Abstract
Background. Human immunodeficiency virus (HIV) infection is associated with increased risk of thromboembolic and cardiovascular comorbid conditions. Although systemic inflammation is linked to cardiovascular risk, direct evidence of vascular inflammation and endothelial dysfunction is lacking.
Methods. We examined by immunofluorescence microscopy thoracic aortas from 16 simian immunodeficiency virus (SIV)– or simian-human immunodeficiency virus (SHIV)–infected and 16 uninfected rhesus macaques.
Results. Focal endothelial proliferation and subendothelial inflammatory cells were found in sections of all infected animals, compared with minimal changes in sections from the 16 uninfected controls. In the infected animals, we detected increased endothelial levels of bacterial 16s ribosomal DNA as well as increased subendothelial accumulation of CD68+ monocytes/macrophages (P < .001) and CD8+ T lymphocytes (P < .001). Endothelial dysfunction was manifested by decreased levels of endothelial nitric oxide synthase (P < .005) and Krüppel-like factor 2 (KLF2) (P < .005). KLF2 expression was decreased in primary human aortic endothelial cells exposed to bacterial lipopolysaccharide or to oxidized low-density lipoprotein in vitro, and this could be prevented by simvastatin.
Conclusions. SIV and SHIV infection lead to endothelial inflammation, dysfunction, and decreased KLF2 expression reflecting early atherosclerotic changes. Translocated bacterial components and lipid oxidation products may induce endothelial dysfunction in HIV infection that could be prevented by statin treatment.
Keywords: SIV, SHIV, endothelial dysfunction, eNOS, KLF2, immunohistochemistry, simvastatin, oxLDL, LPS
Endothelium is a highly reactive surface, responding to microenvironmental stress–inducing factors resulting from turbulence, tissue injury, and inflammation. A bidirectional relationship between coagulation pathways and vascular inflammation modulating endothelial function is also recognized [1]. Despite dramatic improvement in survival in the antiretroviral therapy (ART) era, there remains an increased risk of thromboembolic and cardiovascular comorbid conditions in ART-treated persons with human immunodeficiency virus (HIV) infection [2, 3]. The core mechanism(s) that contribute to this increased risk in HIV disease have not been fully elucidated but may be related to chronic immune activation [4, 5]. Moreover, although systemic indices of inflammation and coagulation have been linked to cardiovascular risk in HIV infection [6] and positron emission tomography-computed tomography (PET/CT) scan has demonstrated metabolic evidence of aortic inflammation in treated HIV infection [7], a detailed histopathologic characterization of vascular disease in HIV infection is lacking.
In the current study, we examined directly descending thoracic aorta specimens collected at necropsy from uninfected rhesus macaques (RMs) and RMs infected with simian immunodeficiency virus (SIV) or chimeric simian/human immunodeficiency virus (SHIV) as models of HIV infection in humans [8]. We hypothesized that expression of the key markers of endothelial function, cell adhesion, and the anti-inflammatory transcriptional factor Krüppel-like factor 2 (KLF2) would be altered in the endothelium of SIV/SHIV-infected RMs in a pattern reflecting vascular dysfunction and predisposing to cardiovascular risk. This study provides, for the first time, direct evidence that SIV/SHIV-infected RMs have subendothelial infiltration of inflammatory cells with significant down-regulation of endothelial nitric oxide (NO) synthase (eNOS) and KLF2 reflecting endothelial dysfunction and early atherosclerosis. In addition, because oxidized lipids and bacterial translocation have been implicated as drivers of inflammation and coagulation in HIV and SIV infection [9–12], we found elevated levels of bacterial 16s ribosomal DNA (rDNA) in the endothelium of SIV/SHIV-infected RMs and found that the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor simvastatin could protect human aortic endothelial cells (HAECs) from the KLF2 down-regulation induced by bacterial products and oxidized low-density lipoprotein (oxLDL).
MATERIALS AND METHODS
Animals and Infection
After rectal exposure to SIVmac239smr (SIVmac239 with a mutation in Nef (n = 10), SHIVSF162P3 (n = 4), or SHIV2873Nip (n = 2) [13–15], animals developed progressive infection (detailed in Supplementary Figure 1). Collections of arterial tissues at necropsy in the infected animals were performed 4 weeks to 16 months after infection. Uncontrolled diarrhea occurred in 3 of 16 infected animals, who were killed at earlier time points (2 weeks, 2 weeks, and 4 months). Nine of the 16 infected animals were male. We also obtained aortic tissue samples from 16 uninfected female RMs. Clinical information on these animals is provided in Supplementary Figure 1.
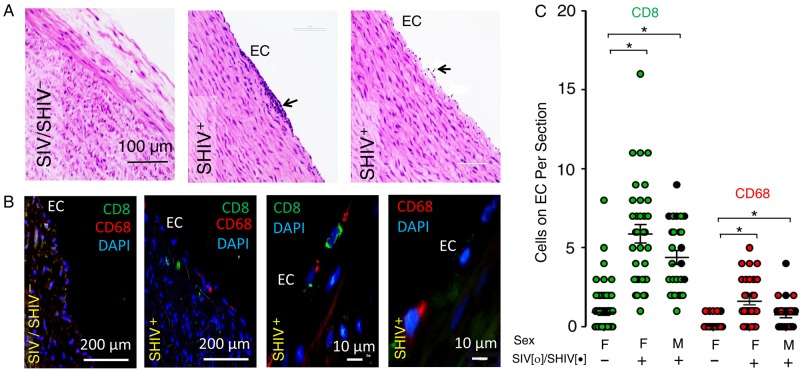
Figure 1.
