Abstract
Aim:
To investigate the effects of docosahexaenoic acid (DHA) on melanin synthesis and related regulatory mechanisms.
Methods:
B16F10 mouse melanoma cells were exposed to DHA for 3 d, and melanin content and tyrosinase activity were measured. Western blot analysis was used to analyze the protein levels in DHA-mediated signal transduction pathways.
Results:
DHA (1–25 μmol/L) did not affect the viability of B16F10 cells, but decreased α-MSH-induced melanin synthesis in a concentration-dependent manner. DHA concentration-dependently reduced tyrosinase activity in the cells, but did not affect mushroom tyrosinase activity in a cell-free system. Furthermore, DHA treatment significantly reduced tyrosinase level without affecting microphthalmia-associated transcription factor (MITF) in the cells. DHA did not activate ERK and Akt in the cells. Pretreatment with the proteasome inhibitor MG132 (80 nmol/L) abolished DHA-induced tyrosinase reduction.
Conclusion:
DHA inhibits melanogenesis in B16F10 cells in vitro through increasing tyrosinase degradation. The results suggest that DHA may be a potential agent for treatment of hyperpigmentary disorders of skin.
Keywords: docosahexaenoic acid, α-MSH; melanin, melanogenesis, tyrosinase, proteasome, MG132, skin, hyperpigmentary disorders
Introduction
Docosahexaenoic acid (DHA) is an omega-3 polyunsaturated fatty acid, and it is the longest and the least saturated fatty acid found in substantial quantities in human tissues1,2. In the field of medicine, DHA has shown numerous health benefits as its dietary presence has been positively linked to decreased risk of and better prognosis in a wide range of human afflictions, including cancer, heart disease, dermatitis, and psoriasis1. It has been reported that DHA decreased basal and UV-induced expression of some proinflammatory cytokines in the skin3. Moreover, DHA inhibited UVB-induced expression of cyclooxygenase-2 in hairless mouse skin4. Although DHA has been tested in skin cells and tissues, its effect on melanogenesis has not been explored. Therefore, in this study, we investigated the effects of DHA on melanin synthesis and related regulatory mechanisms.
Melanogenesis is the process of producing and distributing melanin, a pigmented biopolymer, which is synthesized in melanocytes within specialized membrane-bound organelles known as melanosomes5,6. Skin, hair and eye color are derived from this process. In the biosynthetic pathway of melanin synthesis, tyrosinase is the rate-limiting enzyme: it catalyzes the conversion of tyrosine to 3,4-dihydroxyphenylalanine (DOPA) and oxidizes DOPA to dopaquinone7,8. Consequently, melanin production is largely regulated by the expression and activity of tyrosinase8. Hence, regulating tyrosinase activity is one approach to control melanin production in order to treat hyperpigmentary problems and reduce skin melanin content.
Melanogenesis can be stimulated by extracellular signals, such as ultraviolet radiation (UV), α-melanocyte stimulating hormone (α-MSH), endothelin-1 (ET-1), cytokines and other growth factors9. α-MSH binds to the melanocortin 1 receptor (MC1R), a melanocyte-specific receptor which activates cAMP, resulting in an increase in microphthalmia-associated transcription factor (MITF) expression9,10. MITF is a key factor in melanogenesis, as it regulates the transcription of the enzymes necessary for melanin synthesis, namely, tyrosinase, tyrosinase-related protein 1 (TRP1), and TRP211,12.
Several reports indicate that signaling pathways, such as the extracellular signal-regulated kinase (ERK) and Akt, are heavily involved in melanogenesis13,14. Activation of the abovementioned pathways has been reported to result in a decrease in melanin synthesis15,16. Therefore, we also investigated whether DHA has an influence on these signaling pathways.
Materials and methods
Materials
DHA, synthetic melanin, 3,4-dihydroxy-L-phenylalanine (L-DOPA), and mushroom tyrosinase were purchased from Sigma-Aldrich Co (St Louis, MO, USA). Antibodies specific for phospho-ERK1/2 (Thr202/Tyr204, #9101S), phospho-specific Akt (Ser473, #9271), and phospho-CREB (ser133, #9198) were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies specific for tyrosinase (C-19), TRP1 (G-17), and actin (I-19) were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA), and an antibody specific for microphthalmia Ab-1 (C5, MS-771-P0) was obtained from NeoMarkers (Fremont, CA, USA). Secondary antibodies anti-goat IgG (PI-9500), anti-mouse IgG (PI-2000), and anti-rabbit IgG (PI-1000) were purchased from Vector Laboratories (Burlingame, CA, USA).
