protein Nedd8 enhances the activity of SCF(skp2) toward p27(kip1). Biochem. Biophys. Res. Commun. 270, 1093–1096 [DOI] [PubMed] [Google Scholar]
- 10. Morimoto M., Nishida T., Nagayama Y., and Yasuda H. (2003) Nedd8-modification of Cul1 is promoted by Roc1 as a Nedd8-E3 ligase and regulates its stability. Biochem. Biophys. Res. Commun. 301, 392–398 [DOI] [PubMed] [Google Scholar]
- 11. Ohh M., Kim W. Y., Moslehi J. J., Chen Y., Chau V., Read M. A., and Kaelin W. G. Jr. (2002) An intact NEDD8 pathway is required for Cullin-dependent ubiquitylation in mammalian cells. EMBO Rep. 3, 177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pan Z. Q., Kentsis A., Dias D. C., Yamoah K., and Wu K. (2004) Nedd8 on cullin: building an expressway to protein destruction. Oncogene 23, 1985–1997 [DOI] [PubMed] [Google Scholar]
- 13. Parry G., and Estelle M. (2004) Regulation of cullin-based ubiquitin ligases by the Nedd8/RUB ubiquitin-like proteins. Semin. Cell Dev. Biol. 15, 221–229 [DOI] [PubMed] [Google Scholar]
- 14. Saha A., and Deshaies R. J. (2008) Multimodal activation of the ubiquitin ligase SCF by Nedd8 conjugation. Mol. Cell 32, 21–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scott D. C., Sviderskiy V. O., Monda J. K., Lydeard J. R., Cho S. E., Harper J. W., and Schulman B. A. (2014) Structure of a RING E3 trapped in action reveals ligation mechanism for the ubiquitin-like protein NEDD8. Cell 157, 1671–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sarkaria I., O-charoenrat P., Talbot S. G., Reddy P. G., Ngai I., Maghami E., Patel K. N., Lee B., Yonekawa Y., Dudas M., Kaufman A., Ryan R., Ghossein R., Rao P. H., Stoffel A., Ramanathan Y., and Singh B. (2006) Squamous cell carcinoma related oncogene/DCUN1D1 is highly conserved and activated by amplification in squamous cell carcinomas. Cancer Res. 66, 9437–9444 [DOI] [PubMed] [Google Scholar]
- 17. Kim A. Y., Bommeljé C. C., Lee B. E., Yonekawa Y., Choi L., Morris L. G., Huang G., Kaufman A., Ryan R. J., Hao B., Ramanathan Y., and Singh B. (2008) SCCRO (DCUN1D1) is an essential component of the E3 complex for neddylation. J. Biol. Chem. 283, 33211–33220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurz T., Chou Y. C., Willems A. R., Meyer-Schaller N., Hecht M. L., Tyers M., Peter M., and Sicheri F. (2008) Dcn1 functions as a scaffold-type E3 ligase for cullin neddylation. Mol. Cell 29, 23–35 [DOI] [PubMed] [Google Scholar]
- 19. Kurz T., Ozlü N., Rudolf F., O'Rourke S. M., Luke B., Hofmann K., Hyman A. A., Bowerman B., and Peter M. (2005) The conserved protein DCN-1/Dcn1p is required for cullin neddylation in C. elegans and S. cerevisiae. Nature 435, 1257–1261 [DOI] [PubMed] [Google Scholar]
- 20. Huang G., Kaufman A. J., Ramanathan Y., and Singh B. (2011) SCCRO (DCUN1D1) promotes nuclear translocation and assembly of the neddylation E3 complex. J. Biol. Chem. 286, 10297–10304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scott D. C., Monda J. K., Grace C. R., Duda D. M., Kriwacki R. W., Kurz T., and Schulman B. A. (2010) A dual E3 mechanism for Rub1 ligation to Cdc53. Mol. Cell 39, 784–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monda J. K., Scott D. C., Miller D. J., Lydeard J., King D., Harper J. W., Bennett E. J., and Schulman B. A. (2013) Structural conservation of distinctive N-terminal acetylation-dependent interactions across a family of mammalian NEDD8 ligation enzymes. Structure 21, 42–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meyer-Schaller N., Chou Y. C., Sumara I., Martin D. D., Kurz T., Katheder N., Hofmann K., Berthiaume L. G., Sicheri F., and Peter M. (2009) The human Dcn1-like protein DCNL3 promotes Cul3 neddylation at membranes. Proc. Natl. Acad. Sci. U.S.A. 106, 12365–12370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bommeljé C. C., Weeda V. B., Huang G., Shah K., Bains S., Buss E., Shaha M., Gönen M., Ghossein R., Ramanathan S. Y., and Singh B. (2014) Oncogenic function of SCCRO5/DCUN1D5 requires its neddylation E3 activity and nuclear localization. Clin. Cancer Res. 20, 372–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma T., Shi T., Huang J., Wu L., Hu F., He P., Deng W., Gao P., Zhang Y., Song Q., Ma D., and Qiu X. (2008) DCUN1D3, a novel UVC-responsive gene that is involved in cell cycle progression and cell growth. Cancer Sci. 99, 2128–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu K., Yan H., Fang L., Wang X., Pfleger C., Jiang X., Huang L., and Pan Z. Q. (2011) Mono-ubiquitination drives nuclear export of the