Abstract
Background. Biofilms affect >80% bacterial infections in human and are usually difficult to eradicate because of their inherent drug resistance.
Methods. We investigated the effectiveness of antimicrobial blue light (aBL) (wavelength, 415 nm) for inactivating Acinetobacter baumannii or Pseudomonas aeruginosa biofilms in 96-well microplates or infected mouse burn wounds.
Results. In vitro, in 96-well microplates, exposure of 24-hour-old and 72-hour-old A. baumannii biofilms to 432 J/cm2 aBL resulted in inactivation of 3.59 log10 and 3.18 log10 colony-forming units (CFU), respectively. For P. aeruginosa biofilms, similar levels of inactivation—3.02 log10 and 3.12 log10 CFU, respectively—were achieved. In mouse burn wounds infected with 5 × 106 CFU of A. baumannii, approximately 360 J/cm2 and 540 J/cm2 aBL was required to inactivate 3 log10 CFU in biofilms when delivered 24 and 48 hours, respectively, after bacterial inoculation. High-performance liquid chromatography analysis revealed the presence of endogenous porphyrins in both A. baumannii and P. aeruginosa. TUNEL assay detected no apoptotic cells in aBL-irradiated mouse skin at up to 24 hours after aBL exposure (540 J/cm2).
Conclusions. aBL has antimicrobial activity in biofilms of A. baumannii and P. aeruginosa and is a potential therapeutic approach for biofilm-related infections.
Keywords: antimicrobial blue light, biofilm, Pseudomonas aeruginosa, Acinetobacter baumannii, endogenous porphyrins, burn wound, mouse model, bioluminescence imaging, HPLC, TUNEL assay
Biofilms affect >80% of bacterial infections in human [1]. In biofilms, live bacteria are clustered together in a highly hydrated extracellular matrix [2, 3]. Depletion of metabolic substances or accumulation of waste products in biofilms causes bacteria to enter a slow or nongrowing (stationary) state [3]. Biofilms, as a consequence, are more tolerant of conventional antimicrobial drugs and host defenses, compared with their planktonic counterpart [4, 5], and are associated with persistent infections [6, 7]. The situation is exacerbated by the increasing emergence of multidrug-resistant bacterial strains, particularly multidrug-resistant gram-negative bacteria [8]. New therapeutic approaches are required to tackle drug resistance in biofilm-related infections [9].
A novel light-based antimicrobial approach, antimicrobial blue light (aBL), has attracted increasing attention because of its intrinsic antimicrobial effect without the involvement of exogenous photosensitizers [10–14]. The mechanism underlying the antimicrobial activity of aBL is still not fully understood. A common hypothesis is that aBL excites the naturally occurring endogenous photosensitizing chromophores (mainly iron-free porphyrins) and subsequently leads to the production of cytotoxic reactive oxygen species (ROS) [14]. In our previous studies, we demonstrated that aBL selectively inactivated planktonic bacterial cells (including multidrug-resistant strains) while preserving host cells and that it successfully eliminated acute infections in mouse wounds [15–17]. In the present study, we further investigated the effectiveness of aBL inactivation of Pseudomonas aeruginosa and Acinetobacter baumannii biofilms in 96-well microplates or mouse burn wounds with established infections.
MATERIALS AND METHODS
Blue Light Source
For aBL irradiation, we used a prototype light-emitting diode (LED; Vielight, Toronto, Canada) with peak emission at 415 nm and full-width at half maximum of 10 nm. The LED was mounted on a heat sink to prevent the thermal effects on the irradiated target. The irradiance on the surface of target was adjusted by manipulating the distance between the light source aperture and the target and was measured using a PM100D power/energy meter (Thorlabs, Newton, New Jersey).
Bacterial Strains
The bacterial strains used in this study were P. aeruginosa ATCC 19660 (strain 180) and a multidrug-resistant clinical isolate of A. baumannii. Both strains were made bioluminescent by transfecting the lux operon into the bacterial strains as described previously [18, 19], allowing real-time monitoring of the bioluminescence from bacteria by using bioluminescence imaging. The bacteria were routinely grown in brain heart infusion (BHI) medium supplemented with 50 µg/mL kanamycin in an orbital incubator (37°C; 1300g).
Correlation of Bacterial Luminescence to Colony-Forming Units (CFU) in Biofilms
Bacterial suspensions in BHI were incubated in 96-well microplates (200 μL/well; approximately 106 CFU/mL) for 24 hours to allow biofilm growth [20–22]. At the end of the incubation period, biofilms were carefully washed twice with phosphate-buffered saline (PBS) to remove nonadhesive bacteria, and 200 μL of PBS was added into each well. Biofilms in different wells were then exposed to aBL at an irradiance of 100 mW/cm2 for different periods. After aBL exposure, biofilms were subjected to both luminescence intensity measuring (in relative light units [RLU]) by using a Victor-2 1420 multilabel plate reader (EG&G Wallac, Gaithersburg, Maryland) and a colony-forming assay. For the colony-forming assay, each well was carefully dashed and then vibrated by sonication for 1 minute. Suspensions were then collected and plated on BHI agar after serial dilutions using the method described previously [23]. Total numbers of CFU in the biofilms were determined and linearly fitted with the corresponding bacterial luminescence.
