Abstract
Aim:
Peroxisome proliferator activated receptors (PPARs) are nuclear transcription factors that regulate numerous genes influencing blood pressure. The aim of this study was to examine the effects of clofibrate, a PPARα ligand, on blood pressure in spontaneously hypertensive rats (SHR).
Methods:
Wistar-Kyoto (WKY) and spontaneously hypertensive rats (SHR), 8–9 weeks old, were randomly allocated into groups treated with vehicle or clofibrate (250 mg·kg−1·d−1, ip for 21 d). Systolic blood pressure (SBP) was measured before and after the study period using tail-cuff plethysmography. Rats were sacrificed under anesthesia and blood, urine and tissue samples were processed for subsequent analysis.
Results:
SHR rats showed significantly higher SBP compared with WKY rats (198±6 mmHg vs 93±7 mmHg), and a 3-fold increase in urinary protein excretion. Clofibrate treatment reduced SBP by 26%±2% and proteinuria by 43%±9% in SHR but not in WKY rats. The urinary nitrite/nitrate excretion in SHR rats was nearly 2-fold greater than that in WKY, and was further increased by 30%±4% and 48%±3%, respectively, following clofibrate treatment. In addition, PPARα protein expression and PPARα activity were significantly lower in SHR than that in WKY rats. Clofibrate treatment significantly increased PPARα protein expression and PPARα activity in SHR rats, but not in WKY rats. Moreover, the vasoconstrictor response of aortic ring was markedly increased in SHRs, which was blunted after clofibrate treatment.
Conclusion:
PPARα contributes to regulation of blood pressure and vascular reactivity in SHR, and clofibrate-mediated reduction in blood pressure and proteinuria is probably through increased NO production.
Keywords: PPARα, clofibrate, hypertension, proteinuria, vascular reactivity
Introduction
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors that belong to the nuclear receptor superfamily and consist of three isoforms named, PPARα, PPARδ (or β), and PPARγ that exhibit tissue-specific distribution and ligand-specific effects. PPARα is expressed in tissues with very active fatty acid metabolism such as the heart, kidney, liver, and the endothelium, and vascular smooth muscle cells (VSMCs)1 suggesting that PPARα may exert direct effects on the vascular wall. Activation of PPARα is important in uptake, utilization, and catabolism of fatty acids through up-regulation of genes involved in fatty acid transport and peroxisomal and mitochondrial fatty acid β-oxidation.
Fibrates, the prototype PPARα ligands, are generally known for their hypolipidemic actions through lowering triglyceride (TG) and elevating high-density lipoprotein cholesterol (HDL-C). However, PPARα ligands possess other actions on the vasculature that are both PPARα-dependent as well as -independent2,3. Amongst these effects most notable one is the effect on nitric oxide (NO). NO plays an important role in the regulation of vascular tone, platelet aggregation, oxidative stress, leukocyte adherence, and smooth muscle cell mitogenesis4. PPARα ligands are known to prevent the development of hypertension and to improve inflammation in angiotensin II (AII)-infused rats5. Similarly, an anti-inflammatory effect of fenofibrate in human endothelial cell cultures (HUVECs)6, and antiproliferative effect in human hepatoma cell line (Huh7)7 confirms their role in vasculature and hypertension. Fibrates also exert reno-protective effects through reduced oxidative stress in glomeruli and via inhibiting the development of albuminuria and glomerular fibrosis8. Nitric oxide system which is compromised of three isoforms (eNOS, iNOS, and nNOS) is responsible for the formation of NO through oxidation of L-arginine. PPARα activation has been reported to stimulate endothelial nitric oxide (NO) synthesis9, improve endothelial-mediated NO vasodilation10, and increase urinary excretion of NO in the rat11. The beneficial effect for PPARα in cardiovascular diseases has therefore been suggested to involve the NO/NO synthase and was ascribed to the anti-atherogenic and anti-inflammatory actions of NO12.
Essential hypertension is a component of metabolic syndrome with multiple etiological factors. One of the most important pathological events in the early phase of hypertension development is the impairment of NO/NOS system which can results in increased vascular tone. Furthermore, proteinuria has been shown to be an early indicator in the pathological process involved in essential hypertension. In our previous studies, we reported a reduced PPARα expression/activity in DOCA-salt hypertension and a reduction in blood pressure and increased NO production by clofibrate, a PPARα ligand2. PPARs, as transcriptional regulators have been implicated as regulator of expression and activity of endogenous vasoconstrictors as well as their receptors. Therefore, it is possible that induction of PPARα may attenuate vasoconstriction response to major endogenous vasoconstrictor such as AII, thromboxane A2 (TXA2) and endothelin 1 (ET-1)2,12.
Based on these observations we proposed to investigate the involvement of PPARα in the early phase of essential hypertension. We hypothesized that reduced PPARα contributes to the early phase of essential hypertension and therefore induction of PPARα may confer protection. Therefore aims of this study were to investigate the contribution of PPARα in reducing blood pressure in hypertensive rats and their interaction with NO system.
Materials and methods
This study was approved by the Texas Southern University Animal Care and Usage Committee and was performed according to NIH guidelines for the Care and Use of Laboratory Animals (NIH publication No 93–23, revised 1985) and Animal Welfare Act.
