Abstract
The last decade has seen major progress at all levels of neuroscience, from genes and molecules up to integrated systems-level models of brain function. In particular, there have been advances in the understanding of cell-type-specific contributions to function, together with a clearer account of how these contributions are coordinated from moment to moment to organise behavior. A major current endeavor is to leverage this knowledge to develop new therapeutic approaches. In Parkinson’s disease, there are a number of promising emerging treatments. Here, we will highlight three ambitious novel therapeutic approaches for this condition, each robustly driven by primary neuroscience. Pharmacogenetics genetically re-engineers neurons to produce neurotrophins that are neuroprotective to vulnerable dopaminergic cells or to directly replace dopamine through enzyme transduction. Deep brain stimulation (DBS) is undergoing a transformation, with adaptive DBS controlled by neural signals resulting in better motor outcomes and significant reductions in overall stimulation that could reduce side effects. Finally, optogenetics presents the opportunity to achieve cell-type-specific control with a high temporal specification on a large enough scale to effectively repair network-level dysfunction.
Introduction
Dr James Parkinson, a London apothecary surgeon, defined the symptomatology, inexorable progression and burden of Parkinson’s disease (PD) in his brilliant treatise An Essay on the Shaking Palsy in 1817 [1]. In this he expressed his optimism “that some remedial process may ere long be discovered, by which, at least, the progress of the disease may be stopped”. Almost two hundred years later we are yet to vindicate his hopes with the discovery of a treatment that ceases progression, but we are beginning to see exciting new means by which the symptoms of this devastating disease may be better ameliorated.
Initially, PD was thought to be an acquired disorder but twin studies have revealed that inheritability plays a particularly strong role in patients who develop the condition below the age of fifty [2]. Subsequent investigation has delineated a number of single gene disorders which account for 5–10% of patients, and some common variants that confer additional risk to many others [3,4]. At the cellular level, idiopathic PD is particularly, but not exclusively, characterised by severe loss of dopaminergic neurons in the substantia nigra pars compacta and the accumulation of intracellular, cytoplasmic alpha-synuclein in the form of Lewy bodies [5,6]. Disease progression is associated with the spread of Lewy-body pathology rostrally up the brainstem to the mesocortex and finally neocortex, with the development of associated clinical signs [7]. Recent work raises the possibility that this spread may reflect a prion-like process whereby alpha-synuclein induces pathological refolding in previously healthy proteins with resultant Lewy-body aggregation [8,9]. The underlying molecular pathology is complex, involving many different molecular pathways, including protein folding and clearance, mitochondrial function/oxidative stress and the ubiquitin–proteasome system [10].
PD can manifest with diverse motor, cognitive, affective and autonomic symptoms. Impairment of voluntary movement may take the form of slowness (bradykinesia), stiffness (rigidity), tremor and postural instability, leading to falls [11]. With the exception of postural instability these motoric symptoms are the most readily treated. To this end, the gold-standard therapy is dopaminergic replacement using oral levodopa, a precursor of dopamine [12]. However, it is increasingly acknowledged that the PD-associated dopaminergic denervation of the basal ganglia is not homogeneous, and exogenous dopaminergic therapy with levodopa or dopamine agonists can itself be associated with side effects, like impulsivity, due to overstimulation in the ventral striatum where dopaminergic innervation remains relatively intact [13]. Moreover, PD is slowly progressive and, during the course of the disease, possibly partly in response to pulsatile oral dopaminergic therapy, paradoxical involuntary movements, termed dyskinesias, and unpredictable fluctuations in medication response develop in the majority of cases [14].
Such complications motivated the development of electrical deep brain stimulation (DBS), which involves the implantation of chronic electrodes into selected, focal basal ganglia structures (Figure 1) and, after connection to an internalised battery-powered stimulator, the delivery of continuous electrical stimulation using brief pulses at a set frequency that is most commonly above 100 Hz. Over 100,000 patients world-wide have undergone DBS, in particular for PD and tremor, with beneficial outcomes that are sustained and exceed those achievable in comparable patients treated only with oral medication [15–19]. Still DBS is by no means perfect, as patients show only partial responses that are complicated by side effects related to both surgery and, as will be discussed, continuous, regular stimulation. Indeed, it is remarkable quite how effective it is, given that stimulation generally consists of continuous high-frequency pulses, regardless of aetiology or patient state.
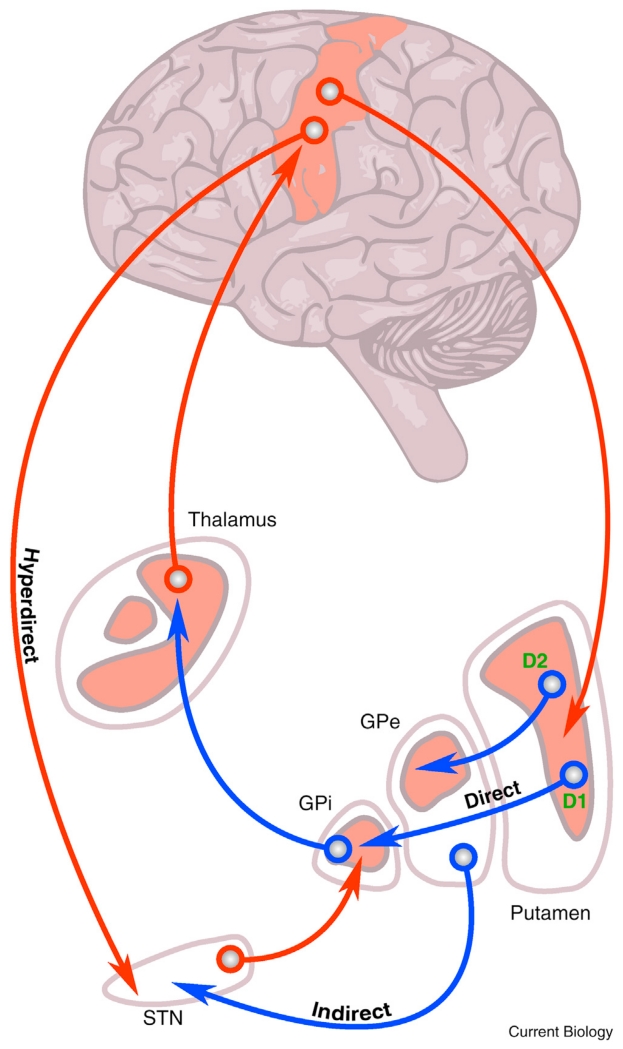
Figure 1.
Schematic of the classic model of motor loops between the cortex and the basal ganglia.
Note the excitatory glutamatergic connections (red) and the inhibitory GABA-ergic neurons (blue). Direct and indirect connections are highlighted with dissociated dopamine receptor associations. The hyperdirect pathway between cortex and subthalamic nucleus (STN) is also shown. DBS treatment for PD involves implantation of electrodes into the STN, globus pallidus interna (GPi) or thalamus depending on the patient phenotype. D1 and D2 dopamine receptors are excited and inhibited by dopamine released from the substantia nigra pars compacta. GPe, globus pallidus externa. Adapted with permission [102].
The limitations of both current pharmacological and surgical therapies highlight the need to interact with the brain with better spatial and temporal control. Here, our aim is to critically review and discuss emerging therapies that could deliver such improved spatiotemporal targeting. Three broad approaches are being taken. Firstly, there are attempts to restore physiologically appropriate spatiotemporal patterns of brain activity through the replacement and protection of dopaminergic neurons or the re-engineering of other cell types to produce dopamine via pharmacogenetics. Secondly, there are moves to improve on electrical DBS techniques so that they impose more appropriate temporal patterning on the nervous system, i.e. adaptive DBS. Finally, we discuss a technique which brings together both spatial and temporal targeting in animal models, namely optogenetics. These advances should improve therapeutic efficacy whilst reducing side effects and could ultimately provide benefit across a wide range of neuropsychiatric disorders.
Pharmacogenetics
One early approach to restore dopaminergic stimulation with a physiologically appropriate spatiotemporal pattern involved the replacement of dopaminergic neurons with embryonic dopaminergic cells [20]. Although the overall results of the clinical trials were negative, they did provide proof of principle that cell transplantation can successfully re-innervate the striatum and provide symptomatic relief in a minority of cases. However, this was at the cost of troublesome dyskinesias (despite withdrawal of levodopa in some patients), believed to be due to anomalous serotoninergic innervation of the striatum [21]. In view of these setbacks and because of additional ethical and practical obstacles in obtaining reliable and homogenous embryonic grafts, attention shifted to the use of stem cells which could potentially provide unlimited standardised dopaminergic neurons for implantation. This shows promise with both embryonic and fibroblast-derived stem cells (neuroblasts) differentiating into dopaminergic neurons, surviving transplantation into rodent models and resulting in a degree of functional recovery [22,23]. Dopaminergic neurons may even be directly converted from fibroblasts. However, such research is still at the preclinical stage and significant further work is required to determine the associated risks of tumor formation, immune reactions and graft-induced dyskinesias before clinical trials can be implemented [24]. Moreover, some of the initial enthusiasm for cell transplantation as a potential cure has dissipated following reports that alpha-synuclein pathology may re-emerge in transplanted cells [15]. For these various reasons, there is now increasing interest in symptomatic treatment through the re-engineering of healthy non-dopaminergic cells either to produce neuroprotective growth factors, to rebalance pathological networks or even to generate dopamine. This is the growing field of pharmacogenetics, which has resulted in a number of new agents progressing to human trials.
