Abstract
Background
BDNF gene polymorphism impacts human motor cortex function and plasticity.
Objective/hypothesis
Using transcranial magnetic stimulation (TMS), we investigated whether BDNF polymorphism influences cortical plastic changes in acute stroke.
Methods
Twenty patients were recruited within 10 days of their first-ever ischemic stroke and genotyped for BDNF polymorphism. Blinded to the latter, we evaluated the excitability of the affected and unaffected hemisphere by measuring resting and active motor threshold and motor-evoked potential amplitude under baseline conditions and after intermittent theta burst stimulation, a protocol of repetitive TMS inducing LTP-like activity. We also computed laterality indexes to assess inter-hemispheric excitability imbalance.
Results
Demographics, threshold and amplitude of motor-evoked potentials did not differ between those with (8 patients) and without polymorphism. Excitability of the unaffected hemisphere was significantly higher than the excitability of the affected hemisphere as probed by each measure. This imbalance was exaggerated in those without polymorphism; laterality indexes of rest motor thresholds were 0.016 ± 0.050 and 0.139 ± 0.028 for patients with and without polymorphism [t = 2.270, P = 0.036]. Exaggerated hemispheric imbalance also persisted after intermittent theta burst stimulation, which failed to induce any difference between groups.
Conclusions
Our results suggest that inter-hemispheric imbalance with greater excitability over unaffected hemisphere, is several times stronger in stroke patients without, as opposed to with, polymorphism.
Keywords: Neurophysiology, Transcranial magnetic stimulation, Stroke, BDNF, Acute cerebral infarction
Introduction
Non-invasive brain stimulation techniques have been extensively employed to investigate motor cortex excitability changes after human stroke. Using single pulse, paired-pulse and repetitive transcranial magnetic stimulation (TMS) protocols it is possible to probe different aspects of excitatory and inhibitory function [1]. A single pulse of TMS applied over motor cortex produces a peripheral muscular response, the motor-evoked potential (MEP). The threshold and recruitment of MEPs at different intensities of stimulation can provide key information about the level of corticospinal excitability. Inhibitory GABA-A circuits acting on pyramidal neurons can be tested by delivering a conditioning subthreshold TMS pulse that precedes the test TMS stimulus by 1–5 ms, a paradigm termed Short Interval Intra-Cortical Inhibition (SICI). GABA-B activity can be tested by means of the cortical silent period, obtained with a single TMS pulse delivered during tonic contralateral hand/arm contraction or by means of the Long-Interval Intra-Cortical Inhibition (LICI) protocol, where a supra-threshold conditioning stimulus precedes the test stimulus by 50–200 ms. Other inhibitory mechanisms can also be evaluated; for instance, pairing a correctly timed peripheral stimulus with a TMS pulse over M1 produces Short-Latency Afferent Inhibition (SAI), which depends on GABAergic and cholinergic circuits.
TMS studies in human stroke patients have shown excitability changes in the cortical circuits of both the affected (AH) and unaffected (UH) hemisphere [2,3] and it has been suggested that the specific functional changes that take place early after stroke in the AH and in remote brain areas are correlated with long term recovery and might represent forms of adaptive or maladaptive cortical plasticity. Absence of motor responses after stimulation of the AH in the first hours or days after the stroke [4] and hyperexcitability of the UH [5] seem to be associated with poor recovery. According to an influential model known as the inter-hemispheric competition model [6], the increase in UH excitability is particularly deleterious for recovery as it entails an increased inhibition of the AH by the UH. The AH is thus said to be “doubly disabled,” since ipsilateral damage is coupled with excess inhibition from the opposite hemisphere. On the other hand, several different changes observed in the affected motor cortex such as reduced SICI [2,7-9], LICI [2], SAI [10], and enhanced long term potentiation (LTP) like activity [5] seem to be associated with a good outcome.