Cell culture
B16F10 murine melanoma cells were obtained from the Korean Cell Line Bank (Seoul, Korea). The cells were maintained in Dulbecco's modified Eagle's medium (DMEM) and were supplemented with 10% (v/v) fetal bovine serum (Hyclone, Logan, UT, USA), 50 μg/mL streptomycin and 50 μg/mL penicillin (Hyclone) in 5% CO2 at 37 °C.
Cell viability assay
Cell viability was determined using a crystal violet assay. After incubating the cells with DHA for 24 h, the medium was removed, and the cells were stained with 0.1% crystal violet in 10% ethanol for 5 min at room temperature. The cells were then rinsed four times with distilled water, and the crystal violet retained by adherent cells was extracted with 95% ethanol. Absorbance was measured at 590 nm using an ELISA reader (VERSAMax; Molecular Devices, Sunnyvale, CA, USA).
Measurement of melanin content
Extracellular melanin release was measured as described previously, with a slight modification17. B16F10 cells were incubated overnight at a density of 1×105 cells in 6-well plates. Then, 1 μmol/L of α-MSH was added, and the cells were treated with increasing concentrations of DHA in phenol red-free DMEM for three days. Next, 200 μL of the sample was placed in 96-well plates, and the optical density (OD) of each culture well was measured at 400 nm using an ELISA reader. The number of cells was then counted using hemocytometry. Melanin production was expressed as the percentage of α-MSH-treated controls.
Tyrosinase activity
Tyrosinase activity was assayed as DOPA oxidase activity. B16F10 cells were incubated at a density of 1×105 cells in 6-well plates and incubated with DHA in DMEM for three days. The cells were washed with phosphate-buffered saline (PBS), lysed with phosphate buffer (pH 6.8) containing 1% Triton X-100 and disrupted by freezing and thawing. The lysates were then clarified by centrifugation at 13 000 r/min for 30 min. After quantifying protein content in the lysate and adjusting with lysis buffer, 90 μL of each lysate (containing the same amount of protein) was pipetted into the wells of a 96-well plate followed by the addition of 10 μL of 10 mmol/L L-DOPA. Control wells contained 90 μL of lysis buffer and 10 μL of 10 mmol/L L-DOPA. After incubation at 37 °C for 20 min, dopachrome formation was monitored by measuring absorbance at 475 nm with an ELISA reader.
A cell-free assay system was used to determine the direct effect of DHA on tyrosinase activity. In each well, 70 μL of phosphate buffer containing DHA was mixed with 20 μL of 53.7 units/mL of mushroom tyrosinase. Then, 10 μL of 10 mmol/L L-DOPA was added. After incubation at 37 °C for 20 min, absorbance was measured at 475 nm.
Western blot analysis
Cells were lysed in cell lysis buffer composed of 62.5 mmol/L Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate (SDS), 5% β-mercaptoethanol, 2 mmol/L phenylmethylsulfonyl fluoride, protease inhibitors (Complete; Roche, Mannheim, Germany), 1 mmol/L Na3 VO4, 50 mmol/L NaF, and 10 mmol/L EDTA. A 20 μg sample of protein per lane was separated by SDS-polyacrylamide gel electrophoresis and blotted onto PVDF membranes, which were then saturated with 5% dried milk in Tris-buffered saline containing 0.5% Tween 20. Blots were then incubated with the appropriate primary antibodies at a dilution of 1:1000 and further incubated with horseradish peroxidase-conjugated secondary antibody. Bound antibodies were detected using an enhanced chemiluminescence plus kit (Amersham International, Little Chalfont, UK). Images of the blotted membranes were obtained using a LAS-1000 Lumino-Image Analyzer (Fuji Film, Tokyo, Japan).
Reverse transcription-polymerase chain reaction (RT-PCR)
To characterize mRNA expression, total RNA was isolated from cells using an RNeasy Mini kit (Qiagen, Valencia, CA, USA). Then, 1 μg of RNA was reverse transcribed using the ImProm II Reverse Transcription System (Promega, Madison, WI, USA). The resulting cDNA was amplified with primers specific for MITF (forward, 5′-CCCGTCTCTGGAAACTTGATCG-3′ and reverse, 5′-CTGTACTCTGAGCAGGTG-3′) and tyrosinase (forward, 5′-GGCCAGCTTTCAGGCAGAGGT-3′ and reverse, 5′-TGGTGCTTCATGGGCAAAATC-3′). The PCR for MITF and tyrosinase were performed for 23 cycles using the following conditions: 1 min at 95 °C, 1 min at 59 °C, and 1 min at 72 °C. The resulting PCR products were visualized by electrophoretic separation on 1.5% agarose gels with Safe-Pinky DNA gel stain (Gendepot, Barker, TX, USA). Primers specific for GAPDH were used for loading control amplifications.