Pharmacogenetics involves the delivery of selected genes to specific targets in the brain with the aim of reprogramming the function of healthy cells to strengthen or take over from the susceptible cell population, here, dopaminergic neurons. Peripheral administration is limited by the impervious nature of the blood–brain barrier to many of the vectors and agents that have therapeutic potential, and thus primary delivery is usually achieved by stereotactic injection, harnessing techniques from the world of DBS. In order to integrate the genes into the host neuron a vector is required and this can take non-viral or viral forms [25]. Non-viral methods, such as polyplex synthetic nanocarriers and immunoliposomes, have shown some success in animal models but demonstrate limited and transient expression of transduced material and are therefore at an early pre-clinical stage of testing [26–28]. In order to achieve continuous, long-lasting gene expression, viral vectors, with their ability to integrate genetic material into the patient’s genome or construct intracellular episomes, currently appear most encouraging. A number of different families of viruses have shown promise for pharmacogenetic manipulation. The most favorable and clinically developed advances relate to adeno-associated virus (AAV) vectors and lentivirus vectors. AAVs are relatively small single-stranded DNA viruses of the Parvoviridae family that have a genome of around 4.7 kilobases. AAVs have been shown to lead to persistent functional integration over a period of years and, for PD, they have the advantage that they can demonstrate a degree of specificity for neurons of the basal ganglia[29–32]. Their small size, however, limits their genetic delivery capacity to a single neurotrophin or enzyme. Ideally, a vector should have the capacity to insert multiple genetic units that could act synergistically to enhance therapeutic efficacy. A larger, more accommodating, class of viruses is the lentivirus family, which is a group of retroviruses with a genome typically of about 10 kilobases. Initial concerns regarding potential oncogenicity due to chromosomal integration have partially been addressed through the creation of non-integrating lentiviruses that can be directed to drive expression transiently in dividing cells or in a sustained manner in non-dividing cells [33]. Both AAVs and lentiviruses have now been tested in patients with PD using a number of different pharmacogenetic approaches, including neuroprotective growth factor transduction, physiological network rebalancing through upregulation of gamma-aminobutyric acid (GABA) and reprogramming of non-dopaminergic neural tissue to produce dopamine.
Pharmacogenetic Neuroprotection
Neurotrophins are a collection of molecules that act on neurons to regulate and promote cell growth and survival and have shown promise in slowing dopaminergic denervation in PD. Glial-cell-derived neurotrophic factor (GDNF) directly injected into the striatum of parkinsonian mice demonstrated both neuroprotective effects and positive motor outcomes, but was subsequently not found to be efficacious in human trials [34,35]. Presuming inadequate neurotrophin penetration in early studies, pharmacogenetic approaches using both lentivirus and AAV vectors have since been employed which could lead to more widespread and longterm transduction of neurotrophins. Initial success with an AAV2–GDNF vector in monkeys rendered parkinsonian by the systemic application of the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) has led to open-label, and hence unblinded, phase I clinical trials, expected to be completed in 2018 [36,37]. Neurturin has functional similarities to GDNF and has also shown efficacy when delivered through an AAV vector in pre-clinical studies, although these have not yet translated into motor improvements or disease modification in robust double-blinded clinical trials[38,39]. A repeat study of AAV–Neurturin with higher dosages and longer follow-up times is currently underway.
Pharmacogenetic Network Compensation
Classically, dopamine depletion within the basal ganglia is believed to lead to underactivity of the direct pathway, which favours movement and bypasses the STN, and overactivity of the indirect pathway, which suppresses movement and involves the STN (Figure 1) [40]. An alternative approach to replacing dopamine then would be to rebalance the two pathways by inhibition of the subthalamic nucleus and indirect pathways, a policy supported by the partial success of lesional surgery and DBS of this target [41,42]. This rebalancing could potentially be achieved pharmacogenetically by enhancing local GABA concentrations within the subthalamic nucleus. Glutamic acid decarboxylase (GAD) catalyses the rate-limiting step of GABA formation and has therefore been the focus for this form of pharmacogenetic manipulation. AAV–GAD treatment of parkinsonian rats demonstrated increased GABA release in the subthalamic nucleus and an improvement in motor output [43]. Following this, an open-label phase I study of patients using unilateral AAV–GAD injection to the subthalamic nucleus was performed and revealed a reduction of ipsilateral thalamic metabolism and improved contralateral motor scores at three and twelve months [44]. A follow-up double-blind randomised study confirmed both the safety and efficacy of bilateral AAV–GAD delivery to the same nucleus, but motor benefit was modest and rather less than that achievable with conventional DBS [45].
Pharmacogenetic Dopaminergic Replacement
It has long been argued that intermittent, pulsatile dopaminergic therapy leads to plastic changes in the motor system that may be responsible for the late emergence of motor fluctuations and dyskinesias [46]. Given that the biochemical pathways that convert L-tyrosine to levodopa are well described, could pharmacogenetics be used to augment endogenous dopamine production through repurposing existing healthy non-dopaminergic neurons? A number of approaches involving all steps of the dopaminergic pathway have been attempted. Aromatic amino acid decarboxylase (AADC) converts levodopa to dopamine; therefore, as dopaminergic neurons containing AADC degenerate in PD, total levels of the enzyme are reduced and the efficacy of levodopa drops. AADC substitution could then both improve motor symptoms and reduce the total exogenous levodopa requirements, reducing side effects. Such long-lasting effects have been shown in MPTP-treated monkeys [47]. In human studies, however, the clinical improvement has so far only been short lived, even though there was evidence that the genetic and biochemical effects were permanent [47,48].
A more advanced and potentially efficacious approach might therefore be to transfect a genetic package containing genes for the complete dopamine synthesis pathway, including AADC but also tyrosine hydroxylase, which converts L-tyrosine to levodopa, and guanosine triphosphate (GTP)-cyclohydrolase-1, which facilitates this conversion by synthesising tetrahydrobiopterin, a cofactor for tyrosine hydroxylase (Figure 2). This represents the most ambitious pharmacogenetic therapy to date, attempting to transduce the entire dopaminergic synthetic pathway into non-dopaminergic neurons of the striatum. For this, a lentivirus was loaded with genes for all three enzymes in the dopamine pathway and the complete package named ProSavin. Pre-clinical studies in MPTP-treated monkeys demonstrated that ProSavin increased extracellular dopamine levels and improved motor function, which was sustained at one year [49]. Recent results from a preliminary study in humans are also encouraging. In this open-label study, 15 patients were treated with stereotactic striatal injections of ProSavin at three different doses [50]. There was a modest-to-moderate improvement in motor performance with the suggestion of a dose-response effect that was sustained at 12 months. This was accompanied by evidence of a sustained increase in endogenous dopamine as revealed through decreased binding of a competitive radionucleotide (11C-raclopride) during positron emission tomography (PET) imaging (Figure 2). Increased levels of dyskinesias were experienced, in keeping with the therapeutic mechanism of ProSavin, and were managed with drug adjustment. Double-blind randomised studies are needed to determine whether this treatment gives clinically meaningful improvements in motor function and how this compares to conventional treatment with medication and DBS.
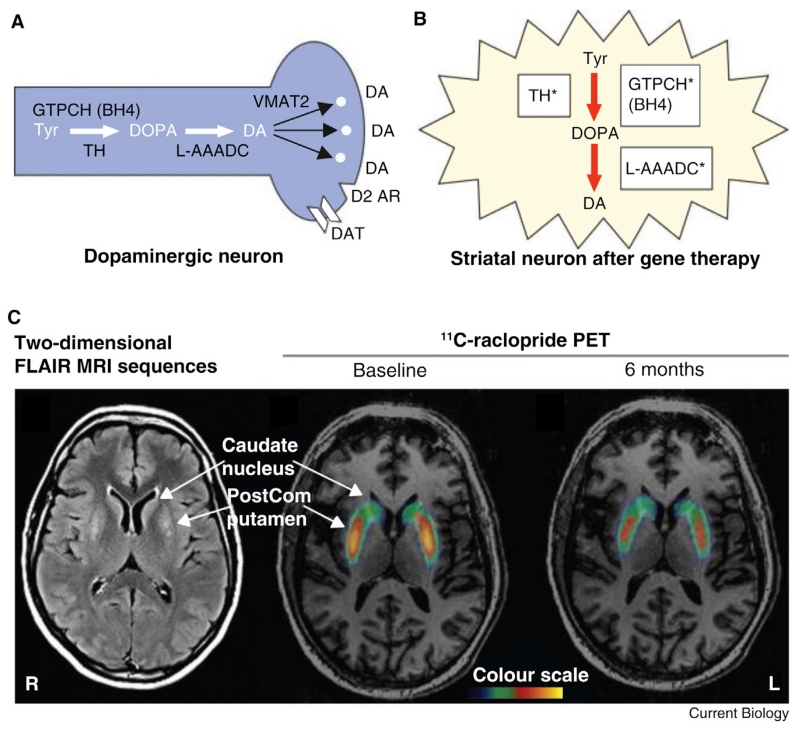
Figure 2.
Schematic of pharmacogenetic replacement of complete dopamine synthesis pathway and radionucleotide evidence of increased endogenous dopamine production following treatment.
