Abstract
Human airway epithelial cells express pannexin 1 (Panx1) channels to release ATP, which regulates mucociliary clearance. Airway inflammation causes mucociliary dysfunction. Exposure of primary human airway epithelial cell cultures to IFN-γ for 48 h did not alter Panx1 protein expression but significantly decreased ATP release in response to hypotonic stress. The IFN-γ-induced functional down-regulation of Panx1 was due to the up-regulation of dual oxidase 2 (Duox2). Duox2 suppression by siRNA led to an increase in ATP release in control cells and restoration of ATP release in cells treated with IFN-γ. Both effects were reduced by the pannexin inhibitor probenecid. Duox2 up-regulation stoichiometrically increases H2O2 and proton production. H2O2 inhibited Panx1 function temporarily by formation of disulfide bonds at the thiol group of its terminal cysteine. Long-term exposure to H2O2, however, had no inhibitory effect. To assess the role of cellular acidification upon IFN-γ treatment, fully differentiated airway epithelial cells were exposed to ammonium chloride to alkalinize the cytosol. This led to a 2-fold increase in ATP release in cells treated with IFN-γ that was also inhibited by probenecid. Duox2 knockdown also partially corrected IFN-γ-mediated acidification. The direct correlation between intracellular pH and Panx1 open probability was shown in oocytes. Therefore, airway epithelial cells release less ATP in response to hypotonic stress in an inflammatory environment (IFN-γ exposure). Decreased Panx1 function is a response to cell acidification mediated by IFN-γ-induced up-regulation of Duox2, representing a novel mechanism for mucociliary dysfunction in inflammatory airway diseases.
Keywords: acidosis, ATP, epithelial cell, hydrogen peroxide, pannexin, dual oxidase 2
Introduction
ATP is a key regulator of the innate pulmonary host defense by activating purinergic P2Y2 receptors, which promote chloride secretion by calcium-activated Cl− channels, inhibit Na+ absorption by epithelial Na+ channels, increase ciliary beat frequency and airway surface liquid volume, and induce mucin release, thereby activating mucociliary clearance (1–4).
Although the effects of ATP on airway epithelial cells have been studied widely, Panx1 channels have only been recognized recently to be involved in ATP release in these tissues (5, 6). Mechanical stress has been shown to be one of the prime stimuli to increase the ATP concentration on the airway surface to a concentration sufficient to activate P2Y2 receptors (7). This ATP release was neither dependent on the intracellular calcium concentration, excluding an exocytotic release mechanism, nor caused by the cystic fibrosis transmembrane conductance regulator. In previous studies, we were the first to show that Panx1, an ortholog of the invertebrate innexin, is expressed at the apical membrane of airway epithelia, contributing to ATP release (5).
Pannexin proteins form pannexons, which are channels that open at resting membrane potential because of mechanical stress and in response to extracellular ATP when co-expressed with P2Y2 receptors (8, 9). In addition to stimulated ATP release, resting ATP levels on the apical surface reflect a steady state that, depending on its range, can help autoregulate the release or metabolism of ATP.
Currently little is known about the physiological regulation of pannexin channels in the airway epithelium. Most studies have been performed on oocytes or transfected HEK cells. These studies demonstrated that ATP itself is a potent regulator by increasing the permeability of Panx1 initially when co-expressed with P2Y2 receptors but, consequently, causing inhibition as a feedback loop (10, 11).
The cytokine IFN-γ is produced in inflammatory airway diseases such as severe asthma (12, 13) and chronic bronchitis with and without airflow obstruction (14). We have shown that IFN-γ can increase H2O2 production via increased expression of Duox2, a member of the NADPH oxidase gene family, in ALI2 cultures (15, 16). In addition to H2O2, Duox also releases cytosolic H+ (17), which could contribute to intracellular acidification. Consequently, a decrease in intracellular pH could decrease mucociliary function by direct inhibition of the ciliary beat frequency (18). In fact, Duox is up-regulated in chronic bronchitis and patients with smoke exposure (15, 19, 20). Preliminary data also suggested that intracellular pH is essential in regulating pannexin channels, pointing to an important regulatory role of pannexin channels in the host defense of airway epithelia.
The data presented here reveal novel regulation mechanisms demonstrating that IFN-γ exposure of airway epithelial cells decreases ATP release by inhibition of Panx1 channels. The inhibition is mediated by Duox2 and caused by intracellular acidification. H2O2 causes a temporary inhibition via oxidation of a terminal cysteine.
Experimental Procedures
Chemicals and Solutions
Unless stated otherwise, all materials were purchased from Sigma-Aldrich (St. Louis, MO).
ALI Cell Culture
Human airways were obtained from organ donors whose lungs were rejected for transplant. Institutional review board-approved consent for research was obtained by the Life Alliance Organ Recovery Agency of the University of Miami or the LifeCenter Northwest and conformed to the Declaration of Helsinki. Airway epithelial cells were isolated and dedifferentiated through expansion. Passage 1 cells were redifferentiated at an ALI on collagen-coated 12- or 24-mm-diameter inserts (Costar, Corning, NY) as described previously (21–24).
RNA Extraction and Quantitative RT-PCR
Total RNA was extracted from ALI-cultured human airway epithelial cells using the RNeasy Protect mini kit (Qiagen, Valencia, CA). Reverse transcription was done using the iScript cDNA synthesis kit (Bio-Rad) with 1 μg of RNA according to the instructions of the manufacturer. Real-time quantitative PCR was performed using the following TaqMan probes: Hs00209790_m1 for Panx1, Hs00213694_m1 for Duox1, Hs00204187_m1 for Duox2, Hs00167309_m1 for SOD2, and Hs02758991_g1 for GAPDH.
Western Blotting
ALI-cultured human airway epithelial cells were lysed in radioimmune precipitation assay buffer containing protease inhibitors. Protein yield was measured by BCA assay (Pierce). Proteins were separated on a 4–20% precast Ready Gel (Bio-Rad) and blotted onto Immobilon-P membranes (Millipore, Billerica, MA). Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline (pH 7.4) with 0.05% Tween 20 (TTBS) for 1 h. Primary antibodies were as follows: rabbit anti-Panx1 antibody (catalog no. 488100, lot no. 1493629A, Invitrogen), used as prescribed previously (5); mouse anti-Duox1 and anti-Duox2 (catalog no. sc-393096, lot no. E2014, and catalog no.. sc-398681, lot no. G3014, Santa Cruz Biotechnology, Dallas, TX); and mouse anti-β-actin antibody (catalog no. A2228, lot no. 123M4887V, Sigma-Aldrich). Secondary antibody was an anti-rabbit (catalog no. 474-1506, lot no. 10147551, KPL, Gaithersburg, MD) or anti-mouse (catalog no. 474-1806, lot no. 10148784, KPL) horseradish peroxidase-linked antibody used at 1:5000 in TTBS for 1 h at room temperature. Positive signals were visualized by chemiluminescence on a ChemiDoc XRS system (Bio-Rad).