Statistical analysis
The statistical significance of between-group differences was assessed by analysis of variance (ANOVA), followed by Student's t-test. P values <0.05 were considered significant.
Results
Effects of DHA on cell viability
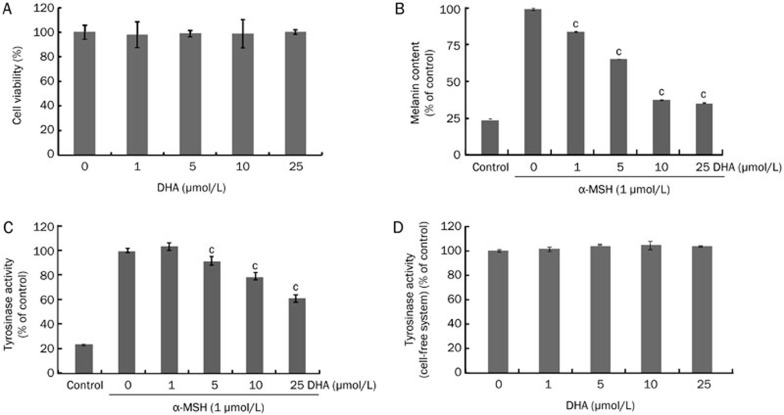
B16F10 cells were treated with DHA at various concentrations between 1 and 25 μmol/L for 24 h. As shown in Figure 1A, DHA treatment did not show any cytotoxic effects within the tested concentration range. Hence, cells were treated with 1–25 μmol/L of DHA for the subsequent experiments.
Figure 1.
Effects of DHA treatment on melanin synthesis and tyrosinase activity. (A) B16F10 cells were treated with DHA (1–25 μmol/L) for 24 h in serum-free media. Cell viabilities were determined using crystal violet assays. Cells were treated with DHA (1–25 μmol/L) in the presence of α-MSH (1 μmol/L) for three days. (B) Melanin content and (C) tyrosinase activity were measured. (D) To test the direct effect on tyrosinase, its activity in a cell-free system was also measured. Each determination was made in triplicate, and the data shown represent the mean±SD.
Effects of DHA on melanin synthesis and tyrosinase activity
To determine the effects of DHA treatment on melanogenesis, B16F10 cells were cultured with 1–25 μmol/L of DHA for three days in the presence of 1 μmol/L α-MSH (which increases melanin synthesis), and the extracellular melanin release was measured. As shown in Figure 1B, DHA reduced α-MSH-induced melanin synthesis in a dose-dependent manner. Because tyrosinase is the rate-limiting enzyme for melanin synthesis7, the effects of DHA on cellular tyrosinase activity were examined by evaluating L-DOPA oxidation activity. DHA-treated cells showed a dose-dependent reduction in tyrosinase activity (Figure 1C), consistent with the reduction of melanin content in DHA-treated cells.
In addition, to investigate whether DHA directly inhibits tyrosinase activity, its effect on mushroom tyrosinase was measured in a cell-free system. DHA showed no direct effect on tyrosinase activity (Figure 1D); these results indicate that the inhibitory activity of DHA on melanogenesis may have resulted from the inhibition of tyrosinase expression and not from the direct inhibition of tyrosinase.
Effects of DHA on melanogenic protein levels
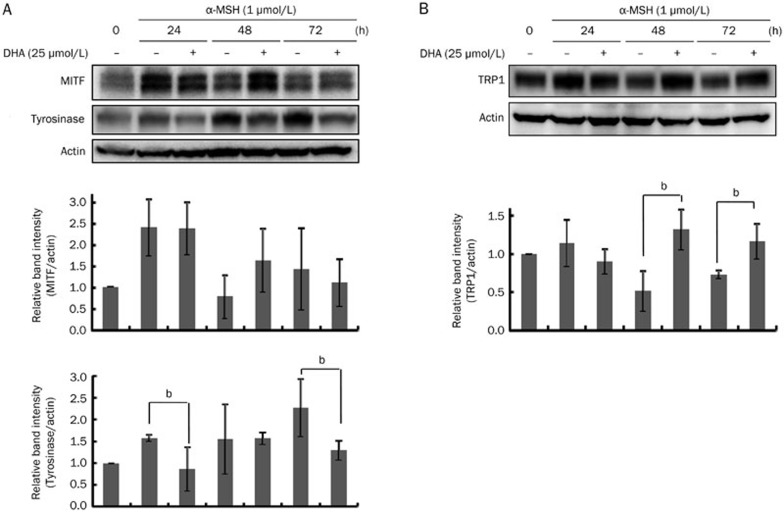
Changes in the levels of MITF, tyrosinase, and TRP1 proteins were evaluated in a time-course experiment following 24–72 h of DHA treatment in the presence of α-MSH (1 μmol/L). Interestingly, MITF protein levels were slightly increased upon DHA treatment at 48 h. By contrast, tyrosinase protein levels were clearly decreased after DHA treatment (Figure 2A). TRP1 protein levels were increased by DHA at 48 h and 72 h (Figure 2B).
