Abstract
Hyaluronan (HA) is synthesized by three HA synthases (HAS1, HAS2, and HAS3) and secreted in the extracellular matrix. In human skin, large amounts of HA are found in the dermis. HA is also synthesized by keratinocytes in the epidermis, although its epidermal functions are not clearly identified yet. To investigate HA functions, we studied the effects of HA depletion on human keratinocyte physiology within in vitro reconstructed human epidermis. Inhibition of HA synthesis with 4-methylumbelliferone (4MU) did not modify the expression profile of the epidermal differentiation markers involucrin, keratin 10, and filaggrin during tissue reconstruction. In contrast, when keratinocytes were incubated with 4MU, cell proliferation was decreased. In an attempt to rescue the proliferation function, HA samples of various mean molecular masses were added to keratinocyte cultures treated with 4MU. These samples were unable to rescue the initial proliferation rate. Furthermore, treatments with HA-specific hyaluronidase, although removing almost all HA from keratinocyte cultures, did not alter the differentiation or proliferation processes. The differences between 4MU and hyaluronidase effects did not result from differences in intracellular HA, sulfated glycosaminoglycan concentration, apoptosis, or levels of HA receptors, all of which remained unchanged. Similarly, knockdown of UDP-glucose 6-dehydrogenase (UGDH) using lentiviral shRNA effectively decreased HA production but did not affect proliferation rate. Overall, these data suggest that HA levels in the human epidermis are not directly correlated with keratinocyte proliferation and differentiation and that incubation of cells with 4MU cannot equate with HA removal.
Keywords: differentiation, epidermis, hyaluronan, hyaluronidase, keratinocyte, proliferation, 4-methylumbelliferone
Introduction
Hyaluronan (HA),2 a large polysaccharide of the extracellular space, is composed of repetitive d-glucuronic acid and d-N-acetylglucosamine dimer units. Although it belongs to the glycosaminoglycan (GAG) family, HA is not synthesized in the Golgi network; the molecule is assembled at the inner face of the plasma membrane, and the newly formed polymer is extruded into the extracellular matrix as it lengthens by addition of the dimer units. This mechanism allows the formation of huge HA molecules with masses ranging from 105 to 107 Da and lengths ranging from 2 to 25 μm (1).
HA synthesis is performed by three different glycosyltransferases called hyaluronan synthases (HAS) located at the plasma membrane. These three enzymes, HAS1, HAS2, and HAS3, differ from each other with respect to temporal expression pattern during development, specific activity, and size of HA polymers generated (2, 3). The degradation of HA is carried out in somatic tissues by HYAL1 and HYAL2, two enzymes belonging to the hyaluronidase family (4).
HA has been identified in most tissues of the organism. Half of the total amount of HA is localized in skin (5). The skin is a large organ making up the first barrier against physical, biological, and chemical damages to the body. In the dermis, the main cell type is represented by fibroblasts, which metabolize connective tissue matrix proteins. The fibrous and amorphous extracellular matrix is composed of numerous collagen and elastic connective fibers forming a network in which large amounts of HA are deposited. In the epidermis, the main cellular components are keratinocytes organized in four distinct layers. Keratinocytes proliferate in the basal layer, the deepest compartment, and then migrate to the surface, forming spinous, granular, and cornified layers corresponding to successive differentiation states (6). HA is also abundant in the epidermis. As shown by Tammi et al. (7) more than 15 years ago, it is localized in the extracellular spaces of basal, spinous, and granular layers, with proliferating basal regions containing the majority of HA. This localization suggests a role of HA in keratinocyte proliferation.
A few studies have explored the roles of HA in the healthy epidermis. HA is generally proposed as a guardian of epidermal hydration through its water retention properties and as a booster of epidermal proliferation and differentiation (8–10). For instance, it has been shown that exogenous HA, through interaction with its cell surface receptor CD44, induces the expression of differentiation markers such as keratin 10, involucrin, and filaggrin in cultured human keratinocytes (11). However, modulation of endogenous epidermal HA results in discrepant effects. On the one hand, decreases in HA concentrations caused by 4-methylumbelliferone (4MU), an inhibitor of HA synthesis, or by tumor growth factor-β correlate with a decrease in proliferation of rat keratinocytes in organotypic cultures (12, 13). On the other hand, incubation of rat and mouse keratinocytes with HA-specific Streptomyces hyaluronidase, which degrades extracellular HA, unexpectedly results in the induction of keratin 10 and filaggrin expression (14, 15). From these observations, it can be surmised that the functions of HA in normal human epidermis have not been clarified yet.
We have recently demonstrated that HAS1 is the main synthase involved in extracellular HA production by normal human keratinocytes, whereas HAS2 and HAS3 are instead implicated during the early steps of keratinocyte culture as well as in atopic dermatitis conditions (16). In those studies, we suppressed HA from keratinocyte monolayer cultures using either 4MU or hyaluronidase. Surprisingly, HA depletion had no influence on the differentiation process (16). In light of those conflicting data, the present study was designed to unravel the role of HA in proliferation and differentiation of human keratinocytes grown in a monolayer model and in an in vitro model that is as close as possible to native human epidermal growth, i.e. reconstructed human epidermis (RHE) cultured on a polycarbonate filter at an air-liquid interface. The results indicate that HA is not a major player in the control of keratinocyte proliferation and differentiation and that 4MU is not a sufficiently specific tool to dissect HA functions.
Experimental Procedures
Chemicals
Streptomyces hyaluronidase, 4MU, and staurosporine were purchased from Sigma-Aldrich (Bornem, Belgium). Biotinylated HA-binding protein) and HA fragments were generously provided by Professor Markku Tammi, University of Kuopio, Finland, and Pierre Fabre Co., Toulouse, France, respectively. HA of 4000 kDa (Healon®) was purchased from Abbott Medical Optics. Mouse anti-human CD44, anti-human RHAMM, and anti-human TLR4, as well as rabbit anti-human RPL13 antibodies were purchased from BD Biosciences, Santa Cruz Biotechnologies (Santa Cruz, CA), Thermo Scientific, and Cell Signaling (Leiden, The Netherlands), respectively. Mouse anti-human keratin 10 was from Dako (Heverlee, Belgium), mouse anti-human involucrin was from Sigma-Aldrich, and mouse anti-BrdU was from BD Biosciences. Anti-PARP-1 was from BD Biosciences, and anti-cleaved caspase-3 was from Cell Signaling.
Keratinocytes
Human keratinocytes were isolated from normal adult abdominal skin leftovers from abdominoplasty surgery as described (Dr. B. Bienfait, Clinique St-Luc, Namur, Belgium) (17). The surgeries were performed with informed consent from patients and were approved by the Medical Ethics Committee of Clinique St-Luc. Proliferating primary cultures were trypsinized, and keratinocytes were amplified into secondary cultures in EpiLife culture medium containing human keratinocyte growth supplement (Life Technologies) (17).
To produce monolayer cultures, third passage keratinocyte cultures were used as described (17). Briefly, when cells reached 60% density, they were harvested by trypsinization and plated at a density of 5000 cells/cm2 in culture dishes to grow keratinocytes in monolayer. When cells reached 50% confluence, the complete medium was changed to a growth factor- and hormone-free medium (EpiLife with amino acids, hydrocortisone, and antibiotics, Invitrogen-Cascade Biologics, Mansfield, UK), corresponding to autocrine conditions. Keratinocytes proliferated until culture confluence, when they began to express differentiation markers such as keratin 10 and involucrin.
Reconstructed Human Epidermis
To produce RHE, third passage keratinocyte cultures were used as described (18). When cells reached 60% density, they were harvested by trypsinization and plated at a density of 150,000 cells/insert on polycarbonate culture inserts (Millipore, Billerica, MA) in EpiLife medium containing human keratinocyte growth supplement and 1.5 mm calcium. After 24 h, the medium under the filter was replaced and keratinocytes were exposed to the air-liquid interface by removing the medium in the upper compartment. The culture medium (EpiLife complemented with human keratinocyte growth supplement, 1.5 mm calcium, 50 μg/ml vitamin C, and keratinocyte growth factor (R&D Systems, Abingdon, UK)) was renewed every other day until the 11th day.
UGDH and CD44 Depletions Using shRNA Lentivirus
UDP-glucose 6-dehydrogenase (UGDH) and CD44 depletions were achieved using transduction of lentiviral particles (MISSION Lentiviral Transduction Particles, Sigma-Aldrich) comprising a puromycin resistance gene and an shRNA under control of U6 promoter. Five types of shRNA were used. One has no known target and was used as non-mammalian shRNA control; one RNA (TRCN0000280945) targets UGDH mRNA (nucleotide positions 1512–1532 on the mRNA sequence); and one RNA (TRCN0000308110) targets CD44 mRNA (nucleotide positions 2692–2715 on the mRNA sequence). Keratinocytes were transduced with lentivirus at a multiplicity of infection of 10 in the presence of 4 μg/ml protamine sulfate (Sigma-Aldrich). After 24 h, the culture medium was replaced with fresh complete medium containing 2 μg/ml puromycin (Sigma-Aldrich) for selection. When cells reached 60% density, they were harvested by trypsinization and seeded in 6-well dishes for evaluation of proliferation, RNA expression, and HA content.
