Abstract
γδ T cells expressing Vδ2 may be instrumental in the control of malaria, because they inhibit the replication of blood-stage parasites in vitro and expand during acute malaria infection. However, Vδ2 T-cell frequencies and function are lower among children with heavy prior malaria exposure. It remains unclear whether malaria itself is driving this loss. Here we measure Vδ2 T-cell frequency, cytokine production, and degranulation longitudinally in Ugandan children enrolled in a malaria chemoprevention trial from 6 to 36 months of age. We observed a progressive attenuation of the Vδ2 response only among children incurring high rates of malaria. Unresponsive Vδ2 T cells were marked by expression of CD16, which was elevated in the setting of high malaria transmission. Moreover, chemoprevention during early childhood prevented the development of dysfunctional Vδ2 T cells. These observations provide insight into the role of Vδ2 T cells in the immune response to chronic malaria.
Keywords: γδ T cells, malaria, Plasmodium falciparum, CD16, immunologic tolerance
Despite decades of eradication efforts, malaria remains one of the leading causes of morbidity and mortality among young children worldwide [1]. Vaccine efforts are hampered by a poor understanding of the immunologic processes driving natural immunity, which only develops after years of consistent exposure. Natural immunity is not sterilizing, because individuals remain susceptible to parasitemia despite no longer suffering from symptomatic disease. Mounting evidence suggests that this “clinical” immunity is not simply due to an adaptive immune response that restricts parasite replication but rather depends in part on mechanisms of immunologic tolerance [2, 3].
γδ T cells expressing the Vγ9 and Vδ2 chains of the T-cell receptor have been implicated in the control of blood-stage infection [4, 5]. These effectors constitute 0.5%–5% of peripheral T cells in primates and recognize small nonpeptidic metabolites known as phosphoantigens, which arise as intermediates of the isoprenoid synthesis pathway occurring in the plasmodial apicoplast [6–8]. This recognition is T-cell receptor dependent but requires neither processing nor presentation by professional antigen-presenting cells. Instead, the ubiquitously expressed molecule butyrophilin 3A1 allows rapid binding and presentation of phosphoantigens by many cell types, including γδ T cells themselves [9, 10]. Vγ9Vδ2 T cells rapidly produce type I cytokines and proliferate in response to plasmodium antigens [11, 12], and they have been shown to inhibit the replication of blood-stage parasites in vitro by the release of cytotoxic granules containing granulysin [5], independent of CD4 activation [13]. Thus, Vγ9Vδ2 T cells can act as ready-made innate effectors, suggesting that the Vδ2 response may be most important during the first infections of infancy before the adaptive immune response to Plasmodium falciparum has developed. Indeed, in malaria-naive adults, experimental infection prompts a robust expansion of Vδ2 cells, with frequencies remaining elevated in the peripheral blood up to 2 months after treatment [14–17].
We recently demonstrated that heavy prior malaria exposure is strongly associated with decreased Vδ2 cell frequency and function among 4-year-old Ugandan children [18]. Low frequencies of P. falciparum–responsive Vδ2 T cells were associated with a reduced probability of symptoms during subsequent infection, suggesting that the loss and dysfunction of Vδ2 T cells may contribute to clinical tolerance to malaria. However, these data were primarily based on cross-sectional measurements of Vδ2 T cells, raising the question of whether repeated P. falciparum exposure causes Vδ2 decline or, instead, whether those with lower numbers of Vδ2 cells are simply more susceptible to malaria early in life. Furthermore, intriguing evidence suggests that there are 2 distinct subpopulations of Vδ2 T cells with unique antigen recognition pathways, phenotype and effector attributes [19–21], which can be distinguished based on their expression of CD16. Vδ2 T cells expressing CD16 are poorly responsive to phosphoantigen but express perforin and are capable of antibody-dependent cell-mediated cytotoxicity [19]. Expression of CD16 on Vδ2 T cells has not been examined in malaria infection.
Here we present a comprehensive longitudinal analysis of Vδ2 T-cell frequency, parasite-specific responsiveness, and CD16 expression in the peripheral blood of Ugandan children aged 6–36 months. We observed a longitudinal decline in both the frequency and P. falciparum-specific effector functions of Vδ2 cells, evident during early infancy and only in those children incurring the highest rates of malaria. CD16 identified Vδ2 T cells unresponsive to P. falciparum antigen stimulation, and the proportion of Vδ2 T cells expressing CD16 increased with age and in the setting of high malaria transmission. In addition, we found that malaria chemoprevention prevented dysfunction of Vδ2 T cells in young children. Together, these results suggest a causative link between repeated malaria episodes and the loss and dysfunction of Vδ2 T cells in the peripheral blood of heavily exposed children.
METHODS
Study Site and Procedures
Samples for this study were obtained from a randomized controlled trial of malaria chemoprevention in Tororo, Uganda, a district with holoendemic transmission and an annual entomologic inoculation rate of 310 infective bites per person-year [22]. Details of this trial have been described elsewhere [23]. In this report, we included data from children randomized to receive chemoprevention from 6 to 24 months of age with monthly sulfadoxine-pyrimethamine, which was found to have no efficacy for prevention of malaria (n = 49; Figures 1–3) , monthly dihydroartemisinin-piperaquine (DP), which had 58% protective efficacy for prevention of malaria (n = 85; Figure 4), or no chemoprevention (n = 88; Figure 4).
