Abstract
Background
In pancreatic cancer, early detection and complete surgical resection with negative margins offers the only cure for the disease. Work up to evaluate resectability includes triple phase helical scan CT of the pancreas and endoscopic ultrasound (EUS). A paucity of data exists in using PET/CT scan as staging work up in early resectable pancreatic cancer. The objective of our study was to determine if PET/CT prevents futile laparotomy by detecting occult metastatic disease in patients with resectable/borderline pancreatic cancer.
Methods
We looked at our institutional PET/CT data base incorporating National Oncologic PET Registry with diagnosis of resectable or borderline resectable pancreatic cancer from 2005 to 2012. Clinical, radiographic, and pathologic follow-up was evaluated, including age, gender, evidence of metastatic disease, and initial CA 19–9 levels. The impact of PET/CT on patient management was estimated by calculating the percentage of patients whose treatment plan was altered due to PET/CT. The confidence interval was computed using the exact binomial distribution. The effect on the change was evaluated by the multiple logistic regression model. The final model was selected using the backward elimination method.
Results
We identified 285 patients with early stage (resectable or borderline) pancreatic cancer who received PET/CT as part of initial staging workup. Upon initial work up (CT + EUS), 62% of patients were considered resectable and 38% were borderline resectable. Addition of PET/CT scan changed the management in 10.9% (n=31) of patients (95% CI: 7.5%–15.1%). Median time from EUS to PET/CT was 5 days. Metastatic lesions were confirmed with biopsy in 19 (61%) patients. The proportion in the change in treatment plan is significantly higher in patients who were initially considered to have borderline resectable compared to resectable malignancy (16.5% vs. 7.4%). In 199 patients who were taken to surgery, 18.1% (n=36) were found to have metastatic disease intraoperatively.
Conclusions
PET/CT helped improve detection of occult metastases, ultimately sparing these patients a potentially unnecessary surgery. The role of PET/CT scan should be validated in prospective study.
Introduction
Pancreatic cancer still remains lethal and is the fourth common most common cause of death of cancer related death among U.S men and Women. In the next decade pancreatic cancer will become the second leading cause of cancer death in the Unites States after lung cancer. In 2014, 46,420 individuals will be diagnosed with pancreatic cancer and approximately 39,590 people will die of pancreatic cancer in the United States.1
Early detection and complete surgical resection of the tumor with negative margins offers the only chance for cure for this disease. Typical work up for pancreatic cancer to evaluate resectability includes triple phase helical scan computed tomography (CT) and endoscopic ultrasound (EUS). Some institutions may add staging laparoscopy however this is not universally accepted due to lack of controlled studies showing benefit compared to radiographic imaging.2 National Comprehensive Cancer Network (NCCN) guideline recommends pancreatic CT or magnetic resonance imaging (MRI), imaging of chest and EUS for workup of pancreatic cancer to determine its resectability. However, NCCN does not recommend fusion positron emission tomography/computed tomography (PET/CT) as staging workup and states that the role remains unclear due to lack of limited data on clinical utility of PET/CT scan.3 Studies in pancreas cancer have shown overall sensitivity of PET scan alone to be high as 92% and potentially can be reliable in detecting metastatic disease in the liver.4–6 However, other studies have shown no benefit of PET in terms of detecting small volume of disease in the liver or the peritoneum.7,8 In other diseases there are data to suggest superiority of PET scan over CT for detecting extra hepatic disease as well.9,10 However due to lack of anatomic localization, PET alone is not commonly used. Instead CT is added to PET to improve anatomic and functional localization.
PET/CT has been utilized in other tumor types such as colorectal and has been used to detect occult metastatic disease and potentially avoid futile laparotomy.11 In pancreatic cancer paucity of data exist using PET/CT scan as staging work up in early resectable pancreatic cancer. Few small studies have shown potential benefit of PET/CT scan. Small prospective data showed PET/CT changed management 16% in pancreatic cancer and another one showed changed management in 11% of patients.12,13
The main objective of our study was to determine if PET/CT prevents futile laparotomy in patients with resectable pancreatic cancer based on CT scan and EUS. Specifically, this study would assess if PET/CT scan detect occult metastatic disease which was not detected by CT scan resulting in altering the management of the patient.
Methods
Patients
Between 2005 and 2012, an institutional review board (IRB) approved retrospective review of prospective data from Moffitt Cancer Center was recorded. We looked at our institutional PET/CT database incorporating National Oncologic PET Registry (NOPR) with diagnosis of pancreatic cancer. We then excluded patients with advanced or locally advanced pancreatic cancer as well as IPMNs.
