Abstract
Aim:
To explore whether icaritin, a prenylflavonoid derivative of the Chinese tonic herb Epimedium, could suppress the proliferation of human osteosarcoma cells in vitro, and to elucidate the mechanisms of the action.
Methods:
Human osteosarcoma SaOS2 cell line was used in the present study. The proliferation of the cells was examined using MTT assay and immunofluorescence DAPI staining. Cell motility was studied with the scratch assay. Cell apoptosis was determined by Annexin V-FITC and PI double staining using flow cytometry. Western blotting and RT-PCR were used to measure the expression of mRNAs and proteins in the cells.
Results:
Icaritin (5–15 μmol/L) suppressed the proliferation of SaOS2 cells in vitro in a dose-dependent manner. Furthermore, the cell motility was significantly decreased after exposure to icaritin. Moreover, icaritin (5 μmol/L) time-dependently induced the apoptosis of SaOS2 cells, markedly suppressed MMP-2 and MMP-9 expression, upregulated caspase-3 and caspase-9 expression, and increased the level of cleaved caspase-3 in the cells. Co-exposure to the caspase-3 inhibitor zVAD-fmk (10 μmol/L) compromised the icaritin-induced caspase-3 expression and apoptosis in SaOS2 cells.
Conclusion:
Icaritin suppresses the proliferation of SaOS2 human osteosarcoma cells by increasing apoptosis and downregulating MMP expression.
Keywords: osteosarcoma, icaritin, apoptosis, MMP-2, MMP-9, caspase-3, zVAD-fmk
Introduction
Icaritin, a prenylflavonoid derivative from the Chinese tonic herb Epimedium, has been shown to have many pharmacological and biological activities, such as antioxidant activity1, enhancement of the osteoblastic differentiation of mesenchymal stem cells2,3,4, inhibition of human prostate carcinoma growth5,6 and neuroprotective effects7,8,9.
Osteosarcoma (OS) is one of the most common malignant bone tumors during childhood and adolescence and is the second leading cause of mortality in this age group10,11. This type of bone tumor is characterized by a highly malignant and metastatic potential, and approximately 80% of osteosarcomas originate in the appendicular skeleton. Multi-drug chemotherapy has greatly increased the five year survival rate from 20% to 70%12,13,14. However, the rate has been stagnant for 30 years, and disease prognosis is particularly poor for patients with recurrent and metastatic disease, partially due to the emergence of chemotherapy resistance. The abnormal control of cell proliferation in osteosarcoma has been linked to the suppression of apoptosis15, increased MMP expression12,16 and the genetic alteration of oncogenes that regulate the cell cycle17,18.
The mechanisms underlying the antitumor activity of icaritin have drawn increasing attention5,6,18,19,20. Although accumulating evidence has shown that icaritin plays a potential role in inhibiting cancer cell growth, the underlying mechanism remains elusive. It has been reported that icaritin can effectively inhibit tumor growth, such as the growth of endometrial cancer cells and human prostate carcinoma growth. Therefore, we aimed to analyze the potential antitumor mechanism of icaritin in osteosarcomas, and we provide clues for developing this compound into a novel therapy.
Materials and reagents
All reagents were of the highest purity. Icaritin was purchased from the National Institute of Control of Pharmaceuticals and Biological Products (Beijing, China); the product ID was 45588, and the purity was >98%, as analyzed by HPLC. A 30 μmol/L stock solution was prepared by dissolving icaritin in 10% DMSO (Sigma-Aldrich, USA) and was stored at 20 °C. The caspase inhibitor zVAD-fmk was prepared and stored at 20 °C (Selleck, USA).
Cell line and cell culture
The SaOS2 human osteosarcoma cell line was obtained from the Global Bioresource Center (ATCC, Rockefeller, Maryland, USA). Cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin, and 100 μg/mL streptomycin in an incubator at 37 °C in the presence of 5% CO2.
Cell proliferation essay
Cells were seeded in a 96-well dish at a final density of 1×104 cells/well and were incubated in DMEM medium containing 10% FBS for 24 h. Then, the cells were treated with different concentrations (0, 1, 2, 3, 5, 10, or 15 μmol/L) of icaritin for 24, 48, and 72 h. The medium was removed, and fresh medium was added to each well along with 20 μL of MTT solution (5 mg/mL). After a 2 h incubation, 150 μL of DMSO was added to each well. The absorption at 570 nm was determined for each sample using an automatic ELISA plate reader. Three replicate wells were used for each treatment, and the experiments were repeated twice21,22.
Scratch assays
Cells were seeded in a 24-well dish at a final density of 1×105 cells/well and were incubated in DMEM medium containing 10% FBS for 24 h. The SaOS2 cells were wounded with a pipette tip to obtain two perpendicular wounds that formed a cross shape. The cells in the experimental group were treated with 5 μmol/L icaritin for 0, 24, 48, and 72 h. The cells in the control group were treated with the same volume of DMSO for the same time. The wounds were photographed at 0, 24, 48, and 72 h using an inverted microscope (Leica, Frankfurt, Germany). The distance migrated was calculated by dividing the distance at the time point by the distance at the beginning. For each experiment, a total of 4 wounds were measured per group, and each experiment was repeated twice.
Immunofluorescence staining
Cells were seeded in a 24-well dish at a final density of 1×105 cells/well and were incubated in DMEM medium containing 10% FBS for 24 h. The cells were then treated with 5 μmol/L icaritin for 24 h; the control group was treated with the same volume of DMSO. Cells cultured on sterile glass coverslips were fixed with 4% paraformaldehyde in PBS for 10 min and then permeabilized with 0.4% Triton X-100 for 15 min at room temperature. A polyclonal rabbit anti-MMP-2 antibody (1:250, Bioworld Technology, Nanjing, China), anti-MMP-9 antibody (1:250, Bioworld Technology, Nanjing, China), anti-caspase-3 antibody (1:100, Santa Cruz Biotechnology, Santa Cruz, USA) or anti-caspase-9 antibody (1:100, Santa Cruz Biotechnology, Santa Cruz, USA) was applied to the sections at 4 °C overnight. Next, the sections were incubated with FITC-conjugated goat anti-rabbit IgG antibody (1:200; Sigma-Aldrich, St Louis, CA, USA). Following immunostaining, all of the sections were mounted with Mowiol anti-fading agent containing DAPI to counterstain the nuclei. Images were taken using a Leica DM3000.