Figure 2.
Effects of DHA treatment on MITF and tyrosinase levels. B16F10 cells were cultured with 25 μmol/L of DHA for 24–72 h. Whole-cell lysates were subjected to Western blot analysis with antibodies against (A) MITF and tyrosinase or (B) TRP1. Equal protein loading was verified by reactivity with an anti-actin antibody. Band intensity relative to the control was determined by densitometric analysis. Values are expressed as the mean±SD (n=3). bP<0.05 compared to α-MSH-treated control.
Effects of DHA on signal transduction pathways
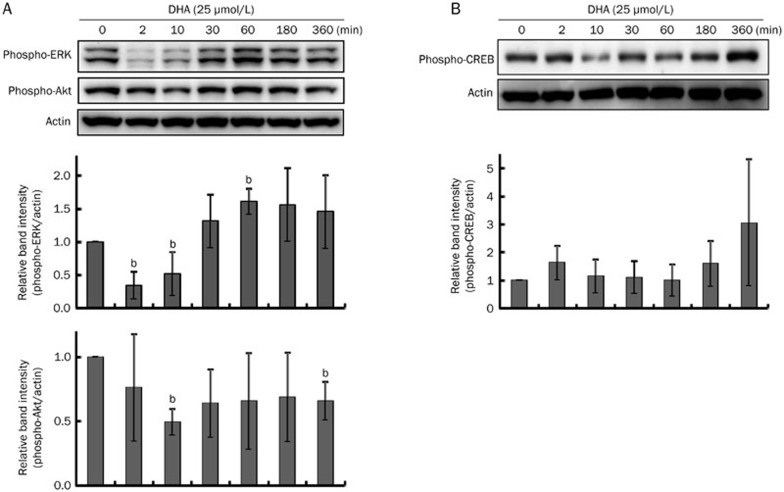
The activation of ERK or Akt reportedly plays a key role in inhibiting melanogenesis14,16. Thus, it was investigated whether DHA-induced hypopigmentation was related to ERK or Akt phosphorylation. However, neither ERK nor Akt was activated by DHA (Figure 3A). Instead, there was a decrease in ERK phosphorylation at 2–10 min. In addition, we examined the PKA pathway and found that cAMP response element-binding protein (CREB) was weakly phosphorylated at 360 min (Figure 3B).
Figure 3.
Effects of DHA treatment on signal transduction pathways. After 24 h serum starvation, B16F10 cells were treated with 25 μmol/L of DHA for the times indicated. Whole-cell lysates were then subjected to Western blot analysis using antibodies against (A) phospho-specific ERK and phospho-specific Akt or (B) phospho-specific CREB. Equal protein loading was verified by reactivity with an anti-actin antibody. Band intensity relative to the control was determined by densitometric analysis. Values are expressed as the mean±SD (n=3). bP<0.05 compared to untreated control.
