Abstract
Deep brain stimulation of the globus pallidus internus alleviates involuntary movements in patients with dystonia. However, the mechanism is still not entirely understood. One hypothesis is that deep brain stimulation suppresses abnormally enhanced synchronized oscillatory activity within the motor cortico-basal ganglia network. Here, we explore deep brain stimulation-induced modulation of pathological low frequency (4–12 Hz) pallidal activity that has been described in local field potential recordings in patients with dystonia. Therefore, local field potentials were recorded from 16 hemispheres in 12 patients undergoing deep brain stimulation for severe dystonia using a specially designed amplifier allowing simultaneous high frequency stimulation at therapeutic parameter settings and local field potential recordings. For coherence analysis electroencephalographic activity (EEG) over motor areas and electromyographic activity (EMG) from affected neck muscles were recorded before and immediately after cessation of high frequency stimulation. High frequency stimulation led to a significant reduction of mean power in the 4–12 Hz band by 24.8 ± 7.0% in patients with predominantly phasic dystonia. A significant decrease of coherence between cortical EEG and pallidal local field potential activity in the 4–12 Hz range was revealed for the time period of 30 s after switching off high frequency stimulation. Coherence between EMG activity and pallidal activity was mainly found in patients with phasic dystonic movements where it was suppressed after high frequency stimulation. Our findings suggest that high frequency stimulation may suppress pathologically enhanced low frequency activity in patients with phasic dystonia. These dystonic features are the quickest to respond to high frequency stimulation and may thus directly relate to modulation of pathological basal ganglia activity, whereas improvement in tonic features may depend on long-term plastic changes within the motor network.
Keywords: deep brain stimulation, dystonia, oscillations, electrophysiology, basal ganglia
Introduction
Deep brain stimulation (DBS) of the globus pallidus internus (GPi) is an established and highly effective therapeutic option for severe, medically-refractory idiopathic dystonia (Coubes et al., 2000; Vidailhet et al., 2005, 2007; Kupsch et al., 2006; Ostrem and Starr, 2008; Volkmann et al., 2012). Excellent short and long term clinical results have been achieved in patients with generalized and segmental dystonia, but also in cervical and other focal dystonia (Krauss et al., 1999; Kiss et al., 2007; Ostrem et al., 2007; Walsh et al., 2013; Volkmann et al., 2014) although the first line treatment in these patients remains botulinum toxin therapy (Albanese et al., 2011). However, responder rate in patients with dystonia to DBS treatment is variable and there are no biomarkers available until now to predict individual clinical outcome (Andrews et al., 2010). Less severely affected patients with shorter disease duration have a more favourable outcome as well as carriers of the genetic status of DYT1 mutation (Krauss et al., 2004; Valldeoriola et al., 2010; Isaias et al., 2011; Markun et al., 2012; Panov et al., 2013). In contrast, patients with secondary dystonia show a smaller reduction of dystonic symptoms with GPi DBS (Tagliati et al., 2004; Vidailhet et al., 2009; Andrews et al., 2010; Air et al., 2011; Koy et al., 2013; Lumsden et al., 2013) with the exception of tardive dystonia (Franzini et al., 2005; Trottenberg et al., 2005; Damier et al., 2007; Sako et al., 2008; Gruber et al., 2009; Mentzel et al., 2012). Irrespective of the type of dystonia, patients with predominant hyperkinesia, i.e. phasic or mobile involuntary movements may respond better and more quickly to pallidal DBS as compared to patients with severe tonic posturing (Krauss, 2002; Volkmann and Benecke, 2002; Coubes et al., 2004; Krauss et al., 2004; Vidailhet et al., 2005, 2009; Wang et al., 2006; Yianni et al., 2006; Hung et al., 2007; Johnson et al., 2008). In general, the maximal effects of pallidal DBS may take up several weeks to months to occur and its precise mechanism of action remains largely unknown (Yianni et al., 2003).
One hypothesis posits that DBS suppresses or over-rides pathological oscillatory network activity within the cortex-basal ganglia loop (Hammond et al., 2007; Brown and Eusebio, 2008; McIntyre and Hahn, 2010; Vitek et al., 2012). Several electrophysiological studies in patients undergoing DBS for movement disorders over the past two decades have revealed evidence for disease-specific oscillatory patterns of neuronal basal ganglia activity (Liu et al., 2002; Silberstein et al., 2003; Dostrovsky and Bergman, 2004; Hutchison et al., 2004; Neumann et al., 2012), that may act as a noisy disruptive signal disturbing both local and distant neuronal network functioning causing characteristic movement disorders (Marsden and Obeso, 1994). Pathological basal ganglia activity is best described in patients with Parkinson’s disease undergoing DBS in the subthalamic nucleus (STN) or GPi: it involves enhanced synchronization in the 13–30 Hz beta band (Brown, 2003) recorded as local field potential (LFP) activity that is reduced by levodopa treatment in parallel with clinical improvement of motor symptoms (Kühn et al., 2006, 2009; Ray et al., 2009). Suppression of subthalamic nucleus beta band activity has been observed immediately after cessation of DBS together with improvement in bradykinesia (Wingeier et al., 2006; Kühn et al., 2008) and directly during STN-DBS (Brown et al., 2004; Eusebio et al., 2011). More recently, controlled adaptive stimulation in patients with Parkinson’s disease applied only during periods of increased beta band activity has been shown superior to standard high frequency stimulation (HFS) (Little et al., 2013) opening up new avenues for advanced treatment methods such as closed loop stimulation, once reliable disease-specific biomarkers have been identified.
The pathophysiology of dystonia is not fully understood, and pathological findings are evident at the cortical, brainstem and basal ganglia levels of the motor and sensory network (Berardelli et al., 1998; Charlesworth and Bhatia, 2013; Quartarone and Hallett, 2013). At the level of the basal ganglia, increasing evidence suggests that neuronal activity is characterized by enhanced synchronized oscillations in the low frequency band (4–12 Hz); (Liu et al., 2002, 2008; Silberstein et al., 2003; Chen et al., 2006a, b; Weinberger et al., 2012). Such synchronization correlates and is coherent with EMG activity during involuntary (mainly phasic) dystonic muscle contractions, suggesting that it may contribute to the pathophysiology of dystonia (Chen et al., 2006a, b; Liu et al., 2006; Sharott et al., 2008). Pallidal low frequency activity significantly drives EMG of the affected muscles (Sharott et al., 2008), increases during involuntary movements and correlates with the strength of the muscle spasms (Liu et al., 2008). It is of interest to note that pallidal low frequency activity can be modulated by a geste manoeuvre along with the alleviation of dystonic posturing as shown in two patients with cervical dystonia (Tang et al., 2007).
So far, there is only indirect evidence for low frequency oscillatory basal ganglia activity being related to the pathophysiology of dystonia. However, if DBS may act by suppressing abnormal oscillatory activity within the motor corticobasal ganglia network it should modulate low frequency activity in dystonia. Here, we explore for the first time the immediate effect of DBS on pallidal LFPs in patients with different types of dystonia by using a specially designed device that allows simultaneous LFP recordings and monopolar HFS (Eusebio et al., 2011). Our aim was to address whether GPi HFS suppresses local activity, and, if so, whether this effect is propagated along the corticobasal ganglia network to reduce involuntary movements. As immediate motor effects in patients with dystonia mainly occur for the mobile component of the involuntary movements, we separately assessed HFS effects in patients with predominant phasic movements, and predominant tonic posturing.
Materials and methods
Patients and surgery
Twelve patients with severe medically-refractory idiopathic (n = 10) or secondary (n = 2) dystonia [age 50 ± 2 years (mean ± SE), six females; disease duration 13 ± 4 years] participated in this study with informed consent and permission of the local ethics committee of the Charité, University Medicine, Berlin, Campus Virchow-Klinikum, according to The Code of Ethics of the World Medical Association (Declaration of Helsinki, 1967). Patients were recruited from the Department of Neurology, Charité University Medicine, Campus Virchow-Klinikum (Cases 1–3 and 5–12) and the Department of Neurosurgery, Medical University, Hannover (Case 4). Clinical details are summarized in Table 1. All patients underwent bilateral implantation of GPi DBS electrodes. One patient additionally underwent bilateral implantation of electrodes in Vim for severe tremor (Case 3). Because previous studies have described a different time course of DBS effects as well as different physiological characteristics for mobile and tonic dystonic features (Wang et al., 2006; Grips et al., 2007; Johnson et al., 2008), we grouped our patients according to their predominant clinical presentation of dystonia into a ‘phasic’ group and a ‘tonic’ group. The classification was done based on preoperative video recordings by a physician specialized on movement disorders and blinded to the electrophysiological data (C.B.).
Table 1. Descriptive patient data.