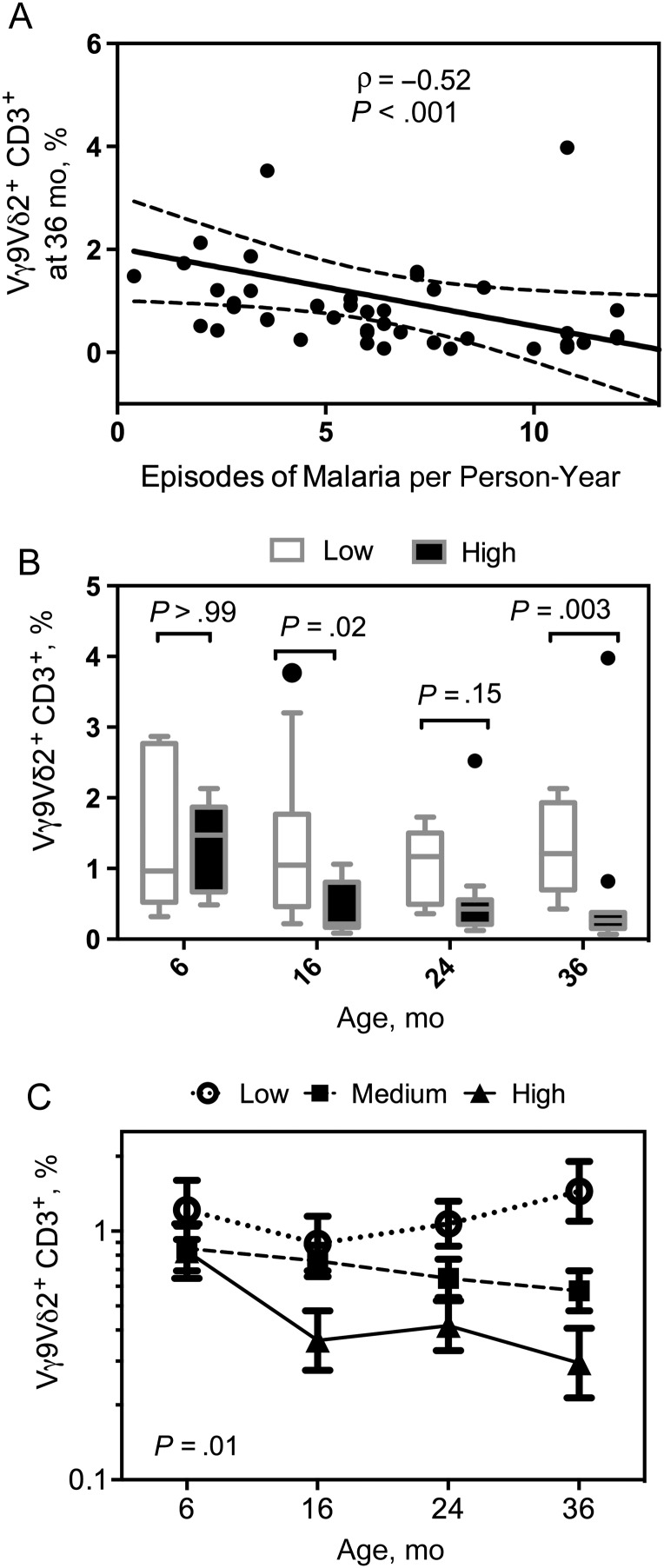
Figure 1.
Early loss of Vγ9Vδ2 T cells concurrent with heavy malaria exposure. Vγ9Vδ2 T-cell frequencies were measured at 6 (n = 26), 16 (n = 49), 24 (n = 42), and 36 (n = 45) months of age. A, Percentage of Vγ9Vδ2 T cells for children at age 36 months plotted against episodes of malaria per person-year. B, Children were divided into 3 categories based on total malaria incidence (low, <3 [n = 12]; medium, ≥3 and <8 [n = 25]; and high, ≥8 [n = 12] episodes per person-year). Shown are Vγ9Vδ2 T-cell frequencies in the lowest and highest groups of malaria exposure plotted by age (box plots with median and Tukey whiskers). C, Log-transformed Vγ9Vδ2 T-cell frequencies were analyzed for each exposure group by generalized estimate equations, accounting for repeated measures, age, and parasite status at the time of sampling. Shown are model-adjusted means with standard errors of the mean at 6, 16, 24, and 36 months of age.
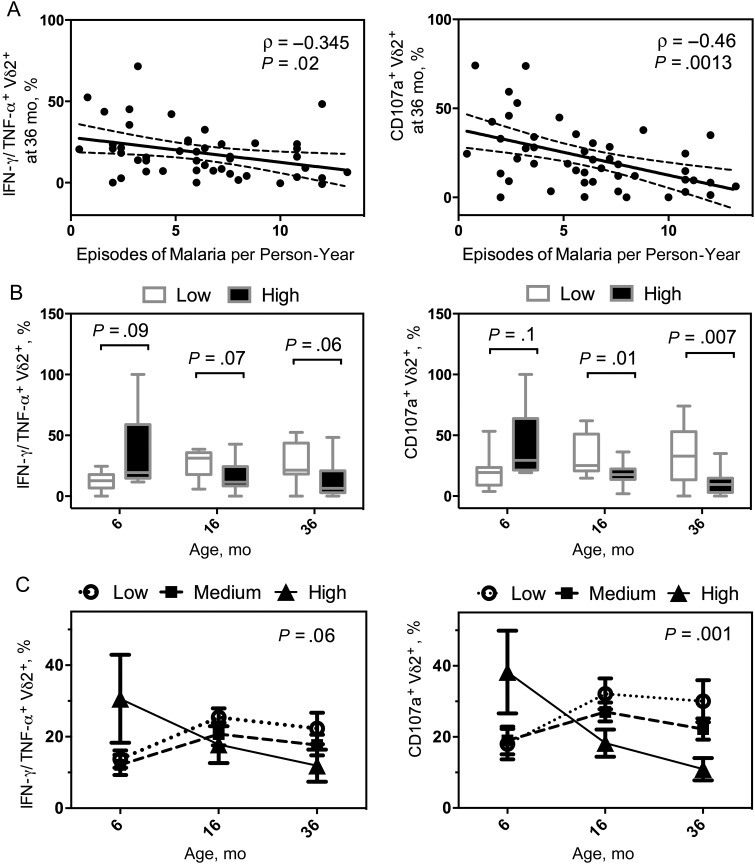
Figure 2.
