Abstract
Oscillations form a ubiquitous feature of the central nervous system. Evidence is accruing from cortical and sub-cortical recordings that these rhythms may be functionally important, although the precise details of their roles remain unclear. The basal ganglia share this predilection for rhythmic activity which, as we see in Parkinson’s disease, becomes further enhanced in the dopamine depleted state. While certain cortical rhythms appear to penetrate the basal ganglia, others are transformed or blocked. Here, we discuss the functional association of oscillations in the basal ganglia and their relationship with cortical activity. We further explore the neural underpinnings of such oscillatory activity, including the important balance to be struck between facilitating information transmission and limiting information coding capacity. Finally, we introduce the notion that synchronised oscillatory activity can be broadly categorised as immutability promoting rhythms that reinforce incumbent processes, and mutability promoting rhythms that favour novel processing.
Keywords: Basal ganglia, Parkinson’s disease, Information theory, Cross-frequency, Immutable, Deep brain stimulation
Introduction
Oscillations form a ubiquitous feature of the central nervous system. Evidence is accruing from cortical and sub-cortical recordings that these rhythms may be functionally important, although the precise details of their roles remain unclear. The basal ganglia share this predilection for rhythmic activity which, as we see in Parkinson’s disease, becomes further enhanced in the dopamine depleted state. While certain cortical rhythms appear to penetrate the basal ganglia, others are transformed or blocked. Here, we discuss the functional association of oscillations in the basal ganglia and their relationship with cortical activity.
Anatomical substrate
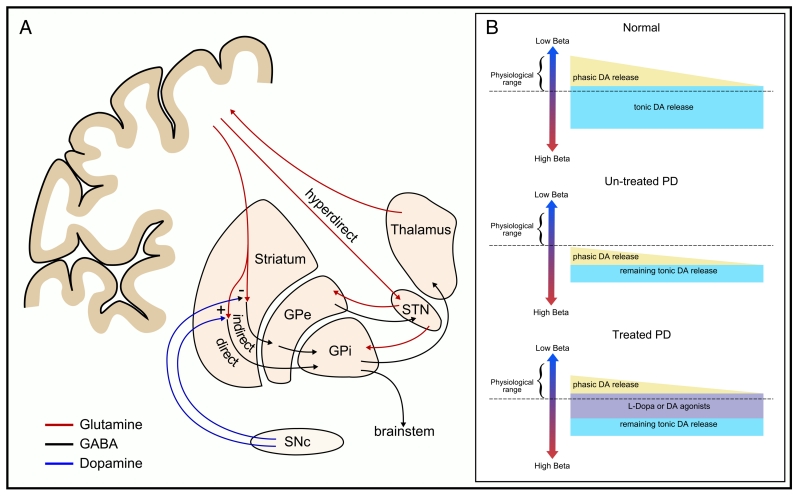
The basal ganglia consist of several parallel, homologous but functionally distinct loops that connect cortical limbic, oculomotor, prefrontal and motor territories (Nambu, 2008). We will focus predominantly on the motor loops — a series of circuits evolved for the control of voluntary movements (Fig. 1A). Convergence of motor projections from cortical territories occurs at the striatal level, with segregated circuits projecting through globus pallidus and substantia nigra to thalamic and brainstem (reticular) nuclei (Kaneda et al., 2002; Nambu, 2008). By far the most dominant source of synaptic inputs to the striatum comes from the corticostriatal pathways (Ingham et al., 1998; Mathai and Smith, 2011; Raju et al., 2008), where cortical afferents directly innervate the output cells of striatum — the medium spiny neurons (MSNs). A major ascending input onto the MSNs is dopaminergic and arises from the substantia nigra pars compacta. MSNs can be divided into those which express dopamine D1-class receptors and project to the globus pallidus pars interna (GPi), and those which express dopamine D2-class receptors and project to the globus pallidus pars externa (GPe). Dopamine release in the striatum leads to excitation of the direct pathway via the excitatory D1-receptors, while simultaneously inhibiting the indirect pathway via inhibitory D2-receptor activation. These pathways form the basis of the classic direct and indirect model of basal ganglia function as proposed by Albin et al. (1989) and DeLong (1990). The subthalamic nucleus (STN), the sole excitatory (glutaminergic) nucleus of the basal ganglia network also receives direct cortical afferents through a cortico-subthalamic projection (the hyperdirect pathway), of which relatively little is known (Mathai and Smith, 2011).
Fig. 1.
Anatomy of the basal ganglia and dopaminergic modulation of basal ganglia oscillations in Parkinson’s disease. [A] Major anatomical connections within and between the basal ganglia and cortex. [B] Schematic of the relationship between dopaminergic activity in the basal ganglia, and beta activity in health and in PD. Upper panel; normal state associated with low levels of beta. Middle panel; untreated PD. Due to the loss of nigral dopaminergic neurones there is less presynaptic dopamine for release in the striatum and STN. Net dopamine, the sum of tonic and phasic release modes, is low and the dynamic range of dopamine variation begins from a lower threshold than in the healthy state. Lower panel; treatment of PD patients with levodopa or dopamine agonists is thought to change the set-point of the system, driving the dynamic range into normal limits.
[B] Adapted with permission, Jenkinson and Brown (2011).
Oscillations — from single units to network activity
Rhythmic activity is a ubiquitous feature of the cerebro-basal ganglia network, having been observed at every level from single-units to extracranial magnetic fields. However, the origin and functional interpretation of such activity and how it relates to neuronal spiking are complex. Single-units can display highly regimented patterns of repetitive firing, with oscillatory dynamics emerging in the background local field potential (LFP). While LFPs undoubtedly result from a complex interaction of synaptic and cellular mechanisms (Logothetis et al., 2001), the major driving influence appears to originate from slow subthreshold currents, primarily post-synaptic potentials (Eccles, 1951). Numerous studies have shown rhythmic multi-unit firing that correlates with oscillations in the LFP (e.g. Kuhn et al., 2005), however the extent to which this relationship holds when moving to macroscales remains unclear (Manning et al., 2009; Ray and Maunsell, 2010; Truccolo et al., 2011; Wyler et al., 1982). Recordings must also consider neuronal morphology and, as they move towards broader spatial realms, the anatomical arrangement of cells (Buzsáki et al., 2012). For instance, magnetoencephalography is known to be sensitive to the orientation of the extensive dendritic trees of Purkinje cells that are thought to dominate the observed field (Okada and Nicholson, 1988).
Cortical rhythms
While oscillations are commonly considered to encode feature binding across various sensory modalities (e.g. visual, auditory, olfactory), their role in motor processing appears less clear (van Wijk and Daffertshofer, 2012). Before addressing the specific role of oscillations in the basal ganglia (a recipient of massive cortical input), some knowledge of their cortical counterparts would seem informative.
Most research into event-related brain oscillations focuses on changes in power that likely reflect the degree, and in the case of extracranial fields the spatial extent, of synchronisation in the neuronal population. Early pioneering work by Jasper and Andrews (1938) demonstrated 20 Hz oscillations close to the central sulcus that desynchronised (i.e. displayed a relative decrease in power, see Pfurtscheller and Lopes da Silva, 1999 for a discussion) upon passive and active manipulation of the limb. Cortical beta activity (13–30 Hz) is now widely associated with static motor control such as tonic or postural contraction. Cortical stimulation at beta frequencies is known to reduce motor output (Joundi et al., 2012a; Pogosyan et al., 2010) and beta has been consistently observed to desynchronise prior to and during movement execution (Lalo et al., 2007). Desynchronisation is often followed by a post movement beta rebound (PMBR), which is generally thought to represent the processing of somatosensory feedback, though this view remains contentious (see, for example, Parkes et al., 2006).
It was soon evident that, cortically at least, a wide range of frequencies react in anticipation of and during voluntary movements. Both beta and mu (8–13 Hz) rhythms demonstrate event-related-desynchronisation (ERD) prior to movement, with sustained suppression during movement execution. Despite initial similarities, differences in their time-course and topography implicate these rhythms as functionally distinct, though their precise roles remain a matter of some debate (Pfurtscheller and Lopes da Silva, 1999).
Several frequencies show event-related synchronisation (ERS; a relative increase in power) upon movement execution. Gamma band activity (40–90 Hz) synchronises just prior to movement onset and has been described as representing active information processing (Pfurtscheller et al., 1993; Salenius et al., 1996). During movement, the increase in power appears more focal than either the mu or beta ERD, and can be more consistently localised to the functional anatomy of the sensorimotor strip (Crone et al., 1998). Gamma band activity is therefore considered to promote movement; indeed cortical stimulation at gamma frequencies increases the rate of force production (Joundi et al., 2012a). Fluctuations in oscillatory dynamics are also regularly observed in the theta band (4–7 Hz) prior to and during movement, though these are generally considered to reflect ongoing cognitive processes, such as attention and memory processing (Kilmesch, 1999).
But what of these oscillations at the sub-cortical level — do they pervade the basal ganglia, are they generated sub-cortically, or are they transformed or even focussed in the basal ganglia? Some oscillations, such as the beta rhythm, appear ubiquitous throughout the basal ganglia network whilst others, such as the mu rhythm, are transformed or attenuated entirely (Klostermann et al., 2007). Transient fluctuations in power and modulation in the strength of coupling with cortex demonstrate a dynamic circuit that selectively propagates oscillatory activity in a prescribed manner. For a more thorough discussion on cortical sensorimotor rhythms see, for example, Cheyne (in press).
