Abstract
The National Service Framework advocates correction of anaemia in patients with chronic kidney disease (CKD). Oral iron is insufficient, while intravenous (IV) supplementation replenishes and maintains iron stores. Previously, effective delivery of iron therapy using available parenteral preparations has been hampered by dosing schedules and the need in some cases of a test dose. The introduction in Europe of newer iron preparations, including iron isomaltoside 1000 (Monofer) and iron carboxymaltose (Ferinject), now offers a potentially safe, effective and time-efficient method of outpatient iron repletion. This may potentially lead to better cost-effectiveness in a resource-limited service.
Keywords: anaemia, chronic kidney disease, cost, ferumoxytol (Feraheme), iron carboxymaltose (Ferinject), iron isomaltoside 1000 (Monofer), iron sucrose (Venofer), low-molecular-weight iron dextran (Cosmofer), patient choice
Introduction
Chronic kidney disease (CKD) is a worldwide public health problem that affects approximately 6% of the UK adult population and is associated with a high prevalence of cardiovascular disease and large economic burden [1]. Anaemia occurs early during the course of CKD and progressively increases as a result of iron and erythropoietin deficiencies. The incidence of pre-dialysis anaemia in patients attending the renal service has been estimated to be around 68% of all patients referred for the first time for assessment with 61% having either absolute (depletion of both circulating and iron stores) or functional (depletion of circulating/available iron) iron deficiency [2,3].
The Renal National Service Framework and Best Practice Guidelines advocate correction of anaemia in patients with CKD not yet on renal replacement therapy [4,5]. Only 26% of these patients, however, receive replacement intravenous (IV) iron therapy.
The increasing use of erythropoiesis-stimulating agents (ESAs) has also led to a rapid increase in the need for adequate iron replacement therapy to maintain sufficient iron stores. This is essential for maximizing the effects of ESA therapy [6,7]. Recent randomized clinical studies have raised safety concerns of using ESA agents and their potential toxicity. Indeed, the risks of pure red cell aplasia (PRCA), thrombotic potential and possibly neoplasm make ESAs less attractive [8–10]. It is not clear from these recent publications whether the increased incidence of adverse cardiac and other events including stroke is due to the actual haemoglobin level achieved (i.e., a normal haemoglobin concentration of 13 g/dL or greater), the drug preparation itself (ESA), the dose of the drug used (i.e. high doses used in patients with possible ESA hyporesponsiveness) or an unrecognized factor which may be impacting on patients’ morbidity and mortality. With these recent data concerning ESA, correction of iron deficiency with IV iron may, perhaps, represent a safer option to optimize anaemia in CKD patients. The NICE guidelines also suggest that iron replacement is required for CKD patients with low haemoglobin prior to ESA use [11]. By optimizing iron stores and iron bioavailability, ESA efficiency can be maximized, its requirement potentially delayed and patient quality of life improved [2,8,12].
What properties should the ideal iron preparation possess?
In considering the potential ‘ideal’ iron preparation, consideration must be given to the requirements of the clinician, health service and most importantly the patient in addition to the drug’s pharmacological characteristics such as method of administration and flexibility of use.
Oral iron, although possibly more convenient, remains a poor substitute because of its poor bioavailability and compliance [13,14]. Iron delivery is restricted by inflammation which is commonly present in CKD. The mechanism is unclear but putatively involves hepcidin, a 25-amino acid synthesized in the liver. Hepcidin acts as a regulator of iron metabolism with effects on absorption, transportation and storage [15]. Hepcidin binds to the iron exporter channel ferroportin which causes internalization and degradation of iron [16]. This subsequently leads to reduced red cell iron absorption and sequestration in the reticulo-endothelial system. To overcome this increased hepcidin synthesis and hence reduced iron absorption leading to anaemia, the most effective method is via the IV route of iron administration [6,7]. Used in isolation, IV iron leads to a significant rise in haemoglobin concentrations in the order of 06–2.7 g/dL [17,18].
