Abstract
Sjögren's syndrome is a chronic autoimmune disorder characterized by lymphocytic infiltration and hypofunction of salivary and lacrimal glands. This loss of salivary function leads to oral dryness, impaired swallowing and speech, and increased infection and is associated with other autoimmune diseases and an increased risk of certain cancers. Despite the implications of this prevalent disease, diagnosis currently takes years, partly due to the diversity in patient presentation. Saliva is a complicated biological fluid with major constituents, including heavily glycosylated mucins MUC5B and MUC7, important for its viscoelastic and hydrating and lubricating properties. This study investigated Sjögren's patient's perception of dryness (bother index questionnaires) along with the rheological, protein composition, and glycan analysis of whole mouth saliva and the saliva on the mucosal surface (residual mucosal saliva) to understand the properties that most affect patient wellbeing. Sjögren's patients exhibited a statistically significant reduction in residual mucosal saliva, salivary flow rate, and extensional rheology, spinnbarkeit (stringiness). Although the concentration of mucins MUC5B and MUC7 were similar between patients and controls, a comparison of protein Western blotting and glycan staining identified a reduction in mucin glycosylation in Sjögren's, particularly on MUC7. LC-MS/MS analysis of O-glycans released from MUC7 by β-elimination revealed that although patients had an increase in core 1 sulfation, the even larger reduction in sialylation resulted in a global decline of charged glycans. This was primarily due to the loss of the extended core 2 disialylated structure, with and without fucosylation. A decrease in the extended, fucosylated core 2 disialylated structure on MUC7, residual mucosal wetness, and whole mouth saliva flow rate appeared to have a negative and cumulative effect on the perception of oral dryness. The observed changes in MUC7 glycosylation could be a potential diagnostic tool for saliva quality and taken into consideration for future therapies for this multifactorial syndrome.
Sjögren's syndrome (SS) is a chronic autoimmune disorder with a global prevalence of between 0.5–2.0%, of which 90% are female (1). The disease is characterized by an infiltration of lymphocytes into exocrine tissue, including the salivary and lacrimal glands, leading to glandular hypofunction and symptoms of dry mouth (xerostomia) and eyes (1). Chronic dry mouth leads to an increased risk of malnutrition (2), owing to problems with mastication, swallowing, and taste (3, 4), as well as an increase in caries (5) and yeast infection (6). These symptoms have a profound effect on psychological and social functioning, resulting in an overall negative effect on quality of life (7). Sjögren's can also occur in conjunction with another autoimmune disease (secondary SS) such as rheumatoid arthritis and systemic lupus erythematosus (8), and patients have a dramatically increased risk of non-Hodgkins lymphoma (relative risk of 13.7) as well as thyroid cancer (relative risk 2.58) (9). This makes diagnosis essential not only for effective disease management but also for the monitoring of other dangerous associated diseases. The heterogeneous presentation of SS, along with patients seeing different doctors for oral and eye complaints, makes diagnosis difficult and often delayed for several years. Although diagnostic criteria have changed over time with the improving knowledge of SS and now include a range of possible diagnostic benchmarks (10), a diagnostic tool that effectively describes the quality of saliva is lacking. As oral dryness is an early and prevalent symptom in SS (11), diagnosis of this symptom would improve the overall diagnosis of SS.
Saliva is a complicated and dynamic non-Newtonian biological fluid containing water, ions, and several protein groups, including mucins, proline rich proteins, amylase, and immunoglobulins (12). It is produced by the parotid, submandibular, and sublingual bilaterally paired major salivary glands as well as the minor salivary glands that are distributed throughout the oral cavity and number in the hundreds (12). To facilitate its boundary (surface) and hydrodynamic (fluid) lubricating roles, saliva comprises whole mouth saliva, which can be stimulated or unstimulated, and residual mucosal saliva (RMS), the saliva that is retained on oral surfaces (13). A mucin-rich basal level of saliva, unstimulated whole mouth saliva (UWMS), is produced at all times with the mucins principally secreted from the submandibular, sublingual, and minor glands. Saliva production can be stimulated by chewing and taste where contribution by the parotid glands, secreting a mucin-free amylase-rich serous fluid, is greatest followed by the submandibular glands (12). The RMS is made up of normal salivary proteins with an increased concentration of secreted mucins (14, 15).
Mucins are large, very heavily O-glycosylated proteins. Two secreted mucins, MUC5B (∼1 MDa) and MUC7 (∼150 kDa), are found in saliva and contain central mucin domains, rich in serine and threonine residues that covalently bind the O-glycosylation, creating an extended linear structure (16). The two salivary mucins hold a different range of O-glycans. Both mucins contain glycans from core 1 and core 2 O-glycans with a high degree of sialylation; however, those observed on MUC7 are shorter relative to MUC5B, which are larger, more complex, and show a greater range (17, 18). Furthermore, MUC5B glycosylation shows greater individual variation due to its presentation of glycans reflecting blood group and secretor status (19). The mucins and their glycosylation are essential to a variety of saliva properties. Their innate role in microbial defense is to act as decoys of microbial binding glycans to hinder pathogens from reaching and binding the mucosal surface (20, 21). They are also important for biolubrication, including boundary lubrication, as their negatively charged glycans give them hydrating and electrostatically repulsive properties (16, 22).
Mucins also contribute to salivary viscoelasticity (23, 24). Early investigations demonstrated attenuation of rheological properties upon removal of mucins and restoration following reconstitution with the mucin fraction (25). Again, it is the glycan component that is important for this property as saliva viscoelasticity is reduced upon degradation of mucin glycans (26). The diverse importance of mucins and their glycosylation shows it is imperative to understand any compositional changes that may occur in SS and lead to negative alterations in the physical properties of saliva.
Many techniques can be used to assess the physical properties of saliva and investigate oral dryness-associated diseases, including SS. Assessment of UWMS flow rate is simple and noninvasive and has been linked to oral dryness below the hyposalivation threshold rate of 0.1 ml/min (27, 28). This link is not always apparent with hyposalivation-independent xerostomia in 20–30% of early stage SS patients (11, 29). Rheological analysis is another approach to understanding saliva. Spinnbarkeit is one such property and measures the ability of a fluid to be drawn into strings, indirectly allowing measurement of elastic and adhesive properties (30) with the additional advantage of correlating with viscosity (31). RMS wetness is another important characteristic, given its involvement in lubrication, that can be measured clinically by the use of specific filter strips for collection (32). Studies have shown an inverse correlation between RMS wetness at specific sites of the mouth, including the palate (32), the anterior tongue (33), and labial mucosa (27) and hyposalivation. Although these physical parameters are important indicators of oral health, they are not effective indicators for all SS patients.
Given the complex nature and the diversity of symptoms observed in SS, it is essential to have a better understanding of salivary changes not only in UWMS but also RMS properties in this disease. To this end, a range of saliva investigations were undertaken in this discovery project, as well as the subjective analysis of the patients' discomfort (Bother Index questionnaires) to ascertain which properties most affect patient well-being. These properties included UWMS flow rate, RMS volume, the rheological property spinnbarkeit, and mucin protein and glycan content. Changes in MUC7 O-glycosylation were also investigated, not only from the UWMS but also from the low abundance (<3 μl saliva) RMS from the lower labial surface.
EXPERIMENTAL PROCEDURES
Ethics Approval
Ethical approval was granted by the National Research Ethics Service (NRES9 Committee London-Brent (11/LO/1121). Informed written consent was obtained from all patients, controls, and volunteer subjects included in the study.
Saliva Sample Collection
Saliva samples were collected from patients diagnosed with Sjögren's syndrome (n = 25) and healthy age-matched control subjects (n = 35). Patients were attending the oral medical clinic at Guy's hospital, London. Diagnosis of patients was achieved using the revised European classification criteria, American-European Consensus Group (34). UWMS was collected using the passive drool technique over a 10 min period following a minimum 1 h fasting period, and flow rates were calculated gravimetrically. Bacterial, cellular, and other debris were removed from UWMS by centrifugation (2000 × g, 10 min, 4 °C) and the supernatant stored at −80 °C. Mucosal hydration was assessed by collecting residual mucosal saliva from the: anterior hard palate (AHP)1, buccal (BUC), anterior tongue (AT), and lower labial (LL) oral surfaces using SialoPaperTM collection strips (surface area 44.15 mm2, Oralflow, NY, USA). Saliva volume was calculated by measuring electrical capacitance of the wet collection strip using a Periotron® 8000 (Oralflow), the values then converted to fluid volume using a standard curve as described previously (14). RMS samples were stored at −80 °C and eluted prior to analysis.
Clinical Oral Dryness Assessment
All subjects were assessed for xerostomia, the subjective feeling of dryness, using a questionnaire-type Bother Index (BI) (35) and for objective signs of dryness using the Clinical Oral Dryness Score (CODS) (36). The CODS comprises 10 clinical features such as sticky mucosa and fissured tongue that are associated with oral dryness. The BI is a two-part questionnaire, the first consisting of five questions, B5, and the second a single visual analogue scale, B1, giving details on a subject's perception of dry mouth (xerostomia). In both assessments, a higher score is indicative of greater levels of oral dryness.
