Abstract
In this review, we briefly describe glutamate (Glu) metabolism and its specific transports and receptors in the central nervous system (CNS). Thereafter, we focus on excitatory amino acid transporters, cystine/glutamate antiporters (system xc-) and vesicular glutamate transporters, specifically addressing their location and roles in CNS and the molecular mechanisms underlying the regulation of Glu transporters. We provide evidence from in vitro or in vivo studies concerning alterations in Glu transporter expression in response to hypoxia or ischemia, including limited human data that supports the role of Glu transporters in stroke patients. Moreover, the potential to induce brain tolerance to ischemia through modulation of the expression and/or activities of Glu transporters is also discussed. Finally we present strategies involving the application of ischemic preconditioning and pharmacological agents, eg β-lactam antibiotics, amitriptyline, riluzole and N-acetylcysteine, which result in the significant protection of nervous tissues against ischemia.
Keywords: glutamate, brain ischemia, excitatory amino acid transporter, cystine/glutamate antiporter, vesicular glutamate transporter, ischemic preconditioning, ceftriaxone
Introduction
Glutamate (Glu) is the most widespread neurotransmitter in the central nervous system (CNS) and is involved in almost every aspect of physiological brain functioning, including memory, learning, cognition, and the control of emotion1. The estimated concentration of Glu in the entire brain is approximately 5–15 mmol per kg wet weight (depending on the brain region), but only a small part of the Glu is released into the synaptic cleft2. Glu is almost exclusively (99.99%) present intracellulary, where the intracellular Glu concentration ([Glu]i) is within 1–10 mmol/L, with the largest concentration observed in synaptic vesicles at the nerve terminals (ca 100 mmol/L), whereas the extracellular Glu concentration ([Glu]e) is only a few μmol/L1. Glu is exocytotically released from both astrocytes and neurons, which possess membrane receptors, susceptible to Glu excitation3. Even though Glu is a predominant neurotransmitter, this compound can be toxic. Although under physiological conditions the majority of Glu is stored intracellulary, under some pathological conditions, the extracellular concentration is dramatically increased. Neuronal injury caused by brain ischemia is mediated through a massive overload with Glu, Glu receptor activation, and Ca2+-dependent excitoxicity4. The [Glu]e is highest just after the onset of ischemia, while the mechanisms underlying the uncontrolled release of Glu are complex. Apart from the dysfunction of excitatory amino acids transporters (EAATs), these mechanisms involve the excessive release of Glu and post-necrotic Glu liberation. Depending on the severity of brain injury, the overactivation of Glu receptors might cause necrosis or apoptosis5. Because of the risk of excitotoxic damage, the precise physiological control must maintain the homeostasis. EAATs localized to both astrocytes and neurons play a major role in system regulation. In this review, we discuss the potential modulation of Glu transporters in the context of brain ischemia and tolerance to this condition. The nomenclature of particular Glu transporters is different for animals and humans. In humans, EAAT1, EAAT2, and EAAT3 are the terms used for these transporters, whereas in rodents GLAST, GLT-1, and EAAC1 are used, respectively. In this review, we use the latter terminology because the majority of the studies cited here were conducted on rodents.
Glutamate metabolism and cycling
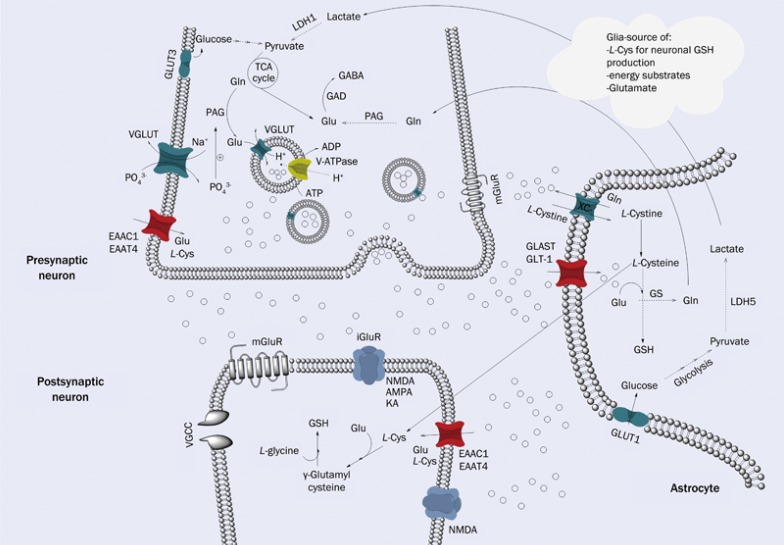
There are only a few ways for the body to produce Glu molecules: 1) the Glu-glutamine cycle; 2) synthesis in neurons and astrocytes from glucose; and 3) synthesis inside neurons from lactate delivered from astrocytes. A fraction of Glu present in the brain participates in the Glu-glutamine cycle in neurons and astrocytes. However, de novo synthesis is necessary because Glu can be oxidized and cannot be entirely regenerated through this cycle. Glu also does not cross the blood brain barrier and hence is not delivered to the CNS through the ingestion of food. Glucose is the major substrate for Glu synthesis in astrocytes and neurons6, and the influx of Na+ (together with Glu through EAATs) stimulates glucose uptake in astrocytes and neurons7. Glucose enters the tricarboxylic acids cycle (TCA cycle) and provides α-ketoglutarate (α-KG) as a carbon backbone of Glu, while the source of the nitrogen used in Glu synthesis is leucine, alanine, isoleucine or asparate8. Glu in neurons can also be synthesized from lactate delivered from astrocytes. In some neurons, Glu might be converted to gamma-aminobutyric acid (GABA) through the action of Glu decarboxylase9. Glu is released from presynaptic neurons into the synaptic cleft, where this compound binds to specific ionotropic (iGluR) or metabotropic receptors (mGluR) located on postsynaptic and presynaptic neurons and glial cells. [Glu]e can also be elevated through the cystine-Glu antiporter (system xc-)10. Because there is no evidence of the extracellular metabolism of Glu and because high Glu concentrations are highly toxic, the mechanism responsible for Glu clearance must be efficient11. Through GLAST and GLT-1, primarily located on astrocytes12,13, Glu is taken up through the inward co-transport of three Na+ molecules, one H+ molecule and the counter transport of one K+ 14,15. The Na+-dependent neuronal Glu transporters include EAAC1 and EAAT4, which exhibit a similar mechanism of action. In astrocytes, Glu is converted to glutamine through glutamine synthetase (a specific enzyme in astrocytes and oligodendrocytes) in an ATP-dependent process. Notably, not every molecule of Glu is converted to glutamine, as a small fraction of Glu is degraded to α-KG and enters the TCA cycle. The glutamine produced from astrocytes is released through the glutamine transporter system N transporter 1 (SN1) and reaches neurons via system A transporter (SAT1). Here, glutamine is converted to Glu through phosphate-activated glutaminase16. Subsequently, transmitter is loaded into vesicles through vesicular glutamate transporters (VGLUTs), and after interaction with soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs), Glu is released into the synaptic cleft where it binds to Glu receptors and EAATs. Thereafter, the Glu-glutamine cycle terminates (Figure 1)17.
Figure 1.
Glutamate cycling and metabolism. When Glu is released into synaptic cleft it binds to various synaptic and extrasynaptic receptors – mGluR and iGluR. While synaptic Glu concentration reaches excessive concentration EAATs start uptake direct into astrocytes (GLAST and GLT-1) and neurons (EAAC1 and EAAT4). Inside astrocytes Glu is subjected into Glu–Gln cycle. First, in glial cell it is converted to glutamine by GS and is directed by means of specific transporters towards neurons. In neurons Gln is reversed to Glu by phosphate activated glutaminase (phosphate is delivered by VGLUTs, the second type of activity of plasma membrane VGLUTs) and by co-operation of VGLUT and V-ATPase is packed into synaptic vesicles, which after interaction with SNARE proteins (not shown on the figure) are released into synaptic cleft and Glu-Gln cycle is closed. However, this mechanism is not efficient enough to maintain constant amount of Glu. Another source of Glu is glucose entering astrocytes and neurons by GLUT1 & GLUT3, respectively. Glucose as a substrate of glycolysis forms pyruvate which after enzymatic reaction catalized by LDH is converted to lactate. From astrocytes lactate diffuse to neurons where after few enzymatic reaction of TCA cycle forms Glu. On this figure there is also demonstrated mechanism of action of xc- system which is in fact cysteine/Glu antiporter. By means of this transporter L-cystine is uptaken from synaptic space to astrocyte where is converted to L-Cys which may be transported to neurons and serve as a substrate to GSH synthesis, or this synthesis may take place in astrocyte. ADP indicates adenosine diphosphate; ATP, adenosine triphosphate; GLAST, GLT-1, EAAC1, and EAAT4, excitatory amino acid transporter 1–4 respectively; Gln, glutamine; Glu, glutamate; iGluR, ionotropic glutamate receptors; GLUT1 & GLUT3, glucose transporters; GS, glutamine synthetase; GSH, glutathione; L-Cys, L-cysteine; LDH1&5, lactate dehydrogenase 1&5; mGluR, metabotropic glutamate receptors; PAG, phosphate activated glutaminase; TCA cycle, tricarboxyl acids cycle; VGLUT, vesicular glutamate transporter; V-ATPase, vacuolar-type H+-ATPase; System xc-/xCT, cystine/glutamate antiporter.
Glutamate neurotransmission and intracellular signaling
Glu is released from presynaptic terminals through two different Ca2+-dependent or Ca2+-independent mechanisms. The first mechanism involves N-type and P/Q-type voltage-dependent Ca2+ channels (VDCC)18. Ca2+-independent release is mediated through the reverse activity of Glu transporters19. Glu acts on two types of receptors, iGluR and mGluR. The activity of iGluR depends on ion influx. iGluRs are ion channels permeable to Ca2+ and Na+ and primarily localized to postsynaptic membranes. However, some subtypes of N-methyl-D-aspartate receptor (NMDA) are extrasynaptically present. NMDA receptors have a tetrameric structure, comprising two GluN1 subunits and at least one GluN2 (A, B, C, or D) or GluN3 (A or B). The formation of the distinct receptors depends on many factors, such as the development regulation and localization of specific neurons. NMDA receptors are characterized according to their affinity for Glu, regulated through binding sites for Mg2+, Zn2+, H+, glycine, and polyamines20. The excessive stimulation of extrasynaptic NMDA receptors might lead to apoptosis signaling and consequent cell death. Nevertheless, the stimulation of synaptic NMDA receptors might promote cell survival through the Ca2+-dependent signal transduction pathway21,22,23. However, recent studies have shown that neurotoxic effects are primarily mediated through synaptic NMDA receptors and that the silencing of extrasynaptic NMDA receptors does not exert any protective effect. In these studies, synaptic, but not extrasynaptic, NMDA receptors were involved in long-term potentiation, and in long-term depression both types of receptors are activated24. The hyperactivation of NMDA receptors is the most important mechanism of excitotoxic Glu-induced neuron damage25. However, other pathways, dependent on the activity of 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propanoic acid/kainate receptors (AMPA/KA receptors), or mGluR are also important. The stimulation of Glu receptors increases the intracellular Ca2+ concentration through NMDA receptors and VDCC, Ca2+ release from internal stores, and the reversal of the Na+/Ca2+ exchanger (NCX)26 Overloading the intracellular space with Ca2+ results in the dysfunction of mitochondria, activation of caspases and calpains, increased production of nitrogen oxide (NO) and reactive oxygen species, and the activation of the arachidonic acid pathway. The progression of these processes results in neuronal cell death through apoptosis or necrosis27,28. AMPA receptors are characterized by the fast generation of excitatory postsynaptic potential and lower affinity to Glu. The short-term binding of Glu to AMPA receptors induces rapid transmission but also rapid current decay. The long-term binding of NMDA receptors to Glu, as a consequence of high affinity to Glu, produces prolonged signaling and delayed current decay. AMPA and kainate (KA) receptors also have tetrameric structures and comprise GluA1–4 and GluK1–5 subunits, respectively. mGluR acts via G-proteins. At least eight subtypes of mGluR, mGluR1–8, have been identified, and each receptor subtype is characterized by different molecular and pharmacological properties. The metabotropic Glu receptor family is divided into three groups: group I (mGluR1 and mGluR5), group II (mGluR2 and mGluR3) and group III (mGluR4, mGluR6, mGluR7, and mGluR8). This classification is based on the similarity of the sequence, mode of the signal transduction, localization and pharmacology of particular receptor groups29,30. Group I mGluRs are coupled to phospholipase C through Gq/11 protein, leading to the production of inositol-1,4,5-triphosphate (IP3) and diacylglycerol. This group is located postsynaptically near ionotropic receptors, such as NMDA and AMPA receptors, and probably presynaptically (according to data from studies on synaptoneurosomes)31,32,33,34,35,36. This localization induces the potentiation of Glu ionotropic receptor activity through mGluR1/537. Groups II and III are negatively coupled to adenylate cyclase and are localized both pre- and postsynaptically, which determines the function of these receptors. Presynaptic receptors inhibit the release of Glu, while postsynaptically localized receptors interfere with the modulation of ion channels and release of other neurotransmitters29,37,38. mGluR5 and mGluR3 are present on the glial cells, particularly astrocytes39,40.
Glu transporters
Location in the brain
GLAST, GLT-1, and EAAC1 are widely distributed in the CNS, while EAAT4 and EAAT5 are predominantly expressed in the cerebellum and retina, respectively41. System xc- is specific to the border of the CNS42, whereas VGLUTs are located in several brain structures43 (Table 1).