Effects of DHA on melanin synthesis in the presence of MG132
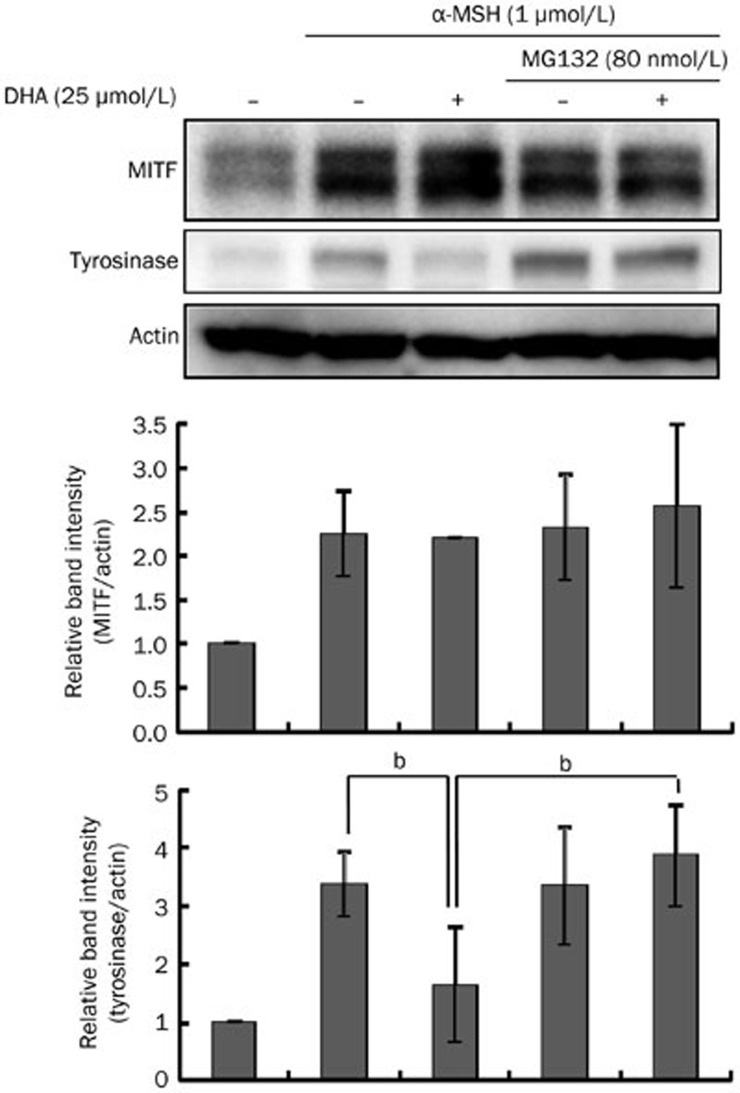
Proteasome-mediated proteolytic degradation was stimulated to determine whether alterations in protein degradation could be responsible for DHA treatment-reduced tyrosinase level. B16F10 cells were treated with DHA (25 μmol/L) in the presence or absence of MG132, a proteasome inhibitor. MG132 treatment completely restored tyrosinase levels that had been downregulated by DHA (Figure 4). By contrast, a change in MITF level was not observed. To exclude the possibility that DHA could reduce the mRNA level of tyrosinase, we investigated the transcription levels of MITF and tyrosinase mRNA by RT-PCR. As shown in Figure 5, however, DHA had no influence on MITF or tyrosinase mRNA level, confirming that DHA affects tyrosinase protein level without affecting mRNA expression.
Figure 4.
Effects of DHA treatment on melanin synthesis in the presence of MG132. B16F10 cells were preincubated with 80 nmol/L MG132 for 3 h before adding 25 μmol/L DHA and then incubated for 24 h. Whole-cell lysates were analyzed by Western blotting with antibodies against MITF and tyrosinase. Equal protein loading was verified by reactivity with an anti-actin antibody. Band intensity relative to the control was determined by densitometric analysis. Values are expressed as the mean±SD (n=3). bP<0.05 compared to α-MSH-treated control.
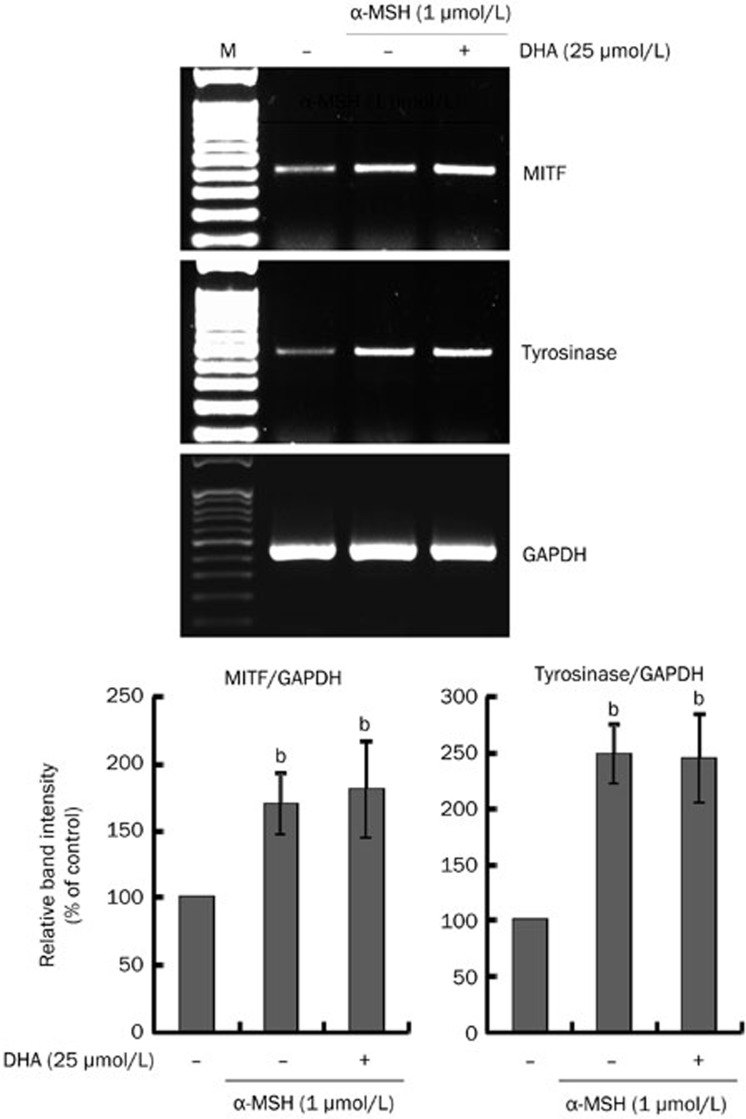
Figure 5.