(A) Dopamine (DA) synthesis pathway in healthy brain. The rate-limiting enzyme for DA synthesis in normal brain is tyrosine hydroxylase (TH), which requires tetrahydrobiopterin (BH4) as cofactor. BH4 synthesis is dependent on GTP cyclohydrolase (GTPCH). Dihydroxyphenylalanine (DOPA) produced by TH is converted to DA by L-aromatic amino acid decarboxylase (L-AAADC), which in the normal brain is packaged into synaptic vesicles by vesicular monoamine transporter type 2 (VMAT2). In the normal brain, the effect of DA released into the synapse is terminated by reuptake through DA transporter (DAT) and synthesis and release are regulated by D2-like autoreceptors (D2 AR). (B) Gene therapy using ProSavin causes striatal neurons to express TH, GTPCH, and L-AAADC, and thereby enables them to produce DA. (C) Two-dimensional fluid attenuated inversion recovery MRI sequences from 1 month after bilateral surgery (left), showing increased signal within the postcommissural (PostCom) putamen corresponding to motor putamen injection sites (lower arrow), and no signal change in the non-injected caudate nucleus (upper arrow). Binding potential parametric maps from 11C-raclopride PET scans of a single patient carried out at baseline (middle) and 6 months after bilateral ProSavin injection (right) within the putamen. Hot colours demonstrate increased raclopride binding and hence diminished endogenous dopamine. Note a decrease in the binding potential index only in the left and right postcommissural putamen area (middle, lower arrow), compared with no binding potential change on either side of the non-injected caudate nucleus (middle, upper arrow), indicating increased endogenous dopamine at sites of ProSavin injections. Adapted with permission [50,103].
One outstanding question related to cell transplantation and pharmacogenetic techniques is the extent to which they may be eventually expected to lead to circuit-specific, temporally defined synaptic interactions with selected, functionally appropriate targets. Still, they may suffice in providing an essential tonic dopaminergic input necessary to maintain physiological function within a circuit which, although dynamic, is predicated on a particular balance of inputs. This is, after all, how pharmacological therapies like levodopa work, and these newer approaches would have the additional advantage of avoiding the coarse fluctuation in dopaminergic input that is implicated in the genesis of dyskinesias and unpredictable motor states experienced in more advanced cases of PD.
Direct Network Modulation
A very different approach is to ameliorate the dysfunctional circuit dynamics that result from dopaminergic denervation. But what are these abnormal circuit dynamics? Early conceptualisations of basal ganglia function invoked an imbalance between two opposing streams: the direct and the indirect pathways [40] (Figure 1). This relatively simple model, which was predicated on a pure rate coding scheme, was remarkably successful in explaining numerous experimental results relating to the basal ganglia, in addition to a number of clinical disorders such as PD. However, objections to a model based simply on average neuronal firing rates have subsequently come from anatomical, physiological and clinical studies, including the paradoxically beneficial effect of DBS on the globus pallidus interna [51–53]. Indeed, basal ganglia recordings from non-human primates demonstrating oscillatory single-unit and population activity in the dopamine-deficient state have since been corroborated by studies in patients with PD that show bursts of similar activity, albeit at slightly higher frequencies, in the beta range (12–35 Hz) [54]. This signature marker of PD poses a challenge to simple rate-based coding accounts of basal ganglia function, with the emergence of bursts of synchronised activity in the dopamine-deficient state demonstrating that dopamine controls patterns of neuronal activation in addition to simply modulating the rate of firing. Synchronisation of activity in the beta band is widely distributed with cortical, subcortical and cortico-subcortical connectivity [55–57]. Beta activity in the cortex temporally precedes that in the basal ganglia and reduced dopaminergic function strengthens effective connectivity in the hyperdirect pathway from cortex to the subthalamic nucleus [58]. Reciprocal connections between the subthalamic nucleus and globus pallidus externa are also dopaminergic dependent, amplifying subcortical oscillations when dopamine is low [59]. The subthalamic nucleus thus emerges as a key node through which to interact with this circuit dysfunction.
Nevertheless, it remains unclear how DBS of the subthalamic nucleus — the most popular target for the surgical treatment of PD — works. Due to anatomical correspondence between DBS and earlier lesional targeting, the mechanism was initially presumed to represent a functional lesion [60]. Although there is some evidence of local somal inhibition, there is also now strong support for both antidromic and orthodromic axonal activation [61]. This suggests that local delivery of DBS pulses leads to a widely distributed modulation of the motor network. In particular, stimulation serves to suppress pathologically synchronised oscillations in a similar manner to levodopa administration [62,63].
Although clinically effective, DBS is still limited by costs, side effects and partial efficacy. Current clinical stimulation is open-loop with fixed stimulation settings leading to relentless interference in motor networks. This inevitably causes disruption of physiological, in addition to pathological, processing. Consequently, DBS may cause motor, speech and executive side effects [64–67]. Might it be possible to limit stimulation to times when circuit disturbance is more pronounced, sparing periods of more physiological processing? The upshot would be reduced side effects and power consumption but also, potentially, improved efficacy, if the benefits of suppressing pathological activity in the motor circuit with conventional DBS were partly offset simultaneously by effects on residual physiological motor processing. Triggering the delivery of DBS by increases in the very pathological activity that it suppresses would offer a simple solution, much more tractable than following the patient’s clinical state with multiple limb-mounted sensors or motion detection systems in the environment. This adaptive approach to brain stimulation has recently been successfully applied in the treatment of epilepsy [68]. However, pathological activity in epilepsy is paroxysmal and discrete, so how might on-demand stimulation fare in disorders with more persistent dysfunction? Fortunately, there is mounting evidence in non-human primate models and patients with PD that this form of adaptive DBS can prove more efficacious for motor control than standard continuous high-frequency DBS, despite reductions in overall stimulation. Furthermore, the opportunity afforded by DBS in patients allows for the investigation of neural network activity within the basal ganglia through the recording of single-cell and local field potential activity in awake human subjects, furthering our understanding of motor and cognitive systems.
Rosin et al. [69] elegantly demonstrated effective adaptive stimulation in a non-human primate model of PD. They tested two MPTP-treated parkinsonian monkeys. Surgical implantation of microelectrodes in the motor cortex and the globus pallidus interna of the monkeys enabled recording from individual neurons at the two sites during stimulation of the globus pallidus interna. The aim of this study was to assess whether delivering stimulation according to the pattern of firing of neurons was able to improve parkinsonism over and above that of standard continuous (open-loop) high-frequency stimulation. In addition, they set out to determine whether pattern-triggered stimulation had dissociable effects on cortico-basal ganglia oscillations and neuronal firing discharge rates. A number of different closed-loop paradigms were examined with the most effective resulting from triggering from the firing of motor cortical neurons and stimulation in the globus pallidus interna with short trains of seven pulses at 130 Hz at a latency of 80 milliseconds (Figure 3). In the MPTP model, the dominant circuit oscillation that results is 12.5 Hz and thus an 80 millisecond delay corresponds to stimulating at the start of the next oscillatory cycle. Neuronal activity was recorded in the globus pallidus interna before, during and after stimulation. The clinical effect of stimulation was a marked reduction in bradykinesia, which was most pronounced in the limb contralateral to stimulation. Notably, the improvement was substantially greater than that achieved with standard high-frequency stimulation despite the significantly lower overall charge delivery. These compelling behavioral improvements were accompanied by a reduction in pallidal discharge rate and oscillatory activity which was more pronounced with adaptive stimulation compared with the standard high-frequency condition, despite the lower overall number of stimuli. Furthermore, they found that if the paradigm were changed so that sensing and stimulating both occurred in the globus pallidus interna, bradykinesia was worsened. Importantly, this occurred in the context of firing rates that were still reduced but with increased oscillatory bursting. Further, the authors found no correlation between pallidal firing rate and oscillatory activity, suggesting independent mechanisms. As such, it appears that bradykinesia relates more strongly to oscillatory activity, not firing rate, affording critical support for the concept that low-frequency oscillations play a key pathophysiological role in PD. Additionally, this seminal experiment provided proof of concept that adaptive DBS can be efficacious and, most notably, can actually be more effective than conventional DBS, despite a reduction in overall stimulation.
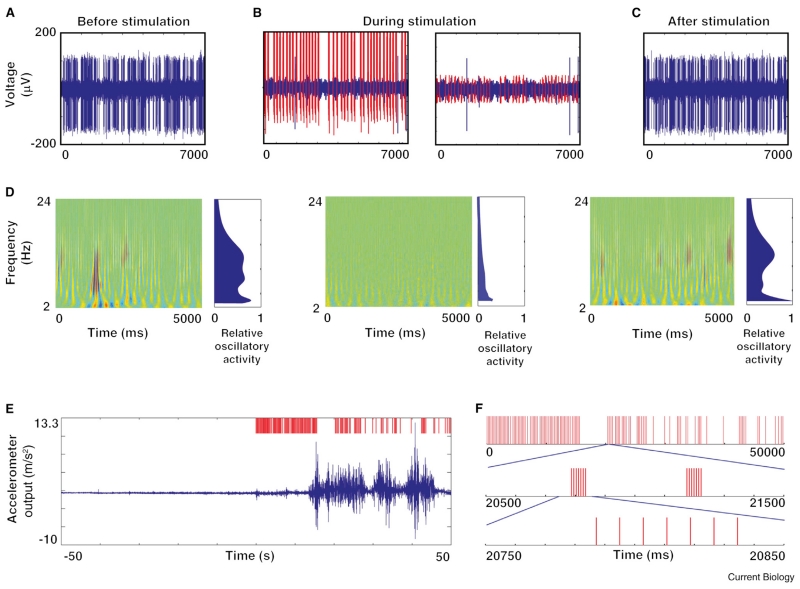
Figure 3.