Male SHR (8–9 weeks old, 200–250 g, Charles River, Houston, TX, USA) and their corresponding control WKY rats were used in this study. Both WKY and SHRs were randomly distributed into two groups (8–10 rats/group): Control (vehicle treatment) and treatment groups (clofibrate, a PPARα ligand, 250 mg/kg, ip) for 21 d. Systolic blood pressure was measured before and after the study period by tail-cuff plethysmography (SC1000, Hatteras Instrument, Cary, NC, USA). Rats were placed in metabolic cages individually to collect 24 h urine to determine urinary excretion of protein, nitrate/nitrite, Na+ and creatinine. Rats were sacrificed under pentobarbital anesthesia (40 mg/kg; ip). Kidney was isolated, homogenized and the tissues were collected for protein expression and activity assay.
Aortic ring study
Rats were anesthetized with pentobarbital sodium (40 mg/kg; ip). The chest cavity was opened, and the thoracic aorta was removed and placed in a petri-dish containing cold Krebs (37 °C) solution of the following composition (mmol/L each): NaCl 113, KCl 4.7, NaHCO3 25.0, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, and glucose 5, pH 7.4, and continuously aerated with 95% O2, 5% CO2. The aorta was cleansed of excess fat and connective tissue and cut into 3–4 mm rings. The aortic ring was then mounted in a 10 mL jacketed bath (World Precision Instruments-WPI, Sarasota, FL, USA) at 37 °C. The ring was suspended in bath solution by means of two hooks, the lower one fixed to the bottom of the bath while the upper one was connected via a transbridge (model TBM4, World Precision Instruments, Sarasota, FL, USA) data-acquisition system (DataQ Instruments, Akron, OH, USA) for recording of isometric tension developed to application of vasoactive agents. The rings were subjected to a resting tension of 2 g. The rings under this tension were allowed to equilibrate for a period of 90 min while being rinsed every 15 min. During the equilibration period, the rings were subjected to two challenges of 10−5 mol/L phenylephrine (PE), 30 min apart. Following the equilibration period, constrictor responses to cumulative doses of PE (10−5–10−1 mol/L), AII (10−6–10−2 mol/L), U46619, a TXA2 mimetic (10−5–10−1 mol/L) or endothelin-1 (ET-1; 10−7–10−3 mol/L) were determined in aortic rings. Before administering each batch of agonist the rings were washed by flushing with Krebs solution at least three times at 15 min interval, so that the tension level can come back to at or near initial resting level. Sequence of agonist use was also randomized between experiments. Except, for ET-1 which was always administered as the last agonist because effect of ET-1 last longer and take more time to stabilize than any other agonists that we used.
PPARα protein expression and PPARα activity
Protein extraction and Western blot analysis of PPARα were performed as described earlier13. For Western analysis of PPARα, rat aortic tissue from the different experimental groups was homogenized in ice cold SET buffer (0.25 mol/L sucrose, 1 mmol/L EDTA and 10 mmol/L Tris HCl, pH 7.4). Organelles were isolated by differential centrifugation. Cytosolic fraction was collected at 40 000×g (60 min) and this fraction was used immediately or kept at −70 °C. Protein concentration was determined in each fraction using a Micro BCA kit (Pierce, Rockford, IL, USA). Protein from the cytosolic fraction (80 μg) was precipitated with 70% ice cold acetone. The pellet was solubilized in 20 μL of SDS sample buffer and heated in boiling water for 3 min. This sample was electrophoresed through a 10% SDS-polyacrylamide gel under reducing condition (Sample buffer containing 10 mmol/L dithiothreitol). Proteins were transferred onto PVDF membrane and the non-specific binding was blocked by overnight incubation of the membrane in 4% dry milk in Tris-Tween-buffered saline at 4 °C. Membrane was incubated subsequently at room temperature in 4% milk-TST (Tris-Tween-saline) containing rabbit polyclonal antiserum against PPARα (Santa Cruz Biotechnology, Santa Cruz, CA, USA), using a 1:500 dilution of a 200 μg/mL stock. After incubation with a secondary, peroxidase-conjugated antibody, signals were be visualized by chemiluminescence (Amersham, Buckinghamshire, UK). PPARα activity was determined in the nuclear fraction by TransM PPARα kit from Active Motif (Carlsbad, CA, USA) following the manufacturers protocol.
Biochemical analysis
Urinary excretion of protein was determined by a colorimetry (Bio-Rad Protein Assay) using BSA as standard. Plasma and urine concentration of nitrite and nitrate was determined by a commercial kit from Assay Design (Ann Arbor, MI, USA). In this ELISA based kit nitrate was converted to nitrite by nitrate reductase and the amount of nitrite was quantified colorimetrically. Plasma 8-isoprostane was determined using an ELISA kit from Cayman Chemicals. Urinary excretion of Na was determined using flame photometer while creatinine was quantitated using a kit from Sigma.
Statistical analysis
Data obtained from this study were compared between the groups for significance difference using one way ANOVA. Values were presented as Mean±SEM and a P value less than 0.05 was considered statistically significant.