Simian immunodeficiency virus (SIV) or simian-human immunodeficiency virus (SHIV) infection in rhesus macaques (RMs) is associated with focal endothelial proliferation and subendothelial migration of inflammatory cells. Sections of formaldehyde-fixed paraffin-embedded descending thoracic aorta from uninfected and SIV/SHIV-infected were processed for hematoxylin-eosin (HE) histochemical, and immunofluorescence staining using anti-CD68, anti-CD8, and DAPI. A, B, Representative data obtained by microscopic examination (×20, ×40, and ×100 objectives) of aortic endothelium from 16 infected and 16 uninfected animals are shown, indicating endothelial adhesion/infiltration of immune cells (arrow) in the aorta of an infected animal (A; HE staining). B, CD8+ and CD68+ cells identified by immunofluorescence staining were detectable more frequently on or below the endothelium of SIV/SHIV-infected animals. C, Summary data were generated by counting the numbers of CD8+ (green) and CD68+ (red) cells adhered to the aortic endothelium of SIV-infected (open circles), SHIV-infected (black circles), and uninfected male (M) and female (F) animals. Five aortic sections were examined from each of 16 infected and 16 uninfected control animals. *P < .001; 2-tailed nonparametric Mann–Whitney U test. Abbreviations: DAPI, 4',6-diamidino-2-phenylindole; EC, endothelial cell.
Viral Quantification
Plasma SIV/SHIV levels were quantified by specific real-time polymerase chain reaction (PCR) for SIV and SHIV RNA, as described elsewhere [16].
Tissue Sampling
At necropsy, portions of the descending thoracic aortas just distal to the aortic arch were collected and were either fixed in 10% buffered formaldehyde for paraffin embedding or flash frozen in dry ice at −80°C.
Antibodies
The following antibodies and peptides were used for immunohistochemical and immunofluorescence studies: rabbit polyclonal anti-eNOS (phospho-S1177, ab75639: AbCam), rabbit polyclonal anti-CD8 (ab4055; AbCam), mouse monoclonal anti-CD68 (KP1, ab955; AbCam), rabbit polyclonal anti-KLF2 (LS-B4570; LifeSpan BioSciences), antibody raised against peptide spanning amino acids 216–265 of human KLF2 (Q9Y5W3; National Center for Biotechnology Information reference sequence NP_057354.1), and the respective KLF2 peptide (LS-E115225; LifeSpan BioSciences).
HAEC Culture Experiments
The HAECs (Clonetics) were purchased from Lonza and cultured in glass slide chambers (Millicell EZ; Millipore) with EGM-2 medium (Lonza), according to the manufacturer's instructions. All experiments were performed using HAECs between the 10th and 12th passages. HAECs were treated with Escherichia coli lipopolysaccharide (LPS; L3024; Sigma Aldrich) at a concentration of 100 ng/mL for 1, 6, and 24 hours or with oxLDL (Intracell) at 100 µg/µL for 1, 6, and 24 hours. In one experimental arm, HAECs were also treated with 10 µmol/L Simvastatin (Sigma Aldrich). At the end of treatment, HAECs were immunostained and examined with fluorescence microscopy.
PCR Amplification of Bacterial 16S rDNA in Endothelium
With maximum precautions taken to avoid contamination, RM endothelium was scraped and DNA was prepared by means of alkaline digestion and phenol-chloroform-isopropanol extraction, as described elsewhere [17]. A 20-µL amplification reaction consisted of 10 µL of Power SYBR Green PCR Master mix (2×) (Invitrogen); 2 µL of 5 µmol/L forward and 2 µL of 5 µmol/L reverse primers, 0008F (5′-AGA GTT TGA TCC TGG CTC AG-3′) and 0532R (5′-TAC CGC GGC TGC TGG CAC-3′; Invitrogen) [18]; and 5 µL containing 1 ng of the endothelial DNA extract. PCR amplification of the first 500 base pairs of the 16S ribosomal RNA gene was performed using the StepOne Plus Real Time PCR System (Applied Biosystems) to determine both directional sequences. E. coli (DH5α) DNA in serial dilutions served as a positive control, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control. The reaction conditions for amplification of DNA were 95°C for 5 minutes, followed by 94°C for 1 minute, 1 minute at 48°C, and 1 minute at 72°C for 40 cycles. The DNA was amplified in triplicate, and amplification products were loaded onto a 1% agarose gel. The DNA bands in the PCR product were resolved and documented using BioRad electrophoresis and gel imaging systems. Mean values of the appropriate band densities were calculated from the gel images using ImageJ (version 2.0.0-rc-23/1.49 m) software.
Histology and Immunostaining
For immunohistochemical and immunofluorescence staining, deparaffinized sections of aorta were rehydrated, fixed in 4% paraformaldehyde, and processed at 90°C for 20 minutes in Tris- ethylenediaminetetraacetic acid buffer (pH 9.0), followed by blocking with 2% bovine serum albumin in Tris-buffered saline plus Triton X-100 0.025% for 1 hour at room temperature. The slides were then incubated overnight at 4°C with primary antibody in blocking buffer, at the manufacturer's recommended concentrations. Samples were washed and exposed to fluorescein isothiocyanate and/or rhodamine red–conjugated secondary antibodies (Jackson ImmunoResearch Laboratories). Slides were then hard mounted in Vectashild with DAPI (4′,6-diamidino-2-phenylindole; Vector Laboratories) and examined using an automated epifluorescence microscope (EVOSFL; Life Technologies).
Microscopy and Image Analysis
Sections of RM aorta were imaged using epifluorescence microscopy (EVOSFL; Life Technologies) using ×20, ×40, and ×100 oil immersion objectives. The public domain software (ImageJ, version 2.0.0-rc-23/1.49 m; http://imagej.nih.gov/ij/) was used to analyze digital images of aortic endothelial cells. Briefly, images of each fluorochrome channel were converted to 8-bit monochrome images, and the background value was subtracted to 0. Each DAPI-stained nucleus was precisely selected by the “oval” selection tool, and 100 such nuclei were selected per image. The fluorescence intensity value was measured for nuclear DAPI and for the respective images containing target signals using the same region of interest (KLF2, eNOS). We determined the target to nuclear fluorescence ratio (Fc/n) according to the formula Fc/n = (Fn − Fb)/(Fc −Fb), where Fb is background autofluorescence [19]. To perform the data processing and statistical analyses, we used GraphPad Prism (v6.02), and Microsoft Excel software (Microsoft Office Professional Plus 2013).