aBL Inactivation of Bacteria in Biofilms In Vitro
Bacterial suspensions in BHI broth were incubated in 96-well microplates (200 μL/well; approximately 106 CFU/mL) for 24 or 72 hours to allow biofilm growth [20–22]. During incubation, culture medium was changed every other day. At the end of incubation, biofilms were carefully washed twice by using PBS, and 200 μL of fresh PBS was added into each well. Biofilms were then irradiated with aBL at an irradiance of 100 mW/cm2. At varying time points after the initiation of aBL irradiation, bacterial luminescence of the biofilms was measured using the multilabel plate reader, and bacterial viability in biofilms was estimated on the basis of the bacterial luminescence. The experiment was performed in 4 replicates for each condition.
aBL Inactivation of Bacterial Biofilms in Mouse Burn Wounds
Adult female BALB/c mice aged 7–8 weeks and weighing 17–19 g were purchased from Charles River Laboratories (Wilmington, MA). All animal procedures were approved by the institutional animal care and use committees of Massachusetts General Hospital (protocol 2014N000009) and accorded with the guidelines of the National Institutes of Health.
Before the incurrence of thermal burn wounds in mice, mice were anesthetized by intraperitoneal injection of a ketamine-xylazine cocktail (100 mg/kg-20 mg/kg). A partial-thickness, third-degree burn wound was made by exposing the depilated area on the back of each mouse for 3 seconds to a brass block (1 cm × 1 cm), which was heated to thermal equilibration with boiling tap water. Sterile saline (0.5 mL intraperitoneally) was administered to support fluid balance during recovery. Five minutes following induction of thermal injury, bacterial suspensions (50 μL) containing 5 × 106 CFU in PBS were inoculated on the burn sites and remained in place while the mice recovered from anesthesia. A. baumannii was used as the model pathogen in this experiment. At 24 or 48 hours after bacterial inoculation, when biofilms in the mouse burn wounds were formed [24–27], aBL was delivered to the infected burn wounds at an irradiance of 100 mW/cm2. Mice were given a total light exposure of up to 360 J/cm2 and 540 J/cm2 in aliquots for 24-hour-old and 48-hour-old burn wounds, respectively, with bioluminescence imaging taking place after each aliquot of light. For each condition (including untreated controls), a group of 8–10 mice were used.
Bioluminescence Imaging In Vivo
The bioluminescence emission of bacteria in mouse burn wounds was detected by using a Hamamatsu bioluminescence imaging system (Hamamatsu Photonics KK, Bridgewater, New Jersey). This system included an intensified charge-coupled device camera (C2400-30H, Hamamatsu) developed for imaging under extremely low light levels (down to photon levels), a camera controller, a specimen chamber, and an image processor (C5510-50, Hamamatsu). When set for photon counting, the camera controller's automatic amplification circuit was used for maximum sensitivity. An integration time of 2 minutes was used for bioluminescence image acquisition. For each measurement, the background signal was subtracted from the bioluminescence signal. The bioluminescence intensity of the infections was quantified using Argus 5.0 software (Hamamatsu).
High-Performance Liquid Chromatography (HPLC) Analysis of the Presence of Endogenous Porphyrins in Bacterial Cells
To determine the presence of endogenous photosensitizing porphyrins in bacterial cells, HPLC analysis was performed by using an Agilent's new 6430 Triple Quad LC/MS System (Agilent Technologies, Lexington, Massachusetts) after the extraction of endogenous porphyrins from bacterial cells. The method of extracting endogenous porphyrins was similar to a protocol reported previously [28]. In brief, overnight bacterial cultures were centrifuged at 13 500g for 5 minutes and then washed using PBS. The pellets of bacteria were collected and resuspended in 1.0 mL of extraction solvent (ratio of ethanol to dimethyl sulfoxide to acetic acid, 80:20:1 [vol/vol/vol]) and stored at −80°C for 24 hours. The cell walls of bacteria were then disrupted by sonication for 20 minutes. After centrifugation (at 13 500 × g for 6 minutes), the supernatant was collected, filtered through a Sep-Pak C18-Cartridge, and injected into a HPLC column for reverse-phase chromatography. Detection of porphyrins from the extracts was performed using a fluorescence spectrophotometer interface with the HPLC column at an excitation spectrum of 405 nm and an emission spectrum of 630 nm. A standard mixture of porphyrins (Porphyrin Products) and a standard protoporphyrin IX (Pp IX; Sigma Aldrich) were used for HPLC peak identification.