Results
Effect of clofibrate on systolic blood pressure and proteinuria
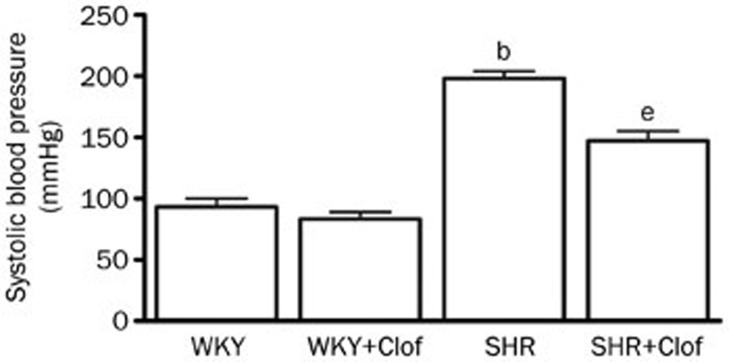
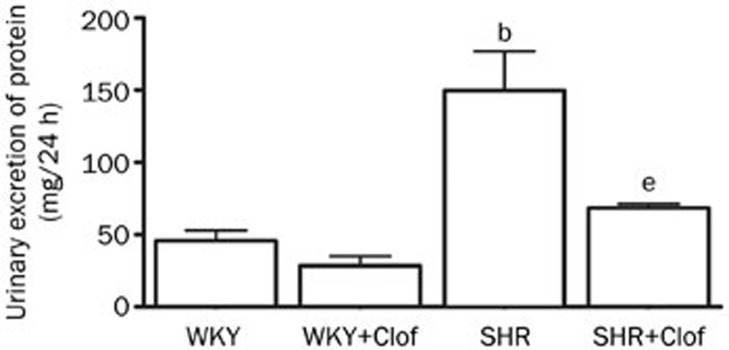
Male SHR and their WKY control rats, 8–9 weeks old, were allocated into groups untreated (WKY and SHR) or treated with clofibrate, a prototype PPARα ligand, for 21 d (WKY+Clof and SHR+Clof). Baseline blood pressure in WKY and SHR rats before any treatment was 85±11 mmHg and 176±9 mmHg, respectively. After 21 d of study period this pressure was changed to 93±11 mmHg and 198±6 mmHg in untreated WKY and SHR. In SHR, this pressure change was 113% higher than the untreated WKY rats. Clofibrate did not alter blood pressure in WKY rats but significantly prevented the increase in SHR (SHR+Clof: 147±8 mmHg, Figure 1). SHRs had a significantly higher proteinuria in 2 months time compared to their WKY counterpart (WKY: 35±7 mg/24 h; SHR 113±21 mg/24 h). In SHR, clofibrate blunted this increase in urinary protein loss by 43% (P<0.05) although without any significant effect in WKY rats (Figure 2).
Figure 1.
Systolic blood pressure in the WKY and SHR treated with clofibrate (Clof: 250 mg/kg; ip) or vehicle for 21 d. Values are mean±SEM. bP<0.05 vs WKY. eP<0.05 vs SHR.
Figure 2.
Urinary excretion of protein in the WKY and SHR treated with clofibrate (Clof: 250 mg/kg; ip) or vehicle for 21 d. Values are mean±SEM. bP<0.05 vs WKY. eP<0.05 vs SHR.
Effect of clofibrate on NO production
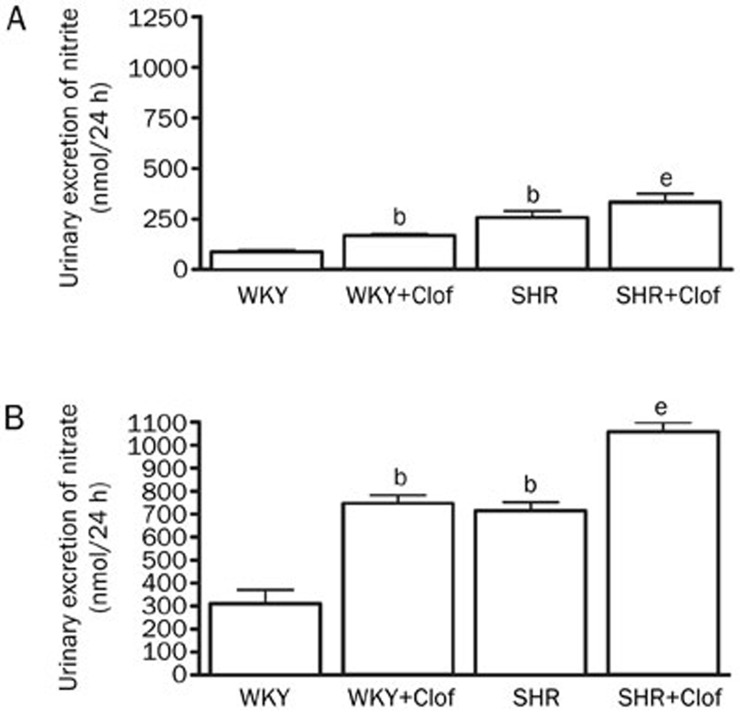
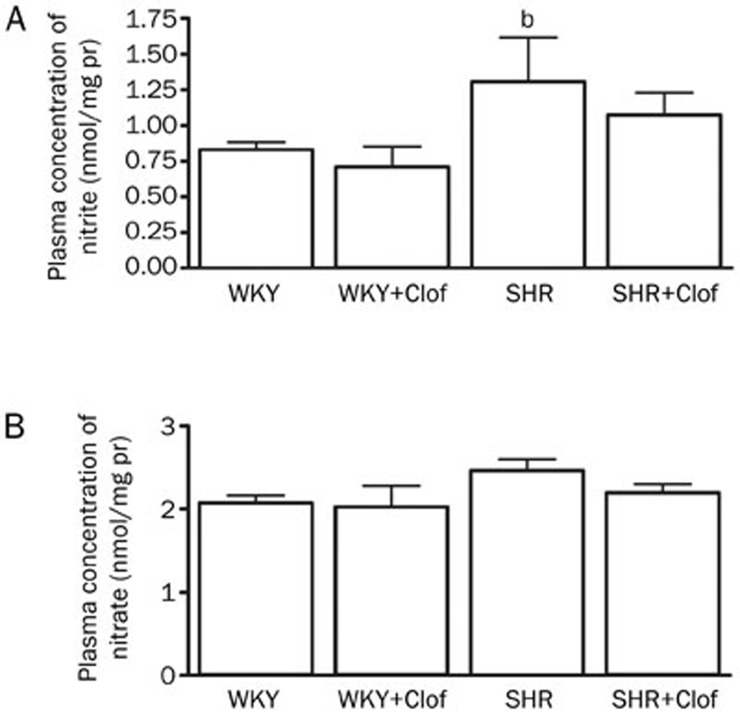
Urinary excretion of 24 h nitrite and nitrate is an indicator of NO production as they are the stable end product of NO. SHRs produced a significantly higher amount of both nitrites (195%) and nitrate (130%) (Figure 3). Clofibrate treatment increased NO production in both WKY and SHRs as it is evident by increased nitrite and nitrate excretion. On the other hand plasma NO was tightly maintained to a distinct level in both WKY and SHRs (Figure 4) except that nitrite was significantly higher in SHR (1.3±0.3 nmol/mg protein) compared to that of WKY rats (0.8±0.06 nmol/mg protein). Similarly, plasma NO was unchanged in both WKY and SHRs when they were treated with clofibrate (Figure 4).