Statistical Analysis
We used the 2-tailed nonparametric Mann–Whitney U test to compare the independent group frequencies of subendothelial inflammatory cells, and fluorescence intensity ratio values. (GraphPad Prism software; version 6.02). Differences were considered significant at P < .05.
RESULTS
Association Between SIV/SHIV Infection in RMs and Focal Endothelial Proliferation and Subendothelial Migration of Inflammatory Cells
The histopathology of vascular lesions in SIV- and SHIV-infected RMs is currently undefined. To bridge this gap, we first systematically screened multiple hematoxylin-eosin–stained sections of thoracic aortas collected at necropsy from 16 SIV- or SHIV-infected RMs and 16 uninfected RMs to ascertain whether SIV/SHIV infection is associated with evidence of vascular inflammation that is suspected in HIV infection [7, 20, 21]. Using light microscopy, we detected foci of endothelial proliferation and infiltrating leukocytes in aortic sections obtained from all 16 infected animals, compared with nearly none in the 16 uninfected controls (Figure 1A).
To characterize these infiltrating inflammatory cells, we analyzed serial sections with antibodies to markers for macrophages (CD68+), and CD8+ T cells. Representative immunofluorescence images demonstrate subendothelial infiltration of CD68+ (macrophages) and CD8+ T cells in SIV/SHIV-infected RMs (Figure 1B). We observed no cellular infiltration and only occasional adhesion of CD8+ and CD68+ cells in the aorta sections from uninfected control animals (Figure 1B). Summary data (Figure 1C) demonstrate significantly greater endothelial adhesion and subendothelial infiltrations of CD8+ and CD68+ cells in the SIV/SHIV-infected animals, compared with the uninfected RMs, irrespective of sex and the strain of the infecting virus.
SIV/SHIV Infection in RMs and Reduced Vascular Endothelial eNOS Expression Reflective of Endothelial Dysfunction
Reduced expression of eNOS leading to diminished bioavailability of vasodilating NO is a hallmark of endothelial dysfunction. Endothelial dysfunction has been imputed in both treated and untreated HIV infection by means of indirect methods [7, 22]. To determine directly whether SIV/SHIV infection is associated with endothelial dysfunction, we immunostained sections of paraffin-embedded thoracic aorta specimens collected from 16 SIV/SHIV-infected and 16 uninfected control RMs, using phospho-S1177 eNOS antibodies that detect an activated form of the enzyme that catalyzes NO production [23].
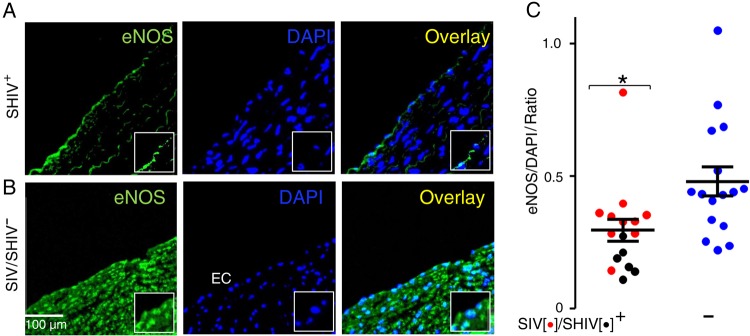
We found reduced S1177 phosphorylated eNOS in the endothelium and subendothelium of the SIV/SHIV-infected aortas (Figure 2A), compared with levels in uninfected control RMs (Figure 2B). We then quantified and analyzed the fluorescence intensity data from 100 endothelial cells from each of 16 infected and 16 uninfected animals. Results were expressed as the mean of the ratio of eNOS to DAPI nuclear fluorescence intensity values for each animal. Vascular staining for eNOS was significantly lower in the SIV/SHIV-infected animals than in the uninfected controls (Figure 2C; P < .005).
Figure 2.
Simian immunodeficiency virus (SIV) and simian-human immunodeficiency virus (SHIV) infection in rhesus macaques (RMs) leads to reduced endothelial nitric oxide synthase (eNOS) expression, reflective of endothelial dysfunction. A, B, Formaldehyde-fixed paraffin-embedded sections of descending thoracic aortas from SIV/SHIV-infected (A) and SIV/SHIV-uninfected (B) RMs were processed for immunofluorescence staining using rabbit polyclonal anti-eNOS (anti-rabbit fluorescein isothiocyanate) antibody and DAPI and analyzed using epifluorescence microscopy (EVOSFL, Life Technologies) with a ×40 objective (inset, ×100). EC, . C, Summary data, expressed as ratio of eNOS to nuclear DAPI fluorescence. In each aortic specimen, 100 endothelial cells were examined, and the mean ratio was summarized for each animal. Data shown represent means of these means in each group (see “Methods” section for details). Aortic specimens were examined from 16 SIV-infected (red) or SHIV-infected (black) and 16 uninfected (blue) animals. Data were analyzed using 2-tailed Mann–Whitney tests; *P < .005. Abbreviations: DAPI, 4',6-diamidino-2-phenylindole; EC, endothelial cell.
Diminished Endothelial KLF2 Levels in SIV and SHIV Infection
KLF2 is a key determinant of the anti-inflammatory and antiatherogenic vascular environment [24–26] and a potent inducer of eNOS [27]. KLF2 blocks expression of the procoagulant tissue factor [28] and also inhibits thrombin-induced activation of endothelial cells by decreasing expression of the thrombin-activated protease-activated receptor type 1 [29]. To determine whether the reduced eNOS expression in SIV/SHIV-infected RMs could be linked to altered expression of endothelial KLF2, we examined nuclear KLF2 levels by immunofluorescence microscopy in these aortic sections, normalizing the digitized nuclear KLF2 fluorescence to nuclear DNA (as measured by DAPI fluorescence).