human Dcn1-like protein hDCNL1. J. Biol. Chem. 286, 34060–34070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang G., Stock C., Bommeljé C. C., Weeda V. B., Shah K., Bains S., Buss E., Shaha M., Rechler W., Ramanathan S. Y., and Singh B. (2014) SCCRO3 (DCUN1D3) antagonizes the neddylation and oncogenic activity of SCCRO (DCUN1D1). J. Biol. Chem. 289, 34728–34742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robertson H. M., Preston C. R., Phillis R. W., Johnson-Schlitz D. M., Benz W. K., and Engels W. R. (1988) A stable genomic source of P element transposase in Drosophila melanogaster. Genetics 118, 461–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Estilo C. L., O-Charoenrat P., Ngai I., Patel S. G., Reddy P. G., Dao S., Shaha A. R., Kraus D. H., Boyle J. O., Wong R. J., Pfister D. G., Huryn J. M., Zlotolow I. M., Shah J. P., and Singh B. (2003) The role of novel oncogenes squamous cell carcinoma-related oncogene and phosphatidylinositol 3-kinase p110α in squamous cell carcinoma of the oral tongue. Clin. Cancer Res. 9, 2300–2306 [PubMed] [Google Scholar]
- 30. Dai X., Schonbaum C., Degenstein L., Bai W., Mahowald A., and Fuchs E. (1998) The ovo gene required for cuticle formation and oogenesis in flies is involved in hair formation and spermatogenesis in mice. Genes Dev. 12, 3452–3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amoyel M., and Bach E. A. (2014) Cell competition: how to eliminate your neighbours. Development 141, 988–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Levayer R., and Moreno E. (2013) Mechanisms of cell competition: themes and variations. J. Cell Biol. 200, 689–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morata G., and Martín F. A. (2007) Cell competition: the embrace of death. Dev. Cell 13, 1–2 [DOI] [PubMed] [Google Scholar]
- 34. Lambertsson A. (1998) The minute genes in Drosophila and their molecular functions. Adv. Genet. 38, 69–134 [DOI] [PubMed] [Google Scholar]
- 35. Ou C. Y., Lin Y. F., Chen Y. J., and Chien C. T. (2002) Distinct protein degradation mechanisms mediated by Cul1 and Cul3 controlling Ci stability in Drosophila eye development. Genes Dev. 16, 2403–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu J. T., Lin H. C., Hu Y. C., and Chien C. T. (2005) Neddylation and deneddylation regulate Cul1 and Cul3 protein accumulation. Nat. Cell Biol. 7, 1014–1020 [DOI] [PubMed] [Google Scholar]
- 37. Reynolds P. J., Simms J. R., and Duronio R. J. (2008) Identifying determinants of cullin binding specificity among the three functionally different Drosophila melanogaster Roc proteins via domain swapping. PLoS ONE 3, e2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim S. H., Kim H. J., Kim S., and Yim J. (2010) Drosophila Cand1 regulates Cullin3-dependent E3 ligases by affecting the neddylation of Cullin3 and by controlling the stability of Cullin3 and adaptor protein. Dev. Biol. 346, 247–257 [DOI] [PubMed] [Google Scholar]
- 39. Giot L., Bader J. S., Brouwer C., Chaudhuri A., Kuang B., Li Y., Hao Y. L., Ooi C. E., Godwin B., Vitols E., Vijayadamodar G., Pochart P., Machineni H., Welsh M., Kong Y., Zerhusen B., Malcolm R., Varrone Z., Collis A., Minto M., Burgess S., McDaniel L., Stimpson E., Spriggs F., Williams J., Neurath K., Ioime N., Agee M., Voss E., Furtak K., Renzulli R., Aanensen N., Carrolla S., Bickelhaupt E., Lazovatsky Y., DaSilva A., Zhong J., Stanyon C. A., Finley R. L. Jr., White K. P., Braverman M., Jarvie T., Gold S., Leach M., Knight J., Shimkets R. A., McKenna M. P., Chant J., and Rothberg J. M. (2003) A protein interaction map of Drosophila melanogaster. Science 302, 1727–1736 [DOI] [PubMed] [Google Scholar]
- 40. de la Cova C, Abril M., Bellosta P, Gallant P, Johnston LA. (2004) Drosophila myc regulates organ size by inducing cell competition. Cell 117, 107–116 [DOI] [PubMed] [Google Scholar]
- 41. Moreno E., Basler K., and Morata G. (2002) Cells compete for decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature 416, 755–759 [DOI] [PubMed] [Google Scholar]
- 42. Morata G., and Lawrence P. A. (1975) Control of compartment development by the engrailed gene in Drosophila. Nature 255, 614–617 [DOI] [PubMed] [Google Scholar]
- 43. Moreno E., and Basler K. (2004) dMyc transforms cells into super-competitors. Cell 117, 117–129 [DOI] [PubMed] [Google Scholar]
- 44. Vincent J. P., Fletcher A. G., and Baena-Lopez L. A. (2013) Mechanisms and mechanics of cell competition in epithelia. Nat Rev. Mol. Cell Biol. 14, 581–591 [DOI] [PubMed] [Google Scholar]