Brain-Derived Neurotrophic Factor (BDNF), secreted in response to neuronal activity, exercise, and motor learning [11], plays an important role in synaptic plasticity [12,13]. In at least a third of Caucasians, activity-dependent secretion of BDNF is reduced by a valine (Val) to methionine (Met) substitution in the precursor of the BDNF protein producing a common haplotype [Val66Met] [14].
Both functional magnetic resonance imaging studies [15] and TMS studies [16] have shown that this polymorphism impacts on human motor cortex function and motor system plasticity. These findings might have implications for the process of recovery after a stroke. The present study aimed to investigate whether BDNF Val66Met polymorphism influenced the cortical plasticity observed in the acute phase of stroke. To this end, we evaluated motor cortex excitability to single pulse TMS and the LTP-like activity induced by a repetitive TMS (rTMS) paradigm, known as intermittent theta burst stimulation (iTBS), in patients with acute stroke and correlated electrophysiological findings with BDNF genotype.
Material and methods
Patients
Twenty patients (11 males, 9 females; mean age: 64.15; standard error: 2.4) with first-ever stroke were recruited. Inclusion criteria were: 1) single ischemic stroke (both cortical and subcortical) involving the middle cerebral artery territory; 2) less than10 days post-stroke; 3) hand weakness; 4) recordable muscle evoked potential (MEP) after TMS of the AH. Exclusion criteria were: 1) history of seizure; 2) hemorrhagic stroke; 3) concomitant neurological or other severe medical problems; 4) complete paralysis of the hand; 5) inability to give informed consent; 6) treatment with drugs acting on the central nervous system; 7) contraindications for TMS studies. In order to identify patients at risk for post-stroke epilepsy all patients underwent an EEG before entering the study [17] and none of them showed any epileptic abnormality. The main clinical, neuroradiological and demographic characteristics of the patients are reported in Table 1. The evaluation of neurological impairment was based on the National Institutes of Health Stroke Scale (NIHSS). All the patients signed a written informed consent form. This study was conducted in accordance with the Helsinki Declaration of 1975 and was approved by the local Ethics Committee.
Table 1.
Epidemiological findings of ValVal and Met carrier groups.
| Patient | Sex | Age | NIHSS | NIHSS – motor arm function | BDNF genotype |
|---|---|---|---|---|---|
| ValVal group | |||||
| 1 | M | 54 | 6 | 2 | ValVal |
| 2 | M | 79 | 2 | 1 | ValVal |
| 3 | F | 74 | 2 | 2 | ValVal |
| 4 | M | 56 | 12 | 4 | ValVal |
| 5 | M | 59 | 3 | 2 | ValVal |
| 6 | F | 66 | 5 | 1 | ValVal |
| 7 | F | 64 | 4 | 2 | ValVal |
| 8 | M | 68 | 6 | 3 | ValVal |
| 9 | M | 70 | 9 | 2 | ValVal |
| 10 | M | 70 | 5 | 2 | ValVal |
| 1 | F | 69 | 9 | 4 | ValVal |
| 12 | M | 83 | 4 | 3 | ValVal |
| Met carrier group | |||||
| 13 | F | 77 | 8 | 3 | ValMet |
| 14 | F | 53 | 7 | 2 | ValMet |
| 15 | M | 60 | 6 | 2 | ValMet |
| 16 | F | 47 | 4 | 1 | ValMet |
| 17 | F | 76 | 3 | 1 | MetMet |
| 18 | M | 54 | 7 | 2 | ValMet |
| 19 | M | 53 | 6 | 2 | ValMet |
| 20 | F | 51 | 9 | 1 | ValMet |
BDNF genotyping
Genomic DNA was extracted from whole blood using a standardized salting-out method [18]. SNP rs6265 (Val66Met) was genotyped using the TaqMan allelic discrimination assay from Applied Biosystems Inc. The predesigned SNP genotyping assay ID is ID C_11592758_10. Real-time PCR was performed in 20 μl volumes with 5 ng of genomic DNA, 1 μl TaqMan SNP genotyping assay (containing two PCR primers and two dye (VIC or FAM)-labeled TaqMan MGB probes) and 10 μl of TaqMan Universal PCR Master Mix (Applied Biosystem) according to the manufacturer’s manual. PCR was performed at 95 °C for 10 min and 40 cycles at 95 °C for 15 s and 60 °C for 1 min using the ABI Prism 7900HT RealTime PCR (Applied Biosystems). Analyzes of amplification products were achieved using SDS software, version 2.4. Two blank controls in each 96-well-plate were used for the assay quality control. Subjects were genotyped as follows: homozygous for the Val allele (ValVal), heterozygotes (Val-Met), and homozygous for the Met allele (MetMet).