Longitudinal decline in Vγ9Vδ2 T-cell function correlates with high incidence of malaria. Proportion of Vγ9Vδ2 T cells producing tumor necrosis factor (TNF) α and interferon (IFN) γ, or expressing CD107a, after Plasmodium falciparum antigen stimulation at 6 (n = 29), 16 (n = 49), and 36 (n = 47) months of age. A, Percentages of cytokine-producing Vγ9Vδ2 T cells and CD107a-expressing cells at 36 months plotted against total episodes of malaria per person-year. B, Children were divided into 3 categories based on total malaria incidence (low, <3 [n = 13]; medium, ≥3 and <8 [n = 25]; and high, ≥8 [n = 11] episodes per person-year). Cytokine-producing and CD107a-expressing Vγ9Vδ2 T-cell frequencies in the lowest and highest groups of malaria exposure plotted by age (box plots with median and Tukey whiskers). C, Proportion of cytokine-producing and CD107a-expressing Vγ9Vδ2 T-cell frequencies were analyzed for each exposure group by generalized estimate equations, accounting for repeated measures, age, and parasite status at the time of sampling. Shown are model-adjusted mean with standard errors of the mean at 6, 16, 24, and 36 months of age.
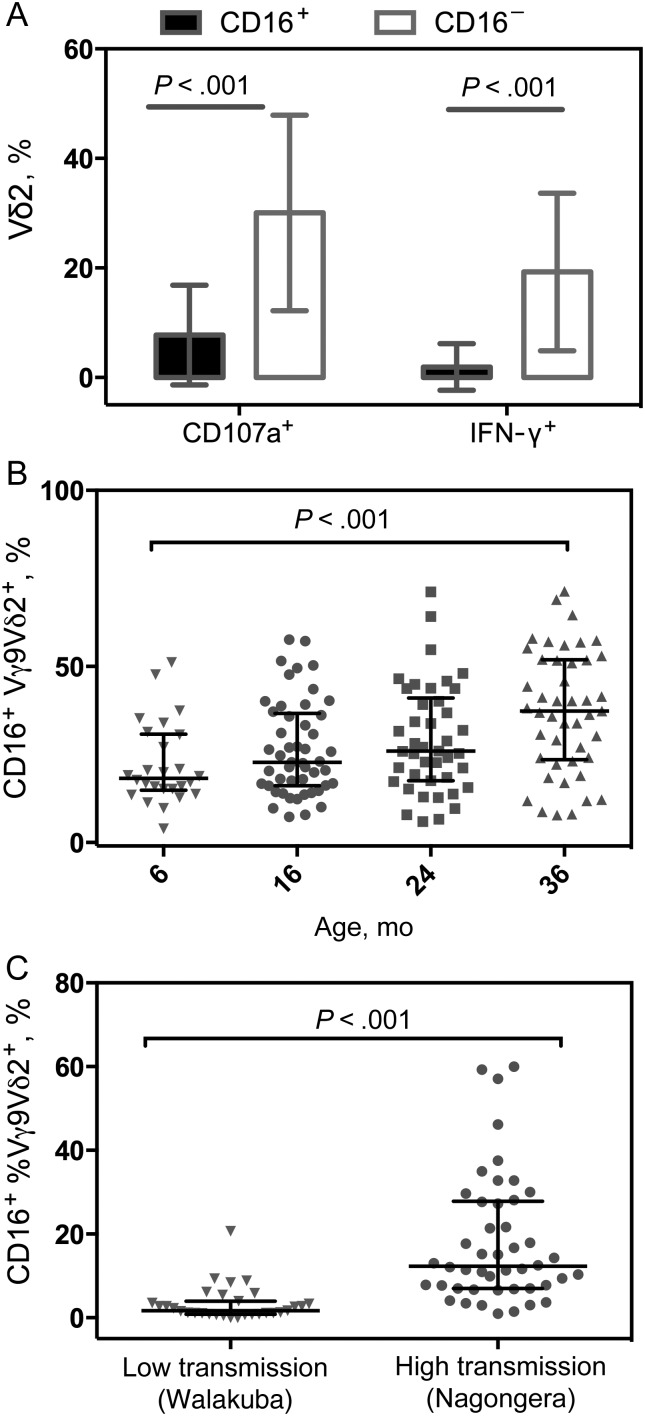
Figure 3.
CD16 expression identifies unresponsive Vγ9Vδ2 T cells and is increased in high-transmission settings. A, Percentage of Vγ9Vδ2 T cells expressing interferon (IFN) γ or CD107a after Plasmodium falciparum antigen stimulation at 36 months of age, grouped by expression of CD16 (n = 47). B, Percentages of Vγ9Vδ2 T cells expressing CD16 without P. falciparum antigen stimulation with increasing age. P values represent overall trend with increasing age using generalized estimating equations, adjusted for repeated measures, parasitemia at the time of the assay, and prior incidence. C, Percentage of Vγ9Vδ2 T cells expressing CD16 in children from a low-transmission setting (n = 31; age range, 1.54 to 10.78 years; mean, 6.26 years) and in children from a high-transmission setting (n = 46; age range, 1.84–12.18 years; mean, 6.66 years).