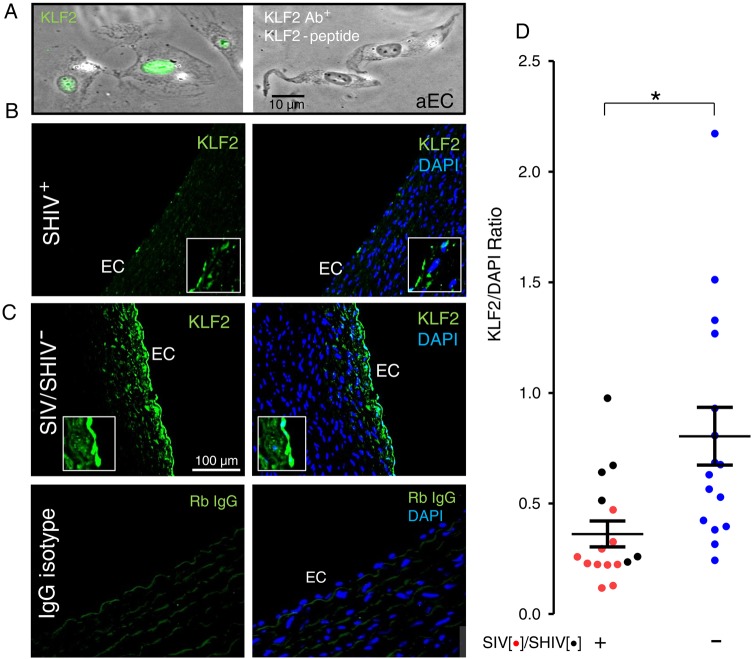
We observed reduced KLF2 expression (Figure 3B) in the SIV/SHIV-infected aortic endothelium compared with that in the uninfected controls (Figure 3C), and when we compared digital image data from 100 endothelial cells from each specimen (Figure 3D), the difference in KLF2 expression between the groups was significant (P < .005). Thus, the vascular environment in SIV and SHIV infection is characterized by a diminished expression of the anti-inflammatory and antiatherogenic transcriptional regulator KLF2.
Figure 3.
Endothelial Krüppel-like factor 2 (KLF2) expression is reduced in the rhesus macaque (RM) aorta infected with simian immunodeficiency virus (SIV) or simian-human immunodeficiency virus (SHIV). A, Anti-KLF2 antibody staining (Ab+) of human primary endothelial nuclei was blocked by incubating with target KLF2 peptide. B, C, Paraffin sections of descending thoracic aortas of SIV/SHIV infected (B), or uninfected (C) RMs were stained with rabbit polyclonal anti-KLF2 (B, C,upper) or rabbit (Rb) immunoglobulin (IgG) control (C, lower) followed by anti-rabbit fluorescein isothyocyanate-conjugated antibody and DAPI and examined using epifluorescence microscopy (EVOSFL; Life Technologies) with a ×40 objective (inset, ×100). D, Summary data showing normalized ratios of nuclear KLF2 (green) to nuclear DAPI (blue) fluorescence from all animals. In each aortic specimen, 100 endothelial cells were examined, and the mean ratio was summarized for each SIV-infected (red), SHIV-infected (black), and uninfected (blue) animal. Data were analyzed using 2-tailed Mann–Whitney tests; *P < .005. Abbreviations: DAPI, 4',6-diamidino-2-phenylindole; EC, endothelial cell.
Detection of Bacterial DNA in SIV/SHIV-infected RM Aortic Endothelium and Effect of Statin in Protecting Human Aortic Endothelial Cells From LPS and oxLDL-Induced Down-modulation of KLF2 Expression
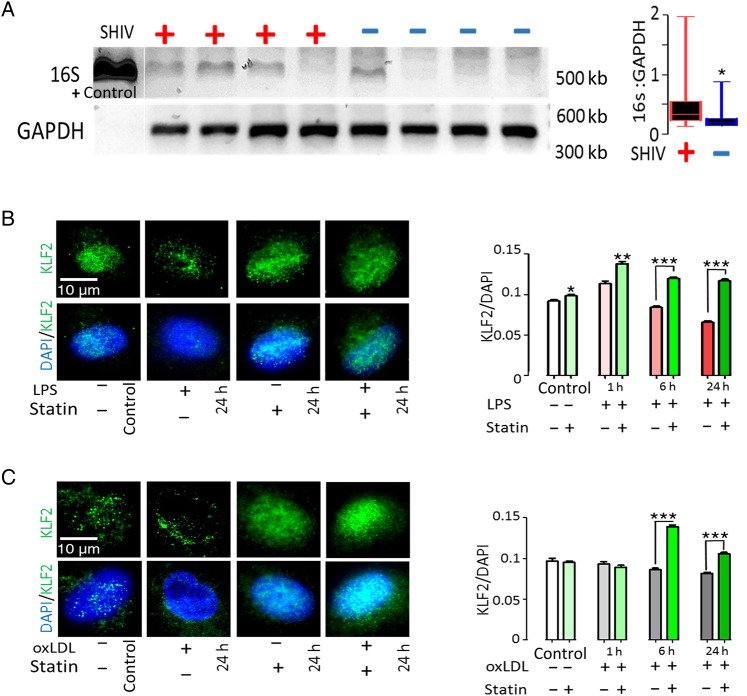
As noted, the transcription factor KLF2 sustains an anti-inflammatory and antithrombotic endothelial microenvironment [30]. Because circulating bacterial products and oxidized lipids may be key triggering factors for vascular inflammation [10, 31], we screened these aortic samples for bacterial 16s rDNA (see Methods for details). We noted significantly increased levels of bacterial 16s rDNA in the endothelium of the infected animals (Figure 4A). To explore strategies that might restore endothelial KLF2 levels, we performed experiments using bacterial LPS and oxLDL to down-modulate KLF2 expression in primary HAECs [32–34]. We asked whether the HMG-CoA reductase inhibitor simvastatin could protect HAECs from LPS and oxLDL-induced KLF2 down-regulation in vitro, using immunofluorescence microscopy to identify endothelium-specific KLF2 expression.