Spinnbarkeit (Rheological) Measurement
A Neva-MeterTM (IMI-0501, Ishikawa Ironworks Co., Japan) was used to carry out spinnbarkeit measurement on neat, fresh UWMS at room temperature. Sample (50 μl) was loaded, and three repeat readings were taken. Saliva was stretched with a constant stretching rate of 5 mm/sec until break point, which was determined by electrical conductivity (31).
Sample Preparation, Electrophoresis, and Visualization
All samples were prepared under reducing conditions with 50 mm DTT, 25% NuPAGE lithium dodecyl sulfate sample buffer (Life Technologies, Calsbad, USA), and water (RMS samples only) and heat denatured (3 min, 100 °C). For RMS samples lithium dodecyl sulfate, DTT, and water were applied directly to the SialoPaperTM, heat denatured, and eluate collected by centrifugation (15,000 × g, 2 min). Protein content was assessed using SDS-PAGE on NuPAGE 4–12% bis-Tris gels (Life Technologies). For salivary mucin (MUC5B and MUC7) analysis, gels were stained for glycosylated mucin detection or transferred to nitrocellulose membranes (for mucin protein immunodetection and lectin detection) or PVDF membrane (for O-glycan analysis).
Periodic Acid Schiff's (PAS) staining
PAS oligosaccharide stain was used to detect the carbohydrate component of the salivary mucins. Electrophoresed gels were fixed (25% methanol and 10% glacial acetic acid) for 1 h, followed by a 20 min water wash and oxidation for 15 min in 2% periodic acid. Gels were then washed in water for 5 min and placed in Schiff's reagent (VWR, Lutterworth, UK) for 30–60 min, in the dark. All steps were carried out under gentle agitation. Gels were destained in water.
Mucin Western Blotting
Detection of MUC5B and MUC7 was carried out using antibodies directed to mucin protein core (37). Saliva was separated by SDS-PAGE and transferred to nitrocellulose membranes for Western blot analysis. Membranes were blocked in 0.1 m TBS-T (TBS- 0.1% Tween®-20) (for MUC5B) or 2% (w/v) skimmed milk powder (LabM Ltd., Lancashire, UK) TBS-T solution (for MUC7) for 60 min. Membranes were incubated for 60 min in monoclonal antibodies (hybridoma supernatant): EU-MUC5Bb 1:100 or EU-MUC7a 1:100 (EU Consortium on mucins in inflammatory disease, gifts of Prof. Dallas Swallow, University College London, UK) or anti-MUC7 HPA006411 at 0.13 μg/ml (Sigma Aldrich, Dorset, UK) in TBS-T or 2% skim milk in TBS-T. Membranes were then incubated with polyclonal secondary antibody, goat anti-mouse immunoglobulins HRP conjugated at 0.5 μg/ml (P0447, Dako) or goat anti-rabbit immunoglobulins HRP conjugated at 0.125 μg/ml (P0448, Dako) in TBS-T for 60 min. Membranes were washed 3 × 5 min with TBS-T between incubations. ClarityTM Western ECL Substrate (Bio-Rad, Hemel Hempstead, UK) was used for signal detection with the aid of a ChemiDocTM MP imaging system (Bio-Rad).
Sialic Acid Detection
Detection of specific sialic acid moieties was carried out using lectins. Saliva was separated by SDS-PAGE and transferred to nitrocellulose for lectin detection. Membranes were processed as for mucin protein detection with the following changes. Membranes were washed in TBS-T for 30 min. Sialic acid residues were detected using Sambucus nigra agglutinin (SNA) lectin, which preferentially binds α-2, 6 linked sialic acid, and Maackia amurensis leukoagglutinin II (MAL II) lectin, which preferentially binds α-2, 3 linked sialic acid (Vector Laboratories, CA, USA), diluted to 0.05 μg/ml and 0.4 μg/ml, respectively, in TBS-T, and incubated for 60 min. Lectin signal was detected using Vectastain® ABC Kit (Vector Laboratories) and the chemiluminescent substrate described above. Lectin staining was validated by digesting saliva with a α-2,3 linked sialic-acid-specific sialidase (New England Biolabs, P0720S) and a general α-2,3, α-2,6, α-2,8 linked sialic-acid-specific sialidase (New England Biolabs, P0728S) before lectin staining.
Mucin Quantification
Densitometry using purified mucin standards (gifts of Prof. Claes Wickström, Malmö University, Sweden) of known concentrations were used to generate a standard curve to calculate mucin protein concentrations in saliva samples. In the case of sialic acids where a purified standard was not used, pixel intensities were used directly. Densitometry measurement for Western blotted MUC5B, MUC7, and sialic acid was achieved using ChemiDocTM complementary software ImageLabTM (Version 4.0 build 16, Bio-Rad). Densitometry measurement for PAS-stained MUC5B and MUC7 was achieved using ImageJ software (38).
Preparation of O-glycans by Reductive β-Elimination
Saliva samples separated by SDS-PAGE and transferred to PVDF membranes were stained for 20–30 min with Alcian blue stain solution (0.125% Alcian blue, 25% ethanol, 10% acetic acid) and destained in methanol overnight. Alcian blue stained MUC7 band (running between 188 and 98 kDa) was excised. Release of O-glycans from excised MUC7 bands was achieved by reductive β-elimination (39). Briefly, samples were incubated for 16 h at 50 °C with 20 μl of fresh reductive β-elimination solution (50 mm sodium hydroxide and 0.5 m sodium borohydride). Samples were neutralized with 1 μl glacial acetic acid.
Desalting of neutralized O-glycan samples was achieved using 40 μl of AG50WX8 resin (BioRad, Hercules, CA) in C18 ZipTips (Millipore, Billerica, USA), recovering glycans with water as an unretarded fraction (39). Glycan eluate was completely dried by vacuum evaporation and further desalted with 5 × 50 μl 1% glacial acetic acid in methanol. Samples were stored at −20 °C and rehydrated in water immediately prior to LC-MS/MS analysis. A pooled sample of saliva was included in each gel to monitor transfer and β-elimination efficiency.
LC-MS/MS of O-Glycans from MUC7
An HTC PAL autosampler (CTC Analytics) and an Agilent 1100 series HPLC were used, attached to a LTQ linear ion trap (Thermo Fisher Scientific) for negative ion mode LC-MS/MS. Fused silica HPLC columns with an inner diameter of 250 μm and 10 cm length were packed with 5 μm porous graphitized carbon (PGC) particles. A 10–15 μl flow rate after passive splitting (from 250 μl/ml) was used for O-glycan separation. The mobile phase used consisted of solvent A (10 mm ammonium bicarbonate) and solvent B (10 mm ammonium bicarbonate with 80% acetonitrile). Initially, 100% of solvent A was run for 5 min duration. This was changed to solvent B to 45% in 41 min followed by 100% solvent B for 8 min and equilibrated to 100% solvent A for 25 min. A standard of glycans released from porcine gastric mucin was run every four samples to monitor instrument performance. Three glycans were specifically monitored and CVs maintained below 10 for consistency. Sample order was randomized, and blanks were run after every sample.
XcaliburTM (Rev.2.0.7, Thermo Fisher Scientific) software was used to visualize data for peak identification and quantification of relative glycan abundance. All glycans were detected as reduced alditols. O-glycans were identified by manual annotation of MS/MS spectra using fragmentation principals as previously described (40) and following guidelines set out by previous, extensive analysis of MUC7 O-glycans released under the same conditions (18). Low-intensity isomers not confirmed in multiple samples were not included in the study. Peaks were considered if they were 5% of the glycan base peak. It was assumed that all reducing ends were GalNAc-ol. All MS/MS were inspected manually to assess spectra quality and fragmentation patterns were also compared with those present in the publically available UniCarb-DB glycan MS/MS and retention time database containing 635 spectra of 451 different structures (41) (www.unicarb-db.org). Structures used in the results and supplementary tables accurately depict the level of annotation, and for instance, where the location of a residue is unknown, it is shown with a bracket. All raw data used for MS analysis in this study were submitted via Proteios (42) for public availability in SweStore, the Swedish data storage infrastructure (links in Supp. 2).
Relative, quantitation was performed as described previously (43). Intensities were determined from integrated single-ion chromatograms that were normalized to total O-glycan ion intensity. All data were converted to relative percentage to allow for comparison between samples with differing intensities. Relative refers to the relative intensity of each glycan or glycan group within a patient sample. Quantitation was completed with no adjustment to the original data. No sodium adducts were apparent during quantitation analysis. Isomers were effectively separated by PGC chromatography. Quantitation was performed on individual isomers, and only after this analysis were isomers grouped to streamline the data where isomers differences were not statistically significant. As patient samples were available in very minute amounts, replicates were not possible. To improve statistical confidence, all patient groups started with 12–14 patients with patients removed when intensity was too low for effective annotation and quantitation. Data are shown in the supplementary tables with the standard error shown.
Statistical Analysis
All data were tested for normality (D'Adostino and Pearson omnibus normality test) to establish the type of analysis (parametric or nonparametric) required. Differences between samples were analyzed using unpaired, two tailed t test and two-way analysis of variance for parametric data. The Mann–Whitney test was applied for nonparametric data. All data analyses were carried out using GraphPad Prism 6 software (La Jolla, California, USA).