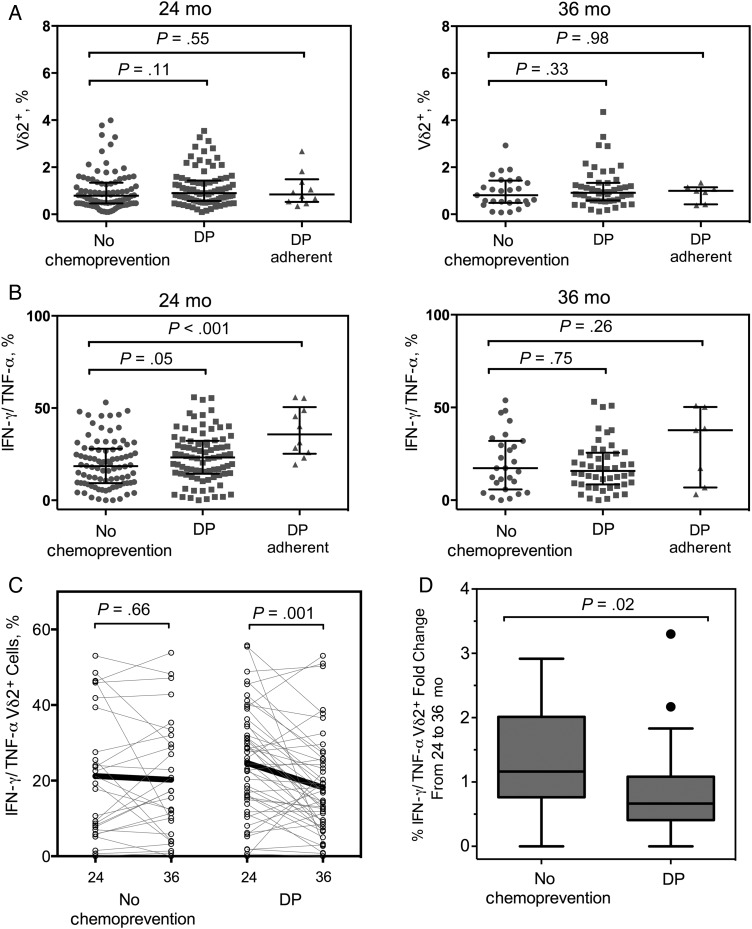
Figure 4.
Chemoprevention postpones the development of Vγ9Vδ2 T-cell dysfunction. Vγ9Vδ2 T-cell frequencies and cytokine secretion after Plasmodium falciparum antigen stimulation from children who did or did not receive monthly dihydroartemisinin-piperaquine (DP) from 6 to 24 months of age, measured by flow cytometry. A, Percentages of Vγ9Vδ2 T cells in the peripheral blood at age 24 months (no chemoprevention, n = 88; DP, n = 85; DP adherent, n = 10) and 36 months (no chemoprevention, n = 27; DP, n = 45; DP adherent, n = 10). B, Proportion of Vγ9Vδ2 T cells in the peripheral blood at age 24 and 36 months. C, Change in the proportion of cytokine-producing Vγ9Vδ2 T cells from 24 to 36 months of age, plotted by individual. D, Fold change of cytokine-producing Vγ9Vδ2 T cells between 24 and 36 months of age in children who did or did not receive chemoprevention. Abbreviations: IFN, interferon; TNF, tumor necrosis factor.
Children were followed up clinically for a year after the intervention ended. Among those randomized to DP, piperaquine levels in plasma were measured at 4–6 random time points during the intervention. Piperaquine was measured using liquid chromatography/tandem mass spectroscopy and demonstrated a lower limit of quantitation of 10 ng/mL, with a calibration range of 10–100 ng/mL [24]. Adherence scores were calculated based on pharmacokinetic models incorporating age, weight, and days since last piperaquine dosing [25]. Subjects were categorized into 2 groups of adherence based on calculated scores: low (0 to <2) and high (≥2).
Children who presented with a fever (tympanic temperature >38.0°C or history of fever in the previous 24 hours) had blood obtained by finger prick for a thick smear. If the smear was positive for P. falciparum parasites, malaria was diagnosed, and the patient was given artemisinin-based combination therapy. Incident episodes of malaria were defined as all febrile episodes accompanied by any parasitemia but not preceded by another treatment in the prior 14 days [1]. The incidence of malaria was calculated as the number of episodes per person-year at risk.
Ethical Approval
Written informed consent was obtained from the parent or guardian of all study participants. Study protocols were approved by the Uganda National Council of Science and Technology, the Makerere University School of Medicine Research and Ethics Committee, and the University of California, San Francisco Committee on Human Research.
Sample Processing
For sampling, 6–10 mL of blood was obtained in acid citrate dextrose tubes. Peripheral blood mononuclear cells (PBMCs) were isolated using density gradient centrifugation (Ficoll-Histopaque; GE Life Sciences) and cryopreserved in liquid nitrogen before analysis.
Malaria Antigens
P. falciparum blood-stage 3D7 parasites were grown by standard methods [26] and harvested at 5%–10% parasitemia. Red blood cells (RBCs) infected with mature asexual stages (iRBC) were purified magnetically and cryopreserved in glycerolyte before use. Uninfected RBCs (uRBCs) were used as controls. Parasites were regularly tested for mycoplasma.
Surface and Intracellular Cytokine Staining
Thawed PBMCs were stained for surface markers or rested overnight for intracellular cytokine staining. Rested cells were kept in 10% fetal bovine serum (Gibco) and counted before stimulation with uRBCs, iRBCs, medium, or phorbol miristate acetate/calcium ionophore at 0.5 × 106 cells/condition. An effector-target cell ratio of 1:2 was used with uRBCs and iRBCs [27]. Anti-CD28 and anti-CD49d were added for costimulation (0.5 µg/mL; BD Pharmingen). Monensin (10 µg/mL) and antibody to CD107a were added with iRBCs. Brefeldin A (10 µg/mL) was added at 6 hours. At 24 hours, cells were fixed and permeabilized according to standard protocols (Invitrogen/Caltag). Surface and/or intracellular staining was done according to standard protocols [28, 29], using the antibodies included in Supplementary Table 1.