Table 1. Expression of the Glu transporters at the mRNA and protein levels in the CNS and in cellular cultures.
| Glu transporter | Brain structures |
Cells in the nervous system |
Cell cultures |
|||
|---|---|---|---|---|---|---|
| mRNA | Protein | mRNA | Protein | mRNA | Protein | |
| GLAST | Cerebellum, cerebral cortex, hippocampus, striatum, thalamus, hypothalamus44 | Throughout the CNS45, retina46 | Astrocytes, Müller cells, pigment epithelia47,48, rat cohlear hair cells (neurons)49 | Bergman glia, Purkinje cells45, Müller cells46 | Astrocytes, microglia, oligodendrocytes50, astrocytes, prepared from mouse cerebral cortex51, neuronal PC12 line52 | Rat hippocampal neurons, microglia53 |
| GLT-1 | Throughout the brain, except the cerebellum54 | Throughout the CNS55 | No data | Glial cells; astrocytes56, neurons, oligodendrocytes-during development57 | Astrocytes, microglia and oligodendrocytes50 | Rat primary hippocampal and cortical neurons58, C57BL/6J mice hippocampal microglia59 |
| EAAC1 | No data | Throughout the CNS60 | No data | Neurons55, oligodendrocytes61 | No data | No data |
| EAAT4 | Cerebellum, slight in cortex and retina54 | Throughout the fore- and midbrain62,63, cortex, cerebellum- during development64 | No data | Purkinje cells1, astrocytes65 | No data | Rat cortical astrocytes66, mouse astrocytes65 |
| EAAT5 | No data | Retina67 | No data | Bipolar cells67 | No data | No data |
| xCt, 4F2hc/xc-* | Cortex, cerebellum68, area postrema, habenular nucleus, subfornical organ, hypothalamus, meninges69 | Leptomeninges, ventricles, choroid plexus70 | Neurons of cortex and cerebellum68, ependymal cells of the lateral wall of the third ventricle69 | Astrocytes71, microglia72, Müller cells73, immature cortical neurons74, vascular endothelial cells, epenndymal cells42 | No data | Glioma75 |
| VGLUT1 | Cortex (layers II-VI), thalamus, some nuclei of the brain stem; vestibular and cochlear nuclei, lateral reticular, external cuneate76, cerebellum, striatum43 | Hippocampus, neocortex (layers I-III), cerebellum, entorhinal and piriform cortex, amygdalia, subiculum43 | Dopaminergic neurons cholinergic neurons GABAergic neurons43 | Photoreceptors and bipolar cells43 | No data | Hippocampal, cerebellar and cerebrocortical neurons77,78,79 |
| VGLUT2 | Cortex (middle layers), thalamus, most nuclei of brain stem76, cerebellum, striatum43 | Cerebral cortex (layer IV), dentate gyrus (granular layer), thalamus, hypothalamus, olfactory bulb, the brain stem43 | Glutamatergic neurons76, dopaminergic neurons, GABAergic neurons, cholinergic neurons43 | Purkinje cells, ganglion cells43 | No data | Hippocampal, cerebellar and cerebrocortical neurons78,79,80,81,82,83 |
| VGLUT3 | Cerebellum, striatum43 | Hippocampus, neocortex, hypothalamus, olfactory bulb, substantia nigra, raphe nuclei, striatum43 | Dopaminergic neurons cholinergic neurons GABAergic neurons43 | Glutamatergic neurons, GABAergic neurons, serotonergic neurons, cholinergic neurons, amacrine cells43 | No data | No data |
* xCT denotes mRNA of the light chain of the xc- antiporter, 4F2hc-mRNA of the heavy chain of the xc- antiporter, xc- -xc- antiporter protein.
Function
The general function of EAATs is to regulate the extracellular Glu concentration and maintain the concentration of Glu at low physiological levels to avoid toxic effects. After release into the synaptic cleft, Glu is rapidly removed through EAATs into glial cells and neurons. The major transporter is the GLT-1, which is responsible for more than 90% of total Glu uptake57. Studies using antisense oligonucleotides directed against GLT-1 have revealed that the loss of GLT-1 results in a substantial increase in [Glu]e80. GLASTs, expressed on Müller glial cells, regulate extracellular Glu levels in the retina55,81. EAAC1 transports cysteine (along with Glu)82, providing a substrate for glutathione (GSH) synthesis in neurons84. The role of the EAAT4 is to regulate neuronal excitability through counteracting the depolarization of neurons41. Both EAAT4 and EAAT5 possess a thermodynamically uncoupled Cl- flux, which involves high Cl- conductance with relatively low Glu uptake85.
VGLUTs mediate the accumulation of Glu in secretory vesicles. VGLUT1 knockout mice show progressive neurological deficits, including blindness, incoordination and an enhanced startle response86,87,88. The synaptic vesicles isolated from these mice exhibit a diminished rate of Glu uptake. These animals also showed decreased Glu neurotransmission in hippocampal neurons87. The genetic inactivation of VGLUT2 results in perinatal lethality, consistent with the predominant expression of VGLUT2 during embryogenesis and early postnatal development83,89. VGLUT3 is involved in auditory function and mechanical hypersensitivity90,91,92.
System xc- mediates the uptake of the sulfur-containing amino acid cystine in exchange for Glu at a 1:1 ratio93. This system comprises two subunits: 4F2hc, required for cell surface expression, and xCT with typical functional activity. Aside from the primary role of system xc-, the maintenance of intracellular GSH levels in astrocytes is essential94. After uptake, cystine is reduced to cysteine, which is the primary rate-limiting amino acid in GSH synthesis95. Therefore, system xc- is involved in the defense against oxidative stress96. The Na+-independent Glu uptake, mediated through the system xc-, likely represents only a small fraction of the total astrocyte Glu uptake under physiological conditions.
Mechanism
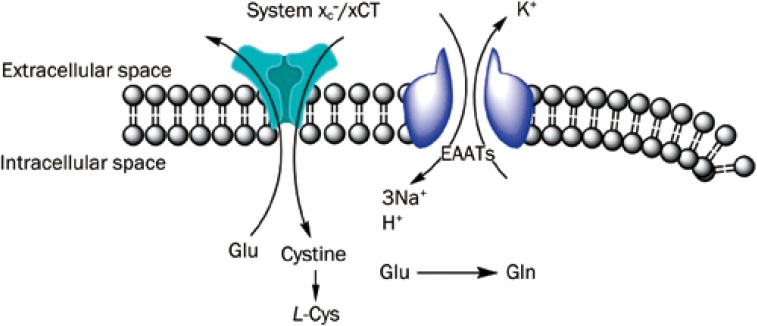
The Na+ and K+ electrochemical gradient is the driving force for Glu transport coupled to EAATs across the cell membrane. This negative membrane potential maintains [Glu]i at a range 5×106-fold greater, compared with the [Glu]e97. An accepted model of EAAT action involves the transport of Glu across the cellular membrane coupled to the inwardly directed electrochemical potential gradients of Na+ and H+, and the outwardly directed K+ gradient. The initiation of this process involves the recruitment of Glu and three Na+ and one H+ from the extracellular space to an outward-facing conformation of the transporter. The binding of these substrates triggers a conformational change, which adopts an inward-facing conformation of EAATs, followed by cargo release into the cytoplasm of the cell. The subsequent step involves the recruitment of K+ ions to an EAAT transporter from the cytoplasm, which evokes the return to an outward-facing conformation and release outside the cell98,99. (Figure 2). The disruption of the electrochemical potential reverses Glu transport. Cerebral ischemia, for example, leads to the Na+/K+-ATPase function impairment, resulting in a decrease in the ratio of the extracellular Na+ concentration ([Na+]e) to the intracellular K+ concentration ([K+]i) and/or an increase in [Na+]i/[K+]e97.
Figure 2.
Glu transporters mode of action. The role of the system xc-/xCT is to uptake L-Cys from synaptic space to intracellular space in change of Glu. The opposite direction of Glu transport mediated by EAATs, depends on co-transport of three Na+, one proton and one Glu molecule in change of one K+. EAATs indicates excitatory amino acids transporters; Gln, glutamine; Glu, glutamate; Cys, L-cysteine; System xc-/xCT, cystine/glutamate antiporter.
EAATs are also involved in substrate-gated anion conductance, mediated through EAAT4 and EAAT5. The Glu uptake capacity of these molecules is relatively poor. The remaining EAATs also demonstrate anion conductance, with decreasing permeability (GLAST>EAAC1>>GLT-1) and selectivity (NO3−>I−>Br−>Cl−>F−). The physiological purpose of anion influx likely compensates for the positive charge influx during Glu transport100.
VGLUTs depend on the existence of a vesicular membrane potential gradient, rather than a pH gradient. The proton electrochemical gradient, which exists across the vesicle membrane, is the driving force for Glu uptake. This potential is created by a vacuolar-type ATPase. These transporters are also highly specific for Glu, however the affinity of these molecules is relatively low (Km=1–3 mmol/L). VGLUTs achieve maximum activity at a low concentration of Cl− (2–5 mmol/L)101,102,103,104,105.
The system xc- is necessary for cystine uptake. The gradient of high [Glu]i and low [Glu]e concentrations is the driving force leading to the cystine import and thus, the intracellular Glu depletion inhibits cystine uptake93. The system xc- is characterized by Na+-independent Glu uptake and typically comprises Cl−-dependent glutamate/cystine antiporters. This system shows sensitivity to Cl− transport blockers, including 4,4′-diiso-thiocyanatostilbene-2,2′-disulfonic acid (DIDS) and furosemide106. Human fibroblasts preloaded with 3H]-labeled cystine and deprived of Glu, release cystine, when Glu is added to the incubating medium, indicating the ability of system xc- to reverse its manner of action93 (Figure 2).
Expression
Distinct genes, encoding eukaryotic Glu transporters, and their chromosomal loci are presented in Table 2. The expression of these genes is cell and tissue specific (Table 1).
Table 2. Genes encoding Glu transporter proteins and their chromosomal loci in humans and rodents.
| Transporter name |
Gene name | Humans | Locus |
||
|---|---|---|---|---|---|
| Humans | Rodents | Rats | Mice | ||
| EAAT1 | GLAST | SLC1A3 | 5p13 | 2q16 | 15A2;15 3.82cM |
| EAAT2 | GLT-1 | SLC1A2 | 11p13-p12 | 3q31 | 2 54.13cM |
| EAAT3 | EAAC1 | SLC1A1 | 9p24 | 1q54 | 19 |
| EAAT4 | EAAT4 | SLC1A6 | 19p13 | 7q11 | 10 39.72cM |
| EAAT5 | EAAT5 | SLC1A7 | 1p32 | 5q35 | 4 |
| xCT | xCT | SLC7A11 | 4q28-q32 | 2q26 | 3 21.72cM |
| 4F2hc | 4F2hc | SLC3A2 | 11q13 | 1q43 | 19 5.44cM |
| VGLUT1 | VGLUT1 | SLC17A7 | 19q13 | 1q22 | 7 |
| VGLUT2 | VGLUT2 | SLC17A6 | 11p14 | 1q22 | 7 |
| VGLUT3 | VGLUT3 | SLC17A8 | 12q23 | 7q13 | 10 |
The expression of EAATs at the mRNA and protein levels does not always occur in parallel, eg, GLT-1 mRNA was observed in some hippocampal neurons, but not GLT-1 transporter protein107. The enormous loss of GLT-1 protein in the motor cortex in amyotrophic lateral sclerosis (ALS) does not occur with the decrease in the corresponding mRNA108. In turn, a nearly simultaneous decrease in both GLT-1 protein and its corresponding mRNA in the rat hippocampus has been observed in transient ischemia109. These findings might suggest that the metabolic turnover rates of Glu transporter proteins and their corresponding mRNAs are regulated through different mechanisms, depending on cell phenotype, signaling pathways and environmental cues110.
Regulation
The regulation of the EAATs can be achieved at the level of gene expression and the modulation of the kinetics of their action. The expression and maintenance of functionally active Glu transporters requires both neuronal and non-neuronal factors, eg, the induced expression of GLT-1 in cultured astrocytes only occurs in co-culture with neurons. Several factors have been demonstrated to induce GLT-1 expression in astroglial cultures, including cAMP66, pituitary adenylate cyclase-activating peptide (PACAP, a neuron-derived peptide)111 and brain-derived neurotrophic factor (BDNF)112 or the activation of epidermal growth factor (EGF) receptor113. Many studies with pharmacological and genetic manipulations imply that the increase of GLT-1 expression might depend on phosphatidylinositol-3-kinase (PI3-K) and nuclear factor kappa B (NF-κB) signaling pathways114,115. NF-κB directly binds to the GLT-1 promoter and thereby regulates its transcription115. NF-κB might act as both a transcriptional activator116 and a repressor117. It has been suggested that the GLT-1 promoter region contains distinct NF-κB sites in the proximal and 5′UTR regions, and this transcription factor might differentially contribute to gene activation or repression, depending on the binding site115. For example, tumor necrosis factor-α (TNF-α) can inhibit GLT-1 transcription, and this inhibition requires NF-κB115. On the other hand, NF-κB, by itself mediates cAMP-, TGF-α, or EGF-induced GLT-1 promoter activation59,115.
Methylation of the promoter region is another mechanism to regulate EAAT expression, eg, the methylation of the GLT-1 CpG promoter region (a regulatory sequence rich in cytosine and guanine) altering the binding properties of nuclear factors to particular DNA sites. The inhibition of the DNA methyltransferases results in a potent GLT-1 mRNA transcription enhancement, and some endocrine substances can modulate the expression of Glu transporters, eg, growth hormone promotes GLT-1 expression in the placenta of mice, while insulin-like growth factor-II (IGF-II) action results in the downregulation of placental EAAT4. At physiological concentrations IGF-II maintains the proper levels of GLT-1, EAAC1, and GLAST transporters. Glu also plays an important role in the regulation of EAATs expression, eg, the disruption of the cortical glutamatergic pathways results in the selective and transient down-regulation of GLT-1 and GLAST transporters in the rat striatum and hippocampus, with no changes in EAAC1 expression118,119. The human and murine xCT genes possess 5′ flanking regions containing multiple putative AP-1 binding sites120,121. A presumed NF-κB binding site was also identified in this region, however, there is no direct evidence that this factor activates the transcription of the xCT gene122. The proximal 5′ flanking region of the murine xCT gene also contains four antioxidant response elements (AREs). One of the AREs, located closest to the 5′ end, is involved in induction of xCT promoter activity, associated with the transcription factor Nrf2 and oxidative stress70. The hippocampal cell line HT22, treated with the Nrf2 inducer tert-butylhydroquinone (tBHQ), showed a strong increase in xCT protein and system xc- activity, following the upregulation of the Nrf2 protein level123. Moreover, a major increase in xCT protein levels was observed in retroviral-transfected rat astrocyte cultures over-expressing Nrf2124.