Effects of DHA treatment on MITF and tyrosinase mRNA expression. B16F10 cells were cultured with 25 μmol/L DHA in the presence of α-MSH (1 μmol/L) for 24 h. mRNA levels of MITF and tyrosinase were determined by RT-PCR assay as described in 'Materials and methods.' GAPDH primers were used as loading controls. The resulting PCR products were analyzed by agarose gel electrophoresis. M, marker. Band intensity relative to the control was determined by densitometric analysis. Values are expressed as the mean±SD (n=3). bP<0.05 compared to untreated control.
Discussion
The search for new therapeutic agents to address hyperpigmentary problems and reduce skin melanin content is ongoing. DHA, which has been shown to have various health benefits, was explored as a possible anti-melanogenic agent. In the present study, DHA inhibited melanin synthesis induced by α-MSH in B16F10 mouse melanoma cells (Figure 1B), and the molecular mechanism for DHA regulated melanogenesis was further investigated. MITF is known to be a major regulator of tyrosinase expression18,19,20. Our results showed that DHA slightly increased MITF level at 48 h, but tyrosinase protein level was markedly decreased in DHA-treated cells (Figure 2A). By contrast, TRP1 protein level was increased by DHA (Figure 2B). This result is interesting because MITF is a major transcriptional regulator for both tyrosinase and TRP118,21,22,23. These results suggest that the DHA-induced inhibition of melanogenesis does not involve MITF but may be affected by tyrosinase expression and/or degradation. Conversely, TRP1 regulation may be dependent on MITF expression. Because tyrosinase is a rate-limiting enzyme, its downregulation may be more important than TRP1 upregulation to modulate melanin synthesis.
An important mechanism to selectively eliminate misfolded, abnormal and normal proteins is known as ubiquitin-mediated proteasomal degradation24. Like other intracellular proteins, tyrosinase protein levels are regulated by a balance between synthesis and degradation8. Under normal conditions, tyrosinase is partly degraded by endogenous proteasomes25. Therefore, regulating tyrosinase degradation is one approach to control melanogenesis8. Several researchers have reported hypopigmentation resulting from increased tyrosinase degradation26,27,28. For example, Kageyama et al (2004) reported that phospholipase D2 decreased melanin synthesis without affecting tyrosinase transcription but showed a decrease in tyrosinase activity. Treatment with proteasome inhibitors upregulated melanogenesis, which strongly suggests that downregulation of melanogenesis is due to proteasomal degradation of tyrosinase. Similarly, DHA did not affect MITF expression or the signaling pathways but was shown in this study to accelerate the degradation of tyrosinase, leading to reduced production of melanin. As shown in Figure 4, the use of a proteasome inhibitor restored tyrosinase level in DHA-treated B16F10 cells. Furthermore, DHA did not alter MITF or tyrosinase mRNA level (Figure 5). These results suggest that the decrease in melanin synthesis can be attributed to the proteasomal degradation of tyrosinase.
Several reports have indicated that the ERK and Akt pathways are involved in melanogenesis13,14,16,29,30. Activation of the ERK and Akt pathways result in suppression of MITF and, consequently, in a decrease of tyrosinase expression13,14. However, DHA did not activate either the ERK or the Akt pathway (Figure 3A), suggesting that this is not the manner in which DHA decreases melanin synthesis. Because CREB phosphorylation increases melanin synthesis10,31, we examined the involvement of CREB. However, CREB was not inactivated by DHA (Figure 3B). In addition, the possibility of direct inhibition of tyrosinase by DHA was analyzed (Figure 1D). DHA did not directly inhibit tyrosinase, although there was a clear decrease in tyrosinase activity (Figure 1C). These results suggest that other signaling pathways might be involved in the DHA-induced melanin reduction. However, the signaling pathways related to tyrosinase degradation were not thoroughly studied here.