TUNEL Assay of Apoptotic Cells in aBL-Irradiated Mouse Skin
aBL-irradiated mouse skin was examined for the presence of apoptotic cells, using a TUNEL assay in which fragmented DNA from apoptotic cells undergoes end labeling with fluorophore. Skin biopsy specimens were collected before and 0 hours and 24 hours after aBL exposure. The biopsy samples were fixed in 10% phosphate-buffered formalin (Fisher Scientific) for 24 hours, processed, and then embedded in paraffin. Serial 4-μm–thick tissue sections were analyzed using the DeadEnd Fluorometric TUNEL System (Promega, Madison, WI) according to the manufacturer's protocol. Briefly, following deparaffinization and rehydration, sections were fixed, permeabilized with proteinase K for 8–10 minutes, and repeatedly fixed. Cover slips of 50 µL of terminal deoxynucleotidyl transferase mix was applied to sections for 1 hour at 37°C in a humidified chamber. After removal of the cover slips, the sections were immersed in SSC buffer (2×) for 15 minutes, washed with PBS, and mounted with medium including DAPI (Vectashield, Vector Laboratories). Fluorescence images were captured using a FluoView FV1000-MPE confocal microscopy (Olympus Corporation, Tokyo, Japan) with fluorescein isothiocyanate as the fluor and DAPI as the nuclear counterstain.
For the positive control, tissue sections were treated with DNase I to induce DNA fragmentation, using RQ1 RNase-free DNase (catalog no. M6101; Promega). Briefly, after proteinase K treatment, the tissue sections were treated with 10 unit/mL of DNase I for 10 minutes at room temperature, and then the rest of the steps of the TUNEL assay were followed.
Statistic Analyses
Data are presented as the mean ± SD, and differences between means were compared for significance by one-way analysis of variance (ANOVA). P values of <.05 were considered statistically significant.
RESULTS
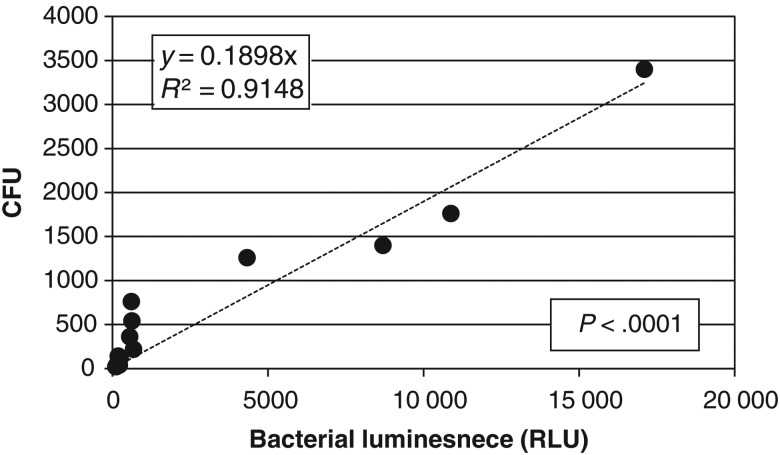
Bacterial Luminescence Linearly Correlated to the Number of CFU in Biofilms
As shown in Figure 1, there was a good linear correlation between the bacterial luminescence of P. aeruginosa biofilms and the number of CFU of bacteria in P. aeruginosa biofilms. The line regression resulted in an R2 value of 0.9148 and a P value of <.0001. A similar result was observed in A. baumannii biofilms (data not shown).