As per Moffitt’s pancreatic cancer pathway, patients with suspected resectable or borderline pancreatic cancer are evaluated with three-phase computed tomography angiogram (CTA) with pancreas protocol, CT of the chest, CA 19–9 and endoscopic ultrasound with histologic confirmation. Patients with potentially resectable/borderline disease are referred for surgical consultation. Finally, prior to surgical resection or neoadjuvant therapy for borderline cancer all of the patients were evaluated with PET/CT scan (Figure 1).
Figure 1.
Treatment algorithm for patients with pancreatic cancer at Moffitt Cancer Center
All patients were discussed in our multidisciplinary gastrointestinal tumor board prior to definitive treatment planning. The decision of borderline resectable vs resectable vs locally advanced was made in the tumor board. The definition of borderline-resectable disease used at our institution includes the following criteria: (1) ≤180° circumferential tumor abutment of superior mesenteric vein (SMV), portal vein (PV), or superior mesenteric artery (SMA); (2) short segment encasement (approximately 1.5 cm) of the PV/SMV amenable to partial vein resection and reconstruction; or (3) gastroduodenal artery encasement up to the origin of the hepatic artery very similar to NCCN definition of borderline pancreatic cancer. Outside biopsies were re-evaluated by our pathologists to confirm definitive diagnosis. Clinical, radiographic, and pathologic follow-up was evaluated for each patient. Clinical information included age, gender, evidence of local regional disease, evidence of metastatic disease, pathologic characteristics, and initial CA 19–9 levels.
PET/CT scan
Metabolic tracer imaging was performed with 2-deoxy-2-[18F] fluoro-D-glucose positron emission tomography integrated with computed tomography, using either a Siemens Biograph Classic PET/CT or a General Electric Healthcare Discovery PET/CT scanner. Imaging was initiated at 90 min after intravenous injection of 296–555 MBq (8–15 mCi) of radiotracer. Both devices provide for the selection of a voxel within a selected volume of interest for the maximum standardized uptake rate (SUVmax), defined as the ratio of tissue radioactivity concentration (e.g., in units of kBq/ml) at time T, C PET(T), and the injected dose (e.g., in units of MBq) at the time of injection divided by body weight (e.g., in units of kg), or SUVbw = C PET(T)/(injected dose/patient’s weight).
Statistical Analysis
The demographic and baseline characteristics of patients were summarized using descriptive statistics. More specifically, categorical data was presented as frequencies and percentages. For continuous data, mean, standard deviation, median and range was presented. The impact of PET/CT on patient management was estimated by calculating the percentage of patients whose treatment plan was altered due to PET/CT. The 95% confidence interval for the proportion of changing the management of the patient by PET/CT scan was computed by the Clopper-Pearson method. The association between categorical variables was evaluated by the Fisher exact test. A multiple logistic regression model was used to assess the association of change in treatment plan by PET/CT or during surgery with predictors. The predictors in the initial model included stage of primary tumor prior to PET, gender, age, T-N stage, location of primary tumor (head or body/tail), time interval between EUS and PET scan and baseline CA 19-9. The final model was determined by backward elimination method. The odds ratio (OR) and its 95% confidence intervals were reported. A two-sided p-value of <0.05 was considered statistically significant. All statistical analyses were performed using SAS 9.2 (SAS Institute Inc, Cary, NC).