Hypotonic Stress-induced ATP Release Assay
Culture media in the basolateral compartment were replaced with media containing IFN-γ (100 ng/ml) or vehicle (distilled H2O), and the cultures were incubated for 48 h at 37 °C. Media were removed, and the filters were transferred to a Turner TD 20/20 luminometer (Turner Biosystems, Sunnyvale, CA), where 26 μl of a luciferin (150 mm) and luciferase (2.0 mg) solution was added gently to the apical compartment. The culture was allowed to equilibrate for 100 s to re-establish basal conditions, and then (time 0), 15 μl of a hypotonic solution (H2O with 1 mm CaCl2 and 1 mm MgCl2) was added to the apical compartment, causing hypotonic stress for an additional 200 s, as described previously (5, 7). ATP release was recorded in arbitrary light units every 0.2 s. ATP release was reported as peak ATP response upon hypotonic stress, with data given as fold increases (or decreases) over the appropriate control increase (measured as the difference between baseline and peak arbitrary light units).
Voltage Clamp Experiments in Oocytes
An mRNA for mouse Panx1 was prepared using the mMessage in vitro transcription kit (Invitrogen) (20). Oocytes were injected with 20–40 nl of mRNA (1 mg/ml) and incubated for 18–48 h at 18 °C. Oocytes were tested using a two-electrode voltage clamp (model OC725C, Warner Instruments, Hamden, CT) under constant perfusion with solutions containing sodium acetate for acidification. Oocytes expressing Panx1 were held at −60 mV, and pulses to +10 mV were applied at a rate of 0.1 Hz to transiently open the channels.
Patch Clamp Experiments in ALI Cultures
The currents were recorded in intact, fully differentiated normal human bronchial epithelial (NHBE) cells in a cell-attached patch configuration using glass patch pipettes of 1–3 μm diameter and of 2.5–4 MΩ resistance. The bath and pipette solution contained 1 mm KCl, 10 mm HEPES, 2 mm CaCl2 and 99 mm NaCl (pH 7.1). The currents were elicited by a symmetric voltage ramp between −60 to +140 mV and returned to −60 mV. The speed of the ramp was 0.4 mV/s. The analog signal was filtered before digitalization with an eight-pole low-pass Bessel filter with a cutoff frequency of 1/5 of the conversion frequency. Experiments were performed at room temperature (25 °C). Data were collected with an Axopatch 200B (Molecular Devices, Sunnyvale, CA) and sampled with a Digidata 1322A interface. The acquisition and basic analysis of data were performed with pClamp 9.2 (Axon Instruments, Inc.)
Intracellular pH Measurement
Intracellular pH (pHi) was measured as described previously (18). Briefly, ALI cultures were loaded with 2.5 μm 2′,7′-bis(2-carboxyethyl)-5,6-carboxyfluorescein acetoxymethyl ester (BCECF)-AM for 45 min at 37 °C and 5% CO2 and rinsed three times. Then filters were cut out of the inserts and mounted in a closed bath imaging perfusion chamber (Warner Instruments, large diamond, RC-21B). All solutions were perfused at a rate of 250 μl/min. For fluorescence measurements, a Lambda DG4 excitation system (Sutter, Novato, CA) was used, with 10-nm-wide excitation filters centered on 495 and 440 nm (Chroma Technology Corp., Brattleboro, VT). At the end of these experiments, the system was perfused with a high K+ HEPES solution with 15 μm of nigericin equilibrated at pH 7.2. The calibration of pH measurements was done according to methods described previously (18).
Knockdown of Duox2 Using Lentiviruses
pLKO.1-Puro plasmids encoding non-targeting or Duox2 shRNA (catalog nos. SHC002 and TRCN0000045963, respectively, Sigma-Aldrich) were amplified. The virus was produced as described previously (15). Briefly, HEK293T cells were co-transfected using third-generation replication-defective lentiviral vectors and packaging plasmids. The viral yield was quantified using a p24 ELISA assay (PerkinElmer Life Sciences). Dedifferentiated NHBE cells from four lung donors were infected with 40 ng/2 × 105 cells of Duox2 shRNA or non-targeting lentiviruses in suspension and selected with 1 μg/ml puromycin. After growth to confluency, cultures were redifferentiated at the ALI for 4–6 weeks, at which point cells exhibited mucus production and beating cilia. mRNA levels were assayed by real-time PCR using TaqMan kits as described previously (15), and Western blotting was done as above. We have been successful in knocking down several proteins in fully differentiated airway epithelial cells (5, 25, 26).
Statistics
Statistical analysis was performed by Student's t test and analysis of variance or Kruskal Wallis test with appropriate post tests for at least three independent experiments, with significance accepted at p < 0.05.
Results
Patch Clamp Confirmation of Panx1 in ALI Cultures
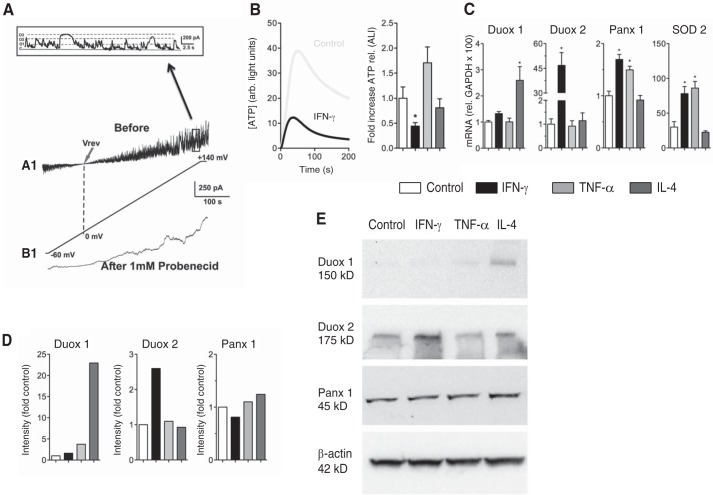
Patch clamp recordings of macroscopic currents of fully differentiated ALI cultures in the cell-attached configuration confirmed Panx1 currents by stimulation with positive membrane potentials (Fig. 1, A and A1). The high single-channel conductance recorded is typical for Panx1. Furthermore, probenecid, an inhibitor of Panx1, caused significant inhibition of the currents (Fig. 1, A and B1). Probenecid discriminates pannexins from connexins. Other known targets include transporters. Although we cannot rule out that probenecid inhibits other channels, it represents a useful tool for studying Panx1 function.
FIGURE 1.