| Case | Surgical centre | Age/gender | Diagnosis / predominant symptoms preOP | Disease duration, years | TWSTRS (severity subscore) preOP/postOP | BFMDRS (movement scale) preOP/postOP | Stimulation settings during recording | DBS parameters at 3 months postOP or later | Contacts within GPi |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Berlin | 33/M | Idiopathic CD, Tonic torticollis | 3 | 23/10 | R: 02, 130Hz, 120μs, 2.5 V |
R: 1−; 60 Hz, 60 μs, 3 V L: 1−; 60 Hz, 60 μs, 3 V |
R: 1, 2, 3 L: 0, 1, 2, 3 |
|
| 2 | Berlin | 52/M | Secondary GD (cerebral palsy), Phasic retrocollis | 52 | 20/16 | 66.5/59 | R: 02, 130Hz, 120μs, 3 V |
R: 2−; 160 Hz, 60 μs, 2.5 V L: 2−; 160 Hz, 60μs, 2.5 V |
R: 2, 3 L: 1, 2, 3 |
| 3 | Berlin | 40/F | Idiopathic CD, Essential tremor, Tonic torticollis |
9 | 31/9 | L: 13, 130Hz, 120μs, 3 V |
L: 1−, 2−; 130 Hz, 60 μs, 2.6 VR: 1−, 2−; 130Hz, 60 μs, 2.6 V |
L: 0, 1, 2, 3R: 1, 2, 3 | |
| 4 | Hannover | 60/F | idiopathic SD, Tonic laterocollis | 6 | 19/15 | 9/6 | R: 02, 130Hz, 120μs, 3 V |
R: 1−; 130 Hz, 90 μs, 2 V L: 1−; 130Hz, 90μs, 2 V |
n.a. n.a. |
| 5 | Berlin | 53/M | Idiopathic SD, Phasic retrocollis | 7 | 25/14 | 30/22 | L: 13, 130Hz, 120μs, 3.5 V |
L: 0−, 1−, 2−; 130Hz, 60 μs, 4 V R: 0−, 1 + , 2 + ; 130 Hz, 60 μs, 1 V |
L: 0, 1, 2, 3 R: 0, 1, 2, 3 |
| 6 | Berlin | 59/F | Idiopathic CD, Phasic laterocollis | 16 | 24/6 | L: 02, 130 Hz, 120 μs, 2.5 V |
L: 1−, 2 + ; 130Hz, 90 μs, 5 V R: 1−; 130Hz, 90μs, 2.7 V |
L: 0, 1, 2, 3 R: 1, 2, 3 |
|
| 7 | Berlin | 51/F | Idiopathic GD, Tonic laterocollis | 5 | 23/17 | 38/6 | L: 02, 130Hz, 120μs, 3 V |
L: 1−; 130Hz, 90 μs, 3.5 V R: 1−; 130Hz, 90μs, 3.5 V |
L: 1, 2, 3 R: 0, 1, 2, 3 |
| 8 | Berlin | 57/M | Idiopathic SD, Phasic laterocollis | 12 | 26/23 | 48/12 | R: 13, 130Hz, 120μs, 3 V L: 13, 130Hz, 120μs, 3 V |
R: 1−; 60 Hz, 150 μs, 3.5 V L: 1−; 60Hz, 150μs, 4 V |
R: 0, 1, 2, 3 L: 0, 1, 2, 3 |
| 9 | Berlin | 47/F | Idiopathic CD, Tonic laterocollis | 4 | 18/12 | R: 02, 130 Hz, 60 μs, 3.5 V L: 02, 130 Hz, 60 μs, 3.5 V |
R: 1−; 130 Hz, 90 μs, 2 V L: 0−, 1−; 130Hz, 90μs, 1.8 V |
R: 0, 1, 2, 3 L: 0, 1, 2, 3 |
|
| 10 | Berlin | 44/M | Tardive CD, Phasic laterocollis | 10 | 20/3 | R: 02, 130 Hz, 90 μs, 4.5 V L: 02, 130 Hz, 90 μs, 4.5 V |
R: 1−, 2−; 130Hz, 90 μs, 2.5 V L: 1−, 2−; 130Hz, 90μs, 2.5 V |
R: 0, 1, 2, 3 L: 0, 1, 2, 3 |
|
| 11 | Berlin | 58/M | Idiopathic SD, Tonic torticollis | 21 | 23/14 | 13.5/6 | R: 13, 130Hz, 90μs, 3 V |
R: 1−, 2−; 130Hz, 90 μs, 2.7 V L: 1−, 2−; 130Hz, 90 μs, 3.3 V |
R: 1, 2, 3 L: 1, 2, 3 |
| 12 | Berlin | 47/F | Idiopathic CD, Phasic troticollis | 5 | 12/9* | R: 13, 130Hz, 90μs, 3 V L: 13, 130Hz, 90μs, 3 V |
R: 0−; 130Hz, 90 μs, 1.9 V L: 0−; 130Hz, 90μs, 1.9 V |
R: 1, 2, 3 L: 0, 1, 2, 3 |
|
| Mean (± SE) | 50 (±2) 6F, 6M | 13 (±4) | |||||||
BFMDRS = Burke-Fahn-Marsden Dystonia rating scale (movement scale, max.120 points); CD = cervical dystonia; F = female; GD = generalized dystonia; L = left; M = male; n.a. = not available; R = right; SD = segmental dystonia;
Tsui = Torticollis Rating Scale (max. 25 points); TWSTRS = Toronto Western spasmodic torticollis rating scale (severity subscore, max. 35 points).
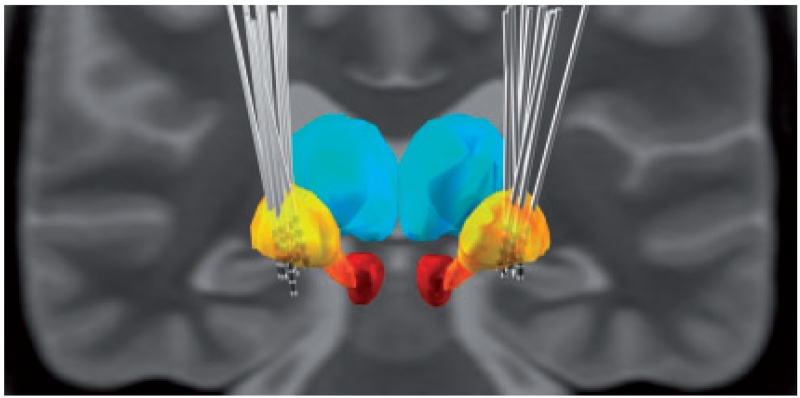
The surgical procedure has been described previously (Kupsch et al., 2006; Brucke et al., 2008). The DBS electrode used was Model 3389 (Cases 1, 2, 5, 6, 8 and 12) or 3387 (Cases 3, 4, 7 and 9–11, Medtronic Neurological Division) with four platinum-iridium cylindrical surfaces (1.27-mm diameter and 1.5-mm length) and a contact-to-contact separation of 0.5 mm (3389) or 1 mm (3387). Contact 0 was the most caudal and contact 3 the most rostral. Target coordinates were based on direct visualization of the GPi in the individual stereotactic T2-weighted MRI. The intended coordinates at the tip of electrode 0 were 17.4–22 mm lateral from the midline, 2–4 mm in front of the midcommissural point, and 2–4 mm below the midcommissural point as determined by MRI adjusted to the individual patient’s anatomy. Correct placement of the DBS electrode was confirmed by intraoperative microelectrode recordings and direct macrostimulation in all patients. Postoperative CT (Hannover) or MRI (Berlin) confirmed correct placement of the macroelectrode in all patients using automated normalization and contact localization in standard Montreal Neurological Institute (MNI) stereotactic space coordinates (methods are described in more detail in Schonecker et al., 2009). All electrode reconstructions are visualized in Fig. 1.
Figure 1.
Reconstruction of DBS-electrode placements for 11 of 12 patients (Case 4 was excluded due to postoperative CT). After normalization of transversal and coronar MR-images into MNI-space, lead-trajectories and DBS-electrode contacts were reconstructed and visualized in MNI-space. Volumes for globus pallidus (yellow), thalamus (blue), subthalamic nucleus (orange) and red nucleus (red) are shown and superimposed onto a standard MNI template. Except for right contact 1 in Case 2 all stimulated contacts lay within the GPi.
Correct placement of DBS electrodes was further supported by significant improvement of dystonic symptoms with chronic DBS at least 3 months after surgery in all patients as assessed using dystonia rating scales [Burke-Fahn-Marsden Dystonia Rating Scale, TWSTRS [Toronto Western Spasmodic Torticollis Rating Scale), Tsui (Torticollis Rating Scale) with a mean improvement of 52.7 ± 7.1% (P < 0.001, paired Student’s t-test). Patients with predominant phasic dystonia showed a mean improvement of 49.7 ± 13.1% (and accordingly 57.3% when excluding Case 2 with secondary dystonia due to cerebral palsy), which was not significantly different from the mean improvement of 55.7 ± 8.3% in patients with predominant tonic dystonia (P = 0.74, Student’s t-test).
Paradigm and recordings
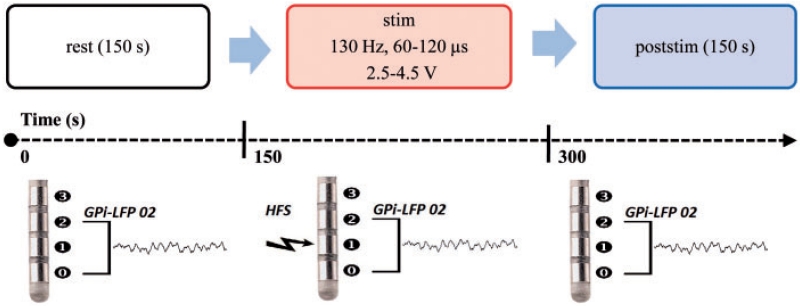
Local field potential recordings were performed 2.4 ± 0.1 (mean ± SE, range 1–3) days after insertion of the DBS macroelectrodes. Patients were comfortably seated in an armchair and instructed neither to speak nor to move voluntarily during ongoing recordings. They were explicitly advised not to try to avoid any dystonic muscle contractions by performing a sensory manoeuvre (geste antagoniste) or by resisting spasm. The paradigm consisted of three conditions: (i) bipolar LFP recordings at rest; (ii) monopolar unilateral stimulation through contact 1 or 2 with simultaneous bipolar LFP recordings from adjacent contact pairs using a specially designed amplifier; and (iii) bipolar LFP recordings from the same contact pair immediately after cessation of stimulation (Fig. 2). These trials are named ‘rest’, ‘stim’ and ‘poststim’. The paradigm always started with a rest recording of 150 s (‘rest’ condition without any prior DBS). ‘Stim’ and ‘poststim’ trials always lasted 150 s and were repeated two to four times per session depending on the patient’s condition without any specific announcement or obvious indicators to prevent distraction, movement induced artefacts and attentional changes.
Figure 2.
Schema of the experimental paradigm consisting of a 150 s prestimulation baseline recording, and at least two runs of 150 s LFP recordings during unilateral monopolar stimulation, followed by 150 s of LFP recordings after cessation of HFS.
Stimulation parameters for the ‘stim’ condition were evaluated during a short test stimulation. The hemisphere used for unilateral stimulation was chosen contralateral to the more prominent clinical features in patients with limb involvement (but did not take into account recent findings of asymmetric pallidal LFP activity pattern in cervical dystonia) (Lee and Kiss, 2014; Moll et al., 2014), or with respect to the occurrence of a distinct peak in the 4–12 Hz band and signal quality of the recorded LFPs. In four patients (Cases 8–10 and 12) both hemispheres were stimulated and recorded in two subsequent sessions. Test stimulation was performed at contacts 1 and 2 to evaluate the threshold for potential immediate effects and, more important, for side effects using an external stimulator (DualScreen Model 3628, Medtronic). Stimulation frequency was set at 130 Hz and was constant for all patients and during all ‘stim’ trials. Mean pulse width used was 103 ± 5 (range 60–120 μs, see Table 1 for details). Unilateral monopolar stimulation was then started at the contact with the higher threshold for side effects and set at 0.2 V below this threshold and to a maximum of 4.5 V. In the case of low signal quality for LFP recordings (which may occur due to large differences in impedance between the bipolar contact pairs most likely related to contact localization) the adjacent second best contact had to be chosen. Stimulation amplitude varied between 2.5–4.5 V, with a mean amplitude of 3.2 ± 0.1 V during LFP-recordings, which was not significantly higher compared to the amplitude of postoperative chronic DBS (3.0 ± 0.2 V; paired Student’s t-test, P = 0.43).