Dopamine dependent rhythms in the basal ganglia
Most of our knowledge regarding the role of oscillatory activity in the basal ganglia comes either from animal models (usually rodent or non-human primate) or patients undergoing deep brain stimulation (DBS). The latter involves the implantation of DBS electrodes into the basal ganglia, thalamic and even brainstem nuclei. Recordings from these sites offer a glimpse into the sub-cortical functioning of specific brain networks, albeit under the guise of disease state. Recordings in patients can be made intra-operatively from the microelectrodes used to aid functional localisation of surgical targets. Microelectrodes afford recording of single neuronal units, the background firing of multiple units, and of the local field potential (LFP). Alternatively LFP recordings can be made post-operatively, with fewer time constraints, directly from the DBS electrode. The most common target for DBS, the STN, is implanted for the treatment of Parkinson’s disease (PD) — a condition caused by the loss of dopaminergic cells in the substantia nigra and hence a marked reduction in the dopaminergic innervation of the basal ganglia, particularly the striatum. The depletion in dopamine levels favours the classic indirect pathway in accordance with the Albin et al. (1989) and DeLong (1990) model. Accordingly, the bradykinesia and akinesia (slowing and paucity of movement, respectively) of PD can be effectively treated through administration of the dopamine precursor levodopa. Thus PD offers a fascinating model through which the functioning of the basal ganglia can be probed with a controllable bias in net dopamine levels (see Fig. 1B).
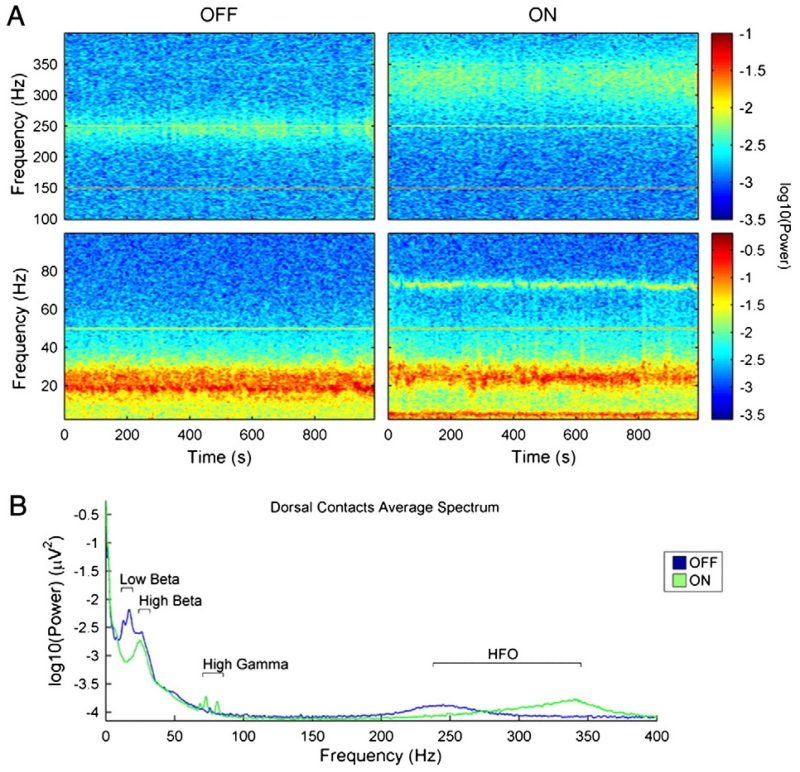
Data from PD patients suggest that 95% of STN LFP recordings made directly from the DBS electrode possess distinguishable peaks in the beta-band (Little and Brown, 2012; Fig. 2). When patients with PD are treated with dopaminergic medication, beta power in the STN LFP is largely dissipated (Hammond et al., 2007) and is instead replaced by increased synchronisation in the theta and gamma bands (Alonso-Frech, 2006; Brown et al., 2001). There is also a suggestion that high-frequency oscillations (about 250 Hz) are replaced by even higher frequency activity (250–350 Hz) in the STN (Foffani et al., 2003; López-Azcárate et al., 2010; Özkurt et al., 2011). In addition, a spectral peak in the alpha band is often seen, although this is not clearly modulated by dopaminergic state (Priori et al., 2004).
Fig. 2.
Grand average spectra from the STN observed ON and OFF dopaminergic medication in a cohort of patients with Parkinson’s disease. [A] Time-evolving spectra centred about the onset of movement during self-initiated wrist-extensions. Beta activity desynchronises prior to and during movement, particularly ON medication. [B] Time-averaged resting power spectrum. Note the reduction in low, but not high frequency beta power ON medication. There are also peaks in the theta/alpha, gamma and high-frequency (250–350 Hz) bands.
[A] and [B] adapted with permission, López-Azcárate et al. (2010).
Within the STN, firing of individual spike trains with spectral peaks in the beta band has been shown to phase-couple to the background multiple unit activity picked-up in micro-electrode recordings in 20–33% of instances across patients (Moran et al., 2008) and non-human primates (Moran et al., 2012). The same consistency was not evident for tremor-band activity (where tremor was clinically evident) despite small pockets of highly synchronised oscillators (Moran et al., 2008). Such differences in the degree of recruitment and phase-consistency are likely to be important determinants of which neuronal activities are best represented in the LFP.
Exaggerated beta activity tends to be most prominent in the motor territories of the Parkinsonian basal ganglia (Levy et al., 2002a,b; Stein and Bar-Gad, in press; Zaidel et al., 2010), and may be subdivided into lower (13–20 Hz) and upper (20–35 Hz) frequency bands (see Fig. 2). This was elegantly illustrated in a study of inter-hemispheric coupling between STN LFPs, in which peak activity was reported in one or both bands indicating that these frequencies are not mutually exclusive within a nucleus (de Solages et al., 2010). STN activity in the lower beta band usually dominates over power in the upper band, and background levels are suppressed more radically by dopaminergic activation (Litvak et al., 2011; López-Azcárate et al., 2010). However, there is sparse evidence that the phasic reduction in beta seen with movement is in-fact underpinned by changes in net dopamine levels. Rather this hypothesis remains to be proven, presumably through techniques capable of directly tracking the moment-to-moment fluctuations in dopamine level, such as fast-scan cyclic voltammetry (see Jenkinson and Brown, 2011 for a discussion).
As with the cortical response, beta recorded in the STN desynchronises in preparation of and during movement, with subsequent PMBR. Voluntary actions promote a desynchronisation of the beta-band of between 30 and 50%. Suppression is sustained during fast rhythmic finger tapping, with interdigitated rebound synchronisation occurring between movements at slower rates (Joundi et al., 2012b). Some degree of suppression is observed bilaterally, with the greatest desynchronisation contralateral to movement (Alegre et al., 2005; Joundi et al., 2012b). Overt speech, and even covert speech albeit to a lesser degree, displays a similar pattern of beta band desynchronisation (Brittain et al., 2012; Hebb et al., 2012). This movement-related reactivity in the beta band is likely a physiological feature, as it can be detected in healthy monkeys (Courtemanche et al., 2003), and non-Parkinsonian patients (Androulidakis et al., 2007; Sochurkova and Rektor, 2003) and displays a similar pattern of activation as its cortical counterpart (Fogelson et al., 2006; Pfurtscheller and Lopes da Silva, 1999).
Beta suppression is not only confined to movement, but may also be seen following cues that are predictive of forthcoming action demands. Observations from the rat demonstrate a global response of beta across the basal ganglia to cue utilisation (Leventhal et al., 2012). Recordings in patients show that salient cues suppress beta activity in the STN even when they cannot directly lead to explicit motor processing, and that this reactivity is promoted by dopaminergic mechanisms (Oswal et al., 2012). Although these recent findings are not inconsistent with the view that beta promotes the status quo or tonic activity at the expense of voluntary movements (Brown, 2007; Engel and Fries, 2010), they have raised the possibility that premovement reductions in the level of beta activity signal the likelihood that a new voluntary action will need to be actuated (for a thorough discussion see Jenkinson and Brown, 2011).
The putative promotion of tonic motor activity by high levels of beta and the failure of beta to suppress under circumstances that predict the need for action provide plausible means by which elevated and less reactive beta activity in Parkinsonism might contribute to bradykinesia, akinesia and rigidity (Fig. 1B). Treatment-induced suppression of beta activity has repeatedly been shown to correlate with improvements in clinical score (Eusebio et al., 2011; Kühn et al., 2006, 2009; Ray et al., 2008; Weinberger et al., 2006); however, it was only recently that spontaneous fluctuation in beta activity was shown to be a reliable cross-patient marker indexing ongoing clinical state (Chen et al., 2010a; Little et al., 2012). Such fluctuation is measured as the coefficient-of-variation or complexity in beta amplitude and could arise through variation in the strength, density and spatial extent of phase synchronisation. The latter can be inferred from the phase consistency of beta across DBS electrode contacts (Pogosyan et al., 2010), which also correlates with clinical state.
In sum, these observations suggest that it is both the degree of synchronisation and the reactivity of synchronisation (most likely in response to salient sensory and internal stimuli) that impact upon the performance of an ensemble of neurons (as, for example, in motor programming).