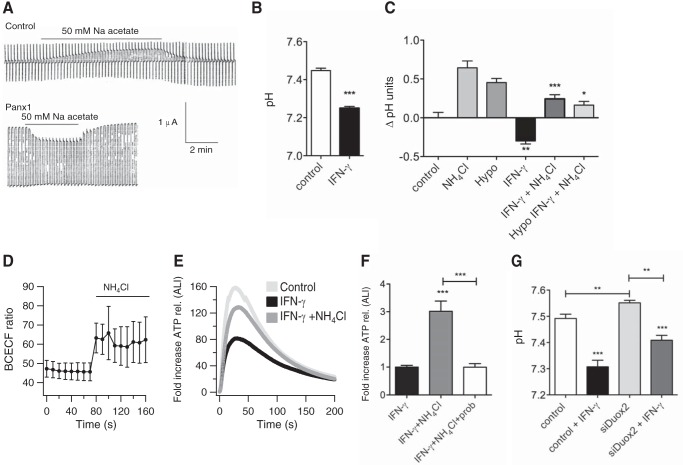
Effect of IFN-γ on hypotonic stress-induced ATP release. A, functional expression and regulation of Panx1 channels in intact (non-trypsinized) NHBE cells cultured at the air-liquid interface (n = 1). A1, ion current flowing through pannexin channels in response to a voltage ramp from −60 to +140 mV. B1, the same patch after 5-min incubation with 1 mm probenecid under the same voltage conditions. Inset, a 25-s period of the trace in A1 (before the probenecid effect) showing the single-channel activity. The variance in the open level corresponds to the subconductance state of the pannexin channel. Vrev, reversal potential. B, left panel, average traces (n = 5 lung donors, 3 replicates each) of ATP release of NHBE cells ± 48 h of preincubation with IFN-γ (100 ng/ml). Hypotonic stress started at time 0. Right panel, mean ± S.E. of -fold increases of ATP release (peak release) after 48 h of different stimuli relative to the control. IFN-γ (100 ng/ml for 48 h); TNF-α (10 ng/ml for 48 h); IL-4 (10 ng/ml for 48 h); all n = 5 lung donors, 3 replicates each; *, p < 0.05. C, mean ± S.E. of mRNA expression (relative to GAPDH × 100) after the same stimuli as shown in B. Shown are data for Duox1, Duox2, Panx1, and SOD2. n = 4 lungs, 3 replicates each; *, p < 0.05. D, quantification Western blotting analyses of samples from three different lung donors, pooled and analyzed in duplicates. A representative pooled sample is shown in E. Intensity was corrected for β-actin and set to 1 for control. E, representative Western blotting for the quantification shown in D for Duox1 and Duox2, pannexin 1 (Panx1), and β-actin.
IFN-γ Reduces Hypotonic Stress-induced ATP Release in Normal Bronchial Epithelial Cells
ALI cultures were exposed basolaterally to 100 ng/ml IFN-γ for 48 h and subjected to hypotonic stress (200 mosmol), then ATP release was assessed as described previously (5, 7). Our results demonstrated that ALI cultures exposed to IFN-γ showed a statistically significant decrease in ATP release compared with control cells (Fig. 1B). INF-γ exposure (100 ng/ml for 48 h) was related to an increase in Duox2 mRNA and protein expression (Fig. 1, C–E). On the other hand, INF-γ exposure did not change Duox1 mRNA or protein expression. Although Panx1 mRNA increased with INF-γ, Panx1 protein did not change (Fig. 1, C–E). In addition, we have shown before that IFN-γ does not change the mRNA levels of the NAPDH oxidases Nox1, 2, 4, and 5, possible off-target oxidases (15).
To show the specificity of INF-γ-induced Duox2 up-regulation, we used other proinflammatory stimuli. TNF-α (10 ng/ml for 48 h), as shown before (19), did not change Duox1 or Duox2 mRNA or protein expression (Fig. 1, C–E). Even though there was a trend for an increased ATP release (Fig. 1B, right panel), it did not reach statistical significance. An increase in superoxide dismutase 2 (SOD2) mRNA (Fig. 1C) was not only seen with TNF-α but also with IFN-γ. Because TNF-α increased ATP release and IFN-γ decreased it (Fig. 1B, right panel), SOD2 was not related to changes in ATP release. IL-4 (10 ng/ml for 48 h) increased expression of Duox1 mRNA and protein (Fig. 1, C–E), as shown previously (19), but did not change ATP release (Fig. 1B, right panel).
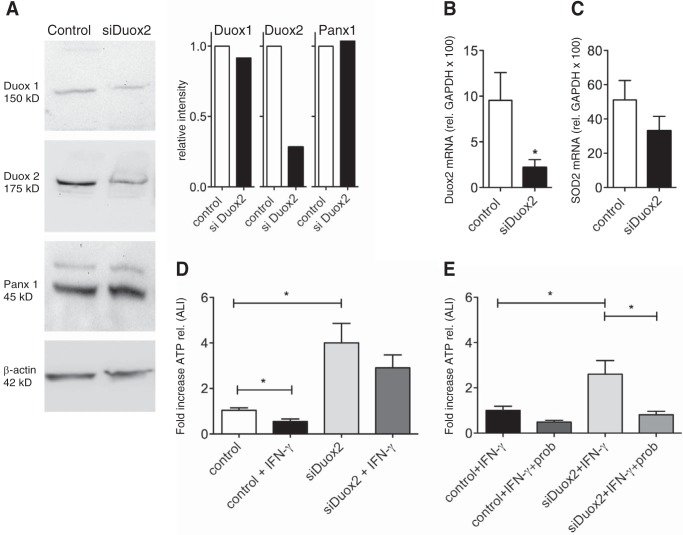
Suppression of Duox2 by shRNA Results in an Increase in ATP Release in ALI Cultures with and without IFN-γ Pretreatment
ALI cultures were infected with Duox2 shRNA or non-targeting control lentiviruses and selected with puromycin as described previously (15). After full differentiation (4–6 weeks at ALI), cultures were stimulated with IFN-γ for 48 h. ATP release was then assessed at baseline and after hypotonic challenge. Cells infected with siDuox2 showed significantly decreased protein (Fig. 2A) and mRNA levels of Duox2 (Fig. 2B) but not SOD2 (Fig. 2C). As shown before, IFN-γ caused a decrease in ATP release in non-targeting, shRNA-infected control cells. Cells infected with siDuox2, however, showed a significant increase in ATP release even at baseline. More importantly, siDuox2 attenuated the decrease in ATP release after IFN-γ pretreatment (Fig. 2D). The increased ATP release was blocked by probenecid, indicating the involvement of Panx1 and not another mechanism (Fig. 2E). Although we have shown previously that Duox2 shRNA can increase Nox4 mRNA (15), this increase would not explain the rescued ATP release if associated with H2O2 production.
FIGURE 2.