Figure 3.
Urinary excretion of nitrite (A) and nitrate (B) in the WKY and SHR treated with clofibrate (Clof: 250 mg/kg; ip) or vehicle for 21 d. Values are mean±SEM. bP<0.05 vs WKY. eP<0.05 vs SHR.
Figure 4.
Plasma nitrite (A) and nitrate (B) in the WKY and SHR treated with clofibrate (Clof: 250 mg/kg; ip) or vehicle for 21 d. Values are mean±SEM. bP<0.05 vs WKY. eP<0.05 vs SHR.
Effect of clofibrate on free radical activity and urinary excretion of Na and creatinine
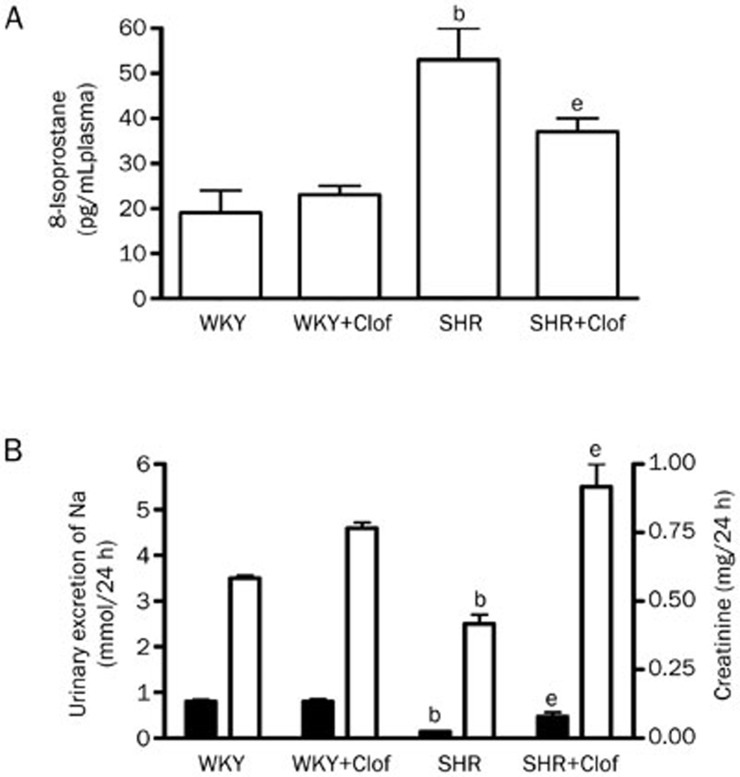
Plasma 8-isoprostane, an indicator of free radical generation was measured in both WKY and SHRs. Basal 8-isoprostane in SHRs was 53±7 pg/mL, which was 178% higher than their WKY control (Figure 5A). Clofibrate reduced 8-isoprostane by 30% in SHR rats to 37±3 pg/mL. Both Na+ and creatinine excretion in SHR were attenuated by 83% and 29%, respectively, compared to that of WKY rats. Clofibrate enhanced Na+ and creatinine excretion in SHR by ∼2–4-fold (Figure 5B).
Figure 5.
Plasma 8-isoprostane (A) and urinary excretion of (B) Na (left Y axis and creatinine (right Y axis) in the WKY and SHR treated with clofibrate (Clof: 250 mg/kg; ip) or vehicle for 21 d. Values are mean±SEM. bP<0.05 vs WKY. eP<0.05 vs SHR.