Magnetic stimulation
Motor cortex excitability to single pulse TMS
Magnetic stimulation was performed with a high-power Magstim 200 (Magstim Co., Whitland, Dyfed). A figure-of-eight coil with external loop diameters of 9 cm was held over the motor cortex at the optimum scalp position to elicit MEPs in the contralateral first dorsal interosseous muscle (FDI). The induced current flowed in a postero-anterior direction.
We evaluated the threshold and amplitude of MEPs. The resting motor threshold (RMT) was defined as the minimum stimulus intensity, expressed as the percentage of the maximal output intensity deliverable by the stimulator, which produced a liminal MEP (about 50 μV in 50% of 10 trials) at rest [19]. The active motor threshold (AMT) was defined as the minimum stimulus intensity that produced a liminal MEP (about 200 μV in 50% of 10 trials) during isometric contraction of the tested muscle. The MEP amplitude was evaluated using a stimulus intensity of 120% RMT with the muscle at rest. Ten data sweeps were collected, and the mean peak-to-peak amplitude of the MEPs was calculated. We evaluated the RMT, AMT and MEP amplitude elicited from the AH and UH.
Intermittent theta burst stimulation
iTBS was delivered over the affected motor cortex “hot spot” for MEPs in the contralateral FDI muscle using a MagPro stimulator (Medtronic A/S Denmark) connected to a figure-of-eight coil (MCF B65). The magnetic stimulus had a biphasic waveform with a pulse width of about 280 μs and a maximum magnetic field strength of 1.5 T. The initial direction of the current induced in the brain was anterior to posterior. The stimulation intensity was defined in relation to the AMT evaluated using the MagPro stimulator. An intensity of 80% AMT was used. We applied the iTBS protocol in which 10 bursts of high frequency stimulation (3 pulses at 50 Hz) are applied at 5 Hz every 10 s, for a total of 600 pulses [20].
MEP amplitude was evaluated before and immediately after iTBS using a stimulus intensity of 120% RMT with the muscle at rest. Subjects were given audio-visual feedback of their electromyographic (EMG) signal at high gain to assist them in maintaining complete relaxation; trials contaminated by EMG activity were discarded. Ten data sweeps were collected, and the mean peak-to-peak amplitude of the MEPs was calculated.
Statistical analysis
Because our cohort included only a single MetMet patient, we identified two groups: a ValVal group and Met carrier group (Val-Met or MetMet). We evaluated if and how BDNF genotype could impact on stroke patients’ brain excitability and plasticity by testing RMT, AMT and MEP amplitude for both hemispheres before and after iTBS. We also characterized, before and after iTBS, inter-hemispheric excitability balance by computing the Laterality Index (LI) [21]. LI, initially implemented in fMRI, is a simple index which can facilitate the description of the excitability imbalance in stroke patients.
In the case of MEP amplitude LI is expressed by the following equation:
Unlike MEP amplitude, AMT and RMT are linked to excitability with a negative correlation: the lower the value, the higher is the excitability. Thus, in order to have a positive LI consistently meaning higher UH excitability, the numerator of the formula was changed so that UH values were subtracted from AH values in the case of AMT and RMT, as expressed by the following equations:
LI ranges from −1 to +1 and the bigger the higher distance from 0, the is the inter-hemispheric imbalance. Positive values denote higher excitability of the UH.