Suppression of Duox2 by lentiviral infection with siDuox2. A, representative Western blotting (pooled from three lung donors) and protein quantification (corrected for β-actin, pooled samples from three donors and run in duplicate) for Duox 1 and 2, pannexin 1 (Panx1), and β-actin after infection with control plasmid or shRNA for Duox2 (see “Experimental Procedures”). Duox2 protein is down-regulated but Duox1 and Panx1 are not. There is a minor band in the panx1 blots. Exclusion or inclusion of this band in the quantification did not make a difference. B and C, mean ± S.E. of mRNA expression (relative to GAPDH × 100) after infection with control plasmid or shRNA for Duox2 (see “Experimental Procedures”). Duox2 mRNA is down-regulated (B, n = 3 lung donors, 3 replicates each) but SOD 2 is not (C, n = 3 lung donors, 3 replicates each). D and E, Duox2 shRNA eliminates the IFN-γ effect on ATP release and leads to an increase in ATP release at baseline. The sustained ATP release in cells expressing siDuox2 can be inhibited by probenecid (E), indicating that this ATP release occurs through Panx1. Data are mean ± S.E. of -fold increases of ATP release (peak release) of control (non-targeting shRNA) or Duox2 shRNA-infected cells ± IFN-γ ± probenecid. n = 3 lung donors, 3 replicates each. *, p < 0.05.
H2O2 Modulates Pannexon Currents but Is Not Responsible for the IFN-γ Effect
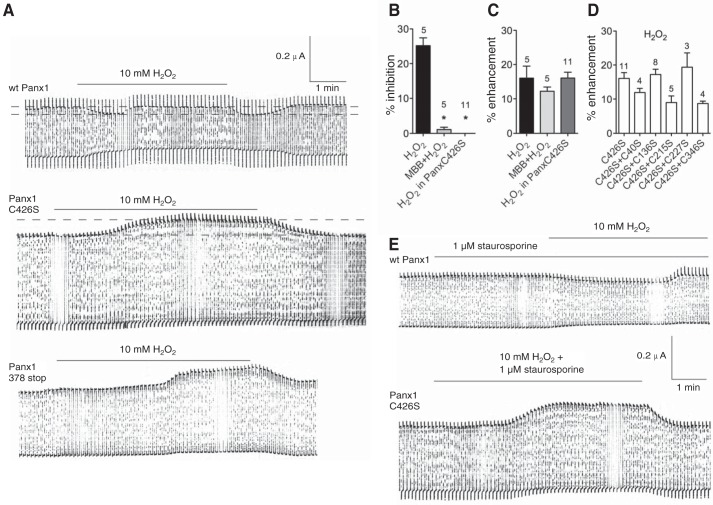
Duox2 produces H2O2 and, simultaneously, an equal amount of H+. The prime function of Duox2 is the generation of extracellular H2O2. Therefore, the effect of H2O2 on pannexons, independent of pHi, was tested in Xenopus oocytes expressing WT Panx1 and a series of cysteine replacement mutants. Panx1 currents were measured by holding the oocytes at −60 mV and applying pulses to +10 mV at a rate of 0.1 Hz to transiently open the channels (27). Oocytes have a lower H2O2 permeability than most other cells, and, therefore, require higher extracellular H2O2 concentrations to obtain cytoplasmic effects (28–30). The reason is most likely the lower aquaporin expression in oocytes. Aquaporins are considered the main pathway for bringing H2O2 through membranes (31, 32). Control plasmids were used by us before, with no effects on the results (e.g. Ref. 33).
H2O2 (10 mm) affected pannexons in a complex way (Fig. 3A). Initially, the currents were attenuated after extracellular application of H2O2. While H2O2 was still present, the currents increased, sometimes exceeding the pretreatment levels. After washout, the currents declined to the levels of maximal inhibition to return to pretreatment levels. Reaction of the (cytoplasmic) terminal cysteine (Cys-426) of Panx1 with the thiol reagent maleimidobutyryl-biocytin prevented the attenuation of the pannexon currents by H2O2 but did not affect the current increase (Fig. 3, B and C). The increase occurred with a similar delay of minutes after the application of H2O2 and led to similar increases of the currents above the original current levels observed for the recovery from H2O2-induced inhibition.
FIGURE 3.
Electrophysiological recordings of membrane currents of an oocyte expressing Panx1. A, membrane currents were measured of oocytes expressing WT Panx1, the replacement mutant Panx1 C426S, or the truncation mutant 378stop. Oocytes were held at −60 mV, and pulses to +10 mV were applied at a rate of 0.1 Hz to transiently open the channels. Application of 10 mm H2O2 resulted in a biphasic response in WT Panx1, where an initial attenuation was followed by recovery to base levels or even a small increase of the currents. Following washout of H2O2, the currents dropped to the level of maximal inhibition but then recovered to the level seen before the interference. With both Panx1 mutants, only an increase in current amplitude was observed that reversed upon washout of H2O2. B and C, quantitative analysis of the inhibitory (B) and enhancing (C) components of the H2O2 effect on currents in oocytes expressing WT Panx1 or Panx1C426S. The inhibitory component was attenuated by pretreatment of the oocytes with 1 mm maleimidobutylyl-biocytin (MBB), known to react with the terminal cysteine Cys-426 of Panx1 (33). Replacement of this cysteine by serine abolished (C426S) the inhibitory component, revealing the full extent of amplification of the currents by H2O2. Percent inhibition or enhancement was determined on the basis of current measurements indicated by the dotted lines in A. Numbers indicate the number of oocytes used. D, series of double cysteine mutants. Because no inhibition was seen, only the enhancement is shown. *, p < 0.05. In C and D, no statistically significant differences were found. E, inhibition of phosphorylation by staurosporine does not affect inhibition or enhancement of pannexin currents in oocytes expressing WT Panx1 or the mutant Panx1C426S.
An extensive cysteine scan has been performed by us before (33). The terminal cysteine Cys-426 was the only one in wild-type Panx1 to react with thiol reagents, including maleimidobutyryl-biocytin. Therefore, replacement of the terminal cysteine by serine was examined. H2O2 did not inhibit pannexon currents but increased them with a delay after H2O2 application (Fig. 3, A–C). Double cysteine replacement, combining C426S with either C40S, C136S, C215S, C227S, or C346S, eliminated the attenuation but not the enhancement of pannexon currents (Fig. 3D). Neither inhibition nor enhancement of the pannexon currents were affected by staurosporine, a nonspecific inhibitor of protein kinases (Fig. 3E).