LFPs were recorded bipolarly from contacts 0–2 or 1–3 of the DBS macroelectrodes, depending on the selected contact for test stimulation (1 or 2 respectively, see Table 1 for selected contacts in individual patients). LFP signals were bandpass filtered at 4–40 Hz using a specially designed high-gain (100 dB) amplifier (Rossi et al., 2008; Eusebio et al., 2011) and sampled at 1 kHz (Cases 1–8) or 5 kHz (Cases 9–12). Monopolar stimulation was delivered via the external stimulator between active contact 1 (when recording from contacts 0/2) or 2 (when recording from contacts 1/3) and a surface skin electrode to allow simultaneous monopolar stimulation and LFP recordings from adjacent contact pairs. The external stimulator was switched on or off corresponding to the experimental condition. Vim electrodes (Case 2) were not stimulated nor recorded from during the entire experiment.
EEG activity was recorded over midfrontal (CZ) and, if possible, from sensorimotor areas (C3/C4) according to the 10/20 system using Ag/AgCl surface-electrodes, referenced to the ears, and bandpass filtered at 0.5–250 Hz. EEG recordings were limited due to surgical dressings or had to be rejected due to mains noise artefacts in some patients and were only available in 12 hemispheres of nine patients (four hemispheres of three patients with tonic movements, Cases 1, 7 and 9; eight hemispheres of six patients with phasic movements, Cases 2, 5, 6, 8, 10 and 12).
EMG activity was recorded from the sternocleidomastoid muscle in all patients using Ag/AgCl surface-electrodes placed at least 2 cm apart on the muscle belly. EMG signals were bandpass filtered at 4–250 Hz (Cases 9–13) and 10–250 Hz (Cases 1–8). EMG signals could only be obtained in 6 of 12 patients either ipsilateral (Cases 1, 3, 5–7 and 10) or contralateral (Cases 1, 3, 6–8 and 10) to LFP muscles. Vertical electrooculography signals were recorded to assess eye-movement artefacts. EEG and EMG activity were amplified (×50 000), sampled at 1 kHz (Cases 1–8) or 5 kHz (Cases 9–12) and monitored on-line using a Digitimer D360 amplifier (Digitimer Ltd.). All signals were digitized through an analogue-to-digital converter CED 1401 (Cambridge Electronic Design) and recorded onto a computer using commercial Spike2 software (Spike2 6.07, CED). No clinical scores were obtained during the recording period.
Analysis and statistics
Power analysis
Files sampled at 5 kHz (Cases 9–12) were down sampled to a common sampling rate of 1 kHz using commercial software (Spike2 software, CED). LFP recordings from six patients (Cases 1, 2, 4–6 and 8) showed a sharp artefact at 24–26 Hz that was induced by the device (subharmonic of the 50 Hz mains noise artefact), which was discarded and interpolated by the average of the two adjacent lower and higher bins. The first 2 s of each trial (‘stim’ and ‘post-stim’ condition) were discarded in all patients due to the artefacts induced by switching on/off the external stimulator and respective time needed for stabilization of the amplifier signal.
Spectral analysis was performed based on methods outlined by Halliday et al. (1995). LFP power was analysed for each trial separately by Fast Fourier Transform (FFT) that was performed on non-overlapping blocks of 1s duration using Spike2 software. The block size used was 2048 data points, resulting in a frequency resolution of 0.49 Hz. Normalization of each power spectrum was performed by dividing each frequency bin by the standard deviation (SD) of the 13–40 Hz power in each condition of each patient (Priori et al., 2004; Neumann et al., 2014).
Normalized power-spectra were further expressed in arbitrary units (au). Resulting autospectra for the conditions ‘stim’ and ‘poststim’ were first averaged across trials for each patient and subsequently averaged across patients for the grand average. To investigate the frequency range of stimulation-related power changes we followed two complimentary approaches. First, the power spectra between the ‘rest’ and ‘stim’ condition were compared using Wilcoxon’s sign rank test for 0.5 Hz bins. Starting from 3 Hz up to 40 Hz, for each 0.5 Hz step, the power was compared between ‘rest’ and ‘stim’ across all patients and across the groups alone. Significant differences are shown if at least four consecutive bins (i.e. 2 Hz band width) reached a significance value of <0.05. Once the major power changes were confirmed to occur in the <12 Hz range, in a second step we used previously defined frequency bands (i.e. the 4–12 and 13–30 Hz range) for the ANOVA to evaluate frequency-specific effects of HFS between conditions and groups. According to previous results in patients with dystonia, we expected significant modulation of low frequency activity (4–12 Hz range), which has been shown to be enhanced in dystonia (Silberstein et al., 2003; Liu et al., 2008) and coupled to dystonic muscle activity (Brown and Williams, 2005). Power in the 0–3 Hz range wasn’t analysed because it is most prone to movement artefacts and potential stimulation-induced artefacts caused by the amplifier (Eusebio et al., 2011). Normalized power was compared between conditions for each frequency band of interest using ANOVA. Statistical analysis was performed using MATLAB (The Mathworks Inc.) and PASW 18 (SPSS Inc.). All data were normally distributed as assessed by the Kolmogorov-Smirnov test (P > 0.05). A repeated-measures ANOVA with the factor ‘condition’ (‘rest’, ‘stim’ and ‘poststim’) and the factor ‘group’ (phasic and tonic) was performed for both 4–12 Hz and 13–30 Hz band. Post hoc paired samples t-tests were applied and corrected using Bonferroni correction. The relative power change was evaluated using Student’s t-test. Clinical parameters before and after GPi-DBS were compared using paired Student’s t-test. All results are given as mean ± SE.
Coherence analysis
Coherence was calculated between simultaneously recorded pallidal LFPs and ipsilateral or contralateral EMG, and between pallidal LFPs and midline or ipsilateral EEG (CZ and/or either C3 or C4) (Halliday et al., 1995). EMG signals were first digitally rectified (Myers et al., 2003). Coherence estimates were calculated over the last 30 s of the ‘rest’ condition and the first 30 s of the ‘poststim’ condition. Record lengths were kept constant in all patients. Analysis was restricted to the conditions without high frequency stimulation due to the stimulation-induced artefacts in EEG and EMG. Coherence estimates were calculated with a block size of 2048 data points resulting in a frequency resolution of 0.488 Hz and 14 segments. Coherence values were averaged for each patient across trials for the ‘poststim’ condition. Coherence was considered significant in individual patients if at least two consecutive bins were above 0.206, i.e. above P = 0.05 as defined by the Halliday method. Coherence estimates were normalized by transforming the square root of the coherence at each frequency bin using the Fisher Transform (Halliday et al., 1995). Mean coherence values were calculated for the respective frequency bands of interest (4–12 Hz, 13–30 Hz) for ‘rest’ and ‘poststim’ condition. As calculated EEG-LFP coherence estimates were normally distributed, as assessed by the Kolmogorov-Smirnov test, a repeated-measures ANOVA with the factors ‘condition’ (‘rest’, and ‘poststim’) and between-subject factor ‘group’ (‘phasic’ and ‘tonic’) was performed separately for the 4–12 Hz and 13–30 Hz frequency band. Post hoc paired samples t-tests were applied and corrected using step-wise correction for multiple comparisons where appropriate. Due to the limited number of patients where EMG from dystonic muscles was available, we only describe the number of significant individual LFP-EMG coherence estimates.
Results
Evaluation of power spectra
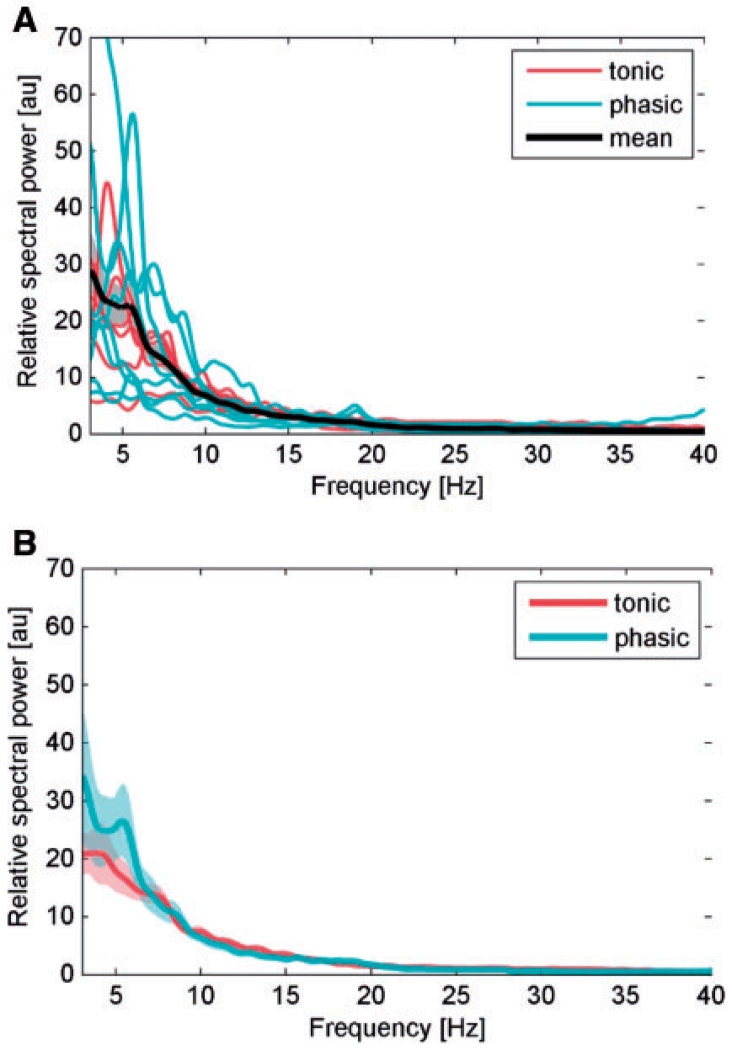
Individual normalized power spectra of rest recordings from the selected contact pair of each patient as well as the grand average of these spectra is shown in Fig. 3A (16 GPi from 12 patients). In line with previous results (Silberstein et al., 2003), the power spectrum is characterized by prominent 4–12 Hz low frequency activity that was similar for patients with phasic and tonic dystonia (Fig. 3B).