Figure 1.
Correlation of bacterial luminescence to the number of colony-forming units (CFU) in Pseudomonas aeruginosa biofilms. Bacterial luminescence is expressed in relative light units (RLU).
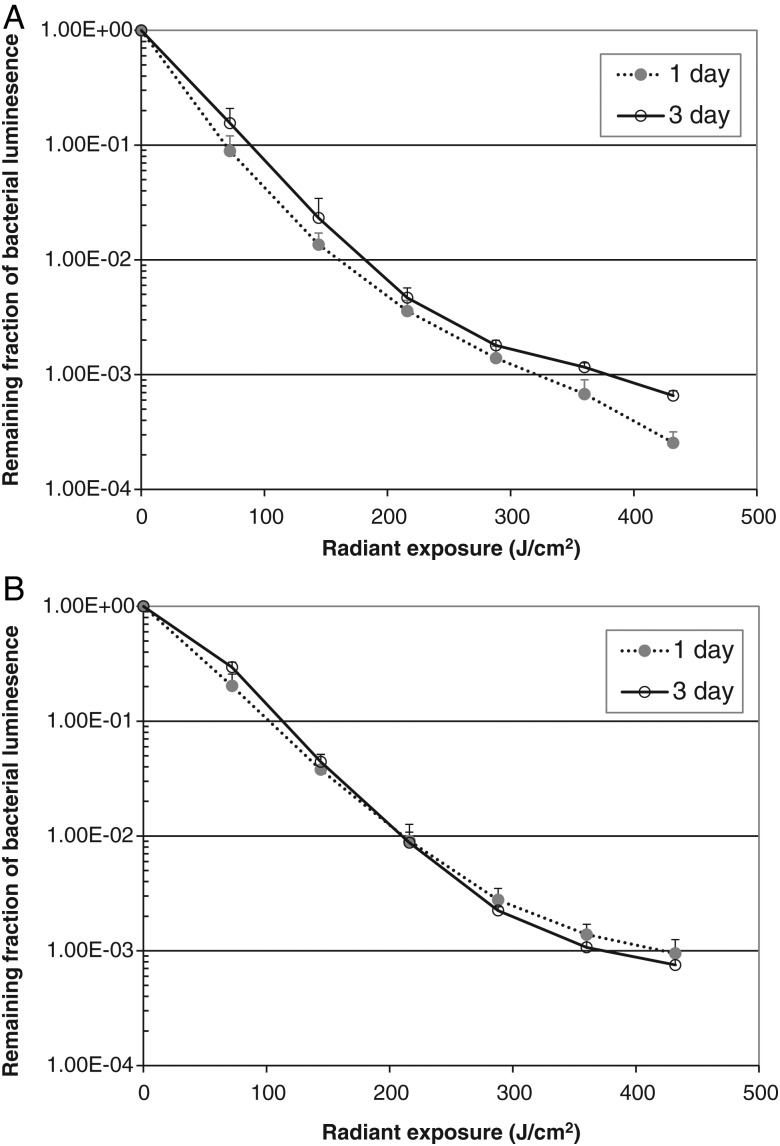
aBL Effectively Inactivated Bacterial Biofilms In Vitro
For A. baumannii, 72-hour-old biofilms were slightly more resistant to aBL than 24-hour-old biofilms. When an exposure of 432 J/cm2 aBL had been delivered (by irradiation for 72 minutes at an irradiance of 100 mW/cm2), inactivation of 3.59 log10 and 3.18 log10 CFU was achieved for 24-hour-old and 72-hour-old biofilms, respectively (Figure 2A). For P. aeruginosa, the extent of aBL-induced inactivation was similar for bacteria in 24-hour-old and 72-hour-old biofilms. When an exposure of 432 J/cm2 aBL had been delivered, inactivation of 3.02 log10 and 3.12 log10 CFU was achieved for bacteria in 24-hour-old and 72-hour-old biofilms, respectively (Figure 2B). In contrast, all biofilms without being exposed to aBL only showed modest viability loss during the equivalent period (<0.27 log10 CFU for A. baumannii biofilms and <0.42 log10 CFU for P. aeruginosa biofilms).
Figure 2.
Antimicrobial blue light inactivation of bacterial biofilms in 96-well microplates. A, Acinetobacter baumannii biofilms. B, Pseudomonas aeruginosa biofilms. Bars denote standard deviations.
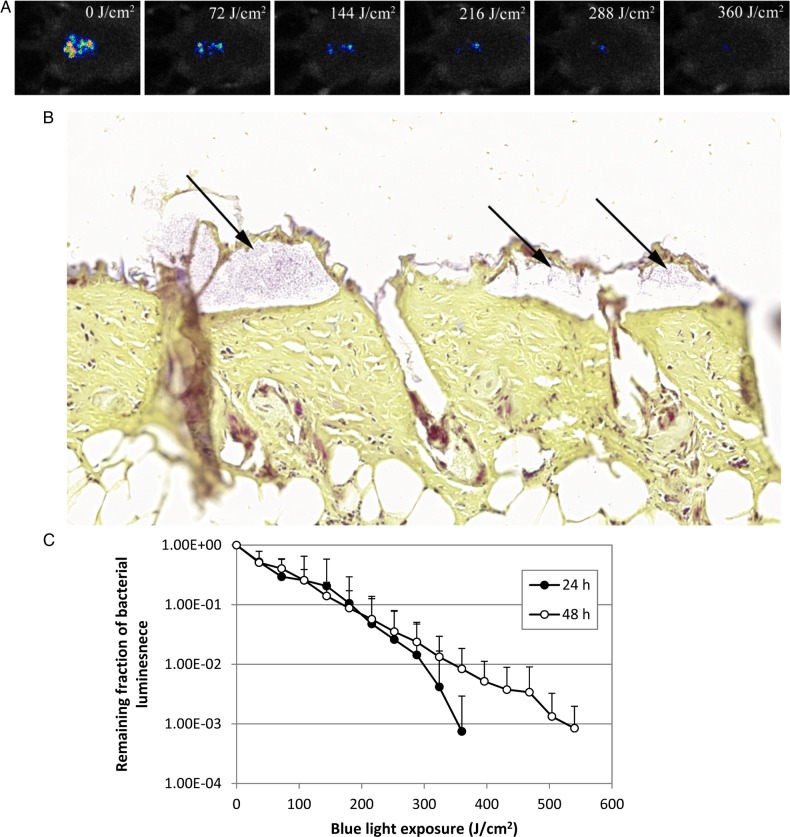
aBL Effectively Inactivated Bacterial Biofilms in Infected Mouse Burn Wounds
Figure 3A is a set of bacterial luminescence images from a representative mouse burn wound infected with 5 × 106 CFU of A. baumannii and exposed to aBL 24 hours after bacterial inoculation. Gram stain of the histological section of a representative skin specimen (harvested 24 hours after inoculation) demonstrated the presence of A. baumannii biofilms on the surface of infected burn wounds 24 hours after inoculation (Figure 3B). The bacterial luminescence was almost completely eradicated after an exposure of 360 J/cm2 aBL had been delivered (by irradiation for 60 minutes at an irradiance of 100 mW/cm2).