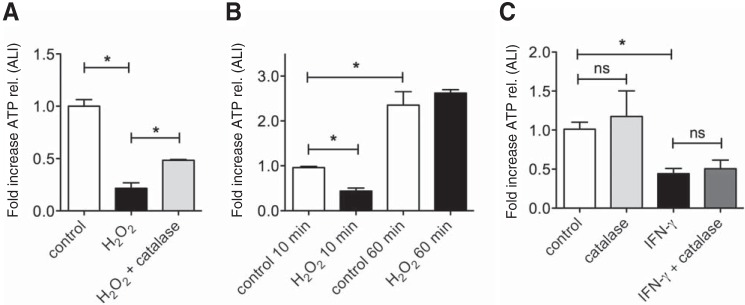
Because Panx1 is constitutively expressed in NHBE cells, experiments with mutants were limited to oocytes. However, similar results were obtained in NHBE cells using H2O2. Duox2 can create at least a concentration of 150 μm H2O2 in the apical fluid above airway cells, and 0.5 mm is a reasonable maximum (16). Therefore, we used 0.5 mm H2O2 for our human cell experiments. A short-term (<10 min) exposure to 0.5 mm H2O2 (preincubation with H2O2 for 10 min and consecutive exposure to hypotonic stress) led to a decrease in ATP release that could be partially rescued by catalase (30 units in 100 μl of PBS apically, proven to be the most effective dose, Fig. 4A). A 60-min preincubation with H2O2 did not affect hypotonic stress-induced ATP release (Fig. 4B). Interestingly, even the control cultures revealed higher ATP release after 60 min of rest, which might be due to less perturbation of the apical membrane for this period of time. On the other hand, catalase had no effect on the IFN-γ-mediated inhibition of ATP release (Fig. 4C).
FIGURE 4.
A, short-term (<10 min) exposure to 0. 5 mm H2O2 decreases ATP release from NHBE cells but can be rescued with 30 units catalase. H2O2 was applied for 10 min before hypotonic stress was induced to elicit ATP release. Data are mean ± S.E. of -fold increases of ATP release (peak release, n = 3 lung donors, 2 replicates each). B, long-term (60-min) exposure to 0.5 mm H2O2 before applying hypotonic stress does not affect ATP release from NHBE cells. Data are mean ± S.E. of -fold increases of ATP release (peak release, n = 3 lung donors, ≥2 replicates each). Interestingly, even control cells show increased ATP release after 60 min of rest. C, catalase (apical incubation of ALI cultures with 30 units in 100 μl for 48 h) does not rescue IFN-γ-mediated decreases in ATP release (n = 2 lung donors, 4 replicates each). *, p < 0.05; ns, not significant.
In summary, short-term H2O2 exposures of oocytes inhibited the currents of expressed Panx1 via oxidation of a terminal cysteine residue. ATP release from NHBE cells was also inhibited by short-term exposure with H2O2. However, longer-term exposures did not inhibit ATP release. In addition, catalase could not reverse IFN-γ-mediated inhibition of ATP release.
pHi Changes Affect the Function of Panx1 Channels
To evaluate the role of intracellular pH on pannexin channels, the Panx1 protein was again expressed in Xenopus oocytes. To show a possible regulation by varying intracellular pH, the oocytes were immersed in sodium acetate for intracellular acidification. Oocytes contain an endogenous pH-sensitive channel that was activated by extracellular acetate (Fig. 5A). In contrast, acetate attenuated the membrane currents in Panx1-expressing oocytes (Fig. 5A). The degree of Panx1 channel inhibition was 28.2% ± 1.8% (n = 8), with correction for the average current increase because of the endogenous channels.
FIGURE 5.
Intracellular acidification inhibits Panx1 currents in oocytes and ATP release from airway epithelial cells. A, top panel, electrophysiological recordings of membrane currents of an oocyte not expressing Panx1. Activation, but not inhibition, of a current carried by endogenous channels is seen. Bottom panel, electrophysiological recordings of membrane currents of an oocyte expressing Panx1. Intracellular acidification by sodium acetate leads to inhibition of Panx1 currents (representative of three independent experiments). B, effect of IFN-γ on the pH of ALI cultures. Shown is pHi in ALI-cultured cells exposed to 100 ng/ml IFN-γ for 48 h compared with control cells (mean ± S.E., n = 5 lung donors, 3 replicates each). C, mean ± S.E. of ΔpHi in ALI cultures ± IFN-γ treatment (100 ng/ml for 48 h) in baseline solution, exposed to NH4Cl ± hypotonic stress (n = 3 lung donors, 2 replicates each; **, p < 0.01 compared with the control group without IFN-γ; *, p < 0.05; ***, p < 0.005 compared with the control group with IFN-γ). D, mean ± S.E. of the BCECF ratio recorded from six representative epithelial cells perfused with NH4Cl. An increase in the BCECF ratio indicates an increase in i (one lung donor). Hypotonic stress did not change the ratio (data not shown). E and F, intracellular alkalization leads to attenuation of IFN-γ induced decreases in ATP release. However, this effect is blocked by probenecid, indicative of Panx1 involvement. Shown is the average ATP release after hypotonic stress of three cultures pretreated with IFN-γ ± NH4Cl (E, n = 3 lung donors, 3 replicates each). Also shown is the mean ± S.E. of -fold increases of ATP release (peak release) comparing IFN-γ ± NH4Cl (F, n = 3 lung donors, ≥2 replicates each). G, Duox2 siRNA partially corrects the pHi in NHBE cells after IFN-γ treatment (n = 3 lung donors, ≥5 replicates each). ** and ***, p < 0.05 compared as indicated; ***, p < 0.001 compared with the appropriate control.
Treatment with IFN-γ Leads to Intracellular Acidification of NHBE Cells
ALI cultures were exposed to 100 ng/ml IFN-γ for 48 h. Then the pH level was measured as described previously (18). Ciliated cells exposed to IFN-γ revealed significantly lower pHi compared with control cells (Fig. 5B).
Intracellular Alkalization with NH4Cl Increases ATP Release in ALI Cultures Exposed to IFN-γ
To further demonstrate that pHi plays a role in the regulation of ATP release, ALI cultures were preincubated basolaterally with IFN-γ for 48 h and then incubated with NH4Cl or vehicle before hypotonic stress. Treatment of cells with NH4Cl increased the pH in control cells as well as in IFN-γ-treated cells (Fig. 5, C and D).
ALI cultures exposed to IFN-γ and NH4Cl showed a significant increase in ATP release compared with cells exposed to IFN-γ alone (Fig. 5, E and F), an effect blocked by probenecid (Fig. 5F). This showed that alkalization of the cytosol led to attenuation of the IFN-γ effect. Furthermore, cells infected with siDuox2 also revealed an increased pHi in the presence of IFN-γ compared with non-infected cells, tying together pH changes and ATP release modified by Duox2 (Fig. 5G).
Discussion
For the first time, we showed the electrophysiological presence and activity of Panx1 in primary normal human bronchial epithelial cells. Our data also depict novel regulation mechanisms of Panx1 channels. Airway epithelial cells release less ATP in response to hypotonic stress in an environment expected during airway inflammation (IFN-γ exposure). This was due to decreased Panx1 function with preserved expression levels in response to cell acidification mediated by increased Duox2 activity (15).
Initially we thought that Duox2 may affect pannexons through the production of H2O2. Indeed, H2O2 was found to affect pannexon currents in oocytes sequentially in opposite ways: a primary inhibition reverted to an enhancement of currents. The inhibitory effect is attributable to the terminal cysteine of Panx1, whereas reaction of its thiol group with maleimidobutyryl-biocytin (33) or its replacement by serine abolished the inhibitory effect of H2O2. The effects of H2O2 on thiol groups can lead to disulfides (reversible) or the formation of sulfinic or sulfonic acid (both irreversible). The reversibility of the effect of H2O2 on pannexon currents therefore indicates a formation of disulfide bonds between the terminal cysteine C426S with other thiol groups. These thiol groups could be on other proteins, or H2O2 may induce disulfide bonding between Panx1 subunits within the same pannexon.