Figure 4.
Bacterial 16s rDNA in the Aortic Endothelium of simian immunodeficiency virus (SIV)/simian humanimmunodeficiency virus (SHIV)-infected rhesus macaques (RMs) and Protection of Human Aortic endothelial cells from lipopolysaccharide (LPS)– and oxidized low-density lipoprotein (oxLDL)–induced modulation of Krüppel-like factor 2 (KLF2) expression. Aorta specimens from rhesus macaques (RMs) were scraped taking precautions to avoid any contamination, and endothelial DNA was isolated for polymerase chain reaction amplification of bacterial 16S ribosomal DNA, as described in “Methods” section. A, Representative DNA gel image and quantitated 16s DNA intensity data from the SIV– or SHIV–infected and uninfected RMs were normalized with respective GAPDH (glyceraldehyde 3-phosphate dehydrogenase) band intensity. B, C, Human primary aortic endothelial cells were grown in sterile chamber slides, exposed to 100 ng/mL LPS (B) or 100 µg/mL oxLDL (C), and examined after 1, 6, and 24 hours, with or without pretreatment with 10 µmol/L simvastatin. The cells were subsequently immunostained and digital images were recorded using epifluorescence microscopy (EVOSFL; Life Technologies) with a ×100 objective (×40 images for large-scale data acquisition and analyses) to detect and analyze nuclear KLF2 expression (see “Methods” section for details). B–D, Quantitative fluorescence intensity ratio (KLF2/ DAPI [4',6-diamidino-2-phenylindole]) data for 100 cells at each time point from 3 independent experiments are presented to the right of each panel. Data were analyzed using 2-tailed Mann–Whitney tests; *P < .05; **P < .001; ***P < .0001.
HAECs were grown on sterile chamber slides, pretreated for 24 hours with simvastatin (10 µmol/L), or reserved as controls and exposed to LPS or oxLDL for 1, 6, and 24 hours. Figure 4A–B show an initial surge of KLF2 expression in the HAEC nuclei during the first hour of exposure to LPS, followed by significant decreases at 24 hours. Preexposure to simvastatin protected HAECs from KLF2 down-modulation induced by LPS (Figure 4B). Exposure of HAECs to oxLDL progressively decreased KLF2 levels, and this was prevented at 6 and 24 hours of exposure by pretreatment with simvastatin (Figure 4C). These in vitro studies confirmed that exposure to both microbial products and oxLDL, elements we have shown to be increased in HIV infection [11, 12], can decrease endothelial KLF2 levels and that this decrease can be prevented by simvastatin.
DISCUSSION
Combination ART has modified dramatically the natural history of HIV disease, prolonging life expectancy as deaths due to opportunistic infections and malignancies have decreased dramatically [35, 36]. With prolonged survival however, HIV-infected persons increasingly are at risk for the morbid effects of aging that include hyperlipidemia, fat redistribution syndrome, insulin resistance, and non–insulin-dependent diabetes mellitus, each of which is linked to increased risk of atherosclerosis and coronary heart disease, the most important noninfectious long-term complication of HIV infection [37–39]. Compared with uninfected persons, HIV-infected patients receiving ART are at 1.5–2-fold higher risk of myocardial infarction and at about 2–10 times greater risk of venous thromboembolism [3]. Uncontrolled HIV replication places HIV-infected persons at even greater risk for cardiovascular events [3, 40, 41], and the determinants of cardiovascular risk in HIV infection treated and untreated remain important targets for study and intervention [42].
Here we provide, for the first time, direct evidence that SIV and SHIV infection of RMs alters the vascular endothelium, triggering inflammatory changes characterized by subendothelial infiltration of immune cells. We also showed a significant reduction in eNOS levels in the aortic endothelium of infected animals, reflective of vascular dysfunction. We also demonstrated for the first time that, in SIV/SHIV-infected RMs, the master regulator of the endothelial antithrombotic antiatherogenic environment—KLF2—is significantly down-modulated in vascular endothelium. Our screening for bacterial components demonstrated higher levels of bacterial DNA within the endothelium of the infected animals (Figure 4A). In addition, we demonstrated that KLF2 down-modulation could be induced in human endothelial cells by a direct local effect of a microbial Toll-like receptor 4 ligand, LPS, and by the lipid oxidation product oxLDL. Finally, we showed that the HMG-CoA reductase inhibitor simvastatin, an anti-inflammatory lipid-lowering agent, could protect the endothelium from LPS- and oxLDL-induced down-modulation of KLF2 expression through mechanisms independent of lipid-lowering effects.
Vascular responses to inflammatory stimuli include induction of adhesion molecules and procoagulant factors (eg, vascular cell adhesion molecule 1, intercellular adhesion molecule 1, E-selectin, tissue factor, and P-selectin) that promote coagulation and leukocyte attachment to the endothelial surface, followed by firm adhesion and transmigration across the endothelium [4, 20, 43]. Endothelial dysfunction, as typically detected with sonographic studies of vascular tone, is common in both untreated and ART-treated HIV infection [22, 44–46]. A key mediator of this endothelial dysfunction is related to reduced bioavailability of vasodilators, particularly NO, and/or an increase in endothelium-derived contracting factors that may result from reduced expression of eNOS. Endothelial dysfunction as we observe it here is characterized by a proinflammatory, proliferative, and prothrombotic state of the endothelium and is considered a key initiating event in the pathogenesis of atherosclerosis [7, 47].