Western blot analysis
For Western blot analysis, the cells were seeded in a 6-well dish to a final concentration of 1×106 cells/well and incubated in DMEM medium containing 10% FBS for 24 h. The cells were then treated with 5 μmol/L icaritin for 24, 48, or 72 h, and the control group was treated with the same volume of DMSO. The cells were washed twice in ice cold PBS. The samples were individually homogenized in 5 mmol/L Tris–HCl buffer containing 4 mmol/L EDTA, pH 7.4, 1 μmol/L pepstatin, 100 μmol/L leupeptin, 100 μmol/L phenylmethyl sulfonylfluoride, and 10 μg/mL aprotinin, and the lysates were cleared by centrifugation at 14 000×g for 10 min at 4 °C. Protein concentrations were determined using the Lowry method. Approximately 100 μg of protein was run on a discontinuous SDS-PAGE gel and transferred to a nitrocellulose membrane. The membranes were blocked with 5% skim milk in TBS containing 0.05% Tween 20 and were incubated with the following primary antibodies overnight at 4 °C: polyclonal rabbit anti-MMP-2 antibody (1:250, Bioworld Technology, Nanjing, China), anti-MMP-9 antibody (1:250, Bioworld Technology, Nanjing, China), or anti-caspase-3 antibody (1:500, Cell signaling, Boston, Massachusetts, USA). The optical density (OD) of the signals was quantified and is expressed as the ratio of the OD of the tested proteins to the OD of β-actin.
Reverse transcription polymerase chain reaction (RT-PCR)
For PCR analysis, the cells were seeded in a 6-well dish at a final density of 1×105 cells/well and were incubated in DMEM medium containing 10% FBS for 24 h. The cells were then treated with 5 μmol/L icaritin for 24 h, and the control group was treated with the same volume of DMSO. Total RNA was extracted from the cultured cells using TRIzol reagent according to the manufacturer's protocol. RNA was quantified using spectroscopy at 260 nm. Five micrograms of RNA was reversely transcribed into cDNA using a standard RT protocol. One microliter (0.4 μg) of cDNA was added to a PCR reaction premix containing 10 pmol/L of each of the corresponding primer pairs. The primers used in this analysis are listed in Table 1. PCR amplification was performed over 35 cycles of 95°C for 30 s, 60 °C for 30 s, and 72 °C for 90 s. The PCR products were separated on 1% agarose gels and were stained with ethidium bromide. The amount of each product was quantified using a gel documentation system (Bio-Rad, Nanjing, China). β-Actin expression was used as an internal reference to verify equal concentrations of cDNA in each sample.
Table 1. Primers of MMP-2, MMP-9, and β-actin.
| Gene | Primers |
|---|---|
| MMP-2 | Forward: 5′-GATAACCTGGATGCCGTCGTG-3′ |
| Reverse: 5′-CAGCCTAGCCAGTCGGATTTG-3′ | |
| MMP-9 | Forward: 5′-AATCTCACCGACAGGCAGCT-3′ |
| Reverse: 5′-CCAAACTGGATGACGATGTC-3′ | |
| β-Actin | Forward: 5′-CGTFGACATCCGTAAAGACC-3′ |
| Reverse: 5′-TAGAGCCACCAATCCACACA-3′ |
Flow cytometry
Apoptosis was analyzed using flow cytometry. For the apoptosis analysis, cells were incubated with 5 μmol/L icaritin for 0, 24, 48, or 72 h, followed by Annexin V-FITC and propidium iodide (PI) double staining performed according to the manufacturer's instructions (Biosea, Beijing, China).
The effect of caspase inhibitor zVAD-fmk on icaritin-induced apoptosis
Cells were seeded in a 6-well dish at a final density of 1×105 cells/well and were incubated in DMEM medium containing 10% FBS for 24 h. The cells were treated with icaritin alone or in combination with the caspase-3 inhibitor 10 μmol/L zVAD-fmk for 24 h, and the lysates were used for the Western blot analysis. The control group was treated with the same volume of 10% DMSO. An MTT assay was performed to investigate the effect of zVAD-fmk on the growth of the SaOS2 cells.
Statistical analysis
Comparisons of the two data sets were analyzed using t-tests, and data with more than two variables were analyzed using two-way repeated-measures ANOVAs with post hoc Tukey's analysis. All data are plotted as the mean±standard deviation.
Results
Icaritin inhibits SaOS2 proliferation in vitro
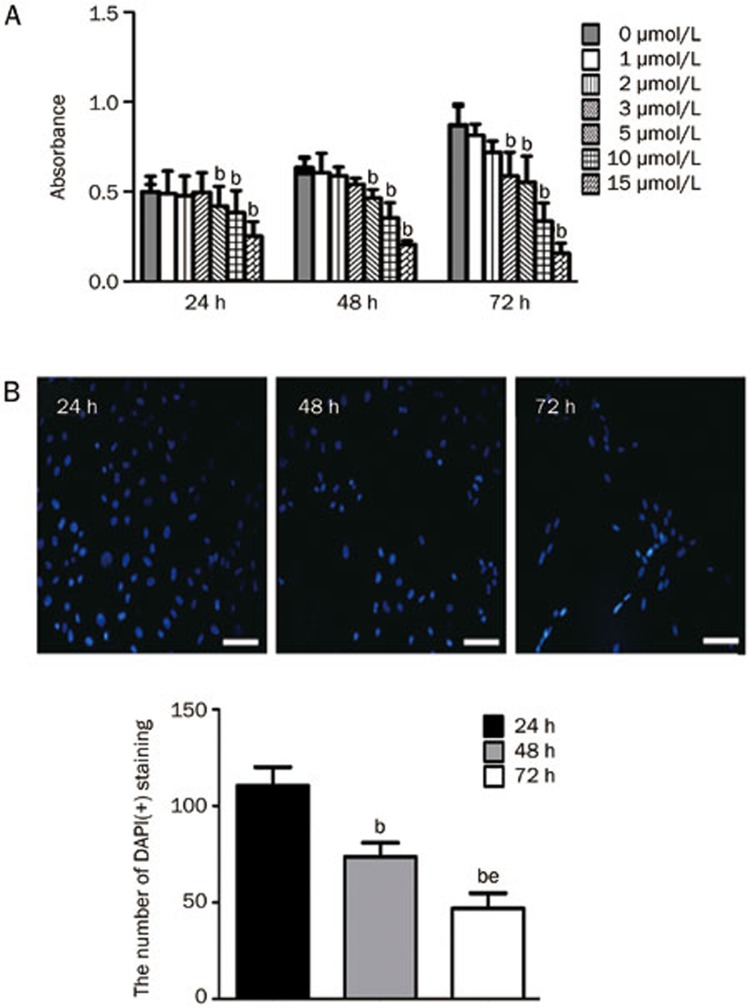
To investigate the effect of icaritin on the inhibition of SaOS2 proliferation, we performed an MTT assay and immunofluorescence DAPI staining. For the MTT analysis, the cells were incubated for various lengths of time (24, 48, or 72 h) in medium containing various concentrations (0, 1, 2, 3, 5, 10, or 15 μmol/L) of icaritin. The control cells were incubated under the same conditions. Administration of 5 μmol/L icaritin significantly decreased SaOS2 proliferation after 24 h, while icaritin treatment exerted inhibitory effects on the SaOS2 cells starting at a dose of 3 μmol/L at 72 h (Figure 1A). For the immunofluorescence DAPI staining, we again confirmed that 5 μmol/L icaritin significantly suppressed SaOS2 proliferation. The number of DAPI(+) cells was significantly reduced after 48 and 72 h of icaritin treatment compared to the number of DAPI(+) cells at 24 h (P<0.05). Meanwhile, a dose of 5 μmol/L icaritin for 72 h obviously decreased the number of DAPI(+) cells compared to the 48 h treatment (P<0.05, Figure 1B).