A mechanism for exaggerated beta in Parkinson’s disease
The enhanced beta rhythm observed in Parkinson’s disease does not proliferate throughout the cortico-basal ganglia loop in uniform fashion. Rather, the beta range can be subdivided into two distinct frequency bands which appear to show disparity in their modulation to dopamine depletion across the basal ganglia and cortex. We now discuss these distinctions and present a theory for the emergence of exaggerated lower frequency beta in the basal ganglia that involves an interaction between high frequency cortical beta and enhanced GPe–STN reciprocal coupling.
Repeated observations implicate the cerebral cortex as the most likely source of the enhanced beta rhythm ubiquitous throughout the basal-ganglia in Parkinson’s disease (Gradinaru et al., 2009; Hammond et al., 2007; Litvak et al., 2011). So is it that the influence of cortical beta upon the basal ganglia is increased or that cortical beta activity itself is also exaggerated? One suggestion is that the cortico-subthalamic pathway is enhanced through disinhibition of the STN by a suppressed GPe, allowing the STN to become entrained by excitatory cortical projections. However, MEG studies suggest that cortical beta over motor areas is increased at rest in patients with moderately advanced PD (Stoffers et al., 2008), and its suppression by movement is attenuated or even reversed in very mild PD (Pollok et al., 2012). Both aspects correlate with motor impairment (Pollok et al., 2012; Stoffers et al., 2008). Recent electrocorticographic recordings made directly from the primary motor cortex have shown that beta activity is increased when PD patients have to stop a movement compared to patients with dystonia, a disorder characterised by muscle spasms, and essential tremor, neither of which are associated with loss of neurons in the substantia nigra (Crowell et al., 2012). However, although this study demonstrated a divergence in resting cortical power between disease types at frequencies above 20 Hz, the elevation in beta activity in PD was not significant.
So cortical beta activity may be elevated in PD, but does this activity then drive the sub-cortical beta? Coherence and directionality analysis between motor cortical areas and basal ganglia nuclei confirm a predominant cortical drive that is, paradoxically, preferentially seen in the upper beta range (Hirschmann et al., 2011; Litvak et al., 2011; Williams et al., 2002). Thus it appears likely that the upper cortical beta range impacts upon basal ganglia activity, despite the emergence of a pronounced lower beta oscillation in the STN LFP in the absence of dopaminergic medication. Dopaminergic state does not, at rest, substantially change the coherence between cortex and STN in the upper beta band (Hirschmann et al., 2011; Litvak et al., 2011). However, beta activities in the upper and lower bands display non-linear interactions suggestive of harmonic relationships, with these interactions being severely attenuated by dopaminergic medication (Marceglia et al., 2006). A picture emerges in which high beta activity in motor cortical areas could drive similar subcortical oscillations that, in the low dopamine state, also lead to oscillations at subharmonic frequencies within the STN. Thus in the OFF medication state there may be a transformation of the descending high beta drive into basal ganglia oscillations in the low beta range. Proof of this hypothesis is awaited in a demonstration of selective bicoherence between cortical activity and STN LFPs, and its modulation by dopamine. Meanwhile interdependence of the two rhythms may serve to explain why treatment induced changes in both low and high beta activities in the STN correlate with improvements in akinesia–rigidity (Kühn et al., 2009).
The potential frequency transduction of cortical beta and the subcortical exaggeration of lower beta band activity could result from a common mechanism. The STN and GPe possess massively reciprocal and opposing connections, with GPe inhibiting STN and STN exciting GPe (Albin et al., 1989). As outlined in Marreiros et al. (2013), this local circuit is theoretically prone to unstable oscillatory activity and strengthening of this circuit in the absence of dopaminergic input is a recurrent theme in studies modelling the exaggerated beta activity seen in the Parkinsonian basal ganglia. Such strengthening has recently been corroborated in experimental models of Parkinson’s disease (Cruz et al., 2011; Fan et al., 2012). While the possibility exists that the intrinsically resonant GPe–STN network oscillates independently, it is just as likely that this network could be at least partially entrained by descending cortical high-beta oscillations. Evidence from non-human primates and medicated patients further suggests that these basal ganglia beta oscillations are prevented from re-innervating the cortex, hence averting a recurrent entrainment loop. This has been interpreted in terms of low pass filtering or damping of cortical input from the basal ganglia, with both effects appearing deficient in the untreated Parkinsonian state suggesting that they are dependent on dopaminergic innervation (Eusebio et al., 2009; Rivelin-Etzion et al., 2008).
Beyond motor control
Unsurprisingly, it has become increasingly apparent that oscillatory synchrony in the basal ganglia does not only impact on motor control. Cognitive and limbic processes may be modulated, although in these cases the responsible oscillations are likely concentrated in non-motor regions (e.g. Kühn et al., 2005; Rodriguez-Oroz et al., 2011).
Theta/alpha-band activity
Theta/alpha STN power is increased during cued button or keyboard presses (Alegre et al., 2013; Klostermann et al., 2007) and externally paced grips (Anzak et al., 2012), although the response in self-paced movements is less consistent (Oswal et al., 2013; Singh et al., 2011). Higher levels of theta and alpha STN power are associated with improved motor performance, at least in PD. Thus theta/alpha power in the STN correlates with force measures during onset, maintenance and release of maximal grips, and with the latency to onset of grip and its release (Anzak et al., 2012; Tan et al., 2013). The breadth of these associations raises the possibility that STN activity in the theta/alpha band might subserve a non-specific function related to movement, such as attention. This would be in line with views about coherent activity at similar frequencies between the STN, parieto-temporal cortex and brainstem of patients with PD (Hirschmann et al., 2011; Litvak et al., 2011). Similar reactivity upon movement in the STN of patients with PD and the GPi of patients with dystonia has led to the suggestion that movement related power increases may be primarily physiological rather than disease specific (Singh et al., 2011). However, this does not exclude a role in disease, and theta activity is especially raised at rest in the motor territory of the STN of PD patients with levodopa-induced dyskinesias (Rodriguez-Oroz et al., 2011) and in the STN and globus pallidus of patients with dystonia (Neumann et al., 2012; Silberstein et al., 2003). Theta activity in the STN LFP is also sensitive to conflict during decision making paradigms (Cavanagh et al., 2011; Fumagalli et al., 2011). Interestingly, resting theta power in the ventral STN is reported to be particularly elevated in PD patients with impulse-control disorders (Rodriguez-Oroz et al., 2011). Alpha band activity in the STN is suppressed by emotional stimuli, with the degree of suppression modulated by the affective state of the patient (Huebl et al., 2011; Kühn et al., 2005).
Beta-band activity
Research into beta activity has remained largely anchored in the motor realm. However, by viewing beta as a neural signature of motor planning in the basal ganglia, important inferences with respect to executive processing can be made. For instance, with regard to decision making, a post-ERD resynchronisation of beta-band activity within the STN (and cortically — see Swann et al., 2009) has now been consistently associated with delayed behavioural responding alongside a concomitant improvement in accuracy (Alegre et al., 2013; Brittain et al., 2012; Ray et al., 2012). A relative increase in STN beta activity is also seen during motor inhibition in the Go–NoGo task (Kühn et al., 2004). One theory posits that the STN plays an important role in inhibiting behaviour by providing a global stopping signal that is mediated through the hyperdirect pathway (Frank, 2006). Structural diffusion imaging has revealed the white matter tracts connecting STN to both inferior frontal cortex (IFC) and pre-supplementary motor area (preSMA; Aron et al., 2007). These sites are preferentially activated in conflict scenarios (Aron et al., 2007) and the integrity of these tracts have been shown to predict stopping performance (as measured, for example, by stop-signal reaction time; Coxon et al., 2012). Current theories posit the rapid resynchronisation of beta as the neural signature of the hyperdirect pathway, mediated through executive processing centres (Brittain et al., 2012). Such findings reveal the temporal interplay of motor preparation and its recruitment during conflict scenarios, further informing contemporary models of response inhibition (see, for instance, Aron, 2007; Frank, 2006). Beyond direct motor consequences, there is emerging evidence that beta-band reactivity may play an important role in semantic encoding strategies (Hanslmayr et al., 2009, 2012).
Gamma-band activity
Gamma activity in the basal ganglia may consist of two general patterns. The first is a narrow band synchronisation that can peak at any frequency between 60 and 90 Hz. This finely-tuned gamma (FTG) activity can be seen in some, but not all, resting spectra, is increased by dopaminergic therapy in PD patients, undergoes phasic increase during voluntary movement and is recorded in several disease syndromes (Kempf et al., 2009). The latter suggests that FTG in the basal ganglia is likely to possess a physiological, rather than largely pathological, role. Furthermore, comparable activity has been detected in the motor cortex of healthy subjects (Muthukumaraswamy, 2010), and this cortical activity is likely related to that at subcortical levels (Litvak et al., 2012). FTG is also dependent on arousal state and is increased after startling stimuli, suggesting that it might relate to the ascending reticular activating system, with the basal ganglia acting as a staging post to cerebral cortex (Jenkinson et al., 2013).