Figure 3.
(A) Individual normalized power spectra of rest recordings from the selected contact pair of each patient (red lines correspond to patients from the tonic group, blue lines to phasic group) and averaged normalized power spectra (bold black line) with 95% confidence limits (shaded areas of power spectra) in the 4–40 Hz range. (B) Mean normalized power spectra at rest separately for both phasic (blue line) and tonic (red line) patient subgroup.
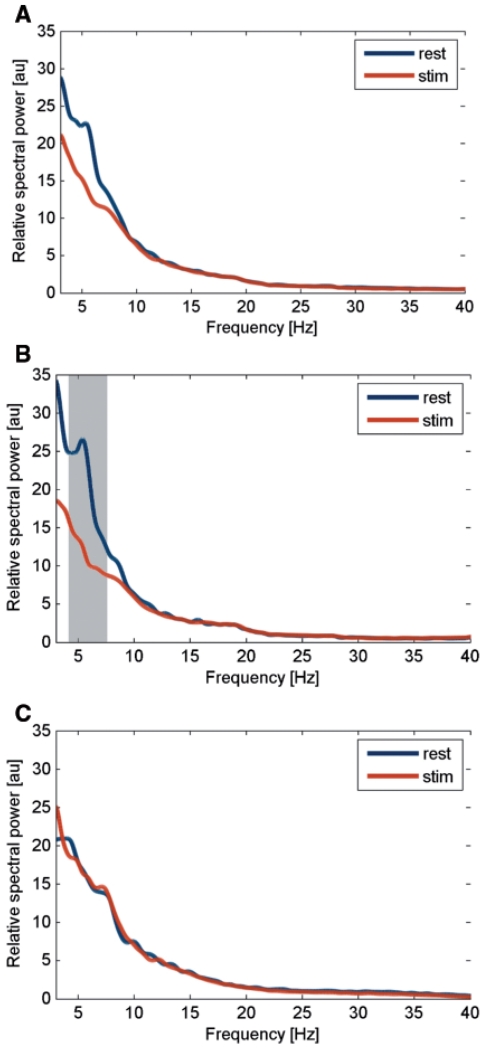
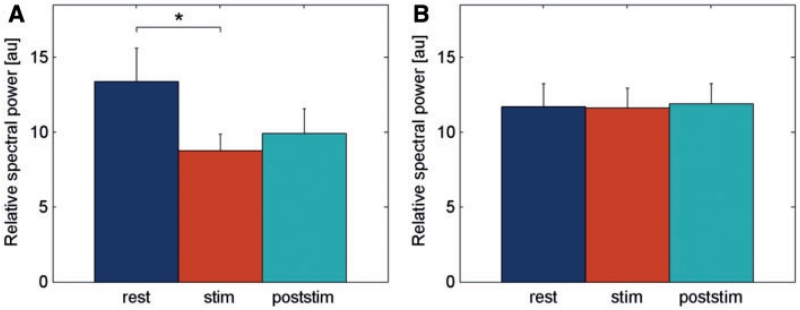
The comparison of power spectra between ‘rest’ and ‘stim’ condition (using Wilcoxon’s sign rank test for 1 Hz bins) did not reveal an overall effect of HFS (Fig. 4A) but showed a significant difference with reduced power during ‘stim’ condition in the frequency range from 4.4 to 7.3 Hz for the phasic group (Fig. 4B). No significant changes were revealed in the tonic group (Fig. 4C). Accordingly, the repeated-measure ANOVA with the factor ‘condition’ (three levels: rest, stim and poststim) and the between-subject factor ‘group’ (two levels: phasic and tonic dystonic movements) for the 4–12 Hz band revealed a significant main effect for condition (F = 5.18, P = 0.01) and a significant interaction between ‘condition’ and ‘group’ (F = 3.44, P = 0.04). According to the significant interaction between ‘condition’ and ‘group’, we explored the different HFS-induced power modulation of 4–12 Hz activity between groups. Post hoc paired samples t-test revealed that in patients with predominantly phasic dystonia 4–12 Hz power was significantly decreased during HFS [8.8 ± 1.1 arbitrary units (a.u.)] compared to the LFP activity at rest (13.4 ± 2.2 a.u., P = 0.04; Fig. 5A). Furthermore, a trend for power reduction was revealed during the ‘poststim’ period (9.9 ± 1.6 a.u., P = 0.096; Fig. 5A). In contrast, in patients with predominantly tonic dystonic movements, no significant power modulation occurred with respect to the stimulation condition (rest 11.7 ± 1.5 a.u.; stim 11.6 ± 1.3 a.u.; poststim 11.9 ± 1.4 a.u.; Fig. 5B). No significant difference was revealed for beta band power using ANOVA (data not shown). An individual example for HFS-induced modulation of 4–12 Hz pallidal activity in a patient with predominant phasic dystonia is shown in Fig. 6 (Case 6).
Figure 4.
Mean relative spectral power during rest condition (blue line) and stimulation (red line) for the overall data (A), the phasic (B) and tonic (C) group. The grey shaded area denotes a significant difference between conditions using Wilcoxon’s sign rank test for 0.5 Hz bins (with at least four consecutive bins at P < 0.05). Note that only the group of patients with predominant phasic dystonia showed a suppression of low frequency activity that was significant for the frequency range from 4.4–7.3 Hz.
Figure 5.
Post hoc analysis of the group × condition interaction of LFP power changes. The bar chart shows the mean normalized 4–12 Hz activity before HFS (‘rest’), during HFS (‘stim’), and after cessation of HFS (‘poststim’) in the phasic (A) and tonic (B) subgroup of patients. Note that 4–12 Hz activity was significantly suppressed during HFS in the subgroup of patients with predominant phasic dystonic movements.
Figure 6.
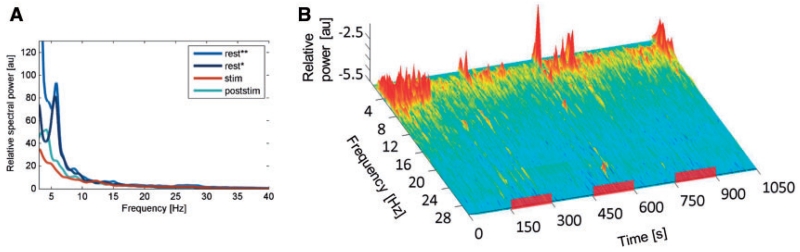
(A) Example of normalized power spectra at rest (dark blue), during HFS stimulation (red) and after cessation of HFS (light blue) of a single patient with primary dystonia and predominant phasic dystonic movements (Case 6). Note that the ~6 Hz peak that occurrs at rest is largely suppressed by HFS. Rest recordings that were performed with the Digitimer D360 (marked by a single asterisk; Digitimer Ltd.) and via the specially designed amplifier (marked by two asterisks) used in the study for simultaneous stimulation and recordings are similar. (B) Time–frequency plot of the same patient with concatenated periods of ‘rest’, ‘stim’ and ‘poststim’ condition. Periods of HFS are marked by red bars (stim condition). Pallidal HFS suppresses low frequency activity (peak ~6 Hz) in the patient that occurred in parallel with the clinical reduction in phasic dystonia.
EEG - GPi–LFP coherence
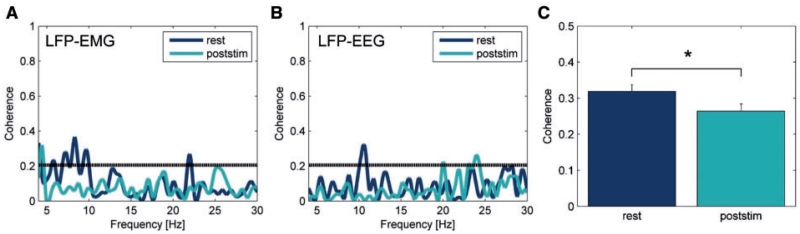
In 8 of 12 hemispheres (seven of nine patients) a significant coherence (at least two consecutive bins) within the 4–12 Hz range was found between pallidal LFPs and EEG recorded over Cz at rest (Cases 1, 6–10 and 12; if available, a significant coherence was also found for LFP and ipsilateral EEG recorded over C3/C4). Fisher transformed normalized mean EEG-LFP coherence from rest recordings in these patients shows a peak in the 4–12 Hz range. Figure 7B shows an example of EEG–LFP coherence from a patient with predominant tonic posturing (Case 9) at rest and after cessation of HFS. A significant coherence in the beta frequency range was revealed in three hemispheres (Cases 6, 7 and 12). To evaluate whether HFS-induced changes are not restricted to local power changes in the 4–12 Hz band but distributed along the cortico-basal ganglia network, coherence values were compared between patient groups at rest and poststim condition. The 2 × 2 ANOVA with within-subject factor ‘condition’ (rest and poststim) and between-subject factor ‘group’ (phasic and tonic) revealed a significant main effect for ‘condition’ (F = 6.010, P = 0.034) with a significant larger coherence at rest (mean Fisher transformed coherence 0.319 ± 0.018) compared to poststim period (0.264 ± 0.020; Fig. 7C). No main effect for group nor interaction was revealed. Similar results were obtained when EEG-LFP coherence values obtained for Cz and C3/C4 were averaged in individual patients (ANOVA: F = 6.972, P = 0.033). No significant effect was obtained for the beta frequency band (P > 0.5).
Figure 7.
Examples of coherence spectra between pallidal LFP and dystonic EMG (A) and ipsilateral EEG (B). The significant 4–12 Hz coherence at rest is reduced in the time period shortly after cessation of HFS. The grey horizontal line indicates the significance threshold of P = 0.206. (C) Statistical analysis (ANOVA) of averaged EEG - GPi–LFP in the 4–12 Hz range revealed a main effect of condition with a significant larger coherence at rest compared to poststim period (*P = 0.034).