Flow Cytometry Data Analysis
Flow cytometry profiles were gated on Aqua-negative, CD19−, CD14−, CD3+ lymphocytes. Approximately 300 000 events were collected. Prior phenotypic work revealed that >80% of peripheral blood Vδ2 T cells also express Vγ9; thus, interferon (IFN) γ, tumor necrosis factor (TNF) α, and CD107a expression was quantified using only Vδ2. Samples were analyzed on an LSR2 flow cytometer (Becton Dickinson) with FACSDiva software (version 6.0). Data were analyzed using FlowJo (Tree Star, version 9.7.6) and Pestle (version 1.7)/SPICE (version 5.3; M. Roederer, Vaccine Research Center, National Institute of Allergy and Infectious Diseases [NIAID]; http://exon.niaid.nih.gov/spice/) software [30]. Gating strategies are presented in Supplementary Figures 1 and 2.
Vδ2 CD16 Expression in High- Versus Low-Transmission Areas
Samples were obtained from children enrolled in a large malaria surveillance survey of 2 Ugandan districts: the suburban town of Walakuba, Jinja district, with an annual entomologic inoculation of 2.8, and the rural region of Nagongera, Tororo district, with an annual entomologic inoculation of 310 [31]. Additional details from this survey have been described elsewhere [22]. The sample included 31 children from Walakuba (age range, 1.54–10.78 years; mean, 6.26 years) and 46 from Nagongera (age range, 1.84–12.18 years; mean, 6.66 years). Before PBMC isolation, 25 µL of blood was removed and incubated with antibodies (described in Supplementary Table 1) for 15 minutes at room temperature. Then 450 µL of BD Pharm Lyse (Becton Dickinson) buffer was added, and cells were incubated for 15 minutes at room temperature. Data was collected on an Accuri C6 2-laser flow cytometer (Becton Dickinson).
Statistical Methods
All statistical analyses were performed using Prism 4.0 (GraphPad), Stata version 12, and/or SPICE v.5.3 (NIAID) software. Frequencies of malaria-specific cytokine-producing T cells are reported after background subtraction of the identically gated cell population from the same sample stimulated with uRBCs. Continuous variables were compared using Spearman correlation. Comparisons of Vδ2Vγ9 frequencies between time points were performed using the paired Wilcoxon matched-pairs signed rank test, and comparisons between malaria incidence or chemoprevention groups were performed using the Wilcoxon rank sum test.
XY scatterplots show best-fit linear regression lines with 95% confidence intervals. Grouped scatterplots show medians and interquartile range, and box plots show medians with Tukey whiskers. To compare trajectories of Vδ2Vγ9 T-cell measurements with age among strata of children with varying intercurrent malaria, we used generalized estimating equations with robust standard errors and adjustment for repeated measures and infection status at the time of sampling. In these multivariate models, nonnormal variables were log-transformed and analyzed for statistically significant interaction between malaria exposure strata and age. In all analyses, differences were considered significant at P < .05 (2 tailed).
RESULTS
Early Loss of Vγ9Vδ2 T Cells Among Children With Heavy Malaria Exposure
To determine whether repeated malaria exposure drives Vγ9Vδ2 T-cell loss or, instead, whether there is a higher propensity for symptomatic among children with lower Vγ9Vδ2 T-cell frequencies, we measured the percentage of CD3+ cells expressing Vδ2 and Vγ9 at 6, 16, 24, and 36 months of age among children randomized to chemoprevention with sulfadoxine-pyrimethamine, which showed no efficacy for preventing malaria [22]. These children had a mean (standard deviation [SD]) malaria incidence of 5.35 (3.1) episodes per person-year during chemoprevention, and 8.22 (3.97) episodes per person-year after chemoprevention. We observed a strong inverse relationship between Vγ9Vδ2 T-cell frequencies at 36 months and the episodes of malaria per person-year for each subject between 6 and 36 months (Figure 1A).
To explore whether longitudinal changes in the frequency of Vγ9Vδ2 T cells over time were related to differing amounts of intercurrent malaria, we stratified the subjects into 3 groups based on their incidence of malaria between 6 and 36 months (low, <3 [n = 12]; medium, ≥3 and <8 [n = 26]; high, ≥8 [n = 11] episodes per person-year). At 6 months, Vγ9Vδ2 T-cell frequencies were similar between children in the highest- and lowest-incidence strata. However, by 16 months these frequencies had diverged significantly, with lower frequencies observed among the highest-incidence children. This trend was maintained at 24 months and again significant at 36 months (Figure 1B). Repeated-measures analysis, using generalized estimating equations and adjusting for age, confirmed that changes in Vδ2 T-cell frequencies over time differed significantly between the high- and low-incidence groups (P = .01; Figure 1C). No relationship was observed between the other major circulating γδ T-cell subset (Vδ1+ cells) and malaria incidence (Supplementary Figure 3). Together, these data suggest that repeated malaria exposure in early childhood drives the loss of circulating Vγ9Vδ2 T cells.
Heavy Malaria Exposure and Progressive Dysfunction of Vδ2 T Cells
Recurrent malaria has been associated not only with reduced Vδ2 T-cell frequencies, but also with the impairment of key effector functions including proliferation and production of inflammatory cytokines IFN-γ and TNF [18]. To assess whether the functional characteristics of Vδ2 T cells decline with repeated malaria episodes within individual children, we stimulated PBMCs obtained at 6, 16, and 36 months of age with P. falciparum–infected RBCs and quantified cytokine production and degranulation (assessed by mobilization of CD107a). As with the frequencies of Vδ2 T cells, we found a significant inverse relationship between the proportion of functional Vδ2 T cells and the episodes of malaria per person-year subjects had sustained by 36 months (Figure 2A).