Use of parenteral iron therapy for the treatment of anaemia dates back to 1932 when it was first administered by Heath et al. [19]. However, it was found to have significant ‘disagreeable symptoms, sometimes severe and possibly dangerous’ even with a modest dose of 40–80 mg [19]. Subsequently, in 1946, Goetsch et al. examined ferric hydroxide but found that it also caused numerous adverse problems [20]. Then, in 1947, Nissim discovered that use of an iron saccharide preparation appeared to be much safer [21]. Finally, Baird and Podmore in 1954 developed an iron dextran preparation (Imferon), which showed some initial promise but was withdrawn in 1992 due to a high incidence of anaphylactic reactions [22]. Since then, there has been resurgence in developing a useful and clinically practical parenteral iron therapy that would overcome the issues regarding safety associated with traditional iron therapies, related to anaphylaxis/immunogenicity and release of free or labile iron. Currently, several iron preparations have been in regular clinical use, but all possess various limitations to the ‘ideal’ situation. More recently, several next generation IV iron preparations have become commercially available. For the clinician, the decision about the qualities of the ‘perfect’ parenteral iron preparation remains to be confirmed, but the future potentially looks bright.
Delivery of iron
For an ‘ideal’ parenteral iron preparation, safety is critical and has been demonstrated in several studies for the current therapies [23–25]. In particular, there should be a low anaphylactic or anaphylactoid potential, and the preparation should not have a significant detrimental effect on renal function.
When IV iron is administrated, it passes to the reticulo-endothelial system (RES). The iron complex splits, and the iron is then combined to ferritin or transferrin which is used in haemoglobin production and storage. However, a small percentage of iron may be released as free iron. Hence, when considering the potential molecular structure of an ideal iron formulation, binding of the iron to the carbohydrate complex is critical to ensure that the complex allows tight binding of iron with minimal release of free iron that does not bind to transferrin. However, critical to iron’s importance in biological processes is its ability to cycle reversibly between its ferrous and ferric oxidation states. This specific property, which is essential for its functions, also makes it hazardous because free iron can catalyze the formation of free radicals leading to cell damage. Labile iron (catalytic iron) consists of chemical forms that can participate in redox cycling. This property enables it to generate powerful oxidant species such as hydroxyl radical and/or reactive iron–oxygen complexes such as ferryl or perferryl ion [26]. These complexes then propagate lipid peroxidation. In addition, iron can directly catalyze lipid peroxidation, the oxidative reaction of polyunsaturated lipids, by removing hydrogen atoms from polyunsaturated fatty acids in the lipid bilayers of organelle membranes [26]. Iron chelators may provide a protective effect, thus establishing a cause–effect relationship, at least in animal models.
In a population of CKD patients, debate remains regarding the effects of IV iron on renal function and proteinuria. Several animal models have demonstrated the potential detrimental effects of free iron on glomerular function [27]. This may be related to the putative toxic effect of iron to cells, in particular glomerular and mesangial cells via oxidative stress (lipid peroxidation) and cell cytotoxicity leading to endothelial dysfunction which leads to proteinuria, accelerated atherosclerosis and potentially an increase in serum creatinine. Previously, Zager et al. demonstrated in vitro in animal studies that the loosely bound parenteral iron formulations may cause direct cytopathic changes to renal cells (particularly mesangial cells and endothelial cells) and this, therefore, may potentially cause renal deterioration [27–29]. There are a few human studies, but Agarwal in a study of 20 CKD patients suggested that there was an increase in oxidative stress markers when IV sucrose was given, and this could, in part, be abrogated by N-acetyl cysteine [30]. However, this intervention did not affect proteinuria, a strong surrogate marker of renal progression leading to the possibility of direct ‘drug-induced toxicity’. Recently, Shah et al. in preliminary studies carried out in India compared catalytic iron in subjects with either no renal disease or diabetes, with patients with diabetes and demonstrated that patients with overt diabetes have an increase in urinary catalytic iron which may be toxic to renal cells [31].