These results exclude thiol reactions to be responsible for the delayed enhancement of pannexon currents. Because this phenomenon also remained unaffected by staurosporine, a phosphorylation event appears to be an unlikely cause. The mechanisms leading to current enhancement could include a reactive alkalization of the cytoplasm or an increase in cytoplasmic calcium ion concentration (9, 34).
Similarly, short-term exposure of NHBE cells to H2O2 also decreased ATP release, whereas longer exposures (60 min) had no effect. However, this adaptive response is blunted in an inflammatory environment because Duox2 up-regulation not only leads to production of H2O2 but also intracellular acidification. Intracellular acidification indeed inhibited both Panx1 currents in oocytes and ATP release in NHBE cells. By changing pHi to a more alkaline environment, IFN-γ-mediated decreases in ATP release were reversed. This indicates that acidification is the main reason of a reduced ATP release under inflammatory conditions.
It has been widely recognized that ATP release from NHBE cells is an essential regulator of airway host defense mechanisms, mainly because of preservation of mucociliary clearance (7, 35). Nevertheless, only a few reports exist that evaluate the exact mechanism. In previous papers, it has been shown that pannexins are proteins and orthologs of the invertebrate innexins that can form “hemichannels” in addition to complete gap junction channels. ATP release in erythrocytes has been found to be regulated by Panx1 channels (36), and our group has shown previously that Panx1 is expressed in the apex of airway epithelial cells (5). Hypotonic stress-induced ATP release in these cultures could be inhibited by carbenoxolone and probenecid but not flufenamic acid, speaking for Panx1 as the main contributor to ATP release (5). Furthermore, fully differentiated NHBE cells infected with an shRNA-expressing lentivirus to Panx1 also led to attenuation of ATP release upon hypotonic stress (5).
A threshold ATP concentration of about 1 μm has to be reached for induction of signaling pathways via P2Y2 receptors (37). Furthermore, other reports illustrated the important role of ATP as a paracrine mediator of airway epithelial cell function (38). Previous studies have shown that the contribution of non-exocytosis-mediated ATP release of ALI cultures is sufficient for the induction of ATP signaling pathways (7), consistent with ATP release through Panx1 (5). Consequently, inhibition of these channels would impair ATP-induced effects. Our studies show that exposure to IFN-γ causes inhibition of hypotonic stress-induced ATP release by ∼78%, which would impair proper mucociliary clearance. Our data fit well with data from a recent publication showing that smokers and patients with chronic bronchitis have not only decreased ATP concentrations in their airway surface liquid but also ATP levels below the ones needed for signaling (39).
In contrast to connexins, pannexins mainly form homomeric membrane channels rather than gap junctions (36, 40, 41). There are also significant differences between connexons and pannexins regarding their regulation. In voltage clamp studies, it has been shown that Panx1 channels can be opened by positive transmembrane potentials (8, 9, 42) and that they are highly mechanosensitive, resulting in increased open probability by mechanical stress (8, 43, 44). Intracellular acidification correlates negatively with the open probability of many connexin channels (45, 46) via identified intracellular regions shown to participate in pH gating as well as pH-induced changes in the conformation of Cx43 hemichannels by atomic force microscopy (47, 48). Interestingly, CO2 perfusion also abolished the conductance of Panx1 currents in oocytes (9), which is in accordance with our findings on hand showing that lowering of pHi leads to an inhibition of Panx1 currents (Fig. 5). Whether pannexon gating by protons is as direct as that shown for connexin channels contained in isolated membrane patches (49, 50) has not been determined.
Connexin channels are also regulated by phosphorylation, and because Panx channels have predicted phosphorylation sites, their regulation could be similar (51). Others have demonstrated a direct inverse correlation between gap junctional permeability and the phosphorylation state of Cx43 (52). However, because staurosporine did not affect the modulation of Panx1 channel activity, phosphorylation events are not likely involved in H2O2-mediated regulation of these channels, at least in oocytes.
We have shown previously that IFN-γ-mediated up-regulation of Duox2 increases H2O2 levels, creating oxidative stress (15). This process by itself can negatively affect pannexon function, at least transiently. Adaptive rebound alkalization is blocked by lowering pHi related to Duox2 activity and, thereby, inhibiting the permeability of Panx1 channels.
It is interesting to note that increases in ATP release were significantly greater in the absence of Duox2 than the change in whole cell pHi (Figs. 2D and 5G). This could indicate that some additional regulation mechanisms are at work that are not addressed here. On the other hand, changes in pH close to the apical membrane, where Duox makes protons, may be larger than changes in whole cell pHi in analogy to calcium influx into a cell (53). Therefore, whole cell pH change measurements may underestimate the pH changes at the apical membrane that are ultimately responsible for Panx1 regulation.
In summary, our data show that IFN-γ induces intracellular acidification by Duox2 up-regulation. Both intracellular acidification and increased H2O2 production by Duox2 synergistically inhibit Panx1 channels, leading to decreased ATP release from the airway (Fig. 6). These are new mechanisms how the loss of pannexin function contributes to mucociliary dysfunction in airway inflammation.
FIGURE 6.
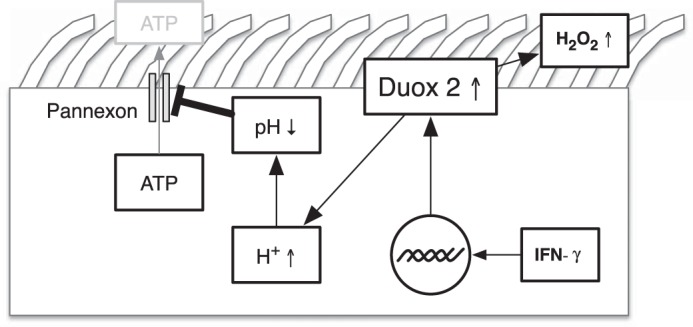
Illustration of IFN-γ medicated mechanisms of decreased ATP release in NHBE cells.
Author Contributions
S. K. contributed to the overall conception of the article, partially designed, performed, and analyzed experiments shown in all figures, and wrote the first draft of the paper. J. W. and G. D. designed, performed, and analyzed all experiments in oocytes. M. S. P. performed and analyzed the pilot experiments and some experiments shown in Fig. 5. C. G. was responsible for the patch clamp experiments. M. S. conceived and supervised the work in airway epithelial cells, finalized the writing of the paper, and contributed to the preparation of the figures. All authors reviewed the results, edited the paper, and approved the final version of the manuscript.
Acknowledgments
We thank Nathalie Baumlin and Dr. Gregory E. Conner for help and assistance.