Closed-loop DBS in the non-human primate with physiological and behavioral results.
(A–C) Examples of 7 second traces of spiking activity in a GPi neuron before (A), during (B), and after (C) closed-loop stimulation of globus pallidus with a brief train of electrical shocks triggered by neuronal spikes in the motor cortex after a delay of 80 milliseconds. The stimulus artifact is shown in red (B, left box), as is the residual artifact after artifact template removal (B, right box). (D) Oscillatory activity depicted through wavelet spectrograms and displayed by frequency as a function of time, with blue to red color indicating the intensity of activity. Spectrograms of activity before (left column), during (middle column), and after (right column) the application of closed-loop stimulation are shown. Estimates of oscillatory activity averaged over time are shown to the right of each panel, and are relative to the maximal oscillatory power in the entire recording from this neuron. (E) 100 second trace of the motion signal from an accelerometer fastened to the primate’s limb contralateral to the stimulating electrodes. Trace starts 50 seconds before the onset of stimulation. Stimulus raster is depicted in red in the upper trace. Movement increases dramatically once stimulation starts. (F) Characteristics of the triggered stimulus pattern. Triggered stimulation consisted of a train of seven pulses of stimulation at 130 Hz (expanded bottom raster). Triggering was episodic (top raster). Adapted with permission [69].
This research in MPTP-treated non-human primates has now been complemented by work in patients with PD; here, the strategy has, however, been subtly different. The study described above used the firing of motor cortical neurons to trigger stimulation in the globus pallidus interna. However, recordings from individual neurons are difficult to sustain over prolonged periods and require additional surgical instrumentation, so are not an ideal feedback signal for adaptive DBS in patients requiring stimulation over many years [70]. An alternative approach is to use a feedback signal recorded directly from the stimulating electrode. Local field potentials principally represent the summation of synaptic currents in the population of neurons local to the electrode [71]. These signals remain robust over many years of recording and potentially contain greater informational content than single neurons [70,72]. Beta oscillations can be recorded in local field potentials from the subthalamic nucleus in the vast majority of patients with PD withdrawn from oral medication and are suppressed by levodopa and DBS in proportion to clinical improvement [63,73,74]. The parallel modulation of beta activity with motor function suggests that beta activity could be used to track clinical state and act as feedback to trigger adaptive DBS. This hypothesis has recently been tested in patients [75]. Here, beta oscillations were amplified and filtered around the patient-specific beta peak and an on-line (i.e. real time) measure of amplitude derived (Figure 4). A threshold was then set which resulted in a trigger signal being sent to a stimulator when beta amplitude crossed above the trigger threshold. Triggering of the stimulator switched on the conventional high-frequency stimulation. The stimulation voltage was ramped up so as to avoid the discomfort sometimes experienced with abrupt stimulation onset. What was surprising about this study was that, despite a 50% reduction in overall stimulation, motor function was 30% better than with conventional continuous stimulation. Supporting the critical role of beta oscillations in PD, beta activity was also suppressed in proportion to clinical improvement across conditions.
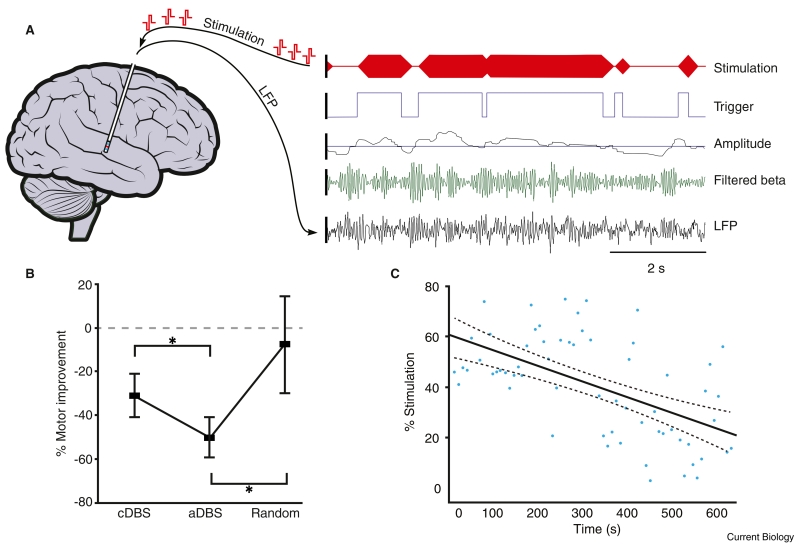
Figure 4.
Adaptive, closed-loop stimulation in humans with PD using a local field potential (LFP) feedback.
(A) Left, schematic demonstrating DBS electrode in situ with simultaneous recording and stimulation. Right, example data demonstrating bipolar LFPs recorded from the blue contacts at the end of the electrode. LFP is shown after wide band (3–37 Hz) filtering (bottom trace) and following narrow band filtering around the patient-specific beta peak (second trace). Rectification and smoothing produced a beta amplitude signal (third trace) with trigger threshold shown overlaid (horizontal blue line). Threshold crossing by beta amplitude resulted in a trigger signal to the stimulator (fourth trace) and ramped high frequency stimulation delivered to patient (top trace). (B) Clinical motor improvement comparing conventional (cDBS), adaptive (aDBS) and random stimulation. Note adaptive DBS is significantly superior to both conventional DBS and random stimulation. (C) Example from one subject demonstrating progressive reduction in percentage time that stimulation is triggered per 10 second block (y-axis) as time elapses (x-axis) despite a constant beta threshold. Thus, beta activity must have been falling over time, leading to less triggering per 10 second block. This suggests short-term plasticity. Adapted with permission [75].
The above study also revealed an unexpected result; there was a progressive reduction in total time on stimulation despite a fixed trigger threshold, pointing to a gradual diminution of the frequency of beta bursts during adaptive stimulation (Figure 4). This suggests that more temporally focused stimulation might encourage beneficial plasticity in the motor circuit. Overall, the study demonstrated that even using a simple control system with a single feedback signal, significant gains can be made in terms of power consumption and motor improvement. Moreover, this simple but tractable system could soon be realised in chronically internalised patients using new devices with the capability to simultaneously sense and stimulate [76]. Further studies are now necessary to establish the long-term viability of adaptive DBS and to establish that side effects are diminished relative to standard treatment, in line with the lower power demands of the former. Other work aimed at optimisation of feedback signals and control algorithms is also warranted before adaptive DBS can realise its full potential. Moreover, evidence is now accumulating that variations in beta band activity alone do not capture all aspects of PD and that beta activity may interact with other signals, such as gamma activity, through excessive phase-amplitude coupling at both cortical and sub-cortical levels [77,78]. It remains to be seen whether more sophisticated spectral biomarkers may capture greater degrees of PD phenomenology and may therefore prove useful in adaptive DBS. However, any potential advantages of such biomarkers may be offset by their higher complexity, which may increase the power consumption necessary for on-line detection and analysis and slow the response of the DBS system to changes in physiological state.
The above form of adaptive DBS relies on the feedback signal, i.e. beta activity in the local field potential, being a faithful correlate of clinical state from moment to moment. Obviously, this link between feedback signal and clinical state will be tighter if pathological beta activity is mechanistically involved in motor impairment, but this is not a prerequisite. Yet, if beta activity were causally involved in motor symptomatology, then stimulation patterns could potentially be chosen that specifically target this rhythm in the hope that these would be more efficacious, and again more selective, than standard high-frequency stimulation. There is certainly some evidence that beta oscillations may be mechanistically important with studies showing that stimulating motor sites within the beta range causes a slowing of movement and an increase in rigidity, but the scale of such deleterious effects is limited [79,80]. Accordingly, it is as yet still not certain whether pathological beta activity is quantitatively important in the generation of bradykinesia and rigidity.