Figure 3.
Antimicrobial blue light (aBL) inactivation of biofilms in infected mouse burn wounds. A, Successive bacterial luminescence images from a representative mouse burn wound infected with 5 × 106 colony-forming units of Acinetobacter baumannii and exposed to 360 J/cm2 aBL 24 hours after bacterial inoculation. B, Gram-stained section of a representative mouse skin burn wound specimen showing the presence of A. baumannii biofilms (arrows). The skin sample was harvested 24 hours after bacterial inoculation. C, Dose-response curves of mean bacterial luminescence of mouse burn wounds infected with 5 × 106 A. baumannii and treated with aBL 24 hours (n = 10) and 48 hours (n = 10) after bacterial inoculation. Bars denote standard deviations.
Figure 3C shows the dose-response curves of mean bacterial luminescence in mouse burn wounds infected with 5 × 106 CFU of A. baumannii and treated with aBL 24 and 48 hours, respectively, after bacterial inoculation. The infections were more resistant to aBL therapy 48 hours after inoculation than 24 hours after inoculation. To achieve inactivation of 3 log10 CFU of A. baumannii in mouse burn wounds, approximately 360 J/cm2 and 540 J/cm2 aBL was required 24 hours and 48 hours, respectively, after inoculation (P = .06). This difference is because infections 48 hours after inoculation were located more deeply in mouse skin than infections 24 hours after inoculation. The bacterial luminescence of the mouse burn wounds without exposure to aBL remained almost unchanged during the equivalent period (data not shown; P < .001).
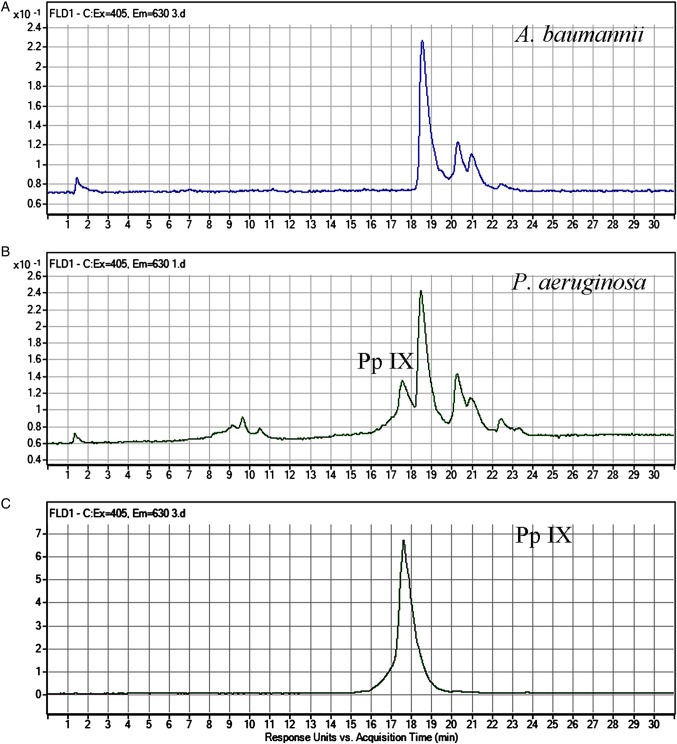
HPLC Analysis Revealed the Presence of Endogenous Porphyrins in Both A. baumannii and P. aeruginosa
HPLC files of the porphyrin extracts from A. baumannii, the porphyrin extracts from P. aeruginosa, and standard Pp IX are shown in Figure 4. The emission intensity peaks in the chromatograms (Figure 4A and 4B) indicate the presence of endogenous porphyrins in both A. baumannii and P. aeruginosa. For A. baumannii, 3 principal emission intensity peaks were detected at the retention times of approximately 18.5 minutes, 20.3 minutes, and 21 minutes, respectively, with a total running time of 30 minutes (Figure 4A). For P. aeruginosa, in addition to the emission intensity peaks observed in the chromatogram of A. baumannii, an emission intensity peak at the retention time of 17.5 minutes, suggesting the presence of Pp IX, was observed (Figure 4B). For both bacterial strains, the emission intensity peak at 18.5 minutes was predominant. However, this emission intensity peak was not detected in the standard mixture of porphyrins composed of uroporphyrin, heptaporphyrin, hexaporphyrin, pentaporphyrin, coproporphyrin, and mesoporphyrin IX (data not shown).
Figure 4.
High-performance liquid chromatography chromatograms of porphyrin extracts from Acinetobacter baumannii (A), porphyrin extracts from Pseudomonas aeruginosa (B), and standard protoporphyrin IX (Pp IX; C).