Effect of clofibrate on vascular reactivity to vasoconstrictors
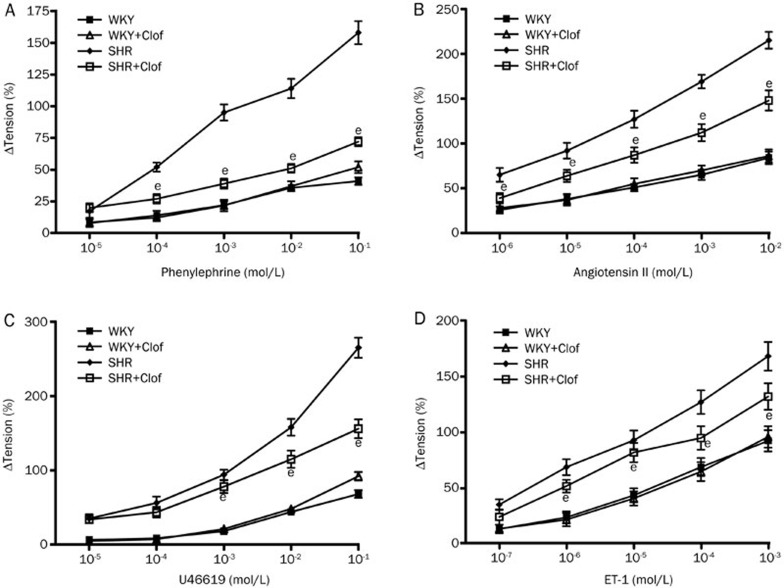
The effect of clofibrate on the vasoconstrictor responses was investigated by measuring reactivity of aortic rings to phenylephrin (PE), angiotensin II (AII), U46619 and endothelin 1 (ET-1). PE vasoconstriction in SHR was almost quadruple compared to that of WKY rats (Figure 6A). This increase was significantly blunted (42%) when SHRs were treated with clofibrate. Clofibrate did not alter PE vasoconstriction in WKY rats.
Figure 6.
Changes in tension in response to PE (A), angiotensin II (B), U46619 (C) and ET-1 (D) in aortic ring isolated from WKY and SHR treated with clofibrate (Clof: 250 mg/kg; ip) or vehicle for 21 d. Values are mean±SEM. bP<0.05 vs SHR.
In SHR group, vasoconstriction induced by AII was increased by 151% compared to WKY rats (Figure 6B). Clofibrate reduced the increased response to AII by 36% in SHRs while it had no effect on WKY rat. Most noticeable vasoconstriction was produced by U46619, a TXA2 mimetic. In SHR, vasoconstriction response to U46619 was >4-fold higher compared to that of WKY rats (Figure 6C). Clofibrate blunted this increase by 27% in SHR group with no effect on WKY rats. SHRs also displayed a 117% increase in vasoconstriction response to ET-1 (Figure 6D) which was reduced by 27% when SHRs were treated with clofibrate.
Effect of clofibrate on PPARα expression and activity
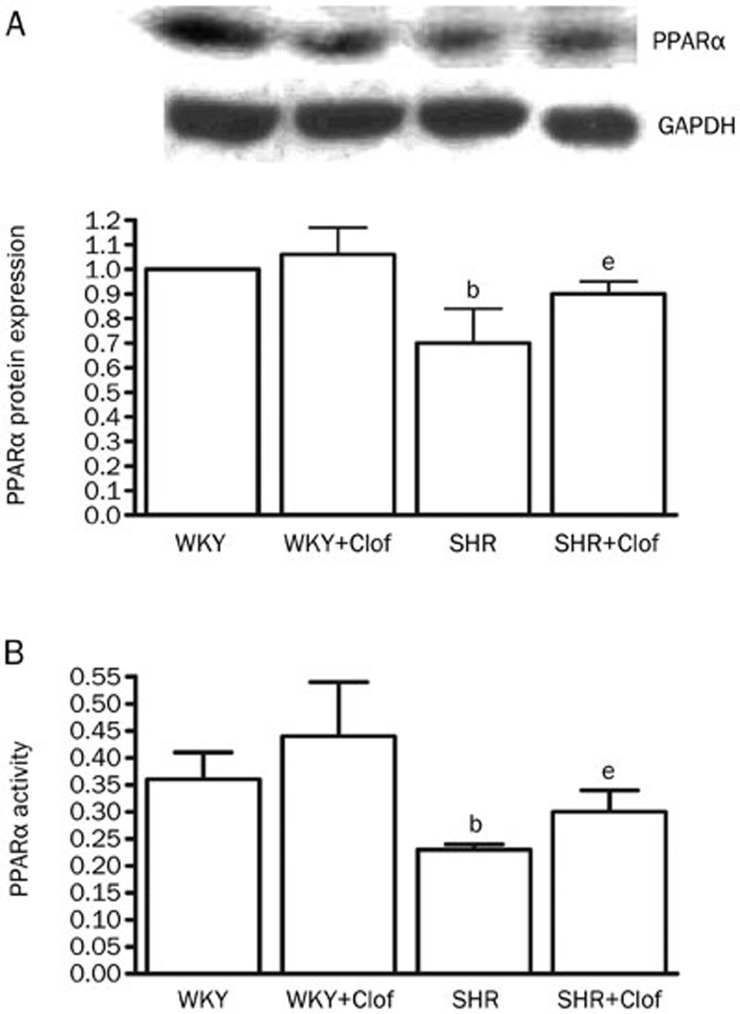
Figure 7A shows effect of clofibrate on PPARα protein expression and activity in SHR and WKY rats. In SHR, PPARα protein expression was significantly reduced (30%) compared to the WKY rats. This was also accompanied by a 36% reduction in PPARα activity (Figure 7B). Although, clofibrate did not influence PPARα activity or expression in WKY rats but both PPARα expression and activity were enhanced by clofibrate in SHRs by 29% and 31%, respectively.
Figure 7.