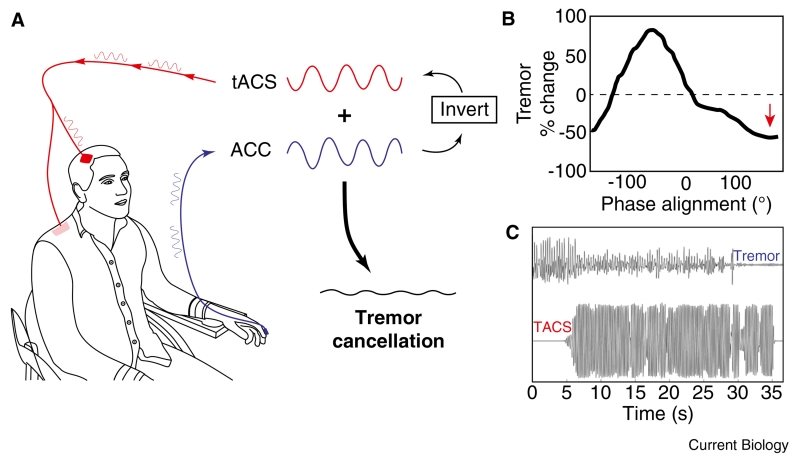
For this reason, attempts to specifically target mechanistically relevant oscillatory activity have focused on tremor in PD. Parkinsonian tremor usually occurs at rest and is likely to have a different pathophysiological basis to bradykinesia and rigidity [81]. In particular, it is locked to oscillatory activity in the cortico-basal ganglia loop at tremor and double tremor frequency [82]. Tremor-related activity in the motor cortex is likely to be intimately related to the generation of tremor because extirpation of the motor cortex or sectioning of the pathways leading from it have been shown to abolish parkinsonian tremor [83]. Attempts to directly interfere with these pathological oscillations have taken two general forms. The first leverages an old technique called active noise cancellation. In order to improve communication with pilots in noisy cockpits, engineers in the 1950s devised a system in which ambient surrounding noise was recorded, inverted and played back to pilots through headphones in real time. Noise-cancelling headphones thus resulted in cancellation of unwanted sounds and a higher signal-to-noise ratio in the audio domain. A similar approach was recently adopted to cancel cortical oscillations within the tremor network in PD [84]. PD patients were fitted with an accelerometer to measure tremor and non-invasive transcranial alternating current stimulation (tACS) delivered through a sponge pad placed over the scalp overlying the motor cortex (Figure 5). The frequency of tACS was chosen to be very close to that of the intrinsic tremor frequency. This resulted in a gradual drifting of the two oscillations in and out of phase with each other every three or so seconds and enabled an off-line analysis of the effect on tremor amplitude of the phase difference between stimulation and tremor. The technique identified phase regions in which tremor was amplified and suppressed (i.e. in line with cancellation), but sizes of the effect were modest, in the range of 10%. Nevertheless, the authors went on to track tremor phase so that they could actively keep the phase difference between the stimulation and tremor at an optimal level for tremor suppression. The effect of stimulation was then much more striking, with an average 50% reduction in tremor amplitude even though stimulation duration was limited to 30 seconds. This approach is now being tested with even longer periods of stimulation. Ultimately, in order to be tractable over years, as is necessary for a clinical intervention, this kind of stimulation would be delivered through subgaleal or extradural electrodes, although this is still considerably safer than DBS, which involves penetrating the brain substance.
Figure 5.
Adaptive transcranial alternating current stimulation (tACS) aiming to cancel oscillatory activity.
(A) Schematic demonstrating recording of tremor signal via accelerometer (blue) and delivery of phase-shifted tACS signal at the same frequency (red). Phase cancellation occurs when the two signals are out of phase and added together (black). (B) Percentage change in tremor amplitude with shifting phase alignment between tremor and stimulation in one subject during 30 seconds of phase tracking in blocks with optimal phase position for suppression shown with red arrow. Note certain phases enhance tremor whereas opposing phases suppress tremor. (C) Example of tremor (top trace) and tACS (bottom trace) delivered at the optimal phase for suppression in one patient. Note that the tremor is completely suppressed by tACS around 30 seconds in to the recording time, suggesting a cumulative effect of stimulation. Adapted with permission [84].
The phase cancellation of pathological oscillations by tACS is ideally suited to structures that are essentially planar, like the cerebral cortex, so that flat stimulating electrodes maintain a more-or-less fixed distance from the relevant laminar elements. However, this is not possible in nuclear structures where neurons are more intermixed so that these may be subject to phase cancellation at one depth but not at others due to field gradients. Indeed, neurons may actually end up driven by the stimulation at levels more proximal to the stimulation source. For such structures it may be more appropriate to attenuate pathological oscillatory activity with pulsatile electrical stimuli that shift oscillators out of their preferred regimes with an all-or-nothing effect. This likely involves two processes. Stimuli delivered at certain phases of a pathological oscillatory activity will phase advance or phase delay neuronal responses in their oscillatory cycle [85,86]. Provided that stimulation is not supramaximal the net result is to partially desynchronise the oscillatory network, and to reduce its peripheral manifestation in the form of tremor. However, cumulative effects may also be expected as a result of short-term, spike-timing-dependent plasticity. Both instantaneous and cumulative effects of phase-locked pulsed stimulation have recently been demonstrated in patients with essential tremor in whom the thalamus was directly stimulated as a treatment for tremor [87]. In addition, cumulative effects were suggested during phase-cancelling tACS for tremor (Figure 5C) [84].
The above approach can potentially be optimised still further by leveraging the multiple contacts of DBS electrodes. Here, pulsed stimulation is differentially patterned over the electrode contacts to fragment a previously synchronised neuronal population into a cluster of subpopulations, the phases of which are shifted with respect to each other [88]. These clusters may then desynchronise further before finally resynchronising unless re-stimulated. The timing of further stimulation can be feedback controlled or pre-determined. The technique, termed coordinated reset, was first developed to treat tremor [88], although more recently it has been piloted for the lower beta band oscillations in the subthalamic nucleus of MPTP-treated monkeys, where it has surprisingly long-lasting effects on movement [89].
These different approaches to more tailored therapeutic brain stimulation are linked by their exploitation of the fluctuation of pathological neuronal activity over time; either the fluctuation inherent within cycles of oscillatory activity or the slower fluctuations in the amplitude of the envelope of such activity. This tailoring to temporal characteristics affords the interventions a degree of specificity as intact physiological activities are not yoked in time to pathological activity. Spatial specificity is, however, still limited. Yet moves are afoot to improve the resolution of electrical interfaces with the brain. Current clinical DBS electrode technology, for instance, employs a quadripolar electrode with four circular contacts of 1.5 mm length. Recent work has introduced a new directional electrode which has been found intra-operatively to improve the therapeutic window of stimulation [90]. Importantly, if this translates into a clinically realisable therapy, this could also be combined with the temporal techniques described above to provide combined spatiotemporally targeted DBS. However, the new field of optogenetics promises a means of combining temporal specificity with the ultimate in spatial specificity — the stimulation of neurons of a specific cell type within a controlled region.
Optogenetics
Opsins are light-sensitive receptors that are naturally present in humans in the form of rod opsins (rhodopsin) and cone opsins (photopsin) in the eye. Reactivity to light converts photic energy into a neuroelectrical signal for downstream visual processing. Similar proteins exist within the prokaryotic world (type I opsins) and these have recently been leveraged for in vivo experimentation on mammals. In 2005, Deisseroth and colleagues [91] demonstrated the functional integration of light-sensitive channelrhodopsin-2 (ChR2) into mammalian neurons using a lentivirus gene vector. ChR2 is a light-gated cation channel that has very rapid temporal kinetics and is sensitive to blue light in the 450–490 nm range. Illumination with an appropriate light source led to abrupt depolarisation with a latency of only 2 milliseconds, sufficient to cause firing of the neuron. These authors were then able to show entrainment of spiking neurons with their extrinsically delivered light pulses. As such, this demonstrated the potential for high-fidelity temporal control of individual neuronal spikes using only a light source. These initial exciting results have been followed up by a wealth of powerful studies that have sought to modulate circuits in freely behaving animals to further elucidate the pathophysiological role of specific brain structures and indeed specific types of neurons.
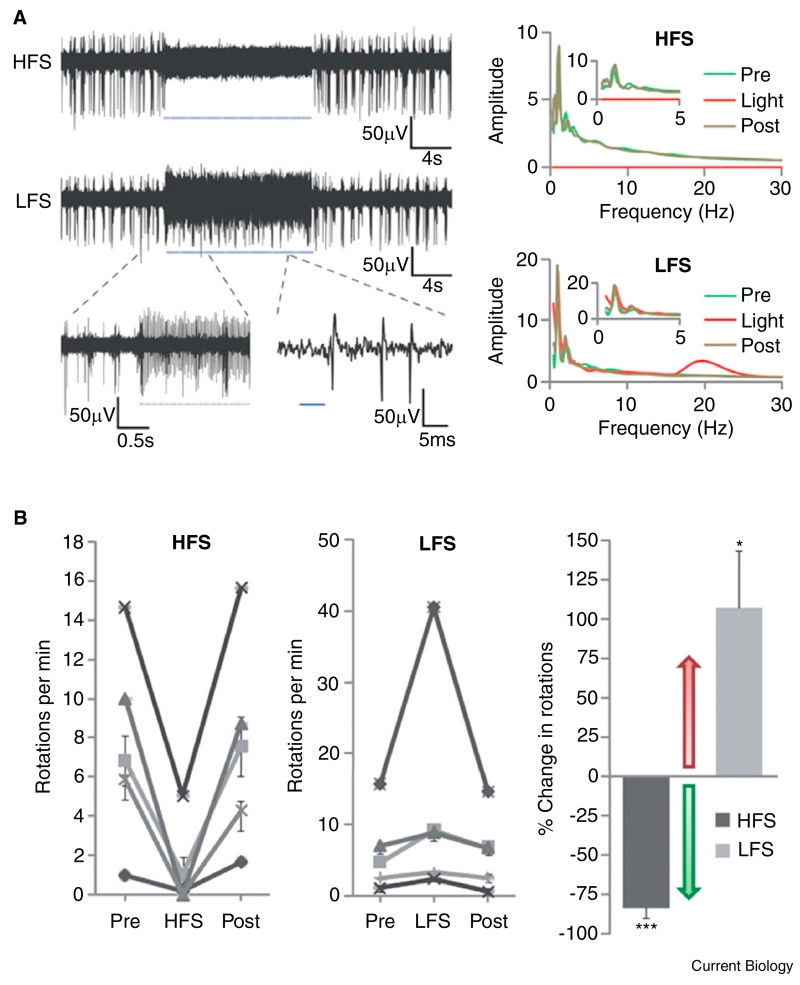
Models of PD were among the first to benefit from such an approach. Here the same group used both ChR2 and a different class of opsins, namely the halorhodopsins (NpHR), involving a light-activated chloride pump that hyperpolarises neurons and inhibits firing of action potentials to differentially control selected cell groups within and around the basal ganglia [92]. First they tested whether direct inhibition of the subthalamic nucleus would have therapeutic potential in a rodent model of PD using NpHR inhibitory modulation. This was physiologically effective, leading to an 80% reduction in spiking in neurons in the subthalamic nucleus. However, whereas electrical stimulation with DBS at the same site led to a marked and significant reduction in pathological rotational activity (their behavioral marker of parkinsonism), NpHR-mediated inhibition of the subthalamic nucleus had no effect on motor output. Instead, they demonstrated that high-frequency optogenetic stimulation of afferent fibres leading to the subthalamic nucleus both greatly reduced spiking within the nucleus and led to a robust improvement in pathological rotation (Figure 6). Further testing revealed that similar motor improvement could also be achieved by optical stimulation of layer 5 projection neurons in the motor cortex, suggesting that the white matter connection between the cortex and subthalamic nucleus, termed the hyperdirect pathway, was critical in mediating the effects of stimulation. Moreover, the effect of stimulation of afferent fibres to the nucleus was frequency specific; optogenetic stimulation at 20 Hz worsened pathological motor activity, consistent with the purported role of exaggerated beta band oscillations in parkinsonian motor impairment.