After stratifying subjects by malaria incidence using the groups defined above, we saw that percentages of IFN-γ/TNF-α+ and CD107a+ Vδ2 T cells were similar at 6 months but lower in the highest-incidence group at 16 and 36 months than in the lowest-incidence group (Figure 2B). Repeated-measures analysis using generalized estimating equations revealed declining percentages of IFN-γ/TNF-α+ and CD107a+ Vδ2 cells over time in the highest-incidence strata, in contrast to an increase in these percentages in the lower-incidence strata (P = .06 and P = .001, respectively; Figure 2C). These data indicate that the ability of Vδ2 T cells to secrete proinflammatory cytokines and degranulate in response to P. falciparum antigen declines within an individual in the setting of repeated malaria infections.
CD16 Expression on Vδ2Vγ9 T-cells in High Transmission Settings
Expression of the low-affinity Fc receptor CD16 (FCγRIII) has been shown to distinguish Vδ2 cells that are less responsive to phosphoantigens [19], and up-regulated expression of CD16 on Vδ2 T cells has been described among children heavily exposed to malaria [18]. To determine how expression of CD16 influences Vδ2 T-cell responsiveness to P. falciparum antigen and how expression changes with increasing age and intercurrent malaria, we measured surface expression of CD16 longitudinally in the context of the flow-based functional assays described above. We found that the percentage of Vδ2 T cells that responded to P. falciparum antigen by producing IFN-γ or degranulating was markedly lower among cells expressing CD16 (Figure 3A). Next we looked at changes in CD16 expression with increasing age and found that the percentage of Vγ9Vδ2 T cells expressing CD16 increased significantly between 6 and 36 months (Figure 3B).
To determine the influence of malaria exposure on CD16 expression, we stratified children by incidence and applied repeated-measures analysis as above. We found no differences between incidence categories in this cohort; however, we went on to compare the percentage of CD16 Vγ9Vδ2 T cells between children from an area of extremely high transmission intensity (Nagongera, Tororo District, Uganda) and children from an area where malaria transmission is >100-fold lower (Walakuba, Jinja District, Uganda) [31]. CD16 expression in children from Nagongera was dramatically higher than in those from Walakuba (Figure 3C), consistent with up-regulated expression of CD16 on Vδ2 cells in response to repeated malaria infections.
The Effect of Chemoprevention in Vδ2 Dysfunction
To determine whether prevention of blood-stage malaria could preserve Vδ2 T-cell frequency and function, we compared Vδ2 frequencies between children randomized to receive monthly DP, or no chemoprevention from 6 to 24 months of age. Children randomized to DP had 58% less malaria (mean [SD], 3.14 [2.71] episodes per person-year) during the intervention than those receiving no chemoprevention (mean, 5.42 [3.1] episodes per person-year) [23]. At 24 months we observed a higher proportion of Vδ2 T cells secreting IFN-γ and TNF-α in the DP group than in children receiving no chemoprevention (Figure 4B), although we did not observe a difference in Vδ2 frequencies (Figure 4A). When subjects in the DP arm were stratified into adherence groups based on pharmacokinetic modeling of serum piperaquine levels [24, 25], the significance of this relationship was strengthened (P < .001; Figure 4B).
We also examined Vδ2 T-cell frequency and function at age 36 months, 1 year after chemoprevention was stopped. The incidence of malaria was similar between groups after cessation of chemoprevention, with a mean (SD) of 7.52 (4.08) episodes per person-year for the DP group and 7.44 (4.08) episodes per person-year for the no-chemoprevention group [23]. At 36 months, we found no difference in the frequency or function of Vδ2 T cells between the 2 study arms (Figure 4A). However, the proportion of Vδ2 T cells secreting cytokine in response to P. falciparum antigen decreased between 24 and 36 months within the group formerly receiving DP, whereas there was no change in the untreated group, as shown by both a paired analysis (Figure 4C) and a comparison of the fold changes between time points for each treatment arm (Figure 4D). Together these data suggest that prevention of blood-stage P. falciparum infection preserves the functional responsiveness of Vδ2 T cells.
DISCUSSION
Prior work by our group suggests that a reduced frequency of Vδ2 T cells in the peripheral blood, along with limited cytokine production and reduced proliferative capacity, may contribute to clinical tolerance on subsequent infection [18]. In the current study, we have built on our previous work by providing strong evidence for a causal relationship between heavy malaria exposure and attenuation of the Vδ2 response. We show a longitudinal decline in Vδ2 cell frequency and cytokine production in heavily exposed children, contrasted with stable or increasing percentages of these cells in less-exposed children from the same cohort.
We identified CD16 expression as a marker for P. falciparum antigen unresponsive Vδ2 T cells, with increased percentages of these cells in high-transmission settings. Moreover, limiting exposure to blood-stage malaria by DP administration prevented the decline in Vδ2 cytokine production experienced by children who did not receive chemoprevention. These data argue against the possibility that children with fewer Vδ2 T cells are simply more susceptible to malaria and make it unlikely that other factors commonly associated with P. falciparum infection (ie, coinfections, socioeconomic and genetic factors) are the actual drivers of Vδ2 loss and dysfunction. Importantly, these observations suggest a possible mechanism by which the immune system is able to reduce immunopathology on reinfection with P. falciparum.