KLF2, a zinc finger family transcription factor, has key regulatory roles in cellular differentiation and tissue development and is recognized to drive an endothelial “atheroprotective phenotype” [28]. When expressed in endothelial cells, KLF2 is a key promoter of an endothelial anti-inflammatory and antithrombotic microenvironment [30]. Its overexpression inhibits proinflammatory and prothrombotic gene expression (eg, vascular cell adhesion molecule 1 and plasminogen activator inhibitor 1) and enhances the expression of eNOS and thrombomodulin [27, 33]. As reported elsewhere, knocking down KLF2 expression markedly reduces basal thrombomodulin and eNOS protein levels, and overexpression of KLF2 inhibits the cytokine-mediated induction of tissue factor in endothelial cells [28, 29]. Moreover, KLF2 was identified as a negative transcriptional regulator of protease-activated receptor type 1 expression and thrombin sensitivity of endothelial cells [29]. Here we report for the first time that endothelial expression of KLF2 is diminished in SIV/SHIV-infected RMs, an observation that implicates this factor as plausibly underlying the vascular inflammation and dysfunction that characterizes HIV [7] and SIV infections [10].
Several studies have implicated both translocated microbial products and oxidized lipids as contributing to the inflammatory and procoagulant phenotype of HIV infection [10, 12, 42]. Other reports indicate that both LPS and oxLDL can attenuate KLF2 expression in monocytes/macrophages and endothelial cells [27, 32–34]. Not only do our observations indicate that levels of bacterial DNA are increased levels in the vascular endothelium of SIV/SHIV-infected RMs, but our in vitro data also indicate that both bacterial LPS and oxLDL can significantly reduce the expression of KLF2 in primary human endothelial cells and that pretreatment with simvastatin can protect endothelial cells from KLF2 down-modulation, and thus possibly from other associated proatherosclerotic changes.
Statins are a class of drugs that inhibit HMG-CoA reductase; their long-term administration confers substantial protection from the progression of atherosclerosis and its cardiovascular complications [30]. This may be related in part to the effects of statins on induction of endothelial cell KLF2, which induces eNOS and relaxes vascular tone [27, 33]. The clinical benefit of these agents also may be related to their ability to lower circulating lipid levels. Like other agents of this class, simvastatin has potent anti-inflammatory effects, decreasing expression of inflammatory cytokines by peripheral blood cells [48]. These effects are thought to be mediated by modulation of transcription factors; simvastatin up-regulates KLF2 expression and reduces signaling via the NF-kB and c-ets (E-twenty-six transcription factor) pathways [48, 49]. In clinical trials, another agent in this class, rosuvastatin, significantly reduced several markers of inflammation and lymphocyte and monocyte activation in ART-treated HIV-infected subjects [50]. Here we show that while both LPS and oxLDL could down-modulate expression of KLF2 in human primary endothelial cells, simvastatin can protect against this proinflammatory procoagulant effect through mechanisms independent of its lipid-lowering effect.
Both SIV and HIV infections are associated with an enteropathy that permits leakage of microbial products into the systemic circulation where they may drive inflammation and promote coagulation [10, 11]. HIV infection is also characterized by increased systemic oxidative stress wherein increased levels of oxidized lipids may also contribute to a procoagulant inflammatory phenotype [12]. These elements (as well as HIV itself) may all promote an atherogenic phenotype that is aided and abetted by an increased prevalence of other conventional cardiovascular risk-factors in HIV-infected persons (Figure 5). A large placebo-controlled trial of pitavastatin administration (REPRIEVE, clintrials.gov NCT02344290) will ascertain whether statin administration confers clinical benefit to HIV-infected persons who do not meet current guidelines for statin administration.
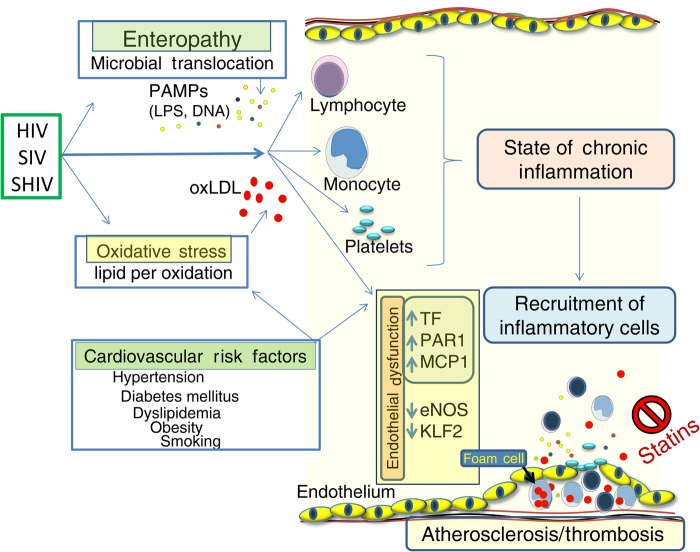
Figure 5.
Schematic model of vascular inflammation and endothelial dysfunction in human immunodeficiency virus (HIV), simian immunodeficiency virus (SIV), and simian-human immunodeficiency virus (SHIV) infection. These infections are associated with enteropathy and translocation of microbial products into systemic circulation, where they and the inflammatory mediators they induce interact with immune cells and endothelium. Low-grade oxidative stress generates bioactive oxidized lipids (eg, oxidized low-density lipoprotein [oxLDL]) that also may activate immune cells and endothelium, each facilitating thrombosis and subendothelial accumulation of lipid-containing foam and other inflammatory cells. As shown here, statins may attenuate these proatherosclerotic events. Abbreviations: eNOS, endothelial nitric oxide synthase; KLF2, Krüppel-like factor 2; LPS, lipopolysaccharide; MCP1, monocyte chemotactic protein 1; PAMPs, pathogen-associated molecular patterns; PAR1, protease-activated receptor 1; TF, tissue factor.