Figure 6.
Selective optical control of afferent fibers in the STN using optogenetics in a 6-OHDA mouse model of Parkinson’s disease.
(A) Optical high-frequency stimulation (HFS) at 130 Hz to afferent fibers in the STN region of an anesthetized mouse with 473 nm light-inhibited STN spikes (top left). Optical low-frequency stimulation (LFS) at 20 Hz produced reliable spiking at 20 Hz (bottom left). Top right panel shows that HFS still allowed very low frequency activity in the STN. Bottom right panel confirms that LFS did drive 20 Hz activity in the STN, but again left very low frequency activity unchanged. The effect of optical stimulation was therefore frequency selective, and only seen during light exposure and not before or after exposure. (B) Optical HFS to STN in these animals produced robust therapeutic effects, reducing apomorphine-induced ipsilateral rotations (a measure of parkinsonism in this model). In contrast, optical LFS exacerbated pathological effects, causing increased ipsilateral rotations. Both effects were reversible and highly statistically significant. The two panels to the left demonstrate effects of different forms of stimulation within animals. The right-hand panel summarises group-level effects as percentage change in rotations per minute. An increase in rotations represents a worsening of the parkinsonian deficit. Adapted from [92]: reprinted with permission from AAAS.
A follow-up study was able to go further and dissect the functional roles of the direct and indirect pathways of the basal ganglia by optical stimulation of virally transduced ChR2 receptors that were under the differential control of regulatory elements for the dopamine D1 or D2 subtypes (Figure 1) [93]. Excitation of the D2-expressing indirect pathway induced parkinsonian symptoms, including freezing and bradykinesia, whereas activation of the D1-expressing direct pathway ameliorated the same symptoms in parkinsonian rodents. This provides some support for the classic model of basal ganglia function [40] and, taken together with earlier studies, suggests that both rate and pattern coding coexist within the basal ganglia, at least in the context of disease. Optogenetic techniques have since been used to explore many features of striatal function and have proven a boon in linking cell-type-specific function to behavioral change in animal models of neuropsychiatric disorders [94,95]. More recent advances have linked opsins not just to ion channels but to a range of intracellular signaling pathways via new G-protein-coupled receptors termed OptoXRs [96]. This allows for the manipulation of the excitability of target populations of neurons without specifically controlling spike timing. Furthermore, flexible optical control of gene transcription has also been achieved, which opens up an ever greater range of possible experimental and potentially therapeutic interventions [97]. Theoretically then, optogenetics holds considerable promise for correcting network level dysfunction in patients with PD and other neuropsychiatric diseases [98].
Although it is clear that optogenetics can play a valuable role in dissecting pathophysiology through discriminative circuit-level control and can specifically ameliorate motor deficits in rodent models of PD, there are a number of technical, practical and regulatory challenges to overcome before this can be translated clinically [99]. Expression of opsins using viral vectors still remains to be proven safe and effective in humans. Additionally, optogenetics is currently vastly more energy inefficient than electrical stimulation techniques and this would, using current technology, greatly limit device longevity and risk unacceptable tissue heating. This could be circumvented by designing opsins with greater light sensitivity and downstream signaling pathway amplification or by engineering solutions [100,101]. Enthusiasm for the therapeutic potential of optogenetics should also be tempered by acknowledgement that the sophisticated level of microcircuit control offered by this technique is not yet fully supported by sufficient understanding of cell-type-specific contributions to aberrant chronocircuitry in many neuropsychiatric diseases, including PD. These issues highlight how clinical translation will require advances in multiple different domains from basic physiology to bioengineering and molecular technologies, and this confluence of technologies will make bridging the gap to patients especially challenging. Additionally, even if successfully translated, the increased safety concerns mean that optogenetics would then have to demonstrate superiority over established treatments, such as medication and electrical interventions, and this remains to be proven.
Conclusions
Neuroscientific advances are driving forward our understanding of neuropsychiatric disease and leading, in particular, to the development of novel treatments for PD. Conceptualisations of disease pathology that are able to include multi-scale formulations from genes to networks are leading to new potential therapeutic approaches that ambitiously attempt to re-engineer aberrant networks at all levels. Pharmacogenetics aims to restore the local milieu through expression of neuroprotective growth factors or replacement of the dopaminergic synthesis pathway by transduction into healthy cells. Adaptive DBS attempts to modify distributed macro-networks using locally detected neural signals to temporally direct stimulation to periods of heightened pathological activity. Furthermore, population-based signals such as local field potentials might not simply be sources of feedback, but might actually reflect population dynamics that are central to the pathophysiology. These could then be direct targets for interventions by phase-cancelling or -disruption techniques that use electricity to interface with the brain. Finally, the micro- and macro-scales are brought together in the field of optogenetics, which has already afforded new insights into the pathophysiology of PD and the mechanisms of its therapy. Translation to the clinical environment for optogenetics and all emerging treatments will require rigorous safety testing and robust trials comparing them with existing clinical therapies. Neither should we underestimate the challenges presented by PD, for as yet the above advances are only targeted at those mechanisms that relate to motor impairment. We have barely begun to consider interventions that might ameliorate other disease features, such as the speech, gait and neuropsychiatric dysfunction that increasingly dominate in advanced cases. There remains much to be done, but we have at least entered into a phase of neuroscientific research that is yielding important insights into pathophysiology and novel therapeutic approaches.
Acknowledgments
S.L. is supported by the Wellcome Trust and is a Rosetrees Trust Clinical Research fellow. P.B. is supported by the Medical Research Council and the National Institute for Health Research Oxford Biomedical Research Centre.