Figure 1.
The effect of icaritin on SaOS2 cell proliferation. The values are expressed as the absorbance (A). Nuclei staining by DAPI (B). SaOS2 cells were stained with DAPI after incubation with 5 μmol/L icaritin for 24, 48, and 72 h. Scale bar=100 μm. n=3. Mean±SD. bP<0.05 vs 24 h. eP<0.05 vs 48 h.
Icaritin regulates osteosarcoma cell motility
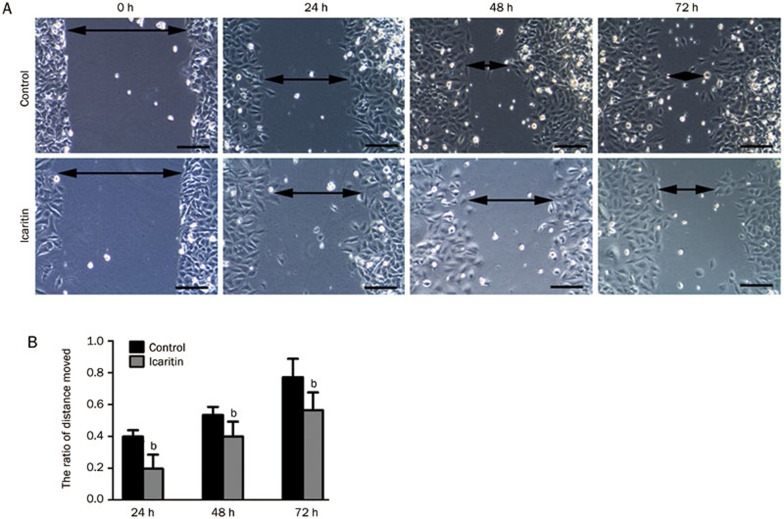
Scratch assays were performed to investigate the changes in cell motility in the icaritin-treated and control groups. The results showed that icaritin treatment decreased the motility of SaOS2 cells after 24, 48, and 72 h of treatment compared to the control group (P<0.05). The average distance that the SaOS2 cells in the icaritin-treated group moved was shorter than that of the cells in the control group. The ratio of the distance moved in the control group was 39.1%, 55.6%, and 78.5% at 24, 48, and 72 h. Meanwhile, for the icaritin-treated group, the ratio was 19.8%, 39.2%, and 56.1% at 24, 48, and 72 h (Figure 2A, 2B).
Figure 2.
(A) Scratch assays to determine the motility of SaOS2 cells. Double headed arrows indicate the wound edges. The cells treated with icaritin showed a decreased motility compared to the control group at the different time points (24, 48, and 72 h). (B) Representative graphical representation of the average distance moved in the icaritin-treated and control groups. n=3. Mean±SD. bP<0.05, compared to the control group. Scale bars=10 μm.
Icaritin inhibits the expression of MMP-2 and MMP-9 in the SaOS2 cell line
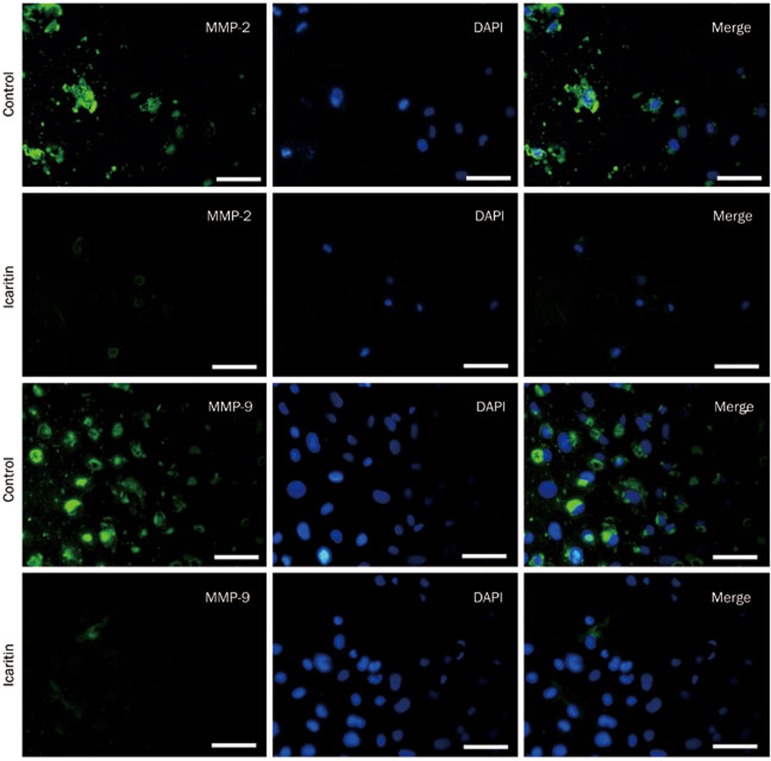
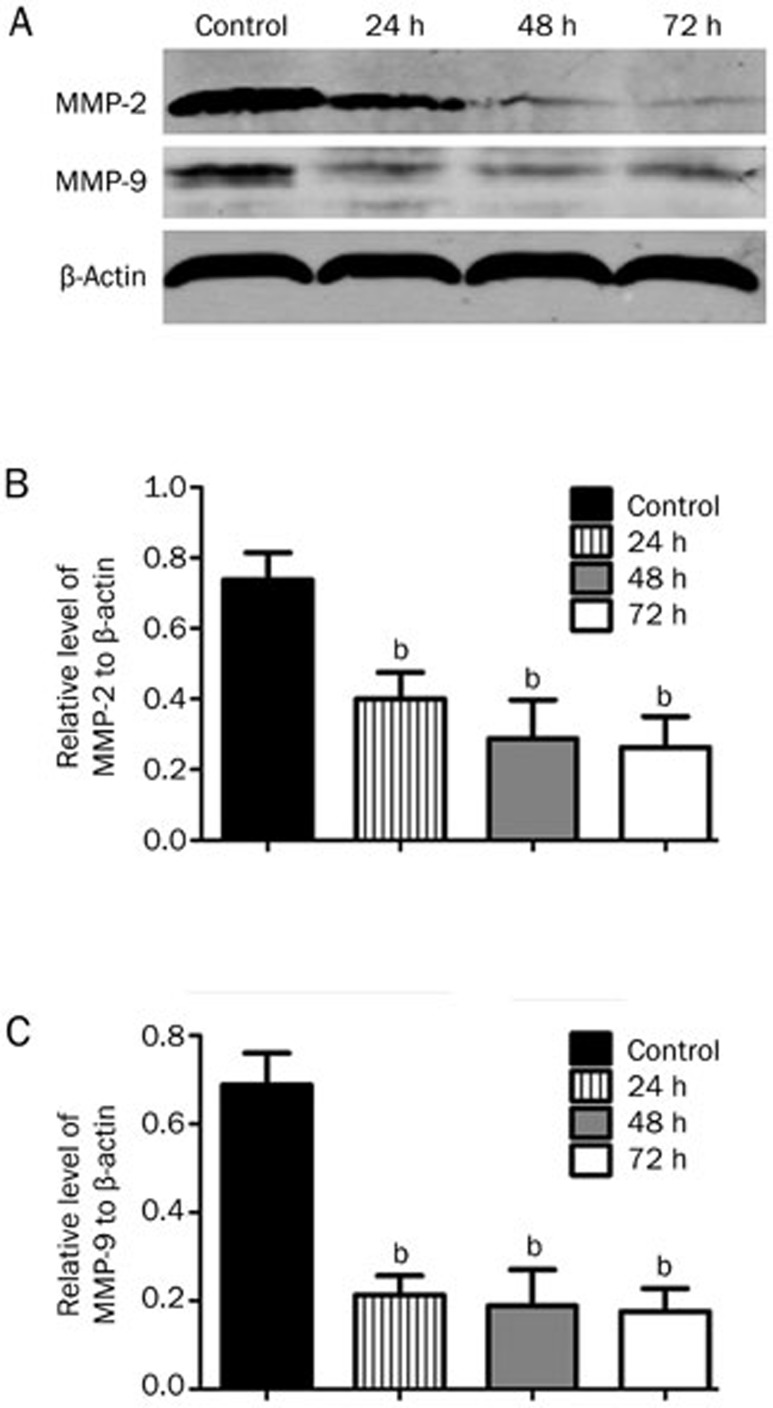
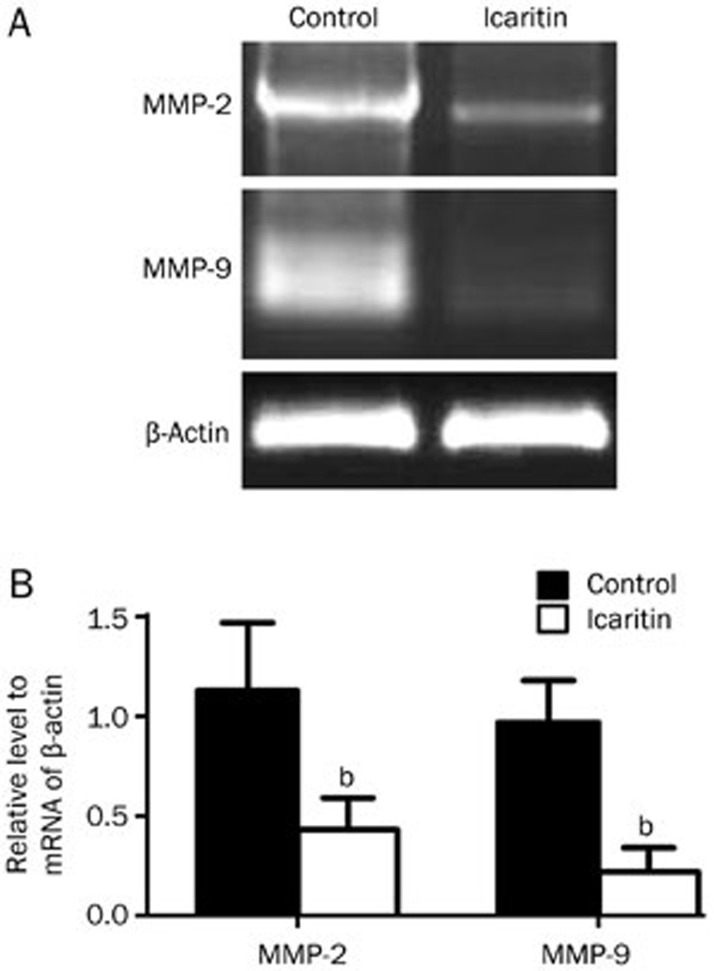