A more broadband form of gamma activity is also present in recordings of the basal ganglia. Unlike FTG this rhythm is not evident at rest, and instead emerges as a movement related synchronisation starting from around 40 Hz. The strength of synchronisation correlates with reaction time (Joundi et al., 2012c) and the size, force and velocity of voluntary movements (Anzak et al., 2012; Brucke et al., 2012; Tan et al., 2013). However, whether these are direct associations or that the primary relationship is with motor effort remains to be determined (see Jenkinson et al., 2013 for a discussion). It has also yet to be resolved whether the broad gamma ERS upon movement, which can extend up to 600 Hz in the STN (Litvak et al., 2012), is the product of multiple, dynamic, phase-coupled neuronal clusters spanning this broad frequency range, or reflects the brief and asynchronous burst of activity hypothesized to be an LFP correlate of population firing (Manning et al., 2009; Miller et al., 2009; Ray and Maunsell, 2010).
Gamma activity in the STN has also been implicated in cognitive processing. It is increased during the performance of paced random number generation and verbal fluency tests. These gamma changes correlate with a measure of randomness which indexes success in switching from automatic counting to a more controlled random generation of numbers (Anzak et al., 2013) and with the ability to switch between sub-categories in verbal fluency tasks (Anzak et al., 2011). Together these studies provide support for STN gamma activity in executive processes such as suppression of habitual or pre-potent responses and switching from automatic to controlled processing in cognitive tasks.
Segregation or multiplexing?
It may be an over-simplification to consider oscillatory synchrony within a given frequency band as having distinct functional associations. In practice task-related changes in synchrony occur across different frequency bands and all these changes may contribute to behaviour. We have, for example, already touched upon the robust correlations that have been reported between treatment induced suppressions of beta LFP activity in the STN and improvements in bradykinesia and rigidity (Kühn et al., 2006, 2008, 2009; Ray et al., 2008; Weinberger et al., 2006). And yet, the proportionate role of modulations in beta activity in the execution of a manual grip task has recently been noted to be relatively modest, with greater effects being seen with changes in the alpha and gamma frequency bands (Anzak et al., 2012; Tan et al., 2013). The latter studies have taken a multivariate approach to correlation, which highlights those LFP features that continue to predict behavioural performance while other features are held constant. Thus the relationship hitherto reported between beta activity and bradykinesia–rigidity might be tightly locked with or even secondary to effects at both lower and higher frequencies. Even at the level of the final effecter, motor-units can display complex cross-frequency associations with motor output (Halliday et al., 1995).
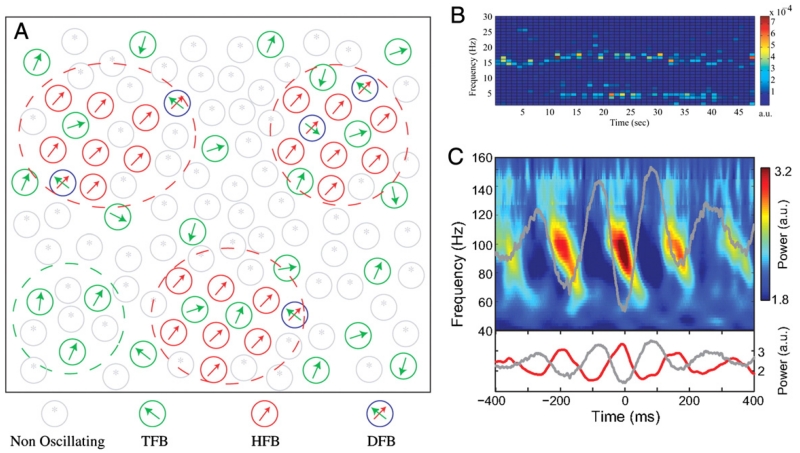
While synchronised pockets of oscillators may explain the presence of multiple spectral peaks in the local field potential, cross-frequency interactions (such as those reported in Lopez-Azcarate et al., 2010; Marceglia et al., 2006) require convergence in these anatomical pathways, and hence the capacity of neurons to encode multiple rhythms (Fig. 3). Such multiplexing behaviour has been observed in single units, where firing rate distributions can possess multiple distinct peaks (Moran et al., 2008, also Fig. 3B). The juxtaposition of anatomical convergence and cross-frequency coupling is further emphasised in a dynamic causal model of the cortical motor circuitry during execution of a manual grip (Chen et al., 2010b). In this study, the inclusion of cross-frequency associations between induced power changes in different cortical territories led to a significant improvement in model performance over the linear solution.
Fig. 3.
Multiplexing and cross-frequency interaction. [A] Functional organisation of a model of the STN in Parkinson’s disease depicting neurons classified by their oscillatory activity. Neurons are described as oscillating at tremor frequency band (TFB), high-frequency [8–20 Hz] band (HFB) or dual-frequency band (DFB). The model involves multiplexing both at the nuclear and neuronal levels. [B] Example of the time–frequency firing pattern of a DFB neuron oscillating simultaneously at both tremor and high (beta-range) frequencies; clear evidence of multiplexing within a neuron. [C] Cross-frequency coupling between theta-phase and gamma-amplitude in the rat striatum obtained during navigation of a T-maze task.
[A] and [B] adapted with permission, Moran et al. (2008), [C] adapted with permission, Tort et al. (2008).
Finally, cross-frequency interactions are not limited to consideration of local power changes. Levodopa treatment causes a frequency-selective change in the reactivity of cortico-subthalamic coherence during movement. The degree of reactivity correlates across the alpha and gamma bands with treatment-related improvement in motor performance (Litvak et al., 2012; Oswal et al., 2013). However, partial coherence analysis suggests that changes in coupling in the two frequency bands explain the same portion of the variance in the clinical response to treatment (Oswal et al., 2013). Interestingly, this is the case despite frequency dependent differences in the cortical topography of the coherences. The implication is that the dopamine dependent disengagement of the STN from its locking to temporal cortex at alpha band frequencies, and the engagement of STN locking to motor cortex in the gamma band are two sides of the same coin. The introduction of techniques such as singular value decomposition and higher-order spectra in the study of the basal ganglia should help define how such function may be underpinned by co-ordinated changes across frequencies.
Concluding speculation
Hitherto, oscillatory activities in circuits involving the basal ganglia have been broadly classified as primarily antikinetic (e.g. beta activity), or prokinetic (e.g. gamma activity), based on their respective behavioural associations in patients with PD (Brown, 2003). Yet this characterisation does not fully capture the nature of the two classes of activity which can, as we have seen, impact on non-motor executive processing. Indeed, we might better consider them as immutability promoting rhythms that reinforce incumbent processes and mutability promoting rhythms that favour novel processing within the broader executive domain.
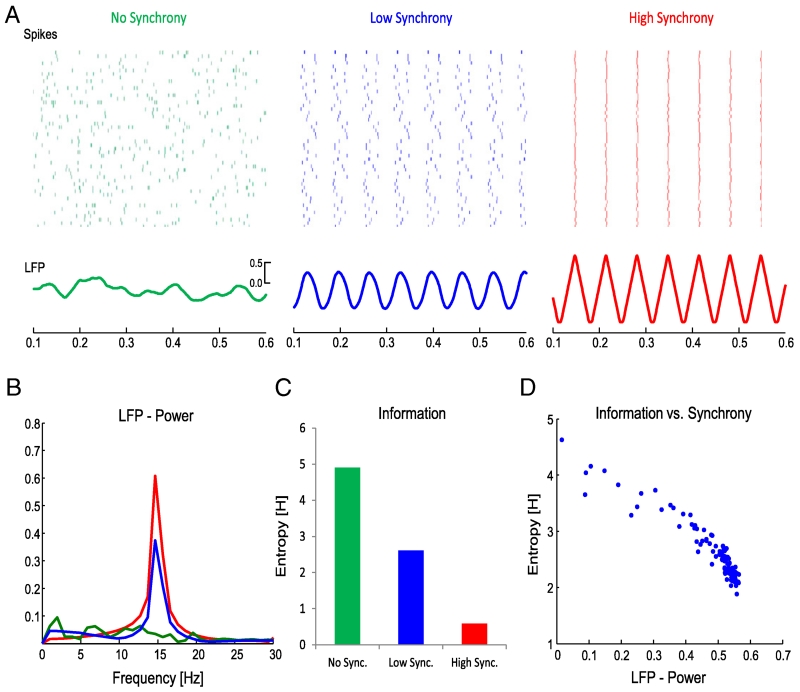
Beta is relatively persistent and can demonstrate phase synchrony over extensive neuronal populations, both locally and between connected regions or nuclei. This constrains neural activity to temporally predictable and spatially amorphous low-entropy signalling that impedes the response to novel demands. At times this may be advantageous, such as when suppressing the repertoire of actions that could potentially be invoked in a given scenario (Kuhn et al., 2004). Likewise, phasic increases in beta activity occur in the STN when a selected response suddenly becomes inappropriate (Alegre et al., 2013) or when the optimum response does not coincide with the pre-potent selection (Brittain et al., 2012). Conversely, there are times when a suppression of beta activity is desirable, such as during a motor response (Hammond et al., 2007). This may liberate circuits to engage in rate coding and more dynamic forms of synchronisation that are played out on a finer spatial resolution. There is good evidence for a reciprocal interaction between beta synchronisation and rate coding. For instance, during movement paradigms, desynchronisation of beta-band activity over primary motor cortex is associated with an increase in firing rate of pyramidal tract neurons, boosting output to the spinal cord (Baker et al., 2001; Spinks et al., 2008; van Wijk and Daffertshofer, 2012), and something very similar has been reported in the striatum of healthy non-human primates (Courtemanche et al., 2003). In short, beta desynchronisation leads to an increase in potential information coding (increased entropy). These concepts, while not new, are currently undergoing resurgence. Inspired by information theory, Barlow (1961) first proposed the efficient coding hypothesis as a model of how neurons encode sensory stimuli. More recently, Hanslmayr et al. (2012) reintroduced these concepts as information via desynchronisation (see Fig. 4).