RESULTS
Saliva Flow Analysis, Rheology, Residual Mucosal Saliva and Oral Dryness Scores
Comparative analysis of salivary physiological properties was carried out between age-matched SS patients and controls as shown in Table I. Patients were assessed using both objective (CODS) and subjective (BI) dryness assessments indicating all SS patients used in the study suffered from oral dryness (p < .001). SS patients UWMS flow rate (0.15 ± 0.03 ml/min, n = 25) was statistically significantly (p < .0001) reduced compared with controls (0.41 ± 0.05 ml/min, n = 35). RMS was measured using filter paper strips on four mucosal surfaces (AHP, BUC, AT, and LL), revealing a characteristic intraoral wetness pattern, with the AHP the least hydrated followed by the LL, BUC, and AT. This pattern was consistent in both patients and controls, suggesting it is independent of UWMS flow rate. RMS was significantly reduced on all four mucosal surfaces of the SS patients (Table I). PAS staining was also performed on RMS samples and showed a trend toward an increased level of PAS staining in SS patients compared with controls (Table I), suggesting that the reduction in RMS volume in SS patients is due to a decrease in water but not mucin.
Table I. Comparison of UWMS flow rate, rheology and dryness assessment for SS patients and healthy controls.
| Sjögren's patients |
Controls |
||||
|---|---|---|---|---|---|
| Mean ± S.E. | n | Mean ± S.E. | n | ||
| Age (years) | 55.00 ± 3.16 | 25 | 55.71 ± 3.01 | 35 | |
| CODS (score) | 4.04 ± 0.42a | 24 | 0.27 ± 0.08 | 30 | |
| BI (score) | B5 | 7.46 ± 1.01a | 24 | 0.13 ± 0.06 | 32 |
| B1 | 5.98 ± 0.63a | 0.03 ± 0.03 | |||
| UWMS Flow Rate (ml/min) | 0.15 ± 0.03a | 25 | 0.41 ± 0.04 | 35 | |
| RMS (μl) | AHP | 0.36 ± 0.05b | 24 | 1.09 ± 0.16 | 35 |
| BUC | 2.08 ± 0.21c | 3.05 ± 0.16 | |||
| AT | 2.27 ± 0.26c | 3.28 ± 0.13 | |||
| LL | 1.20 ± 0.18c | 2.18 ± 0.22 | 34 | ||
| RMS MUC7 PAS (staining intensity) | AHP | 1504 ± 886b | 23 | 292 ± 40 | 30 |
| BUC | 488 ± 137 | 23 | 271 ± 43 | 30 | |
| AT | 721 ± 154 | 23 | 266 ± 34 | 30 | |
| LL | 877 ± 200 | 14 | 669 ± 171 | 29 | |
| Spinnbarkeit (mm) | 4.67 ± 0.78d | 21 | 28.54 ± 5.66 | 30 | |
Objective dryness assessment was achieved using the Clinical Oral Dryness Score (CODS). Bother Index (BI), a two-part questionnaire (B1 and B5), was used for subjective dryness assessment. Unstimulated whole mouth saliva (UWMS) flow rate was calculated gravimetrically. Residual mucosal saliva (RMS) was collected and measured from four oral mucosal surfaces: anterior hard palate (AHP), buccal (BUC), anterior tongue (AT), and lower labial (LL) and stained by PAS. Extensional rheological was quantified by measuring saliva spinnbarkeit. Statistical significance is shown as a p ≤ .0001, b p ≤ .05, c p ≤ .001, d p ≤ .01.
Extensional rheology, or spinnbarkeit, is the stringiness of a substance and is an indirect measure of the adhesive properties of a solution (30). Patient spinnbarkeit was statistically significantly lower (p < .05), forming much shorter filaments than the controls. Alteration in this viscoelastic property of saliva, in addition to reduced salivary flow rate and mucosal hydration, indicate the quality of saliva was affected in addition to its quantity. Thus, further investigation of saliva composition was undertaken.
UWMS Mucin Composition
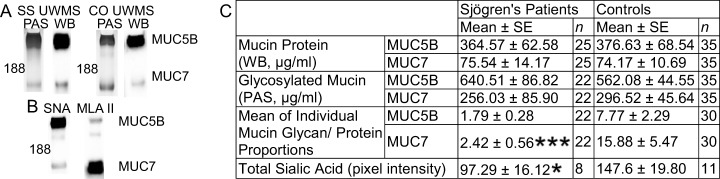
Salivary MUC5B and MUC7 are important contributors to saliva viscoelasticity, thus analysis of these proteins was performed. The detection of MUC5B and MUC7 protein and glycan components was carried out using antibodies directed to nonglycosylated regions of the protein core (37) and PAS stain (Fig. 1A). Mean MUC5B and MUC7 protein concentrations were similar in patients and controls, as were the PAS glycan staining results, with no statistically significant difference between patients and controls (Fig. 1C). An estimate of the level of mucin glycosylation per unit protein was calculated for each sample and the mean results shown in Fig. 1C. The proportion of MUC7 glycan/protein was significantly reduced (p = .0001) in SS (2.42 ± 0.56) compared with controls (15.88 ± 5.47). A similar trend (p = .053) was observed in MUC5B with patients showing lower glycosylation (1.79 ± 0.28) than controls (7.77 ± 2.29).
Fig. 1.
Comparison of UWMS MUC5b and MUC7 from SS patients and controls. (A) Mucins were detected using PAS stain for mucin glycosylation detection (PAS) and protein detected using Western blotting (WB) with antibodies directed to a nonglycosylated region of the protein. (B) Sialic acid was assessed by SNA (MUC5B) and MALII (MUC7) lectins. (C) Quantification of mucins used serially diluted purified mucin standards and the mucin glycan/protein proportion calculated individually and the mean calculated. Statistical significance is shown as * p ≤ .05, **** p ≤ .0001.
Sialic acid content of UWMS MUC5B and MUC7 was assessed using SNA lectin, which preferentially binds α-2,6 linked sialic acid residues, and MAL II lectin, which preferentially binds α-2,3 linked sialic acid. MUC5B preferentially bound SNA, and this lectin was used for MUC5B sialic acid content determination (Fig. 1B). MAL II was used for MUC7 as this lectin bound favorably (Fig. 1B). Total UWMS mucin sialic acid content (sum of MUC7 MAL II and MUC5B SNA) was significantly reduced (p = .041) in patients (97.29 ± 16.12) compared with controls (147.6 ± 19.80, Fig. 1C). This reduction in sialylation, in conjunction with a reduction in the proportion of mucin glycan/protein, indicates altered mucin glycosylation in SS.
MUC7 O-Glycan LC-MS/MS analysis of Sjögren's Patients and Controls
Given the difference observed in mucin sialylation between SS patients and controls, further investigation of MUC7 O-glycosylation was undertaken. UWMS and RMS from the lower labial surface were analyzed as pathology in lower labial gland biopsies has been well described in SS (44). The analysis of O-glycans from RMS has not previously been undertaken given the small amount of saliva sampled as an RMS collection strip is only able to hold a maximum volume of ∼3 μl of total saliva. The amount of saliva sampled here ranged from 1.2 ± 0.2 in SS samples to 2.2 ± 0.2 in controls. The most intense glycans were analyzed by LC-MS/MS analysis using PGC chromatography to separate isomers. Relative quantitation O-glycans for each sample was then carried out (Supp. 1). The highly sensitive nature of this method allowed the analysis of all 13 SS RMS.LL samples; however, only four of the control samples provided quantifiable data, reducing the usefulness of this data particularly given the high variation present between the four samples. This lack of intensity in the control RMS.LL suggests that, although the SS samples had a reduced volume of RMS (Table I), MUC7 was more concentrated compare with controls. PAS staining in SS RMS samples (877 ± 200 staining pixel intensity) also tended toward an increased level compared with controls (669 ± 171 staining pixel intensity, Table I) particularly when the reduced volume in SS samples (SS 1.2 ± 0.2, CO 2.2 ± 0.2) is considered.
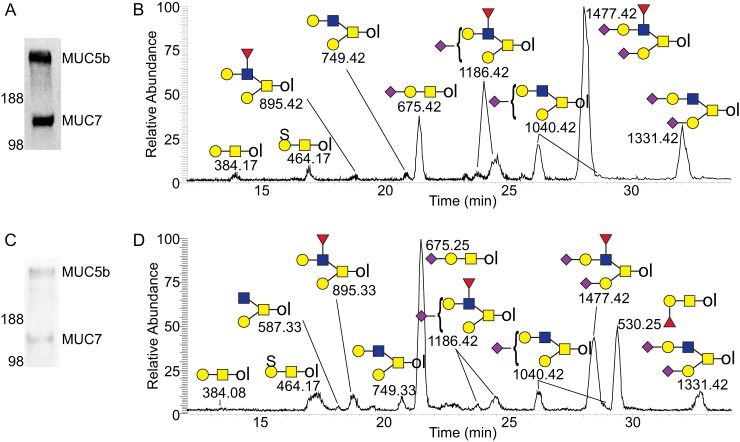
The types of O-glycan structures identified did not vary between UWMS and RMS samples or between control and SS samples and are glycans previously reported on salivary MUC7(18). Considerable variation in individual glycan abundance was observed between individuals (Supp. 1), irrespective of their health status in both UWMS and RMS.LL. The identified glycans were made up of core 1 (Gal β1–3 GalNAc-ol, m/z 384.1) and core 2 (GlcNAc β1–6 (Gal β1–3) GalNAc-ol, m/z 587.2) structures as shown in the UWMS SS and control base peak chromatograms in Fig. 2. The core 1 structures included the sulfated (m/z 464.1), sialylated (branched and linear structures, m/z 675.2), and disialylated structures (m/z 966.3). Many MUC7 samples also carried the small blood group H trisaccharide Fuc α1–2 Gal β1–3 GalNAc-ol (m/z 530.2). The core 2 structures included the neutral structure with the addition of a galactose (Gal β1–4 GlcNAc β1–6 (Gal β1–3) GalNAc-ol, m/z 749.3) from which all other core 2 structures extended, including the further addition of a fucose to the GlcNAc residue (m/z 895.3). The remaining core 2 structures were the sialylated (two isomers, m/z 1040.4), disialylated (m/z 1331.5) and the largest fucosylated disialylated structure core 2 structure (m/z 1477.5).