In conclusion, this study illustrated that DHA is involved in the regulation of melanin synthesis via tyrosinase degradation. As inhibitors of tyrosinase activity have been long sought as a treatment for hyperpigmentary disorders of the skin8, DHA presents itself as a potential agent to address this problem.
Author contribution
Dong-Seok KIM designed the research; Marie Carmel BALCOS performed the experiments and wrote the paper; Su Yeon KIM, Hyo-Soon JEONG performed the experiments; Hye-Young YUN, Kwang Jin BAEK, Nyoun Soo KWON, Kyoung-Chan PARK contributed to the data analysis and interpretation; Dong-Seok KIM provided supervision, performed the data analysis, and wrote the paper.
Abbreviations
DHA, docosahexaenoic acid; DOPA, 3,4-dihydroxyphenylalanine; ERK, extracellular signal-regulated kinase; MITF, microphthalmia-associated transcription factor; α-MSH, α-melanocyte stimulating hormone; PVDF, polyvinylidene fluoride; TRP, tyrosinase-related protein; UV, ultraviolet.
Acknowledgments
This study was supported by a grant (A100179) from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea.
References
- Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids 2003; 126: 1–27. [DOI] [PubMed] [Google Scholar]
- Stillwell W. Docosahexaenoic acid: a most unusual fatty acid. Chem Phys Lipids 2008; 153: 1–2. [DOI] [PubMed] [Google Scholar]
- Storey A, McArdle F, Friedmann PS, Jackson MJ, Rhodes LE. Eicosapentaenoic acid and docosahexaenoic acid reduce UVB- and TNF-alpha-induced IL-8 secretion in keratinocytes and UVB-induced IL-8 in fibroblasts. J Invest Dermatol 2005; 124: 248–55. [DOI] [PubMed] [Google Scholar]
- Rahman M, Kundu JK, Shin JW, Na HK, Surh YJ. Docosahexaenoic acid inhibits UVB-induced activation of NF-kappaB and expression of COX-2 and NOX-4 in HR-1 hairless mouse skin by blocking MSK1 signaling. PLoS One 2011; 6: e28065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Hearing VJ. Update on the regulation of mammalian melanocyte function and skin pigmentation. Expert Rev Dermatol 2011; 6: 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino MV. Signaling pathways in melanosome biogenesis and pathology. Int J Biochem Cell Biol 2010; 42: 1094–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing VJ, Tsukamoto K. Enzymatic control of pigmentation in mammals. FASEB J 1991; 5: 2902–9. [PubMed] [Google Scholar]
- Ando H, Kondoh H, Ichihashi M, Hearing VJ. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J Invest Dermatol 2007; 127: 751–61. [DOI] [PubMed] [Google Scholar]
- Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J 2007; 21: 976–94. [DOI] [PubMed] [Google Scholar]
- Busca R, Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res 2000; 13: 60–9. [DOI] [PubMed] [Google Scholar]
- Vachtenheim J, Borovansky J. Transcription physiology” of pigment formation in melanocytes: central role of MITF. Exp Dermatol 2010; 19: 617–27. [DOI] [PubMed] [Google Scholar]
- Levy C, Khaled M, Fisher DE. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med 2006; 12: 406–14. [DOI] [PubMed] [Google Scholar]
- Jang JY, Lee JH, Kang BW, Chung KT, Choi YH, Choi BT. Dichloromethane fraction of Cimicifuga heracleifolia decreases the level of melanin synthesis by activating the ERK or AKT signaling pathway in B16F10 cells. Exp Dermatol 2009; 18: 232–7. [DOI] [PubMed] [Google Scholar]
- Kim DS, Park SH, Kwon SB, Park ES, Huh CH, Youn SW, et al. Sphingosylphosphorylcholine-induced ERK activation inhibits melanin synthesis in human melanocytes. Pigment Cell Res 2006; 19: 146–53. [DOI] [PubMed] [Google Scholar]
- Kim DS, Jeong YM, Park IK, Hahn HG, Lee HK, Kwon SB, et al. A new 2-imino-1,3-thiazoline derivative, KHG22394, inhibits melanin synthesis in mouse B16 melanoma cells. Biol Pharm Bull 2007; 30: 180–3. [DOI] [PubMed] [Google Scholar]