Fig. 4.
Neural synchrony and its relationship to information theory. Note drop in information coding capacity (as formally indexed by entropy in C and D) with increased synchronisation (or its surrogate LFP power).
Reproduced with permission, Hanslmayr et al. (2012).
The idea that phasic increases in oscillatory activity, such as task related increases in the gamma band, can facilitate novel processing, such as that underpinning a voluntary movement, sits well with current views about how synchronisation promotes interaction between neuronal populations. These posit that synchronisation acts to strengthen relevant information coding channels through processes such as temporal summation and spike-timing dependent plasticity. Note however that implicit in this schema is spatial selectivity and a limit on the strength of synchronisation. The latter arises because at some point the loss of information coding space entailed by synchronisation will outstrip any advantage gained through temporal patterning (see Fig. 5). Indeed, it is precisely this feature that may potentially be exploited by immutability promoting rhythms, as typified by beta.
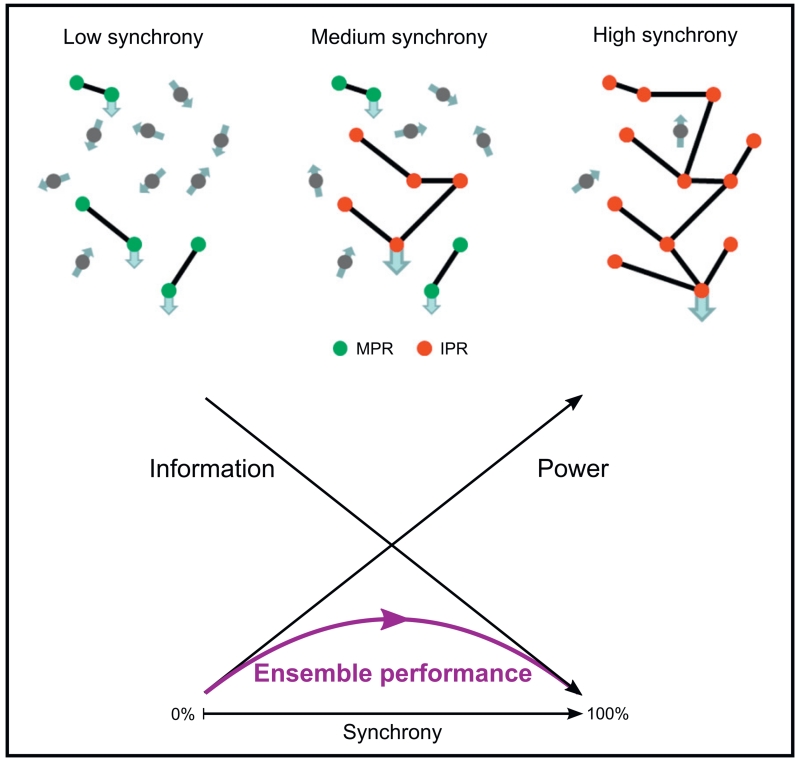
Fig. 5.
Idealised relationship between ensemble performance and neural synchrony. As sub-populations of neurons become correlated, the signal-to-noise ratio of that cluster relative to the population increases. At the same time the amount of information that can be transmitted by the ensemble decreases. The result is an inverted U-shape to ensemble performance as synchronisation increases. Mutability promoting rhythms (MPR), such as those in the gamma band, operate to the left of the ensemble performance curve, as dictated by their low power, weakly and locally synchronised nature. Increases in synchronisation in these activities improve ensemble performance and the ability to react to changing circumstances. Immutability promoting rhythms (IPR) such as alpha and beta, tend to operate to the right of the ensemble performance curve in keeping with their higher power, more extensively synchronised nature. This degree of synchronisation is more likely to be supported by recurrent networks where reinforcement of the ongoing oscillations leads to a further reduction in reactivity to external perturbation. Increases in synchronisation in more synchronised immutability promoting rhythms diminish ensemble performance and the ability to react to changing circumstances. This may, in turn, be exaggerated by plastic reconfiguration of networks by the rhythmic activities themselves (see text).
However, there is one empirical observation that suggests that beta band synchronisation may not merely impose its effects through a limitation on coding space. There is now a considerable evidence that high frequency DBS of basal ganglia targets drives output at stimulation frequency and subharmonics thereof, reducing the entropy of neuronal firing and restricting coding space (Dorval et al., 2008; Moran et al., 2011). This form of stimulation improves motor performance in PD, in contrast to exaggerated beta band synchrony which seems to impede motor processing. The implication is that the effects of synchrony do not simply relate to their impact on information coding capacity as they are partially frequency selective. Thus prominent beta synchronisation may have additional frequency specific consequences such as effects on plasticity. In a coupled oscillator model, low-frequency stimulation not only drove the neural populous to heightened beta-band synchronisation, but also actively reinforced the rhythm through long term potentiation (LTP; Tass and Majtanik, 2006). The GPi, being the key outflow structure of the basal ganglia, displays precisely the arrangement of converging and synchronised inhibitory striatal and excitatory subthalamic input that favours LTP (Schneidman et al., 2011), with an analogous arrangement at GPe. High-frequency stimulation in the above model has little effect on plasticity, with synaptic weights remaining fixed in their heightened pathological state (Tass and Majtanik, 2006). The favouring of the status quo by immutability promoting rhythms like beta therefore appears to be both passive, through a restriction on information coding capacity, and active through the reinforcement of current network relationships. DBS may therefore be effective because it drives neural output at higher frequencies away from beta-specific plasticity effects, albeit still at the expense of compromised information coding capacity. Thus DBS seems to possess both beneficial and deleterious effects on behaviour, depending on whether it is delivered in the setting of excessive beta synchrony or not (Chen et al., 2006; Ray et al., 2009).
Although oscillatory synchrony is evident in the operations of the basal ganglia, there remain many unknowns. Both synchronisation and desynchronisation appear to characterise active basal ganglia networks as they become engaged in task-specific actions, and at the very least these processes allow us to track the flow of information within these circuits. However, the field has suffered from a reductionist view in which function has been sought for specific frequencies rather than in cross-frequency patterns of modulation. Moreover, with respect to the basal ganglia, many of our insights have come from the study of diseased systems so extrapolation to normal function must remain necessarily tentative. Nevertheless, it is this link to disease that makes the study of oscillatory synchrony in the basal ganglia particularly important, and one that is already beginning to bear fruit (Rosin et al., 2011).
Acknowledgments
This work was supported by the Medical Research Council and the NIHR Oxford Biomedical Research Centre.