To evaluate the effect of BDNF Val66Met polymorphism on cortical excitability, we separately applied a mixed model ANOVA with Hemisphere (Affected and Unaffected) as within subjects factor and Genotype (ValVal and Met carriers) as between subjects factor to RMT, AMT and MEP amplitude. An independent sample t-test was used to test LI differences between the two groups. We tested iTBS effects by a mixed model ANOVA with Hemisphere (Affected and Unaffected) and iTBS (pre and post) as within subjects factor and Genotype (ValVal and Met carrier) as between subjects factor, separately applied to RMT, AMT and MEP amplitude, and LI. For LI the ANOVA model did not include the factor Hemisphere. The significance level was set to P < 0.05. Descriptive statistics are reported as mean ± standard error of the mean (SER).
Results
The two groups were matched with respect to sex, age and NIHSS before iTBS (Chi-Square P = 0.199, and independent sample t-tests P = 0.066 and P = 0.594, respectively). Moreover, there was no significant difference in upper limb motor function before iTBS, as evaluated by the relevant item of the NIHSS (P = 0.167) (Table 1).
BDNF haplotype affects motor cortex excitability changes
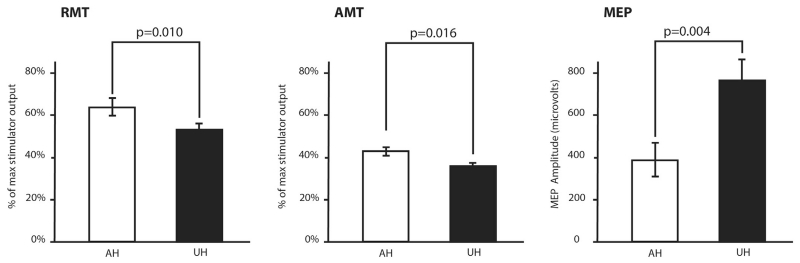
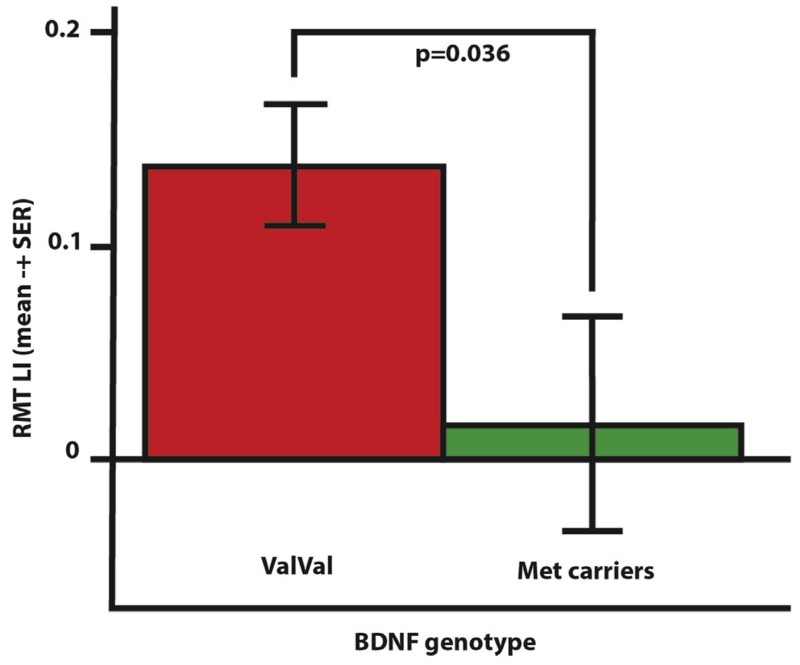
Recordings in two representative patients are shown in Fig. 1. Considering all patients together, UH excitability was higher than AH excitability as probed by RMT, AMT and MEP [Factor Hemisphere: P = 0.010, P = 0.016, P = 0.004, respectively; Fig. 2]. RMT, AMT and MEP did not differ between groups, but RMT LI showed a significant difference between groups, being 0.139 ± 0.028 for ValVal and 0.016 ± 0.050 for Met carriers [t = 2.270, P = 0.036; Fig. 3]. No differences between groups were found when considering MEP LI and AMT LI.