Similarly, tandem amino acid response elements (AAREs) have been observed in the murine xCT gene. These regulatory elements are completely conserved among xCT 5′ flanking regions in humans, mice, rats and bovines. The AARE located closest to the xCT 5′ end binds the transcription factor ATF4. The upregulation of ATF4 intensifies xCT mRNA synthesis with an increase in xCT protein levels and system xc- activity in the HT22 hippocampal neuronal cell line, mouse embryonic fibroblasts and the rat PC12 cell line123. Both AAREs together mediate xCT promoter activation through amino acid starvation125.
The kinetic regulation of EAATs includes membrane translocation, amino acid phosphorylation, sulfhydryl-based redox reactions, interactions with arachidonic acid (AA), Zn2+ or Glu transporter associated proteins (GTRAP) or transporter multimerization126.
Many factors affect the membrane translocation of Glu transporters. EAAC1-mediated transport is activated through platelet-derived growth factor (PDGF), associated with the redistribution of EAAC1 from the intracellular compartment to the cell surface. Glu also initiates the movement of internalized GLAST to the cell membrane surface and the rapid increase in the maximal transport rate (Vmax) for Glu uptake127. The action of protein kinase C (PKC) might also affect the membrane trafficking of Glu transporters. PKC activation causes the rapid intracellular capture of GLAST, resulting in reduced activity128. In addition, EAAC1, expressed in Xenopus oocytes, was downregulated in response to PKC activation, associated with the movement of EAAC1 protein from the cell plasma into the intracellular compartment, without any changes in the EAAC1 affinity for Glu129. However, in the C6 glioma cell line, PKC activation resulted in EAAC1 mobilization in the plasma membrane, followed by an increase in Glu transport activity130.
Glu transporter activity might also be affected through Glu-associated proteins. Thus, Glu transporter-associated protein 41 (GTRAP41) and Glu transporter-associated protein 48 (GTRAP48) modulate EAAT4 activity, and the expression of these proteins is associated with an increase in the Vmax of Glu131. EAAC1 cooperates with GTRAP3–18. However, the latter protein action results in a decrease of EAAC1 affinity for Glu and the reduced transport of neurotransmitter mediated through EAAC1132.
Both GLT-1 and GLAST transporter proteins contain cysteine-associated sulfhydryl groups sensitive to free radical species. The actions of these compounds result in the formation of cystine bridges, thereby inhibiting Glu flux through transporters133, as demonstrated for superoxide anion (O2−), hydrogen peroxide (H2O2), NO and peroxynitrite anion (ONOO−)134,135. However, the overexpression of superoxide dismutase 1 protected Glu transporters from inhibition136.
AA significantly increased substrate-activated currents in oocytes expressing EAAT4137. AAs also modulate the actions of other EAATs, eg, increasing GLT-1 substrate affinity and reducing the maximal rate of GLAST uptake138.
Zn2+ ions regulate astrocytic EAATs through interactions with transporter proteins. This direct interaction with the GLAST transporter leads to potent and selective inhibition139. It has been hypothesized that the Zn2+ released during cerebral ischemia might induce excitotoxic neuronal death via GLAST inhibition126.
The activity of Glu transporters might also be regulated through the activation of diverse receptors. For example, Glu uptake in astrocytes is modulated through adrenergic receptors. The norepinephrine (an agonist of α- and β- receptor subtypes) and phenylephrine (an α-receptor agonist) increase the Glu uptake140. However, isoproterenol (a β-receptor agonist) decreases Glu uptake in astrocyte primary cultures141. Glucocorticoids reduce GLAST and GLT-1 affinities to Glu in astrocytes and thus inhibit Glu transport142. Another hormone, melatonin, was shown to stimulate the high-affinity Glu uptake in the retina143. The kinetic activity of Glu transporters is also regulated through Glu receptor activation, eg, the Glu uptake rate in crude synaptosomes from the mouse cerebral cortex was slightly decreased after treatment with phencyclidine, a noncompetitive antagonist of NMDA receptors. Stronger inhibition was demonstrated through an antagonist of mGluR, (R,S)-2-amino-3-phosphonopropionic acid144. In addition, kainate stimulates high-affinity Glu uptake in the crude synaptosomal preparation145.
It has been well documented that Cl− plays a regulatory role in VGLUT activity. The permeant Cl− increased vesicular Glu uptake at low concentrations, whereas higher concentrations of Cl− have an inhibitory effect on this uptake105. The exact mechanism of the Cl− effect is unclear. At low concentrations of Cl−, this regulatory effect has been associated with the presence of a positive allosteric regulatory binding site. The hypothesis explaining the role of high Cl- concentrations is associated with a buffering role. VGLUT bioenergetics primarily rely on Δψ, the main driving force for Glu accumulation. The high luminal concentration of Cl− will buffer H+, stimulating V-ATPase and thus decrease Δψ and increase ΔpH, which might consequently reduce VGLUT activity146. The stimulation of VGLUT through Cl− is completely inhibited through keto acids and pyruvate and acetoacetate147. Moreover, fatty acids, amino acids, quinolines, azodyes, alkaloids, kynurenic acid and quinoline-related compounds have been reported to inhibit VGLUTs. AAs and polyunsaturated fatty acids, released under pathological conditions, such as ischemia, also inhibit the vesicular uptake of Glu92. The treatment of cultured neurons with subtoxic levels of NMDA resulted in VGLUT1 mRNA upregulation148.
Both, extra- and intracellular levels of Glu are critical for the cystine uptake through the system xc-. Thus, the pathways that govern Glu concentrations might affect the activity of system xc-. EAATs participate in the regulation of system xc-122. An α-aminoadipate (a molecule, which can be transported through system xc- and a metabolite of lysine metabolism in the brain) inhibits this system149. In addition, acidosis might also affect system xc-. A study on cultured human fibroblasts revealed that extracellular acidosis (pH=6.5) inhibits the transport of cystine via system xc-, while the Glu transport through this exchanger is much less affected150. Acidosis in the brain, subsequent to cerebral ischemia, is often associated with prominent lactate accumulation151. A study using rat cortical astrocytes indicated the inhibition of system xc-activity through lactate152. However, a study using the hippocampal cell line HT22 did not show any influence of the latter compound on xc-activity153.
Glutamate transporters in brain ischemia
The predominant mode of Glu release depends on the strength of the injury. In fact, the reversal of Glu transporters has been shown to play a major role in the release of Glu after severe ischemia154. However, other pathways are also involved in Glu efflux, eg, gap-junction, hemichannel-mediated and P2X channel-dependent release from astrocytes155 and release from neurons through exocytosis156,157.
The neurotoxicity of Glu in the brain ischemia has been associated with the reversal of Glu transporters, presumably neuronal Glu transport154. The depletion of the energy supply, leads to the disruption of transmembrane electrochemical gradients, resulting in decreased Glu uptake or Glu efflux via uptake reversal31. Glu transport reversal, due to ATP depletion, has been demonstrated in primary astrocyte cultures158 and slice preparations159.
In vitro models of ischemia
Several data indicate the up-regulation of EAATs following hypoxia in vitro, eg, rat pheochromocytoma (PC12) cell exposure to hypoxia resulted in the significant up-regulation of EAAC1 and GLT-1 expression, without any influence on GLAST expression at both the mRNA and protein levels. Moreover, an increase in Na+-dependent Glu uptake was shown in response to hypoxia160. In addition, exposure to hypoxic conditions resulted in a significant increase in the EAAC1 mRNA level in the C6 rat glioma cell line and the murine GLT-1 mRNA level in GT1-7 cells in an RNAse protection assay. In C6 hypoxic cells a decreased uptake of D-aspartate from incubating medium was shown despite the increased expression of Glu transporters161. One may speculate, that this reduction in the D-aspartate uptake may results from the reversed mode of Glu transporters action. These results reflect an attempt to maintain ion and Glu homeostasis under restricted energy and oxygen supply162,163,164.
The results of studies using an oxygen-glucose deprivation (OGD) model of in vitro global ischemia suggest that Glu transporters reduce the [Glu]e during the early stages of ischemia. However, in the later phases, Glu transporters become the source of extracellular Glu, acting in a reversed manner. Numerous studies using different EAAT blockers were performed to explain this phenomenon, eg, L-trans-pyrrolidine-2,4-dicarboxylic acid (PDC), a competitive, transportable inhibitor of GLAST, GLT-1, EAAC1 and EAAT4, and a non-transportable inhibitor of EAAT5, and DL-threo-β-benzyloxyaspartate (DL-TBOA), a potent competitive inhibitor of Na+-dependent Glu/aspartate transporters (including GLAST, GLT-1, and EAAC1), significantly augmented Glu efflux from cerebrocortical slice cultures during OGD for 30 min. These results suggest that the activity of EAATs was in forward operation. However, after a 60 min incubation with PDC under OGD conditions, Glu accumulation was reduced, suggesting a transition into the reverse mode of operation of Glu transporters165. This evidence corresponds with the results of another experiment, where the incubation of adult corticostriatal slices with DL-TBOA during OGD for 30 min did not attenuate increases in Glu166. In turn, hippocampal slices incubated with DL-TBOA under OGD conditions for the same period of time showed the reduced OGD-induced damage of CA1 pyramidal neurons167. Similarly, the DL-TBOA-treatment of cultured astrocytes and neurons subjected to ATP depletion significantly reduced Glu release168. The 30-min application of OGD to adult corticostriatal slices resulted in a significant increase in the Glu level, whereas incubation with dihydrokainate (DHK), a selective inhibitor of GLT-1, attenuated this increase166. Similar results were obtained in studies involving acute hippocampal slices169. Moreover, the virus-induced increased expression of GLT-1 significantly augmented the vulnerability to Glu in hippocampal slice cultures170. However, there are also data suggesting that under ischemic conditions inhibitors of Glu transporters may differ in some respects of their action. For example, PDC in high concentrations can induce the release of Glu from astrocytes and neurons, whereas DL-TBOA not171. Thus, PDC may affect ischemia-induced Glu efflux mediated by Glu transporters.
In addition, an increase in GLAST and GLT-1 expression was demonstrated after incubation of the hippocampal slice culture with glial cell line-derived neurotrophic factors (GDNF). The exposure of the GDNF-treated hippocampal slice culture to OGD for 30 min resulted in an increase in OGD-induced cell death172. These results indicate the involvement of GLT-1 in OGD-induced extracellular Glu accumulation and the reversed transport of this neurotransmitter via Glu transporters is involved in OGD-induced Glu accumulation.
In vivo models of ischemia
GLT-1 expression in the ischemic brain
Many studies have demonstrated the downregulation of GLT-1 expression after cerebral ischemia in rodents. A significant reduction in GLT-1 mRNA expression has been demonstrated in the CA1 hippocampal area at 6, 12, and 24 h following transient global brain ischemia in rats173. A similar decrease in GLT-1 mRNA expression was observed in the ipsilateral hippocampus at 24 h after middle cerebral artery occlusion (MCAO) in mice174. This result was consistent with the GLT-1 in situ hybridization assay, showing a significant decrease in GLT-1 mRNA expression in the CA1 hippocampal region from 1 d after bilateral common carotid arteries occlusion (BCCAO) in rats up to 21 d of reperfusion. In the CA3 hippocampal region, a decrease in the GLT-1 mRNA expression level was recorded on d 1175. Consistent results were obtained in the Western blot analysis, demonstrating a significant decrease in the GLT-1 protein level in the rodent hippocampus at the early (6 h) and later (3 d) phases of reperfusion after transient global brain ischemia173,176,177. Similarly, an immunohistochemical study revealed a significant decrease in GLT-1 in the CA1 hippocampal region from day 2 to 4 after ischemia-reperfusion in the BCCAO model in rodents175. A histopathological assessment revealed the progressive loss of neurons in the CA1 hippocampal region from 3 d of reperfusion, followed by 5 and 7 d after ischemia. Altogether, these results suggest that GLT-1 dysfunction might contribute to neuronal death in the gerbil hippocampus following BCCAO173,176,177. However, a significant increase in immunoreactivity has been demonstrated during the early stage (0.5–12 h) after reperfusion in the CA1 hippocampal subfield in global ischemia in gerbils178. In addition, a progressive rise in GLT-1 immunoreactivity was observed from d 1 to 21 after global ischemia in the CA3 hippocampal region in rats175. Inconsistencies in these results might reflect the use of various analytical methods and animal models of ischemia.
In the ipsilateral cortex a significant decrease in GLT-1 mRNA expression was observed at 24 and 72 h after MCAO in rodents174,179. An immunohistochemical analysis confirmed these latter findings, showing a parallel significant reduction in GLT-1 protein levels in the rodent cerebral cortex174,178. In the rat striatum, the GLT-1 expression level was reduced at 1 h after transient global ischemia and remained decreased for 6 h after ischemia, returning to basal levels after 24 h of reperfusion176. Surprisingly, in the rat subcortical white matter an increase in GLT-1 expression was demonstrated at 1 and 3 d after MCAO using immunohistochemistry and Western blot analysis180. These results show that post-ischemic changes in the expression of GLT1 depend on the brain structure location.
EAAC1 expression in the ischemic brain
The results of studies concerning EAAC1 expression are inconsistent. In the murine hippocampus, EAAC1 mRNA and protein levels did not change at 24 h after MCAO174. Similarly, no changes in EAAC1 expression in the rat CA1 hippocampal region were observed after1 to 24 h at 3 and 7 d following transient global ischemia in rats176. However, a decrease in EAAC1 protein expression in the CA1 area at 30 min after BCCAO was demonstrated in gerbils, followed by transient enhancement at 3–12 h after reperfusion and a final reduction at 24 h after ischemia, remaining reduced for 10 d177,178.