More recent clinical data on the neutral effects of IV iron on renal function are encouraging [32]. Indeed, 1-year data demonstrate this trend for low-molecular-weight iron dextran (Cosmofer) (Figure 1) [32]. Also, post hoc sub-analysis of the FAIR-HF study in a population of NYHA class II/III heart failure patients with iron deficiency anaemia and a mean eGFR (MDRD) of 64 ± 21 mL/min/1.73 m2 demonstrated a modest improvement in eGFR over a 24-week period after iron repletion in the order of 44 ± 1.7 mL/min in comparison to placebo [33]. Again, short-term data on iron isomaltoside 1000 (Monofer) are encouraging in relation to renal function [34]. These next generation preparations will be subjected to future ongoing scrutiny in longer-term studies.
Fig. 1.
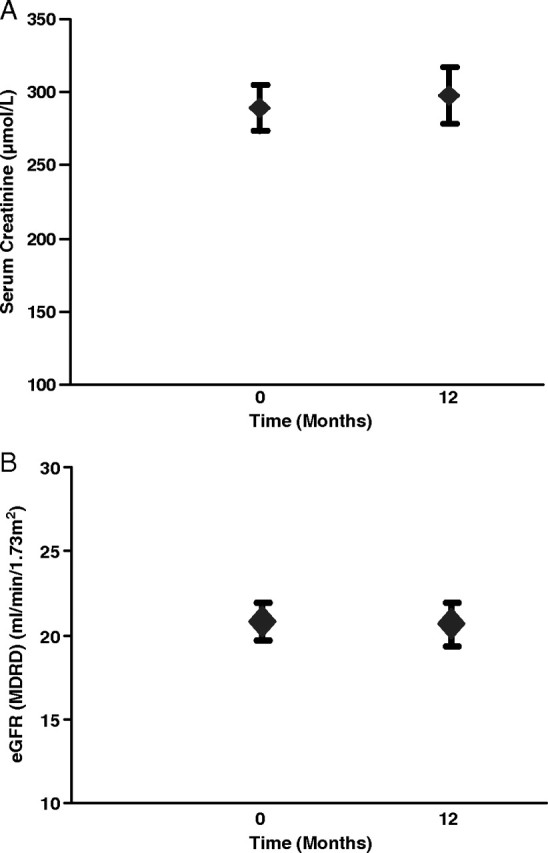
A. Serum creatinine at baseline and after 1 year of intravenous iron therapy. Data are mean ± SEM. B. eGFR (MDRD) at baseline and after 1 year of intravenous iron therapy. Data are mean ± SEM.
The carbohydrate used in these next generation preparations mechanistically vary slightly but in essence assist in isolating the bioactive iron from plasma components until the iron–carbohydrate complexes enter the RES for subsequent entry into the intracellular storage iron pool (ferritin) or transfer to plasma transferrin for haemoglobin production. For iron carboxymaltose (Ferinject), the unique carboxymaltose shell leads to a highly stable, type I polynuclear iron (III)–hydroxide carbohydrate complex that completely surrounds the iron core with the release of minimal amounts of iron into the circulation, while for iron isomaltoside 1000, rather than surrounding the iron core, the iron is enmeshed within the carbohydrate complex forming a matrix which may allow a more gradual release of iron. These novel mechanisms ensure that only minimal quantities of ionic iron are released from their carbohydrate shell/matrix which seem to ensure that free iron adverse effects and subsequent tissue toxicity from reactive oxygen species are negated.