Results
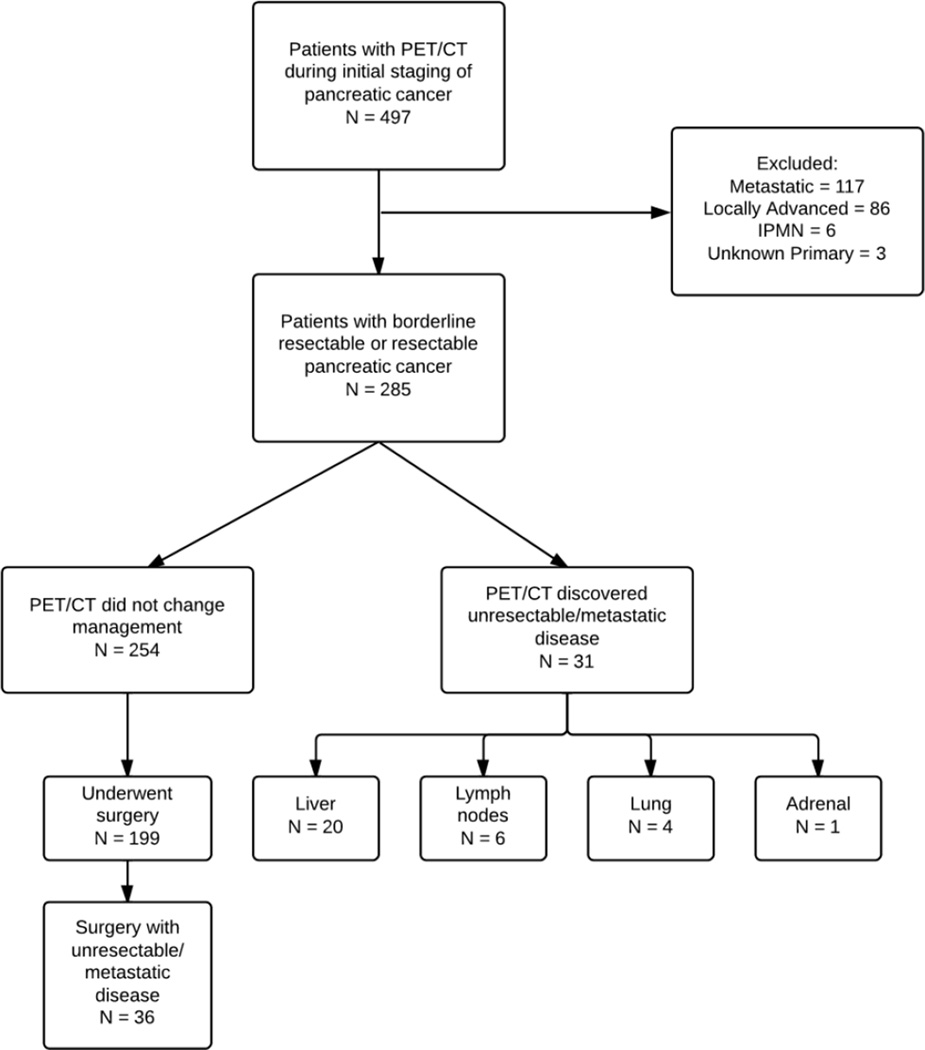
From 2005 until 2012, we initially identified 497 who underwent PET/CT imaging for newly diagnosed pancreatic cancer (Figure 2). Out of 497 patients, we identified 285 patients who were initially found to be resectable or borderline resectable by CT scan and EUS. Patients’ characteristics are listed in Table 1. The median age of our patient cohort was 69.1 years (Range: 35.5–90.6 years). All of the patients had pancreas protocol CT scan and EUS as part of the initial workup which was followed up by PET/CT scan. At the GI multidisciplinary Tumor board prior to PET scan, 109 (38%) patients were considered to have borderline resectable pancreatic cancer and 176 (62%) patients were deemed resectable. There were 8 patients who were initially considered to have T4 lesion. However, the review of CT scan and EUS at the tumor board conference determined that 5 patients had borderline resectable pancreatic cancer and 3 patients had resectable disease. Stages could not be determined in 10 % of patients after EUS for several reasons, the most common being artifact from the calcifications in the head of the pancreas and artifact from metal stents. No FDG uptake of the primary pancreatic lesion was observed in 37 (13%) patients. The median SUV uptake was 6.1 (1.9–21.4).
Figure 2.
Flowchart detailing chart review process of patients in data set
Table 1.
Baseline Characteristics of Patients
| Variables | n (%) or median (range) |
|---|---|
| Gender | |
| Male | 159 (56.5) |
| Female | 126 (43.5) |
| Age (years) | 69.1 (35.5 – 90.6) |
| Stage of primary Tumor prior to PET scan | |
| T1N0 | 24 (8.4) |
| T2N0 | 52 (18.2) |
| T3N0 | 71 (24.9) |
| T4N0 | 4 (1.4) |
| T1N1 | 9 (3.1) |
| T2N1 | 28 (9.8) |
| T3N1 | 63 (22.1) |
| T4N1 | 4 (1.4) |
| NA | 30 (10.5) |
| Location of the tumor | |
| Head | 225 (78.9) |
| Body/tail | 60 (21.1) |
| Resectability | |
| Borderline resectable | 109 (38.2) |
| Resectable | 176 (61.8) |
| FDG uptake of the pancreas lesion | |
| FDG negative | 37 (13.1) |
| FDG positive | 246 (86.9) |
| Baseline CA 19-9 U/ml | 166 (0 – 53800) |
| Median SUV | 6.1 (1.9 – 21.4) |
| Days between CT and PET Scan | 5 (0–108) |
N/A : Not available
PET/CT in the Evaluation of Metastatic Disease
The primary objective of this study was to determine if PET/CT helped improve detection of occult metastases, ultimately sparing these patients a potentially unnecessary surgery. PET/CT findings changed the management in 31 patients (10.9%, 95% CI: 7.5% – 15.1%) with pancreatic cancer who were considered to be resectable (n=13) or borderline resectable (n=18) after routine staging (Figure 1). Most of these occult lesions were found in liver (64%) followed by lung (13%), lymph node (19%) and adrenal gland (3%) (Table 2). Out of these 31 patients, 19 (61%) patients underwent biopsy for pathologic confirmation of metastatic disease. The median time from CT to PET scan was 13 days (Range: 0–108 days). All patients were treated with palliative chemotherapy without surgical resection.