In addition, the data regarding cortical EAAC1 expression is ambiguous. Rao et al181 reported a significant decrease in the mRNA and protein levels at 24 and 72 h after rat MCAO, while Ketheeswaranathan et al174 did not observe any changes in the EAAC1 expression at 24 h in the same ischemia model in mice.
In the rat striatum, the EAAC1 protein level was basically undetectable at 1 h to 7 d after transient global ischemia176. In the rat subcortical white matter, a slight increase in the EAAC1 protein expression was shown at 1 and 3 d after MCAO180. These findings might suggest that EAAC1 expression in brains subjected to ischemia depends on the experimental paradigm (global vs focal ischemia) and is structure specific (striatum vs white matter).
GLAST expression in the ischemic brain
No changes in the GLAST mRNA levels were observed in the hippocampus, cerebral cortex and striatum in rodents at 6 to 24 h following transient global or focal ischemia173,174. An immunohistochemical study showed a markedly increased GLAST level in the CA1 hippocampal area at 0.5 to 12 h after BCCAO, followed by a decrease in expression at 24 h, which remained decreased at 10 d after reperfusion178. No significant changes in the GLAST protein level have been determined in the CA1 hippocampal area between 6 h and 7 d after a transient global ischemia in rats nor gerbils173,176,177. In addition, global and focal brain ischemia did not significantly influence cortical GLAST mRNA and protein expression between 6 h and 7 d in rodents174,181. In the rat subcortical white matter, MCAO caused a significant increase in GLAST protein expression on days 1 to 3 according to the immunohistochemical and Western blot analyses180. These results suggest that changes in GLAST expression depend on brain structure and time after reperfusion.
VGLUTs in ischemic brain.
Little is known about changes in VGLUT proteins and mRNA after brain ischemia in rodents. VGLUT1 was increased in the caudate-putamen and cortex within 3 d after reperfusion and then decreased at 7 d after MCAO in rats. VGLUT2 and VGLUT3 were drastically reduced within this time period182. In addition, a significant decrease in the VGLUT-2 protein level was recorded under excitotoxic conditions in vivo and in vitro following MCAO183. This change was associated with increased calpains activity, as these enzymes play a key role in neuronal death following excitotoxic or ischemic insults184. Calpains are also activated following MCAO in striatum and cerebral cortex, leading to neuronal death185. Increased VGLUT 2 and 3 activity was observed in reactive astrocytes of the ischemic corpus callosum and cortex. In the same study, it was shown that GLT-1 expression was parallel to VGLUT1 expression in the caudate-putamen, suggesting the potential interplay between these two transporters in the regulation of Glu levels182. However, other studies did not show any changes in VGLUT1 expression after focal cerebral ischemia, while VGLUT2 and VGLUT3 mRNAs were downregulated186. Another study on focal cerebral ischemia demonstrated reduction in mRNA level of VGLUT1-3 in the hippocampus and cerebral cortex of young rats187, suggesting an age-dependent modification in VGLUTs expression. Indeed, in young animals the level of VGLUT1 protein was reduced in the hippocampus and cortex, whereas VGLUT2 protein level was increased in these structures, but reduced in the dentate gyrus. Interestingly, the VGLUT2 protein level in adult animals was lower after ischemia in CA3 hippocampal region, dentate gyrus and cortex, but higher in the CA1 compared with the control. VGLUT3 mRNA was reduced in all areas after focal ischemia187. These findings suggested that changes in the expression of VGLUT proteins, following focal cerebral ischemia, are age-related.
In conclusion, brain ischemia modulates the expression of the Glu transporters in CNS cells (Table 3). However, there are some inconsistencies among results of different studies, potentially reflecting the diverse animal species, models of ischemia and types of analytical methods applied in these experiments. Thus, it is difficult to draw reliable conclusions, and further investigations are required.
Table 3. Expression of the Glu transporters in the ischemic brain in rodents.
| Glu transporter | Time after reperfusion | Hippocampus |
Cerebral cortex |
Striatum |
Subcortical white matter |
||||
|---|---|---|---|---|---|---|---|---|---|
| mRNA | Protein | mRNA | Protein | mRNA | Protein | mRNA | Protein | ||
| GLT-1 | ≤24 h after reperfusion | ↓ (CA1) global ischemia193 ↓ Focal ischemia174 ↓ (CA3) global ischemia175 | ↓ (CA1) global ischemia173,176 ↓ Global ischemia177 ↓ (CA1) global ischemia180 ↑ (CA1) global ischemia180 ↑ (CA3) global ischemia175 | ↓ Focal ischemia174,178 | ↓ Focal ischemia174,178 | ↔ Focal ischemia174 | ↓ Global ischemia176 | No data | ↑ Focal ischemia179 |
| >24 h after reperfusion | ↓ (CA1) global ischemia175 ↔ (CA1) global ischemia173 ↔ (CA1) global ischemia176 | ↓ (CA1) global ischemia175 ↑ (CA3) global ischemia175 ↔ (CA1) global ischemia173 (CA1) global ischemia176 | ↓ Focal ischemia174,178 | ↓ Focal ischemia174,178 | No data | No data | No data | ↑ Focal ischemia179 | |
| EAAC1 | ≤24 h after reperfusion | ↔ Focal ischemia174 | ↔ Focal ischemia174 ↔ (CA1) global ischemia176 ↓ (CA1) global ischemia176,180 | ↓ Focal ischemia181 ↔ Focal ischemia174 | ↓ Focal ischemia181 | No data | Undetectable176 | No data | ↔ Focal ischemia179 |
| >24 h after reperfusion | ↔ (CA1) global ischemia176 ↓ (CA1) global ischemia176,180 | ↓ Focal ischemia181 | ↓ Focal ischemia181 | No data | Undetectable176 | No data | ↔ Focal ischemia179 | ||
| GLAST | ≤24 h after reperfusion | ↔ Global ischemia173 ↔ Focal ischemia174 | ↑ (CA1) global ischemia180 ↓ (CA1) global ischemia180 ↔ (CA1) global ischemia173,176 ↔ global ischemia177 | ↔ Global ischemia173 ↔ Focal ischemia174,181 | ↔ Focal ischemia174,181 | ↔ Global ischemia173 ↔ Focal ischemia174 | ↔ Global ischemia176 | No data | ↑ Focal ischemia179 |
| >24 h after reperfusion | No data | ↓ (CA1) global ischemia180 ↔ (CA1) global ischemia173,176 ↔ global ischemia177 | ↔ Focal ischemia181 | ↔ Focal ischemia174,181 | No data | ↔ Global ischemia176 | No data | ↑ Focal ischemia179 | |
↑ - significantly higher compared to control; ↓ - significantly lower compared to control; ↔ - no significant change compared to control.
Role of Glu transporters in ischemia
Several studies using cerebral ischemia animal models indicate a protective role for Glu transporters in the reduction of [Glu]e levels, decreased cell loss and infarct volume and improvements in behavioral recovery.
A whole-cell patch-clamp recording of hippocampal CA1 astrocytes in post-ischemic slices demonstrated a significant reduction in the maximal amplitude of Glu transporter currents at 6 to 24 h after global ischemia compared with the control173. Rats infected with adeno-associated viral vectors, expressing the rat GLT-1 cDNA (AAV-GLT1) showed a significant decrease in ischemia-triggered Glu overflow in a microdialysis assay. This infection also resulted in a significant decrease of brain infarct and DNA fragmentation in the region of AAV-GLT1 injection and an improvement in post-ischemic behavioral recovery188. In contrast, significantly higher Glu concentrations were measured in the dialysates collected from the C57BL/6 mice lacking GLT-1 gene expression after BCCAO for 5 min. GLT-1 knockouts also displayed more pronounced delayed neuronal death in the CA1 hippocampal region. Surprisingly, the Glu levels measured in wild-type mice were significantly higher compared with GLT-1-lacking mice after 20 min of ischemia. In addition, acute neuronal death in the CA1 hippocampal region was present in wild-type mice189. Rao and co-workers178 conducted experiments using GLT-1 antisense oligodeoxynucleotides infusions in cerebrospinal fluid (CSF), followed by 1 h transient MCAO in rats. The results showed an increased infarct volume, neurological deficit and mortality compared with GLT-1 sense/random oligodeoxynucleotide-infused control rats. The administration of EAAC1 antisense oligodeoxynucleotides did not influence the above parameters181. Similarly, the GLT-1 heterozygous knockout mice (GLT-1+/−), subjected to MCAO, displayed significantly increased brain swelling, and the intraperitoneal administration of DHK to these animals intensified this effect190.
The C57BL/6 mice, lacking EAAT4 expression, did not display any reduction of the Purkinje cell number in the cerebellum after 5-min ischemia. In contrast, in C57BL/6 mice, lacking GLAST expression, a significant loss of the Purkinje cells was demonstrated. This loss was associated with EAAT4-low zones of cerebellum in GLAST mutants, suggesting that in cooperation with EAAT4, GLAST participates in preventing the excitotoxic damage of the cerebellum following ischemia191.
Human data
Data from studies on human tissue are sparse because of several limitations. Analyzed patients differed in terms of age, location of the infarct and time of death after stroke.
An immunohistochemical study of brains obtained from adult patients with ischemic stroke, showed a significant reduction of both GLAST and GLT-1 cortical expression in the lesion within 24 h after stroke onset. However, a large increase in the quantity of the white matter GLAST-positive cells was observed within 24 h in the lesion and this increase was maintained for months in both adjacent and remote infarction areas. During the first week after stroke (eg, ramified and amoeboid), microglia expressed GLAST and subsequently astrocytes were predominantly GLAST-positive, potentially reflecting the neuroprotective potential of microglia and astrocytes after brain ischemia192.
Data on the glutamatergic system from studies performed in stroke patients are limited. In persons with large brain infarcts, the Glu concentration in plasma and CSF was significantly increased. Moreover, the Glu levels in plasma and CSF were higher in patients with greater neurological deficits193,194.
Recently, it has been suggested that some individuals might be more susceptible to stroke severity. Mallolas and co-workers195 have shown the GLT-1 promoter polymorphism (an A-to-C change at -181 bp) is associated with higher Glu plasma levels after stroke and with a higher risk of early neurological deterioration. This polymorphism is associated with the downregulation of GLT-1 expression after stroke195.
Modulation of Glu transporters and brain tolerance to ischemia
Preconditioning induces brain tolerance to severe episodes of ischemia. This neuroprotective process can be mediated either through smaller amounts of Glu released into the synaptic cleft or increased Glu uptake by EAATs. Ischemic preconditioning, ie, short episodes of ischemia, downregulated NMDA and AMPA receptors, thereby ameliorating excitotoxicity196,197,198. The introduction of 5-min preconditioning with OGD prior to 1 h exposure to OGD resulted in a significant reduction of neuron damage. This reduction might reflect the increased expression of GLT-1 protein199. However, depending on the type of animal model of ischemia and preconditioning stimulus used, reports on Glu transporter expression and activity are inconsistent, eg, 10 min of ischemic preconditioning caused the upregulation of GLT-1 and EAAC1, but not GLAST, expression in rats200. Cortical spreading depression (CSD) in the same species resulted in the downregulation of GLAST and GLT-1 at 1–7 d after preconditioning with CSD201. In contrast, hypoxic preconditioning after a 3 h exposure to 8% oxygen in newborn rats, followed by severe brain ischemia evoked at 24 h later, diminished brain injury in some regions, namely in the cortex, striatum and hippocampus. In addition, the expression of GLAST was unaffected, while the expression of GLT-1 either increased in the cortex or decreased in the striatum202.
Recently, it has been reported that cerebral ischemic preconditioning caused significant upregulation of GLT-1a in the CA1 hippocampus area in rats, whereas severe brain ischemia, without preconditioning, caused the down-regulation of transporter expression and a corresponding increase in the concentration of Glu and cellular damage. This downregulation of GLT-1a was prevented when preconditioning preceded long-term brain ischemia, and this beneficial effect was inhibited through the intracerebroventricular administration of GLT-1a antisense oligodeoxynucleotides. These findings might suggest that GLT-1a participates in the induction of brain tolerance203. Data from in vitro experiments suggest that the role of EAATs in ischemic tolerance of the brain is indirect. In cortical cultures exposed to low-oxygen concentrations, ischemic tolerance was associated with the up-regulation of GLT-1 and EAAC1204. The co-culture of cortical neurons with astrocytes demonstrated the down-regulation of GLT-1 after OGD preconditioning, associated with the increased resistance of these co-cultures to more severe ischemic episodes through OGD205. Mild ischemia might reverse neuronal Glu transport through EAAC1, while a severe ischemia episode, causing brain damage, might also affect glial transporters, including GLT-1. Thus, inducing the overexpression of GLT-1 could also be malicious under some conditions206.
Pharmacological preconditioning with β-lactam antibiotics changes the expression and activity of EAATs204,207,208. Rothstein and co-workers208 discovered that β-lactam antibiotics, including ceftriaxone (CEF), increase the uptake of excessive Glu through the stimulation of the expression of GLT-1 but not of other Glu transporters (GLAST, EAAC1 or EAAT4). In an in vitro model of dissociated embryonic cortical cultures, pretreatment for 48 h with CEF before 1 h OGD, the number of death cells decreased of 60%. CEF also reversed the effect of the Glu transporter inhibitors, threo-β-hydroxyasparate (THA) and TBOA, which overload the synaptic cleft with Glu. Treatment with CEF in a dose-dependent manner protected neurons from the harmful effects of THA or TBOA, which reduce [Glu]e. Another study reported that pretreatment for 48 h with CEF before focal cerebral ischemia or OGD, induced neuroprotective effects. In MCAO, pretreatment with CEF upregulated the mRNA and protein levels of GLT-1 and EAAC1 and reduced the infarct volume in rats204. The neuroprotective mechanism underlying the effects of CEF depends on the NF-κB pathway and GLT-1 promoter activation209.