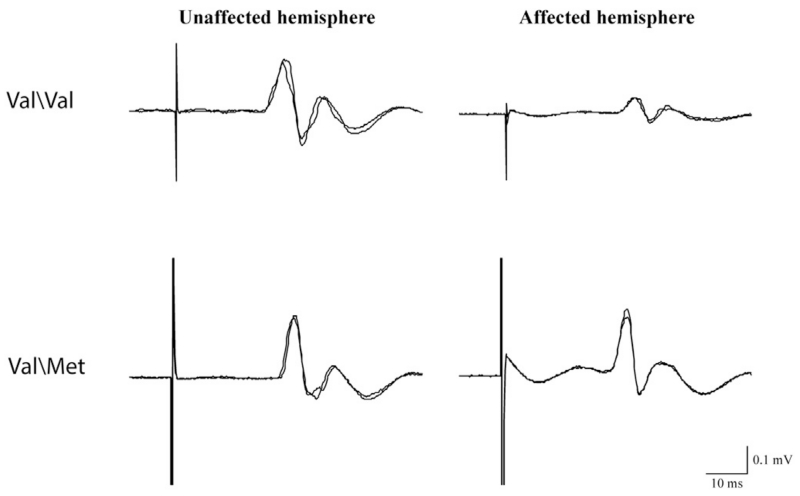
Figure 1.
Motor evoked potentials evoked by the stimulation of the Unaffected and Affected hemispheres in a Val\Val (first row) and a Met carrier (second row) representative subject. Each of the two superimposed traces is the average of five trials.
Figure 2.
Interhemispheric excitability imbalance as probed by resting motor threshold (RMT), active motor threshold (AMT) and motor-evoked potential (MEP) mean values for the affected (AH) and unaffected (UH) hemisphere.
Figure 3.
RMT Laterality Index for ValVal and Met carrier groups. In the acute phase of stroke ValVal patients show a significantly higher inter-hemispheric excitability imbalance compared to Met carrier patients.
BDNF haplotype does not affect iTBS-related LTP-LTD-like phenomena
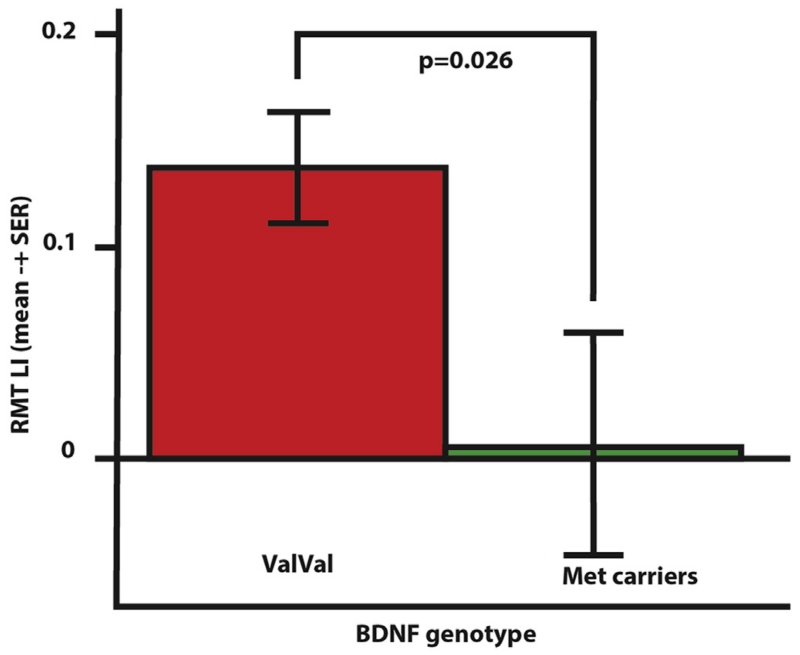
Considering all the patients together, an overall iTBS effect was evident only on AMT [Factor iTBS: F(1,18) = 5.997, P = 0.025] and AMT LI [Factor iTBS F(1,18) = 6.780, P = 0.018]. None of the considered measures were differently modulated between ValVal and Met carriers. In particular, iTBS did not change the RMT LI difference between groups [iTBS by Genotype interaction P = 0.406]. Thus RMT LI remained different between ValVal [0.150 ± 0.029] and Met carriers [0.016 ± 0.050] after neuromodulation [t = 2.424, P = 0.026; Fig. 4]. Taken together, the results suggest that inter-hemispheric imbalance with higher excitability over the UH, as probed by RMT LI, was about 9 times stronger for the ValVal group than the Met carriers group, both before and after iTBS.