HA Assay
HA content was quantified in the medium of monolayer and RHE cultures and in RHE tissue using the Hyaluronan DuoSet kit (R&D Systems). Values were normalized to the amount of protein of the corresponding cell extract measured using the Pierce Protein Assay (Thermo Scientific).
Histological Staining and Fluorescent Labeling
RHE were fixed in 4% formalin in 70% ethanol and 5% glacial acetic acid (acid-formalin/EtOH) as recommended (19), embedded in paraffin, and sectioned with a microtome. The tissue sections were processed for histology. For morphological analysis, hematoxylin-eosin staining was performed. For fluorescence, RHE sections or fixed keratinocyte monolayers were washed with PBS and saturated by immersion in PBS, 0.2% BSA (PAA Laboratories GmbH, Pasching, Austria) with 0.02% Triton X-100 (Merck, Darmstadt, Germany), and then incubated with anti-keratin 10 (1:100), anti-involucrin (1:500), or HA-binding protein (1:25) in a moist chamber for 1 h. After washing, the slices were incubated in Alexa Fluor 488-conjugated secondary antibodies (anti-mouse 1:200) or Alexa Fluor 488-conjugated streptavidin (1:500) (Vector Laboratories, Burlingame, CA) for 1 h. Nuclei were counterstained with Hoechst 33258 (Molecular Probes, Eugene, OR). Finally, sections were mounted with Mowiol (Sigma-Aldrich) and observed with an Olympus AX70 microscope.
Immunofluorescence Staining
Cells seeded on coverslips were washed once with PBS, fixed for 15 min with 4% formaldehyde, permeabilized with 1% Triton X-100 for 5 min, and incubated overnight with murine anti-active caspase 3 primary antibody (1:250) in PBS, BSA 1%. After three washes with PBS, cells were incubated during 1 h with Alexa Fluor 488 nm-conjugated goat mouse anti-IgG secondary antibody (1:1000 in PBS, BSA 1%, Molecular Probes). Cells were washed extensively with PBS, BSA 1%. Nuclei were counterstained with 30 μl of TO-PRO-3 (Sigma-Aldrich) diluted 1:80 in PBS containing 2 mg/ml RNase for 35 min. Finally, sections were mounted with Mowiol (Sigma-Aldrich) and observed with an Olympus AX70 microscope.
Proliferation Assay
Proliferation was evaluated using BrdU incorporation between 4 and 2 days before confluence in monolayer cultures or between the 3th and 5th day of epidermis reconstruction. The samples were incubated with 1 mm BrdU for 24 h before labeling, and then fixed with 4% formalin. After washing and blocking steps, cells and slides were incubated with anti-BrdU (1:100) during 2 h, and then incubated with Alexa Fluor 488-conjugated secondary antibodies (anti-mouse immunoglobulins, 1:200) and Hoechst 33258 for 1 h. Labeling was observed with an Olympus AX70 microscope. Positive BrdU nuclei were normalized to the total number of nuclei.
Quantitative PCR after Reverse Transcription for Analysis of mRNA Expression
RNA extraction was performed using the High Pure RNA Isolation Kit (Roche Diagnostics, Vilvoorde, Belgium), and concentration was measured with the NanoDrop 1000 (Thermo Scientific). SuperScript III RNase H reverse transcriptase (Invitrogen, Merelbeke, Belgium) was used to reverse-transcribe RNA into cDNA, which was amplified using a 7300 real-time PCR system (Applied Biosystems, Lennik, Belgium) after mixing with FastStart Universal SYBR Green Master (Roche, Basel, Switzerland) and sense transcript-specific and antisense primers (300 nm/liter; Sigma-Aldrich). The primers used were: CD44 sense, 5′-CATTGCAGTCAACAGTCGAAGAA-3′; CD44 antisense, 5′ATTGCCACTGTTGATCACTAGCTT-3′; UGDH sense, 5′-GAGGTAGCAACAGCGATTGGA-3′; UGDH antisense, 5′-ACCCAACACTGGCTTTTAGAAACT-3′; HAS1 sense, 5′-CCTCACCAACCGCATGCT-3′; HAS1 antisense, 5′-GGACGAGGGCGTCTCTGA-3′; HAS3 sense, 5′-GCCCTCGGCGATTCG-3′; HAS3 antisense, 5′-TGGATCCAGCACAGTGTCAGA-3′; β-defensin 2 (β-def2) sense, 5′-ATCAGCCATGAGGGTCTTGT-3′; β-def2 antisense, 5′-GAGACCACAGGTGCCAATTT-3′, RPL13a sense, 5′-CTCAAGGTCGTGCGTCTGAA-3′; RPL13aantisense, 5′-TGGCTGTCACTGCCTGGTACT-3′; TBP sense, 5′-TCAAACCCAGAATTGTTCTCCTTAT-3′; and TBPantisense, 5′-CCTGAATCCCTTTAGAATAGGGTAGA-3′. The mean amount of reference gene mRNA levels was used to normalize the mRNA level of interest.
Western Blotting
Proteins were extracted from keratinocyte monolayer cultures with lysis buffer (62.5 mm Tris-HCl, 2% SDS, 8.7% glycerol, 0.2% dithiothreitol) (Sigma-Aldrich). Their concentration was measured using the Pierce 660nm Protein Assay Reagent (catalog number 22660) supplemented with anti-SDS (Thermo Scientific). Twenty μg of proteins were separated using 10% SDS-PAGE and transferred to a polyvinylidene fluoride membrane (GE Healthcare Life Sciences, Diegem, Belgium). The membrane was saturated with 5% powdered milk diluted in PBS, 0.1% Tween 20 and then incubated with primary antibodies against CD44, RHAMM, TLR4, caspase 3, PARP-1, or RPL13A (1:1000) for 1 h. After washing, membranes were incubated with the HRP-conjugated secondary antibodies (anti-mouse immunoglobulins, 1:5000 or anti-rabbit immunoglobulins, 1:5000) for 1 h. Finally, the membrane was incubated with POD Chemiluminescence Substrate (Roche Diagnostics, Mannheim, Germany), and emitted light was detected using the ImageQuant Las4000mini system (GE Healthcare Life Sciences).
Sulfated GAG Measurement
Total GAG content was determined using the colorimetric dimethylmethylene blue dye binding assay (20). Sulfated GAGs in the medium were expressed as μg of sulfated GAG/μg of cellular proteins.
DNA Fragmentation Assay
DNA fragmentation was assayed using an ELISA test based on immobilized anti-histone murine monoclonal antibodies to capture the oligonucleosomal fragments, which are then revealed with an anti-DNA monoclonal antibody (Cell Death Detection ELISA kit, Roche). Briefly, after incubation with apoptotic inducers, cells in 24-well plates were incubated for 30 min at 25 °C with 200 μl of incubation buffer. The cell lysate was then collected and centrifuged at 13,000 rpm for 7 min. The supernatant was collected and conserved at −20 C° before assaying DNA fragmentation, which was carried out following the manufacturer instructions.
Lactate Dehydrogenase (LDH) Release
LDH release was measured with the CytoTox-ONETM Homogeneous Membrane Integrity Assay from Promega (Leiden, The Netherlands) according to the manufacturer's protocol. Fluorescence was measured using a fluorescence plate reader (Thermo Scientific), and LDH release was determined using background control and positive control cell lysis in Triton X-100 (maximum LDH release).
Statistics
Variations in HA concentration, proliferation assays, and mRNA expression data were analyzed to identify statistically significant differences using t test, one-way analyses of variance, or two-way analyses of variance on results as appropriate. Each experiment was performed independently on three samples (technical replicates) of keratinocytes from three separate patients (biological replicates).
Results
Hyaluronidase Treatment Decreases HA Production without Affecting Cell Proliferation
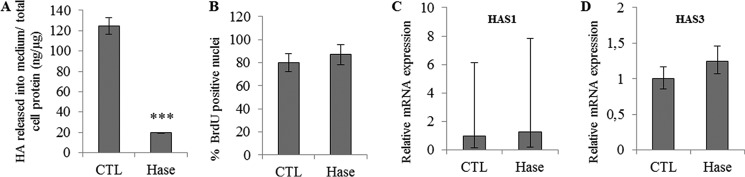
Previously, we demonstrated that hyaluronidase treatment does not affect the differentiation of keratinocyte monolayer models (16). Here, we investigated the effect of HA degradation on proliferation in the same model. Keratinocytes from different donors were grown in the presence or absence of 1 unit/ml Streptomyces hyaluronidase during 48 h. HA concentration in the culture medium decreased markedly after hyaluronidase treatment (Fig. 1A). However, the degradation of HA did not change the proportion of BrdU-positive cells (Fig. 1B). Furthermore, the mRNA and protein levels of HAS1 (Fig. 1C) and HAS3 (Fig. 1D; HAS2 was not detected) and those of HA receptors remained unchanged (shown and discussed later, in Fig. 12, A–D). In addition, knockdown of CD44 expression in keratinocyte cultures using shRNA-bearing lentiviral particles did not influence cell proliferation (Fig. 2), ruling out an important role of CD44 in the control of human keratinocyte proliferation. These results suggest that the amount of HA in human keratinocyte monolayer cultures does not influence cell proliferation.