PPARα protein expression (A) and PPARα activity (B) in the aortic homogenates from WKY and SHR treated with clofibrate (250 mg/kg; ip) or vehicle for 21 d. Values are mean±SEM. bP<0.05 vs WKY. eP<0.05 vs SHR.
Discussion
This study focused on an integrated approach to the regulation of blood pressure by PPARα ligand, clofibrate, in spontaneously hypertensive rats. Our results provided the evidence that transcriptional regulation of blood pressure is involved and PPARα contributes to this regulation of blood pressure in SHR. We further provided the evidence that clofibrate-mediated regulation of blood pressure involves regulation of vasoactive components in the resistance vessels. We are also proposing that the blood pressure regulatory response by clofibrate in whole animal is NO-mediated and may also have PPARα involvement.
High blood pressure is one of the critical risk factors in the development of cardiovascular diseases and stroke14. The endogenous regulation of arterial pressure is not completely understood, but several mechanisms have been identified. An imbalance between reduced production of NO and increased production of reactive oxygen species (ROS), mainly superoxide, may contribute to endothelial dysfunction and development of high blood pressure. Our findings of reduced free radical generation and increased NO production in SHR by clofibrate supports this notion. These observations from our study also suggest a common ROS-NO-dependent pathology in apparently different models of hypertension that may have a similar transcriptional regulation by PPARα.
PPARs are ligand-activated transcription factors that belong to the nuclear receptor superfamily. The role of the different PPAR isoforms in hypertension has been extensively investigated. PPAR agonists have been used to treat cardiovascular diseases due to their additional effects on endothelial function, inflammation and thrombosis. In this context, it is proposed that PPAR activators are capable of reducing blood pressure and attenuating the development of atherosclerosis and cardiac hypertrophy. PPARα, one of the three isoforms of PPARs, has been shown to have a direct effect on vascular wall through up-regulation of several genes involved in fatty acid transport and peroxisomal and mitochondrial fatty acid β-oxidation15. A direct vasorelaxation effect of PPARα highlights its potential in a possible therapeutic use as an anti-hypertensive agent16. Therefore, clofibrate-induced reduction in vasoconstriction response to endogenous vasoconstrictor and a reduction in blood pressure in SHR may be attributed to an induction of PPARα. This notion is in agreement with earlier studies in deoxycorticosterone acetate (DOCA)-salt model 2, SHR model17, and chronic AII infusion model18 of hypertension where PPARα ligand exerted a similar effect. Based on these observations, several mechanisms for anti-hypertensive effect of PPARα can be postulated. We previously reported that PPARα activation, through increased NO generation, promotes renal excretion of Na+ through reduced Na+-K+ ATPase activity in the proximal tubular probably via post translational modification of Na+-K+-ATPase11. Furthermore, in DOCA-salt induced hypertension, clofibrate but not fenofibrate was able to reduce blood pressure through inhibition of endothelial ET-1 production2. It was further validated that increased cytochrome P450 4A (CYP4A) expression is the mechanism responsible for this effect of PPARα in high-salt fed diet rats19. In AII-infused model, docosahexaenoic acid (DHA), a PPARα agonist, reduced blood pressure and attenuated vascular remodeling by inhibiting NAD(P)H oxidase-induced endothelial dysfunction20. Clofibrate-mediated reduction in blood pressure observed in this study is further corroborated by others where fenofibrate reduced blood pressure in SHR21 via reducing expression of vascular inflammatory mediators5.
Involvement of reactive oxygen species (ROS) in pathophysiology of hypertension has been extensively verified but its connection with PPARα was not. Reduction of mean arterial pressure and plasma IL-6 by fenofibrate in an acute model of DOCA-salt hypertension suggested a crosstalk relationship between PPARα and IL-6 in regulating blood pressure22. Increased renal tubular 20-HETE production with a natriuretic effect for PPARα has also been proposed in regulation of blood pressure in DOCA-salt-treated mice23.
Endothelial dysfunction is a hallmark of various cardiovascular diseases including hypertension. In the vascular endothelium, NO, produced by eNOS, is a principal mediator of normal endothelial function24. Blunted endothelium-mediated vasodilatation has been reported in various cardiovascular diseases, including hypertension25,26. In vascular diseases, bioavailability of NO depends on its rate of production along with its rate of removal which can result in the lost of NO signaling and increased production of new radicals which can lead to endothelial dysfunction. Since, vascular tone is a balance of dilatory and constrictor response, impaired vascular relaxation and endothelial dysfunction can result in increased vasoconstrictor response thus may ultimately increase blood pressure. In this study, an increased vasoconstrictor response in SHRs suggests a reduced activity of vasodilators which is validated by a reduced production of NO, major endogenous vasodilator. Clofibrate-mediated improvement in vasoconstriction and accompanying increase in NO confirms the hypothesis that clofibrate-mediated reduction in blood pressure involves NO. At the same time reduced ROS generation in SHR by clofibrate agrees with earlier studies showing an increased ROS generation and NAD(P)H oxidase activity in SHR rats where eNOS “uncoupling” in hypertension was proposed27. At the same time, reduced vasoconstriction response to endogenous vasoconstrictor in SHR by clofibrate propose that clofibrate-mediated induction of PPARα may have a role on PE, AII, TxA2 and ET-1. This notion is corroborated by studies that showed reduced vasoconstrictor activity of these endogenous vasoconstrictors after activation of PPARα28,29,30. Additionally, Jonkers et al reported a reduction in blood pressure in patients treated with bezafibrate (a PPARα agonist) and correlated that with an improvement in endothelial function and increased in plasma cGMP31. They further suggested that bezafibrate-induced improvement of endothelial function was the reason for the observed effects. In the present report we have provided further in vivo evidence for the beneficial effect of clofibrate in blood pressure reduction through normalization of vascular responses to vasoconstrictors in SHR. Similarly, Iglarz et al reported in DOCA-salt-treated rats, that both fenofibrate and rosiglitazone modulated endogenous production of ET-1 and provided beneficial vascular effect in endothelin-dependent hypertension without improving endothelial dysfunction29. This data confirms potential benefit of clofibrate in blood pressure regulation in both endothelium-dependent as well as endothelium-independent mechanisms. Increased production of ROS contributes to diminishing NO bioavailability and it is a leading cause of endothelial dysfunction and hypertrophy of vascular cells32. Our previous studies observed an increased plasma 8-isoprostane level and NAD(P)H oxidase activity in L-NAME-induced high blood pressure, which was blunted by bezafibrate2. In a similar study fenofibrate reduced blood pressure in mice with an implanted osmotic mini-pump releasing AII through increased cytochrome P450 metabolite33,34. Other groups in our laboratory have also reported lowering effect of clofibrate on L-NAME induced high blood pressure in rats through modulation of NO and AII receptor30.