To assess the effect of icaritin on the expression of MMP-2 and MMP-9 in the SaOS2 cells, we performed Western blot, RT-PCR and immunofluorescence staining analyses. For the immunofluorescence staining, we observed that the intensity of MMP-2 and MMP-9 expression in the SaOS2 cells was very high without icaritin treatment. Then, after treatment with 5 μmol/L icaritin, we found that the levels of MMP-2 and MMP-9 expression in the icaritin-treated SaOS2 cells were significantly lower than in the control group (Figure 3). For the Western blot analysis, we observed that 5 μmol/L icaritin significantly suppressed MMP-2 and MMP-9 expression at the protein levels from 75.2% at the 24 h time point to 27.1% at the 72 h time point (P<0.05) (Figure 4). For the RT-PCR analysis, the mRNA of MMP-2 and MMP-9 was also decreased after 24 h of treatment (Figure 5).
Figure 3.
Immunofluorescence staining analysis for MMP-2 and MMP-9 expression in the SaOS2 cells after 24 h of treatment. The immunofluorescence staining showed that the expression levels of MMP-2 and MMP-9 were significantly decreased after treatment with 5 μmol/L icaritin compared to the control group. Scale bar=50 μm.
Figure 4.
Western blot analysis of MMP-2 and MMP-9 expression in the SaOS2 cells after 24, 48, and 72 h of treatment. Western blot analysis confirmed that icaritin could significantly downregulate MMP-2 and MMP-9 expression at 24, 48, and 72 h. n=3. Mean±SD. bP<0.05 vs the control group. β-Actin protein levels indicate that an equal amount of protein was loaded into each lane.
Figure 5.
RT-PCR analysis of MMP-2 and MMP-9 mRNA expression in the SaOS2 cells after 24 h of treatment. RT-PCR analysis confirmed that icaritin could significantly decrease the mRNA expression of MMP-2 and MMP-9. n=3. Mean±SD. bP<0.05 vs control group.