References
- 1.Parkinson J. An Essay on the Shaking Palsy. Sherwood, Neely and Jones; London: 1817. [Google Scholar]
- 2.Tanner CM, Ottman R, Goldman SM, Ellenberg J, Chan P, Mayeux R, Langston JW. Parkinson disease in twins: an etiologic study. JAMA. 1999;281:341–346. doi: 10.1001/jama.281.4.341. [DOI] [PubMed] [Google Scholar]
- 3.Lesage S, Brice A. Parkinson’s disease: from monogenic forms to genetic susceptibility factors. Hum. Mol. Genet. 2009;18:R48–R59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- 4.Singleton AB, Farrer MJ, Bonifati V. The genetics of Parkinson’s disease: progress and therapeutic implications. Movement Disorders. 2013;28:14–23. doi: 10.1002/mds.25249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassler R. Zur Pathologie der Paralysis agitans und des post-enzephalitschen Parkinsonismus. J. Psychol. Neurol. 1938;48:387–476. [Google Scholar]
- 6.Gibb WRG, Poewe WH. The centenary of Friedreich H. Lewy 1885–1950. Neuropathol. Applied Neurobiol. 1986;12:217–221. doi: 10.1111/j.1365-2990.1986.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 7.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 8.Luk KC, Lee VM-Y. Modeling Lewy pathology propagation in Parkinson’s disease. Parkinsonism Related Disorders. 2014;20:S85–S87. doi: 10.1016/S1353-8020(13)70022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM-Y. Exogenous a-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson’s disease. Annu. Rev. Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 11.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosur. Psych. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hornykiewicz O. A brief history of levodopa. J. Neurol. 2010;257:S249–S252. doi: 10.1007/s00415-010-5741-y. [DOI] [PubMed] [Google Scholar]
- 13.Voon V, Fox SH. Medication-related impulse control and repetitive behaviors in Parkinson disease. Arch. Neurol. 2007;64:1089–1096. doi: 10.1001/archneur.64.8.1089. [DOI] [PubMed] [Google Scholar]
- 14.Schrag A. Dyskinesias and motor fluctuations in Parkinson’s disease: A community-based study. Brain. 2000;123:2297–2305. doi: 10.1093/brain/123.11.2297. [DOI] [PubMed] [Google Scholar]
- 15.Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 16.Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Rothlind J, Sagher O, Reda D, Moy CS, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R, Scott R, Ives N, Rick C, Daniels J, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial. Lan. Neurol. 2010;9:581–591. doi: 10.1016/S1474-4422(10)70093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castrioto A, Lozano AM, Poon Y-Y, Lang AE, Fallis M, Moro E. Ten-year outcome of subthalamic stimulation in Parkinson disease: a blinded evaluation. Arch. Neurol. 2011;68:1550–1556. doi: 10.1001/archneurol.2011.182. [DOI] [PubMed] [Google Scholar]
- 19.Weaver FM, Follett KA, Stern M, Luo P, Harris CL, Hur K, Marks WJ, Rothlind J, Sagher O, Moy C, et al. Randomized trial of deep brain stimulation for Parkinson disease: thirty-six-month outcomes. Neurology. 2012;79:55–65. doi: 10.1212/WNL.0b013e31825dcdc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N. Eng. J. Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 21.Politis M, Wu K, Loane C, Quinn NP, Brooks DJ, Rehncrona S, Bjorklund A, Lindvall O, Piccini P. Serotonergic neurons mediate dyskinesia side effects in Parkinson’s patients with neural transplants. Sci. Transl. Med. 2010;2:38–46. doi: 10.1126/scitranslmed.3000976. [DOI] [PubMed] [Google Scholar]
- 22.Kim J, Auerbach J, Rodríguez-Gómez J. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- 23.Hargus G, Cooper O, Deleidi M, Levy A, Lee K, Marlow E, Yow A, Soldner F, Hockemeyer D, Hallett PJ, et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc. Nat. Acad. Sci. USA. 2010;107:15921–15926. doi: 10.1073/pnas.1010209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Politis M, Lindvall O. Clinical application of stem cell therapy in Parkinson’s disease. BMC Med. 2012;10:1. doi: 10.1186/1741-7015-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douglas MR. Gene therapy for Parkinson’s disease: state-of-theart treatments for neurodegenerative disease. Expert Rev. Neurotherapeut. 2013;13:695–705. doi: 10.1586/ern.13.58. [DOI] [PubMed] [Google Scholar]
- 26.Huang R, Han L, Li J, Ren F, Ke W, Jiang C, Pei Y. Neuroprotection in a 6-hydroxydopamine-lesioned Parkinson model using lactoferrin-modified nanoparticles. J. Gene Med. 2009;11:754–763. doi: 10.1002/jgm.1361. [DOI] [PubMed] [Google Scholar]
- 27.Pardridge WM. Gene targeting in vivo with pegylated immunoliposomes. Methods Enzymol. 2003;373:507–528. doi: 10.1016/S0076-6879(03)73032-8. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Fong D, Bannon MJ, Trudeau L-E, Gonzalez-Barrios JA, Arango-Rodriguez ML, Hernandez-Chan NG, Reyes-Corona D, Armendáriz-Borunda J, Navarro-Quiroga I. NTS-Polyplex: a potential nanocarrier for neurotrophic therapy of Parkinson’s disease. Nanomedicine. 2012;8:1052–1069. doi: 10.1016/j.nano.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo WD, Qu G, Sferra TJ, Clark R, Chen R, Johnson PR. Adeno-associated virus-mediated gene transfer to the brain: duration and modulation of expression. Hum. Gene Therapy. 1999;10:201–213. doi: 10.1089/10430349950018995. [DOI] [PubMed] [Google Scholar]
- 30.Schnepp BC, Clark KR, Klemanski DL, Pacak CA, Johnson PR. Genetic fate of recombinant adeno-associated virus vector genomes in muscle. J. Virol. 2003;77:3495–3504. doi: 10.1128/JVI.77.6.3495-3504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitzsimons HL, Riban V, Bland RJ, Wendelken JL, Sapan CV, During MJ. Biodistribution and safety assessment of AAV2-GAD following intrasubthalamic injection in the rat. J. Gene Med. 2010;12:385–398. doi: 10.1002/jgm.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolo-tukhin S, Reier PJ, Mandel RJ, Muzyczka N. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol. Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 33.Wanisch K, Yáñez-Muñoz RJ. Integration-deficient lentiviral vectors: a slow coming of age. Mol. Ther. 2009;17:1316–1332. doi: 10.1038/mt.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- 35.Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- 36.Kells AP, Eberling J, Su X, Pivirotto P, Bringas J, Hadaczek P, Narrow WC, Bowers WJ, Federoff HJ, Forsayeth J, et al. Regeneration of the MPTP-lesioned dopaminergic system after convection-enhanced delivery of AAV2-GDNF. J. Neurosci. 2010;30:9567–9577. doi: 10.1523/JNEUROSCI.0942-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- 38.Marks WJ, Bartus RT, Siffert J, Davis CS, Lozano A, Boulis N, Vitek J, Stacy M, Turner D, Verhagen L, et al. Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. Lan. Neurol. 2010;9:1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- 39.Kordower JH, Herzog CD, Dass B, Bakay RAE, Stansell J, Gasmi M, Bartus RT. Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Ann. Neurol. 2006;60:706–715. doi: 10.1002/ana.21032. [DOI] [PubMed] [Google Scholar]
- 40.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trend. Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 41.Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- 42.Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N. Eng. J. Med. 1998;339:1105–1111. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- 43.Lee B, Lee H, Nam YR, Oh JH, Cho YH, Chang JW. Enhanced expression of glutamate decarboxylase 65 improves symptoms of rat parkinsonian models. Gene Ther. 2005;12:1215–1222. doi: 10.1038/sj.gt.3302520. [DOI] [PubMed] [Google Scholar]
- 44.Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 45.LeWitt PA, Rezai AR, Leehey MA, Ojemann SG, Flaherty AW, Eskandar EN, Kostyk SK, Thomas K, Sarkar A, Siddiqui MS, et al. AAV2-GAD gene therapy for advanced Parkinson’s disease: a double-blind, sham-surgery controlled, randomised trial. Lan. Neurol. 2011;10:309–319. doi: 10.1016/S1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]
- 46.Olanow CW, Obeso JA, Stocchi F. Continuous dopaminereceptor treatment of Parkinson’s disease: scientific rationale and clinical implications. Lan. Neurol. 2006;5:677–687. doi: 10.1016/S1474-4422(06)70521-X. [DOI] [PubMed] [Google Scholar]
- 47.Hadaczek P, Eberling JL, Pivirotto P, Bringas J, Forsayeth J, Bankiewicz KS. Eight years of clinical improvement in MPTP-lesioned primates after gene therapy with AAV2-hAADC. Mol. Ther. 2010;18:1458–1461. doi: 10.1038/mt.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mittermeyer G, Christine CW, Rosenbluth KH, Baker SL, Starr P, Larson P, Kaplan PL, Forsayeth J, Aminoff MJ, Bankiewicz KS. Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson’s disease. Hum. Gene Ther. 2012;23:377–381. doi: 10.1089/hum.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jarraya B, Boulet S, Ralph GS, Jan C, Bonvento G, Azzouz M, Miskin JE, Shin M, Delzescaux T, Drouot X, et al. Dopamine gene therapy for Parkinson’s disease in a nonhuman primate without associated dyskinesia. Sci. Transl. Med. 2009;1:2ra4. doi: 10.1126/scitranslmed.3000130. [DOI] [PubMed] [Google Scholar]
- 50.Palfi S, Gurruchaga JM, Ralph GS, Lepetit H, Lavisse S, Buttery PC, Watts C, Miskin J, Kelleher M, Deeley S, et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: a dose escalation, open-label, phase 1/2 trial. Lancet. 2014;383:1138–1146. doi: 10.1016/S0140-6736(13)61939-X. [DOI] [PubMed] [Google Scholar]
- 51.Marsden CD, Obeso JA. The functions of the basal ganglia and the paradox of stereotaxic surgery in Parkinson’s disease. Brain. 1994;117:877–897. doi: 10.1093/brain/117.4.877. [DOI] [PubMed] [Google Scholar]
- 52.Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J. Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- 53.Nambu A. A new dynamic model of the cortico-basal ganglia loop. Prog. Brain res. 2004;143:461–466. doi: 10.1016/S0079-6123(03)43043-4. [DOI] [PubMed] [Google Scholar]
- 54.Levy R. Dependence of subthalamic nucleus oscillations on movement and dopamine in Parkinson’s disease. Brain. 2002;125:1196–1209. doi: 10.1093/brain/awf128. [DOI] [PubMed] [Google Scholar]
- 55.Stoffers D, Bosboom JLW, Deijen J, Wolters EC, Stam C, Be-rendse HW. Increased cortico-cortical functional connectivity in early-stage Parkinson’s disease: an MEG study. Neuroimage. 2008;41:212–222. doi: 10.1016/j.neuroimage.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 56.Litvak V, Jha A, Eusebio A, Oostenveld R, Foltynie T, Limousin P, Zrinzo L, Hariz M, Friston KJ, Brown P. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson’s disease. Brain. 2011;134:359–374. doi: 10.1093/brain/awq332. [DOI] [PubMed] [Google Scholar]