Pallidal LFP–EMG coherence
In four of seven patients a significant coherence within the 4–12 Hz range was found between pallidal LFPs and contralateral EMG (except for Case 5, where only ipsilateral EMG was available) at rest. Significant pallidal LFP–EMG coherence was found more often in the ‘phasic’ group (three of four patients) over a broad frequency range within the 4–12 Hz band (Cases 5, 6 and 8) and only for two bins in a single patient (Case 3, one of three patients) from the ‘tonic’ group, which is similar to previous results by Wang et al. (2006) pointing to a more prominent GPi–LFP - EMG coherence with rhythmic EMG activity (Wang et al., 2006). Fisher transformed normalized mean pallidal LFP–EMG coherence from rest recordings shows a peak in the 4–12 Hz range that disappears after HFS. A single example is given in Fig. 7A. Only two patients from the tonic group (Cases 3 and 7) showed a significant GPi–LFP - EMG coherence in the beta frequency range. Numbers were too small to perform any statistical analysis.
Discussion
We have shown that pallidal 4–12 Hz oscillatory activity is suppressed during HFS of the GPi in patients with dystonia. This low frequency activity is evidenced by a peak in baseline spectra of LFP activity in dystonia (Silberstein et al., 2003; Liu et al., 2008; Sharott et al., 2008). The reduction in pallidal LFP activity was frequency selective and occurred only in the subgroup of patients with predominant phasic dystonic movements suggesting a link between improvement in phasic dystonia and reduction of low frequency activity. The implication is that HFS acts by reducing oscillatory pallidal 4–12 Hz LFP activity in dystonia, thereby mirroring an effect that has been described during a positive geste manouvre in two patients with cervical dystonia (Tang et al., 2007). The functional consequences of chronic suppression of pallidal 4–12 Hz activity are most likely not confined to the basal ganglia. The suppression of EEG - GPi–LFP coherence and GPi–LFP - EMG coherence in the 4–12 Hz frequency band that was ongoing shortly after HFS supports the notion of a modulation of network activity during GPi HFS. The main clinical effect of HFS in dystonia occurs only after days to weeks and months with reduction of dystonic posturing and tonic abnormal movements (Yianni et al., 2003). Thus, the reduction in pallidal 4–12 Hz activity may be propagated along the different nuclei of the basal ganglia and to the cerebral cortex, so that HFS influences neuronal activity both locally at the site of stimulation and also over other functionally connected elements of the cortex–basal ganglia network inducing long-term plastic changes at cortical level that may allow for compensatory strategies to re-establish normal movements.
Low frequency pallidal activity in dystonia
There is strong evidence that the GPi plays a pivotal role in the pathophysiology of dystonia since dystonic symptoms can be alleviated by pallidotomy and pallidal DBS (Coubes et al., 2000; Vidailhet et al., 2005; Kupsch et al., 2006); with good longterm results during continuous HFS (Vidailhet et al., 2007; Volkmann et al., 2012). It has been suggested that not only reduced pallidal discharge rates (Albin et al., 1989; DeLong, 1990) but more important abnormal discharge patterns (Vitek et al., 1999) and increased low frequency oscillatory activity in the basal ganglia may contribute to the generation of abnormal movements (Silberstein et al., 2003; Liu et al., 2008; Neumann et al., 2012). Enhanced low frequency pallidal activity occurred in association with prolonged dystonic spasms and periods of increased involuntary movements (Liu et al., 2008). Furthermore, functional coupling of low frequency activity with dystonic muscle activity has been established in several studies from different laboratories (Liu et al., 2002; Wang et al., 2004, 2006; Chen et al., 2006a; Foncke et al., 2007; Sharott et al., 2008). Pallidal LFP–EMG coherence occurs most prominently during rhythmic involuntary muscle bursts (Wang et al., 2004). This low frequency coupling between pallidal output and dystonic EMG activity is bidirectional but shows a dominant drive from pallidum as revealed by directionality analysis (Sharott et al., 2008). Muscle spasms were preceded by increased 3–20 Hz pallidal activity in a single patient and the latter correlated with the EMG magnitude of spasms (Liu et al., 2008) further supporting the assumption that excessive synchronization in pallidal LFP activity may play a causal role in generation of abnormal hypertonic muscle activity in dystonia; similar to the dyskinesias described with enhanced theta band activity in the subthalamic nucleus of patients with Parkinson’s disease (Alonso-Frech et al., 2006; Alegre et al., 2012). Taken together with these previous findings, it is reasonable to speculate that enhanced low frequency activity may contribute to the generation of abnormal movements in dystonia, especially the occurrence of phasic movements reflecting the association with rhythmic dystonic EMG activity.
Our findings confirm that enhanced pallidal low frequency activity (4–12 Hz) is present in patients with dystonia and is coherent with EMG activity from affected muscles. Moreover, we demonstrate coherence at low frequency between EEG activity over motor areas and GPi-LFP in our patients extending the pathological network activity to cortical motor areas. The most intriguing result of the present study is the suppression of low frequency pallidal activity during HFS in the subgroup of patients with mobile dystonia. Above we have gathered evidence for what the synchronized pallidal activity represents. Our results of HFS-induced reduction of low frequency activity are in line with a generative role of abnormal GPi activity in dystonia. More specifically, suppression of 4–12 Hz activity occurred in the sub-group of patients with predominant phasic dystonia supporting the concept of a causal link of low frequency activity for hypertonic rhythmic spasms in dystonia. Although we did not consistently measure the clinical improvement in our patients, it is noteworthy that in two of our six patients with phasic dystonia there was an immediate clinical effect of HFS leading to almost complete cessation of phasic movements within seconds to minutes. This was never noted in the tonic group. Moreover, patients also showed reduced GPi–LFP to EMG coherence in a short period after cessation of HFS. It could be speculated that this observation is due to an over-lasting effect of HFS on pallidal drive to dystonic EMG activity. Unfortunately, our set-up only allowed for simultaneous stimulation and recording of pallidal LFP but not EEG or EMG activity so that the latter activities had to be discarded during HFS due to stimulation-induced artefacts. However, these after-effects of DBS have previously been described in patients with Parkinson’s disease after subthalamic nucleus HFS (Kuhn et al., 2008) and most likely represent HFS-induced changes within the motor cortex - basal ganglia network and its drive to muscle activity. It is of note that directionality analysis has not been feasible in our study and HFS-induced changes in pallidal LFP activity and LFP–EEG and LFP–EMG coherence might be influenced by the clinical reduction in phasic movements and thus reduced re-afferent drive or movement artefacts. Three arguments are in favour of our interpretation of the results: (i) we have been careful to avoid frequencies below 4 Hz to limit artefacts from dystonic head movements; (ii) our results parallel the findings from previous studies that have shown a pallidal drive of 4–12 Hz LFP over muscle activity in dystonia (Foncke et al., 2007; Sharott et al., 2008); and (iii) EEG - GPi-LFP coherence was significantly reduced also in the tonic subgroup where patients did not show any clinical effect of short term HFS. It has to be considered that patients were not formally blinded to the ‘stim’ and ‘poststim’ condition and attentional changes may influence our main results. However, task condition was not specifically announced to the patients and stimulation settings did not induce side effects, which limits the possibility that subjects were able to identify the stimulation condition during the repeated trials. Moreover, only the subgroup of patients with predominantly phasic dystonia showed a significant reduction in low frequency LFP activity, which argues in favour of a specific stimulation-induced effect rather than general changes in focused attention that should be similar across patient groups.
Our results further fit with clinical observations of early improvement of phasic movements in dystonia patients after pallidal DBS (sometimes even seen as a so-called stun effect after electrode implantation) as compared to later improvement in tonic dystonic features (Krauss, 2002, Volkmann and Benecke, 2002; Coubes et al., 2004; Krauss et al., 2004; Vidailhet et al., 2005, 2009; Wang et al., 2006; Yianni et al., 2006; Hung et al., 2007; Johnson et al., 2008) although this more rapid improvement was not specifically demonstrated in our patients.
Thus, it could be speculated that the frequency-specific suppression of 4–12 Hz activity by pallidal HFS may directly suppress the abnormal pallidal drive to dystonic muscles and thereby improve hyperkinetic movements; an effect that is more confined to local abnormal basal ganglia activity and its pathological drive to muscle activity. In contrast, more complex long-term plastic changes at multiple levels of the basal ganglia–motor cortical network may lead to improvement in tonic features. It is interesting to note that tardive dystonia, which is most often characterized by predominant mobile dystonia (Skidmore and Reich, 2005), is known to show a rapid and sustained motor improvement up to 87% (Franzini et al., 2005; Trottenberg et al., 2005; Cohen et al., 2007; Damier et al., 2007; Sako et al., 2008; Gruber et al., 2009; Mentzel et al., 2012) that is faster and superior to that of idiopathic dystonia although large randomized clinical trials are still missing. Moreover, in patients with secondary dystonia that usually have a poorer clinical outcome of DBS, the best clinical results are obtained for suppression of hyperkinetic movements (Vidailhet et al., 2009; Vidailhet and Grabli, 2011) that could be due to the efficacious local effect of DBS on pathological basal ganglia activity but reduced capacity for DBS-induced long term compensatory motor cortical network changes in those patients with cerebral lesions. Finally, an interesting clinical observation comprises the development of bradykinesia in some patients with dystonia under chronic DBS (Ostrem et al., 2007; Berman et al., 2009) that is still not understood. The suppression of prokinetic 4–12 Hz pallidal activity during long-term DBS may lead to an imbalance among different frequency bands resulting in slowness of movement (Brown and Williams, 2005). In line with this, increased theta band activity was associated with faster reaction time in patients with Parkinson’s disease (Anzak et al., 2012). It is interesting to note that theta/gamma cross frequency coupling has been observed in cervical dystonia (Moll et al., 2014), the latter activity also being associated with scaling of movement in dystonia patients (Brucke et al., 2012).