Figure 4.
RMT Laterality Index for ValVal and Met carrier groups after iTBS.
Discussion
Several authors have reported an abnormal increase in UH M1 excitability [8,9,22,23] and in the inhibitory drive of the UH on the AH [6,24], after stroke. This is the first study evaluating the effects of BDNF polymorphism on the changes of human brain excitability observed in the acute phase of stroke. Remarkably, the presence of the Val66Met BDNF polymorphism was associated with a 9 fold weaker inter-hemispheric imbalance in cortical excitability as evaluated by comparing the RMT of the AH and the UH. RMT is a neurophysiological parameter that depends on glutamatergic synaptic excitability [1]. Experimental studies have shown that changes in glutamate signaling impact on recovery after stroke and that the effect is, at least in part, mediated by BDNF release [25]. Indeed, through tyrosine kinase receptor signaling, BDNF can induce potentiation of presynaptic glutamate release and is able to increase the response to glutamate at postsynaptic sites [26]. The Val66Met BDNF genotype, by influencing the intracellular trafficking and secretion of the neurotrophin BDNF, impairs glutamate-dependent plasticity as evidenced by reports of poorer episodic memory and abnormal hippocampal activation [14], reductions in hippocampal gray matter [27] and absence of training-induced expansion of the cortical motor map [28]. However, it should be stressed that BDNF has a pleiotropic effect in ischemic tissue: it promotes neuronal survival and differentiation [29], produces angiogenesis [30] and induces synaptic plasticity [31].
In a previous study we found that hyperexcitability of the UH was associated with poor long-term recovery and might represent a form of maladaptive plasticity [5]. The present results could suggest the possibility that the presence of BDNF polymorphism could prevent development of this form of maladaptive plasticity, thereby facilitating recovery. Plasticity in the motor cortex appears partly genetically determined [31], with subjects with BDNF polymorphism showing a smaller expansion in motor map size after training as revealed by TMS [28]. Likewise, individuals with Val/Met demonstrate reduced short-term plasticity in response to a variety of non-invasive brain stimulation protocols [32], and smaller activation volume in cortical areas after motor training [15]. All these effects, like those reported here, suggest diminished plasticity in subjects with BDNF polymorphism.
However, can the imbalance in cortical excitability attenuated by BDNF polymorphism always be considered deleterious? A recent study suggested that whether compensatory activity from the UH contributes to, or interferes with, paretic limb function depends on the level of impairment of the latter [33]. UH over-activity might interfere with paretic limb function in patients with less severe damage, as those with preserved MEPs included in this and our previous study [5], while it might have a compensatory role in severely affected patients [33,35]. This hypothesis might explain why some studies evaluating the effects of BDNF haplotype on recovery after stroke demonstrate a poorer outcome in patients with BDNF polymorphism [34]. Functional changes taking place in the UH might be adaptive or maladaptive depending on the degree of corticospinal tract damage.