A potential limitation of this study is that the SIV/SHIV-infected animals were younger than the uninfected controls. Although this might have resulted in unintended differences in vascular disease, we suspect that, if anything, the relative atherogenic phenotype of SIV/SHIV infection might have been underestimated as a result. Furthermore, all the control animals were female whereas 9 of 16 infected animals were male. This does not alter our conclusions, because both eNOS and KLF2 levels were significantly lower in both male and female infected animals than in the uninfected female animals (Supplementary Figure 2). We show here that both SIV infection and SHIV infection are characterized by immunohistologic evidence of vascular inflammation, endothelial dysfunction and decreased levels of the antiatherogenic endothelial transcription factor KLF2. Although this phenotype might be driven in part by systemic translocation of microbial products and increased levels of oxidized lipids, an HMG-CoA reductase inhibitor can protect vascular endothelial cells from these effects independently from an effect on lipid metabolism and may afford protection from cardiovascular risk in HIV infection, as in uninfected subjects [9, 50].
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the Cleveland Immunopathogenesis Consortium for helpful comments and discussions related to this project, and we thank Mary Wenzel, Janet Robinson, Brian Clagett, Brian Ferarri, and Douglas Bazdar for technical assistance.
Author contributions. S. P. designed, performed, and analyzed experiments and wrote the manuscript with guidance from S. F. S., D. A. Z., M. P., F. V., L. H. C., R. M. R., M. K. J., and M. M. L. M. P. and F. V. provided the aortic tissues and related technical assistance, data analysis, and critical reading of the manuscript. S. P. performed statistical analysis. All authors contributed to general design and discussion of the project and reviewed and approved the manuscript.
Disclaimer. The funding sources had no role in study design, data collection or analysis, the preparation of the manuscript, or the decision to publish it.
Financial support. This work was supported by the Fasenmyer Foundation, the Case Western Reserve University Center for AIDS Research (grant AI-36219), and the Cleveland Immunopathogenesis Consortium (CLIC) (grants AI-76174, R00HL108743, and HL126563). The Yerkes National Primate Research Center is supported by the National Institutes of Health (base grant P51 OD11132).
Potential conflicts of interest. R. M. R., an employee of Biogen since October 2014, provided input on these studies before his employment there. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Levi M, van der Poll T, Buller HR. Bidirectional relation between inflammation and coagulation. Circulation 2004; 109:2698–704. [DOI] [PubMed] [Google Scholar]
- 2.Stein JH, Hsue PY. Inflammation, immune activation, and CVD risk in individuals with HIV infection. JAMA 2012; 308:405–6. [DOI] [PubMed] [Google Scholar]
- 3.Triant VA. Epidemiology of coronary heart disease in patients with human immunodeficiency virus. Rev Cardiovasc Med 2014; 15(suppl 1):S1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Funderburg NT, Lederman MM. Coagulation and morbidity in treated HIV infection. Thromb Res 2014; 133(suppl 1):S21–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol 2008; 214:231–41. [DOI] [PubMed] [Google Scholar]
- 6.Funderburg NT, Mayne E, Sieg SF et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood 2010; 115:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subramanian S, Tawakol A, Burdo TH et al. Arterial inflammation in patients with HIV. JAMA 2012; 308:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chahroudi A, Bosinger SE, Vanderford TH, Paiardini M, Silvestri G. Natural SIV hosts: showing AIDS the door. Science 2012; 335:1188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estes JD, Harris LD, Klatt NR et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog 2010; 6:e1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandrea I, Cornell E, Wilson C et al. Coagulation biomarkers predict disease progression in SIV-infected nonhuman primates. Blood 2012; 120:1357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenchley JM, Price DA, Schacker TW et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12:1365–71. [DOI] [PubMed] [Google Scholar]
- 12.Zidar DA, Juchnowski S, Ferrari B et al. Oxidized LDL levels are increased in HIV infection and may drive monocyte activation. J Acquir Immune Defic Syndr 2015; 69:154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naidu YM, Kestler HW III, Li Y et al. Characterization of infectious molecular clones of simian immunodeficiency virus (SIVmac) and human immunodeficiency virus type 2: persistent infection of rhesus monkeys with molecularly cloned SIVmac. J Virol 1988; 62:4691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harouse JM, Gettie A, Tan RC, Blanchard J, Cheng-Mayer C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science 1999; 284:816–9. [DOI] [PubMed] [Google Scholar]
- 15.Siddappa NB, Song R, Kramer VG et al. Neutralization-sensitive R5-tropic simian-human immunodeficiency virus SHIV-2873Nip, which carries env isolated from an infant with a recent HIV clade C infection. J Virol 2009; 83:1422–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amara RR, Villinger F, Altman JD et al. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 2001; 292:69–74. [DOI] [PubMed] [Google Scholar]
- 17.Campos PF, Gilbert TM. DNA extraction from formalin-fixed material. Methods Mol Biol 2012; 840:81–5. [DOI] [PubMed] [Google Scholar]
- 18.Imrit K, Goldfischer M, Wang J et al. Identification of bacteria in formalin-fixed, paraffin-embedded heart valve tissue via 16S rRNA gene nucleotide sequencing. J Clin Microbiol 2006; 44:2609–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagstaff KM, Glover DJ, Tremethick DJ, Jans DA. Histone-mediated transduction as an efficient means for gene delivery. Mol Ther 2007; 15:721–31. [DOI] [PubMed] [Google Scholar]