Footnotes
Conflicts of interest
PB has been a consultant for Medtronic Inc.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alegre M, Alonso-Frech F, Rodríguez-Oroz MC, Guridi J, Zamarbide I, Valencia M, Manrique M, Obeso JA, Artieda J. Movement-related changes in oscillatory activity in the human subthalamic nucleus: ipsilateral vs. contralateral movements. Eur. J. Neurosci. 2005;22:2315–2324. doi: 10.1111/j.1460-9568.2005.04409.x. [DOI] [PubMed] [Google Scholar]
- Alegre M, Lopez-Azcarate J, Obeso I, Wilkinson L, Rodriguez-Oroz MC, Valencia M, Garcia-Garcia D, Guridi J, Artieda J, Jahanshahi M, Obeso JA. The subthalamic nucleus is involved in successful inhibition in the stop-signal task: a local field potential study in Parkinson’s disease. Exp. Neurol. 2013;239:1–12. doi: 10.1016/j.expneurol.2012.08.027. [DOI] [PubMed] [Google Scholar]
- Alonso-Frech F. Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson’s disease. Brain. 2006;129:1748–1757. doi: 10.1093/brain/awl103. [DOI] [PubMed] [Google Scholar]
- Androulidakis AG, Doyle LMF, Yarrow K, Litvak V, Gilbertson TP, Brown P. Anticipatory changes in beta synchrony in the human corticospinal system and associated improvements in task performance. Eur. J. Neurosci. 2007;25:3758–3765. doi: 10.1111/j.1460-9568.2007.05620.x. [DOI] [PubMed] [Google Scholar]
- Anzak A, Gaynor L, Beigi M, Limousin P, Hariz M, Zrinzo L, Foltynie T, Brown P, Jahanshahi M. A gamma band specific role of the subthalamic nucleus in switching during verbal fluency tasks in Parkinson’s disease. Exp. Neurol. 2011;232:136–142. doi: 10.1016/j.expneurol.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Anzak A, Tan H, Pogosyan A, Foltynie T, Limousin P, Zrinzo L, Hariz M, Ashkan K, Bogdanovic M, Green AL, Aziz T, Brown P. Subthalamic nucleus activity optimizes maximal effort motor responses in Parkinson’s disease. Brain. 2012;135:2766–2778. doi: 10.1093/brain/aws183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzak A, Gaynor L, Beigi M, Foltynie T, Limousin P, Zrinzo L, Brown P, Jahanshahi M. Subthalamic nucleus gamma oscillations mediate a switch from automatic to controlled processing: a study of random number generation in Parkinson’s disease. NeuroImage. 2013;64:284–289. doi: 10.1016/j.neuroimage.2012.08.068. [DOI] [PubMed] [Google Scholar]
- Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J. Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, Spinks R, Jackson A, Lemon RN. Synchronization in monkey motor cortex during a precision grip task. I. Task-dependent modulation in single-unit synchrony. J. Neurophysiol. 2001;85:869–885. doi: 10.1152/jn.2001.85.2.869. [DOI] [PubMed] [Google Scholar]
- Barlow H. Possible principles underlying the transformation of sensory messages. Sensory Communication. 1961:217–234. [Google Scholar]
- Brittain J-S, Watkins KE, Joundi RA, Ray NJ, Holland P, Green AL, Aziz TZ, Jenkinson N. A role for the subthalamic nucleus in response inhibition during conflict. J. Neurosci. 2012;32:13396–13401. doi: 10.1523/JNEUROSCI.2259-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease. Mov. Disord. 2003;18:357–363. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- Brown P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr. Opin. Neurobiol. 2007;17:656–664. doi: 10.1016/j.conb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Lazzaro VD. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J. Neurosci. 2001;21:1033–1038. doi: 10.1523/JNEUROSCI.21-03-01033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brücke C, Huebl J, Schönecker T, Neumann W-J, Yarrow K, Kupsch A, Blahak C, Lütjens G, Brown P, Krauss JK, Schneider G-H, Kühn AA. Scaling of movement is related to pallidal γ oscillations in patients with dystonia. J. Neurosci. 2012;32:1008–1019. doi: 10.1523/JNEUROSCI.3860-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents - EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 2012;13:407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Wiecki TV, Cohen MX, Figueroa CM, Samanta J, Sherman SJ, Frank MJ. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat. Neurosci. 2011;14:1462–1467. doi: 10.1038/nn.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Brücke C, Kempf F, Kupsch A, Lu CS, Lee ST, Tisch S, Limousin P, Hariz M, Brown P. Deep brain stimulation of the subthalamic nucleus: a two-edged sword. Curr. Biol. 2006;16:R952–R953. doi: 10.1016/j.cub.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Chen CC, Hsu YT, Chan HL, Chiou SM, Tu PH, Lee ST, Tsai CH, Lu CS, Brown P. Complexity of subthalamic 13–35 Hz oscillatory activity directly correlates with clinical impairment in patients with Parkinson’s disease. Exp. Neurol. 2010a;224:234–240. doi: 10.1016/j.expneurol.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Chen CC, Kilner JM, Friston KJ, Kiebel SJ, Jolly RK, Ward NS. Nonlinear coupling in the human motor system. J. Neurosci. 2010b;30:8393–8399. doi: 10.1523/JNEUROSCI.1194-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne DO. MEG studies of sensorimotor rhythms: a review. Exp. Neurol. 2013 doi: 10.1016/j.expneurol.2012.08.030. in press, PMID: 22981841. [DOI] [PubMed] [Google Scholar]
- Courtemanche R, Fujii N, Graybiel AM. Synchronous, focally modulated β-band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. J. Neurosci. 2003;23:11741–11752. doi: 10.1523/JNEUROSCI.23-37-11741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coxon JP, Impe AV, Wenderoth V, Swinnen SP. Aging and inhibitory control of action: cortico-subthalamic connection strength predicts stopping performance. J. Neurosci. 2012;32:8401–8412. doi: 10.1523/JNEUROSCI.6360-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;121:2301–2315. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- Crowell AL, Ryapolova-Webb ES, Ostrem JL, Galifianakis NB, Shimamoto S, Lim DA, Starr PA. Oscillations in sensorimotor cortex in movement disorders: an electrocorticography study. Brain. 2012;135:615–630. doi: 10.1093/brain/awr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz AV, Mallet N, Magill PJ, Brown P, Averbeck BB. Effects of dopamine depletion on information flow between the subthalamic nucleus and external globus pallidus. J. Neurophysiol. 2011;106:2012–2023. doi: 10.1152/jn.00094.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Solages C, Hill BC, Koop MM, Henderson JM, Bronte-Stewart H. Bilateral symmetry and coherence of subthalamic nuclei beta band activity in Parkinson’s disease. Exp. Neurol. 2010;221:260–266. doi: 10.1016/j.expneurol.2009.11.012. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Dorval AD, Russo GS, Hashimoto T, Xu W, Grill WM, Vitek JL. Deep brain stimulation reduces neuronal entropy in the MPTP-primate model of Parkinson’s disease. J. Neurophysiol. 2008;100:2807–2818. doi: 10.1152/jn.90763.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC. Interpretation of action potentials evoked in the cerebral cortex. Electroencephalogr. Clin. Neurophysiol. 1951;3:449–464. doi: 10.1016/0013-4694(51)90033-8. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations — signalling the status quo? Curr. Opin. Neurobiol. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Eusebio A, Pogosyan A, Wang S, Averbeck B, Gaynor LD, Cantiniaux S, Witjas T, Limousin P, Azulay J-P, Brown P. Resonance in subthalamo-cortical circuits in Parkinson’s disease. Brain. 2009;132:2139–2150. doi: 10.1093/brain/awp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusebio A, Thevathasan W, Gaynor LD, Pogosyan A, Bye E, Foltynie T, Zrinzo L, Ashkan K, Aziz T, Aziz T, Brown P. Deep brain stimulation can suppress pathological synchronisation in Parkinsonian patients. J. Neurol. Neurosurg. Psychiatry. 2011;82:569–573. doi: 10.1136/jnnp.2010.217489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan KY, Baufreton J, Surmeier DJ, Chan CS, Bevan MD. Proliferation of external globus pallidus–subthalamic nucleus synapses following degeneration of midbrain dopamine neurons. J. Neurosci. 2012;32:13718–13728. doi: 10.1523/JNEUROSCI.5750-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foffani G, Priori A, Egidi M, Rampini P, Tamma F, Caputo E, Moxon KA, Cerutti S, Barbieri S. 300-Hz subthalamic oscillations in Parkinson’s disease. Brain. 2003;126:2153–2163. doi: 10.1093/brain/awg229. [DOI] [PubMed] [Google Scholar]
- Fogelson N, Williams D, Tijssen M, van Bruggen G, Speelman H, Brown P. Different functional loops between cerebral cortex and the subthalamic area in Parkinson’s disease. Cereb. Cortex. 2006;16:64–75. doi: 10.1093/cercor/bhi084. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Netw. 2006;19:1120–1136. doi: 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Fumagalli M, Giannicola G, Rosa M, Marceglia S, Lucchiari C, Mrakic-Sposta S, Servello D, Pacchetti C, Porta M, Sassi M, Zangaglia R, Franzini A, Albanese A, Romito L, Piacentini S, Zago S, Pravettoni G, Barbieri S, Priori A. Conflict-dependent dynamic of subthalamic nucleus oscillations during moral decisions. Soc. Neurosci. 2011;6:243–256. doi: 10.1080/17470919.2010.515148. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of Parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data — theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog. Biophys. Mol. Biol. 1995;64:237–278. doi: 10.1016/s0079-6107(96)00009-0. [DOI] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci. 2007;30:357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Spitzer B, Bäuml K-H. Brain oscillations dissociate between semantic and nonsemantic encoding of episodic memories. Cereb. Cortex. 2009;19:1631–1640. doi: 10.1093/cercor/bhn197. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Staudigl T, Fellner M-C. Oscillatory power decreases and long-term memory: the information via desynchronization hypothesis. Front. Hum. Neurosci. 2012;6:74. doi: 10.3389/fnhum.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb AO, Darvas F, Miller KJ. Transient and state modulation of beta power in human subthalamic nucleus during speech production and finger movement. Neuroscience. 2012;202:218–233. doi: 10.1016/j.neuroscience.2011.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschmann J, Özkurt TE, Butz M, Homburger M, Elben S, Hartmann CJ, Vesper J, Wojtecki L, Schnitzler A. Distinct oscillatory STN-cortical loops revealed by simultaneous MEG and local field potential recordings in patients with Parkinson’s disease. NeuroImage. 2011;55:1159–1168. doi: 10.1016/j.neuroimage.2010.11.063. [DOI] [PubMed] [Google Scholar]