Another plausible attempt to modulate Glu transport within the CNS involves the xc- system. Upregulation of xc- and activation of the antioxidant defense might diminish ischemic damage, even more than EAATs modulation. Oxidative stress during ischemia induces the phosphorylation of Nrf2, resulting in the increased transcription and translation of xc- system elements after translocation into the nucleus and binding to ARE70. It was recently reported that CEF produced an increased Nrf-2 and xCT expression in HT22 hippocampal cells. In vitro studies have shown the induction of xc-, with the simultaneous inhibition of EAATs, exacerbated Glu-induced cell death210,211,212, suggesting potential cooperation between EAATs and the xc- system. The data from in vivo studies support the idea of neuroprotective activity of the xc- system. Mice exposed to hypoxic preconditioning showed the increased expression of xc-, particularly in the hippocampus213. In this context, it is reasonable to speculate that in severe brain ischemia, preceded by pretreatment with CEF, the up-regulation of both EAATs and xc- might prevent excitotoxicity and inhibit the deactivation of membrane xCT214.
Recent data suggest that the administration of amitriptyline, a tricyclic antidepressant drug, before transient MCAO remarkably reduced infarct volume and neurological deficits and significantly reduced the levels of Glu, Asp, and Gly, but augmented the level of GABA and Tau in rats215,216. Amitriptyline might enhance the expression and activity of Glu transporters. This drug also increases IκBα phosphorylation and NF-κB p65 translocation to the nucleus. The injection of Ro106-9920 (NFκB inhibitor) prevented this translocation, inhibited GLAT and GLT-1 upregulation and restored EAAC1 expression in rats217. However, contrary to previous reports, an in vitro study using amitriptyline showed the inhibition of EAAC1 activity and enhanced Glu neurotransmission218.
Riluzole, the drug approved for ALS treatment, is another compound with confirmed modulatory activity on Glu transporters. Riluzole, both significantly increased Glu uptake in vitro and in vivo. The mechanism underlying the action of riluzole are likely associated with changes in the affinity of GLAST, GLT-1, and EAAC1 to Glu219. However, it was also reported that riluzole elevates the expression and activity of GLT-1, but no other transporters, in striatal astrocytes. In the MCAO model, a single dose of riluzole, injected up to 3 h after reperfusion, reduced the infarct volume 75% and improved neurological deficits220. Moreover, the in vitro model of ischemia, OGD, showed that small doses of riluzole prevented neuronal death221.
N-acetylcysteine (NAC) is widely used as a mucolytic agent and in paracetamol intoxification. This compound contains sulfhydryl groups and acts as a free radical scavenger. NAC is also a membrane permeable cysteine precursor222,223,224 that might enhance the activity of system xc- and thus increase GSH synthesis. These results improve the oxidative status in nervous tissue225. It has been demonstrated that the administration of NAC after cerebral ischemia exhibits neuroprotective effects in rats226,227,228. However, the intensification of xc- might increase [Glu]−e, enhancing excitotoxicity225. NAC also reduces oxidative stress in the neurons of EAAC1-deficient mice subjected to MCAO. EAAC1 possesses the most potent ability to transport cysteine into neurons, but because NAC is membrane permeable, this compound can enhance GSH synthesis without the mediation of EAAC1229,230,231. There are no reports about the direct modulation of these transporters through NAC; however, it is reasonable to speculate that the enhanced activity of system xc- and thus increased [Glu]e could drive the activity of EAAC1.
Recent studies have shown the interesting activity of maslinic acid (MA), a natural triterpene and ingredient of Olea europaea. The administration of MA before MCAO was neuroprotective, reduced the infarct volume, improved neurological scores and enhanced the expression of GLT-1 at the protein and mRNA levels in rats. The observed enhanced expression of GLT-1 (mRNA and protein) was likely mediated through NFκB suppression. Similarly, MA promoted neuron survival at high Glu concentrations what might be correlated with an enhanced expression of the Glu transporters, GLAST and GLT-1, in vitro232,233.
Conclusions
Despite many intensive studies, the pharmacotherapy and prophylaxis of stroke remains unsatisfactory. The only registered drug for the treatment of ischemic stroke is the recombinant tissue plasminogen activator. Unfortunately, the use of this drug is limited by many contraindications, including a narrow therapeutic time window of 4.5 h. Moreover, there is no specific stroke-dedicated prophylaxis. Neuroprotective drugs are also lacking. Thus, there is a need to identify and introduce into routine clinical practice new strategies that would facilitate a decrease in ischemia-induced brain injury. One of the attractive ideas is the induction of brain tolerance to ischemia. Another interesting research area, in the context of brain ischemia, is the modulation of the glutamatergic system, as Glu plays an important, but harmful, role in ischemia-induced cerebral injury. Little is known about the potential significance of Glu transporters and their modulation in the induction of brain tolerance to ischemia. The results of studies using in vitro and in vivo models of brain ischemia indicated that this approach is promising. Some of the strategies modulating Glu transporters have shown significant protection of the nervous tissue after ischemia. Interestingly, some of pharmacological modulators of Glu transporters are drugs registered and used in clinical practice, eg, β-lactam antibiotics, NAC, amitriptyline, and riluzole. However, stroke is not an indication for the use of these drugs. In summary, the results of many studies have provided convincing data that the modulation of Glu transporters is a promising to induce brain tolerance to ischemia. The opportunity to increase resistance to cerebral ischemia is attractive to clinicians and patients. Particularly, persons showing high-risk stroke could targets for this type of therapy. However, a mechanism for avoiding stroke remains unknown, but perhaps a decrease in stroke-related brain injury can be achieved.
Acknowledgments
The work of the team is supported by National Science Centre grant No 2011/01/B/NZ4/00783 and by Ministry of Science and Higher Education: K/DSC/000793 and K/DSC/000779, Poland.
References
- Danbolt NC. Glutamate uptake. Prog Neurobiol 2001; 65: 1–105. [DOI] [PubMed] [Google Scholar]
- Choi DW. Cerebral hypoxia: some new approaches and unanswered questions. J Neurosci 1990; 10: 2493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malarkey EB, Parpura V. Mechanisms of glutamate release from astrocytes. Neurochem Int 2008; 52: 142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, et al. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci 2004; 7: 613–20. [DOI] [PubMed] [Google Scholar]
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, et al. Glutamate-induced neuronal death: A succession of necrosis or apoptosis depending on mitochondrial function. Neuron 1995; 15: 961–73. [DOI] [PubMed] [Google Scholar]
- Zou J, Wang YX, Dou FF, Lu HZ, Ma ZW, Lu PH, et al. Glutamine synthetase down-regulation reduces astrocyte protection against glutamate excitotoxicity to neurons. Neurochem Int 2010; 56: 577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A 1994; 91: 10625–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Neuroenergetics: Calling upon astrocytes to satisfy hungry neurons. Neuroscientist 2004; 10: 53–62. [DOI] [PubMed] [Google Scholar]
- Martin DL. Mechanisms controlling ced synthesis and degradation in the brain. In: Martin DL, editor. GABA in the nervous system. Philadelphia: Lippincott Williams & Wilkins; 2000. p 25–41.
- McBean GJ. Cerebral cystine uptake: a tale of two transporters. Trends Pharmacol Sci 2002; 23: 299–302. [DOI] [PubMed] [Google Scholar]
- Westbrook GL. Glutamate receptors and excitotoxicity. Res Publ Assoc Res Nerv Ment Dis 1993; 71: 35–50. [PubMed] [Google Scholar]
- Zheng K, Scimemi A, Rusakov DA. Receptor actions of synaptically released glutamate: the role of transporters on the scale from nanometers to microns. Biophys J 2008; 95: 4584–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Kelmendi B, Sanacora G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol Biochem Behav 2012; 100: 656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beretta S, Begni B, Ferrarese C. Pharmacological manipulation of glutamate transport. Drug News Perspect 2003; 16: 435–45. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature 1996; 383: 634–7. [DOI] [PubMed] [Google Scholar]
- Fremeau RT. Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci 2004; 27: 98–103. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Silver IA. Metabolism and role of glutamate in mammalian brain. Prog Neurobiol 1990; 35: 245–96. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L, Campbell KP, Catterall WA, Harpold MM, Hofmann F, Horne WA, et al. The naming of voltage-gated calcium channels. Neuron 1994; 13: 505–6. [DOI] [PubMed] [Google Scholar]
- Levy LM, Warr O, Attwell D. Stoichiometry of the glial glutamate transporter GLT-1 expressed inducibly in a Chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. J Neurosci 1998; 18: 9620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev 1999; 51: 7–61. [PubMed] [Google Scholar]
- Papadia S, Soriano FX, Leveille F, Martel M, Dakin KA, Hansen HH, et al. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci 2008; 11: 476–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJL, Bading H. A calcium microdomain near NMDA receptors: On switch for ERK-dependent synapse-to-nucleus communication. Nat Neurosci 2001; 4: 565–6. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJL, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat Neurosci 2001; 4: 261–7. [DOI] [PubMed] [Google Scholar]
- Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, et al. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 2012; 150: 633–46. [DOI] [PubMed] [Google Scholar]
- Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science 1969; 164: 719–21. [DOI] [PubMed] [Google Scholar]
- Cano-Abad MF, Villarroya M, Garcia AG, Gabilan NH, Lopez MG. Calcium entry through L-type calcium channels causes mitochondrial disruption and chromaffin cell death. J Biol Chem 2001; 276: 39695–704. [DOI] [PubMed] [Google Scholar]
- Kostandy BB. The role of glutamate in neuronal ischemic injury: the role of spark in fire. Neurol Sci 2012; 33: 223–37. [DOI] [PubMed] [Google Scholar]
- Celsi F, Pizzo P, Brini M, Leo S, Fotino C, Pinton P, et al. Mitochondria, calcium and cell death: A deadly triad in neurodegeneration. Biochim Biophys Acta Bioenerg 2009; 1787: 335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 1997; 37: 205–37. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res 2006; 326: 483–504. [DOI] [PubMed] [Google Scholar]
- Pilc A, Chaki S, Nowak G, Witkin JM. Mood disorders: regulation by metabotropic glutamate receptors. Biochem Pharmacol 2008; 75: 997–1006. [DOI] [PubMed] [Google Scholar]
- Francesconi A, Duvoisin RM. Opposing effects of protein kinase C and protein kinase A on metabotropic glutamate receptor signaling: selective desensitization of the inositol trisphosphate/Ca2+ pathway by phosphorylation of the receptor-G protein-coupling domain. Proc Natl Acad Sci U S A 2000; 97: 6185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M. Molecular organization of the postsynaptic specialization. Proc Natl Acad Sci U S A 2001; 98: 7058–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero I, Miras-Portugal MT, Sanchez-Prieto J. Functional switch from facilitation to inhibition in the control of glutamate release by metabotropic glutamate receptors. J Biol Chem 1998; 273: 1951–8. [DOI] [PubMed] [Google Scholar]
- Bruno V, Battaglia G, Copani A, Cespedes VM, Galindo MF, Cena V, et al. An activity-dependent switch from facilitation to inhibition in the control of excitotoxicity by group I metabotropic glutamate receptors. Eur J Neurosci 2001; 13: 1469–78. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat Rev Neurosci 2008; 9: 423–36. [DOI] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev 1999; 29: 83–120. [DOI] [PubMed] [Google Scholar]
- Wieronska JM, Pilc A. Metabotropic glutamate receptors in the tripartite synapse as a target for new psychotropic drugs. Neurochem Int 2009; 55: 85–97. [DOI] [PubMed] [Google Scholar]
- Tamaru Y, Nomura S, Mizuno N, Shigemoto R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience 2001; 106: 481–503. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Romano C, Ghosh P. Metabotropic glutamate receptor mGluR5 subcellular distribution and developmental expression in hypothalamus. J Comp Neurol 1995; 362: 134–50. [DOI] [PubMed] [Google Scholar]
- Kim K, Lee SG, Kegelman TP, Su ZZ, Das SK, Dash R, et al. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics. J Cell Physiol 2011; 226: 2484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo J, Dargusch R, Schubert D. Distribution of the cystine/glutamate antiporter system xc- in the brain, kidney, and duodenum. J Histochem Cytochem 2006; 54: 549–57. [DOI] [PubMed] [Google Scholar]