This study reiterates the transcriptional regulation of blood pressure by PPARα in a genetic model of hypertension. We are suggesting an integrated NO response in hypertension which is both PPARα dependent as well as PPARα independent. This study also identifies the role of PPARα in modulating endogenous vasoconstrictor response which is critical in maintaining a normal blood pressure.
References
- Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J Adv Pharm Technol Res 2011; 2: 236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newaz M, Blanton A, Fidelis P, Oyekan A. NAD(P)H oxidase/nitric oxide interactions in peroxisome proliferator activated receptor (PPAR) α –mediated cardiovascular effects. Mutat Res 2005; 579: 163–71. [DOI] [PubMed] [Google Scholar]
- Newaz M, Ranganna K, Truong LD, Oyekan A. Effect of peroxisome proliferator-activated receptor-alpha siRNA on hypertension and renal injury in the rat following nitric oxide withdrawal and high salt diet. J Hypertens 2009; 27: 2223–31. [DOI] [PubMed] [Google Scholar]
- Cooke JP, Dzau VJ. Nitric oxide synthase: role in the genesis of vascular disease. Annu Rev Med 1997; 48: 489–509. [DOI] [PubMed] [Google Scholar]
- Diep QN, Benkirane K, Amiri F, Cohn JS, Endermann D, Schiffrin EL. PPARα activator fenofibrate inhibits myocardial inflammation and fibrosis in angiotensin II infused rats. J Mol Cell Cardiol 2004; 36: 295–304. [DOI] [PubMed] [Google Scholar]
- Price ET, Welder GJ, Zineh I. Modulatory effect of fenofibrate on endothelial production of neutrophil chemokines IL-8 and ENA-78. Cardiovasc Drugs Ther 2012; 26: 95–9. [DOI] [PubMed] [Google Scholar]
- Yamasaki D, Kawabe N, Nakamura H, Tachibana K, Ishimoto K, Tanaka T, et al. Fenofibrate suppresses growth of the human hepatocellular carcinoma cell via PPARα-independent mechanisms. Eur J Cell Biol 2011; 90: 657–64. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kume S, Araki S, Isshiki K, Chin-Kanasaki M, Sakaguchi M, et al. Fenofibrate, a PPARα agonist, has renoprotective effects in mice by enhancing renal lipolysis. Kidney Int 2011; 79: 871–82. [DOI] [PubMed] [Google Scholar]
- Cervantes-Pérez LG, Ibarra-Lara Mde L, Escalante B, Del Valle-Mondragón L, Vargas-Robles H, Pérez-Severiano F, et al. Endothelial nitric oxide synthase impairment is restored by clofibrate treatment in an animal model of hypertension. Eur J Pharmacol 2012; 685: 108–15. [DOI] [PubMed] [Google Scholar]
- Tabernero A, Schoonjans K, Jesel L, Carpusca I, Auwerx J, Andriantsitohaina R. Activation of the peroxisome proliferator-activated receptor alpha protects against myocardial ischaemic injury and improves endothelial vasodilatation. BMC Pharmacol 2002; 2: 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newaz MA, Ranganna K, Oyekan AO. Relationship between PPARalpha activation and NO on proximal tubular Na+ transport in the rat. BMC Pharmacol 2004; 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra-Lara L, Cervantes-Pérez LG, Pérez-Severiano F, Del Valle L, Rubio-Ruíz E, Soria-Castro E, et al. PPARalpha stimulation exerts a blood pressure lowering effect through different mechanisms in a time-dependent manner. Eur J Pharmacol 2010; 627: 185–93. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay N, Singh DP, Heese O, Godbole MM, Sinohara T, Black PM, et al. Expression of peroxisome proliferator-activated receptors in human astrocytic cells: PPARgamma agonists as inducers of apoptosis. J Neurosci Res 2000; 61: 67–74. [DOI] [PubMed] [Google Scholar]
- Whitworth JA. World Health Organization, International Society of Hypertension Writing 1. Group. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 2003; 21: 1983–92. [DOI] [PubMed] [Google Scholar]
- Iacobazzi V, Convertini P, Infantino V, Scarcia P, Todisco S, Palmieri F. Statins, fibrates and retinoic acid upregulate mitochondrial acylcarnitine carrier gene expression. Biochem Biophys Res Commun 2009; 388: 643–7. [DOI] [PubMed] [Google Scholar]
- Goya K, Sumitani S, Xu X, Kitamura T, Yamamoto H, Kurebayashi S, et al. Peroxisome proliferator-activated receptor alpha agonists increase nitric oxide synthase expression in vascular endothelial cells. Arterioscler Thromb Vasc Biol 2004; 24: 658–63. [DOI] [PubMed] [Google Scholar]