Fig. 2.
LC(PGC)-MS/MS analysis of UWMS MUC7 O-glycans from SS patients and controls. Control (A) and SS patient (C) saliva samples separated by SDS-PAGE, transferred to PVDF membrane, and stained with Alcian blue. Base peak chromatograms of the UWMS MUC7 O-glycans from a control (B) and SS patient (D). O-glycans from MUC7 bands were released using reductive β-elimination and analyzed using LC(PGC)-MS/MS. O-glycan alditols are labeled with observed m/z and appropriate structural definition. ♦ Sialic acid, ▴ fucose, ● galactose, ■ N-acetylgalactosamine (GalNAc), ■ N-acetylglucosamine (GlcNAc), and S sulfate.
The comparison of UWMS and RMS.LL O-glycan LC-MS/MS data showed some trends consistent between the patient and control groups (Fig. 3). RMS.LL samples had a greater proportion of total sialylated structures compared with UWMS (p < .05) in both patients (RMS.LL 79.1 ± 3.7, UWMS 56.8 ± 8.3) and controls (RMS.LL 90.5 ± 1.4, UWMS 81.0 ± 3.1), an increase predominantly due to core 2 disialylated structures (Table II). Sulfation, however, showed a trend toward reduction in RMS.LL compared with UWMS in both patients (RMS.LL 6.9 ± 3.0, UWMS 12.1 ± 3.4) and controls (RMS.LL 2.2 ± 0.3, UWMS 3.3 ± 0.6). Overall, RMS.LL had a greater abundance of longer, sialylated sugar structures and reduced sulfation compared with UWMS.
Fig. 3.
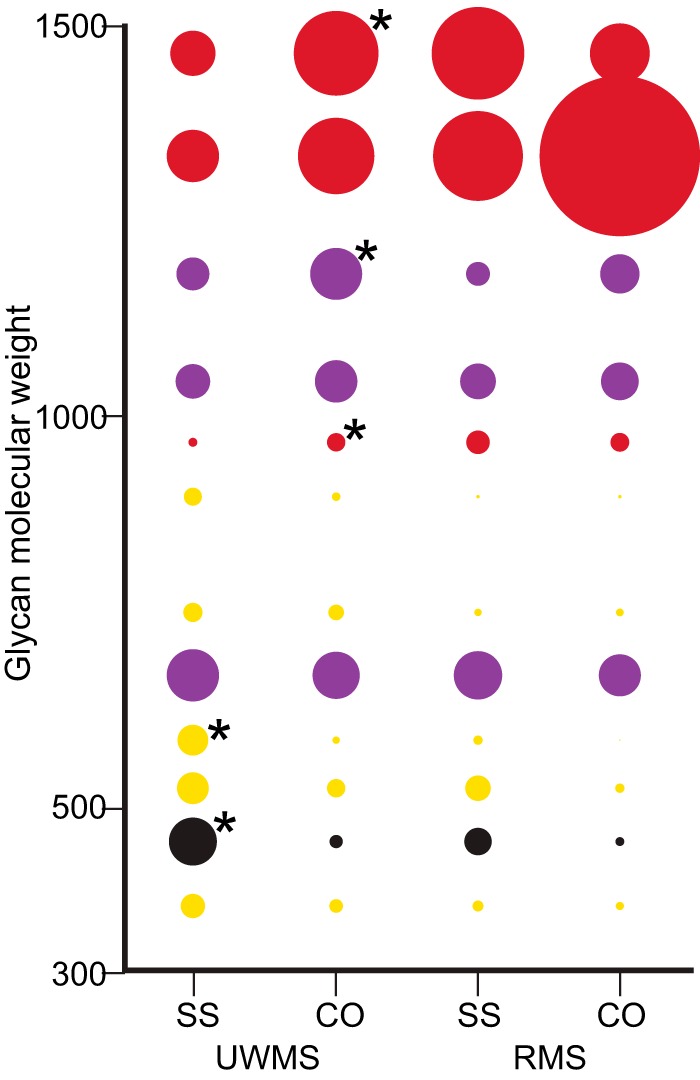
Relative percentage abundance of MUC7 O-glycans in UWMS and RMS. LL of SS patients and controls. Relative percentage abundance is shown by size of circle with all isomers grouped. UWMS SS patients (n = 12) and controls (n = 13) and RMS.LL SS patients (n = 12) and controls (n = 4) are shown. Neutral glycans are in yellow, sulfated in black, monosialylated in purple, and disialylated in red.
Table II. Relative percentage abundance of MUC7 O-glycan groups in UWMS and RMS.LL of SS patients and controls.
| UWMS |
RMS.LL |
||||
|---|---|---|---|---|---|
| Sjögren's |
Controls |
Sjögren's |
Controls |
||
| mean ± S.E. | mean ± S.E. | mean ± S.E. | mean ± S.E. | ||
| n | 12 | 13 | 13 | 4 | |
| Core 1 | 41.6 ± 6.2 | 27.7 ± 2.8 | 34.0 ± 2.8 | 21.9 ± 4.4 | |
| Core 2 | 58.4 ± 6.2 | 72.3 ± 2.8 | 66.0 ± 2.8 | 78.1 ± 4.4 | |
| Neutral glycans | Total | 31.1 ± 7.0 | 15.7 ± 3.0 | 14.0 ± 1.4a | 7.3 ± 1.6 |
| Core 1 | 14.1 ± 4.2 | 8.0 ± 1.6 | 9.1 ± 1.2a | 4.3 ± 1.8 | |
| Core 2 | 17.0 ± 3.4a | 7.8 ± 2.1 | 4.9 ± 1.2 | 3.0 ± 0.9 | |
| Sialylated glycans | Total | 56.8 ± 8.3a | 81.0 ± 3.1 | 79.1 ± 3.7a | 90.5 ± 1.4 |
| Core 1 | 15.4 ± 2.3 | 16.5 ± 1.5 | 18.1 ± 2.0 | 15.3 ± 4.7 | |
| Core 2 | 41.4 ± 8.8a | 64.5 ± 3.9 | 61.0 ± 2.9a | 75.2 ± 5.0 | |
| mono- | 30.2 ± 3.0 | 35.7 ± 1.1 | 27.1 ± 1.4 | 30.0 ± 4.3 | |
| di- | 26.7 ± 6.6a | 45.3 ± 3.8 | 52.0 ± 3.7 | 60.5 ± 4.0 | |
| Sulfated Core 1 | 12.1 ± 3.4a | 3.3 ± 0.6 | 6.9 ± 3.0 | 2.2 ± 0.3 | |
Relative quantification is based on LC-MS/MS data with intensities determined and concerted to relative percentage. Glycans are grouped according to core (1 or 2) and charge, including neutral structures and negatively charged sialylated and sulfated structures. Monosialylated structures are (m/z) 675.2, 1040.4, and 1186.4 and disialylated structures are (m/z) 966.3, 1331.5, and 1477.5. Statistical significance is shown as a where p ≤ .05.
Comparison of the SS patient and control MUC7 O-glycan abundance data is shown for individual glycans in Fig. 3 and glycan groups in Table II with all individual patient data shown in Supp. 1. Patient UWMS had a higher abundance of core 1 structures (41.6 ± 6.2) compared with controls (27.7 ± 2.8), which had higher core 2 abundance. The sulfated core 1 (m/z 464.1, S(Gal β1–3 GalNAc)) was greater in patients (12.1 ± 3.4, p < .05) than in controls (3.3 ± 0.6). The abundance of sialylated core 2 structures was statistically significantly lower in patients (SS 41.4 ± 8.8, CO 64.5 ± 3.9, p < .05) with this reduction primarily due to a reduction in disialylated structures (SS 26.7 ± 6.6, CO 45.3 ± 3.8, p < .05). As shown in Fig. 3, the most obvious and statistically significant reduction (p = .04) in core 2 disialylated structures is the largest glycan included in the study, the fucosylated, disialylated extended core 2 structure (m/z 1477.5), where SS patients' UWMS MUC7 contained almost half (11.2 ± 3.2) of that observed in controls (21.4 ± 3.3). Together, these results indicate a statistically significant reduction in extended core 2 disialylated structures in patients UWMS compared with controls.