- Oka M, Nagai H, Ando H, Fukunaga M, Matsumura M, Araki K, et al. Regulation of melanogenesis through phosphatidylinositol 3-kinase-Akt pathway in human G361 melanoma cells. J Invest Dermatol 2000; 115: 699–703. [DOI] [PubMed] [Google Scholar]
- Smalley K, Eisen T. The involvement of p38 mitogen-activated protein kinase in the alpha-melanocyte stimulating hormone (alpha-MSH)-induced melanogenic and anti-proliferative effects in B16 murine melanoma cells. FEBS Lett 2000; 476: 198–202. [DOI] [PubMed] [Google Scholar]
- Bentley NJ, Eisen T, Goding CR. Melanocyte-specific expression of the human tyrosinase promoter: activation by the microphthalmia gene product and role of the initiator. Mol Cell Biol 1994; 14: 7996–8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotto C, Abbe P, Hemesath TJ, Bille K, Fisher DE, Ortonne JP, et al. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J Cell Biol 1998; 142: 827–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M. MITF: a stream flowing for pigment cells. Pigment Cell Res 2000; 13: 230–40. [DOI] [PubMed] [Google Scholar]
- Yasumoto K, Yokoyama K, Takahashi K, Tomita Y, Shibahara S. Functional analysis of microphthalmia-associated transcription factor in pigment cell-specific transcription of the human tyrosinase family genes. J Biol Chem 1997; 272: 503–9. [DOI] [PubMed] [Google Scholar]
- Bertolotto C, Busca R, Abbe P, Bille K, Aberdam E, Ortonne JP, et al. Different cis-acting elements are involved in the regulation of TRP1 and TRP2 promoter activities by cyclic AMP: pivotal role of M boxes (GTCATGTGCT) and of microphthalmia. Mol Cell Biol 1998; 18: 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavuzer U, Keenan E, Lowings P, Vachtenheim J, Currie G, Goding CR. The microphthalmia gene product interacts with the retinoblastoma protein in vitro and is a target for deregulation of melanocyte-specific transcription. Oncogene 1995; 10: 123–34. [PubMed] [Google Scholar]
- Glickman MH. Getting in and out of the proteasome. Semin Cell Dev Biol 2000; 11: 149–58. [DOI] [PubMed] [Google Scholar]
- Halaban R, Cheng E, Zhang Y, Moellmann G, Hanlon D, Michalak M, et al. Aberrant retention of tyrosinase in the endoplasmic reticulum mediates accelerated degradation of the enzyme and contributes to the dedifferentiated phenotype of amelanotic melanoma cells. Proc Natl Acad Sci U S A 1997; 94: 6210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Yoshida M, Uchiwa H, Kawa Y, Mizoguchi M. Down-regulation of melanin synthesis by a biphenyl derivative and its mechanism. Pigment Cell Res 2003; 16: 494–500. [DOI] [PubMed] [Google Scholar]
- Kageyama A, Oka M, Okada T, Nakamura S, Ueyama T, Saito N, et al. Down-regulation of melanogenesis by phospholipase D2 through ubiquitin proteasome-mediated degradation of tyrosinase. J Biol Chem 2004; 279 27774–80. [DOI] [PubMed] [Google Scholar]
- Hall AM, Krishnamoorthy L, Orlow SJ. 25-Hydroxycholesterol acts in the Golgi compartment to induce degradation of tyrosinase. Pigment Cell Res 2004; 17: 396–406. [DOI] [PubMed] [Google Scholar]
- Kono M, Dunn IS, Durda PJ, Butera D, Rose LB, Haggerty TJ, et al. Role of the mitogen-activated protein kinase signaling pathway in the regulation of human melanocytic antigen expression. Mol Cancer Res 2006; 4: 779–92. [DOI] [PubMed] [Google Scholar]
- Kawano M, Matsuyama K, Miyamae Y, Shinmoto H, Kchouk ME, Morio T, et al. Antimelanogenesis effect of Tunisian herb Thymelaea hirsuta extract on B16 murine melanoma cells. Exp Dermatol 2007; 16: 977–84. [DOI] [PubMed] [Google Scholar]
- Price ER, Horstmann MA, Wells AG, Weilbaecher KN, Takemoto CM, Landis MW, et al. alpha-Melanocyte-stimulating hormone signaling regulates expression of microphthalmia, a gene deficient in Waardenburg syndrome. J Biol Chem 1998; 273: 33042–7. [DOI] [PubMed] [Google Scholar]