Although evidence suggests that γδ T cells play a beneficial role in restricting parasite growth, they may also play a detrimental role in driving inflammation that leads to clinical symptoms. IFN-γ [32] and TNF-α [33] produced by γδ T cells early during infection are associated with protection from clinical malaria, yet later work indicates that higher TNF-α production by γδ T cells on ex vivo stimulation is associated with severe malaria [34]. Thus, dampening an exuberant antimalarial response could serve to protect from symptomatic disease while simultaneously limiting one's ability to clear parasites and prevent reinfection. Because both severe malaria and asymptomatic parasitemia were very infrequent in the 6–36-month age range of this trial, we were unable to assess whether the loss of Vδ2 T cells affected these clinical outcomes.
Although we have limited our exploration of Vδ2 function to cytokine production and degranulation after P. falciparum antigen stimulation, other potential effector functions may be elicited by malaria infection. It is known that a subset of Vδ2 T cells express and can be activated through the low-affinity Fc receptor CD16, independent of the T-cell receptor [19]. Activation through CD16 by opsonized antigen has been shown to mediate antibody-dependent cell-mediated cytotoxicity [35], phagocytosis of E. coli [36], IFNγ release [20], and licensing for professional antigen presentation ([37, 38]). This prompts the question of whether Vδ2 T cells can be activated by opsonized parasite during P. falciparum infection to perform antiparasitic functions. Notably, in our study, CD16 was negatively associated with P. falciparum antigen-induced cytokine production, and the proportion of Vδ2 cells expressing CD16 was higher in children from an area of high malaria transmission than in children from a low-transmission area. This suggests that an increased proportion of Vδ2 T cells may be preferentially stimulated through CD16 as individuals age or as they accumulate a more comprehensive humoral response to P. falciparum.
Participants in this study lived in a region of extremely high malaria transmission. Even children in the lowest malaria incidence tertile experienced a median of 2.3 malaria episodes per year, which may have limited our ability to resolve the influence of malaria exposure on CD16 expression in our longitudinal cohort. Similarly, the finding that administration of DP postponed the development of Vδ2 dysfunction but did not affect the frequency of Vδ2 T cells may indicate that we were underpowered in the extremes of low incidence within this group. Alternatively, different mechanisms might be responsible for Vδ2 loss and for dysfunction. An additional limitation of this study was the restriction of sampling to the peripheral blood. Because of this, we cannot exclude the possibility that functional Vδ2 T cells instead relocate to the spleen, liver, or other pertinent tissue site in response to repeated malaria infections.
Our data strongly indicate that there is a causal link between malaria exposure and the loss of functional Vδ2 T cells from the peripheral blood. This adds to growing body of work indicating that frequent malaria infection has a profound impact on the cellular immune response. Together, these findings stress the importance of evaluating correlates of immune protection from malaria in the context of infection history, because Vδ2-mediated proinflammatory responses may indicate limited prior malaria exposure, rather than actual protection from infection. These are important considerations, both for future field-based studies of malaria immunology and for vaccine design.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We are grateful to all the parents and guardians for kindly giving their consent, and to the study participants for their cooperation. We thank all the members of the study team for their tireless effort and excellent work.
Financial support. Support for this work was provided by National Institute of Child Health and Human Development (grant PO1 HD059454-03), National Institute of Allergy and Infectious Diseases (grants R01AI093615 and K24AI113002 to M. E. F. and K23 AI100949 to P. J.), the Burroughs Wellcome Fund/American Society of Tropical Medicine and Hygiene (P. J.), and the Australian government National Health and Medical Research Council (early-career fellowship to M. J. B.). The Burnet Institute is supported by the National Health and Medical Research Council Infrastructure for Research Institutes Support Scheme and Victorian State Government Operational Infrastructure Support.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 2005; 434:214–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Portugal S, Moebius J, Skinner J et al. Exposure-dependent control of malaria-induced inflammation in children. PLoS Pathog 2014; 10:e1004079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crompton PD, Moebius J, Portugal S et al. Malaria immunity in man and mosquito: insights into unsolved mysteries of a deadly infectious disease*. Annu Rev Immunol 2014; 32:157–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elloso MM, van der Heyde HC, vande Waa JA, Manning DD, Weidanz WP. Inhibition of Plasmodium falciparum in vitro by human γδ T cells. J Immunol 1994; 153:1187–94. [PubMed] [Google Scholar]
- 5.Costa G, Loizon S, Guenot M et al. Control of Plasmodium falciparum erythrocytic cycle: T cells target the red blood cell-invasive merozoites. Blood 2011; 118:6952–62. [DOI] [PubMed] [Google Scholar]
- 6.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev 2007; 215:59–76. [DOI] [PubMed] [Google Scholar]
- 7.Eberl M, Hintz M, Reichenberg A, Kollas AK, Wiesner J, Jomaa H. Microbial isoprenoid biosynthesis and human γδ T cell activation. FEBS Lett 2003; 544:4–10. [DOI] [PubMed] [Google Scholar]
- 8.Behr C, Poupot R, Peyrat MA et al. Plasmodium falciparum stimuli for human gammadelta T cells are related to phosphorylated antigens of mycobacteria. Infect Immun 1996; 64:2892–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vavassori S, Kumar A, Wan GS et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nat Immunol 2013; 14:908–16. [DOI] [PubMed] [Google Scholar]