Methods of administration of parenteral iron preparations
Flexibility of iron administration is important in all clinical situations. Iron has expanded its use beyond dialysis, predialysis and in conservative CKD patients. It is currently a useful therapy in patients with anaemia and chronic transplant dysfunction [35]. Currently, a number of other specialities are utilizing IV iron but often seek assistance from their colleagues within the renal department for its administration. These include cardiology (heart failure) [36], gastroenterology (inflammatory bowel disease) [37,38], haematology, oncology, gynaecology [39], post-partum anaemia [40], and both pre- and post-operative use. In oncology, with the continued controversies of ESAs and carcinoma, use of iron represents an ideal solution as mild to moderate anaemia occurs in up to 75% of cancer patients undergoing therapy [41]. Again, in inflammatory bowel disease, anaemia occurs in between 30% and 70% of patients. This may be due to a number of factors including chronic diarrhoea, vitamin B12 deficiency, folate deficiency and medication-induced bone marrow suppression, but the most common cause is iron deficiency from a combination of intestinal blood loss and reduced absorption from the gut as a result of hepcidin and chronic illness [42]. Indeed, there have been several studies of iron carboxymaltose use in these populations [33,38,39,43,44], and in all cases, the introduction of parenteral iron has allowed a reduction of transfusions and thus reduced the inherent risks associated with blood transfusions such as infection, antibody development and transfusion resistance. Indeed, there is also a reduced risk of panel-reactive antibody development, critical in future transplantation success, which increases more than 2-fold with between one and five blood transfusions administered. Hence, reversion back to giving blood transfusions, unless absolutely clinically imperative, should be avoided in the future.
When examining the currently available iron preparations, several administration regimes are possible, including giving iron as a single total replacement dose, as repeat low doses or as a bolus. Previously, only low-molecular-weight iron dextran could be given as a total dose infusion of ≥ 1000 mg in a single episode, thus sparing veins and time and being more ideal for a non-captive population such as a CKD group. However, the three newer parenteral iron preparations appear to possess this property. For example, Feraheme (ferumoxytol), a semi-synthetic polysaccharide-coated iron oxide, can be administered over 17 s in doses of up to 510 mg and repeated within a week [45–47]. Iron carboxymaltose allows administration as a single dose of up to 1000 mg (not exceeding 15 mg/body weight) in at least 15 min [43,44,48]. More recently, iron isomaltoside 1000 as a single dose of ≥ 1000 mg (not exceeding 20 mg/kg bodyweight) can be administered over 30–60 min [34,49]. Table 1 summarizes the modes and dosage regimes of administration.
Table 1.
Modes and dosage regimes of administration
| Preparation | Methods of administration (dosing regime) | ||
|---|---|---|---|
| Repeated low dose and bolus administration | Intravenous drip | Intravenous drip | |
| Moderate dose infusion | Total dose infusions | ||
| (100–510 mg) | (200–1000 mg) | > 1000 mg | |
| Low-molecular-weight iron dextran (Cosmofer) | ≤ 200 mg | ≤ 1000 mg | 1500 mg ~ 4–6 h (3 ha), Up to 20 mg iron/kg ~ 4–6 h + test dose |
| Iron sucrose (Venofer) | ≤ 200 mg | ≤ 200 mg | Not applicable |
| Iron gluconate (Ferelecit) | 125 mg | Not applicable | Not applicable |
| Iron isomaltoside 1000 (Monofer) | ≤ 200 mg | ≤ 1000 mg not exceeding 20 mg iron/kg | 1000–2000 mg ~ 1 h |
| 0–10 mg iron/kg ~ 30 min | |||
| 11–20 mg iron/kg ~ 60 min | |||
| Iron carboxymaltose (Ferinject) | ≤ 200 mg | ≤ 1000 mg not exceeding 15 mg iron/kg | Not applicable |
| Feraheme (ferumoxytol) | = 510 mg | 510 mg | Not applicable |
aAccelerated dose infusion regime used by the author and several other units—Sinha S, Chiu D, Peebles G, Fenwick S, Bhandari S, Kalra P. Accelerated total dose infusion of low-molecular-weight iron dextran is safe and efficacious in chronic kidney disease patients. Quarterly Journal of Medicine, Advanced access published 18 October 2010
The removal of the need for a test dose for iron administration is an essential prerequisite of an ‘ideal’ iron preparation to optimize efficiency of administration and cost. The ability for total dose infusion (TDI) replacement is also desirable. Currently, there appears to be no detrimental effect of TDI iron apart from those recognized in its side effect profile [26,32]. If we compare the properties of the iron preparations currently available in Europe and the UK, the newer iron preparations ‘tick the boxes’ in a clinician’s wish list for the ideal iron preparation (Table 2).