In contrast to the relative hemispheric excitabilities, we did not find a difference between the level of AH LTP-like activity, as evidenced by the response to iTBS, and the presence of BDNF polymorphism. It might be that this latter form of brain plasticity is more complex and less dependent on a single gene, but influenced by the interaction of multiple genes [34].
Finally, some limitations of the present study should be considered in the interpretation of our results. The main shortcomings of our research are the small simple size and its heterogeneity. For instance, patients were not stratified for the extent and the location of the ischemic lesion. We cannot rule out that these factors, in addition to genetic features, could have some influence on the inter-hemispheric imbalance we report. Moreover, additional, specifically-designed studies are required to demonstrate the role of such genetic and neurophysiological features in determining the outcome in stroke patients. In summary, we present the first demonstration of decreased inter-hemispheric imbalance in cortical excitability in stroke patients carrying the Val66Met BDNF haplotype compared to controls. This has potential implications for the development of non-invasive neuromodulation techniques to promote recovery in stroke, in that it suggests an individually tailored strategy that takes into consideration genetic determinants together with neuroradiological and neurophysiological features.
Acknowledgments
Funding: None.
References
- [1].Paulus W, Classen J, Cohen LG, et al. State of the art: pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimul. 2008 Jul;1:151–63. doi: 10.1016/j.brs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- [2].Swayne OB, Rothwell JC, Ward NS, Greenwood RJ. Stages of motor output reorganization after hemispheric stroke suggested by longitudinal studies of cortical physiology. Cereb Cortex. 2008;18:1909–22. doi: 10.1093/cercor/bhm218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Talelli P, Greenwood R, Rothwell J. Arm function after stroke: neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clin Neurophysiol. 2006;117:1641–59. doi: 10.1016/j.clinph.2006.01.016. [DOI] [PubMed] [Google Scholar]
- [4].Hendricks HT, Zwarts MJ, Plat EF, van Limbeek J. Systematic review for the early prediction of motor and functional outcome after stroke by using motor-evoked potentials. Arch Phys Med Rehabil. 2002 Sep;83:1303–8. doi: 10.1053/apmr.2002.34284. [DOI] [PubMed] [Google Scholar]
- [5].Di Lazzaro V, Profice P, Pilato F, et al. Motor cortex plasticity predicts recovery in acute stroke. Cereb Cortex. 2010;20:1523–8. doi: 10.1093/cercor/bhp216. [DOI] [PubMed] [Google Scholar]
- [6].Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–9. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- [7].Liepert J, Storch P, Fritsch A, Weiller C. Motor cortex disinhibition in acute stroke. Clin Neurophysiol. 2000;111:671. doi: 10.1016/s1388-2457(99)00312-0. [DOI] [PubMed] [Google Scholar]
- [8].Manganotti P, Patuzzo S, Cortese F, Palermo A, Smania N, Fiaschi A. Motor disinhibition in affected and unaffected hemisphere in the early period of recovery after stroke. Clin Neurophysiol. 2002;113:936–43. doi: 10.1016/s1388-2457(02)00062-7. [DOI] [PubMed] [Google Scholar]
- [9].Cicinelli P, Pasqualetti P, Zaccagnini M, Traversa R, Oliveri M, Rossini PM. Interhemispheric asymmetries of motor cortex excitability in the postacute stroke stage a paired-pulse transcranial magnetic stimulation study. Stroke. 2003;34:2653–8. doi: 10.1161/01.STR.0000092122.96722.72. [DOI] [PubMed] [Google Scholar]
- [10].Di Lazzaro V, Profice P, Pilato F, et al. The level of cortical afferent inhibition in acute stroke correlates with long-term functional recovery in humans. Stroke. 2012 Jan;43:250–2. doi: 10.1161/STROKEAHA.111.631085. [DOI] [PubMed] [Google Scholar]