Comparative analysis of patient and control RMS.LL samples was less informative given the number of control samples (n = 4) was too small and the variation too large to give sufficient power to any statistical analysis. These data do, however, show trends that may be used as a guide for further research (Fig. 3). Similar to the trends observed in UWMS, patient RMS had a higher abundance of neutral core 1 structures (9.1 ± 1.2) compared with controls (4.3 ± 1.8). An overall reduction in core 2 sialylated structures on SS MUC7 (61.0 ± 2.9) was also apparent when compared with controls (75.2 ± 5.0); however, the alteration in distribution of the individual glycans (above molecular weight 1000, Fig. 3) in control RMS.LL is perhaps more to do with the variation in this subject group. The sulfated core 1 showed a trend toward an increase in patients' RMS.LL (Fig. 3). These data suggest that like UWMS, RMS.LL samples display changes in the distribution of MUC7 O-glycans in SS and that these changes may be similar to those observed in UWMS, including a reduction in core 2 disialylated structures, a reciprocal increase in neutral core 1 glycans, and an increase in core 1 sulfation.
Saliva Properties, the Perception of Dryness and Diagnostic Markers of Saliva Quality
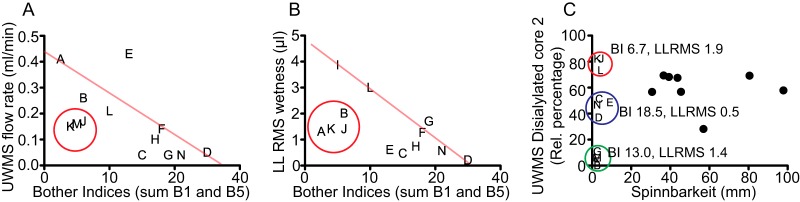
The diagnosis and disease monitoring of SS are exacerbated by the lack of markers that effectively explains the dryness felt by the patient. Thus, the development of salivary markers of oral dryness in SS patients could potentially expedite its diagnosis. The sum of the two Bother Indices (B1 and B5) is a semi-quantitative descriptor of the subjective perception of dryness felt by the SS patient. Both UWMS flow rate (Fig. 4A) and LL.RMS (Fig. 4B) show a general trend that, as these properties are reduced, the patient feels more uncomfortable, giving a higher Bother Index. However, not all patients follow this trend. Some have an unexpectedly low Bother Index sum despite a low UWMS flow rate (red circle, patients K, M, and J) and LL.RMS wetness (red circle, patients A, K, J, and B). That is, these patients do not feel the symptoms of dry mouth as severely as would be expected. This suggests that it is not only the volume of saliva that defines the perception of oral dryness in SS.
Fig. 4.
Saliva properties and the perception of dryness. BI is a subjective measure of dry mouth based on a two-part patient questionnaire with a larger number denoting greater discomfort. BI of SS patients plotted against (A) UWMS flow rate and (B) LL.RMS wetness. An estimation of the clinically expected trend lines are shown on A and B. The red circles represent the area where patients have a lower BI than would be expected from their UWMS flow rate and LL.RMS wetness, designated the sialyl-compensated group. (C) The relative percentage of the disialylated core 2 structure in UWMS plotted against spinnbarkeit of SS patients and controls. BI is the average bother index score of SS patients in the circle and LL.RMS the average volume (μl) of lower labial RMS from patients in the red (sialyl-compensated group), blue (sialyl/flow reduced group), and green (sialyl/flow deficient group) circles. Letter plot points (A-K) denote specific SS patients and black circles are controls.
Spinnbarkeit and content of disialylated core 2 O-glycans on UWMS MUC7 both showed clear differences between controls and patients and are plotted in Fig. 4C and produce interesting patient subgroups. Spinnbarkeit is shown in this patient population to very effectively separate patients and controls. The average spinnbarkeit of patients is statistically significantly reduced (p value ≤ .01) by almost six times (4.67 ± 0.78 compared with 28.54 ± 5.66).
Core 2 disialylation groups controls into a single group while the SS patients form three small subgroups, one with a core 2 disialylation content higher than the controls and two with a lower content. The higher subgroup (red circle, patients K, J, and L) has a lower average BI (6.7) than the two low sialic acid groups (BIs of 13.0 and 18.5) as well as a similar LL.RMS as the lowest group (1.9 μl compared with 1.4 μl), suggesting that the higher content of core 2 disialylation has improved the patient's feeling of discomfort from dryness. This group is designated the sialyl-compensation group. It is interesting to note that patients K and J were also in the groups of patients with BI lower than expected for their UWMS flow rate and LL.RMS wetness. The other two groups, blue, sialyl/flow reduced group and green sialyl/flow deficient, both have lower core 2 disialylation than the controls and differ in BI as well as LL.RMS. A lower core 2 disialylation content as well as a low LL.RMS produces a higher Bother Index. It is essential to note, however, that these subgroups are very small and further analysis is needed to confirm and further understand this interesting observation.
Overall, this shows that SS is multifactorial and that the three properties of UWMS flow rate, LL.RMS wetness, and sialic acid content, give additive effects so that, with the loss of each additional property, the perception of dryness by the patient is worsened. Also, the sialic acid content of MUC7 appears to compensate for a reduced flow rate or LL.RMS and may be a potential diagnostic indicator of saliva quality and, along with other diagnostics such as UWMS flow rate and RMS volume, may be a useful descriptor of dryness.
DISCUSSION
The complicated nature of dry mouth in SS has led to difficulty in assigning a biochemical or biophysical property that is an effective and easy monitor descriptor for the symptoms felt by the patient. This is due to a lack of understanding as to what changes occur in SS and which of these changes are the cause of disease and which an effect. As oral dryness is an early and prominent symptom of SS, effective diagnosis of this aspect would accelerate the clinical assessment of SS. The study presented here was undertaken to ascertain the physical and biochemical properties of saliva that correlate with the subjective perception of dry mouth. Saliva from SS showed negative changes in quantity and physical properties as well as a reduction in mucin glycosylation content. An examination of the glycosylation of MUC7 showed dramatic differences in SS, particularly a reduction in core 2 disialylated structures. A comparison of physical and glycosylation changes and the patients' perception of dryness was undertaken in an attempt to better understand this difficult-to-define property. This analysis suggested that UWMS flow rate, RMS, and core 2 disialylated O-glycan content may have a cumulative impact on the feeling of dry mouth. It was also shown that the rheological properties of saliva are dramatically and consistently lost in SS patients.
All patients expressed discomfort from the feeling of dry mouth and showed objective signs of oral dryness (CODS) as well as significantly reduced UWMS flow rate and mucosal hydration (RMS volume). Changes in salivary gland histology and diminished salivary gland output are well established features of SS (45). A significant decrease in UWMS (46, 47) and minor labial gland (46) flow rates have been reported in SS; however, no change has been observed in the flow rates from minor palatal (47) or buccal (46) glands. This suggests that glands may be affected differently, and as mucosal hydration is reduced in SS, the affected gland secretions may be important for RMS production. The novel use of spinnbarkeit showed a reduction in extensional viscosity in SS UWMS. The spinnbarkeit of SS patients was consistently low, making it clear that not only the amount of RMS and UWMS but the functional lubricating properties of saliva are also altered in SS.
Saliva protein composition changes have been observed in SS; however, variation among patients did not correlate with clinical, serological, or histological disease severity (48). The removal of high molecular weight proteins leads to a near complete fall in saliva lubrication (49), suggesting the high molecular weight mucins are important for oral lubrication. Here, using Western blot quantification, no change in the amount of MUC5B and MUC7 protein in UWMS was observed in SS patients; similar findings have been shown in histological sections of minor glands (50). Given that glycosylation accounts for up to 80% of the mass of salivary mucins (17), it is essential to investigate this component of the glycoproteins. Deglycosylation results in a reduction in the viscoelasticity and lubricating properties of mucins (26, 51). Thus, the proportion of glycans held on the mucin protein backbone was analyzed, and a statically significant reduction in O-glycosylation was observed for MUC7, as well as a decrease in sialic acid residues. Sialic acids were detected using two lectins: SNA and MALII, which showed binding preferences that have been reported previously (52) and coincides with the observation that nearly all sialic acids are α-2,3 linked on MUC7 (18). The reduction of MUC7 glycosylation in SS indicated their altered structure, and further detailed MUC7 O-glycan analysis was undertaken.
Much of the work on oral lubrication has focused on the gel-forming mucin MUC5B; however, purified fractions of salivary mucin (MUC5B) at normal physiological concentrations do not replicate the gel-forming network of saliva (53). Although MUC5B is important for oral lubrication, it is insufficient on its own; other salivary components are essential. Interestingly, it is MUC7 that has a positive correlation with saliva spinnbarkeit (23), not MUC5B (54), suggesting the former may be more important for hydrodynamic lubrication than previously thought. The occurrence of MUC7, along with MUC5B, in the RMS suggests it may also play a role in boundary lubrication; therefore, both UWMS and RMS MUC7 were analyzed by LC-MS/MS.