A potential limitation of our study is the lack of an overall effect of HFS on pallidal LFP. Also, we did not quantify the clinical effect of stimulation at the time of LFP recordings. Subgroup analysis was performed according to previously described clinical observations regarding the time course of DBS effects in dystonia. Experimental settings did not differ between the subgroups of patients and the mean stimulation amplitude during LFP-recordings (3.2 ± 0.1 V) was not significantly different from the amplitude of postoperative chronic DBS (3.0 ± 0.2 V). Moreover, chronic DBS at least 3 months after surgery led to an overall mean improvement of >50% on the respective dystonia rating scales in all patients without a significant difference between the two subgroups that further confirms the correct electrode placement in our patients. Statistical analysis revealed no difference between the two subgroups of patients according to their age, gender, disease duration, severity and distribution of symptoms (P > 0.05, paired Student’s t-test). We have to consider that two patients in the subgroup of mobile dystonia had a different aetiology, namely tardive (Case 11) and secondary dystonia due to cerebral palsy (Case 2); however, both showed a response to chronic DBS. Recent findings by Sanghera et al. (2003) who analysed neuronal firing patterns from intraoperative microelectrode recordings in patients with primary and secondary dystonia failed to reveal significant differences in the rate or pattern of neuronal discharge between primary and secondary dystonia. Thus, the main distinguishing feature in our patients was the presence or not of mobile dystonia that has been classified by an experienced movement disorder specialist blinded to the neurophysiological results of our study. There is abundant clinical evidence for a differential response of phasic and tonic dystonic features to DBS in terms of time course with faster improvement of phasic movements in the order of minutes and hours (Volkmann and Benecke, 2002; Coubes et al., 2004; Krauss et al., 2004; Vidailhet et al., 2005; Hung et al., 2007) that has led to subgroup analysis of clinical data. Here, we present for the first time a neurophysiological marker that reflects the time course of the clinical observation of fast HFS-induced suppression of hyperkinetic movements in dystonia, namely the HFS-induced modulation in 4–12 Hz pallidal LFP activity. It can only be speculated if enhanced pallidal low frequency activity may serve as a biomarker for adaptive control stimulation in dystonia as has been shown recently for subthalamic beta band activity in Parkinson’s disease (Little et al., 2013).
Our results lead to the conclusion that phasic movements are suppressed immediately by local suppression of enhanced low frequency pallidal activity, whereas sustained tonic activity has a delayed response to pallidal HFS. How may the clinical effect on tonic posturing be induced if there is no change in local pallidal activity? It is interesting to note that EEG - GPi–LFP coherence was modulated by HFS with sustained reduction of 4–12 Hz EEG - GPi–LFP coherence after cessation of HFS in our patients pointing to a more widespread network effect of pallidal DBS. Accordingly, a descending drive from cortical activity to dystonic muscle spasms has been described in the same low frequency range in idiopathic dystonia (Tijssen et al., 2000, 2002; Grosse et al., 2004). More importantly, changes in motor cortex excitability, intracortical inhibition and induced plasticity as revealed with transcranial magnetic stimulation have been observed after DBS in parallel with the time course of reduction in dystonic symptoms over several months of chronic DBS (Kuhn et al., 2003; Tisch et al., 2006; Ruge et al., 2011a, b) pointing to DBS-induced compensatory modulation of activity in other brain areas along the pallido-thalamo-cortical network. These results imply that chronic pallidal DBS induces functional reorganization of the motor cortex–basal ganglia circuits possibly overcoming the postulated maladaptive plasticity in dystonia (Edwards et al., 2006; Quartarone and Hallett, 2013).
Together, our study provides new evidence that GPi HFS reduces abnormal low frequency activity in dystonia. We could show immediate effects of HFS on local synchronized pallidal activity in patients with mobile dystonia as well as modulation of network activity to motor cortical areas and dystonic muscles. The suppression of 4–12 Hz pallidal activity and GPi–LFP - EMG coherence was associated with the reduction of phasic dystonic features that are known to be the quickest to respond to DBS. Long-term plastic changes may be related to modulation of cortico–basal ganglia network activity as shown for low frequency EEG - GPi–LFP coherence. This is consistent with the hypothesis that disruption of abnormally synchronized activity both locally in the GPi and along the cortex - basal ganglia loop may thus represent one of the potential mechanisms of DBS in dystonia.
Acknowledgments
Funding
This work was supported by the Else-Kröner-Fresenius Foundation, EKMS 08/22 to A.A.K. and the German Research Foundation [DFG, grant KFO 247].
Abbreviations
- DBS
deep brain stimulation
- GPi
globus pallidus internus
- HFS
high-frequency stimulation
- LFP
local field potential
References
- Air EL, Ostrem JL, Sanger TD, Starr PA. Deep brain stimulation in children: experience and technical pearls. J Neurosurg Pediatr. 2011;8:566–74. doi: 10.3171/2011.8.PEDS11153. [DOI] [PubMed] [Google Scholar]
- Albanese A, Asmus F, Bhatia KP, Elia AE, Elibol B, Filippini G, et al. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur J Neurol. 2011;18:5–18. doi: 10.1111/j.1468-1331.2010.03042.x. [DOI] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders [Review] Trends Neurosci. 1989;12:366–75. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alegre M, Lopez-Azcarate J, Alonso-Frech F, Rodriguez-Oroz MC, Valencia M, Guridi J, et al. Subthalamic activity during diphasic dyskinesias in Parkinson’s disease. Mov Disord. 2012;27:1178–81. doi: 10.1002/mds.25090. [DOI] [PubMed] [Google Scholar]
- Alonso-Frech F, Zamarbide I, Alegre M, Rodriguez-Oroz MC, Guridi J, Manrique M, et al. Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson’s disease. Brain. 2006;129:1748–57. doi: 10.1093/brain/awl103. [DOI] [PubMed] [Google Scholar]
- Andrews C, Aviles-Olmos I, Hariz M, Foltynie T. Which patients with dystonia benefit from deep brain stimulation? A metaregression of individual patient outcomes. J Neurol Neurosurg Psychiatry. 2010;81:1383–9. doi: 10.1136/jnnp.2010.207993. [DOI] [PubMed] [Google Scholar]
- Anzak A, Tan H, Pogosyan A, Foltynie T, Limousin P, Zrinzo L, et al. Subthalamic nucleus activity optimizes maximal effort motor responses in Parkinson’s disease. Brain. 2012;135:2766–78. doi: 10.1093/brain/aws183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia [Review] Brain. 1998;121:1195–212. doi: 10.1093/brain/121.7.1195. [DOI] [PubMed] [Google Scholar]
- Berman BD, Starr PA, Marks WJ, Jr, Ostrem JL. Induction of bradykinesia with pallidal deep brain stimulation in patients with cranial-cervical dystonia. Stereotact Funct Neurosurg. 2009;87:37–44. doi: 10.1159/000195718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease [Review] Mov Disord. 2003;18:357–63. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- Brown P, Eusebio A. Paradoxes of functional neurosurgery: clues from basal ganglia recordings [Review] Mov Disord. 2008;23:12–20. doi: 10.1002/mds.21796. [DOI] [PubMed] [Google Scholar]
- Brown P, Williams D. Basal ganglia local field potential activity: character and functional significance in the human [Review] Clin Neurophysiol. 2005;116:2510–9. doi: 10.1016/j.clinph.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Brown P, Mazzone P, Oliviero A, Altibrandi MG, Pilato F, Tonali PA, et al. Effects of stimulation of the subthalamic area on oscillatory pallidal activity in Parkinson’s disease. Exp Neurol. 2004;188:480–90. doi: 10.1016/j.expneurol.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Brucke C, Huebl J, Schonecker T, Neumann WJ, Yarrow K, Kupsch A, et al. Scaling of movement is related to pallidal gamma oscillations in patients with dystonia. J Neurosci. 2012;32:1008–19. doi: 10.1523/JNEUROSCI.3860-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucke C, Kempf F, Kupsch A, Schneider GH, Krauss JK, Aziz T, et al. Movement-related synchronization of gamma activity is lateralized in patients with dystonia. Eur J Neurosci. 2008;27:2322–9. doi: 10.1111/j.1460-9568.2008.06203.x. [DOI] [PubMed] [Google Scholar]
- Charlesworth G, Bhatia KP. Primary and secondary dystonic syndromes: an update. Curr Opin Neurol. 2013;26:406–12. doi: 10.1097/WCO.0b013e3283633696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Kuhn AA, Hoffmann KT, Kupsch A, Schneider GH, Trottenberg T, et al. Oscillatory pallidal local field potential activity correlates with involuntary EMG in dystonia. Neurology. 2006a;66:418–20. doi: 10.1212/01.wnl.0000196470.00165.7d. [DOI] [PubMed] [Google Scholar]
- Chen CC, Kuhn AA, Trottenberg T, Kupsch A, Schneider GH, Brown P. Neuronal activity in globus pallidus interna can be synchronized to local field potential activity over 3-12 Hz in patients with dystonia. Exp Neurol. 2006b;202:480–6. doi: 10.1016/j.expneurol.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Cohen OS, Hassin-Baer S, Spiegelmann R. Deep brain stimulation of the internal globus pallidus for refractory tardive dystonia. Parkinsonism Relat Disord. 2007;13:541–4. doi: 10.1016/j.parkreldis.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Coubes P, Cif L, El Fertit H, Hemm S, Vayssiere N, Serrat S, et al. Electrical stimulation of the globus pallidus internus in patients with primary generalized dystonia: long-term results. J Neurosurg. 2004;101:189–94. doi: 10.3171/jns.2004.101.2.0189. [DOI] [PubMed] [Google Scholar]