This work was supported by National Institutes of Health Grants R01 HL-89399 and R01 HL-60644, the Flight Attendant Medical Research Institute (CIA103027 and CIA13033), the Cystic Fibrosis Foundation (SALATH14G0; to M. S.), and Chilean Grants 1120802 and ACT1104 and Millennium Initiative P09–022-F (to C. G.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- ALI
- air-liquid interface
- NHBE
- normal human bronchial epithelial.
References
- 1. Davis C. W., Dowell M. L., Lethem M., and Van Scott M. (1992) Goblet cell degranulation in isolated canine tracheal epithelium: response to exogenous ATP, ADP, and adenosine. Am. J. Physiol. 262, C1313–C1323 [DOI] [PubMed] [Google Scholar]
- 2. Lethem M. I., Dowell M. L., Van Scott M., Yankaskas J. R., Egan T., Boucher R. C., and Davis C. W. (1993) Nucleotide regulation of goblet cells in human airway epithelial explants: normal exocytosis in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 9, 315–322 [DOI] [PubMed] [Google Scholar]
- 3. Geary C. A., Davis C. W., Paradiso A. M., and Boucher R. C. (1995) Role of CNP in human airways: cGMP-mediated stimulation of ciliary beat frequency. Am. J. Physiol. 268, L1021–L1028 [DOI] [PubMed] [Google Scholar]
- 4. Schmid A., Clunes L. A., Salathe M., Verdugo P., Dietl P., Davis C. W., and Tarran R. (2011) Nucleotide-mediated airway clearance. Subcell. Biochem. 55, 95–138 [DOI] [PubMed] [Google Scholar]
- 5. Ransford G. A., Fregien N., Qiu F., Dahl G., Conner G. E., and Salathe M. (2009) Pannexin 1 contributes to ATP release in airway epithelia. Am. J. Respir. Cell Mol. Biol. 41, 525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seminario-Vidal L., Okada S. F., Sesma J. I., Kreda S. M., van Heusden C. A., Zhu Y., Jones L. C., O'Neal W. K., Penuela S., Laird D. W., Boucher R. C., and Lazarowski E. R. (2011) Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J. Biol. Chem. 286, 26277–26286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Okada S. F., Nicholas R. A., Kreda S. M., Lazarowski E. R., and Boucher R. C. (2006) Physiological regulation of ATP release at the apical surface of human airway epithelia. J. Biol. Chem. 281, 22992–23002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bao L., Locovei S., and Dahl G. (2004) Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 572, 65–68 [DOI] [PubMed] [Google Scholar]
- 9. Locovei S., Wang J., and Dahl G. (2006) Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 580, 239–244 [DOI] [PubMed] [Google Scholar]
- 10. Qiu F., and Dahl G. (2009) A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP. Am. J. Physiol. Cell. Physiol. 296, C250–C255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ma W., Hui H., Pelegrin P., and Surprenant A. (2009) Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J. Pharmacol. Exp. Ther. 328, 409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kumar R. K., Webb D. C., Herbert C., and Foster P. S. (2006) Interferon-γ as a possible target in chronic asthma. Inflamm. Allergy Drug Targets 5, 253–256 [DOI] [PubMed] [Google Scholar]
- 13. Kumar R. K., Yang M., Herbert C., and Foster P. S. (2012) Interferon-γ, pulmonary macrophages and airway responsiveness in asthma. Inflamm. Allergy Drug Targets 11, 292–297 [DOI] [PubMed] [Google Scholar]
- 14. Panzner P., Lafitte J. J., Tsicopoulos A., Hamid Q., and Tulic M. K. (2003) Marked up-regulation of T lymphocytes and expression of interleukin-9 in bronchial biopsies from patients with chronic bronchitis with obstruction. Chest 124, 1909–1915 [DOI] [PubMed] [Google Scholar]
- 15. Gattas M. V., Forteza R., Fragoso M. A., Fregien N., Salas P., Salathe M., and Conner G. E. (2009) Oxidative epithelial host defense is regulated by infectious and inflammatory stimuli. Free Radic. Biol. Med. 47, 1450–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Conner G. E., Ivonnet P., Gelin M., Whitney P., and Salathe M. (2013) H2O2 stimulates cystic fibrosis transmembrane conductance regulator through an autocrine prostaglandin pathway, using multidrug-resistant protein-4. Am. J. Respir. Cell Mol. Biol. 49, 672–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwarzer C., Machen T. E., Illek B., and Fischer H. (2004) NADPH oxidase-dependent acid production in airway epithelial cells. J. Biol. Chem. 279, 36454–36461 [DOI] [PubMed] [Google Scholar]
- 18. Sutto Z., Conner G. E., and Salathe M. (2004) Regulation of human airway ciliary beat frequency by intracellular pH. J. Physiol. 560, 519–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harper R. W., Xu C., Eiserich J. P., Chen Y., Kao C. Y., Thai P., Setiadi H., and Wu R. (2005) Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett. 579, 4911–4917 [DOI] [PubMed] [Google Scholar]
- 20. Nagai K., Betsuyaku T., Suzuki M., Nasuhara Y., Kaga K., Kondo S., and Nishimura M. (2008) Dual oxidase 1 and 2 expression in airway epithelium of smokers and patients with mild/moderate chronic obstructive pulmonary disease. Antioxid. Redox Signal. 10, 705–714 [DOI] [PubMed] [Google Scholar]
- 21. Fulcher M. L., Gabriel S., Burns K. A., Yankaskas J. R., and Randell S. H. (2005) Well-differentiated human airway epithelial cell cultures. Methods Mol. Med. 107, 183–206 [DOI] [PubMed] [Google Scholar]
- 22. Nlend M. C., Schmid A., Sutto Z., Ransford G. A., Conner G. E., Fregien N., and Salathe M. (2007) Calcium-mediated, purinergic stimulation and polarized localization of calcium-sensitive adenylyl cyclase isoforms in human airway epithelia. FEBS Lett. 581, 3241–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fragoso M. A., Fernandez V., Forteza R., Randell S. H., Salathe M., and Conner G. E. (2004) Transcellular thiocyanate transport by human airway epithelia. J. Physiol. 561, 183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manzanares D., Srinivasan M., Salathe S. T., Ivonnet P., Baumlin N., Dennis J. S., Conner G. E., and Salathe M. (2014) IFN-γ-mediated reduction of large-conductance, Ca2+-activated, voltage-dependent K+ (BK) channel activity in airway epithelial cells leads to mucociliary dysfunction. Am. J. Physiol. Lung Cell. Mol. Physiol. 306, L453–L462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manzanares D., Krick S., Baumlin N., Dennis J. S., Tyrrell J., Tarran R., and Salathe M. (2015) Airway surface dehydration by transforming growth factor β (TGF-β) in cystic fibrosis is due to decreased function of a voltage-dependent potassium channel and can be rescued by the drug pirfenidone. J. Biol. Chem. 290, 25710–25716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Manzanares D., Gonzalez C., Ivonnet P., Chen R. S., Valencia-Gattas M., Conner G. E., Larsson H. P., and Salathe M. (2011) Functional apical large conductance, Ca2+-activated, and voltage-dependent K+ channels are required for maintenance of airway surface liquid volume. J. Biol. Chem. 286, 19830–19839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Silverman W. R., de Rivero Vaccari J. P., Locovei S., Qiu F., Carlsson S. K., Scemes E., Keane R. W., and Dahl G. (2009) The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J. Biol. Chem. 284, 18143–18151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li A., Ségui J., Heinemann S. H., and Hoshi T. (1998) Oxidation regulates cloned neuronal voltage-dependent Ca2+ channels expressed in Xenopus oocytes. J. Neurosci. 