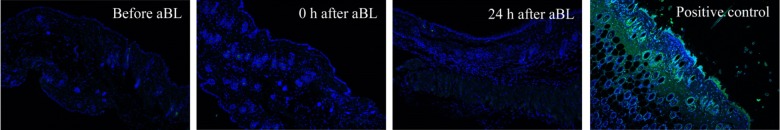
No Apoptotic Cells Were Detected in aBL-Irradiated Mouse Skin
Immunofluorescence pictures of representative mouse skin before and 0 hours and 24 hours after a single aBL exposure (540 J/cm2), shown in Figure 5, revealed no presence of apoptotic cells in aBL-irradiated mouse skin. Fluorescence detected in the positive control (mouse skin section treated with DNase I) indicated the presence of apoptotic cells.
Figure 5.
TUNEL assay of apoptotic cells in mouse skin before and 0 hours and 24 hours after antimicrobial blue light exposure (540 J/cm2). The positive control was treated with DNase I. Nuclei were stained blue with DAPI.
DISCUSSION
Our previous studies showed that aBL irradiation at a wavelength of 415 nm successfully inactivated planktonic bacterial cells (A. baumannii, P. aeruginosa, and Staphylococcus aureus) in suspensions and significantly reduced the bacterial burden in acute infections of mouse burn wounds or skin abrasion wounds when aBL was initiated shortly (ie, 30 minutes) after bacterial inoculation [15–17]. In the present study, we first demonstrated that aBL (wavelength, 415 nm) successfully eradicated A. baumannii or P. aeruginosa in biofilms in vitro. Our results are supported by a recent study from Mckenzie et al [29], showing the successful inactivation of P. aeruginosa in biofilms by using aBL at a wavelength of 405 nm. In that study, the investigators observed inactivation of 3.72 log10 CFU of P. aeruginosa in biofilms when 168 J/cm2 aBL had been delivered. We then further demonstrated, for the first time, the successful aBL inactivation of A. baumannii in biofilms in infected mouse burn wounds by using only a single exposure of aBL. To the best of our knowledge, the present study is the first to show the effectiveness of aBL inactivation of gram-negative bacteria in biofilms in vivo. In addition, our results showed that, under the aBL exposure for treating infections in mice, no apoptosis was activated by aBL in mouse skin.
It is well known that biofilms can be difficult to eradicate by using traditional antibiotics and that a 100–1000-fold increase in antimicrobial tolerance/resistance in biofilm cells, compared to planktonic cells, has been reported [30]. Many mechanisms operate together to produce a high total biofilm-specific tolerance/resistance [3], including failure of antibiotics to penetrate the biofilm [31], slow growth rate [31], altered metabolism [31], oxygen gradients [32], persister cells [33], subpopulations in biofilms [34], sub–minimum inhibitory concentrations of antibiotic [35], mutation [36], quorum sensing [37], and genetic transfer [38]. The results from the present study are promising in that aBL is biofilm-penetrating for both early stage (24-h old) and mature (>48-h old) biofilms.
A common hypothesis for the effect of aBL is that aBL excites the endogenous photosensitizing chromophores (mainly iron-free porphyrins) and subsequently leads to the production of cytotoxic ROS [14]. Where do the iron-free porphyrins in bacterial cells come from? There are 2 possible sources: (1) bacteria synthesize the free porphyrins as a byproduct of heme biosynthesis [39] and (2) bacteria (especially heme auxotrophs) take up heme as a source of iron, with iron-free porphyrins remaining after the iron is removed. In the present study, we are the first to reveal the presence of endogenous porphyrins in both A. baumannii and P. aeruginosa cells by using HPLC analysis. Studies by other investigators demonstrated the presence of coproporphyrin, uroporphyrin, heptacarboxyl porphyrin, and Pp IX in other bacterial species that are sensitive to aBL, including Propionibacterium acnes [40], Helicobacter pylori [41], Porphyromonas gingivalis [42], Prevotella intermedia [42], and Aggregatibacter actinomycetemcomitans [43]. However, none of these porphyrins, except a small amount of Pp IX in P. aeruginosa, were detected in our study. This difference is probably due to the different bacterial species investigated and the different growth media used. In most of the previous studies, either slowly growing bacterial species [40, 41] were investigated or blood/hemin-supplemented growth media [42, 44] were used. Therefore, in previous studies there might be enough nutrition to support bacterial heme synthesis, whereas in our study both A. baumannii and P. aeruginosa were fast growing and BHI without additional nutrition was used as the medium. The bacteria were tested in a stationary phase of growth and the process of heme production might be limited because of nutrition restriction. Nonetheless, the fluorescence detected by HPLC indicated the presence of endogenous porphyrins that can be excited by aBL. The predominant emission intensity peak at 18.5 minutes was close to that of Pp IX (at 17.5 minutes), suggesting that these porphyrins might be Pp IX–like derivatives. Further studies, such as the use of electrospray ionization tandem mass spectrometry, are warranted to identify the structures of these Pp IX–like derivatives.
On the other hand, there is a possibility that porphyrins are not the only endogenous photosensitizing chromophores presenting in bacteria. Other endogenous photosensitizing chromophores (eg, flavins and cytochromes) may also exist in bacteria and contribute to the effect of aBL inactivation [14]. Further studies are needed to investigate this possibility.