- Coubes P, Roubertie A, Vayssiere N, Hemm S, Echenne B. Treatment of DYT1-generalised dystonia by stimulation of the internal globus pallidus. Lancet. 2000;355:2220–1. doi: 10.1016/S0140-6736(00)02410-7. [DOI] [PubMed] [Google Scholar]
- Damier P, Thobois S, Witjas T, Cuny E, Derost P, Raoul S, et al. Bilateral deep brain stimulation of the globus pallidus to treat tardive dyskinesia. Arch Gen Psychiatry. 2007;64:170–6. doi: 10.1001/archpsyc.64.2.170. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin [Review] Trends Neurosci. 1990;13:281–5. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Dostrovsky J, Bergman H. Oscillatory activity in the basal ganglia—relationship to normal physiology and pathophysiology. Brain. 2004;127:721–2. doi: 10.1093/brain/awh164. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Huang YZ, Mir P, Rothwell JC, Bhatia KP. Abnormalities in motor cortical plasticity differentiate manifesting and nonmanifesting DYT1 carriers. Mov Disord. 2006;21:2181–6. doi: 10.1002/mds.21160. [DOI] [PubMed] [Google Scholar]
- Eusebio A, Thevathasan W, Doyle Gaynor L, Pogosyan A, Bye E, Foltynie T, et al. Deep brain stimulation can suppress pathological synchronisation in parkinsonian patients. J Neurol Neurosurg Psychiatry. 2011;82:569–73. doi: 10.1136/jnnp.2010.217489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foncke EM, Bour LJ, Speelman JD, Koelman JH, Tijssen MA. Local field potentials and oscillatory activity of the internal globus pallidus in myoclonus-dystonia. Mov Disord. 2007;22:369–76. doi: 10.1002/mds.21284. [DOI] [PubMed] [Google Scholar]
- Franzini A, Marras C, Ferroli P, Zorzi G, Bugiani O, Romito L, et al. Longterm high-frequency bilateral pallidal stimulation for neuroleptic-induced tardive dystonia. Report of two cases. J Neurosurg. 2005;102:721–5. doi: 10.3171/jns.2005.102.4.0721. [DOI] [PubMed] [Google Scholar]
- Grips E, Blahak C, Capelle HH, Bazner H, Weigel R, Sedlaczek O, et al. Patterns of reoccurrence of segmental dystonia after discontinuation of deep brain stimulation. J Neurol Neurosurg Psychiatry. 2007;78:318–20. doi: 10.1136/jnnp.2006.089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse P, Edwards M, Tijssen MA, Schrag A, Lees AJ, Bhatia KP, et al. Patterns of EMG-EMG coherence in limb dystonia. Mov Disord. 2004;19:758–69. doi: 10.1002/mds.20075. [DOI] [PubMed] [Google Scholar]
- Gruber D, Trottenberg T, Kivi A, Schoenecker T, Kopp UA, Hoffmann KT, et al. Long-term effects of pallidal deep brain stimulation in tardive dystonia. Neurology. 2009;73:53–8. doi: 10.1212/WNL.0b013e3181aaea01. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data-theory and application to the study of physiological tremor, single motor unit discharges and electromyograms [Review] Prog Biophys Mol Biol. 1995;64:237–78. doi: 10.1016/s0079-6107(96)00009-0. [DOI] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson’s disease: networks, models and treatments [Review] Trends Neurosci. 2007;30:357–64. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Hung SW, Hamani C, Lozano AM, Poon YY, Piboolnurak P, Miyasaki JM, et al. Long-term outcome of bilateral pallidal deep brain stimulation for primary cervical dystonia. Neurology. 2007;68:457–9. doi: 10.1212/01.wnl.0000252932.71306.89. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Dostrovsky JO, Walters JR, Courtemanche R, Boraud T, Goldberg J, et al. Neuronal oscillations in the basal ganglia and movement disorders: evidence from whole animal and human recordings [Review] J Neurosci. 2004;24:9240–3. doi: 10.1523/JNEUROSCI.3366-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaias IU, Volkmann J, Kupsch A, Burgunder JM, Ostrem JL, Alterman RL, et al. Factors predicting protracted improvement after pallidal DBS for primary dystonia: the role of age and disease duration. J Neurol. 2011;258:1469–76. doi: 10.1007/s00415-011-5961-9. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Miocinovic S, McIntyre CC, Vitek JL. Mechanisms and targets of deep brain stimulation in movement disorders [Review] Neurotherapeutics. 2008;5:294–308. doi: 10.1016/j.nurt.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss ZH, Doig-Beyaert K, Eliasziw M, Tsui J, Haffenden A, Suchowersky O. The Canadian multicentre study of deep brain stimulation for cervical dystonia. Brain. 2007;130:2879–86. doi: 10.1093/brain/awm229. [DOI] [PubMed] [Google Scholar]
- Koy A, Hellmich M, Pauls KA, Marks W, Lin JP, Fricke O, et al. Effects of deep brain stimulation in dyskinetic cerebral palsy: a meta-analysis. Mov Disord. 2013;28:647–54. doi: 10.1002/mds.25339. [DOI] [PubMed] [Google Scholar]
- Krauss JK. Deep brain stimulation for dystonia in adults. Overview and developments [Review] Stereotact Funct Neurosurg. 2002;78:168–82. doi: 10.1159/000068963. [DOI] [PubMed] [Google Scholar]
- Krauss JK, Pohle T, Weber S, Ozdoba C, Burgunder JM. Bilateral stimulation of globus pallidus internus for treatment of cervical dystonia. Lancet. 1999;354:837–8. doi: 10.1016/S0140-6736(99)80022-1. [DOI] [PubMed] [Google Scholar]
- Krauss JK, Yianni J, Loher TJ, Aziz TZ. Deep brain stimulation for dystonia [Review] J Clin Neurophysiol. 2004;21:18–30. doi: 10.1097/00004691-200401000-00004. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Kempf F, Brucke C, Gaynor Doyle L, Martinez-Torres I, Pogosyan A, et al. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J Neurosci. 2008;28:6165–73. doi: 10.1523/JNEUROSCI.0282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn AA, Kupsch A, Schneider GH, Brown P. Reduction in subthalamic 8-35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur J Neurosci. 2006;23:1956–60. doi: 10.1111/j.1460-9568.2006.04717.x. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Meyer BU, Trottenberg T, Brandt SA, Schneider GH, Kupsch A. Modulation of motor cortex excitability by pallidal stimulation in patients with severe dystonia. Neurology. 2003;60:768–74. doi: 10.1212/01.wnl.0000044396.64752.4c. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Tsui A, Aziz T, Ray N, Brucke C, Kupsch A, et al. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp Neurol. 2009;215:380–7. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Kupsch A, Benecke R, Muller J, Trottenberg T, Schneider GH, Poewe W, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;355:1978–90. doi: 10.1056/NEJMoa063618. [DOI] [PubMed] [Google Scholar]
- Lee JR, Kiss ZH. Interhemispheric difference of pallidal local field potential activity in cervical dystonia. J Neurol Neurosurg Psychiatry. 2014;85:306–10. doi: 10.1136/jnnp-2013-305476. [DOI] [PubMed] [Google Scholar]
- Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M, et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol. 2013;74:449–57. doi: 10.1002/ana.23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Griffin IC, Parkin SG, Miall RC, Rowe JG, Gregory RP, et al. Involvement of the medial pallidum in focal myoclonic dystonia: a clinical and neurophysiological case study. Mov Disord. 2002;17:346–53. doi: 10.1002/mds.10038. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang S, Yianni J, Nandi D, Bain PG, Gregory R, et al. The sensory and motor representation of synchronized oscillations in the globus pallidus in patients with primary dystonia. Brain. 2008;131:1562–73. doi: 10.1093/brain/awn083. [DOI] [PubMed] [Google Scholar]
- Liu X, Yianni J, Wang S, Bain PG, Stein JF, Aziz TZ. Different mechanisms may generate sustained hypertonic and rhythmic bursting muscle activity in idiopathic dystonia. Exp Neurol. 2006;198:204–13. doi: 10.1016/j.expneurol.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Lumsden DE, Kaminska M, Gimeno H, Tustin K, Baker L, Perides S, et al. Proportion of life lived with dystonia inversely correlates with response to pallidal deep brain stimulation in both primary and secondary childhood dystonia. Dev Med Child Neurol. 2013;55:567–74. doi: 10.1111/dmcn.12117. [DOI] [PubMed] [Google Scholar]
- Markun LC, Starr PA, Air EL, Marks WJ, Jr, Volz MM, Ostrem JL. Shorter disease duration correlates with improved long-term deep brain stimulation outcomes in young-onset DYT1 dystonia. Neurosurgery. 2012;71:325–30. doi: 10.1227/NEU.0b013e318258e21b. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Obeso JA. The functions of the basal ganglia and the paradox of stereotaxic surgery in Parkinson’s disease [Review] Brain. 1994;117:877–97. doi: 10.1093/brain/117.4.877. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Hahn PJ. Network perspectives on the mechanisms of deep brain stimulation [Review] Neurobiol Dis. 2010;38:329–37. doi: 10.1016/j.nbd.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentzel CL, Tenback DE, Tijssen MA, Visser-Vandewalle VE, van Harten PN. Efficacy and safety of deep brain stimulation in patients with medication-induced tardive dyskinesia and/or dystonia: a systematic review [Review] J Clin Psychiatry. 2012;73:1434–8. doi: 10.4088/JCP.12r07643. [DOI] [PubMed] [Google Scholar]
- Moll CK, Galindo-Leon E, Sharott A, Gulberti A, Buhmann C, Koeppen JA, et al. Asymmetric pallidal neuronal activity in patients with cervical dystonia. Front Syst Neurosci. 2014;8:15. doi: 10.3389/fnsys.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers LJ, Lowery M, O’Malley M, Vaughan CL, Heneghan C, St Clair Gibson A, et al. Rectification and non-linear pre-processing of EMG signals for cortico-muscular analysis. J Neurosci Methods. 2003;124:157–65. doi: 10.1016/s0165-0270(03)00004-9. [DOI] [PubMed] [Google Scholar]