- Liguz-Lecznar M, Skangiel-Kramska J. Vesicular glutamate transporters (VGLUTs): the three musketeers of glutamatergic system. Acta Neurobiol Exp (Wars) 2007; 67: 207–18. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Nakagawa T, Shige K, Minami M, Satoh M. Changes in the expression of glial glutamate transporters in the rat brain accompanied with morphine dependence and naloxone-precipitated withdrawal. Brain Res 2001; 905: 254–8. [DOI] [PubMed] [Google Scholar]
- Storck T, Schulte S, Hofmann K, Stoffel W. Structure, expression, and functional analysis of a Na+-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci U S A 1992; 89: 10955–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Harada C, Watanabe M, Inoue Y, Sakagawa T, Nakayama N, et al. Functions of the two glutamate transporters GLAST and GLT-1 in the retina. Proc Natl Acad Sci U S A 1998; 95: 4663–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouiche A, Rauen T. Coincidence of L-glutamate/L-aspartate transporter (GLAST) and glutamine synthetase (GS) immunoreactions in retinal glia: evidence for coupling of GLAST and GS in transmitter clearance. J Neurosci Res 1995; 42: 131–43. [DOI] [PubMed] [Google Scholar]
- Otori Y, Shimada S, Tanaka K, Ishimoto I, Tano Y, Tohyama M. Marked increase in glutamate-aspartate transporter (GLAST/GluT-1) mRNA following transient retinal ischemia. Brain Res Mol Brain Res 1994; 27: 310–4. [DOI] [PubMed] [Google Scholar]
- Li HS, Niedzielski AS, Beisel KW, Hiel H, Wenthold RJ, Morley BJ. Identification of a glutamate/aspartate transporter in the rat cochlea. Hear Res 1994; 78: 235–42. [DOI] [PubMed] [Google Scholar]
- Kondo K, Hashimoto H, Kitanaka J, Sawada M, Suzumura A, Marunouchi T, et al. Expression of glutamate transporters in cultured glial cells. Neurosci Lett 1995; 188: 140–2. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Civenni G, Racagni G, Danbolt NC, Schousboe I, Schousboe A. Glutamate receptor agonists up-regulate glutamate transporter GLAST in astrocytes. Neuroreport 1996; 8: 261–5. [DOI] [PubMed] [Google Scholar]
- Ramachandran B, Houben K, Rozenberg YY, Haigh JR, Varpetian A, Howard BD. Differential expression of transporters for norepinephrine and glutamate in wild type, variant, and WNT1-expressing PC12 cells. J Biol Chem 1993; 268: 23891–7. [PubMed] [Google Scholar]
- Perego C, Vanoni C, Bossi M, Massari S, Basudev H, Longhi R, et al. The GLT-1 and GLAST glutamate transporters are expressed on morphologically distinct astrocytes and regulated by neuronal activity in primary hippocampal cocultures. J Neurochem 2000; 75: 1076–84. [DOI] [PubMed] [Google Scholar]
- Ward MM, Jobling AI, Puthussery T, Foster LE, Fletcher EL. Localization and expression of the glutamate transporter, excitatory amino acid transporter 4, within astrocytes of the rat retina. Cell Tissue Res 2004; 315: 305–10. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflugers Arch 2004; 447: 469–79. [DOI] [PubMed] [Google Scholar]
- Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int 2007; 51: 333–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragakis NJ, Dietrich J, Wong V, Xue H, Mayer-Proschel M, Rao MS, et al. Glutamate transporter expression and function in human glial progenitors. Glia 2004; 45: 133–43. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Dhond RP, Benz A, Xu W, Rothstein JD, Danbolt NC, et al. Neuronal expression of the glutamate transporter GLT-1 in hippocampal microcultures. J Neurosci 1998; 18: 4490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Takeuchi H, Doi Y, Kawanokuchi J, Sonobe Y, Jin S, et al. Excitatory amino acid transporter expression by astrocytes is neuroprotective against microglial excitotoxicity. Brain Res 2008; 1210: 11–9. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, et al. Localization of neuronal and glial glutamate transporters. Neuron 1994; 13: 713–25. [DOI] [PubMed] [Google Scholar]
- Gottlieb M, Domercq M, Matute C. Altered expression of the glutamate transporter EAAC1 in neurons and immature oligodendrocytes after transient forebrain ischemia. J Cereb Blood Flow Metab 2000; 20: 678–87. [DOI] [PubMed] [Google Scholar]
- Dehnes Y, Chaudhry FA, Ullensvang K, Lehre KP, Storm-Mathisen J, Danbolt NC. The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate-gated chloride channel concentrated near the synapse in parts of the dendritic membrane facing astroglia. J Neurosci 1998; 18: 3606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie A, Cnops L, Smolders I, McCullumsmith R, Kooijman R, Kwak S, et al. High-affinity Na+/K+-dependent glutamate transporter EAAT4 is expressed throughout the rat fore- and midbrain. J Comp Neurol 2008; 511: 155–72. [DOI] [PubMed] [Google Scholar]
- Bar-Peled O, Ben-Hur H, Biegon A, Groner Y, Dewhurst S, Furuta A, et al. Distribution of glutamate transporter subtypes during human brain development. J Neurochem 1997; 69: 2571–80. [DOI] [PubMed] [Google Scholar]
- Hu WH, Walters WM, Xia XM, Karmally SA, Bethea JR. Neuronal glutamate transporter EAAT4 is expressed in astrocytes. Glia 2003; 44: 13–25. [DOI] [PubMed] [Google Scholar]
- Schlag BD, Vondrasek JR, Munir M, Kalandadze A, Zelenaia OA, Rothstein JD, et al. Regulation of the glial Na+-dependent glutamate transporters by cyclic AMP analogs and neurons. Mol Pharmacol 1998; 53: 355–69. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci U S A 1997; 94: 4155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishige K, Chen Q, Sagara Y, Schubert D. The activation of dopamine D4 receptors inhibits oxidative stress-induced nerve cell death. J Neurosci 2001; 21: 6069–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, et al. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain. J Neurosci 2002; 22: 8028–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, et al. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002; 277: 44765–71. [DOI] [PubMed] [Google Scholar]
- Allen JW, Shanker G, Aschner M. Methylmercury inhibits the in vitro uptake of the glutathione precursor, cystine, in astrocytes, but not in neurons. Brain Res 2001; 894: 131–40. [DOI] [PubMed] [Google Scholar]
- Piani D, Fontana A. Involvement of the cystine transport system xc- in the macrophage-induced glutamate-dependent cytotoxicity to neurons. J Immunol 1994; 152: 3578–85. [PubMed] [Google Scholar]
- Kato S, Ishita S, Sugawara K, Mawatari K. Cystine/glutamate antiporter expression in retinal Muller glial cells: implications for DL-alpha-aminoadipate toxicity. Neuroscience 1993; 57: 473–82. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Schnaar RL, Coyle JT. Immature cortical neurons are uniquely sensitive to glutamate toxicity by inhibition of cystine uptake. FASEB J 1990; 4: 1624–33. [PubMed] [Google Scholar]
- Ye ZC, Rothstein JD, Sontheimer H. Compromised glutamate transport in human glioma cells: reduction-mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine-glutamate exchange. J Neurosci 1999; 19: 10767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, Takahata T, Balaram P. VGLUT1 and VGLUT2 mRNA expression in the primate auditory pathway. Hear Res 2011; 274: 129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelks KB, Wylie R, Floyd CL, McAllister AK, Wise P. Estradiol targets synaptic proteins to induce glutamatergic synapse formation in cultured hippocampal neurons: critical role of estrogen receptor-alpha. J Neurosci 2007; 27: 6903–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund U, Persson M, Ronnback L, Hansson E. Primary cultures from cerebral cortex and hippocampus enriched in glutamatergic and GABAergic neurons. Neurochem Res 2010; 35: 1733–42. [DOI] [PubMed] [Google Scholar]
- Nunzi MG, Russo M, Mugnaini E. Vesicular glutamate transporters VGLUT1 and VGLUT2 define two subsets of unipolar brush cells in organotypic cultures of mouse vestibulocerebellum. Neuroscience 2003; 122: 359–71. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 1996; 16: 675–86. [DOI] [PubMed] [Google Scholar]
- Rauen T, Rothstein JD, Wassle H. Differential expression of three glutamate transporter subtypes in the rat retina. Cell Tissue Res 1996; 286: 325–36. [DOI] [PubMed] [Google Scholar]
- Lee SA, Choi JG, Zuo Z. Volatile anesthetics attenuate oxidative stress-reduced activity of glutamate transporter type 3. Anesth Analg 2009; 109: 1506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moechars D, Weston MC, Leo S, Callaerts-Vegh Z, Goris I, Daneels G, et al. Vesicular glutamate transporter VGLUT2 expression levels control quantal size and neuropathic pain. J Neurosci 2006; 26: 12055–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, et al. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci 2006; 9: 119–26. [DOI] [PubMed] [Google Scholar]
- Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature 1995; 375: 599–603. [DOI] [PubMed] [Google Scholar]
- Fremeau RT. Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, et al. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science 2004; 304: 1815–9. [DOI] [PubMed] [Google Scholar]
- Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, et al. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci U S A 2004; 101: 7158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordera RM, Totterdell S, Wojcik SM, Brose N, Elizalde N, Lasheras B, et al. Enhanced anxiety, depressive-like behaviour and impaired recognition memory in mice with reduced expression of the vesicular glutamate transporter 1 (VGLUT1). Eur J Neurosci 2007; 25: 281–90. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Fukaya M, Shimizu H, Watanabe M. Subtype switching of vesicular glutamate transporters at parallel fibre-Purkinje cell synapses in developing mouse cerebellum. Eur J Neurosci 2003; 17: 2563–72. [DOI] [PubMed] [Google Scholar]
- Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, et al. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron 2008; 57: 263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, et al. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature 2009; 462: 651–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omote H, Miyaji T, Juge N, Moriyama Y. Vesicular neurotransmitter transporter: bioenergetics and regulation of glutamate transport. Biochemistry 2011; 50: 5558–65. [DOI] [PubMed] [Google Scholar]
- Bannai S. Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem 1986; 261: 2256–63. [PubMed] [Google Scholar]
- Cho Y, Bannai S. Uptake of glutamate and cysteine in C-6 glioma cells and in cultured astrocytes. J Neurochem 1990; 55: 2091–7. [DOI] [PubMed] [Google Scholar]
- Dringen R, Hirrlinger J. Glutathione pathways in the brain. Biol Chem 2003; 384: 505–16. [DOI] [PubMed] [Google Scholar]
- Tan S, Schubert D, Maher P. Oxytosis: A novel form of programmed cell death. Curr Top Med Chem 2001; 1: 497–506. [DOI] [PubMed] [Google Scholar]
- Grewer C, Gameiro A, Zhang Z, Tao Z, Braams S, Rauen T. Glutamate forward and reverse transport: from molecular mechanism to transporter-mediated release after ischemia. IUBMB Life 2008; 60: 609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MB. The family of sodium-dependent glutamate transporters: a focus on the GLT-1/EAAT2 subtype. Neurochem Int 1998; 33: 479–91. [DOI] [PubMed] [Google Scholar]
- Billups B, Rossi D, Oshima T, Warr O, Takahashi M, Sarantis M, et al. Physiological and pathological operation of glutamate transporters. Prog Brain Res 1998; 116: 45–57. [DOI] [PubMed] [Google Scholar]
- Sonders MS, Amara SG. Channels in transporters. Curr Opin Neurobiol 1996; 6: 294–302. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature 2000; 407: 189–94. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of differentiation-associated brain-specific phosphate transporter as a second vesicular glutamate transporter (VGLUT2). J Neurosci 2001; 21: RC182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Malherbe P, Broger C, Jahn R. Molecular cloning and functional characterization of human vesicular glutamate transporter 3. EMBO Rep 2002; 3: 798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disbrow JK, Gershten MJ, Ruth JA. Uptake of L-3H] glutamic acid by crude and purified synaptic vesicles from rat brain. Biochem Biophys Res Commun 1982; 108: 1221–7. [DOI] [PubMed] [Google Scholar]
- Naito S, Ueda T. Characterization of glutamate uptake into synaptic vesicles. J Neurochem 1985; 44: 99–109. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Ishibashi T, Baba A. Increase in chloride-dependent L-glutamate transport activity in synaptic membrane after in vitro ischemic treatment. J Neurochem 1995; 65: 1798–804. [DOI] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci 1995; 15: 1835–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristol LA, Rothstein JD. Glutamate transporter gene expression in amyotrophic lateral sclerosis motor cortex. Ann Neurol 1996; 39: 676–9. [DOI] [PubMed] [Google Scholar]
- Torp R, Lekieffre D, Levy LM, Haug FM, Danbolt NC, Meldrum BS, et al. Reduced postischemic expression of a glial glutamate transporter, GLT1, in the rat hippocampus. Exp Brain Res 1995; 103: 51–8. [DOI] [PubMed] [Google Scholar]
- Palos TP, Ramachandran B, Boado R, Howard BD. Rat C6 and human astrocytic tumor cells express a neuronal type of glutamate transporter. Brain Res Mol Brain Res 1996; 37: 297–303. [DOI] [PubMed] [Google Scholar]
- Figiel M, Engele J. Pituitary adenylate cyclase-activating polypeptide (PACAP), a neuron-derived peptide regulating glial glutamate transport and metabolism. J Neurosci 2000; 20: 3596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegelashvili G, Dehnes Y, Danbolt NC, Schousboe A. The high-affinity glutamate transporters GLT1, GLAST, and EAAT4 are regulated via different signalling mechanisms. Neurochem Int 2000; 37: 163–70. [DOI] [PubMed] [Google Scholar]