18, 6740–6747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schlief T., and Heinemann S. H. (1995) H2O2-induced chloride currents are indicative of an endogenous Na+-Ca2+ exchange mechanism in Xenopus oocytes. J. Physiol. 486, 123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hua D., Wang C., He J., Liao H., Duan Y., Zhu Z., Guo Y., Chen Z., and Gong Z. (2012) A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 24, 2546–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bienert G. P., and Chaumont F. (2014) Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 1840, 1596–1604 [DOI] [PubMed] [Google Scholar]
- 32. Miller E. W., Dickinson B. C., and Chang C. J. (2010) Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. U.S.A. 107, 15681–15686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang J., and Dahl G. (2010) SCAM analysis of Panx1 suggests a peculiar pore structure. J. Gen. Physiol. 136, 515–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murali S., Zhang M., and Nurse C. A. (2014) Angiotensin II mobilizes intracellular calcium and activates pannexin-1 channels in rat carotid body type II cells via AT1 receptors. J. Physiol. 592, 4747–4762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Button B., Okada S. F., Frederick C. B., Thelin W. R., and Boucher R. C. (2013) Mechanosensitive ATP release maintains proper mucus hydration of airways. Sci. Signal. 6, ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Locovei S., Bao L., and Dahl G. (2006) Pannexin 1 in erythrocytes: function without a gap. Proc. Natl. Acad. Sci. U.S.A. 103, 7655–7659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mason S. J., Paradiso A. M., and Boucher R. C. (1991) Regulation of transepithelial ion transport and intracellular calcium by extracellular ATP in human normal and cystic fibrosis airway epithelium. Br. J. Pharmacol. 103, 1649–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tarran R., Gray M. A., Evans M. J., Colledge W. H., Ratcliff R., and Argent B. E. (1998) Basal chloride currents in murine airway epithelial cells: modulation by CFTR. Am. J. Physiol. 274, C904–C913 [DOI] [PubMed] [Google Scholar]
- 39. Anderson W. H., Coakley R. D., Button B., Henderson A. G., Zeman K. L., Alexis N. E., Peden D. B., Lazarowski E. R., Davis C. W., Bailey S., Fuller F., Almond M., Qaqish B., Bordonali E., Rubinstein M., Bennett W. D., Kesimer M., and Boucher R. C. (2015) The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. Am. J. Respir. Crit. Care Med. 192, 182–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Isakson B. E., and Thompson R. J. (2014) Pannexin-1 as a potentiator of ligand-gated receptor signaling. Channels 8, 118–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sosinsky G. E., Boassa D., Dermietzel R., Duffy H. S., Laird D. W., MacVicar B., Naus C. C., Penuela S., Scemes E., Spray D. C., Thompson R. J., Zhao H. B., and Dahl G. (2011) Pannexin channels are not gap junction hemichannels. Channels 5, 193–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bruzzone R., Hormuzdi S. G., Barbe M. T., Herb A., and Monyer H. (2003) Pannexins, a family of gap junction proteins expressed in brain. Proc. Natl. Acad. Sci. U.S.A. 100, 13644–13649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Negoro H., Urban-Maldonado M., Liou L. S., Spray D. C., Thi M. M., and Suadicani S. O. (2014) Pannexin 1 channels play essential roles in urothelial mechanotransduction and intercellular signaling. PLoS ONE 9, e106269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Beckel J. M., Argall A. J., Lim J. C., Xia J., Lu W., Coffey E. E., Macarak E. J., Shahidullah M., Delamere N. A., Zode G. S., Sheffield V. C., Shestopalov V. I., Laties A. M., and Mitchell C. H. (2014) Mechanosensitive release of adenosine 5′-triphosphate through pannexin channels and mechanosensitive upregulation of pannexin channels in optic nerve head astrocytes: a mechanism for purinergic involvement in chronic strain. Glia 62, 1486–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. González-Nieto D., Gómez-Hernández J. M., Larrosa B., Gutiérrez C., Muñoz M. D., Fasciani I., O'Brien J., Zappalà A., Cicirata F., and Barrio L. C. (2008) Regulation of neuronal connexin-36 channels by pH. Proc. Natl. Acad. Sci. U.S.A. 105, 17169–17174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Morley G. E., Taffet S. M., and Delmar M. (1996) Intramolecular interactions mediate pH regulation of connexin43 channels. Biophys. J. 70, 1294–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thimm J., Mechler A., Lin H., Rhee S., and Lal R. (2005) Calcium-dependent open/closed conformations and interfacial energy maps of reconstituted hemichannels. J. Biol. Chem. 280, 10646–10654 [DOI] [PubMed] [Google Scholar]
- 48. Duffy H. S., Ashton A. W., O'Donnell P., Coombs W., Taffet S. M., Delmar M., and Spray D. C. (2004) Regulation of connexin43 protein complexes by intracellular acidification. Circ. Res. 94, 215–222 [DOI] [PubMed] [Google Scholar]
- 49. Pfahnl A., and Dahl G. (1998) Localization of a voltage gate in connexin46 gap junction hemichannels. Biophys. J. 75, 2323–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Trexler E. B., Bukauskas F. F., Bennett M. V., Bargiello T. A., and Verselis V. K. (1999) Rapid and direct effects of pH on connexins revealed by the connexin46 hemichannel preparation. J. Gen. Physiol. 113, 721–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Penuela S., Bhalla R., Gong X. Q., Cowan K. N., Celetti S. J., Cowan B. J., Bai D., Shao Q., and Laird D. W. (2007) Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J. Cell Sci. 120, 3772–3783 [DOI] [PubMed] [Google Scholar]
- 52. Kim D. Y., Kam Y., Koo S. K., and Joe C. O. (1999) Gating connexin 43 channels reconstituted in lipid vesicles by mitogen-activated protein kinase phosphorylation. J. Biol. Chem. 274, 5581–5587 [DOI] [PubMed] [Google Scholar]
- 53. Braiman A., and Priel Z. (2001) Intracellular stores maintain stable cytosolic Ca2+ gradients in epithelial cells by active Ca2+ redistribution. Cell Calcium 30, 361–371 [DOI] [PubMed] [Google Scholar]