Table 2.
Characteristics of the patients whose staging was changed after PET scan or during surgery.
| PET Scan, N (%) | Surgery, N (%) | |
|---|---|---|
| Staging/management changed | 31 (10.9%) | 36 (18.1%) |
| Pre-PET staging | ||
| Borderline resectable | 18 (16.5%) | 17 (33.3%) |
| Resectable | 13 (7.4%) | 19 (12.8%) |
| Gender | ||
| Male | 22 (71%) | 23 (63.9%) |
| Female | 9 (29%) | 13 (36.1%) |
| Site of metastases | ||
| Liver | 20 (64.5%) | 12 (33.3%) |
| Lung | 4 (12.9%) | 0 |
| Lymph Node | 6 (19.3%) | 4 (11.1%) |
| Involvement of major blood vessels | 0 | 10 (27.8%) |
| Peritoneum | 0 | 9 (25%) |
| Other | 1 (3.2%) | 1 (2.8%) |
| Location of primary tumor | ||
| Head | 25 (80.7%) | 23 (63.9%) |
| Body/Tail | 6 (19.3%) | 13 (36.1%) |
| Tissue confirmation | 19 (61.3%) | 36 (100%) |
| CA 19-9 (X1000 U/ml), median (range) | 0.5 (0–14.3) | 0.4 (0–14.6) |
| SUV of pancreatic lesion, median (range) | 7.7 (3.7–19.9) | 6.9 (2.9–13.9) |
| Days between CT and PET scan, median (range) | 13 (0–108) | 3.5 (0–51) |
In addition, four patients had suspicious lesions for metastatic disease on the PET scan prior to surgery but underwent surgical resection based on tumor board recommendation. Three patients were found to have metastatic disease upon surgical exploration; liver was involved in 2 patients and omentum in one patient. Hypermetabolic activity was seen on the PET scan in the kidney in one patient. Upon surgical exploration, patient was found to have renal cell carcinoma in addition to pancreatic cancer. Patient underwent resection of pancreatic primary along with nephrectomy.
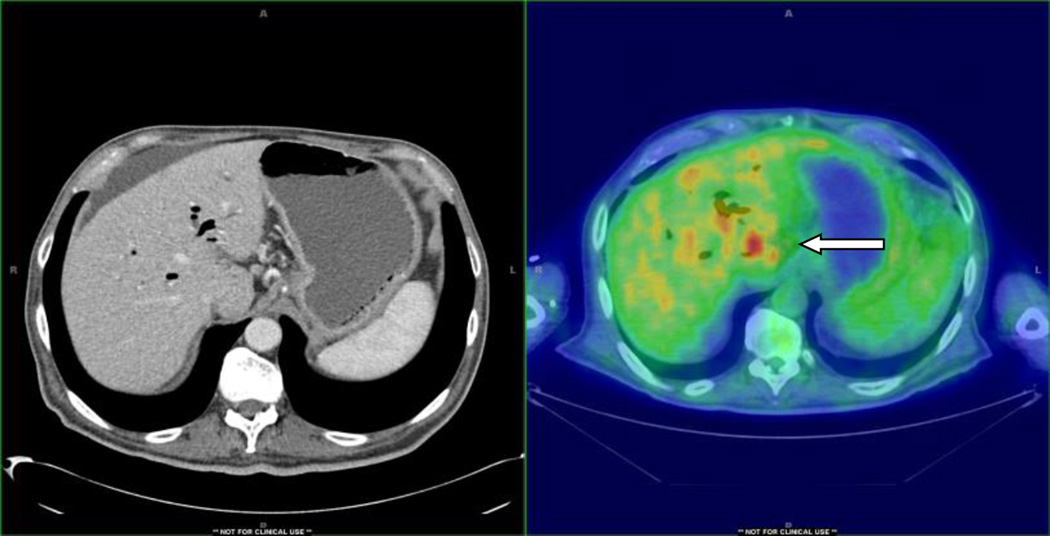
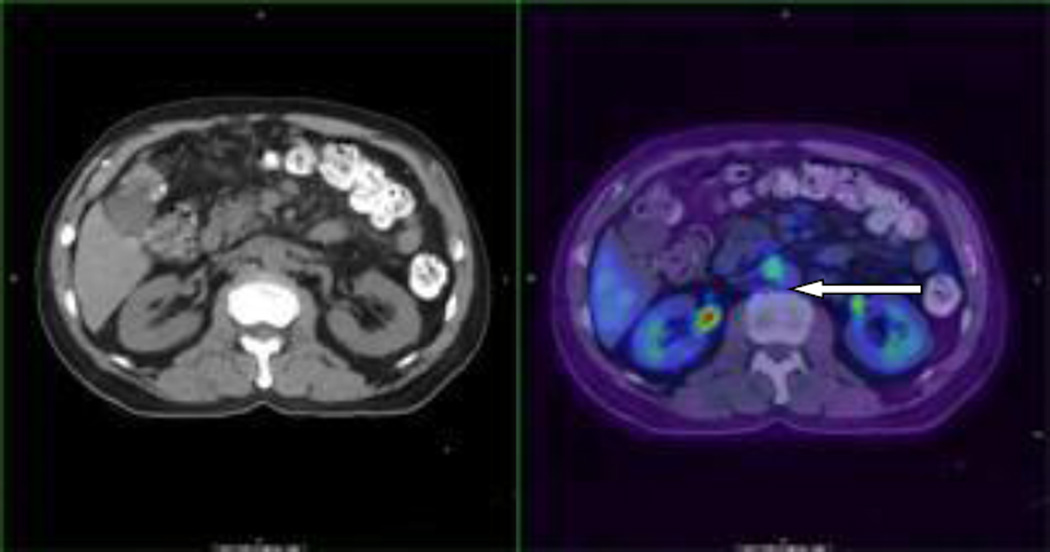
Figure 1 is an example where liver lesion was not detected by routine CT scan but was detected by PET/CT scan which was positive by fine needle aspiration for metastatic pancreatic cancer. Figure 2 is an example where occult lesion was found in the retroperitoneal lymph node which was also confirmed by a biopsy.