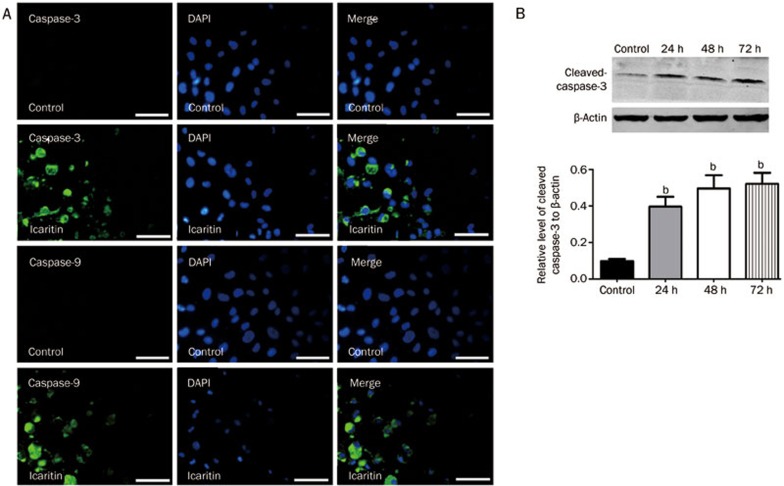
Icaritin increases the expression of caspase-3 and caspase-9 in the SaOS2 cell line
To investigate caspase-3 and caspase-9 expression after icaritin treatment, we performed immunofluorescence staining and Western blot analyses. For the immunofluorescence staining, the results showed that icaritin treatment for 24 h significantly upregulated the expression of caspase-3 and caspase-9 to increase apoptosis. For the SaOS2 cells that were not treated with icaritin, the intensity of caspase-3 and caspase-9 expression was very low (Figure 6A). For the Western blot analysis of cleaved caspase-3, we found that icaritin treatment increased the levels of cleaved caspase-3 by approximately 4-fold after 24, 48, and 72 h compared to the control group (Figure 6B and 6C).
Figure 6.
(A) Immunofluorescence staining analysis for caspase-3 and caspase-9 expression after 24 h of treatment. Immunofluorescence staining analysis revealed that treatment with 5 mol/L icaritin could increase the expression of caspase-3 and caspase-9 in the SaOS2 cells. Scale bar=50 μm. (B & C) Western blot analysis of cleaved caspase-3. The expression of cleaved caspase-3 was significantly upregulated after 24, 48, and 72 h compared to the control group. bP<0.05, compared to the control group. n=3. Mean±SD. β-Actin protein levels indicate that an equal amount of protein was loaded into each lane.
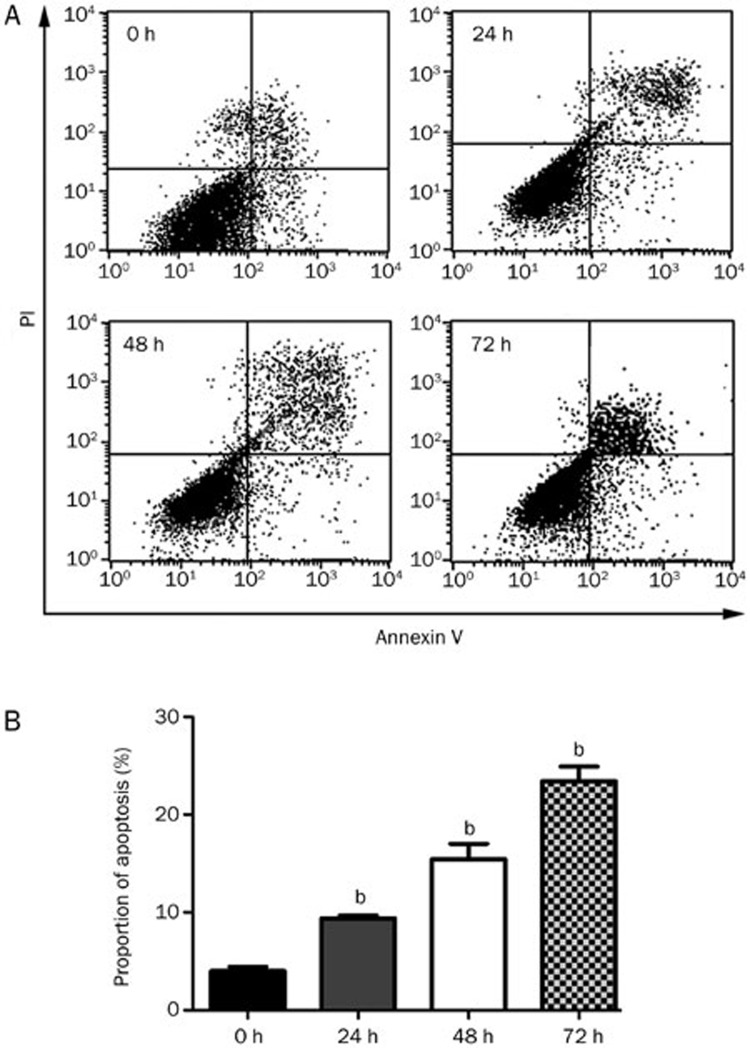
The flow cytometry analysis for apoptosis induced by 5 μmol/L icaritin treatment
To reveal the effect of icaritin on the apoptosis rate of SaOS2 cells, we performed flow cytometry following AnnexinV-FITC and PI double staining. The percentage of apoptotic cells after icaritin treatment for 24, 48, and 72 h (9.9%, 16.5%, and 23.8%) was obviously increased compared with to that of the control group (Figure 7A and 7B).
Figure 7.
Flow cytometry analysis of the apoptosis rate of SaOS2 cells after icaritin treatment. Apoptosis-inducing effect of CEP on SaOS2 cells. After treatment with 5 μmol/L icaritin for 0, 24, 48, and 72 h, the apoptotic rates of SaOS2 cells were determined by Annexin V-FITC and PI double staining using flow cytometry. n=3. Mean±SD. bP<0.05, compared to the control group.