- Huebl J, Schoenecker T, Siegert S, Brücke C, Schneider GH, Kupsch A, Yarrow K, Kühn AA. Modulation of subthalamic alpha activity to emotional stimuli correlates with depressive symptoms in Parkinson’s disease. Mov. Disord. 2011;26:477–483. doi: 10.1002/mds.23515. [DOI] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, Taggart P, Arbuthnott GW. Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. J. Neurosci. 1998;18:4732–4743. doi: 10.1523/JNEUROSCI.18-12-04732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper H, Andrews H. Electroencephalography. III. Normal differentiation of occipital and precentral regions in man. Arch. Neurol. Psychiatry. 1938;39:96–115. [Google Scholar]
- Jenkinson N, Brown P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci. 2011;34:611–618. doi: 10.1016/j.tins.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Jenkinson N, Kühn AA, Brown P. Gamma oscillations in the human basal ganglia. Exp. Neurol. 2013;245:72–76. doi: 10.1016/j.expneurol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Joundi RA, Jenkinson N, Brittain J-S, Aziz TZ, Brown P. Driving oscillatory activity in the human cortex enhances motor performance. Curr. Biol. 2012a;22:403–407. doi: 10.1016/j.cub.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joundi RA, Brittain J-S, Green AL, Aziz TZ, Brown P, Jenkinson N. Oscillatory activity in the subthalamic nucleus during arm reaching in Parkinson’s disease. Exp. Neurol. 2012b;236:319–326. doi: 10.1016/j.expneurol.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Joundi RA, Brittain JS, Green AL, Aziz TZ, Brown P, Jenkinson N. Persistent suppression of subthalamic beta-band activity during rhythmic finger tapping in Parkinson’s disease. Clin. Neurophysiol. 2012c;124:565–573. doi: 10.1016/j.clinph.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Kaneda K, Nambu A, Tokuno H, Takada M. Differential processing patterns of motor information via striatopallidal and striatonigral projections. J. Neurophysiol. 2002;88:1420–1432. doi: 10.1152/jn.2002.88.3.1420. [DOI] [PubMed] [Google Scholar]
- Kempf F, Brucke C, Salih F, Trottenberg T, Kupsch A, Schneider G-H, Doyle Gaynor LMF, Hoffmann K-T, Vesper J, Wöhrle J, Altenmüller D-M, Krauss JK, Mazzone P, Di Lazzaro V, Yelnik J, Kühn AA, Brown P. Gamma activity and reactivity in human thalamic local field potentials. Eur. J. Neurosci. 2009;29:943–953. doi: 10.1111/j.1460-9568.2009.06655.x. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klostermann F, Nikulin VV, Kühn AA, Marzinzik F, Wahl M, Pogosyan A, Kupsch A, Schneider G-H, Brown P, Curio G. Task-related differential dynamics of EEG alpha- and beta-band synchronization in cortico-basal motor structures. Eur. J. Neurosci. 2007;25:1604–1615. doi: 10.1111/j.1460-9568.2007.05417.x. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider G-H, Yarrow K, Brown P. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 2004;127:735–746. doi: 10.1093/brain/awh106. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Trottenberg T, Kivi A, Kupsch A, Schneider G-H, Brown P. The relationship between local field potential and neuronal discharge in the subthalamic nucleus of patients with Parkinson’s disease. Exp. Neurol. 2005;194:212–220. doi: 10.1016/j.expneurol.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Kupsch A, Schneider G-H, Brown P. Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur. J. Neurosci. 2006;23:1956–1960. doi: 10.1111/j.1460-9568.2006.04717.x. [DOI] [PubMed] [Google Scholar]
- Kühn AA, Kempf F, Brücke C, Doyle LG, Martinez-Torres I, Pogosyan A, Trottenberg T, Kupsch A, Schneider G-H, Hariz MI, Vandenberghe W, Nuttin B, Brown P. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory β activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J. Neurosci. 2008;28:6165–6173. doi: 10.1523/JNEUROSCI.0282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn AA, Tsui A, Aziz T, Ray N, Brücke C, Kupsch A, Schneider G-H, Brown P. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp. Neurol. 2009;215:380–387. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Lalo E, Gilbertson T, Doyle L, Di Lazzaro V, Cioni B, Brown P. Phasic increases in cortical beta activity are associated with alterations in sensory processing in the human. Exp. Brain Res. 2007;177:137–145. doi: 10.1007/s00221-006-0655-8. [DOI] [PubMed] [Google Scholar]
- Leventhal DK, Gage GJ, Schmidt R, Pettibone JR, Case AC, Berke JD. Basal ganglia beta oscillations accompany cue utilization. Neuron. 2012;73:523–536. doi: 10.1016/j.neuron.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Ashby P, Hutchison WD, Lang AE, Lozano AM, Dostrovsky JO. Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson’s disease. Brain. 2002a;125:1196–1209. doi: 10.1093/brain/awf128. [DOI] [PubMed] [Google Scholar]
- Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. Synchronized neuronal discharge in the basal ganglia of Parkinsonian patients is limited to oscillatory activity. J. Neurosci. 2002b;22:2855–2861. doi: 10.1523/JNEUROSCI.22-07-02855.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S, Brown P. What brain signals are suitable for feedback control of deep brain stimulation in Parkinson’s disease? Ann. N. Y. Acad. Sci. 2012;1265:9–24. doi: 10.1111/j.1749-6632.2012.06650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S, Pogosyan A, Kuhn AA, Brown P. Beta band stability over time correlates with Parkinsonian rigidity and bradykinesia. Exp. Neurol. 2012;236:383–388. doi: 10.1016/j.expneurol.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak V, Jha A, Eusebio A, Oostenveld R, Foltynie T, Limousin P, Zrinzo L, Hariz MI, Friston K, Brown P. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson’s disease. Brain. 2011;134:359–374. doi: 10.1093/brain/awq332. [DOI] [PubMed] [Google Scholar]
- Litvak V, Eusebio A, Jha A, Oostenveld R, Barnes G, Foltynie T, Limousin P, Zrinzo L, Hariz MI, Friston K, Brown P. Movement-related changes in local and long-range synchronization in Parkinson’s disease revealed by simultaneous magnetoencephalography and intracranial recordings. J. Neurosci. 2012;32:10541–10553. doi: 10.1523/JNEUROSCI.0767-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- López-Azcárate J, Tainta M, Rodríguez-Oroz MC, Valencia M, González R, Guridi J, Iriarte J, Obeso JA, Artieda J, Alegre M. Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson’s disease. J. Neurosci. 2010;30:6667–6677. doi: 10.1523/JNEUROSCI.5459-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in local field potential power spectra are correlated with single-neuron spiking in humans. J. Neurosci. 2009;29:13613–13620. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceglia S, Foffani G, Bianchi AM, Baselli G, Tamma F, Egidi M, Priori A. Dopamine-dependent non-linear correlation between subthalamic rhythms in Parkinson’s disease. J. Physiol. 2006;571:579–591. doi: 10.1113/jphysiol.2005.100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marreiros AC, Cagnan H, Moran RJ, Friston KJ, Brown P. Basal ganglia–cortical interactions in Parkinsonian patients. NeuroImage. 2013;66:301–310. doi: 10.1016/j.neuroimage.2012.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai A, Smith Y. The corticostriatal and corticosubthalamic pathways: two entries, one target. So what? Front. Syst. Neurosci. 2011;5:64. doi: 10.3389/fnsys.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Sorensen LB, Ojemann JG, Den Nijs M. Power-law scaling in the brain surface electric potential. PLoS Comput. Biol. 2009;5:e1000609. doi: 10.1371/journal.pcbi.1000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A, Bergman H, Israel Z, Bar-Gad I. Subthalamic nucleus functional organization revealed by Parkinsonian neuronal oscillations and synchrony. Brain. 2008;131:3395–3409. doi: 10.1093/brain/awn270. [DOI] [PubMed] [Google Scholar]
- Moran A, Stein E, Bar-Gad I. Dynamic stereotypic responses of basal ganglia neurons to subthalamic nucleus high-frequency stimulation in the Parkinsonian primate. Front. Syst. Neurosci. 2011;5:21. doi: 10.3389/fnsys.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A, Stein E, Tischler H, Bar-Gad I. Decoupling neuronal oscillations during subthalamic nucleus stimulation in the Parkinsonian primate. Neurobiol. Dis. 2012;45:583–590. doi: 10.1016/j.nbd.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD. Functional properties of human primary motor cortex gamma oscillations. J. Neurophysiol. 2010;104:2873–2885. doi: 10.1152/jn.00607.2010. [DOI] [PubMed] [Google Scholar]