FIGURE 1.
Effect of HA depletion using Streptomyces Hase on proliferation of keratinocyte monolayer cultures. Proliferative keratinocytes were grown in monolayer and treated 4 days before confluence with 1 unit/ml Hase during 48 h. A, newly synthesized HA in the medium was measured at the end of treatment and normalized with respect to the total amount of cellular proteins. Data are shown as means ± S.D. n = 3, t test, ***, p < 0.001. B, cells were incubated with BrdU for 24 h, and then fixed and immunostained for BrdU and nuclei. BrdU-positive nuclei were normalized to the total number of nuclei. Data are shown as means ± S.D. C and D, the mRNA expression levels of HAS1 (C) and HAS3 (D) were measured using quantitative PCR after reverse transcription. Expression levels were normalized to the average expression level of two reference genes, RPLP0 and TBP. The mRNA levels in control (CTL) conditions were taken as references and arbitrarily fixed at 1. The error bars represent 95% confidence intervals. Experiments were performed independently on three samples (technical replicates) of keratinocytes from three separate patients (biological replicates).
FIGURE 12.
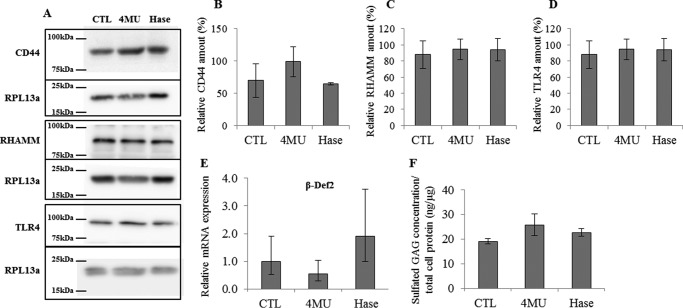
Effect of 4MU or Streptomyces Hase on HA receptors, relative β-def2 mRNA expression, and sulfated GAG abundance in proliferative keratinocytes. Proliferative keratinocytes were grown in monolayer and treated 4 days before confluence with 0.5 mm 4MU or 1 unit/ml Hase during 48 h. A, the abundance of RHAMM, CD44, and TLR4 was evaluated by Western blotting. Twenty μg of proteins were separated using SDS-PAGE, and RPL13a was used as loading control. B–D, relative quantification of Western blotting detections. CTL values were arbitrarily fixed at 1. Error bars represent standard deviations (n = 3). E, the mRNA expression level of β-def2 was measured using quantitative PCR after reverse transcription. Expression levels were normalized to the average expression level of two reference genes, RPLP0 and TBP. The mRNA levels in control conditions were taken as references and arbitrarily fixed at 1. The error bars represent 95% confidence intervals (n = 3, one-way ANOVA analysis of variance). F, the sulfated GAG content in the medium was assessed using dimethylmethylene blue assay after 48 h of treatment. Data were normalized by total protein content of the cell lysate. Data are shown as means ± S.D. (n = 3). Experiments were performed independently on three samples (technical replicates) of keratinocytes from three separate patients (biological replicates).
FIGURE 2.
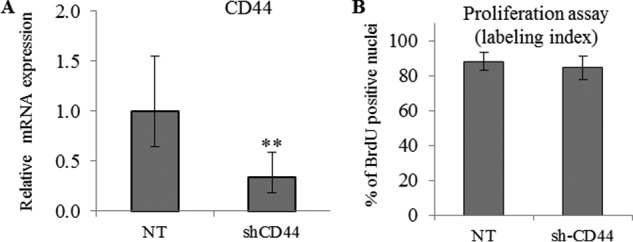
CD44 knockdown does not effect keratinocyte proliferation. CD44 expression knockdown was achieved using shRNA in keratinocytes grown in monolayer. A non-target (NT) shRNA was used as control. A, 2 days before confluence, the expression of CD44 was measured by quantitative PCR after reverse transcription. Expression levels were normalized to the average expression level of two reference genes, RPLP0 and TBP. The mRNA levels in control conditions were taken as references and arbitrarily fixed at 1. The error bars represent 95% confidence intervals (n = 3, paired t test, **, p < 0.01). B, to evaluate proliferation, keratinocytes were incubated with BrdU for 24 h, and then fixed and immunostained for BrdU and nuclei. BrdU-positive nuclei were normalized to the number of total nuclei. Data are shown as means ± S.D. (n = 3, paired t test). Experiments were performed independently on three samples (technical replicates) of keratinocytes from three separate patients (biological replicates).
UGDH Knockdown Decreases HA Production without Affecting Cell Proliferation
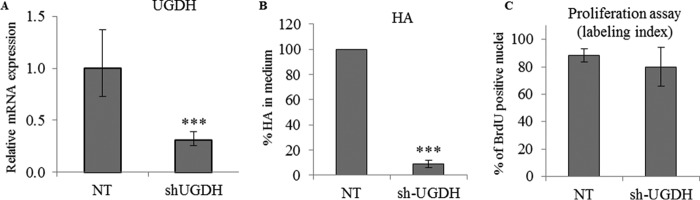
To decrease HA levels using a different method, we targeted UGDH, the enzyme that catalyzes the oxidation of UDP-glucose to UDP-glucuronic acid. UGDH expression knockdown was achieved using lentiviral particles expressing an shRNA targeting UGDH (sh-UGDH). The expression levels of UGDH decreased by 75% (Fig. 3A), and the amount of secreted HA decreased by 90% (Fig. 3B), in keratinocytes infected by sh-UGDH lentivirus particles, confirming that HA synthesis depends on efficient production of glucuronic acid by keratinocytes. However, there was no difference in BrdU labeling between control and UGDH knockdown cells (Fig. 3C). We conclude that inhibition of HA synthesis does not influence keratinocyte proliferation in the monolayer model.
FIGURE 3.
Effect of UGDH knockdown on proliferation and HA secretion of keratinocyte monolayer cultures. UGDH expression knockdown was achieved using shRNA in keratinocytes grown in monolayer. A non-target (NT) shRNA was used as control in the three graphs. A, 2 days before confluence, the expression of UGDH was measured by quantitative PCR after reverse transcription. Expression levels were normalized to the average expression level of two reference genes, RPLP0 and TBP. The mRNA levels in control conditions were taken as references and arbitrarily fixed at 1. The error bars represent 95% confidence intervals. B, the percentage of newly synthesized HA in the medium was measured at the same time. Data are shown as means ± S.D. C, to evaluate proliferation, keratinocytes were incubated with BrdU for 24 h, and then fixed and immunostained for BrdU and nuclei. BrdU-positive nuclei were normalized to the number of total nuclei. Data are shown as means ± S.D. (n = 3, paired t test, **, p < 0.01, ***, p < 0.001). Experiments were performed independently on three samples (technical replicates) of keratinocytes from three separate patients (biological replicates).
Influence of Different Molecular Mass HA on Keratinocyte Proliferation and Differentiation
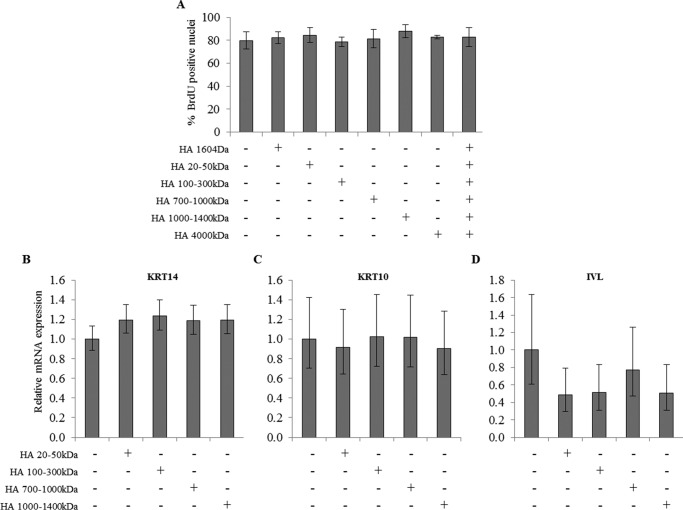
Previously, adding HA was shown to influence the differentiation of keratinocyte monolayer cultures (11) or the proliferation of RHE (10). Therefore, we investigated the role of extracellular HA on the proliferation and differentiation processes in the keratinocyte monolayer model. Subconfluent or confluent keratinocytes were grown in the presence of a high concentration (50 μg/ml) of HA of different molecular masses during 48 h. We observed no difference in proliferation index (Fig. 4A) or in mRNA level of three differentiation markers, keratin 14 (KRT14), keratin 10 (KRT10), and involucrin (IVL), in the presence of HA of different molecular masses (Fig. 4, B–D).