- [11].Klintsova AY, Dickson E, Yoshida R, Greenough WT. Altered expression of BDNF and its high-affinity receptor TrkB in response to complex motor learning and moderate exercise. Brain Res. 2004 Nov 26;1028:92–104. doi: 10.1016/j.brainres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- [12].Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- [13].Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H. BDNF function and intracellular signaling in neurons. Histol Histopathol. 2010 Feb;25:237–58. doi: 10.14670/HH-25.237. [DOI] [PubMed] [Google Scholar]
- [14].Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- [15].McHughen SA, Rodriguez PF, Kleim JA, et al. BDNF val66met polymorphism influences motor system function in the human brain. Cereb Cortex. 2010 May;20:1254–62. doi: 10.1093/cercor/bhp189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cheeran B, Ritter C, Rothwell J, Siebner H. Mapping genetic influences on the corticospinal motor system in humans. Neuroscience. 2009;164:156–63. doi: 10.1016/j.neuroscience.2009.01.054. [DOI] [PubMed] [Google Scholar]
- [17].Rossini PM, Johnston CS. Facilitating acute stroke recovery with magnetic fields? Neurology. 2005 Aug 9;65:353–4. doi: 10.1212/01.wnl.0000173428.48059.c7. [DOI] [PubMed] [Google Scholar]
- [18].Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rossini PM, Barker AT, Berardelli A, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994 Aug;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- [20].Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005 Jan 20;45:201–6. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- [21].Cramer SC, Nelles G, Benson RR, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997 Dec;28:2518–27. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- [22].Liepert J, Hamzei F, Weiller C. Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle Nerve. 2000 Nov;23:1761–3. doi: 10.1002/1097-4598(200011)23:11<1761::aid-mus14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- [23].Shimizu T, Hosaki A, Hino T, et al. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125:1896–907. doi: 10.1093/brain/awf183. [DOI] [PubMed] [Google Scholar]
- [24].Traversa R, Cicinelli P, Bassi A, Rossini PM, Bernardi G. Mapping of motor cortical reorganization after stroke A brain stimulation study with focal magnetic pulses. Stroke. 1997;28:110–7. doi: 10.1161/01.str.28.1.110. [DOI] [PubMed] [Google Scholar]
- [25].Clarkson AN, Overman JJ, Zhong S, Mueller R, Lynch G, Carmichael ST. AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. J Neurosci. 2011 Mar 9;31:3766–75. doi: 10.1523/JNEUROSCI.5780-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013 Jan;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- [27].Pezawas L, Verchinski BA, Mattay VS, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kleim JA, Chan S, Pringle E, et al. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006 Jun;9:735–7. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- [29].Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003 Apr;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- [30].Kermani P, Hempstead B. Brain-derived neurotrophic factor: a newly described mediator of angiogenesis. Trends Cardiovasc Med. 2007 May;17:140–3. doi: 10.1016/j.tcm.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Missitzi J, Gentner R, Geladas N, et al. Plasticity in human motor cortex is in part genetically determined. J Physiol. 2011 Jan 15;589:297–306. doi: 10.1113/jphysiol.2010.200600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cheeran B, Talelli P, Mori F, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008 Dec 1;586:5717–25. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bradnam LV, Stinear CM, Byblow WD. Ipsilateral motor pathways after stroke: implications for non-invasive brain stimulation. Front Hum Neurosci. 2013;7:184. doi: 10.3389/fnhum.2013.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Manso H, Krug T, Sobral J, et al. Evidence for epistatic gene interactions between growth factor genes in stroke outcome. Eur J Neurol. 2012 Aug;19:1151–3. doi: 10.1111/j.1468-1331.2011.03625.x. [DOI] [PubMed] [Google Scholar]
- [35].Di Pino G, Pellegrino G, Assenza G, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. 2014 Sep;:9. doi: 10.1038/nrneurol.2014.162. http://dx.doi.org/10.1038/nrneurol.2014.162. [DOI] [PubMed]