We performed the multivariate analysis to find the variables that are associated with change in staging by PET/CT scan (Table 3). In the final model, pre-PET scan staging (resectable or borderline), gender, SUV of primary pancreatic lesion and time interval between CT and PET scan were included. These four variables were found to be statistically significant factors in the multivariate analysis. For every 2 weeks delay in PET scan evaluation the risk of finding unresectable disease by PET scan increased by factor of 1.38. PET scan changed the staging in 18 (16.5%) out of 109 patients who were initially considered to have borderline disease and 13 (7.4%) out of 176 patients who were deemed to have resectable cancer. Interestingly, CA 19-9 level was not significantly associated with change in resectability status by PET scan on both univariate and multivariate analysis.
Table 3.
Multivariate analysis for association between change in staging by PET scan and clinical factors
| Odds Ratio Estimates (OR) | ||||
|---|---|---|---|---|
| Effect | OR Point Estimate |
95% Wald Confidence Limits |
P value | |
| Pre-PET staging, Resectable vs Borderline | 0.40 | 0.16 | 0.98 | 0.0440 |
| Sex, M vs F | 3.85 | 1.33 | 11.08 | 0.0126 |
| SUV (per 1 unit increase) | 1.19 | 1.05 | 1.34 | 0.0054 |
| Time interval between CT and PET (Per 2 weeks increase) | 1.38 | 1.04 | 1.83 | 0.0278 |
Accuracy of EUS, CT and PET/CT as initial staging workup for resectable pancreatic cancer
Majority of patients with borderline resectable cancer underwent neoadjuvant chemotherapy with GTX (gemcitabine, docetaxel and capecitabine) for 3 cycles followed by radiation therapy followed by surgery as per Moffitt pancreatic cancer pathway. Patients with resectable pancreatic cancer directly proceeded to surgery. Surgical resection was performed in a total of 199 patients. Among 176 patients who were initially staged as resectable pancreatic cancer, 148 (84.1%) patients underwent surgery and 51 (46.8%) patients out of 109 patients staged as borderline resectable pancreatic cancer underwent surgery. The primary reason for not undergoing surgical resection was finding of metastatic disease on PET scan, progression of disease (borderline resectable group), unfit for surgery or patients’ decision.