- Zelenaia O, Schlag BD, Gochenauer GE, Ganel R, Song W, Beesley JS, et al. Epidermal growth factor receptor agonists increase expression of glutamate transporter GLT-1 in astrocytes through pathways dependent on phosphatidylinositol 3-kinase and transcription factor NF-kappaB. Mol Pharmacol 2000; 57: 667–78. [DOI] [PubMed] [Google Scholar]
- Li LB, Toan SV, Zelenaia O, Watson DJ, Wolfe JH, Rothstein JD, et al. Regulation of astrocytic glutamate transporter expression by Akt: evidence for a selective transcriptional effect on the GLT-1/EAAT2 subtype. J Neurochem 2006; 97: 759–71. [DOI] [PubMed] [Google Scholar]
- Sitcheran R, Gupta P, Fisher PB, Baldwin AS. Positive and negative regulation of EAAT2 by NF-kappaB: a role for N-myc in TNFalpha-controlled repression. EMBO J 2005; 24: 510–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke S, Rabson AB, Germino JF, Gallo MA, Tian Y. Mechanism of suppression of cytochrome P-450 1A1 expression by tumor necrosis factor-alpha and lipopolysaccharide. J Biol Chem 2001; 276: 39638–44. [DOI] [PubMed] [Google Scholar]
- Levy LM, Lehre KP, Walaas SI, Storm-Mathisen J, Danbolt NC. Down-regulation of glial glutamate transporters after glutamatergic denervation in the rat brain. Eur J Neurosci 1995; 7: 2036–41. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Martin LJ, Rothstein JD. Regional deafferentation down-regulates subtypes of glutamate transporter proteins. J Neurochem 1995; 65: 2800–3. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem 1984; 43: 1369–74. [DOI] [PubMed] [Google Scholar]
- Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc-. Antioxid Redox Signal 2000; 2: 665–71. [DOI] [PubMed] [Google Scholar]
- Sato H, Kuriyama-Matsumura K, Hashimoto T, Sasaki H, Wang H, Ishii T, et al. Effect of oxygen on induction of the cystine transporter by bacterial lipopolysaccharide in mouse peritoneal macrophages. J Biol Chem 2001; 276: 10407–12. [DOI] [PubMed] [Google Scholar]
- Lewerenz J, Maher P, Methner A. Regulation of xCT expression and system x (c) (-) function in neuronal cells. Amino Acids 2012; 42: 171–9. [DOI] [PubMed] [Google Scholar]
- Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem 2009; 284: 1106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, Erb H, Sun X, Toda S, Kalivas PW, Murphy TH. Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J Neurosci 2006; 26: 10514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation. Biochem Biophys Res Commun 2004; 325: 109–16. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 2000; 32: 1–14. [PubMed] [Google Scholar]
- Duan S, Anderson CM, Stein BA, Swanson RA. Glutamate induces rapid upregulation of astrocyte glutamate transport and cell-surface expression of GLAST. J Neurosci 1999; 19: 10193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correale DM, Kalandadze A, Zelenaia O, Robinson MB. Comparison of the regulation of cell surface expression of the GLT-1, GLAST, and EAAC1 subtypes of glutamate (Glu) transporters. Soc Neurosci Abs 1998; 24: 826.14. [Google Scholar]
- Trotti D, Peng JB, Dunlop J, Hediger MA. Inhibition of the glutamate transporter EAAC1 expressed in Xenopus oocytes by phorbol esters. Brain Res 2001; 914: 196–203. [DOI] [PubMed] [Google Scholar]
- Gonzalez MI, Bannerman PG, Robinson MB. Phorbol myristate acetate-dependent interaction of protein kinase Calpha and the neuronal glutamate transporter EAAC1. J Neurosci 2003; 23: 5589–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M, Song W, Liu MY, Jin L, Dykes-Hoberg M, Lin CI, et al. Modulation of the neuronal glutamate transporter EAAT4 by two interacting proteins. Nature 2001; 410: 89–93. [DOI] [PubMed] [Google Scholar]
- Lin CI, Orlov I, Ruggiero AM, Dykes-Hoberg M, Lee A, Jackson M, et al. Modulation of the neuronal glutamate transporter EAAC1 by the interacting protein GTRAP3–18. Nature 2001; 410: 84–8. [DOI] [PubMed] [Google Scholar]
- Trotti D, Danbolt NC, Volterra A. Glutamate transporters are oxidant-vulnerable: a molecular link between oxidative and excitotoxic neurodegeneration? Trends Pharmacol Sci 1998; 19: 328–34. [DOI] [PubMed] [Google Scholar]
- Pogun S, Dawson V, Kuhar MJ. Nitric oxide inhibits 3H]-glutamate transport in synaptosomes. Synapse 1994; 18: 21–6. [DOI] [PubMed] [Google Scholar]
- Volterra A, Trotti D, Tromba C, Floridi S, Racagni G. Glutamate uptake inhibition by oxygen free radicals in rat cortical astrocytes. J Neurosci 1994; 14: 2924–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ying W, Simma V, Copin JC, Chan PH, Swanson RA. Overexpression of Cu, Zn superoxide dismutase attenuates oxidative inhibition of astrocyte glutamate uptake. J Neurochem 2000; 75: 939–45. [DOI] [PubMed] [Google Scholar]
- Fairman WA, Sonders MS, Murdoch GH, Amara SG. Arachidonic acid elicits a substrate-gated proton current associated with the glutamate transporter EAAT4. Nat Neurosci 1998; 1: 105–13. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Arriza JL, Amara SG, Kavanaugh MP. Differential modulation of human glutamate transporter subtypes by arachidonic acid. J Biol Chem 1995; 270: 6433–5. [DOI] [PubMed] [Google Scholar]
- Vandenberg RJ, Mitrovic AD, Johnston GA. Molecular basis for differential inhibition of glutamate transporter subtypes by zinc ions. Mol Pharmacol 1998; 54: 189–96. [DOI] [PubMed] [Google Scholar]
- Fahrig T. Receptor subtype involved and mechanism of norepinephrine-induced stimulation of glutamate uptake into primary cultures of rat brain astrocytes. Glia 1993; 7: 212–8. [DOI] [PubMed] [Google Scholar]
- Hansson E, Ronnback L. Receptor regulation of the glutamate, GABA and taurine high-affinity uptake into astrocytes in primary culture. Brain Res 1991; 548: 215–21. [DOI] [PubMed] [Google Scholar]
- Virgin C. Jr, Ha TP, Packan DR, Tombaugh GC, Yang SH, Horner HC, et al. Glucocorticoids inhibit glucose transport and glutamate uptake in hippocampal astrocytes: implications for glucocorticoid neurotoxicity. J Neurochem 1991; 57: 1422–8. [DOI] [PubMed] [Google Scholar]
- Faillace MP, Keller Sarmiento MI, Rosenstein RE. Melatonin effect on 3H]glutamate uptake and release in the golden hamster retina. J Neurochem 1996; 67: 623–8. [DOI] [PubMed] [Google Scholar]
- Saransaari P, Lillrank SM, Oja SS. Phencyclidine treatment in mice: effects on phencyclidine binding sites and glutamate uptake in cerebral cortex preparations. J Neural Transm Gen Sect 1993; 93: 47–59. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci 1994; 14: 5559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mestikawy S, Wallen-Mackenzie A, Fortin GM, Descarries L, Trudeau LE. From glutamate co-release to vesicular synergy: vesicular glutamate transporters. Nat Rev Neurosci 2011; 12: 204–16. [DOI] [PubMed] [Google Scholar]
- Juge N, Gray JA, Omote H, Miyaji T, Inoue T, Hara C, et al. Metabolic control of vesicular glutamate transport and release. Neuron 2010; 68: 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni B, Rosteck PR Jr, Nadi NS, Paul SM. Cloning and expression of a cDNA encoding a brain-specific Na+-dependent inorganic phosphate cotransporter. Proc Natl Acad Sci U S A 1994; 91: 5607–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YF. Lysine metabolism in the human and the monkey: demonstration of pipecolic acid formation in the brain and other organs. Neurochem Res 1982; 7: 577–88. [DOI] [PubMed] [Google Scholar]
- Bannai S, Kitamura E. Role of proton dissociation in the transport of cystine and glutamate in human diploid fibroblasts in culture. J Biol Chem 1981; 256: 5770–2. [PubMed] [Google Scholar]
- Folbergrova J, Zhao Q, Katsura K, Siesjo BK. N-tert-butyl-(alpha)-phenylnitrone improves recovery of brain energy state in rats following transient focal ischemia. Proc Natl Acad Sci U S A 1995; 92: 5057–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama Y, Kimura Y, Hashimoto H, Matsuda T, Baba A. L-lactate inhibits L-cystine/L-glutamate exchange transport and decreases glutathione content in rat cultured astrocytes. J Neurosci Res 2000; 59: 685–91. [DOI] [PubMed] [Google Scholar]
- Lewerenz J, Dargusch R, Maher P. Lactacidosis modulates glutathione metabolism and oxidative glutamate toxicity. J Neurochem 2010; 113: 502–14. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature 2000; 403: 316–21. [DOI] [PubMed] [Google Scholar]
- Parpura V, Scemes E, Spray DC. Mechanisms of glutamate release from astrocytes: gap junction “hemichannels”, purinergic receptors and exocytotic release. Neurochem Int 2004; 45: 259–64. [DOI] [PubMed] [Google Scholar]
- Dawson LA, Djali S, Gonzales C, Vinegra MA, Zaleska MM. Characterization of transient focal ischemia-induced increases in extracellular glutamate and aspartate in spontaneously hypertensive rats. Brain Res Bull 2000; 53: 767–76. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Kawamata T, Tamura T, Hovda DA, Becker DP, Tsubokawa T. Calcium-dependent glutamate release concomitant with massive potassium flux during cerebral ischemia in vivo. Brain Res 1991; 558: 136–40. [DOI] [PubMed] [Google Scholar]
- Zeevalk GD, Davis N, Hyndman AG, Nicklas WJ. Origins of the extracellular glutamate released during total metabolic blockade in the immature retina. J Neurochem 1998; 71: 2373–81. [DOI] [PubMed] [Google Scholar]
- Li S, Mealing GA, Morley P, Stys PK. Novel injury mechanism in anoxia and trauma of spinal cord white matter: glutamate release via reverse Na+-dependent glutamate transport. J Neurosci 1999; 19: RC16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Millhorn DE. Hypoxia regulates glutamate metabolism and membrane transport in rat PC12 cells. J Neurochem 2001; 76: 1935–48. [DOI] [PubMed] [Google Scholar]
- Hsu L, Rockenstein E, Mallory M, Hashimoto M, Masliah E. Altered expression of glutamate transporters under hypoxic conditions in vitro. J Neurosci Res 2001; 64: 193–202. [DOI] [PubMed] [Google Scholar]
- Sher PK, Hu SX. Increased glutamate uptake and glutamine synthetase activity in neuronal cell cultures surviving chronic hypoxia. Glia 1990; 3: 350–7. [DOI] [PubMed] [Google Scholar]
- Kelleher JA, Gregory GA, Chan PH. Effect of fructose-1,6-bisphosphate on glutamate uptake and glutamine synthetase activity in hypoxic astrocyte cultures. Neurochem Res 1994; 19: 209–15. [DOI] [PubMed] [Google Scholar]
- Stanimirovic DB, Ball R, Durkin JP. Stimulation of glutamate uptake and Na,K-ATPase activity in rat astrocytes exposed to ischemia-like insults. Glia 1997; 19: 123–34. [DOI] [PubMed] [Google Scholar]
- Fujimoto S, Katsuki H, Kume T, Kaneko S, Akaike A. Mechanisms of oxygen glucose deprivation-induced glutamate release from cerebrocortical slice cultures. Neurosci Res 2004; 50: 179–87. [DOI] [PubMed] [Google Scholar]
- Jung HH, Lee JJ, Washington JM, Zuo Z. Inability of volatile anesthetics to inhibit oxygen-glucose deprivation-induced glutamate release via glutamate transporters and anion channels in rat corticostriatal slices. Brain Res 2008; 1227: 234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonde C, Noraberg J, Noer H, Zimmer J. Ionotropic glutamate receptors and glutamate transporters are involved in necrotic neuronal cell death induced by oxygen-glucose deprivation of hippocampal slice cultures. Neuroscience 2005; 136: 779–94. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Bridges RJ, Chamberlin AR, Shimamoto K, Yasuda-Kamatani Y, Swanson RA. Differing effects of substrate and non-substrate transport inhibitors on glutamate uptake reversal. J Neurochem 2001; 79: 1207–16. [DOI] [PubMed] [Google Scholar]
- Djali S, Dawson LA. Characterization of endogenous amino acid efflux from hippocampal slices during chemically-induced ischemia. Neurochem Res 2001; 26: 135–43. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Shimamoto K, Schousboe A. Comparison of effects of DL-threo-(beta)-benzyloxyaspartate (DL-TBOA) and L-trans-pyrrolidine-2,4-dicarboxylate (t-2,4-PDC) on uptake and release of 3H]D-aspartate in astrocytes and glutamatergic neurons. Neurochem Res 2001; 26: 661–6. [DOI] [PubMed] [Google Scholar]
- Selkirk JV, Stiefel TH, Stone IM, Naeve GS, Foster AC, Poulsen DJ. Over-expression of the human EAAT2 glutamate transporter within neurons of mouse organotypic hippocampal slice cultures leads to increased vulnerability of CA1 pyramidal cells. Eur J Neurosci 2005; 21: 2291–6. [DOI] [PubMed] [Google Scholar]
- Bonde C, Sarup A, Schousboe A, Gegelashvili G, Noraberg J, Zimmer J. GDNF pre-treatment aggravates neuronal cell loss in oxygen-glucose deprived hippocampal slice cultures: a possible effect of glutamate transporter up-regulation. Neurochem Int 2003; 43: 381–8. [DOI] [PubMed] [Google Scholar]
- Yeh TH, Hwang HM, Chen JJ, Wu T, Li AH, Wang HL. Glutamate transporter function of rat hippocampal astrocytes is impaired following the global ischemia. Neurobiol Dis 2005; 18: 476–83. [DOI] [PubMed] [Google Scholar]
- Ketheeswaranathan P, Turner NA, Spary EJ, Batten TF, McColl BW, Saha S. Changes in glutamate transporter expression in mouse forebrain areas following focal ischemia. Brain Res 2011; 1418: 93–103. [DOI] [PubMed] [Google Scholar]
- Bruhn T, Levy LM, Nielsen M, Christensen T, Johansen FF, Diemer NH. Ischemia induced changes in expression of the astrocyte glutamate transporter GLT1 in hippocampus of the rat. Neurochem Int 2000; 37: 277–85. [DOI] [PubMed] [Google Scholar]
- Chen JC, Hsu-Chou H, Lu JL, Chiang YC, Huang HM, Wang HL, et al. Down-regulation of the glial glutamate transporter GLT-1 in rat hippocampus and striatum and its modulation by a group III metabotropic glutamate receptor antagonist following transient global forebrain ischemia. Neuropharmacology 2005; 49: 703–14. [DOI] [PubMed] [Google Scholar]