MUC7 O-glycans identified were the core 1 (Galβ1–3GalNAc) and core 2 (GlcNAcβ1–6(Galβ1–3)GalNAc) structures observed previously (18). MUC7 glycans in UWMS from SS patients had a significantly lower abundance of sialylated glycans, which was primarily due to a reduction in core 2 disialylated structures (m/z 1331.5/665.7 and 1477.5/738.8). The negative charge conferred by sialic acid serves a range of roles, including the interaction with water molecules, creating a hydration shell and improved hydration and lubrication (16). Thus, the loss of negatively charged glycan residues is a proposed mechanism for oral dryness through the reduced water retention capacity of mucins, leading to reduced mucosal hydration (50). The abundance of negative charge also confers electrostatic repulsion properties suggested to give the mucins their rigid linear structure (16). Atomic force microscopy analysis has shown that the deglycosylation of porcine gastric mucin results in tightly packed globular aggregates rather than the usual extended fibers (51). Furthermore, degradation of sialic acid is a proposed mechanism for reduced mucin viscoelasticity in saliva (26), as well as the presentation of decreased mucin viscosity and thinner discharge in women with bacterial vaginosis due to increased sialidase activity (55). A reduction in sialic acid, as shown here in SS, has a range of potential effects on the physical rheological properties of saliva as they relate to hydration and lubrication. Interestingly, it is extended core 2 and disialylated structures that are altered, with no change observed in core 1 sialylation, suggesting it is not only sialic acid content but perhaps the extended nature of the glycans that is also important.
RMS O-glycan analysis was difficult due to the very low quantity collected clinically (1–2 μl whole saliva). Analysis of control RMS samples was more difficult, suggesting SS patients have a higher concentration of MUC7 at the mucosal surface, also shown by PAS staining. These findings coincide with previous observations of concentrated mucin on the mucosal surface of patients with salivary gland hypofunction and reduced mucosal wetness (14, 15). The filter paper adsorption method for RMS analysis could also collect a minor amount of sloughed epithelial cells, which show an enrichment of proteins, including mucins (56). The general enrichment in mucins observed at mucosal surfaces in SS could be the result of altered glycosylation reducing the mucin hydration shell and creating a general reduction in the water component of the RMS. Comparison of UWMS and RMS analysis is tentative given the limited control samples analyzed; however, RMS showed a trend toward a greater relative percentage of extended core 2 sialylated glycans in both controls and patients. The lower turnover of RMS increases the exposure of mucin glycans to bacterial sialidases (57) capable of degrading these glycans (58). Interestingly, analysis of total O-glycans from buccal epithelial cells also showed a greater relative abundance in sialylated glycans relative to whole saliva (20), indicating a higher abundance of sialic acid residues at the mucosal interface. The electrostatic repulsion properties of the central mucin domain have been suggested to confer essential boundary lubrication properties, not just for salivary mucins but other similar lubricating glycoproteins such as lubricin found in the synovial fluid of the joint (16). Lubricin has positively charged terminal regions flanking the central negatively charged domain, and these hydrophobic regions are suggested to be important for the adherence of lubricin to the cartilage surface (59). A similar mechanism has been proposed for the boundary lubricating layer of the oral mucosa (60).
Biophysical salivary properties in SS were investigated to ascertain if these could be linked to patients' oral symptoms. Oral symptoms of SS are early onset, and saliva is easily sampled, making this an ideal route to improving the current severe delays in SS diagnosis. Although the sample numbers used in this study are too low to make further groupings certain, the incomplete understanding of this disease makes initial analyses necessary to direct further research. Tothis end, physical properties were compared with the Bother Indices, which describes the severity of the patient's discomfort from dry mouth symptoms. While UWMS flowrate and RMS volume showed a general correlation with Bother Index, not all patients followed this trend with some feeling better and others worse than would be expected from these parameters. When the proportion of the extended disialylated core 2 structure was included, this parameter explained the Bother Index for many of the outlying sialyl-compensated or sialyl/flow reduced or deficient patients. This suggests that these factors are cumulative and that the retention of one property may, to some extent, alleviate the patient's feeling of dry mouth, and the loss of one may exacerbate the condition despite the maintenance of the other properties. The characterization of dry mouth associated with a range of differing conditions and disease states (61, 62) has proven challenging due, in part, to its occurrence with (28, 63) and without (4, 63) reduced salivary gland output. Hyposalivation-independent xerostomia is reported in 4–50% of oral dryness cases (4, 32, 63). This suggests that the investigation of the MUC7 glycan changes observed here are worth investigating in a larger subset of xerostomia patients to ascertain if this descriptor may be a helpful addition to flow rate analysis as an initial examination of saliva quality.
Sjögren's syndrome patients do not have oral symptoms limited to dry mouth but also have an increased incidence of recurring candidiasis (6). Salivary glycans have been shown to have a protective effect against Candida albicans—an organism able to bind a range of mucin oligosaccharide structures, acting as binding decoys and removing the organisms in the saliva before they can attach to the oral surface (20). Pathogenic Candida glabrata, the second most common cause of candidiasis, has been shown, by glycan microarray analysis, to bind to terminal galactose residues (64). The Western blotting and PAS staining analyses in our study suggested a reduction in glycosylation on salivary mucins, and LC-MS/MS analysis showed an increase in neutral structures with terminal galactose residues, both of which are likely to increase candidiasis. These glycan changes may result in a reduction in Candida clearance and allow increased binding to RMS MUC7, which, like UWMS, showed an increase in terminal galactose residues leading to easier attachment to buccal cells.
A statistically significant increase in the amount of MUC7 core 1 sulfation was observed. This same core 1 3-linked gal sulfation has been shown to be increased in another systemic inflammatory disease, rheumatoid arthritis (65). Although sulfate, like sialic acid, carries a negative charge, given the low abundance of the sulfated core 1 structure compared with the highly abundant sialic acid, it is unlikely that they play a major role in lubrication but, instead, may have immunological and/or binding functions. A recent study has shown that structures including this motif, human salivary MUC5B, and the Sulfo-Lewis A antigen (SO3-3Galβ1–3GlcNAc) may be able to induce proinflammatory cytokines interleukin 6 and tumor necrosis factor alpha through a Toll-like receptor-4 mediated pathway (66). This suggests that changes in glycosylation may be involved in disease processes rather than simply a response to the inflammatory state, an idea that warrants further research. A reduction in Sulfo-Lewis A on MUC5B has been shown in SS by Western blotting (50), suggesting that glycan changes may differ between the salivary mucins in SS. Overall, it is clear that the salivary MUC7 O-glycosylation changes observed in SS could be responsible for a range of physical, immunological, and pathological problems associated with this complex syndrome.
Concluding Remarks
This study was undertaken to ascertain the salivary physical, protein, and glycan compositional changes that occur in SS as well as the patients' perception of their discomfort from dry mouth symptoms. A reduction in UWMS flow rate, RMS volume, and spinnbarkeit (extensional rheology) was observed as well as changes in the salivary mucins MUC5B and, especially, MUC7. The observation of reduced glycosylation on MUC7 led to the detailed LC-MS/MS O-glycan analysis of MUC7 from UWMS, as well as RMS to determine the specific changes during SS. MUC7 from SS, both UWMS and RMS, showed a reduction in extended core 2 disialylated structures, resulting in an overall decrease in negatively charged sialic acid residues. A lack of negatively charged glycans is a proposed mechanism for reduced water retention, leading to less hydrated and lubricating mucins. The use of this property, along with UWMS flow rate and RMS volume, suggested a cumulative impact of these three parameters on the symptom of dry mouth. Overall, this suggests that MUC7 O-glycan changes in SS may alter the physical properties of saliva, leading to the symptom of dry mouth.
Supplementary Material
Acknowledgments
The LTQ mass spectrometer was obtained by a grant from the Swedish Research Council (342–2004-4434). We would like to thank Prof. Claes Wickström (Malmö University, Sweden) for the purified mucin and Prof. Dallas Swallow (University College London, UK) for EU consortium antibody and to all of the patients and volunteers for their participation in the study.
Footnotes
Author contributions: G.B.P., N.G.K., G.H.C., and S.A.F. designed the research; N.M.C. and S.A.F. performed the research; N.M.C., N.G.K., and S.A.F. analyzed data; and N.M.C., G.B.P., N.G.K., and S.A.F. wrote the paper.
* N.C., G.C., and G.P. acknowledge funding from Biotechnology and Biological Sciences Research Council (BB/H015922/1) and Colgate-Palmolive in partnership with King's College London. SF and NK acknowledge funding from the Swedish Research Council (2013-5895 and 2010-5322) and Hedlund's foundation.
1 The abbreviations used are:
- AHP
- anterior hard palate
- AT
- anterior tongue
- BI
- Bother Index
- BUC
- buccal
- CODS
- Clinical Oral Dryness Score
- CO
- control
- LL
- lower labial
- MAL II
- Maackia amurensis leukoagglutinin II
- PAS
- periodic acid-Schiff
- PGC
- porous graphitized carbon
- RMS
- residual mucosal saliva
- SNA
- Sambucus nigra agglutinin
- SS
- Sjögren's syndrome
- TBS-T
- TBS-Tween® 20
- UWMS
- unstimulated whole mouth saliva.