Metastatic or unresectable disease was found in 36 (17.9%) patients during surgery, primarily due to involvement of peritoneum (33%), liver (25%) and large blood vessel (28%).
In the final multivariate model evaluating the association between change in staging during surgery and clinical factors, location of the primary tumor (head or body/tail) and pre-PET staging (resectable or borderline resectable) were included and both were found to be statistically significant (Table 4). Baseline CA19-9 level was significant in univariate model but did not affect the final multivariate model. Among the 51 patients who were diagnosed with borderline resectable pancreatic cancer based on imaging studies, 17 (33.3%) patients were found to have metastatic or unresectable disease during surgery. Similarly, 19 (12.8%) out of 148 patients who were diagnosed with resectable disease were found to have unresectable during surgery.
Table 4.
Multivariate analysis for association between change in staging during surgery and clinical factors
| Odds Ratio Estimates | ||||
|---|---|---|---|---|
| Effect | Point Estimate |
95% Wald Confidence Limits |
P value |
|
| Pre-PET staging, Resectable vs Borderline | 0.17 | 0.07 | 0.44 | 0.0003 |
| Location, Head vs Body/Tail | 0.25 | 0.09 | 0.68 | 0.0068 |
Discussion
Surgery remains the only curative treatment for pancreatic cancer. Therefore, proper initial staging has become more increasingly important not only to access local and regional disease extent but to evaluate potential distant metastatic disease to avoid futile laparotomy. The goal of our study was to see if PET/CT scan prevents futile laparotomy after standard initial staging workup with EUS and CT scan by detecting metastatic disease. PET involves the use of radionuclide tagged molecules that are chosen to measure specific functions. Combining PET with computed tomography (PET/CT) allows for more accurate anatomic localization and for attenuation correction of the PET data. PET/CT with 2-{fluorine-18]fluoro-2-deoxy-D-glucose (FDG) allows estimation of metabolic activity with regards to glucose utilization.14 However, whether the PET or PET/CT adds anything to initial workup in borderline or resectable pancreas cancer is unclear. Current data are conflicting. Earlier data show that PET scan or PET/CT does not add additional information to that derived from standard imaging.15,16 Study by Frolich et al attempted to investigate the role of PET scan in resectable disease.5 The study demonstrated sensitivity of 68% and specificity of 95% to detect liver lesions. However, these studies included heterogeneous population including both malignant and benign disease along with early and advanced disease. Prospective study to determine the impact of PET/CT on the management of resectable suspected pancreatic cancer was conducted by Heinrich et al with 59 patients.12 In this study, PET/CT scan was able to change management in 16% (6/37) of the patients. Furthermore, authors demonstrated that PET/CT was cost effective as well. In another study, Bang et al reported that out of 72 pancreatic cancer cases that were considered resectable, distant metastases were found in additional 17 patients (24%) with PET/CT. Liver was the most common site of occult metastases.17 However, EUS was not utilized for staging and not all the patients were histologically confirmed as adenocarcinoma.
Our study only included patients with biopsy proven resectable or borderline resectable pancreatic cancer and all patients had histologic diagnosis of adenocarcinoma. In our study, we evaluated if additional testing with PET/CT scan changed our management of these patients by detecting small volume metastatic disease not detected by standard imaging. We have previously published a 10% rate of upstaging in a small series with 82 patients with pancreatic cancer which also included intraductal papillary mucinous neoplasm (IPMN).13 In this study, PET/CT scan changed the management in 10.9% of the patients by detecting metastases not initially picked up by standard imaging and included both EUS and CT scan. In patients initially considered to have borderline resectable cancer, PET scan changed the resectability status in 16.5% of the patients and helped avoid futile laparotomy. In patents with resectable disease addition of PET/CT scan avoided surgery in 7.4% of the patients. This was found to be statistically significant based on multivariate analysis. In this study, delay between CT and PET scan was associated with finding of metastatic disease by PET scan, demonstrating the importance of completing staging workup in timely fashion. Higher SUV of the primary pancreatic lesion increased the likelihood of finding metastatic disease. Majority of the