Table 2.
The newer iron preparations in a clinician’s wish list for the ideal iron preparation
| Product | Low-molecular-weight iron dextran (Cosmofer) | Iron sucrose (Venofer) | Iron gluconate (Ferelecit) | Iron isomaltoside 1000 (Monofer) | Iron carboxymaltose (Ferinject) | Ferumoxytol (Feraheme) |
|---|---|---|---|---|---|---|
| Molecular weight, kDa | 165 | 34–60 | 290–440 | 150 | 150 | 750a |
| pH | 5.2–6.5 | 10.5–11.1 | 7.7–9.7 | 5.0–7.0 | 5.0–7.0 | 6.0–8.0 |
| Carbohydrate | Dextran branched polysaccharide | Sucrose disaccharide | Gluconate monosaccharide | Isomatoside unbranched linear oligosaccharide | Carboxymaltose branched polysaccharide | Oxide coated with polyglucose, sorbitol, carboxymethylether |
| Maximum single dose | 20 mg/kg | 200 mg | 125 mg | 20 mg/kg | 15 mg/kg single dose limit 1000 mg | 510 mg |
| Maximum single dose administration 80 kg male | 1600 mg | 200 mg | 125 mg | 1600 mg | 1000 mg | 510 mg |
| Maximum single dose administration 60 kg female | 1200 mg | 200 mg | 125 mg | 1200 mg | 900 mg | 510 mg |
| TDI repletion | Yes | No | No | YES | No | No |
| Infusion within 1 h | No | NA | NA | Yes | Yes | Yes |
| Iron concentration, mg/mL | 50 | 20 | 12.5 | 100 | 50 | 50 |
| Test dose required | Yes | No/Yes (only Europe) | No | No | No | No |
| Minimally analytical free iron | < 1% | 10% | 10–20% | < 0.1% | < 1% | < 0.1% |
| Dose adjustment on dialysis | None | None | None | None | None | None |
aMolecular weight for ferumoxytol is not comparable to the other iron values because of measurement to a different standard
Convenience and cost-effectiveness
On the clinician’s ‘wish list’, the flexibility of the iron preparation is one important factor in the choice of iron preparation, while other factors potentially influencing choice relate to convenience to patients and staff. We have previously shown that these have significant influences on choice in addition to cost [32]. An important factor is time. Patients have found that the time saved for them as a result of more effective and efficient delivery of iron therapy has led, for them, to benefits including less disruption to their personal life, less time away from both their home environment and work, less time away from their family and friends, and a reduced amount of time spent travelling for therapy as a result of more local administration of treatment and less treatment episodes [32]. These factors have all had a beneficial financial impact on patients and potentially to the wider community with less absence from work. Development of local services where the newer preparations can be administered may enhance this further. The ability to administer flexibly a total repletion dose of parenteral iron and the future costs of the parent drug may determine future prescribing practices as the other added clinical beneficial properties or parenteral iron between iron preparations become nullified.
For patients suffering from CKD or any other pathology linked to iron deficiency, quality of life is critical and can be objectively improved with parenteral iron therapy [41]. Indeed, iron therapy has reduced the risk to patients as a result of fewer blood transfusions, which carry their inherent risk of transmission of infection and Creutzfeldt–Jakob disease, fewer side effects and importantly for those with progressive renal disease preservation of veins for future vascular access.