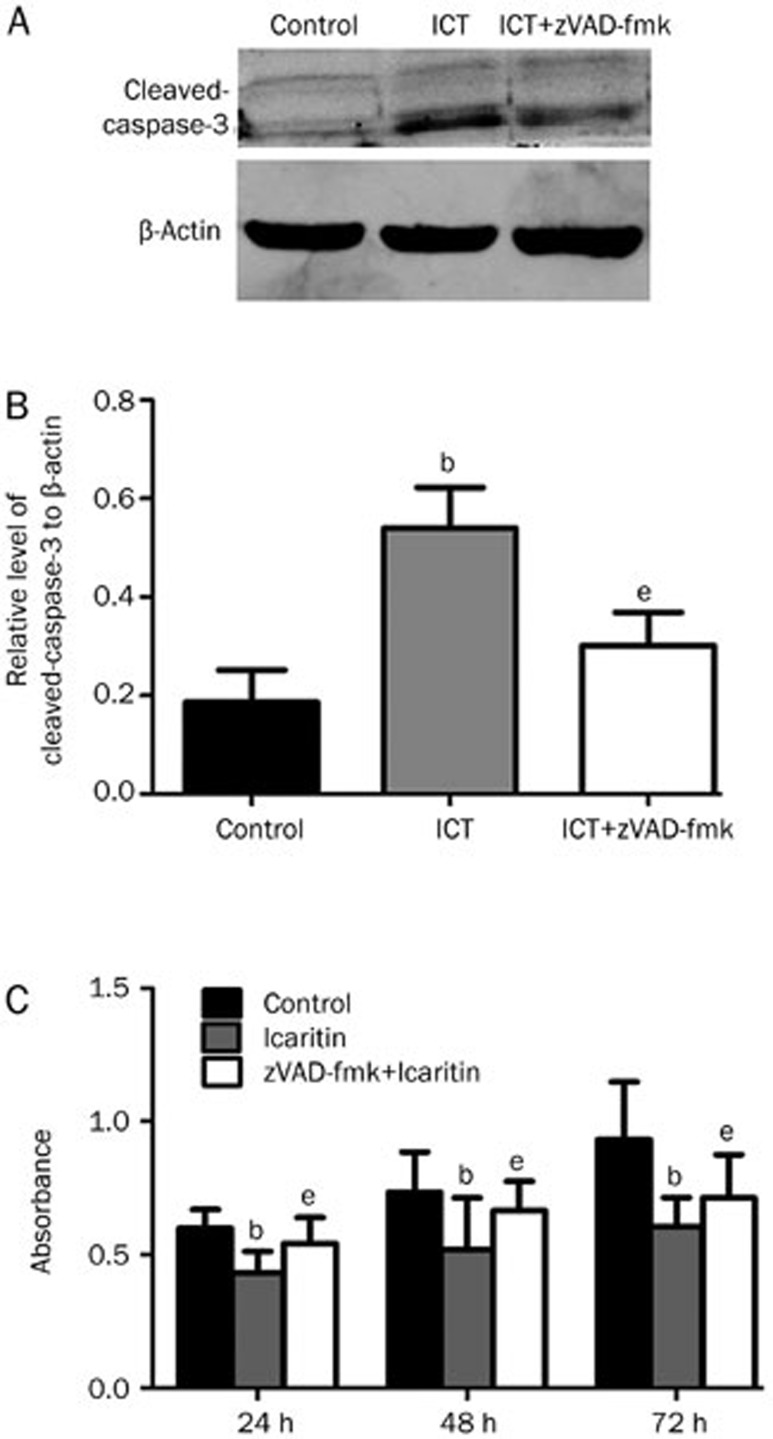
The effect of the caspase-3 inhibitor zVAD-fmk on the icaritin-induced apoptosis
To investigate the effect of the caspase inhibitor zVAD-fmk on the icaritin-induced apoptosis, we performed Western blot analysis to evaluate the levels of cleaved caspase-3. We found that the caspase inhibitor zVAD-fmk could decrease the icaritin-induced caspase-3 expression in the zVAD-fmk+icaritin-treated group compared to the icaritin-treated group (P<0.05) (Figure 8A, 8B). For the MTT assay, we found that zVAD-fmk could compromise the icaritin-induced apoptosis at 24, 48, and 72 h. The absorbance of the zVAD-fmk+icaritin-treated group at 24, 48 and 72 h was higher compared to that of the icaritin-treated group at 24, 48, and 72 h (P<0.05, Figure 8C).
Figure 8.
(A and B) Western blot analysis examining the effect of zVAD-fmk on the icaritin-induced apoptosis. The caspase inhibitor zVAD-fmk could significantly decrease the icaritin-induced cleaved caspase-3 expression. β-Actin protein levels indicate that an equal amount of protein was loaded into each lane. (C) An MTT assay examining the effect of zVAD-fmk. n=3. Mean±SD. bP<0.05, compared to the control group. eP<0.05, compared to the icaritin-treated group.
Discussion
The current study was the first to systemically demonstrate that icaritin, which is extracted from the Chinese tonic herb Epimedium, enhanced the caspase-dependent apoptosis and suppressed the motility and MMP-2/9 expression in the SaOS2 human osteosarcoma cell line in vitro. Therefore, icaritin may have good therapy prospects for treatment of osteosarcoma patients.
Icaritin has been shown to inhibit growth and induce apoptosis in many tumor cells, such as human endometrial cancer cells6, HepG2 cells20, and human prostatic smooth muscle cells19. Icaritin caused sustained ERK1/2 activation and induced apoptosis in human endometrial cancer cells, and icaritin could also induce apoptosis in HepG2 cells via the JNK1 signaling pathway. Icaritin may be useful as an alternative therapeutic choice for imatinib-resistant forms of CML23. The literature has shown that icaritin can suppress the proliferation of many types of cells. The antitumor effect was not specific to human osteosarcoma cells. In our study, we revealed that icaritin could suppress cell growth and induce apoptosis in the SaOS2 human osteosarcoma cell line (Figure 1 and 7).
Metastasis involves many important processes that enable tumor cells to invade into the surrounding tissues, including the coordination of several signaling pathways that allow the detachment of tumor cells, the degradation of the extracellular matrix, invasion, migration, adhesion to endothelial cells, and the reestablishment of tumor growth at a distant site24. It is now well established that metalloproteases have a major influence on the development, invasion, and metastasis of cancer. The metalloproteases are divided into six categories: collagenase (MMP-1, MMP-8, and MMP-13), gelatinase (MMP-2, MMP-9), interstitial lysing (MMP-3, MMP-7, and MMP-10), membrane type matrix metalloproteinase (MMP-14, MMP-15, MMP-16, MMP-17, MMP-24, and MMP-25), lytic enzyme substrate (MMP-7, MMP-26) and other types25,26,27,28. Because MMP-2 and MMP-9 are the major matrix metalloproteinases (MMPs), their activity is increased in many malignant cancers. MMP-2 and MMP-9 also play key roles in tumor cell invasion and metastasis due to their ability to degrade type IV collagen, a major component of the ECM29,30,31.
Overexpression of various MMPs, particularly MMP-2, is correlated with poor prognosis in many types of cancer, including adrenocortical cancer, breast cancer, and thyroid malignancies32,33. In many types of tumors, the expression level of MMP-9 is markedly higher than in normal tissues34,35. Moreover, patients with higher MMP-9 expression have a significantly shorter overall survival time than patients with low MMP-9 expression36. In our study, the observed inhibitory effects of icaritin on the expression of MMP-2 and MMP-9 in SaOS2 cells suggested that icaritin may modulate the invasion and migration of osteosarcoma cells (Figure 3,4,5). In the scratch assays used to determine the motility of the SaOS2 cells, the cells treated with icaritin had decreased motility compared to those in the control group (Figure 2). Osteosarcoma patients frequently undergo lung and liver metastases. Based on the inhibitory effects of icaritin on the expression of MMP-2 and MMP-9, icaritin may have potential as a treatment for patients with advanced osteosarcoma.