metastatic lesions were found in the liver by PET/CT scan but missed by standard imaging. To our knowledge, this is the largest report to evaluate the role of PET/CT as initial staging work up in addition to standard imaging in patients with resectable pancreatic cancer. Further, this is the first study that to assess the value of PET/CT in patients with resectable and borderline resectable pancreatic cancers separately as an initial staging work up. Currently, NCCN guidelines do not recommend PET scan as staging modality for pancreatic cancer. However, in advanced colon cancer, PET/CT prior to liver resection is generally recommended by NCCN guidelines. Prospective data demonstrated that PET scan additional to CT scan can decrease futile laparotomy by 17%.18
This study also demonstrates the limitations of PET/CT scan in evaluating resection status of pancreatic cancer patients. In patients who underwent surgery, additional 18.1% of patients were found to have metastatic or unresectable disease during the surgery. Most of the metastatic lesions were found in the liver and peritoneum. Another important reason for unresectability was involvement of blood vessels. Prior studies have demonstrated that PET/CT is not effective in detecting metastatic disease in the peritoneum.7 Our findings in this study shows that despite our modern technology about one out of 5 of patients are not accurately staged and may undergo unnecessary surgery even with PET/CT. Prior studies have shown that up 30% of the patients who were deemed resectable were found to be unresectable during the laparoscopic findings.19–21 However, none of those studies used PET/CT as initial staging. One can argue that diagnostic laparoscopy may further improve the accuracy of staging; however, to this date there are no controlled studies demonstrating benefit of diagnostic laparoscopy. There are data to support that patients with pancreas tumor in the body or tail, or high initial tumor marker have very high chance of occult peritoneal disease.22,23 In our series, patients with tumors located in body or tail and initial pre-surgical staging of borderline pancreatic cancer were found to have higher rates of peritoneal disease as well compared to resectable disease.
Our study has definite limitations due to its retrospective in nature, and subjectivity in interpretation of resectable pancreatic cancer. For example, there were eight patients with T4 lesions but were considered borderline resectable or resectable when the cases were reviewed at the tumor board. Also, tissue confirmation for metastatic disease was only done for 61% of the patients. The reason for this relatively low rate was that most of the patients received treatment with the local oncologist after PET/CT confirmation of metastatic disease and did not follow up at our institute. In our series, not all the patients who were deemed resectable after PET/CT scan underwent surgery primarily secondary to being medically unfit for surgery.
In conclusion our study of using PET/CT, significantly changed the overall management of patients with pancreatic cancer compared with standard staging. This is especially true for patients with borderline resectable pancreatic cancer where PET/CT scan prevented resection in one out of 6 patients. Additional imaging with PET/CT scan avoided futile laparotomy and potential financial burden by detecting occult metastatic disease. It is important to perform PET scan in timely manner to decrease the likelihood of finding unresectable disease. Furthermore, our study suggests that better staging workup is needed. Despite our current modern imaging techniques with PET/CT, CT and EUS, 18% of the patients (33% of patients with borderline resectable disease) were found to have metastatic or unresectable disease during surgery, which raises potential question of staging laparoscopy as part of the initial workup. These findings will be required to be validated in a prospective study along with cost effective analysis. Future trials should not only incorporate PET/CT scan but diagnostic laparoscopy as well, especially in high risk patients in order to avoid futile laparotomy.
Figure 3.
PET/CT detection of occult liver metastasis.
Figure 4.
PET/CT detection of occult retroperitoneal lymph node
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Zamboni GA, Kruskal JB, Vollmer CM, et al. Pancreatic adenocarcinoma: value of multidetector CT angiography in preoperative evaluation. Radiology. 2007;245:770–778. doi: 10.1148/radiol.2453061795. [DOI] [PubMed] [Google Scholar]
- 3.NCCN. NCCN Clinical Practice Guidelines in Oncology. Pancreatic cancer. Version 2.2012. http://www.nccn.org. [Google Scholar]