- 20.Mu H, Chai H, Lin PH, Yao Q, Chen C. Current update on HIV-associated vascular disease and endothelial dysfunction. World J Surg 2007; 31:632–43. [DOI] [PubMed] [Google Scholar]
- 21.Gresele P, Falcinelli E, Sebastiano M, Baldelli F. Endothelial and platelet function alterations in HIV-infected patients. Thromb Res 2012; 129:301–8. [DOI] [PubMed] [Google Scholar]
- 22.Nolan D, Watts GF, Herrmann SE, French MA, John M, Mallal S. Endothelial function in HIV-infected patients receiving protease inhibitor therapy: does immune competence affect cardiovascular risk? QJM 2003; 96:825–32. [DOI] [PubMed] [Google Scholar]
- 23.Kolluru GK, Siamwala JH, Chatterjee S. eNOS phosphorylation in health and disease. Biochimie 2010; 92:1186–98. [DOI] [PubMed] [Google Scholar]
- 24.Nayak L, Goduni L, Takami Y et al. Kruppel-like factor 2 is a transcriptional regulator of chronic and acute inflammation. Am J Pathol 2013; 182:1696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atkins GB, Wang Y, Mahabeleshwar GH et al. Hemizygous deficiency of Kruppel-like factor 2 augments experimental atherosclerosis. Circ Res 2008; 103:690–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahabeleshwar GH, Qureshi MA, Takami Y, Sharma N, Lingrel JB, Jain MK. A myeloid hypoxia-inducible factor 1α-Kruppel-like factor 2 pathway regulates gram-positive endotoxin-mediated sepsis. J Biol Chem 2012; 287:1448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.SenBanerjee S, Lin Z, Atkins GB et al. KLF2 is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med 2004; 199:1305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin Z, Kumar A, SenBanerjee S et al. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ Res 2005; 96:e48–57. [DOI] [PubMed] [Google Scholar]
- 29.Lin Z, Hamik A, Jain R, Kumar A, Jain MK. Kruppel-like factor 2 inhibits protease activated receptor-1 expression and thrombin-mediated endothelial activation. Arterioscler Thromb Vasc Biol 2006; 26:1185–9. [DOI] [PubMed] [Google Scholar]
- 30.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov 2005; 4:977–87. [DOI] [PubMed] [Google Scholar]
- 31.Gordon SN, Klatt NR, Bosinger SE et al. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J Immunol 2007; 179:3026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Das H, Kumar A, Lin Z et al. Kruppel-like factor 2 (KLF2) regulates proinflammatory activation of monocytes. Proc Natl Acad Sci U S A 2006; 103:6653–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sen-Banerjee S, Mir S, Lin Z et al. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation 2005; 112:720–6. [DOI] [PubMed] [Google Scholar]
- 34.van Tits LJ, Stienstra R, van Lent PL, Netea MG, Joosten LA, Stalenhoef AF. Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages: a crucial role for Kruppel-like factor 2. Atherosclerosis 2011; 214:345–9. [DOI] [PubMed] [Google Scholar]
- 35.Palella FJ Jr, Delaney KM, Moorman AC et al. HIV Outpatient Study Investigators. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med 1998; 338:853–60. [DOI] [PubMed] [Google Scholar]
- 36.Detels R, Munoz A, McFarlane G et al. Multicenter AIDS Cohort Study Investigators. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. JAMA 1998; 280:1497–503. [DOI] [PubMed] [Google Scholar]
- 37.Calza L, Manfredi R, Pocaterra D, Chiodo F. Risk of premature atherosclerosis and ischemic heart disease associated with HIV infection and antiretroviral therapy. J Infect 2008; 57:16–32. [DOI] [PubMed] [Google Scholar]
- 38.Knudsen A, Mathiasen AB, Worck RH et al. Angiographic features and cardiovascular risk factors in human immunodeficiency virus-infected patients with first-time acute coronary syndrome. Am J Cardiol 2013; 111:63–7. [DOI] [PubMed] [Google Scholar]
- 39.Bozzette SA, Ake CF, Tam HK et al. Long-term survival and serious cardiovascular events in HIV-infected patients treated with highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2008; 47:338–41. [DOI] [PubMed] [Google Scholar]
- 40.Duprez DA, Neuhaus J, Kuller LH et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One 2012; 7:e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller CJ, Baker JV, Bormann AM et al. Adjudicated morbidity and mortality outcomes by age among individuals with HIV infection on suppressive antiretroviral therapy. PLoS One 2014; 9:e95061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nordell AD, McKenna M, Borges AH et al. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc 2014; 3:e000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 1994; 76:301–14. [DOI] [PubMed] [Google Scholar]
- 44.Torriani FJ, Komarow L, Parker RA et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: the ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol 2008; 52:569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stein JH, Brown TT, Ribaudo HJ et al. Ultrasonographic measures of cardiovascular disease risk in antiretroviral treatment-naive individuals with HIV infection. AIDS 2013; 27:929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lebech AM, Kristoffersen US, Wiinberg N et al. Coronary and peripheral endothelial function in HIV patients studied with positron emission tomography and flow-mediated dilation: relation to hypercholesterolemia. Eur J Nucl Med Mol Imaging 2008; 35:2049–58. [DOI] [PubMed] [Google Scholar]
- 47.Graham SM, Mwilu R, Liles WC. Clinical utility of biomarkers of endothelial activation and coagulation for prognosis in HIV infection: a systematic review. Virulence 2013; 4:564–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuomisto TT, Lumivuori H, Kansanen E et al. Simvastatin has an anti-inflammatory effect on macrophages via upregulation of an atheroprotective transcription factor, Kruppel-like factor 2. Cardiovasc Res 2008; 78:175–84. [DOI] [PubMed] [Google Scholar]
- 49.Parmar KM, Nambudiri V, Dai G, Larman HB, Gimbrone MA Jr, Garcia-Cardena G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J Biol Chem 2005; 280:26714–9. [DOI] [PubMed] [Google Scholar]
- 50.Funderburg NT, Jiang Y, Debanne SM et al. Rosuvastatin reduces vascular inflammation and T cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr 2015; 68:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.