- Raghavendra Rao VL, Rao AM, Dogan A, Bowen KK, Hatcher J, Rothstein JD, et al. Glial glutamate transporter GLT-1 down-regulation precedes delayed neuronal death in gerbil hippocampus following transient global cerebral ischemia. Neurochem Int 2000; 36: 531–7. [DOI] [PubMed] [Google Scholar]
- Kim DS, Kwak SE, Kim JE, Jung JY, Won MH, Choi SY, et al. Transient ischaemia affects plasma membrane glutamate transporter, not vesicular glutamate transporter, expressions in the gerbil hippocampus. Anat Histol Embryol 2006; 35: 265–70. [DOI] [PubMed] [Google Scholar]
- Rao VL, Dogan A, Bowen KK, Todd KG, Dempsey RJ. Antisense knockdown of the glial glutamate transporter GLT-1 exacerbates hippocampal neuronal damage following traumatic injury to rat brain. Eur J Neurosci 2001; 13: 119–28. [PubMed] [Google Scholar]
- Arranz AM, Gottlieb M, Perez-Cerda F, Matute C. Increased expression of glutamate transporters in subcortical white matter after transient focal cerebral ischemia. Neurobiol Dis 2010; 37: 156–65. [DOI] [PubMed] [Google Scholar]
- Rao VL, Bowen KK, Dempsey RJ. Transient focal cerebral ischemia down-regulates glutamate transporters GLT-1 and EAAC1 expression in rat brain. Neurochem Res 2001; 26: 497–502. [DOI] [PubMed] [Google Scholar]
- Sanchez-Mendoza E, Burguete MC, Castello-Ruiz M, Gonzalez MP, Roncero C, Salom JB, et al. Transient focal cerebral ischemia significantly alters not only EAATs but also VGLUTs expression in rats: relevance of changes in reactive astroglia. J Neurochem 2010; 113: 1343–55. [DOI] [PubMed] [Google Scholar]
- Lobo AC, Gomes JR, Catarino T, Mele M, Fernandez P, Inacio AR, et al. Cleavage of the vesicular glutamate transporters under excitotoxic conditions. Neurobiol Dis 2011; 44: 292–303. [DOI] [PubMed] [Google Scholar]
- Bevers MB, Neumar RW. Mechanistic role of calpains in postischemic neurodegeneration. J Cereb Blood Flow Metab 2008; 28: 655–73. [DOI] [PubMed] [Google Scholar]
- Takagaki Y, Itoh Y, Aoki Y, Ukai Y, Yoshikuni Y, Kimura K. Inhibition of ischemia-induced fodrin breakdown by a novel phenylpyrimidine derivative NS-7: an implication for its neuroprotective action in rats with middle cerebral artery occlusion. J Neurochem 1997; 68: 2507–13. [DOI] [PubMed] [Google Scholar]
- Michalski D, Hartig W, Krugel K, Edwards RH, Boddener M, Bohme L, et al. Region-specific expression of vesicular glutamate and GABA transporters under various ischaemic conditions in mouse forebrain and retina. Neuroscience 2013; 231: 328–44. [DOI] [PubMed] [Google Scholar]
- Llorente IL, Perez-Rodriguez D, Burgin TC, Gonzalo-Orden JM, Martinez-Villayandre B, Fernandez-Lopez A. Age and meloxicam modify the response of the glutamate vesicular transporters (VGLUTs) after transient global cerebral ischemia in the rat brain. Brain Res Bull 2013; 94: 90–7. [DOI] [PubMed] [Google Scholar]
- Harvey BK, Airavaara M, Hinzman J, Wires EM, Chiocco MJ, Howard DB, et al. Targeted over-expression of glutamate transporter 1 (GLT-1) reduces ischemic brain injury in a rat model of stroke. PLoS One 2011; 6: e22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani A, Tanaka K. Functional changes of glial glutamate transporter GLT-1 during ischemia: an in vivo study in the hippocampal CA1 of normal mice and mutant mice lacking GLT-1. J Neurosci 2003; 23: 7176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namura S, Maeno H, Takami S, Jiang XF, Kamichi S, Wada K, et al. Inhibition of glial glutamate transporter GLT-1 augments brain edema after transient focal cerebral ischemia in mice. Neurosci Lett 2002; 324: 117–20. [DOI] [PubMed] [Google Scholar]
- Yamashita A, Makita K, Kuroiwa T, Tanaka K. Glutamate transporters GLAST and EAAT4 regulate postischemic Purkinje cell death: an in vivo study using a cardiac arrest model in mice lacking GLAST or EAAT4. Neurosci Res 2006; 55: 264–70. [DOI] [PubMed] [Google Scholar]
- Beschorner R, Simon P, Schauer N, Mittelbronn M, Schluesener HJ, Trautmann K, et al. Reactive astrocytes and activated microglial cells express EAAT1, but not EAAT2, reflecting a neuroprotective potential following ischaemia. Histopathology 2007; 50: 897–910. [DOI] [PubMed] [Google Scholar]
- Castillo J, Davalos A, Naveiro J, Noya M. Neuroexcitatory amino acids and their relation to infarct size and neurological deficit in ischemic stroke. Stroke 1996; 27: 1060–5. [DOI] [PubMed] [Google Scholar]
- Castillo J, Davalos A, Noya M. Progression of ischaemic stroke and excitotoxic aminoacids. Lancet 1997; 349: 79–83. [DOI] [PubMed] [Google Scholar]
- Mallolas J, Hurtado O, Castellanos M, Blanco M, Sobrino T, Serena J, et al. A polymorphism in the EAAT2 promoter is associated with higher glutamate concentrations and higher frequency of progressing stroke. J Exp Med 2006; 203: 711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenman E, Sinor JD, Brimecombe JC, Herin GA. Alterations of N-methyl-D-aspartate receptor properties after chemical ischemia. J Pharmacol Exp Ther 2000; 295: 572–7. [PubMed] [Google Scholar]
- Tanaka H, Calderone A, Jover T, Grooms SY, Yokota H, Zukin RS, et al. Ischemic preconditioning acts upstream of GluR2 down-regulation to afford neuroprotection in the hippocampal CA1. Proc Natl Acad Sci U S A 2002; 99: 2362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave KR, Lange-Asschenfeldt C, Raval AP, Prado R, Busto R, Saul I, et al. Ischemic preconditioning ameliorates excitotoxicity by shifting glutamate/gamma-aminobutyric acid release and biosynthesis. J Neurosci Res 2005; 82: 665–73. [DOI] [PubMed] [Google Scholar]
- Romera C, Hurtado O, Mallolas J, Pereira MP, Morales JR, Romera A, et al. Ischemic preconditioning reveals that GLT1/EAAT2 glutamate transporter is a novel PPAR(gamma) target gene involved in neuroprotection. J Cereb Blood Flow Metab 2007; 27: 1327–38. [DOI] [PubMed] [Google Scholar]
- Pradillo JM, Hurtado O, Romera C, Cardenas A, Fernandez-Tome P, Alonso-Escolano D, et al. TNFR1 mediates increased neuronal membrane EAAT3 expression after in vivo cerebral ischemic preconditioning. Neuroscience 2006; 138: 1171–8. [DOI] [PubMed] [Google Scholar]
- Douen AG, Akiyama K, Hogan MJ, Wang F, Dong L, Chow AK, et al. Preconditioning with cortical spreading depression decreases intraischemic cerebral glutamate levels and down-regulates excitatory amino acid transporters EAAT1 and EAAT2 from rat cerebal cortex plasma membranes. J Neurochem 2000; 75: 812–8. [DOI] [PubMed] [Google Scholar]
- Cimarosti H, Jones NM, O'Shea RD, Pow DV, Salbego C, Beart PM. Hypoxic preconditioning in neonatal rat brain involves regulation of excitatory amino acid transporter 2 and estrogen receptor alpha. Neurosci Lett 2005; 385: 52–7. [DOI] [PubMed] [Google Scholar]
- Liu YX, Zhang M, Liu LZ, Cui X, Hu YY, Li WB. The role of glutamate transporter-1a in the induction of brain ischemic tolerance in rats. Glia 2012; 60: 112–24. [DOI] [PubMed] [Google Scholar]
- Romera C, Hurtado O, Botella SH, Lizasoain I, Cardenas A, Fernandez-Tome P, et al. In vitro ischemic tolerance involves upregulation of glutamate transport partly mediated by the TACE/ADAM17-tumor necrosis factor-alpha pathway. J Neurosci 2004; 24: 1350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi T, Kawahara K, Yamada T, Nakajima T, Tanaka M. Functional significance of the preconditioning-induced down-regulation of glutamate transporter GLT-1 in neuron/astrocyte co-cultures. Neurochem Res 2005; 30: 1109–16. [DOI] [PubMed] [Google Scholar]
- Selkirk JV, Nottebaum LM, Vana AM, Verge GM, Mackay KB, Stiefel TH, et al. Role of the GLT-1 subtype of glutamate transporter in glutamate homeostasis: the GLT-1-preferring inhibitor WAY-855 produces marginal neurotoxicity in the rat hippocampus. Eur J Neurosci 2005; 21: 3217–28. [DOI] [PubMed] [Google Scholar]
- Chu K, Lee ST, Sinn DI, Ko SY, Kim EH, Kim JM, et al. Pharmacological induction of ischemic tolerance by glutamate transporter-1 (EAAT2) upregulation. Stroke 2007; 38: 177–82. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 2005; 433: 73–7. [DOI] [PubMed] [Google Scholar]
- Lee SG, Su ZZ, Emdad L, Gupta P, Sarkar D, Borjabad A, et al. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J Biol Chem 2008; 283: 13116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz J, Klein M, Methner A. Cooperative action of glutamate transporters and cystine/glutamate antiporter system xc- protects from oxidative glutamate toxicity. J Neurochem 2006; 98: 916–25. [DOI] [PubMed] [Google Scholar]
- Lewerenz J, Albrecht P, Tien ML, Henke N, Karumbayaram S, Kornblum HI, et al. Induction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone in vitro. J Neurochem 2009; 111: 332–43. [DOI] [PubMed] [Google Scholar]
- Fogal B, Li J, Lobner D, McCullough LD, Hewett SJ. System xc- activity and astrocytes are necessary for interleukin-1(beta)-mediated hypoxic neuronal injury. J Neurosci 2007; 27: 10094–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims B, Clarke M, Francillion L, Kindred E, Hopkins ES, Sontheimer H. Hypoxic preconditioning involves system xc- regulation in mouse neural stem cells. Stem Cell Res 2012; 8: 285–91. [DOI] [PubMed] [Google Scholar]
- Seib TM, Patel SA, Bridges RJ. Regulation of the system x-C cystine/glutamate exchanger by intracellular glutathione levels in rat astrocyte primary cultures. Glia 2011; 59: 1387–401. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jia D, Liu Z, Chen X, Hu X. Effects of amitriptyline on monoamines of brain tissue in rats with focal cerebral ischemia-reperfusion injury. Chin Pharmacol Bull 2007; 23: 1077–80. [Google Scholar]
- Liu Z, Zhang Y, Jia D, Hu X, Chen X, Li J. Effects of amitriptyline on amino acids in ischemic areas in rats subjected to focal cerebral ischemia reperfusion. Chin Pharm J (China) 2008; 43: 1305. [Google Scholar]
- Tai YH, Tsai RY, Wang YH, Cherng CH, Tao PL, Liu TM, et al. Amitriptyline induces nuclear transcription factor-kappaB-dependent glutamate transporter upregulation in chronic morphine-infused rats. Neuroscience 2008; 153: 823–31. [DOI] [PubMed] [Google Scholar]
- Baik HJ, Lee SA, Washington JM, Zuo ZY. Amitriptyline inhibits the activity of the rat glutamate transporter EAAT3 expressed in Xenopus oocytes. J Pharm Pharmacol 2009; 61: 577–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli E, Funicello M, Rauen T, Gobbi M, Mennini T. Riluzole enhances the activity of glutamate transporters GLAST, GLT1 and EAAC1. Eur J Pharmacol 2008; 578: 171–6. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Laigle C, Blondeau N, Jarretou G, Lazdunski M. Alpha-linolenic acid and riluzole treatment confer cerebral protection and improve survival after focal brain ischemia. Neuroscience 2006; 137: 241–51. [DOI] [PubMed] [Google Scholar]
- Siniscalchi A, Zona C, Sancesario G, D'Angelo E, Zeng YC, Mercuri NB, et al. Neuroprotective effects of riluzole: an electrophysiological and histological analysis in an in vitro model of ischemia. Synapse 1999; 32: 147–52. [DOI] [PubMed] [Google Scholar]
- Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim Biophys Acta 2013; 1830: 4117–29. [DOI] [PubMed] [Google Scholar]
- Cotgreave IA. N-acetylcysteine: pharmacological considerations and experimental and clinical applications. Adv Pharmacol 1997; 38: 205–27. [PubMed] [Google Scholar]
- Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci 2003; 60: 6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci 2005; 25: 6389–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Zhang Q, Li H, Zhang G. Antioxidant N-acetylcysteine and AMPA/KA receptor antagonist DNQX inhibited mixed lineage kinase-3 activation following cerebral ischemia in rat hippocampus. Neurosci Res 2003; 47: 47–53. [DOI] [PubMed] [Google Scholar]
- Khan M, Sekhon B, Jatana M, Giri S, Gilg AG, Sekhon C, et al. Administration of N-acetylcysteine after focal cerebral ischemia protects brain and reduces inflammation in a rat model of experimental stroke. J Neurosci Res 2004; 76: 519–27. [DOI] [PubMed] [Google Scholar]
- Zhang QG, Tian H, Li HC, Zhang GY. Antioxidant N-acetylcysteine inhibits the activation of JNK3 mediated by the GluR6-PSD95-MLK3 signaling module during cerebral ischemia in rat hippocampus. Neurosci Lett 2006; 408: 159–64. [DOI] [PubMed] [Google Scholar]
- Won SJ, Yoo BH, Brennan AM, Shin BS, Kauppinen TM, Berman AE, et al. EAAC1 gene deletion alters zinc homeostasis and exacerbates neuronal injury after transient cerebral ischemia. J Neurosci 2010; 30: 15409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang BG, Won SJ, Kim JH, Choi BY, Lee MW, Sohn M, et al. EAAC1 gene deletion alters zinc homeostasis and enhances cortical neuronal injury after transient cerebral ischemia in mice. J Trace Elem Med Biol 2012; 26: 85–8. [DOI] [PubMed] [Google Scholar]
- Cao L, Li L, Zuo Z. N-acetylcysteine reverses existing cognitive impairment and increased oxidative stress in glutamate transporter type 3 deficient mice. Neuroscience 2012; 220: 85–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan T, Qian Y, Tang X, Huang M, Huang L, Li Y, et al. Maslinic acid, a natural inhibitor of glycogen phosphorylase, reduces cerebral ischemic injury in hyperglycemic rats by GLT-1 up-regulation. J Neurosci Res 2011; 89: 1829–39. [DOI] [PubMed] [Google Scholar]
- Qian Y, Guan T, Tang X, Huang L, Huang M, Li Y, et al. Maslinic acid, a natural triterpenoid compound from Olea europaea, protects cortical neurons against oxygen-glucose deprivation-induced injury. Eur J Pharmacol 2011; 670: 148–53. [DOI] [PubMed] [Google Scholar]