FIGURE 4.
Effect of HA fragments on keratinocyte proliferation and differentiation. A, proliferative keratinocytes were grown in monolayer and treated at 4 days before confluence with 50 μg/ml of various molecular mass HA preparations as indicated during 48 h. Cells were incubated with BrdU for 24 h, and then fixed and immunostained for BrdU and nuclei. BrdU-positive nuclei were normalized to the total number of nuclei. Data are shown as means ± S.D. B–D, confluent keratinocytes were grown in monolayer and treated with 50 μg/ml of various molecular mass HA preparations as indicated during 48 h. The mRNA expression levels of differentiation markers (keratin 14 (B), keratin 10 (C), and involucrin (D)) were measured using quantitative PCR after reverse transcription. Expression levels were normalized to the average expression level of two reference genes, RPLP0 and TBP. The mRNA levels in control conditions were taken as references and arbitrarily fixed at 1. The error bars represent 95% confidence intervals. (n = 3, one-way ANOVA). Experiments were performed independently on three samples (technical replicates) of keratinocytes from three separate patients (biological replicates).
Hyaluronidase Treatment Decreases HA Production without Affecting Cell Proliferation or Differentiation in RHE
The most obvious way to explore the role of HA in the epidermis is to inhibit its synthesis or remove it from the epithelium while observing cell proliferation and differentiation during the morphogenesis of the epidermis. To this aim, a primary culture model of RHE on polycarbonate filter was used (21). RHE from different donors were grown for increasing times at an air-liquid interface in the presence or absence of 1 unit/ml hyaluronidase from the 3rd until the 11th day of reconstruction (Fig. 5A). Fluorescent detection of HA at day 11 (full differentiation of RHE) showed a decrease in HA staining following hyaluronidase treatment (Fig. 5, A and B). However, KRT10 and IVL remained localized at the expected locations within RHE and exhibited similar intensities after hyaluronidase treatment, suggesting unaltered differentiation (Fig. 5A). To determine whether the proliferation process was altered, a BrdU incorporation assay was performed between the 3rd and the 5th day of reconstruction, when the proliferation rate is highest in RHE (21). As shown in Fig. 5C, on the 5th day, the proportion of BrdU-positive basal cells was the same in control and hyaluronidase-treated tissues despite almost complete depletion of HA in the latter.
FIGURE 5.
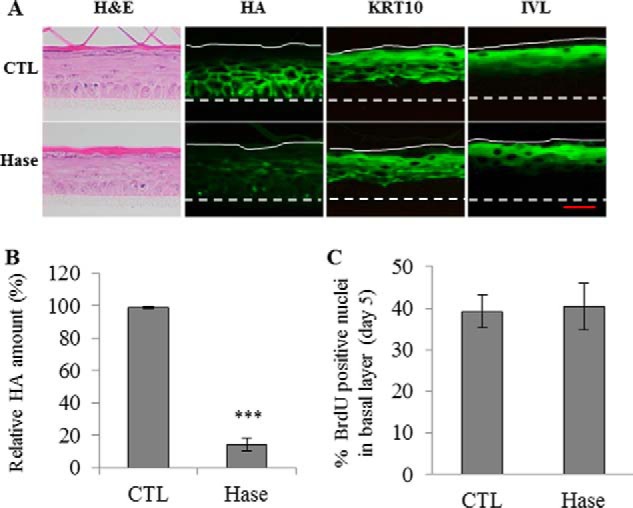
Effect of HA depletion using Streptomyces Hase on morphology, proliferation, and keratinization of a reconstructed human epidermis. RHE were analyzed after 11 days of growth on polycarbonate filter at the air-liquid interface. A, RHE were incubated in the presence of 1 unit/ml Hase or in CTL solutions between the 3th and 11th days. At day 11, RHE were fixed and stained for morphology (H&E), HA, and the differentiation markers KRT10 and IVL. Red bar = 15 μm. B, relative quantification of HA staining. CTL values were arbitrarily fixed at 1. Error bars represent standard deviations (n = 3, one-way ANOVA: ***, p < 0.001). C, proliferation was evaluated using BrdU incorporation between the 3th and 5th days. On the 5th day, RHE were fixed and immunostained for BrdU and nuclei. Nuclei positive for BrdU were normalized to the total number of nuclei in the basal layer. Data are shown as means ± S.D. (n = 3, t test: ***, p < 0.001). Experiments were performed independently on three samples (technical replicates) of keratinocytes from three separate patients (biological replicates).
Influence of Different Molecular Weight HA on RHE Differentiation
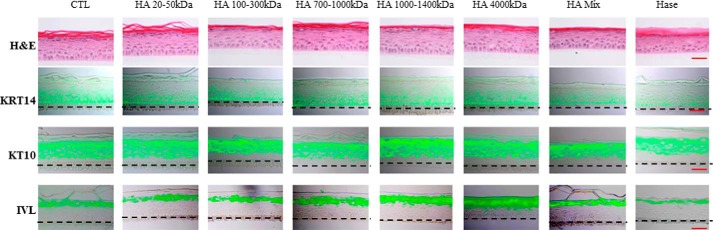
We next investigated the role of extracellular HA on the differentiation process. Epidermis tissues were grown for increasing times at an air-liquid interface in the presence of a high concentration (50 μg/ml) of HA of different molecular masses from the 3rd until the 11th day of reconstruction (Fig. 6). No difference in morphology or in staining patterns of three differentiation markers (KRT14, KRT10, and IVL) was observed.
FIGURE 6.
Effect of HA fragments on morphology and keratinization of a reconstructed human epidermis. RHE were analyzed after 11 days of growth on polycarbonate filter at the air-liquid interface. RHE were incubated between the 3th and 11th days in the presence of 50 μg/ml of various molecular mass HA preparations as indicated. At day 11, RHE were fixed and stained for morphology (H&E), HA, and the differentiation markers KRT14, KRT10, and IVL. Red bar = 15 μm. Experiments were performed independently on three samples (technical replicates) of keratinocytes from three separate patients (biological replicates).
4MU Inhibits Cell Proliferation in Subconfluent Keratinocytes
To support the results obtained using exogenous hyaluronidase, we performed experiments with 4MU, a widely used inhibitor of HA synthesis that decreases the availability of intracellular UDP-glucuronic acid, the precursor of HA synthesis by HAS enzymes, and down-regulates the expression of various HAS (3, 22, 23). We have previously shown, using concentration-response experiments, that 4MU at 0.5 mm is both effective in removing HA and relatively non-toxic in human keratinocytes cultured as monolayers (21). Subconfluent keratinocytes from different donors were thus grown in the presence or absence of 0.5 mm 4MU during 48 h. After treatment, the HA concentration decreased markedly (Fig. 7A). As shown in Fig. 7B, 2 days before the confluence, 80% of the cells were BrdU-positive in the control, whereas only 15% of cells were positive when keratinocytes were cultured in the presence of 4MU. As expected for 4MU (24), this anti-proliferative effect was accompanied by reduced expression of both HAS1 and HAS3 mRNAs. (Fig. 7, C and D).
FIGURE 7.
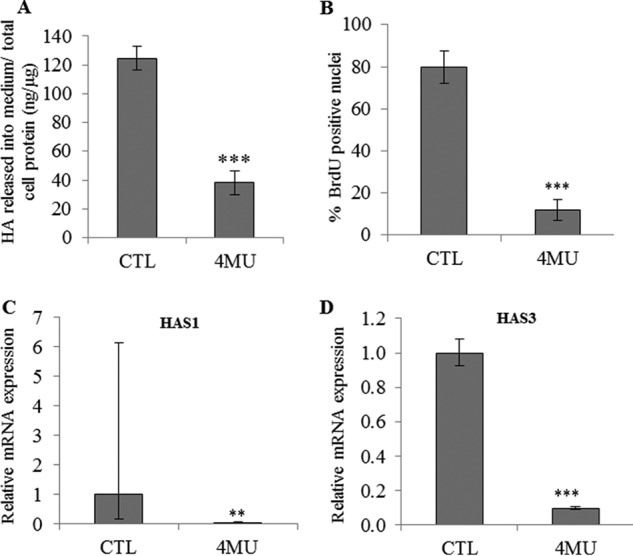
Effect of HA depletion using 4MU on proliferation of keratinocyte monolayer cultures. Proliferative keratinocytes were grown in monolayer and treated 4 days before confluence with 0.5 mm 4MU during 48 h. A, newly synthesized HA in the medium was measured at the end of treatment and normalized with respect to the total amount of cellular protein. Data are shown as means ± S.D. B, cells were incubated with BrdU for 24 h, and then fixed and immunostained for BrdU and nuclei. BrdU-positive nuclei were normalized to the total number of nuclei. Data are shown as means ± S.D. C and D, the mRNA expression levels of HAS1 (C) and HAS3 (D) were measured using quantitative PCR after reverse transcription. Expression levels were normalized to the average expression level of two reference genes, RPLP0 and TBP. The mRNA levels in control conditions were taken as references and arbitrarily fixed at 1. The error bars represent 95% confidence intervals. (n = 3, t test, **, p < 0.01, ***, p < 0.001). Experiments were performed independently on three samples (technical replicates) of keratinocytes from three separate patients (biological replicates).