REFERENCES
- 1. González S., Sung H., Sepúlveda D., Gonzalez M., and Molina C. (2014) Oral manifestations and their treatment in Sjogren's syndrome. Oral Diseases 20, 153–161 [DOI] [PubMed] [Google Scholar]
- 2. Dormenval V., Budtz-Jørgensen E., Mojon P., Bruyère A., and Rapin C. H. (1998) Associations between malnutrition, poor general health and oral dryness in hospitalized elderly patients. Age Ageing 27, 123–128 [DOI] [PubMed] [Google Scholar]
- 3. Ohara Y., Hirano H., Yoshida H., and Suzuki T. (2011) Ratio and associated factors of dry mouth among community-dwelling elderly Japanese women. Geriat. Gerontol. Int. 11, 83–89 [DOI] [PubMed] [Google Scholar]
- 4. Narhi T. O. (1994) Prevalence of subjective feelings of dry mouth in the elderly. J. Dental. Res. 73, 20–25 [DOI] [PubMed] [Google Scholar]
- 5. Papas A. S., Joshi A., MacDonald S. L., Maravelis-Splagounias L., Pretara-Spanedda P., and Curro F. A. (1993) Caries prevalence in xerostomic individuals. J. Can. Dent. Assoc. 59, 171–174, 177–179 [PubMed] [Google Scholar]
- 6. Yan Z., Young A. L., Hua H., and Xu Y. (2011) Multiple oral Candida infections in patients with Sjogren's syndrome—Prevalence and clinical and drug susceptibility profiles. The J. Rheumatol. 38, 2428–2431 [DOI] [PubMed] [Google Scholar]
- 7. Meijer J. M., Meiners P. M., Huddleston Slater J. J., Spijkervet F. K., Kallenberg C. G., Vissink A., and Bootsma H. (2009) Health-related quality of life, employment and disability in patients with Sjogren's syndrome. Rheumatology 48, 1077–1082 [DOI] [PubMed] [Google Scholar]
- 8. Patel R., and Shahane A. (2014) The epidemiology of Sjogren's syndrome. Clin. Epidemiol. 6, 247–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liang Y., Yang Z., Qin B., and Zhong R. (2014) Primary Sjogren's syndrome and malignancy risk: A systematic review and meta-analysis. Ann. Rheumat. Dis. 73, 1151–1156 [DOI] [PubMed] [Google Scholar]
- 10. Shiboski S. C., Shiboski C. H., Criswell L., Baer A., Challacombe S., Lanfranchi H., Schiødt M., Umehara H., Vivino F., Zhao Y., Dong Y., Greenspan D., Heidenreich A. M., Helin P., Kirkham B., Kitagawa K., Larkin G., Li M., Lietman T., Lindegaard J., McNamara N., Sack K., Shirlaw P., Sugai S., Vollenweider C., Whitcher J., Wu A., Zhang S., Zhang W., Greenspan J., Daniels T., and Sjogren's International Collaborative Clinical Alliance Research Groups. (2012) American College of Rheumatology classification criteria for Sjogren's syndrome: A data-driven, expert consensus approach in the Sjogren's International Collaborative Clinical Alliance cohort. Arthritis Care Res. 64, 475–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalk W. W., Vissink A., Spijkervet F. K., Bootsma H., Kallenberg C. G., and Nieuw Amerongen A. V. (2001) Sialometry and sialochemistry: Diagnostic tools for Sjogren's syndrome. Ann. Rheumat. Dis. 60, 1110–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carpenter G. H. (2013) The secretion, components, and properties of saliva. Annu. Rev. Food Sci. Tech. 4, 267–276 [DOI] [PubMed] [Google Scholar]
- 13. Dawes C. (1983) A mathematical model of salivary clearance of sugar from the oral cavity. Caries Res. 17, 321–334 [DOI] [PubMed] [Google Scholar]
- 14. Pramanik R., Osailan S. M., Challacombe S. J., Urquhart D., and Proctor G. B. (2010) Protein and mucin retention on oral mucosal surfaces in dry mouth patients. Eur. J. Oral Sci. 118, 245–253 [DOI] [PubMed] [Google Scholar]
- 15. Lee S. K., Lee S. W., Chung S. C., Kim Y. K., and Kho H. S. (2002) Analysis of residual saliva and minor salivary gland secretions in patients with dry mouth. Arch. Oral Biol. 47, 637–641 [DOI] [PubMed] [Google Scholar]
- 16. Coles J. M., Chang D. P., and Zauscher S. (2010) Molecular mechanisms of aqueous boundary lubrication by mucinous glycoproteins. Curr. Opin. Colloid Interface Sci. 15, 406–416 [Google Scholar]
- 17. Thomsson K. A., Prakobphol A., Leffler H., Reddy M. S., Levine M. J., Fisher S. J., and Hansson G. C. (2002) The salivary mucin MG1 (MUC5B) carries a repertoire of unique oligosaccharides that is large and diverse. Glycobiology 12, 1–14 [DOI] [PubMed] [Google Scholar]
- 18. Karlsson N. G., and Thomsson K. A. (2009) Salivary MUC7 is a major carrier of blood group I type O-linked oligosaccharides serving as the scaffold for sialyl Lewis x. Glycobiology 19, 288–300 [DOI] [PubMed] [Google Scholar]
- 19. Thomsson K. A., Schulz B. L., Packer N. H., and Karlsson N. G. (2005) MUC5B glycosylation in human saliva reflects blood group and secretor status. Glycobiology 15, 791–804 [DOI] [PubMed] [Google Scholar]
- 20. Everest-Dass A. V., Jin D., Thaysen-Andersen M., Nevalainen H., Kolarich D., and Packer N. H. (2012) Comparative structural analysis of the glycosylation of salivary and buccal cell proteins: innate protection against infection by Candida albicans. Glycobiology 22, 1465–1479 [DOI] [PubMed] [Google Scholar]
- 21. Prakobphol A., Tangemann K., Rosen S. D., Hoover C. I., Leffler H., and Fisher S. J. (1999) Separate oligosaccharide determinants mediate interactions of the low-molecular-weight salivary mucin with neutrophils and bacteria. Biochemistry 38, 6817–6825 [DOI] [PubMed] [Google Scholar]
- 22. Veeregowda D. H., Busscher H. J., Vissink A., Jager D. J., Sharma P. K., and van der Mei H. C. (2012) Role of structure and glycosylation of adsorbed protein films in biolubrication. PLoS ONE 7, e42600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inoue H., Ono K., Masuda W., Inagaki T., and Yokota M. (2008) Rheological properties of human saliva and salivary mucins. J. Oral Biosci. 50, 134–141 [Google Scholar]
- 24. Park M. S., Chung J. W., Kim Y. K., Chung S. C., and Kho H. S. (2007) Viscosity and wettability of animal mucin solutions and human saliva. Oral Diseases 13, 181–186 [DOI] [PubMed] [Google Scholar]
- 25. Veerman E. C., Valentijn-Benz M., and Nieuw Amerongen A. V. (1989) Viscosity of human salivary mucins: Effect of pH and ionic strength and role of sialic acid. J. Biol. Buccale 17, 297–306 [PubMed] [Google Scholar]
- 26. Ito F., Yamada S., Mizuno Y., Sugihara N., and Chen L. S. (1988) Correlation between viscosity and sialic acid content of whole human saliva. Aichi-Gakuin Dental Sci. 1, 21–27 [PubMed] [Google Scholar]
- 27. Eliasson L., Birkhed D., and Carlén A. (2009) Feeling of dry mouth in relation to whole and minor gland saliva secretion rate. Arch. Oral Biol. 54, 263–267 [DOI] [PubMed] [Google Scholar]
- 28. Navazesh M., Christensen C., and Brightman V. (1992) Clinical criteria for the diagnosis of salivary gland hypofunction. J. Dental. Res. 71, 1363–1369 [DOI] [PubMed] [Google Scholar]
- 29. Daniels T. E., Cox D., Shiboski C. H., Schiodt M., Wu A., Lanfranchi H., Umehara H., Zhao Y., Challacombe S., Lam M. Y., De Souza Y., Schiødt J., Holm H., Bisio P. A., Gandolfo M. S., Sawaki T., Li M., Zhang W., Varghese-Jacob B., Ibsen P., Keszler A., Kurose N., Nojima T., Odell E., Criswell L. A., Jordan R., Greenspan J. S., and Sjogren's International Collaborative Clinical Alliance Research Groups. (2011) Associations between salivary gland histopathologic diagnoses and phenotypic features of Sjogren's syndrome among 1,726 registry participants. Arthritis Rheumat. 63, 2021–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lai S. K., Wang Y. Y., Wirtz D., and Hanes J. (2009) Micro- and macrorheology of mucus. Adv. Drug Delivery Rev. 61, 86–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gohara K., Ansai T., Koseki T., Ishikawa M., Kakinoki Y., Shibuya K., Nishihara T., and Takehara T. (2004) A new automatic device for measuring the spinnbarkeit of saliva: The Neva Meter. J. Dentistry 32, 335–338 [DOI] [PubMed] [Google Scholar]
- 32. Wolff M., and Kleinberg I. (1998) Oral mucosal wetness in hypo- and normosalivators. Arch. Oral Biol. 43, 455–462 [DOI] [PubMed] [Google Scholar]
- 33. Osailan S., Pramanik R., Shirodaria S., Challacombe S. J., and Proctor G. B. (2011) Investigating the relationship between hyposalivation and mucosal wetness. Oral Diseases 17, 109–114 [DOI] [PubMed] [Google Scholar]