The health service and staff also reap potential benefits from the newer iron therapies. More efficient use of the health service with a reduced treatment waiting time and total number of visits (from less frequent iron administration) all lead to savings in both nursing and medical time. It will allow an increased capacity to give treatment and have significant economic benefits which will allow more patients to receive treatment with a finite and often limited budget. This potential cost-effectiveness has already been demonstrated in several studies of IV iron use [32,47]. There is a growing wealth of clinical data to reassure, but this remains the most critical element for the future use of newer iron preparations.
Conclusion
Clinical practice is normally not based on cost alone but includes medical and patient considerations. The clinical evaluation of the efficacy (IV iron can work) and effectiveness (IV iron does work) without any detriment to quality of life or increase in side effects is important, but maximizing convenience and cost-effectiveness are equally important. This ethos is in line with the views on ‘patient choice’ and more importantly the concept of providing a service for patients without compromising on quality of care given. Therefore, in this new era of patient-directed therapy, the profile of the ‘ideal’ iron preparation should be capable of delivering sufficient quantities of IV iron to correct iron deficiency rapidly, with minimal potential side effects including low catalytic/labile iron release and negligible immunogenicity (risk of anaphylaxis). For preservation of veins, the IV preparations should have a neutral pH and have a wide dosing range to allow a single repletion dose, with no requirement for a test dose. Finally, it should be convenient and cost-effective for the patient, doctor and staff with maximal time efficiency.
Conflict of interest statement. S.B. has received honorarium for lecturing on behalf of Pharmacosmos, Pfizer, Roche and BMS. S.B. also has received funding for travel to conferences from Amgen and Novartis, and has acted as an Investigator in multicentre studies designed and funded by Amgen, Astra-Zeneca, Novartis, Roche, Pharmacosmos and Vifor.
References
- 1.Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:112–119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 2.Besarab A, et al. Optimization of epoietin therapy with intravenous iron therapy in haemodialysis patient. J Am Soc Nephrol. 2000;11:530–538. doi: 10.1681/ASN.V113530. [DOI] [PubMed] [Google Scholar]
- 3.Hsu C, McCulloch CE, Curhan GC. Epidemiology of anaemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2002;13:504–510. doi: 10.1681/ASN.V132504. [DOI] [PubMed] [Google Scholar]
- 4.Health D. In: The National Service Framework for Renal Service (Part 2, Chronic Kidney Disease) Health D, editor. London: 2005. [Google Scholar]
- 5.Locatelli F, et al. Revised European Best Practice Guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant. 2004:1–47. doi: 10.1093/ndt/gfh1032. [DOI] [PubMed] [Google Scholar]
- 6.Bhandari S, Brownjohn A, Turney J. Effective utilization of erythropoietin with intravenous iron therapy. J Clin Pharm Ther. 1998;23:73–78. doi: 10.1046/j.1365-2710.1998.00147.x. [DOI] [PubMed] [Google Scholar]
- 7.Valderrabano F, et al. Pre-dialysis survey on anaemia management (PRESAM) Nephrol Dial Transplant. 2003;18:89–100. doi: 10.1093/ndt/18.1.89. [DOI] [PubMed] [Google Scholar]
- 8.Drueke T, et al. Normalisation of haemoglobin level in patients with chronic kidney disease and anaemia. N Engl J Med. 2006;355:2017–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 9.Pfeffern M, et al. A trial of darbepoetin in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 10.Singh A, et al. Correction of anaemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 11.Guidelines N. In: Anaemia management in people with chronic kidney disease (CKD). Clinical Guidance. N.C.c.f.C. Conditions, editor. London: Royal College of Physicians; 2006. pp. 1–172. [Google Scholar]