Decreased Cell Proliferation Rates following 4MU Treatment Are Not Restored by HA Fragments
The previous results showed that 4MU decreases cell proliferation (but not differentiation) in both keratinocyte monolayer cultures and RHE. This could be due to a lack of secreted HA. To investigate the role of extracellular HA on proliferation, monolayer cultures were used as they allow easier access to all the cells. Subconfluent keratinocyte cultures were treated for 48 h with 4MU, and simultaneously treated with a high concentration (50 μg/ml) of HA of different molecular masses (Fig. 8). This concentration was between 5- and 10-fold higher than the endogenous HA concentration measured in RHE culture medium. In monolayers, similar to RHE, the proportion of BrdU-positive cells decreased markedly after 4MU treatment. This effect was not rescued by the addition of HA fragments of molecular masses ranging from 1.6 to 4000 kDa. Together these observations suggest that 4MU reduces keratinocyte proliferation through HA-independent mechanisms. We then tried to confirm this observation using RHE.
FIGURE 8.
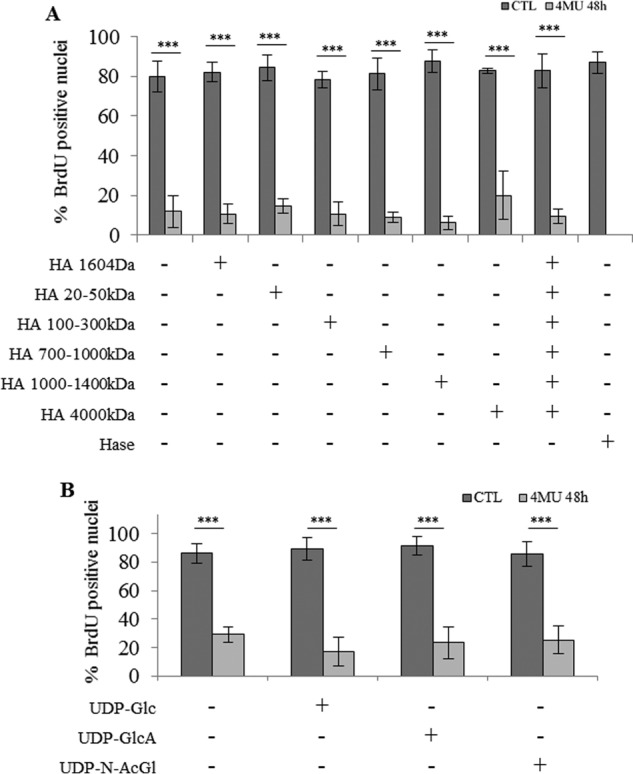
Effect of HA fragments or UDP-sugars on keratinocyte proliferation during incubation with 4MU. Proliferative keratinocytes were grown in monolayer and treated 4 days before confluence with 0.5 mm 4MU and 50 μg/ml of various molecular mass HA preparations as indicated during 48 h. Treatment with 1 unit/ml Streptomyces Hase was used as a control. Cells were incubated with BrdU for 24 h, and then fixed and immunostained for BrdU and nuclei. BrdU-positive nuclei were normalized to the total number of nuclei. Data are shown as means ± S.D. (n = 3, two-way ANOVA: ***, p < 0.001). Experiments were performed independently on three samples (technical replicates) of keratinocytes from three separate patients (biological replicates).
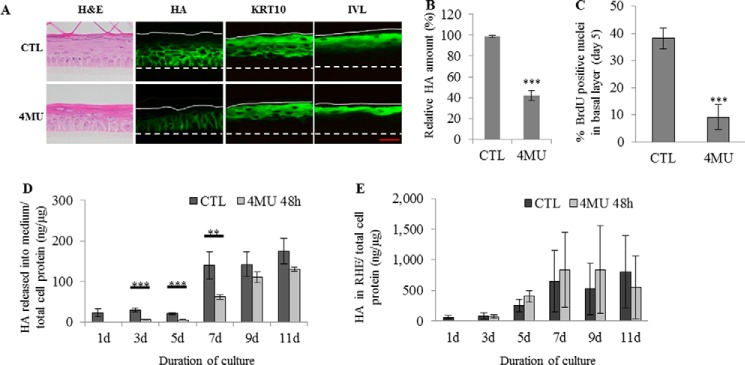
4MU Inhibits Cell Proliferation in RHE despite Limited Effect on HA Content
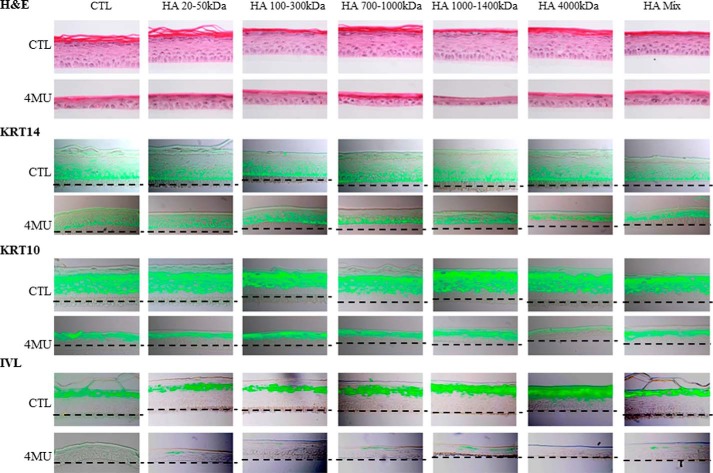
4MU was used at the same concentration (0.5 mm) in RHE because higher concentrations induced cell mortality in the tissue. Fluorescent detection of HA at day 11 (full RHE differentiation) showed a decrease in HA staining in 4MU-treated samples versus controls (Fig. 9, A and B). To determine whether the differentiation process was altered by 4MU, immunodetection of KRT10 and IVL was performed. Signals for both markers were localized at the expected locations within RHE and exhibited similar intensities with or without 4MU treatment, suggesting unaltered differentiation (Fig. 9A). Nevertheless, 4MU-treated RHE appeared thinner than both control RHE. To determine whether the proliferation process was altered, a BrdU-incorporation assay was performed between the 3rd and the 5th days of reconstruction. As shown in Fig. 9C, on the 5th day, 40% of the basal cells were BrdU-positive in control and hyaluronidase-treated tissues, whereas only 10% of basal cells were positive when RHE were cultured in the presence of 4MU. Moreover, RHE thinning following 4MU treatment was not restored by HA fragments (data not shown) and did not influence the pattern of proliferation markers (Fig. 10). It is striking that, despite little effect on HA content, incubation with 4MU markedly decreased RHE proliferation, whereas the hyaluronidase treatment, which almost completely depletes HA, had no effect.
FIGURE 9.
Effect of HA depletion using 4MU on morphology, proliferation and keratinization of a reconstructed human epidermis. RHE were analyzed after 11 days of growth on polycarbonate filter at the air-liquid interface. A, RHE were incubated in the presence of 0.5 mm 4MU or in CTL solutions between the 3th and 11th days. At day 11, RHE were fixed and stained for morphology (H&E), HA, and the differentiation markers KRT10 and IVL. Red bar = 15 μm. B, relative quantification of HA staining. CTL values were arbitrarily fixed at 1. Error bars represent standard deviations (n = 3, one-way ANOVA: ***, p < 0.001). C, proliferation was evaluated using BrdU incorporation between the 3th and 5th days. On the 5th day, RHE were fixed and immunostained for BrdU and nuclei. Nuclei positive for BrdU were normalized to the total number of nuclei in the basal layer. Data are shown as means ± S.D. (n = 3, one-way ANOVA: ***, p < 0.001). D and E, RHE were incubated with 0.5 mm 4MU starting on the 1st, 3rd, 5th, 7th, or 9th day of the tissue reconstruction. Newly synthesized HA in medium (D) and in tissue (E) was measured 48 h later and normalized with respect to total cellular protein concentration. Data are shown as means ± S.D. (n = 3, two-way ANOVA: **, p < 0.01, ***, p < 0.001). Experiments were performed independently on three samples (technical replicates) of keratinocytes from three separate patients (biological replicates).
FIGURE 10.