There is a limitation of aBL in its extent of light penetration, like all other light-based therapeutic and diagnostic approaches. In this study, we only tested the efficacy of aBL inactivation of biofilms on the surface of burned mouse skin, a site at which systemic drug delivery itself may be limited, suggesting that light-based therapies may complement systemic antibacterial therapies in settings of burn wound and other skin infections. For deeply seated biofilms, interstitially delivered light may also be a consideration, and we are in the process of developing a microneedle-array patch that can help deliver aBL interstitially to deeply seated biofilms [45].
Notes
Acknowledgments. We thank Tayyaba Hasan, PhD, at the Wellman Center for her co-mentorship of Y. W., J. C., and R. A.
Disclaimer. The views expressed herein are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the US Government.
Financial support. This work was supported by the Center for Integration of Medicine and Innovative Technology, under a US Army Medical Research Acquisition Activity Cooperative Agreement (award 14-1894 to T. D.); the National Institutes of Health (1R21AI109172 to T. D.); and the American Society for Laser Medicine and Surgery (student research grant BS.S02.15 to Y. W.).
Potential conflicts of interest. C. K. M. is an employee of the US Army. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Romling U, Balsalobre C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J Intern Med 2012; 272:541–61. [DOI] [PubMed] [Google Scholar]
- 2.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 2002; 15:167–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olsen I. Biofilm-specific antibiotic tolerance and resistance. Eur J Clin Microbiol Infect Dis 2015; 34:877–86. [DOI] [PubMed] [Google Scholar]
- 4.Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 2010; 35:322–32. [DOI] [PubMed] [Google Scholar]
- 5.Lebeaux D, Ghigo JM, Beloin C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev 2014; 78:510–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akers KS, Mende K, Cheatle KA et al. Biofilms and persistent wound infections in United States military trauma patients: a case-control analysis. BMC Infect Dis 2014; 14:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burmolle M, Thomsen TR, Fazli M et al. Biofilms in chronic infections - a matter of opportunity - monospecies biofilms in multispecies infections. FEMS Immunol Med Microbiol 2010; 59:324–36. [DOI] [PubMed] [Google Scholar]
- 8.Sommer R, Joachim I, Wagner S, Titz A. New approaches to control infections: anti-biofilm strategies against gram-negative bacteria. Chimia (Aarau) 2013; 67:286–90. [DOI] [PubMed] [Google Scholar]
- 9.Hoiby N, Bjarnsholt T, Moser C et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect 2015; 21:S1–25. [DOI] [PubMed] [Google Scholar]
- 10.Enwemeka CS, Williams D, Enwemeka SK, Hollosi S, Yens D. Blue 470-nm light kills methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Photomed Laser Surg 2009; 27:221–6. [DOI] [PubMed] [Google Scholar]
- 11.Enwemeka CS, Williams D, Hollosi S, Yens D, Enwemeka SK. Visible 405 nm SLD light photo-destroys methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers Surg Med 2008; 40:734–7. [DOI] [PubMed] [Google Scholar]
- 12.Maclean M, MacGregor SJ, Anderson JG, Woolsey G. Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array. Appl Environ Microbiol 2009; 75:1932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonald R, Macgregor SJ, Anderson JG, Maclean M, Grant MH. Effect of 405-nm high-intensity narrow-spectrum light on fibroblast-populated collagen lattices: an in vitro model of wound healing. J Biomed Opt 2011; 16:048003. [DOI] [PubMed] [Google Scholar]
- 14.Dai T, Gupta A, Murray CK, Vrahas MS, Tegos GP, Hamblin MR. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist Updat 2012; 15:223–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai T, Gupta A, Huang YY et al. Blue light eliminates community-acquired methicillin-resistant Staphylococcus aureus in infected mouse skin abrasions. Photomed Laser Surg 2013; 31:531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai T, Gupta A, Huang YY et al. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: efficacy, safety, and mechanism of action. Antimicrob Agents Chemother 2013; 57:1238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Zhu Y, Gupta A et al. Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: implications for prophylaxis and treatment of combat-related wound infections. J Infect Dis 2014; 209:1963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamblin MR, Zahra T, Contag CH, McManus AT, Hasan T. Optical monitoring and treatment of potentially lethal wound infections in vivo. J Infect Dis 2003; 187:1717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai T, Tegos GP, Lu Z et al. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob Agents Chemother 2009; 53:3929–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cieplik F, Spath A, Regensburger J et al. Photodynamic biofilm inactivation by SAPYR--an exclusive singlet oxygen photosensitizer. Free Radic Biol Med 2013; 65:477–87. [DOI] [PubMed] [Google Scholar]