- 12.Agarwal R. Nonhematological benefits of iron. Am J Nephrol. 2007;27:565–571. doi: 10.1159/000107927. [DOI] [PubMed] [Google Scholar]
- 13.Bhandari S, Brownjohn AM, Turney JH. Response of mean reticulocyte haemoglobin content to intravenous iron therapy in haemodialysis patients. Nephrol Dial Transplant. 1998;13:3276–3277. doi: 10.1093/ndt/13.12.3276. [DOI] [PubMed] [Google Scholar]
- 14.Macdougall I, Tucker B, Thompson J. A randomized controlled study of iron supplementation in patients with erythropoietin. Kidney Int. 1996;50:1694–1699. doi: 10.1038/ki.1996.487. [DOI] [PubMed] [Google Scholar]
- 15.Ganz T. Molecular control of iron transport. J Am Soc Nephrol. 2007;18:394–400. doi: 10.1681/ASN.2006070802. [DOI] [PubMed] [Google Scholar]
- 16.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783–788. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 17.Mircescu G, et al. Intravenous iron supplementation for the treatment of anaemia in pre-dialyzed chronic renal failure patients. Nephrol Dial Transplant. 2006;21:120–124. doi: 10.1093/ndt/gfi087. [DOI] [PubMed] [Google Scholar]
- 18.Rozen-Zvi B, et al. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: systematic review and meta-analysis. Am J Kidney Dis. 2008;52:897–906. doi: 10.1053/j.ajkd.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 19.Heath C, Strauss M, Castle W. Quantitative aspects of iron deficiency in hypochronic anaemia. J Clin Invest. 1932;1:1293–1312. doi: 10.1172/JCI100478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goetsch A, Moore C, Minnich V. Observations on the effects of massive doses of iron given intravenously to patients with hyperchromic anemia. Blood. 1946;1:129–142. [PubMed] [Google Scholar]
- 21.Nissim J. Intravenous administration of iron. Lancet. 1947;2:49–51. doi: 10.1016/s0140-6736(47)90053-6. [DOI] [PubMed] [Google Scholar]
- 22.Baird I, Podmore D. Intra-muscular iron therapy in iron deficiency anemia. Lancet. 1954;2:942–946. doi: 10.1016/s0140-6736(54)92555-6. [DOI] [PubMed] [Google Scholar]
- 23.Auerbach M, Winchester J, Wahab A. A randomized trial of three iron dextran infusion methods for anaemia in EPO-treated dialysis patients. Am J Kidney Dis. 1998;31:81–86. doi: 10.1053/ajkd.1998.v31.pm9428456. [DOI] [PubMed] [Google Scholar]
- 24.Moniem K, Bhandari S. Tolerability and efficacy of parenteral iron therapy in haemodialysis patients a comparison of two preparations. Transfus Altern Transfus Med. 2007;9:37–42. [Google Scholar]
- 25.Pharmaceuticals V. Cosmofer summary of product characteristics. [Google Scholar]
- 26.Halliwell B, Gutteridge J. Role of free radicals and catalytic metal ions in human disease: an overview. Meth Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 27.Zager R, et al. Parenteral iron formulations: a comparative toxicological analysis and mechanisms of cell injury. Am J Kidney Dis. 2002;40:90–103. doi: 10.1053/ajkd.2002.33917. [DOI] [PubMed] [Google Scholar]
- 28.Carlini RG, et al. Apoptotic stress pathway activation mediated by iron on endothelial cells in vitro. Nephrol Dial Transplant. 2006;21:3055–3061. doi: 10.1093/ndt/gfl341. [DOI] [PubMed] [Google Scholar]
- 29.Zager R, Johnson A, Hanson S. Parenteral iron nephrotoxicity, potential mechanisms and consequences. Kidney Int. 2004;66:114–156. doi: 10.1111/j.1523-1755.2004.00716.x. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal R, et al. Oxidative stress and renal injury with intravenous iron in patients with chronic kidney disease. Kidney Int. 2004;65:2279–2289. doi: 10.1111/j.1523-1755.2004.00648.x. [DOI] [PubMed] [Google Scholar]