Effect of HA fragments on morphology and keratinization of a reconstructed human epidermis during incubation with 4MU. RHE were analyzed after 11 days of growth on polycarbonate filter at the air-liquid interface. RHE were incubated between the 3th and 11th days in the presence of 0.5 mm 4MU or in CTL solutions, together with 50 μg/ml of various molecular mass HA preparations as indicated. At day 11, RHE were fixed and stained for morphology (H&E), HA, and the differentiation markers KRT14, KRT10, and IVL. Red bar = 15 μm. Experiments were performed independently on three samples (technical replicates) of keratinocytes from three separate patients (biological replicates).
We also tested whether 4MU was able to reduce HA synthesis over the course of epidermal reconstruction. As shown in Fig. 9D, incubation of developing RHE with 0.5 mm 4MU for 48 h starting on days 1, 3, 5, 7, or 9 decreased the HA levels measured in culture medium. Day 1 corresponds to the time when keratinocytes seeded for 24 h on polycarbonate filter are exposed to air. HA levels were measured in the medium on day 2 of treatment and were significantly reduced by 4MU until day 7 of reconstruction. Later on, the HA content in the medium was not significantly altered by 4MU treatment (Fig. 9E). Overall, these results suggest that 4MU has only a transient effect on the amount of extracellular HA in RHE.
Decreased Cell Proliferation Rates following 4MU Treatment Are Not due to Apoptosis or Loss of Integrity of Cell Membrane
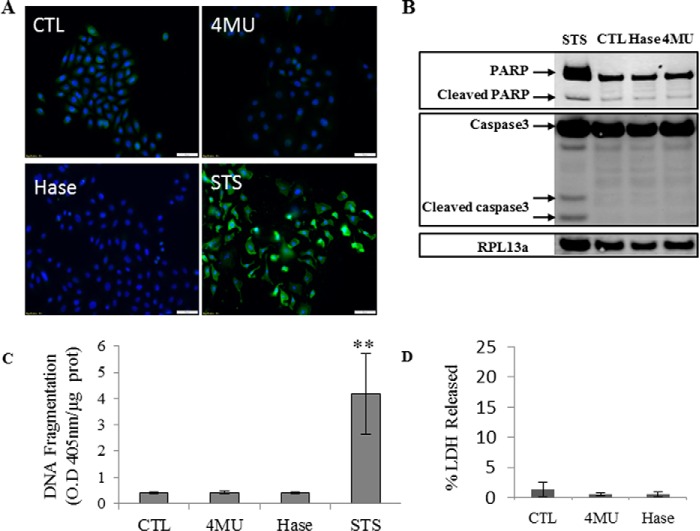
4MU has been reported to induce apoptosis (26), which could explain its anti-proliferative effects. To evaluate the effect of 4MU on keratinocyte apoptosis, we performed immunolocalization of cleaved caspase-3 in monolayer keratinocyte cultures treated with either 0.5 mm 4MU or 1 unit/ml hyaluronidase for 48 h. Keratinocytes were then incubated with 500 nm staurosporine to induce apoptosis as a positive control. We observed no signal of cleaved caspase-3 under 4MU or hyaluronidase (Hase) conditions (Fig. 11A). These results were confirmed by the absence of cleaved caspase-3 or cleaved PARP-1, its substrate, in Western blots (Fig. 11B). The inter-nucleosomal DNA cleavage (final apoptotic step) was also quantified, and no difference was observed between our conditions (Fig. 11C). Plasma membrane integrity tested using LDH release was not altered by 4MU or hyaluronidase treatment (Fig. 11D). In summary, the anti-proliferative effect of 4MU on human keratinocyte cultures is not due to apoptosis.
FIGURE 11.
Effect of 4MU or Streptomyces Hase on apoptosis in proliferative keratinocytes. Proliferative keratinocytes were grown in monolayer and treated 4 days before confluence with 0.5 mm 4MU or 1 unit/ml Hase during 48 h. Staurosporine (STS) at 500 nm during 24 h was used as a positive control of apoptosis induction. A, cells were fixed, permeabilized, stained for caspase-3 (green) and nuclei (blue), and observed under fluorescent microscopy. B, the abundance of caspase-3 and PARP was evaluated by Western blotting. Twenty μg of proteins were separated using SDS-PAGE, and RPL13a was used as loading control. C, nucleosomal DNA fragmentation was quantified by the Cell Death Detection ELISA assay (Roche). O. D. 405 nm, optical density at 405 nm. D, LDH released into the medium was measured; the results are expressed as percentages of the maximum LDH release ± S.D. (n = 3). Experiments were performed independently on three samples (technical replicates) of keratinocytes from three separate patients (biological replicates).
4MU and Hyaluronidase Treatments Do Not Affect Abundance of HA Receptors, β-Defensin 2 mRNA Expression, or the Amount of Sulfated GAGs
4MU but not hyaluronidase may affect other factors, which could themselves have an impact on keratinocyte proliferation, e.g. abundance of HA receptors, β-defensin 2 (the expression of which is increased by HA fragments (27)), or sulfated GAGs. For instance, 4MU is known to decrease the mRNA expression not only of HAS but also, in some cases, of CD44 (28). In the current study, the abundance of TLR4, RHAMM, and CD44, the three major HA receptors in monolayer keratinocyte cultures, was unchanged when measured by Western blotting after 0.5 mm 4MU or, by comparison, after 1 unit/ml hyaluronidase incubation for 48 h (Fig. 12, A–D). Similarly, β-defensin 2 mRNA expression level was not influenced by 4MU or Hase treatment (Fig. 12E). Although it is established that 4MU usually inhibits HA synthesis without affecting the production of other GAGs (3), in rare cases, 4MU might inhibit GAG production (22). Therefore, we measured the total sulfated GAG content in the medium using the dimethylmethylene blue assay. The abundance of sulfated GAGs was similar in control and treated conditions (Fig. 12F).
Discussion
Despite the high abundance of HA in the epidermis, its functions within this cutaneous tissue have not been clearly identified yet. In the present study, we investigated the potential role of HA in controlling keratinocyte proliferation and differentiation, using a model that is as close as possible to native epidermis. Our analysis of RHE firstly revealed that continuous exposure of the developing epidermis to a HA-specific hyaluronidase degrades almost all HA but does not affect proliferation or differentiation of keratinocytes embedded in this tissue. This observation was somewhat surprising based on previous studies suggesting a strong connection between HA and keratinocyte proliferation (8, 9) or differentiation (14). However, when we tried to replicate these results using a classical HA synthesis inhibitor, namely 4MU, instead of removing HA through hyaluronidase activity, we observed a marked decrease in proliferation, leading to a thinner epidermis, whereas the differentiation markers were preserved. However, despite a strong negative influence of 4MU on HAS1 and HAS3 expression (HAS2 expression is negligible in the RHE system), 4MU was less efficient than hyaluronidase in removing tissue HA, probably because pre-existing HA has a slow turnover, at least in RHE. Differences between the effects produced by hyaluronidase and by 4MU have been observed in other studies of keratinocytes. For example, high concentrations of 4MU block the cellular proliferation of rat keratinocyte monolayers (12), but treatment with hyaluronidase is unable to affect proliferation in a rat organotypic model (14). Another surprising observation in the current study was the absence of any effect of adding HA fragments, regardless of their molecular mass, on keratinocyte proliferation and differentiation, contrary to previous observations (10, 11).
Because the sizes of HA fragments may influence keratinocyte proliferation (10, 29), we tried to rescue the anti-proliferative effect of 4MU using HA samples of different molecular masses. However, we were unable to restore the proliferation rate of keratinocyte monolayers using various forms of exogenous HA. Similar observations have been made by Rilla et al. (12) in 2004 using high molecular mass HA. Conversely, in 4MU-treated smooth muscle cells, cell proliferation can be restored by adding exogenous HA (30).
Based on these elements, we reasoned that 4MU but not hyaluronidase could increase apoptosis, a known effect of 4MU in e.g. aortic smooth muscle cells (26). However, the typical apoptotic markers, cleaved caspase-3, PARP-1, or DNA nucleosomes, remained stable after incubation with 4MU or hyaluronidase. Similarly, there was no effect of 4MU on sulfated GAG production, in line with observations made in smooth muscle cells (30), nor on the expression of HA receptors. The effect of 4MU on HA receptors has been variable depending on the cell types investigated (24, 28).