According to previous studies, the mechanisms underlying the antitumor activity of icaritin include the induction of apoptosis by multiple signaling pathways5,6,20,23. The caspase (cysteinyl aspartate specific protease) family is involved in apoptotic signal transduction and plays an important role in regulating apoptosis37,38. Caspase-3 is the final performer, and zVAD-fmk inhibits its activity and blocks cell death. The mechanism by which zVAD-fmk blocks caspase activity is through the induction of caspase-3 alkylation and the suppression of its ability to induce apoptosis39. In our study, we tested whether the icaritin-induced apoptosis was caspase-dependent using the caspase inhibitor zVAD-fmk. The results revealed that the effect of icaritin-induced apoptosis was decreased when the cells were treated with icaritin and zVAD-fmk. This finding illustrated that zVAD-fmk could override the icaritin-induced apoptosis and increase SaOS2 growth (Figure 8C). Thus, these results illustrated that the icaritin-induced apoptosis was associated with the caspase cascade. Caspase-3 and caspase-9, which are the key factors in the caspase cascade, were upregulated after icaritin treatment for 24 h. These findings demonstrated that icaritin treatment increased both the apoptosis rate of the tumors and the activation of caspase-3 and caspase-9.
It has been reported that icaritin is relatively stable in the stomach. However, the drug begins to be metabolized in the intestine40. The oral bioavailability of icaritin in rats was found to be low, and first-pass metabolism might exist. A large amount of icariside II, which showed more potential pharmacological activity than icaritin, was found in rat plasma after intragastric administration of icaritin41. One study reported that icaritin has many metabolized products, including icariside I, icariside II, and icariside II 7-o-GLC UA. Icariside II, which is the degradation product of icaritin, is absorbed in the intestine and then undergoes C7-OH glucuronidation in the liver. The glucuronic acid product is released into the small intestine via the bile. Meanwhile, icariside II, which is not metabolized, is released into the urine through the kidneys. Thus, a literature review revealed that the first-pass effect of icaritin exists in the body. In our study, we revealed that the antitumor effect of icaritin is obvious. However, taking the first-pass effect of icaritin into consideration, a special modified method should be used to reduce the extent of the hepatic first-pass effect.
There are some limitations to our findings. In our study, we did not investigate the in vivo effect of icaritin on osteosarcomas. Thus, in future research, we will assess the antitumor effect of icaritin in an animal model. Furthermore, we could not reveal the mechanism by which icaritin suppresses MMP-2 and MMP-9 expression in the osteosarcoma cells. In further studies, we will investigate the underlying mechanisms of how icaritin affects MMP-2 and MMP-9 expression and the antitumor effect of icaritin in vivo.
In conclusion, we found that icaritin inhibited cell growth and could induce apoptosis and suppress MMP-2 and MMP-9 expression in the SaOS2 human osteosarcoma cell line in vitro. We speculated that the underlying mechanisms involved in the icaritin-induced apoptosis were dependent on the initiation of a caspase cascade. Moreover, icaritin suppressed the proliferation of SaOS2 cells by increasing apoptosis and decreasing cell motility through the downregulation of MMP-2 and MMP-9 expression.
Author contribution
Jun WANG designed research; Xiao-fang WANG performed research, analyzed data and wrote the paper.
Acknowledgments
This work was supported by grants to Yun-long MA from Department of Orthopedics, Peking University Third Hospital in China.
References
- Wo Y, Zhu D, Hu Y, Wang Z, Liu J, Lou Y. Reactive oxygen species involved in prenylflavonoids, icariin and icaritin, initiating cardiac differentiation of mouse embryonic stem cells. J Cell Biochem 2008; 103: 1536–50. [DOI] [PubMed] [Google Scholar]
- Sheng H, Rui XF, Sheng CJ, Li WJ, Cheng XY, Jhummon NP, et al. A novel semisynthetic molecule icaritin stimulates osteogenic differentiation and inhibits adipogenesis of mesenchymal stem cells. Int J Med Sci 2013; 10: 782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D, Xie XH, Wang XL, Wan C, Lee YW, Chen SH, et al. Icaritin, an exogenous phytomolecule, enhances osteogenesis but not angiogenesis — an in vitro efficacy study. PLoS One 2012; 7: e 41264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Yuan L, Wang X, Zhang TL, Wang K. Icaritin and its glycosides enhance osteoblastic, but suppress osteoclastic, differentiation and activity in vitro. Life Sci 2007; 81: 832–40. [DOI] [PubMed] [Google Scholar]
- Guo Y, Zhang X, Meng J, Wang ZY. An anticancer agent icaritin induces sustained activation of the extracellular signal-regulated kinase (ERK) pathway and inhibits growth of breast cancer cells. Eur J Pharmacol 2011; 658: 114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong JS, Zhang QH, Huang X, Fu XQ, Qi ST, Wang YP, et al. Icaritin causes sustained ERK1/2 activation and induces apoptosis in human endometrial cancer cells. PLoS One 2011; 6: e 16781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhang X, Wang H, Qi L, Lou Y. Neuroprotective effects of icaritin against beta amyloid-induced neurotoxicity in primary cultured rat neuronal cells via estrogen-dependent pathway. Neuroscience 2007; 145: 911–22. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang H, Wu J, Zhu D, Zhang X, Ou L, et al. Enhanced co-expression of beta-tubulin III and choline acetyltransferase in neurons from mouse embryonic stem cells promoted by icaritin in an estrogen receptor-independent manner. Chem Biol Interact 2009; 179: 375–85. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang X, Wang H, Qi L, Lou Y. Neuroprotective effects of icaritin against beta amyloid-induced neurotoxicity in primary cultured rat neuronal cells via estrogen-dependent pathway. Neuroscience 2007; 145: 911–22. [DOI] [PubMed] [Google Scholar]
- Sharili AS, Allen S, Smith K, Price J, McGonnell IM. Snail2 promotes osteosarcoma cell motility through remodelling of the actin cytoskeleton and regulates tumor development. Cancer Lett 2013; 333: 170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Ren Y, Han EQ, Li H, Chen D, Jacobs JJ. Inhibition of the Wnt-beta-catenin and Notch signaling pathways sensitizes osteosarcoma cells to chemotherapy. Biochem Biophys Res Commun 2013; 431: 274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JS, Lin CW, Hsieh YS, Cheng HL, Lue KH, Yang SF, et al. Selaginella tamariscina (Beauv.) possesses antimetastatic effects on human osteosarcoma cells by decreasing MMP-2 and MMP-9 secretions via p38 and Akt signaling pathways. Food Chem Toxicol 2013; 59: 801–7. [DOI] [PubMed] [Google Scholar]