- 4.Zimny M, Buell U. 18FDG-positron emission tomography in pancreatic cancer. Ann Oncol. 1999;10(Suppl 4):28–32. [PubMed] [Google Scholar]
- 5.Frohlich A, Diederichs CG, Staib L, et al. Detection of liver metastases from pancreatic cancer using FDG PET. Journal of Nuclear Medicine. 1999;40:250–255. [PubMed] [Google Scholar]
- 6.Maemura K, Takao S, Shinchi H, et al. Role of positron emission tomography in decisions on treatment strategies for pancreatic cancer. J Hepatobiliary Pancreat Surg. 2006;13:435–441. doi: 10.1007/s00534-006-1102-8. [DOI] [PubMed] [Google Scholar]
- 7.Izuishi K, Yamamoto Y, Sano T, et al. Impact of 18-fluorodeoxyglucose positron emission tomography on the management of pancreatic cancer. J Gastrointest Surg. 2010;14:1151–1158. doi: 10.1007/s11605-010-1207-x. [DOI] [PubMed] [Google Scholar]
- 8.Diederichs CG, Staib L, Vogel J, et al. Values and limitations of 18F-fluorodeoxyglucose-positron-emission tomography with preoperative evaluation of patients with pancreatic masses. Pancreas. 2000;20:109–116. doi: 10.1097/00006676-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Ruers TJ, Langenhoff BS, Neeleman N, et al. Value of positron emission tomography with [F-18]fluorodeoxyglucose in patients with colorectal liver metastases: a prospective study. J Clin Oncol. 2002;20:388–395. doi: 10.1200/JCO.2002.20.2.388. [DOI] [PubMed] [Google Scholar]
- 10.Fong Y, Saldinger PF, Akhurst T, et al. Utility of 18F-FDG positron emission tomography scanning on selection of patients for resection of hepatic colorectal metastases. Am J Surg. 1999;178:282–287. doi: 10.1016/s0002-9610(99)00187-7. [DOI] [PubMed] [Google Scholar]
- 11.Ruers TJM, Wiering B, van der Sijp JRM, et al. Improved Selection of Patients for Hepatic Surgery of Colorectal Liver Metastases with F-18-FDG PET: A Randomized Study. Journal of Nuclear Medicine. 2009;50:1036–1041. doi: 10.2967/jnumed.109.063040. [DOI] [PubMed] [Google Scholar]
- 12.Heinrich S, Goerres GW, Schafer M, et al. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg. 2005;242:235–243. doi: 10.1097/01.sla.0000172095.97787.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farma JM, Santillan AA, Melis M, et al. PET/CT fusion scan enhances CT staging in patients with pancreatic neoplasms. Ann Surg Oncol. 2008;15:2465–2471. doi: 10.1245/s10434-008-9992-0. [DOI] [PubMed] [Google Scholar]
- 14.Kapoor V, McCook BM, Torok FS. An introduction to PET-CT imaging. Radiographics. 2004;24:523–543. doi: 10.1148/rg.242025724. [DOI] [PubMed] [Google Scholar]
- 15.Kauhanen SP, Komar G, Seppanen MP, et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg. 2009;250:957–963. doi: 10.1097/SLA.0b013e3181b2fafa. [DOI] [PubMed] [Google Scholar]
- 16.Lytras D, Connor S, Bosonnet L, et al. Positron emission tomography does not add to computed tomography for the diagnosis and staging of pancreatic cancer. Dig Surg. 2005;22:55–61. doi: 10.1159/000085347. discussion 62. [DOI] [PubMed] [Google Scholar]
- 17.Bang S, Chung HW, Park SW, et al. The clinical usefulness of 18-fluorodeoxyglucose positron emission tomography in the differential diagnosis, staging, and response evaluation after concurrent chemoradiotherapy for pancreatic cancer. Journal of clinical gastroenterology. 2006;40:923–929. doi: 10.1097/01.mcg.0000225672.68852.05. [DOI] [PubMed] [Google Scholar]
- 18.Ruers TJ, Wiering B, van der Sijp JR, et al. Improved selection of patients for hepatic surgery of colorectal liver metastases with (18)F-FDG PET: a randomized study. Journal of Nuclear Medicine. 2009;50:1036–1041. doi: 10.2967/jnumed.109.063040. [DOI] [PubMed] [Google Scholar]
- 19.Thomson BN, Parks RW, Redhead DN, et al. Refining the role of laparoscopy and laparoscopic ultrasound in the staging of presumed pancreatic head and ampullary tumours. Br J Cancer. 2006;94:213–217. doi: 10.1038/sj.bjc.6602919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayo SC, Austin DF, Sheppard BC, et al. Evolving Preoperative Evaluation of Patients with Pancreatic Cancer: Does Laparoscopy Have a Role in the Current Era? Journal of the American College of Surgeons. 2009;208:87–95. doi: 10.1016/j.jamcollsurg.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Kadhim LA, Dholakia AS, Herman JM, et al. The role of 18F-fluorodeoxyglucose positron emission tomography in the management of patients with pancreatic adenocarcinoma. Journal of Radiation Oncology. 2013;2:341–352. doi: 10.1007/s13566-013-0130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez-del Castillo C, Warshaw AL. Laparoscopy for staging in pancreatic carcinoma. Surg Oncol 2 Suppl. 1993;1:25–29. doi: 10.1016/0960-7404(93)90055-4. [DOI] [PubMed] [Google Scholar]
- 23.del Castillo CF, Warshaw L. Peritoneal metastases in pancreatic carcinoma. Hepatogastroenterology. 1993;40:430–432. [PubMed] [Google Scholar]