At this stage, we must conclude that the anti-proliferative effect of 4MU on human keratinocytes cannot be ascribed to HA removal or even to prevention of HA actions. Treatment with hyaluronidase is effective in removing extracellular HA but has no effect on proliferation. Several published studies have used either 4MU or hyaluronidase to manipulate endogenous HA levels, but very few have analyzed the two substances in a comparative fashion. 4MU is known to decrease the proliferation rate of many cancer cells (24, 31–34), as well as smooth muscle cells (30), without clear evidence of apoptosis and/or toxicity. However, this effect has not systematically been linked to HA removal. To confirm that HA removal is not involved in the anti-proliferative effect of 4MU on human keratinocytes, we infected monolayers with lentiviral particles expressing one shRNA targeting UGDH. The UGDH enzyme catalyzes oxidation of UDP-glucose into UDP-glucuronic acid required for HAS functioning. We observed that UGDH knockdown in keratinocytes correlates with a decrease in HA production, similar to the decrease obtained in human smooth muscle cells with the same shRNA (35). However, there was no simultaneous effect on keratinocyte proliferation. Conversely, transient UGDH inhibition is able to decrease cell proliferation in some cancer cell types due to HA removal (22). Finally, we observed that CD44 knockdown does not affect human keratinocyte proliferation, whereas keratinocyte-specific deletion of CD44 in mice does inhibit the proliferation of these cells (36).
The anti-proliferative effect of 4MU on human keratinocytes is thus likely dependent on a mechanism other than HA-CD44 signaling. Recently, Mizunuma and co-workers (31) have demonstrated a marked anti-proliferative effect of 4MU on the HRA ovarian adenocarcinoma cell line (with no effect on cell migration or invasion) despite very low levels of expression of HAS and CD44 in those cells. In that case, 4MU inhibited the expression of thymidine phosphorylase, an enzyme with both proliferative and angiogenic activities (37). A similar inhibitory effect may exist in human keratinocytes and could explain our current observations.
Our study does not support an important role of the HA-CD44 axis in normal epidermal proliferation or differentiation, in line with a least one previous study (13). Some observations in mouse skin also suggest a limited role of HA in epidermal turnover. For instance, keratinocyte proliferation was unaltered in knock-out mice lacking functional HAS1, HAS3, or both, even during wound healing conditions (38–40) and despite some compensatory effects produced by HAS2. Furthermore, mice overexpressing hyaluronidase-1 in the skin exhibit no differentiation or proliferation abnormalities in the epidermis despite lowered HA content (41). Finally, CD44-deficient mice exhibit a normal rate of proliferation in the epidermis (11).
HA may indeed have a more critical role in the upper part of the epidermis, just below the stratum granulosum, as an additional protection layer or, once degraded by various invaders, as a damage-associated molecular pattern (42). Further studies are needed to unravel these roles of HA in the skin.
Besides demonstrating that HA does not regulate human epidermal keratinocyte proliferation and differentiation, our study also shows that experimental results obtained using 4MU should not automatically equate with lack of HA, but should definitely be confirmed using other approaches able to decrease HA content, because 4MU independently affects cell proliferation and perhaps other cell functions.
Author Contributions
J. M., Y. P., and B. F. conceived the study. J. M., C. L., Y. P., and B. F. designed the experiments. J. M., V. P., F. H., V. D. G., and D. V. V. performed the research. J. M., V. P., F. H., V. D. G., D. V. V., M. S., C. L., Y. P., and B. F. analyzed the data and wrote the paper. All the authors gave approval for the submitted version.
Acknowledgments
We thank K. Kathleen De Swert and B. Balau for expert technical assistance. We thank the Pierre Fabre Company (Toulouse, France) and especially Sandrine Bessou-Touya for kindly providing the HA fragments.
Note Added in Proof
Several changes were made to this article that was originally published as a Paper in Press on December 1, 2015. Fig. 1E was removed because this figure was a repetition of data presented in Fig. 12A. There was an error in the labeling of the columns in Fig. 12A: the “4MU” and “Hase” labels were inverted. Under “Experimental Procedures,” for the Western blots, there was no bromphenol blue in the lysis buffer used for protein extraction. These errors have been corrected and do not affect the conclusions of the paper.
This work was supported by FRFC Grant 2.4522.10F and FNRS Grant 1.5.003.06F (to Y. P.). The authors declare that they have no conflicts of interest with the contents of this article.
- HA
- hyaluronan
- HAS
- HA synthase
- Hase
- hyaluronidase
- GAG
- glycosaminoglycan
- 4MU
- 4-methylumbelliferone
- UGDH
- UDP-glucose 6-dehydrogenase
- LDH
- lactate dehydrogenase
- PARP
- poly(ADP-ribose) polymerase
- RHAMM
- receptor for hyaluronan-mediated motility
- RHE
- reconstructed human epidermis(es)
- β-defensin 2
- β-def2
- TBP
- TATA box-binding protein
- IVL
- involucrin
- ANOVA
- analysis of variance
- KRT10
- keratin 10
- KRT14
- keratin 14
- CTL
- control.
References
- 1. Weigel P. H., Hascall V. C., and Tammi M. (1997) Hyaluronan synthases. J. Biol. Chem. 272, 13997–14000 [DOI] [PubMed] [Google Scholar]
- 2. Itano N., Sawai T., Yoshida M., Lenas P., Yamada Y., Imagawa M., Shinomura T., Hamaguchi M., Yoshida Y., Ohnuki Y., Miyauchi S., Spicer A. P., McDonald J. A., and Kimata K. (1999) Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem. 274, 25085–25092 [DOI] [PubMed] [Google Scholar]
- 3. Tammi R. H., Passi A. G., Rilla K., Karousou E., Vigetti D., Makkonen K., and Tammi M. I. (2011) Transcriptional and post-translational regulation of hyaluronan synthesis. FEBS J. 278, 1419–1428 [DOI] [PubMed] [Google Scholar]
- 4. Csoka A. B., Frost G. I., and Stern R. (2001) The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 20, 499–508 [DOI] [PubMed] [Google Scholar]
- 5. Fraser J. R., Laurent T. C., and Laurent U. B. (1997) Hyaluronan: its nature, distribution, functions and turnover. J. Intern. Med. 242, 27–33 [DOI] [PubMed] [Google Scholar]
- 6. Simpson C. L., Patel D. M., and Green K. J. (2011) Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nat. Rev. Mol. Cell Biol. 12, 565–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tammi R., Ripellino J. A., Margolis R. U., and Tammi M. (1988) Localization of epidermal hyaluronic acid using the hyaluronate binding region of cartilage proteoglycan as a specific probe. J. Invest. Dermatol. 90, 412–414 [DOI] [PubMed] [Google Scholar]
- 8. Stern R., and Maibach H. I. (2008) Hyaluronan in skin: aspects of aging and its pharmacologic modulation. Clin. Dermatol. 26, 106–122 [DOI] [PubMed] [Google Scholar]
- 9. Tammi R., and Tammi M. (1991) Correlations between hyaluronan and epidermal proliferation as studied by [3H]glucosamine and [3H]thymidine incorporations and staining of hyaluronan on mitotic keratinocytes. Exp. Cell Res. 195, 524–527 [DOI] [PubMed] [Google Scholar]
- 10. Gu H., Huang L., Wong Y. P., and Burd A. (2010) HA modulation of epidermal morphogenesis in an organotypic keratinocyte-fibroblast co-culture model. Exp. Dermatol. 19, e336–e339 [DOI] [PubMed] [Google Scholar]
- 11. Bourguignon L. Y., Ramez M., Gilad E., Singleton P. A., Man M. Q., Crumrine D. A., Elias P. M., and Feingold K. R. (2006) Hyaluronan-CD44 interaction stimulates keratinocyte differentiation, lamellar body formation/secretion, and permeability barrier homeostasis. J. Invest. Dermatol. 126, 1356–1365 [DOI] [PubMed] [Google Scholar]
- 12. Rilla K., Pasonen-Seppanen S., Rieppo J., Tammi M., and Tammi R. (2004) The hyaluronan synthesis inhibitor 4-methylumbelliferone prevents keratinocyte activation and epidermal hyperproliferation induced by epidermal growth factor. J. Invest. Dermatol. 123, 708–714 [DOI] [PubMed] [Google Scholar]
- 13. Pasonen-Seppanen S., Karvinen S., Torronen K., Hyttinen J. M., Jokela T., Lammi M. J., Tammi M. I., and Tammi R. (2003) EGF upregulates, whereas TGF-β downregulates, the hyaluronan synthases Has2 and Has3 in organotypic keratinocyte cultures: correlations with epidermal proliferation and differentiation. J. Invest. Dermatol. 120, 1038–1044 [DOI] [PubMed] [Google Scholar]