- Nambu A. Seven problems on the basal ganglia. Curr. Opin. Neurobiol. 2008;18:595–604. doi: 10.1016/j.conb.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Neumann W-J, Huebl J, Brücke C, Ruiz MH, Kupsch A, Schneider G-H, Kühn AA. Enhanced low-frequency oscillatory activity of the subthalamic nucleus in a patient with dystonia. Mov. Disord. 2012;27:1063–1066. doi: 10.1002/mds.25078. [DOI] [PubMed] [Google Scholar]
- Okada YC, Nicholson C. Magnetic evoked field associated with transcortical currents in turtle cerebellum. Biophys. J. 1988;53:723–731. doi: 10.1016/S0006-3495(88)83153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswal A, Litvak V, Sauleau P, Brown P. Beta reactivity, prospective facilitation of executive processing, and its dependence on dopaminergic therapy in Parkinson’s disease. J. Neurosci. 2012;32:9909–9916. doi: 10.1523/JNEUROSCI.0275-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswal A, Brown P, Litvak V. Movement related dynamics of subthalmo-cortical alpha connectivity in Parkinson’s disease. NeuroImage. 2013;70:132–142. doi: 10.1016/j.neuroimage.2012.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özkurt TE, Butz M, Homburger M, Elben S, Vesper J, Wojtecki L, Schnitzler A. High frequency oscillations in the subthalamic nucleus: a neurophysiological marker of the motor state in Parkinson’s disease. Exp. Neurol. 2011;229:324–331. doi: 10.1016/j.expneurol.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Parkes LM, Bastiaansen MCM, Norris DG. Combining EEG and fMRI to investigate the post-movement beta rebound. NeuroImage. 2006;29:685–696. doi: 10.1016/j.neuroimage.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin. Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Kalcher J. 40-Hz oscillations during motor behavior in man. Neurosci. Lett. 1993;164:179–182. doi: 10.1016/0304-3940(93)90886-p. [DOI] [PubMed] [Google Scholar]
- Pogosyan A, Yoshida F, Chen CC, Martinez-Torres I, Foltynie T, Limousin P, Zrinzo L, Hariz MI, Brown P. Parkinsonian impairment correlates with spatially extensive subthalamic oscillatory synchronization. Neuroscience. 2010;171:245–257. doi: 10.1016/j.neuroscience.2010.08.068. [DOI] [PubMed] [Google Scholar]
- Pollok B, Krause V, Martsch W, Wach C, Schnitzler A, Südmeyer M. Motor-cortical oscillations in early stages of Parkinson’s disease. J. Physiol. 2012;590:3203–3212. doi: 10.1113/jphysiol.2012.231316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori A, Foffani G, Pesenti A, Tamma F, Bianchi AM, Pellegrini M, Locatelli M, Moxon KA, Villani RM. Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson’s disease. Exp. Neurol. 2004;189:369–379. doi: 10.1016/j.expneurol.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Raju DV, Ahern TH, Shah DJ, Wright TM, Standaert DG, Hall RA, Smith Y. Differential synaptic plasticity of the corticostriatal and thalamostriatal systems in an MPTP-treated monkey model of parkinsonism. Eur. J. Neurosci. 2008;27:1647–1658. doi: 10.1111/j.1460-9568.2008.06136.x. [DOI] [PubMed] [Google Scholar]
- Ray S, Maunsell JHR. Differences in gamma frequencies across visual cortex restrict their possible use in computation. Neuron. 2010;67:885–896. doi: 10.1016/j.neuron.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray NJ, Jenkinson N, Wang S, Holland P, Brittain JS, Joint C, Stein JF, Aziz T. Local field potential beta activity in the subthalamic nucleus of patients with Parkinson’s disease is associated with improvements in bradykinesia after dopamine and deep brain stimulation. Exp. Neurol. 2008;213:108–113. doi: 10.1016/j.expneurol.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Ray NJ, Jenkinson N, Brittain J, Holland P, Joint C, Nandi D, Bain PG, Yousif N, Green A, Stein JS, Aziz TZ. The role of the subthalamic nucleus in response inhibition: evidence from deep brain stimulation for Parkinson’s disease. Neuropsychologia. 2009;47:2828–2834. doi: 10.1016/j.neuropsychologia.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Ray NJ, Brittain J-S, Holland P, Joundi RA, Stein JF, Aziz TZ, Jenkinson N. The role of the subthalamic nucleus in response inhibition: evidence from local field potential recordings in the human subthalamic nucleus. NeuroImage. 2012;60:271–278. doi: 10.1016/j.neuroimage.2011.12.035. [DOI] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Marmor O, Saban G, Rosin B, Haber SN, Vaadia E, Prut Y, Bergman H. Low-Pass filter properties of basal ganglia–cortical–muscle loops in the normal and MPTP primate model of Parkinsonism. J. Neurosci. 2008;28:633–649. doi: 10.1523/JNEUROSCI.3388-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Oroz MC, López-Azcárate J, Garcia-Garcia D, Alegre M, Toledo J, Valencia M, Guridi J, Artieda J, Obeso JA. Involvement of the subthalamic nucleus in impulse control disorders associated with Parkinson’s disease. Brain. 2011;134:36–49. doi: 10.1093/brain/awq301. [DOI] [PubMed] [Google Scholar]
- Rosin B, Slovik M, Mitelman R, Rivlin-Etzion M, Haber SN, Israel Z, Vaadia E, Bergman H. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron. 2011;72:370–384. doi: 10.1016/j.neuron.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Salenius S, Salmelin R, Neuper C, Pfurtscheller G. Human cortical 40 Hz rhythm is closely related to EMG rhythmicity. Neurosci. Lett. 1996;213:75–78. doi: 10.1016/0304-3940(96)12796-8. [DOI] [PubMed] [Google Scholar]
- Schneidman E, Puchalla JL, Segev R, Harris RA, Bialek W, Berry MJ. Synergy from silence in a combinatorial neural code. J. Neurosci. 2011;31:15732–15741. doi: 10.1523/JNEUROSCI.0301-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein P, Kühn AA, Kupsch A, Trottenberg T, Krauss JK, Wöhrle JC, Mazzone P, Insola A, Lazzaro VD, Oliviero A, Aziz T, Brown P. Patterning of globus pallidus local field potentials differs between Parkinson’s disease and dystonia. Brain. 2003;126:2597–2608. doi: 10.1093/brain/awg267. [DOI] [PubMed] [Google Scholar]
- Singh A, Levin J, Mehrkens JH, Bötzel K. Alpha frequency modulation in the human basal ganglia is dependent on motor task. Eur. J. Neurosci. 2011;33:960–967. doi: 10.1111/j.1460-9568.2010.07577.x. [DOI] [PubMed] [Google Scholar]
- Sochurkova D, Rektor I. Event-related desynchronization/synchronization in the putamen. An SEEG case study. Exp. Brain Res. 2003;149:401–404. doi: 10.1007/s00221-003-1371-2. [DOI] [PubMed] [Google Scholar]
- Spinks RL, Kraskov A, Brochier T, Umilta MA, Lemon RN. Selectivity for grasp in local field potential and single neuron activity recorded simultaneously from M1 and F5 in the awake macaque monkey. J. Neurosci. 2008;28:10961–10971. doi: 10.1523/JNEUROSCI.1956-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein E, Bar-Gad I. Beta oscillations in the cortico-basal ganglia loop during parkinsonism. Exp. Neurol. 2013 doi: 10.1016/j.expneurol.2012.07.023. in press, PMID: 22921537. [DOI] [PubMed] [Google Scholar]
- Stoffers D, Bosboom JLW, Deijen JB, Wolters EC, Stam CJ, Berendse HW. Increased cortico-cortical functional connectivity in early-stage Parkinson’s disease: a magnetoencephalography study. NeuroImage. 2008;41:212–222. doi: 10.1016/j.neuroimage.2008.02.027. [DOI] [PubMed] [Google Scholar]
- Swann N, Tandon N, Canolty R, Ellmore TM, McEvoy LK, Dreyer S, DiSano M, Aron AR. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J. Neurosci. 2009;29:12675–12685. doi: 10.1523/JNEUROSCI.3359-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Pogosyan A, Anzak A, Foltynie T, Limousin P, Zrinzo L, Ashkan K, Bogdanovic M, Green AL, Aziz T, Brown P. Frequency specific activity in subthalamic nucleus correlates with hand bradykinesia in Parkinson’s disease. Exp. Neurol. 2013;240:122–129. doi: 10.1016/j.expneurol.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tass PA, Majtanik M. Long-term anti-kindling effects of desynchronizing brain stimulation: a theoretical study. Biol. Cybern. 2006;94:58–66. doi: 10.1007/s00422-005-0028-6. [DOI] [PubMed] [Google Scholar]
- Tort ABL, Kramer MA, Thorn C, Gibson DJ, Kubota Y, Graybiel AM, Kopell NJ. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. PNAS. 2008;105:20517–20522. doi: 10.1073/pnas.0810524105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truccolo W, Donoghue JA, Hochberg LR, Eskandar EN, Madsen JR, Anderson WS, Brown EN, Halgren E, Cash SS. Single-neuron dynamics in human focal epilepsy. Nat. Neurosci. 2011;14(5):635–641. doi: 10.1038/nn.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk BCM, Daffertshofer A. Neural synchrony within the motor system: what have we learned so far? Front. Hum. Neurosci. 2012;6:252. doi: 10.3389/fnhum.2012.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger M, Mahant N, Hutchison WD, Lozano AM, Moro E, Hodaie M, Lang AE, Dostrovsky JO. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson’s disease. J. Neurophysiol. 2006;96:3248–3256. doi: 10.1152/jn.00697.2006. [DOI] [PubMed] [Google Scholar]
- Williams D, Tijssen M, van Bruggen G, Bosch A, Insola A, Lazzaro VD, Mazzone P, Oliviero A, Quartarone A, Speelman H, Brown P. Dopamine-dependent changes in the functional connectivity between basal ganglia and cerebral cortex in humans. Brain. 2002;125:1558–1569. doi: 10.1093/brain/awf156. [DOI] [PubMed] [Google Scholar]
- Wyler AR, Ojemann GA, Ward AA. Neurons in human epileptic cortex: correlation between unit and EEG activity. Ann. Neurol. 1982;11(3):301–308. doi: 10.1002/ana.410110311. [DOI] [PubMed] [Google Scholar]
- Zaidel A, Spivak A, Grieb B, Bergman H, Israel Z. Subthalamic span of β oscillations predicts deep brain stimulation efficacy for patients with Parkinson’s disease. Brain. 2010;133:2007–2021. doi: 10.1093/brain/awq144. [DOI] [PubMed] [Google Scholar]