- 31.Shah S, et al. Oxidants in chronic kidney disease. J Am Soc Nephrol. 2007;18:16–28. doi: 10.1681/ASN.2006050500. [DOI] [PubMed] [Google Scholar]
- 32.Bhandari S, Naudeer S. Improving efficiency and value in health care. Intravenous iron management for anaemia associated with chronic kidney disease: linking treatment to an outpatient clinic, optimizing service provision and patient choice. J Eval Clin Pract. 2008;14:996–1001. doi: 10.1111/j.1365-2753.2007.00932.x. [DOI] [PubMed] [Google Scholar]
- 33.Ankers SD, et al. FAIR-HF Trial: ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:3426–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 34.Wakström B, et al. Monofer® (iron isomaltoside 1000), a novel intravenous iron for treatment of iron deficiency in chronic kidney disease (CKD) patients. J Nephrol. 2011 doi: 10.5301/JN.2011.6248. In press. [DOI] [PubMed] [Google Scholar]
- 35.Zheng S. Iron deficiency anemia and iron losses after renal transplantation. Transpl Int. 2009;22:434–440. doi: 10.1111/j.1432-2277.2008.00814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolger A. Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J Am Coll Cardiol. 2006;48:1225–1227. doi: 10.1016/j.jacc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Kulnigg S, et al. A novel intravenous iron treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol. 2008;103:1182–1192. doi: 10.1111/j.1572-0241.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- 38.Munoz M, Gomez-Ramirez S, Garcia-Erce J. Intravenous iron in inflammatory bowel disease. World J Gastroenterol. 2009;15:4666–4674. doi: 10.3748/wjg.15.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wyck DV, et al. Large-dose intravenous ferric carboxymaltose injection for iron deficiency anemia in heavy uterine bleeding: a randomized, controlled trial. Transfusion. 2009;49:2719–2728. doi: 10.1111/j.1537-2995.2009.02327.x. [DOI] [PubMed] [Google Scholar]
- 40.Seid M, et al. Ferric carboxymaltose injection in the treatment of postpartum iron deficiency anemia: a randomized controlled clinical trial. AmJ Obstet Gynecol. 2008;199:435–437. doi: 10.1016/j.ajog.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 41.Auerbach M, Ballard H, T. JR. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: a multicenter, open-label, randomized trial. J Clin Oncol. 2004;22:1301–1307. doi: 10.1200/JCO.2004.08.119. [DOI] [PubMed] [Google Scholar]
- 42.Gasche C, et al. Iron, anaemia, and inflammatory bowel diseases. Gut. 2004;53:1190–1197. doi: 10.1136/gut.2003.035758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tagboto S, et al. The efficacy of a single dose if intravenous ferric carboxymaltose (FERINJECT(r)) on anaemia in a pre-dialysis population of CKD patients. J Ren Care. 2009;35:18–23. doi: 10.1111/j.1755-6686.2009.00075.x. [DOI] [PubMed] [Google Scholar]
- 44.Covic A, Mircescu G. The safety and efficacy of intravenous ferric carboxymaltose in anaemic patients undergoing haemodialysis: a multi-centre, open-label, clinical study. Nephrol Dial Transplant. 2010;25:2722–2730. doi: 10.1093/ndt/gfq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Landry R, et al. Pharmacokinetic study of ferumoxytol: a new iron replacement therapy in normal subjects and hemodialysis patients. Am J Nephrol. 2005;25:400–410. doi: 10.1159/000087212. [DOI] [PubMed] [Google Scholar]
- 46.Singh A, et al. Safety of ferumoxytol in patients with anemia and CKD. Am J Kidney Dis. 2008;52:907–915. doi: 10.1053/j.ajkd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Spinowitz BS, et al. Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol. 2008;19:1599–1605. doi: 10.1681/ASN.2007101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.(SPC), F.-S.o.P.C. Ferinject—summary of product characteristics (SPC) 2001. [Google Scholar]
- 49.A/S, P. 2010. Monofer Core SPC, data on file. [Google Scholar]