- Mamnoor PK, Hegde P, Datla SR, Damarla RK, Rajagopalan R, Chakrabarti R. Antihypertensive effect of ragaglitazar: a novel PPARalpha and gamma dual activator. Pharmacol Res 2006; 54: 129–35. [DOI] [PubMed] [Google Scholar]
- Wilson JL, Duan R, El-Marakby A, Alhashim A, Lee DL. Peroxisome proliferator activated receptor-α agonist slows the progression of hypertension, attenuates plasma interleukin-6 levels and renal inflammatory markers in angiotensin II infused mice. PPAR Res 2012: 2012; 645969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Zhao X, Wang MH, Imig JD, Pollock DM. Peroxisome proliferator-activated receptor-α activation reduces salt-dependent hypertension during chronic endothelin B receptor blockade. Hypertension 2005; 46: 366–371. [DOI] [PubMed] [Google Scholar]
- Diep QN, Amiri F, Touyz RM, Cohn JS, Endemann D, Neves MF, et al. PPARalpha activator effects on Ang II-induced vascular oxidative stress and inflammation. Hypertension 2002; 40: 866–71. [DOI] [PubMed] [Google Scholar]
- Li CB, Li XX, Chen YG, Zhang C, Zhang MX, Zhao XQ, et al. Effects and mechanisms of PPARalpha activator fenofibrate on myocardial remodeling in hypertension. J Cell Mol Med 2009; 13: 4444–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L, Wilson JL, Duan R, Hudson T, El-Marakby A. Peroxisome proliferator activated receptor-alpha activation decreases mean arterial pressure, plasma interleukin-6 and COX-2, while increasing renal CYP4A expression in an acute model of DOCA-salt hypertension. PPAR Res 2011; 2011: 502631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Luo P, Chang HH, Huang H, Yang T, Dong Z, et al. Induction of renal 20-hydroxyeicosatetraenoic acid by clofibrate attenuates high-fat diet-induced hypertension in rats. J Pharmacol Exp Ther 2006; 317: 11–8. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation 1998; 97: 2222–9. [DOI] [PubMed] [Google Scholar]
- Kung CF, Luscher TF. Different mechanisms of endothelial dysfunction with aging and hypertension in rat aorta. Hypertension 1995; 25: 194–200. [DOI] [PubMed] [Google Scholar]
- Cui X, Liu X, Feng H, Zhao S, Gao H. Grape seed proanthocyanidin extracts enhance endothelial nitric oxide synthase expression through 5′-AMP activated protein kinase/Surtuin 1-rüpple like factor 2 pathway and modulate blood pressure in ouabain induced hypertensive rats. Biol Pharm Bull 2012; 35: 2192–7. [DOI] [PubMed] [Google Scholar]
- Li H, Witte K, August M, Brausch I, Gödtel-Armbrost J, Habermeier A, et al. Reversal endothelial nitric oxide synthase uncoupling and up-regulation of endothelial nitric oxide synthase expression lowers blood pressure in hypertensive rats. J Am Coll Cardiol 2006; 47: 2536–44. [DOI] [PubMed] [Google Scholar]
- Bulhak AA, Sjöquist PO, Xu CB, Edvinsson L, Pernow J. Protection against myocardial ischaemia/reperfusion injury by PPAR-alpha activation is related to production of nitric oxide and endothelin-1. Basic Res Cardiol 2006; 101: 244–52. [DOI] [PubMed] [Google Scholar]
- Iglarz M, Touyz RM, Amiri F, Lavoie MF, Diep QN, Schiffrin EL. Effect of peroxisome proliferator-activated receptor-α and -γ activators on vascular remodeling in endothelin-dependent hypertension. Arterioscler Thromb Vasc Biol 2003; 23: 45–51. [DOI] [PubMed] [Google Scholar]
- Banks T, Oyekan A. Peroxisome proliferator-activated receptor alpha activation attenuated angiotensin type 1-mediated but enhanced angiotensin type 2-mediated hemodynamic effects to angiotensin II in the rat. J Hypertens 2008; 26: 468–77. [DOI] [PubMed] [Google Scholar]
- Jonkers IJ, de Man FH, van der Laarse A, Frölich M, Gevers Leuven JA, Kamper AM, et al. Bezafibrate reduces heart rate and blood pressure in patients with hypertriglyceridemia. J Hypertens 2001; 19: 749–55. [DOI] [PubMed] [Google Scholar]
- Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res 2006; 71: 247–58. [DOI] [PubMed] [Google Scholar]
- Walusimbi-Kisitu M, Harrison EH. Fluorometric assay for rat liver peroxisomal fatty acyl-coenzyme A oxidase activity. J Lipid Res 1983; 24: 1077–84. [PubMed] [Google Scholar]
- Vera T, Taylor M, Bohman Q, Flasch A, Roman RJ, Stec DE. Fenofibrate prevents the development of angiotensin II-dependent hypertension in mice. Hypertension 2005; 45: 730–5. [DOI] [PubMed] [Google Scholar]