- Adamski J, Price A, Dive C, Makin G. Hypoxia-induced cytotoxic drug resistance in osteosarcoma is independent of HIF-1Alpha. PLoS One 2013; 8: e 65304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattmann C, Thiemann M, Stenzinger A, Christmann A, Roth E, Ehemann V, et al. Radiosensitization by histone deacetylase inhibition in an osteosarcoma mouse model. Strahlenther Onkol 2013; 189: 957–66. [DOI] [PubMed] [Google Scholar]
- Han J, Tian R, Yong B, Luo C, Tan P, Shen J, et al. Gas6/Axl mediates tumor cell apoptosis, migration and invasion and predicts the clinical outcome of osteosarcoma patients. Biochem Biophys Res Commun 2013; 435: 493–500. [DOI] [PubMed] [Google Scholar]
- Roomi MW, Kalinovsky T, Niedzwiecki A, Rath M. Modulation of u-PA, MMPs and their inhibitors by a novel nutrient mixture in pediatric human sarcoma cell lines. Int J Oncol 2013; 43: 1027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Huang C, Yang YL, Ding Y, Ou-Yang HQ, Zhang YY, et al. Inhibition of the STAT3 signaling pathway is involved in the antitumor activity of cepharanthine in SaOS2 cells. Acta Pharmacol Sin 2012; 33: 101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Zhu D, Lou Y. A novel anticancer agent, icaritin, induced cell growth inhibition, G1 arrest and mitochondrial transmembrane potential drop in human prostate carcinoma PC-3 cells. Eur J Pharmacol 2007; 564: 26–36. [DOI] [PubMed] [Google Scholar]
- Chen MF, Qi L, Li Y, Zu XB, Dai YQ, Zhang P. Icaritin induces growth inhibition and apoptosis of human prostatic smooth muscle cells in an estrogen receptor-independent manner. Amino Acids 2010; 38: 1505–13. [DOI] [PubMed] [Google Scholar]
- He J, Wang Y, Duan F, Jiang H, Chen MF, Tang SY. Icaritin induces apoptosis of HepG2 cells via the JNK1 signaling pathway independent of the estrogen receptor. Planta Med 2010; 76: 1834–9. [DOI] [PubMed] [Google Scholar]
- Wang J, Rong W, Hu X, Liu X, Jiang L, Ma Y, et al. Hyaluronan tetrasaccharide in the cerebrospinal fluid is associated with self-repair of rats after chronic spinal cord compression. Neuroscience 2012; 210: 467–80. [DOI] [PubMed] [Google Scholar]
- Wang J, Ma C, Rong W, Jing H, Hu X, Liu X, et al. Bog bilberry anthocyanin extract improves motor functional recovery by multifaceted effects in spinal cord injury. Neurochem Res 2012; 37: 2814–25. [DOI] [PubMed] [Google Scholar]
- Zhu J, Li Z, Zhang G, Meng K, Kuang W, Li J, et al. Icaritin shows potent anti-leukemia activity on chronic myeloid leukemia in vitro and in vivo by regulating MAPK/ERK/JNK and JAK2/STAT3/AKT signalings. PLoS One 2011; 6: e 23720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Liu L, Yang W, Zang L, Xi Y. RNAi-mediated knockdown of relaxin decreases in vitro proliferation and invasiveness of osteosarcoma MG-63 cells by inhibition of MMP-9. Eur Rev Med Pharmacol Sci 2013; 17: 1102–9. [PubMed] [Google Scholar]
- Reeves C, Charles-Horvath P, Kitajewski J. Studies in mice reveal a role for anthrax toxin receptors in matrix metalloproteinase function and extracellular matrix homeostasis. Toxins 2013; 5: 315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdigao J, Reis A, Loguercio AD. Dentin adhesion and MMPs: a comprehensive review. J Esthet Restor Dent 2013; 25: 219–41. [DOI] [PubMed] [Google Scholar]
- Shuman Moss LA, Jensen-Taubman S, Stetler-Stevenson WG. Matrix metalloproteinases: changing roles in tumor progression and metastasis. Am J Pathol 2012; 181: 1895–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010; 141: 52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosa JP, Kis A, Bacsi K, Balla B, Nagy Z, Takacs I, et al. The protective role of bone morphogenetic protein-8 in the glucocorticoid-induced apoptosis on bone cells. Bone 2011; 48: 1052–7. [DOI] [PubMed] [Google Scholar]
- Ma X, Yang Y, Wang Y, An G, Lv G. Small interfering RNA-directed knockdown of S100A4 decreases proliferation and invasiveness of osteosarcoma cells. Cancer Lett 2010; 299: 171–81. [DOI] [PubMed] [Google Scholar]
- Blyth K, Vaillant F, Jenkins A, McDonald L, Pringle MA, Huser C, et al. Runx2 in normal tissues and cancer cells: a developing story. Blood Cells Mol Dis 2010; 45: 117–23. [DOI] [PubMed] [Google Scholar]
- Xu L, Ding X, Tan H, Qian J. Correlation between B7-H3 expression and matrix metalloproteinases 2 expression in pancreatic cancer. Cancer Cell Int 2013; 13: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson WG. The tumor microenvironment: regulation by MMP-independent effects of tissue inhibitor of metalloproteinases-2. Cancer Metastasis Rev 2008; 27: 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lau G, Chen L, Yuan YF, Huang J, Luk JM, et al. Interleukin 23 promotes hepatocellular carcinoma metastasis via NF-kappa B induced matrix metalloproteinase 9 expression. PLoS One 2012; 7: e 46264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Kohda F, Nakahara T, Chiba T, Tsuji G, Hachisuka J, et al. Aberrant expression of S100A6 and matrix metalloproteinase 9, but not S100A2, S100A4, and S100A7, is associated with epidermal carcinogenesis. J Dermatol Sci 2013; 72: 311–9. [DOI] [PubMed] [Google Scholar]
- Liu Z, Li L, Yang Z, Luo W, Li X, Yang H, et al. Increased expression of MMP9 is correlated with poor prognosis of nasopharyngeal carcinoma. BMC Cancer 2010; 10: 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Li J. Caspase blockade induces RIP3-mediated programmed necrosis in Toll-like receptor-activated microglia. Cell Death Dis 2013; 4: e716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazawa M, Kudo M, Iwata T, Saito K, Takahashi K, Igarashi J, et al. Caspase-independent apoptosis induction of quorum-sensing autoinducer analogs against chronic myeloid leukemia K562. Invest New Drugs 2012; 30: 862–9. [DOI] [PubMed] [Google Scholar]
- Schulz J, Weller M, Moskowitz M. Caspases as treatment targets in stroke and neurodegenerative diseases. Ann Neurol 1999; 45: 421–9. [DOI] [PubMed] [Google Scholar]
- Zhang YP, Xu W, Li N, Li HY, Shen ZY, Zhang XM, et al. LC-MS-MS method for simultaneous determination of icariin and its active metabolite icariside II in human plasma. Chromatographia 2008; 68: 245–50. [Google Scholar]
- Liu J, Lou YJ. Determination of icariin and metabolites in rat serum by capillary zone electrophoresis: rat pharmacokinetic studies after administration of icariin. J Pharm Biomed Anal 2004; 36: 365–70. [DOI] [PubMed] [Google Scholar]