- 34. Vitali C., Bombardieri S., Jonsson R., Moutsopoulos H. M., Alexander E. L., Carsons S. E., Daniels T. E., Fox P. C., Fox R. I., Kassan S. S., Pillemer S. R., Talal N., Weisman M. H., and European Study Group on Classification Criteria for Sjogren's, Syndrome. (2002) Classification criteria for Sjogren's syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheumat. Dis. 61, 554–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Challacombe S. J., Osailan S. M., and Proctor G. B. (2015) Clinical scoring scales for assessment of dry mouth. In: Carpenter G., ed. Dry Mouth: A Clinical Guide on Causes, Effects and Treatments. pp. 119–132, Springer-Verlag, Berlin Heidelberg [Google Scholar]
- 36. Osailan S. M., Pramanik R., Shirlaw P., Proctor G. B., and Challacombe S. J. (2012) Clinical assessment of oral dryness: Development of a scoring system related to salivary flow and mucosal wetness. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 114, 597–603 [DOI] [PubMed] [Google Scholar]
- 37. Rousseau K., Wickstrom C., Whitehouse D. B., Carlstedt I., and Swallow D. M. (2003) New monoclonal antibodies to non-glycosylated domains of the secreted mucins MUC5B and MUC7. Hybridoma Hybridomics 22, 293–299 [DOI] [PubMed] [Google Scholar]
- 38. Abramoff M. D., Magalhaes P. J., and Ram S. J. (2004) Image processing with ImageJ. Biophoton. Int. 11, 36–42 [Google Scholar]
- 39. Schulz B. L., Packer N. H., and Karlsson N. G. (2002) Small-scale analysis of O-linked oligosaccharides from glycoproteins and mucins separated by gel electrophoresis. Anal. Chem. 74, 6088–6097 [DOI] [PubMed] [Google Scholar]
- 40. Karlsson N. G., Schulz B. L., and Packer N. H. (2004) Structural determination of neutral O-linked oligosaccharide alditols by negative ion LC-electrospray-MSn. J. Am. Soc. Mass. Spectrom. 15, 659–672 [DOI] [PubMed] [Google Scholar]
- 41. Hayes C. A., Karlsson N. G., Struwe W. B., Lisacek F., Rudd P. M., Packer N. H., and Campbell M. P. (2011) UniCarb-DB: A database resource for glycomic discovery. Bioinformatics 27, 1343–1344 [DOI] [PubMed] [Google Scholar]
- 42. Häkkinen J., Vincic G., Mansson O., Wårell K., and Levander F. (2009) The Proteios software environment: An extensible multiuser platform for management and analysis of proteomics data. J. Proteome Res. 8, 3037–3043 [DOI] [PubMed] [Google Scholar]
- 43. Schulz B. L., Sloane A. J., Robinson L. J., Sebastian L. T., Glanville A. R., Song Y., Verkman A. S., Harry J. L., Packer N. H., and Karlsson N. G. (2005) Mucin glycosylation changes in cystic fibrosis lung disease are not manifest in submucosal gland secretions. Biochem. J. 387, 911–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Delli K., Vissink A., and Spijkervet F. K. (2014) Salivary gland biopsy for Sjogren's syndrome. Oral Maxillofacial Surg. Clinics N. Am. 26, 23–33 [DOI] [PubMed] [Google Scholar]
- 45. Pereira D. L., Vilela V. S., Dos Santos T. C., and Pires F. R. (2014) Clinical and laboratorial profile and histological features on minor salivary glands from patients under investigation for Sjogren's syndrome. Med. Oral Patol. Oral Cir. Bucal 19, e237–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eliasson L., Almståhl A., Lingström P., Wikström M., and Carlén A. (2005) Minor gland saliva flow rate and proteins in subjects with hyposalivation due to Sjogren's syndrome and radiation therapy. Arch. Oral Biol. 50, 293–299 [DOI] [PubMed] [Google Scholar]
- 47. Márton K., Boros I., Varga G., Zelles T., Fejérdy P., Zeher M., and Nagy G. (2006) Evaluation of palatal saliva flow rate and oral manifestations in patients with Sjogren's syndrome. Oral Diseases 12, 480–486 [DOI] [PubMed] [Google Scholar]
- 48. Fleissig Y., Deutsch O., Reichenberg E., Redlich M., Zaks B., Palmon A., and Aframian D. J. (2009) Different proteomic protein patterns in saliva of Sjogren's syndrome patients. Oral Diseases 15, 61–68 [DOI] [PubMed] [Google Scholar]
- 49. Bongaerts J. H. H., Rossetti D., and Stokes J. R. (2007) The lubricating properties of human whole saliva. Tribol. Lett. 27, 277–287 [Google Scholar]
- 50. Alliende C., Kwon Y. J., Brito M., Molina C., Aguilera S., Pérez P., Leyton L., Quest A. F., Mandel U., Veerman E., Espinosa M., Clausen H., Leyton C., Romo R., and Gonzalez M. J. (2008) Reduced sulfation of muc5b is linked to xerostomia in patients with Sjogren syndrome. Ann. Rheumat. Dis. 67, 1480–1487 [DOI] [PubMed] [Google Scholar]
- 51. Hong Z., Chasan B., Bansil R., Turner B. S., Bhaskar K. R., and Afdhal N. H. (2005) Atomic force microscopy reveals aggregation of gastric mucin at low pH. Biomacromolecules 6, 3458–3466 [DOI] [PubMed] [Google Scholar]
- 52. Shori D. K., Genter T., Hansen J., Koch C., Wyatt H., Kariyawasam H. H., Knight R. A., Hodson M. E., Kalogeridis A., and Tsanakas I. (2001) Altered sialyl- and fucosyl-linkage on mucins in cystic fibrosis patients promotes formation of the sialyl-Lewis X determinant on salivary MUC-5B and MUC-7. Pflugers Arch. 443, S55–S61 [DOI] [PubMed] [Google Scholar]
- 53. Raynal B. D., Hardingham T. E., Thornton D. J., and Sheehan J. K. (2002) Concentrated solutions of salivary MUC5B mucin do not replicate the gel-forming properties of saliva. Biochem. J. 362, 289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Takehara S., Yanagishita M., Podyma-Inoue K. A., and Kawaguchi Y. (2013) Degradation of MUC7 and MUC5B in human saliva. PLoS ONE 8, e69059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Olmsted S. S., Meyn L. A., Rohan L. C., and Hillier S. L. (2003) Glycosidase and proteinase activity of anaerobic gram-negative bacteria isolated from women with bacterial vaginosis. Sex. Transmit. Diseases 30, 257–261 [DOI] [PubMed] [Google Scholar]
- 56. Gibbins H. L., Proctor G. B., Yakubov G. E., Wilson S., and Carpenter G. H. (2014) Concentration of salivary protective proteins within the bound oral mucosal pellicle. Oral Diseases 20, 707–713 [DOI] [PubMed] [Google Scholar]
- 57. Lewis A. L., and Lewis W. G. (2012) Host sialoglycans and bacterial sialidases: a mucosal perspective. Cell Microbiol. 14, 1174–1182 [DOI] [PubMed] [Google Scholar]
- 58. Ali L., Kenny D. T., Hayes C. A., and Karlsson N. G. (2012) Structural identification of O-linked oligosaccharides using exoglycosidases and MSn together with UniCarb-DB fragment spectra comparison. Metabolites 2, 648–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ali L., Flowers S. A., Jin C., Bennet E. P., Ekwall A. K., and Karlsson N. G. (2014) The O-glycomap of lubricin, a novel mucin responsible for joint lubrication, identified by site-specific glycopeptide analysis. Mol. Cell Proteomics 13, 3396–3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gibbins H. L., Yakubov G. E., Proctor G. B., Wilson S., and Carpenter G. H. (2014) What interactions drive the salivary mucosal pellicle formation? Colloids Surf. B Biointerfaces 120, 184–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Scully C. (2003) Drug effects on salivary glands: Dry mouth. Oral Diseases 9, 165–176 [DOI] [PubMed] [Google Scholar]
- 62. Nederfors T. (2000) Xerostomia and hyposalivation. Adv. Dental Res. 14, 48–56 [DOI] [PubMed] [Google Scholar]
- 63. Longman L. P., Higham S. M., Rai K., Edgar W. M., and Field E. A. (1995) Salivary gland hypofunction in elderly patients attending a xerostomia clinic. Gerodontology 12, 67–72 [DOI] [PubMed] [Google Scholar]
- 64. Maestre-Reyna M., Diderrich R., Veelders M. S., Eulenburg G., Kalugin V., Brückner S., Keller P., Rupp S., Mösch H. U., and Essen L. O. (2012) Structural basis for promiscuity and specificity during Candida glabrata invasion of host epithelia. Proc. Natl. Acad. Sci. U.S.A. 109, 16864–16869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Flowers S. A., Ali L., Lane C. S., Olin M., and Karlsson N. G. (2013) Selected reaction monitoring to differentiate and relatively quantitate isomers of sulfated and unsulfated core 1 O-glycans from salivary MUC7 protein in rheumatoid arthritis. Mol. Cell. Proteomics 12, 921–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Barrera M. J., Aguilera S., Veerman E., Quest A. F., Diaz-Jimenez D., Urzua U., Cortes J., Gonzalez S., Castro I., Molina C., Bahamondes V., Leyton C., Hermoso M. A., and Gonzalez M. J. (2015) Salivary mucins induce a Toll-like receptor 4-mediated pro-inflammatory response in human submandibular salivary cells: Are mucins involved in Sjogren's syndrome? Rheumatology. 54, 1518–1527 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.