- Neumann WJ, Huebl J, Brucke C, Gabriels L, Bajbouj M, Merkl A, et al. Different patterns of local field potentials from limbic DBS targets in patients with major depressive and obsessive compulsive disorder. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.2. Advance Access published on February 11, 2014, doi: 10.1038/mp.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann WJ, Huebl J, Brucke C, Ruiz MH, Kupsch A, Schneider GH, et al. Enhanced low-frequency oscillatory activity of the subthalamic nucleus in a patient with dystonia. Mov Disord. 2012;27:1063–6. doi: 10.1002/mds.25078. [DOI] [PubMed] [Google Scholar]
- Ostrem JL, Starr PA. Treatment of dystonia with deep brain stimulation [Review] Neurotherapeutics. 2008;5:320–30. doi: 10.1016/j.nurt.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrem JL, Marks WJ, Jr, Volz MM, Heath SL, Starr PA. Pallidal deep brain stimulation in patients with cranial-cervical dystonia (Meige syndrome) Mov Disord. 2007;22:1885–91. doi: 10.1002/mds.21580. [DOI] [PubMed] [Google Scholar]
- Panov F, Gologorsky Y, Connors G, Tagliati M, Miravite J, Alterman RL. Deep brain stimulation in DYT1 dystonia: a 10-year experience. Neurosurgery. 2013;73:86–93. doi: 10.1227/01.neu.0000429841.84083.c8. [DOI] [PubMed] [Google Scholar]
- Priori A, Foffani G, Pesenti A, Tamma F, Bianchi AM, Pellegrini M, et al. Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson’s disease. Exp Neurol. 2004;189:369–79. doi: 10.1016/j.expneurol.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Hallett M. Emerging concepts in the physiological basis of dystonia [Review] Mov Disord. 2013;28:958–67. doi: 10.1002/mds.25532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray NJ, Jenkinson N, Brittain J, Holland P, Joint C, Nandi D, et al. The role of the subthalamic nucleus in response inhibition: evidence from deep brain stimulation for Parkinson’s disease. Neuropsychologia. 2009;47:2828–34. doi: 10.1016/j.neuropsychologia.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Rossi L, Marceglia S, Foffani G, Cogiamanian F, Tamma F, Rampini P, et al. Subthalamic local field potential oscillations during ongoing deep brain stimulation in Parkinson’s disease. Brain Res Bull. 2008;76:512–21. doi: 10.1016/j.brainresbull.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Ruge D, Cif L, Limousin P, Gonzalez V, Vasques X, Hariz MI, et al. Shaping reversibility? Long-term deep brain stimulation in dystonia: the relationship between effects on electrophysiology and clinical symptoms. Brain. 2011a;134:2106–15. doi: 10.1093/brain/awr122. [DOI] [PubMed] [Google Scholar]
- Ruge D, Tisch S, Hariz MI, Zrinzo L, Bhatia KP, Quinn NP, et al. Deep brain stimulation effects in dystonia: time course of electro-physiological changes in early treatment. Mov Disord. 2011b;26:1913–21. doi: 10.1002/mds.23731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako W, Goto S, Shimazu H, Murase N, Matsuzaki K, Tamura T, et al. Bilateral deep brain stimulation of the globus pallidus internus in tardive dystonia. Mov Disord. 2008;23:1929–31. doi: 10.1002/mds.22100. [DOI] [PubMed] [Google Scholar]
- Sanghera MK, Grossman RG, Kalhorn CG, Hamilton WJ, Ondo WG, Jankovic J. Basal ganglia neuronal discharge in primary and secondary dystonia in patients undergoing pallidotomy. Neurosurgery. 2003;52:1358–70. doi: 10.1227/01.neu.0000064805.91249.f5. discussion 70-3. [DOI] [PubMed] [Google Scholar]
- Schonecker T, Kupsch A, Kuhn AA, Schneider GH, Hoffmann KT. Automated optimization of subcortical cerebral MR imaging-atlas coregistration for improved postoperative electrode localization in deep brain stimulation. AJNR Am J Neuroradiol. 2009;30:1914–21. doi: 10.3174/ajnr.A1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharott A, Grosse P, Kuhn AA, Salih F, Engel AK, Kupsch A, et al. Is the synchronization between pallidal and muscle activity in primary dystonia due to peripheral afferance or a motor drive? Brain. 2008;131:473–84. doi: 10.1093/brain/awm324. [DOI] [PubMed] [Google Scholar]
- Silberstein P, Kuhn AA, Kupsch A, Trottenberg T, Krauss JK, Wohrle JC, et al. Patterning of globus pallidus local field potentials differs between Parkinson’s disease and dystonia. Brain. 2003;126:2597–608. doi: 10.1093/brain/awg267. [DOI] [PubMed] [Google Scholar]
- Skidmore F, Reich SG. Tardive Dystonia [Review] Curr Treat Options Neurol. 2005;7:231–6. doi: 10.1007/s11940-005-0016-0. [DOI] [PubMed] [Google Scholar]
- Tagliati M, Shils J, Sun C, Alterman R. Deep brain stimulation for dystonia [Review] Expert Rev Med Devices. 2004;1:33–41. doi: 10.1586/17434440.1.1.33. [DOI] [PubMed] [Google Scholar]
- Tang JK, Mahant N, Cunic D, Chen R, Moro E, Lang AE, et al. Changes in cortical and pallidal oscillatory activity during the execution of a sensory trick in patients with cervical dystonia. Exp Neurol. 2007;204:845–8. doi: 10.1016/j.expneurol.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Tijssen MA, Marsden JF, Brown P. Frequency analysis of EMG activity in patients with idiopathic torticollis. Brain. 2000;123:677–86. doi: 10.1093/brain/123.4.677. [DOI] [PubMed] [Google Scholar]
- Tijssen MA, Munchau A, Marsden JF, Lees A, Bhatia KP, Brown P. Descending control of muscles in patients with cervical dystonia. Mov Disord. 2002;17:493–500. doi: 10.1002/mds.10121. [DOI] [PubMed] [Google Scholar]
- Tisch S, Limousin P, Rothwell JC, Asselman P, Quinn N, Jahanshahi M, et al. Changes in blink reflex excitability after globus pallidus internus stimulation for dystonia. Mov Disord. 2006;21:1650–5. doi: 10.1002/mds.20899. [DOI] [PubMed] [Google Scholar]
- Trottenberg T, Volkmann J, Deuschl G, Kuhn AA, Schneider GH, Muller J, et al. Treatment of severe tardive dystonia with pallidal deep brain stimulation. Neurology. 2005;64:344–6. doi: 10.1212/01.WNL.0000149762.80932.55. [DOI] [PubMed] [Google Scholar]
- Valldeoriola F, Regidor I, Minguez-Castellanos A, Lezcano E, Garcia-Ruiz P, Rojo A, et al. Efficacy and safety of pallidal stimulation in primary dystonia: results of the Spanish multicentric study. J Neurol Neurosurg Psychiatry. 2010;81:65–9. doi: 10.1136/jnnp.2009.174342. [DOI] [PubMed] [Google Scholar]
- Vidailhet M, Grabli D. New approaches in dystonia (clinical features, genetic issues and pathophysiology) [Review] Bull Acad Natl Med. 2011;195:921–34. [PubMed] [Google Scholar]
- Vidailhet M, Grabli D, Roze E. Pathophysiology of dystonia [Review] Curr Opin Neurol. 2009;22:406–13. doi: 10.1097/WCO.0b013e32832d9ef3. [DOI] [PubMed] [Google Scholar]
- Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Benabid AL, Cornu P, et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. 2005;352:459–67. doi: 10.1056/NEJMoa042187. [DOI] [PubMed] [Google Scholar]
- Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Lagrange C, Yelnik J, et al. Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet Neurol. 2007;6:223–9. doi: 10.1016/S1474-4422(07)70035-2. [DOI] [PubMed] [Google Scholar]
- Vitek JL, Chockkan V, Zhang JY, Kaneoke Y, Evatt M, DeLong MR, et al. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann Neurol. 1999;46:22–35. doi: 10.1002/1531-8249(199907)46:1<22::aid-ana6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Vitek JL, Zhang J, Hashimoto T, Russo GS, Baker KB. External pallidal stimulation improves parkinsonian motor signs and modulates neuronal activity throughout the basal ganglia thalamic network. Exp Neurol. 2012;233:581–6. doi: 10.1016/j.expneurol.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann J, Benecke R. Deep brain stimulation for dystonia: patient selection and evaluation [Review] Mov Disord. 2002;17(Suppl 3):S112–5. doi: 10.1002/mds.10151. [DOI] [PubMed] [Google Scholar]
- Volkmann J, Wolters A, Kupsch A, Muller J, Kuhn AA, Schneider GH, et al. Pallidal deep brain stimulation in patients with primary generalised or segmental dystonia: 5-year follow-up of a randomised trial. Lancet Neurol. 2012;11:1029–38. doi: 10.1016/S1474-4422(12)70257-0. [DOI] [PubMed] [Google Scholar]
- Volkmann J, Mueller J, Deuschl G, Kühn AA, Krauss JK, Poewe W, et al. Pallidal neurostimulation in patients with medication-refractory cervical dystonia: a randomised, sham-controlled trial. Lancet Neurol. 2014;13:875–84. doi: 10.1016/S1474-4422(14)70143-7. [DOI] [PubMed] [Google Scholar]
- Walsh RA, Sidiropoulos C, Lozano AM, Hodaie M, Poon YY, Fallis M, et al. Bilateral pallidal stimulation in cervical dystonia: blinded evidence of benefit beyond 5 years. Brain. 2013;136:761–9. doi: 10.1093/brain/awt009. [DOI] [PubMed] [Google Scholar]
- Wang S, Liu X, Yianni J, Green AL, Joint C, Stein JF, et al. Use of surface electromyography to assess and select patients with idiopathic dystonia for bilateral pallidal stimulation. J Neurosurg. 2006;105:21–5. doi: 10.3171/jns.2006.105.1.21. [DOI] [PubMed] [Google Scholar]
- Wang SY, Liu X, Yianni J, Aziz TZ, Stein JF. Extracting burst and tonic components from surface electromyograms in dystonia using adaptive wavelet shrinkage. J Neurosci Methods. 2004;139:177–84. doi: 10.1016/j.jneumeth.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Weinberger M, Hutchison WD, Alavi M, Hodaie M, Lozano AM, Moro E, et al. Oscillatory activity in the globus pallidus internus: comparison between Parkinson’s disease and dystonia. Clin Neurophysiol. 2012;123:358–68. doi: 10.1016/j.clinph.2011.07.029. [DOI] [PubMed] [Google Scholar]
- Wingeier B, Tcheng T, Koop MM, Hill BC, Heit G, Bronte-Stewart HM. Intra-operative STN DBS attenuates the prominent beta rhythm in the STN in Parkinson’s disease. Exp Neurol. 2006;197:244–51. doi: 10.1016/j.expneurol.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Yianni J, Bain PG, Gregory RP, Nandi D, Joint C, Scott RB, et al. Post-operative progress of dystonia patients following globus pallidus internus deep brain stimulation. Eur J Neurol. 2003;10:239–47. doi: 10.1046/j.1468-1331.2003.00592.x. [DOI] [PubMed] [Google Scholar]
- Yianni J, Wang SY, Liu X, Bain PG, Nandi D, Gregory R, et al. A dominant bursting electromyograph pattern in dystonic conditions predicts an early response to pallidal stimulation. J Clin Neurosci. 2006;13:738–46. doi: 10.1016/j.jocn.2005.07.022. [DOI] [PubMed] [Google Scholar]