- 21.Coenye T, Nelis HJ. In vitro and in vivo model systems to study microbial biofilm formation. J Microbiol Methods 2010; 83:89–105. [DOI] [PubMed] [Google Scholar]
- 22.Pratten J, Wills K, Barnett P, Wilson M. In vitro studies of the effect of antiseptic-containing mouthwashes on the formation and viability of Streptococcus sanguis biofilms. J Appl Microbiol 1998; 84:1149–55. [DOI] [PubMed] [Google Scholar]
- 23.Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. Biotechniques 1997; 23:648–50. [DOI] [PubMed] [Google Scholar]
- 24.Uppu DS, Samaddar S, Ghosh C, Paramanandham K, Shome BR, Haldar J. Amide side chain amphiphilic polymers disrupt surface established bacterial bio-films and protect mice from chronic Acinetobacter baumannii infection. Biomaterials 2016; 74:131–43. [DOI] [PubMed] [Google Scholar]
- 25.Thompson MG, Black CC, Pavlicek RL et al. Validation of a novel murine wound model of Acinetobacter baumannii infection. Antimicrob Agents Chemother 2014; 58:1332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brisbois EJ, Bayliss J, Wu J et al. Optimized polymeric film-based nitric oxide delivery inhibits bacterial growth in a mouse burn wound model. Acta Biomater 2014; 10:4136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tran PL, Huynh E, Hamood AN et al. The ability of quaternary ammonium groups attached to a urethane bandage to inhibit bacterial attachment and biofilm formation in a mouse wound model. Int Wound J 2015; doi:10.1111/iwj.12554. [DOI] [PMC free article] [PubMed]
- 28.Fotinos N, Convert M, Piffaretti JC, Gurny R, Lange N. Effects on gram-negative and gram-positive bacteria mediated by 5-aminolevulinic Acid and 5-aminolevulinic acid derivatives. Antimicrob Agents Chemother 2008; 52:1366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKenzie K, Maclean M, Timoshkin IV, Endarko E, Macgregor SJ, Anderson JG. Photoinactivation of bacteria attached to glass and acrylic surfaces by 405 nm light: potential application for biofilm decontamination. Photochem Photobiol 2013; 89:927–35. [DOI] [PubMed] [Google Scholar]
- 30.Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol 1999; 37:1771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walters MC III, Roe F, Bugnicourt A, Franklin MJ, Stewart PS. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother 2003; 47:317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borriello G, Werner E, Roe F, Kim AM, Ehrlich GD, Stewart PS. Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob Agents Chemother 2004; 48:2659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis K. Persister cells: molecular mechanisms related to antibiotic tolerance. Handb Exp Pharmacol 2012; 211:121–33. [DOI] [PubMed] [Google Scholar]
- 34.Klausen M, Aaes-Jorgensen A, Molin S, Tolker-Nielsen T. Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol Microbiol 2003; 50:61–8. [DOI] [PubMed] [Google Scholar]
- 35.Jorgensen KM, Wassermann T, Jensen PO et al. Sublethal ciprofloxacin treatment leads to rapid development of high-level ciprofloxacin resistance during long-term experimental evolution of Pseudomonas aeruginosa. Antimicrob Agents Chemother 2013; 57:4215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macia MD, Perez JL, Molin S, Oliver A. Dynamics of mutator and antibiotic-resistant populations in a pharmacokinetic/pharmacodynamic model of Pseudomonas aeruginosa biofilm treatment. Antimicrob Agents Chemother 2011; 55:5230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bjarnsholt T, Jensen PO, Burmolle M et al. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology 2005; 151:373–83. [DOI] [PubMed] [Google Scholar]
- 38.Olsen I, Tribble GD, Fiehn NE, Wang BY. Bacterial sex in dental plaque. J Oral Microbiol 2013; 5:20736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lesage S, Xu H, Durham L. The occurrence and roles of porphyrins in the environment—possible implications for bioremediation. Hydrol Sci J-J Sci Hydrol 1993; 38:343–54. [Google Scholar]
- 40.Romiti R, Schaller M, Jacob K, Plewig G. High-performance liquid chromatography analysis of porphyrins in Propionibacterium acnes. Arch Dermatol Res 2000; 292:320–2. [DOI] [PubMed] [Google Scholar]
- 41.Hamblin MR, Viveiros J, Yang C, Ahmadi A, Ganz RA, Tolkoff MJ. Helicobacter pylori accumulates photoactive porphyrins and is killed by visible light. Antimicrob Agents Chemother 2005; 49:2822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soukos NS, Som S, Abernethy AD et al. Phototargeting oral black-pigmented bacteria. Antimicrob Agents Chemother 2005; 49:1391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cieplik F, Spath A, Leibl C et al. Blue light kills Aggregatibacter actinomycetemcomitans due to its endogenous photosensitizers. Clin Oral Investig 2014; 18:1763–9. [DOI] [PubMed] [Google Scholar]
- 44.Fontana CR, Song X, Polymeri A, Goodson JM, Wang X, Soukos NS. The effect of blue light on periodontal biofilm growth in vitro. Lasers Med Sci 2015; 30:2077–86. [DOI] [PubMed] [Google Scholar]
- 45.Guimarães C, An J, Humar M, Goth W, Yun A. Biocompatible optical needle array for antibacterial blue light therapy. Presented at: SPIE 9341: Bioinspired, biointegrated, bioengineered photonic devices III, 93410R doi:10.117/12.2083384. [Google Scholar]