- 57.Little S, Tan H, Anzak A, Pogosyan A, Kühn A, Brown P. Bilateral functional connectivity of the Basal Ganglia in patients with Parkinson’s disease and its modulation by dopaminergic treatment. PLoS One. 2013;8:e82762. doi: 10.1371/journal.pone.0082762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marreiros AC, Cagnan H, Moran RJ, Friston KJ, Brown P. Basal ganglia–cortical interactions in Parkinsonian patients. Neuro-Image. 2013;66:301–310. doi: 10.1016/j.neuroimage.2012.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holgado AJN, Terry JR, Bogacz R. Conditions for the generation of beta oscillations in the subthalamic nucleus-globus pallidus network. J. Neurosci. 2010;30:12340–12352. doi: 10.1523/JNEUROSCI.0817-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benabid AL, Benazzous A, Pollak P. Mechanisms of deep brain stimulation. Movement Disorders. 2002;17(Suppl 3):S73–S74. doi: 10.1002/mds.10145. [DOI] [PubMed] [Google Scholar]
- 61.Deniau JM, Degos B, Bosch C, Maurice N. Deep brain stimulation mechanisms: beyond the concept of local functional inhibition. Eur. J. Neurosci. 2010;32:1080–1091. doi: 10.1111/j.1460-9568.2010.07413.x. [DOI] [PubMed] [Google Scholar]
- 62.Moran A, Stein E, Tischler H, Bar-Gad I. Decoupling neuronal oscillations during subthalamic nucleus stimulation in the parkinsonian primate. Neurobiol. Dis. 2012;45:583–590. doi: 10.1016/j.nbd.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 63.Eusebio A, Cagnan H, Brown P. Does suppression of oscillatory synchronisation mediate some of the therapeutic effects of DBS in patients with Parkinson’s disease? Front. Int. Neurosci. 2012;6:47. doi: 10.3389/fnint.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tripoliti E, Zrinzo L, Martinez-Torres I, Frost E, Pinto S, Foltynie T, Holl E, Petersen E, Roughton M, Hariz MI, et al. Effects of subthalamic stimulation on speech of consecutive patients with Parkinson disease. Neurology. 2011;76:80–86. doi: 10.1212/WNL.0b013e318203e7d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen CC, Brücke C, Kempf F, Kupsch A, Lu CS, Lee ST, Tisch S, Limousin P, Hariz M, Brown P. Deep brain stimulation of the subthalamic nucleus: a two-edged sword. Curr. Biol. 2006;16:952–953. doi: 10.1016/j.cub.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 66.Ray NJ, Jenkinson N, Brittain J, Holland P, Joint C, Nandi D, Bain PG, Yousif N, Green A, Stein JS, et al. The role of the subthalamic nucleus in response inhibition: Evidence from deep brain stimulation for Parkinson’s disease. Neuropsychologia. 2009;47:2828–2834. doi: 10.1016/j.neuropsychologia.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 67.Frank MJ. Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Networks. 2006;19:1120–1136. doi: 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 68.Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 69.Rosin B, Slovik M, Mitelman R, Rivlin-Etzion M, Haber SN, Israel Z, Vaadia E, Bergman H. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron. 2011;72:370–384. doi: 10.1016/j.neuron.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 70.Andersen R, Musallam S, Pesaran B. Selecting the signals for a brain-machine interface. Curr. Opin. Neurobiol. 2004;14:720–726. doi: 10.1016/j.conb.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 71.Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents — EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 2012;13:407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giannicola G, Rosa M, Servello D, Menghetti C, Carrabba G, Pacchetti C, Zangaglia R, Cogiamanian F, Scelzo E, Marceglia S, et al. Subthalamic local field potentials after seven-year deep brain stimulation in Parkinson’s disease. Exp. Neurol. 2012;237:312–317. doi: 10.1016/j.expneurol.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 73.Little S, Brown P. What brain signals are suitable for feedback control of deep brain stimulation in Parkinson’s disease? Ann. N. Y. Acad. Sci. 2012;1265:9–24. doi: 10.1111/j.1749-6632.2012.06650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kühn AA, Kempf F, Brücke C, Doyle L, Martinez-Torres I, Pogosyan A, Trottenberg T, Kupsch A, Schneider G-H, Hariz M, et al. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J. Neurosci. 2008;28:6165–6173. doi: 10.1523/JNEUROSCI.0282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M, Foltynie T, Limousin P, Ashkan K, Fitzgerald J, et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann. Neurol. 2013;74:449–457. doi: 10.1002/ana.23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Afshar P, Khambhati A, Stanslaski S, Carlson D, Jensen R, Linde D, Dani S, Lazarewicz M, Cong P, Giftakis J, et al. A translational platform for prototyping closed-loop neuromodulation systems. Front. Neural Circuits. 2012;6:117. doi: 10.3389/fncir.2012.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.López-Azcárate J, Tainta M, Rodríguez-Oroz MC, Valencia M, Gonzá-lez R, Guridi J, Iriarte J, Obeso JA, Artieda J, Alegre M. Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson’s disease. J. Neurosci. 2010;30:6667–6677. doi: 10.1523/JNEUROSCI.5459-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Hemptinne C, Ryapolova-Webb ES, Air EL, Garcia PA, Miller KJ, Ojemann JG, Ostrem JL, Galifianakis NB, Starr PA. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc. Nat. Acad. Sci. USA. 2013;110:4780–4785. doi: 10.1073/pnas.1214546110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Timmermann L, Florin E. Parkinson’s disease and pathological oscillatory activity: Is the beta band the bad guy? - New lessons learned from low-frequency deep brain stimulation. Exp. Neurol. 2012;233:123–125. doi: 10.1016/j.expneurol.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 80.Little S, Joundi R, Tan H, Pogosyan A, Forrow B, Joint C, Green A, Aziz T, Brown P. A torque-based method demonstrates increased rigidity in Parkinson’s disease during low-frequency stimulation. Exp. Brain Res. 2012;219:499–506. doi: 10.1007/s00221-012-3107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trend. Neurosci. 2007;30:357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 82.Timmermann L, Gross J, Dirks M, Volkmann J, Freund H-J, Schnitzler A. The cerebral oscillatory network of parkinsonian resting tremor. Brain. 2003;126:199–212. doi: 10.1093/brain/awg022. [DOI] [PubMed] [Google Scholar]
- 83.Bucy P, Case T. Tremor: physiologic mechanism and abolition by surgical means. Arch. Neurol. Psych. 1939;41:721–746. [Google Scholar]
- 84.Brittain J-S, Probert-Smith P, Aziz TZ, Brown P. Tremor suppression by rhythmic transcranial current stimulation. Curr. Biol. 2013;23:436–440. doi: 10.1016/j.cub.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smeal RM, Ermentrout GB, White JA. Phase-response curves and synchronized neural networks. Phil. Trans. R. Soc. Lon. B, Biol. sci. 2010;365:2407–2422. doi: 10.1098/rstb.2009.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ermentrout B. Type I membranes, phase resetting curves, and synchrony. Neural Computation. 1996;8:979–1001. doi: 10.1162/neco.1996.8.5.979. [DOI] [PubMed] [Google Scholar]
- 87.Cagnan H, Brittain J-S, Little S, Foltynie T, Limousin P, Zrinzo L, Hariz M, Joint C, Fitzgerald J, Green AL, et al. Phase dependent modulation of tremor amplitude in essential tremor through thalamic stimulation. Brain. 2013;136:3062–3075. doi: 10.1093/brain/awt239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tass PA. Stochastic phase resetting of stimulus-locked responses of two coupled oscillators: transient response clustering, synchronization, and desynchronization. Chaos. 2003;13:364–376. doi: 10.1063/1.1505813. [DOI] [PubMed] [Google Scholar]
- 89.Tass PA, Qin L, Hauptmann C, Dovero S, Bezard E, Boraud T, Meissner WG. Coordinated reset has sustained aftereffects in Parkinsonian monkeys. Ann. Neurol. 2012;72:816–820. doi: 10.1002/ana.23663. [DOI] [PubMed] [Google Scholar]
- 90.Pollo C, Kaelin-Lang A, Oertel MF, Stieglitz L, Taub E, Fuhr P, Lozano AM, Raabe A, Schüpbach M. Directional deep brain stimulation: an intraoperative double-blind pilot study. Brain. 2014;137:2015–2026. doi: 10.1093/brain/awu102. [DOI] [PubMed] [Google Scholar]
- 91.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 92.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kravitz AV, Freeze BS, Parker PRL, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lenz JD, Lobo MK. Optogenetic insights into striatal function and behavior. Behav. Brain Res. 2013;255:44–54. doi: 10.1016/j.bbr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 95.Burguière E, Monteiro P, Feng G, Graybiel AM. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science. 2013;340:1243–1246. doi: 10.1126/science.1232380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- 97.Konermann S, Brigham MD, Trevino AE, Hsu PD, Heidenreich M, Cong L, Platt RJ, Scott DA, Church GM, Zhang F. Optical control of mammalian endogenous transcription and epigenetic states. Nature. 2013;500:472–476. doi: 10.1038/nature12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chow BY, Boyden ES. Physiology. Synthetic physiology. Science. 2011;332:1508–1509. doi: 10.1126/science.1208555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Williams JC, Denison T. From optogenetic technologies to neuromodulation therapies. Sci. Transl. Med. 2013;5:177ps6. doi: 10.1126/scitranslmed.3003100. [DOI] [PubMed] [Google Scholar]
- 100.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 101.Kim T, McCall JG, Jung YH, Huang X, Siuda ER, Li Y, Song J, Song YM, Pao HA, Kim R-H, et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science. 2013;340:211–216. doi: 10.1126/science.1232437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Obeso JA, Rodríguez-Oroz MC, Benitez-Temino B, Blesa FJ, Guridi J, Marin C, Rodriguez M. Functional organization of the basal ganglia: Therapeutic implications for Parkinson’s disease. Movement Disorders. 2008;23(Suppl 3):S548–S559. doi: 10.1002/mds.22062. [DOI] [PubMed] [Google Scholar]
- 103.Stoessl AJ. Gene therapy for Parkinson’s disease: a step closer? Lancet. 2014;383:1107–1109. doi: 10.1016/S0140-6736(13)62108-X. [DOI] [PubMed] [Google Scholar]