- 10.Guenot M, Loizon S, Howard J et al. Phosphoantigen burst upon plasmodium falciparum schizont rupture can distantly activate Vγ9-Vδ2 T-cells. Infect Immun 2015; 83:3816–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behr C, Dubois P. Preferential expansion of Vγ9 Vδ2 T cells following stimulation of peripheral blood lymphocytes with extracts of Plasmodium falciparum. Int Immunol 1992; 4:361–6. [DOI] [PubMed] [Google Scholar]
- 12.Goodier MR, Lundqvist C, Hammarström ML, Troye-Blomberg M, Langhorne J. Cytokine profiles for human Vγ9+ T cells stimulated by Plasmodium falciparum. Parasite Immunol 1995; 17:413–23. [DOI] [PubMed] [Google Scholar]
- 13.Horowitz A, Newman KC, Evans JH, Korbel DS, Davis DM, Riley EM. Cross-talk between T cells and NK cells generates rapid effector responses to Plasmodium falciparum-infected erythrocytes. J Immunol 2010; 184:6043–52. [DOI] [PubMed] [Google Scholar]
- 14.Martini F, Paglia MG, Montesano C et al. Vγ9Vδ2 T-cell anergy and complementarity-determining region 3-specific depletion during paroxysm of nonendemic malaria infection. Infect Immun 2003; 71:2945–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roussilhon C, Agrapart M, Guglielmi P, Bensussan A, Brasseur P, Ballet JJ. Human TcRγδ+ lymphocyte response on primary exposure to Plasmodium falciparum. Clin Exp Immunol 1994; 95:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz E, Shapiro R, Shina S, Bank I. Delayed expansion of Vδ2+ and Vδ1+ γδ T cells after acute Plasmodium falciparum and Plasmodium vivax malaria. J Allergy Clin Immunol 1996; 97:1387–92. [DOI] [PubMed] [Google Scholar]
- 17.Teirlinck AC, McCall MBB, Roestenberg M et al. Longevity and composition of cellular immune responses following experimental Plasmodium falciparum malaria infection in humans. PLoS Pathog 2011; 7:e1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jagannathan P, Kim CC, Greenhouse B et al. Loss and dysfunction of Vδ2+ γδ T cells are associated with clinical tolerance to malaria. Sci Transl Med 2014; 6:251ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angelini DF, Borsellino G, Poupot M et al. FcγRIII discriminates between 2 subsets of Vγ9Vδ2 effector cells with different responses and activation pathways. Blood 2004; 104:1801–7. [DOI] [PubMed] [Google Scholar]
- 20.Couzi L, Pitard V, Sicard X et al. Antibody-dependent anti-cytomegalovirus activity of human γδ T cells expressing CD16 (FcγRIIIa). Blood 2012; 119:1418–27. [DOI] [PubMed] [Google Scholar]
- 21.He X, Liang H, Hong K et al. The potential role of CD16+ Vγ2Vδ2 T cell-mediated antibody-dependent cell-mediated cytotoxicity in control of HIV type 1 disease. AIDS Res Hum Retroviruses 2013; 29:1562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamya MR, Arinaitwe E, Wanzira H et al. Malaria transmission, infection, and disease at three sites with varied transmission intensity in Uganda: implications for Malaria control. Am J Trop Med Hyg. Am J Trop Hyg 2015; 92:903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bigira V, Kapisi J, Clark TD et al. Protective efficacy and safety of three antimalarial regimens for the prevention of malaria in young Ugandan children: a randomized controlled trial. PLoS Med 2014; 11:e1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kjellin LL, Dorsey G, Rosenthal PJ, Aweeka F, Huang L. Determination of the antimalarial drug piperaquine in small volume pediatric plasma samples by LC-MS/MS. Bioanalysis 2014; 6:3081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sundell K, Jagannathan P, Huang L et al. Variable piperaquine exposure significantly impacts protective efficacy of monthly dihydroartemisinin-piperaquine for the prevention of malaria in Ugandan children. Malar J 2015; 14:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trager W, Jensen JB. Human malaria parasites in continuous culture. 1976 J Parasitol 2005; 91:484–6. [DOI] [PubMed] [Google Scholar]
- 27.Boyle MJ, Jagannathan P, Bowen K et al. Effector phenotype of Plasmodium falciparum-specific CD4+ T cells is influenced by both age and transmission intensity in naturally exposed populations. J Infect Dis 2015; 212:416–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maecker HT, Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry 2006; 69:1037–42. [DOI] [PubMed] [Google Scholar]
- 29.Lamoreaux L, Roederer M, Koup R. Intracellular cytokine optimization and standard operating procedure. Nat Protoc 2006; 1:1507–16. [DOI] [PubMed] [Google Scholar]
- 30.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry 2011; 79:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kilama M, Smith DL, Hutchinson R et al. Estimating the annual entomological inoculation rate for Plasmodium falciparum transmitted by Anopheles gambiae s.l. using three sampling methods in three sites in Uganda. Malar J 2014; 13:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D'Ombrain MC, Robinson LJ, Stanisic DI et al. Association of early interferon-γ production with immunity to clinical malaria: a longitudinal study among Papua New Guinean children. Clin Infect Dis 2008; 47:1380–7. [DOI] [PubMed] [Google Scholar]
- 33.Robinson LJ, D'Ombrain MC, Stanisic DI et al. Cellular tumor necrosis factor, gamma interferon, and interleukin-6 responses as correlates of immunity and risk of clinical Plasmodium falciparum malaria in children from Papua New Guinea. Infect Immun 2009; 77:3033–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanisic DI, Cutts J, Eriksson E et al. γδ T cells and CD14+ monocytes are predominant cellular sources of cytokines and chemokines associated with severe malaria. J Infect Dis 2014; 210:295–305. [DOI] [PubMed] [Google Scholar]
- 35.Gertner-Dardenne J, Bonnafous C, Bezombes C et al. Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood 2009; 113:4875–84. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y, Wu W, Wong WM et al. Human γδ T cells: a lymphoid lineage cell capable of professional phagocytosis. J Immunol 2009; 183:5622–9. [DOI] [PubMed] [Google Scholar]
- 37.Himoudi N, Morgenstern DA, Yan M et al. Human γδ T lymphocytes are licensed for professional antigen presentation by interaction with opsonized target cells. J Immunol 2012; 188:1708–16. [DOI] [PubMed] [Google Scholar]
- 38.Brandes M. Professional antigen-presentation function by human γδ T cells. Science 2005; 309:264–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.