- 14. Passi A., Sadeghi P., Kawamura H., Anand S., Sato N., White L. E., Hascall V. C., and Maytin E. V. (2004) Hyaluronan suppresses epidermal differentiation in organotypic cultures of rat keratinocytes. Exp. Cell Res. 296, 123–134 [DOI] [PubMed] [Google Scholar]
- 15. Maytin E. V., Chung H. H., and Seetharaman V. M. (2004) Hyaluronan participates in the epidermal response to disruption of the permeability barrier in vivo. Am. J. Pathol. 165, 1331–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malaisse J., Bourguignon V., De Vuyst E., Lambert de Rouvroit C., Nikkels A. F., Flamion B., and Poumay Y. (2014) Hyaluronan metabolism in human keratinocytes and atopic dermatitis skin is driven by a balance of hyaluronan synthases 1 and 3. J. Invest. Dermatol. 134, 2174–2182 [DOI] [PubMed] [Google Scholar]
- 17. Minner F., Herphelin F., and Poumay Y. (2010) Study of epidermal differentiation in human keratinocytes cultured in autocrine conditions. Methods Mol. Biol. 585, 71–82 [DOI] [PubMed] [Google Scholar]
- 18. De Vuyst E., Charlier C., Giltaire S., De Glas V., de Rouvroit C. L., and Poumay Y. (2014) Reconstruction of normal and pathological human epidermis on polycarbonate filter. Methods Mol. Biol. 1195, 191–201 [DOI] [PubMed] [Google Scholar]
- 19. Lin W., Shuster S., Maibach H. I., and Stern R. (1997) Patterns of hyaluronan staining are modified by fixation techniques. J. Histochem. Cytochem. 45, 1157–1163 [DOI] [PubMed] [Google Scholar]
- 20. Farndale R. W., Buttle D. J., and Barrett A. J. (1986) Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta 883, 173–177 [DOI] [PubMed] [Google Scholar]
- 21. Frankart A., Malaisse J., De Vuyst E., Minner F., de Rouvroit C. L., and Poumay Y. (2012) Epidermal morphogenesis during progressive in vitro 3D reconstruction at the air-liquid interface. Exp. Dermatol. 21, 871–875 [DOI] [PubMed] [Google Scholar]
- 22. Wang T. P., Pan Y. R., Fu C. Y., and Chang H. Y. (2010) Down-regulation of UDP-glucose dehydrogenase affects glycosaminoglycans synthesis and motility in HCT-8 colorectal carcinoma cells. Exp. Cell Res. 316, 2893–2902 [DOI] [PubMed] [Google Scholar]
- 23. Kakizaki I., Kojima K., Takagaki K., Endo M., Kannagi R., Ito M., Maruo Y., Sato H., Yasuda T., Mita S., Kimata K., and Itano N. (2004) A novel mechanism for the inhibition of hyaluronan biosynthesis by 4-methylumbelliferone. J. Biol. Chem. 279, 33281–33289 [DOI] [PubMed] [Google Scholar]
- 24. Kultti A., Pasonen-Seppanen S., Jauhiainen M., Rilla K. J., Karna R., Pyoria E., Tammi R. H., and Tammi M. I. (2009) 4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP-glucuronic acid and downregulation of hyaluronan synthase 2 and 3. Exp. Cell Res. 315, 1914–1923 [DOI] [PubMed] [Google Scholar]
- 25. Jokela T. A., Karna R., Makkonen K. M., Laitinen J. T., Tammi R. H., and Tammi M. I. (2014) Extracellular UDP-glucose activates P2Y14 receptor and induces signal transducer and activator of transcription 3 (STAT3) Tyr705 phosphorylation and binding to hyaluronan synthase 2 (HAS2) promoter, stimulating hyaluronan synthesis of keratinocytes. J. Biol. Chem. 289, 18569–18581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vigetti D., Rizzi M., Moretto P., Deleonibus S., Dreyfuss J. M., Karousou E., Viola M., Clerici M., Hascall V. C., Ramoni M. F., De Luca G., and Passi A. (2011) Glycosaminoglycans and glucose prevent apoptosis in 4-methylumbelliferone-treated human aortic smooth muscle cells. J. Biol. Chem. 286, 34497–34503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gariboldi S., Palazzo M., Zanobbio L., Selleri S., Sommariva M., Sfondrini L., Cavicchini S., Balsari A., and Rumio C. (2008) Low molecular weight hyaluronic acid increases the self-defense of skin epithelium by induction of β-defensin 2 via TLR2 and TLR4. J. Immunol. 181, 2103–2110 [DOI] [PubMed] [Google Scholar]
- 28. Keller K. E., Sun Y. Y., Vranka J. A., Hayashi L., and Acott T. S. (2012) Inhibition of hyaluronan synthesis reduces versican and fibronectin levels in trabecular meshwork cells. PLoS ONE 7, e48523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaya G., Tran C., Sorg O., Hotz R., Grand D., Carraux P., Didierjean L., Stamenkovic I., and Saurat J. H. (2006) Hyaluronate fragments reverse skin atrophy by a CD44-dependent mechanism. PLoS Med. 3, e493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vigetti D., Rizzi M., Viola M., Karousou E., Genasetti A., Clerici M., Bartolini B., Hascall V. C., De Luca G., and Passi A. (2009) The effects of 4-methylumbelliferone on hyaluronan synthesis, MMP2 activity, proliferation, and motility of human aortic smooth muscle cells. Glycobiology 19, 537–546 [DOI] [PubMed] [Google Scholar]
- 31. Tamura R., Yokoyama Y., Yoshida H., Imaizumi T., and Mizunuma H. (2014) 4-Methylumbelliferone inhibits ovarian cancer growth by suppressing thymidine phosphorylase expression. J. Ovarian Res. 7, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lokeshwar V. B., Lopez L. E., Munoz D., Chi A., Shirodkar S. P., Lokeshwar S. D., Escudero D. O., Dhir N., and Altman N. (2010) Antitumor activity of hyaluronic acid synthesis inhibitor 4-methylumbelliferone in prostate cancer cells. Cancer Res. 70, 2613–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Urakawa H., Nishida Y., Wasa J., Arai E., Zhuo L., Kimata K., Kozawa E., Futamura N., and Ishiguro N. (2012) Inhibition of hyaluronan synthesis in breast cancer cells by 4-methylumbelliferone suppresses tumorigenicity in vitro and metastatic lesions of bone in vivo. Int. J. Cancer 130, 454–466 [DOI] [PubMed] [Google Scholar]
- 34. Piccioni F., Malvicini M., Garcia M. G., Rodriguez A., Atorrasagasti C., Kippes N., Piedra Buena I. T., Rizzo M. M., Bayo J., Aquino J., Viola M., Passi A., Alaniz L., and Mazzolini G. (2012) Antitumor effects of hyaluronic acid inhibitor 4-methylumbelliferone in an orthotopic hepatocellular carcinoma model in mice. Glycobiology 22, 400–410 [DOI] [PubMed] [Google Scholar]
- 35. Vigetti D., Ori M., Viola M., Genasetti A., Karousou E., Rizzi M., Pallotti F., Nardi I., Hascall V. C., De Luca G., and Passi A. (2006) Molecular cloning and characterization of UDP-glucose dehydrogenase from the amphibian Xenopus laevis and its involvement in hyaluronan synthesis. J. Biol. Chem. 281, 8254–8263 [DOI] [PubMed] [Google Scholar]
- 36. Kaya G., Rodriguez I., Jorcano J. L., Vassalli P., and Stamenkovic I. (1997) Selective suppression of CD44 in keratinocytes of mice bearing an antisense CD44 transgene driven by a tissue-specific promoter disrupts hyaluronate metabolism in the skin and impairs keratinocyte proliferation. Genes Dev. 11, 996–1007 [DOI] [PubMed] [Google Scholar]
- 37. Akiyama S., Furukawa T., Sumizawa T., Takebayashi Y., Nakajima Y., Shimaoka S., and Haraguchi M. (2004) The role of thymidine phosphorylase, an angiogenic enzyme, in tumor progression. Cancer Sci. 95, 851–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bai K. J., Spicer A. P., Mascarenhas M. M., Yu L., Ochoa C. D., Garg H. G., and Quinn D. A. (2005) The role of hyaluronan synthase 3 in ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 172, 92–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kobayashi N., Miyoshi S., Mikami T., Koyama H., Kitazawa M., Takeoka M., Sano K., Amano J., Isogai Z., Niida S., Oguri K., Okayama M., McDonald J. A., Kimata K., Taniguchi S., and Itano N. (2010) Hyaluronan deficiency in tumor stroma impairs macrophage trafficking and tumor neovascularization. Cancer Res. 70, 7073–7083 [DOI] [PubMed] [Google Scholar]
- 40. Mack J. A., Feldman R. J., Itano N., Kimata K., Lauer M., Hascall V. C., and Maytin E. V. (2012) Enhanced inflammation and accelerated wound closure following tetraphorbol ester application or full-thickness wounding in mice lacking hyaluronan synthases Has1 and Has3. J. Invest. Dermatol. 132, 198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Muto J., Morioka Y., Yamasaki K., Kim M., Garcia A., Carlin A. F., Varki A., and Gallo R. L. (2014) Hyaluronan digestion controls DC migration from the skin. J. Clin. Invest. 124, 1309–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nikitovic D., Berdiaki A., Galbiati V., Kavasi R. M., Papale A., Tsatsakis A., Tzanakakis G. N., and Corsini E. (2014) Hyaluronan regulates chemical allergen-induced IL-18 production in human keratinocytes. Toxicol. Lett. 232, 89–97 [DOI] [PubMed] [Google Scholar]