Abstract
Patients with chronic kidney disease (CKD) often suffer from iron deficiency anaemia necessitating treatment with intravenous (IV) iron. Several studies demonstrate that oral iron is insufficient in these patients and that IV supplementation is a more effective treatment. Until now, use of available parenteral iron preparations has been limited by dosing schedules and the need, in some cases, for a test dose, and despite the availability of a range of different IV iron compounds, there is still a need for improved compounds. The new IV iron, iron isomaltoside 1000 Monofer®, is composed of iron and chemically modified isomalto-oligosaccharides which have a mean molecular weight of 1000 Da and consist predominantly of 3–5 glucose units. In contrast to dextrans, the carbohydrate isomaltoside 1000 is a linear and unbranched structure with theoretically a low immunological potential. Hence, a test dose is not necessary. Iron isomaltoside 1000 contains strongly bound iron within the iron–isomaltoside formulation, which enables a controlled slow release of bioavailable iron to the iron-binding proteins, with potentially a reduced risk of free iron toxicity. This allows flexible dosing including high and rapid dosing securing convenient iron therapy for a wide range of patients. The development of Monofer® has been enthusiastically acknowledged by clinicians, and in 2009, there has been fast approval by European authorities via a decentralized registration procedure. This new IV iron is currently being marketed in several European countries.
This article describes the development rationale and summarizes the clinical data assessing the use of iron isomaltoside 1000 administered without a test dose by either repeated bolus injections or fast high single iron infusions [defined as total dose infusion (TDI)] to patients suffering from CKD. Since CKD is associated with a high prevalence of cardiovascular disease, data from a small trial applying high single doses of iron isomatoside 1000 in patients with chronic heart failure (CHF) are also reviewed. Collectively, the available data demonstrate adequate efficacy and a good safety profile of iron isomaltoside 1000 in CKD and CHF patients even when administered without a test dose and as single rapid high-dose infusions.
Keywords: anaemia, chronic kidney disease, iron deficiency, parenteral iron
Introduction
Anaemia is common in chronic kidney disease (CKD) [1] and is associated with increased morbidity and mortality [2–4]. Diagnosing both absolute and functional iron deficiency in these patients is crucial for correct anaemia management [5]. Use of erythropoiesis-stimulating agents (ESAs) and intravenous (IV) iron repletion have played central roles in the optimal correction of this anaemia [6–10]. A significant proportion of patients can benefit from anaemia correction with IV iron alone [11]. Although different IV iron compounds are available [12], there is still a need for improved preparations as use of existing products is limited by the need for a test dose or dosing schedules.
The first parenteral iron compound was synthesized in the early 1950s [13]. The first pharmaceutical iron product, Imferon®, based on high-molecular-weight dextran, was withdrawn from the market in 1992 due to manufacturing issues [14]. This IV iron dextran preparation carried a risk of anaphylaxis, but the more recently introduced lower-molecular-weight iron dextran preparation (Infed®/Cosmofer®) has significantly lowered risk level [15], although a test dose is recommended during the administration schedule. Other marketed products such as iron gluconate (Ferrlecit®) and iron sucrose (Venofer®) both contain loosely bound iron and can, therefore, only be administered in relatively low doses [16,17]. Hence, there is a need for efficacious IV iron formulations without dosing limitations and with optimized administration convenience and patient safety.
Recently, newer IV iron preparations have been developed, including ferumoxytol (Feraheme®), ferric carboxymaltose (Ferinject®) and now iron isomaltoside 1000 (Monofer®).
The present article describes the development rationale of iron isomaltoside 1000 and summarizes the clinical data assessing its efficacy and safety when administered in clinical trials to patients suffering from CKD. Since CKD is associated with a high prevalence of cardiovascular disease, data from a small trial applying high single doses of iron isomatoside 1000 in chronic heart failure (CHF) are also reviewed.
Development rationale of iron isomaltoside 1000
New iron preparations should ideally be capable of delivering sufficient quantities of IV iron to correct iron deficiency rapidly within one visit with minimal potential side effects including low catalytic/free iron release and negligible immunogenicity (risk of anaphylaxis). For preservation of veins, the IV preparations should have a neutral pH and a wide dosing range to allow a single repletion dose with no requirement for a test dose. Finally, the product should be convenient and cost-effective for the patient, the health care professional and the health service. The intention behind the development of iron isomaltoside 1000 was to fulfil all of these requirements. Hence, the ambition with iron isomaltoside 1000 (Monofer®) was to develop an efficacious IV iron without dose limitations and without test dose requirement in order to optimize user convenience, dosing flexibility and patient safety.
All IV iron agents consist of iron and carbohydrate. The carbohydrate used in Monofer® is isomaltoside 1000 which has a mean molecular weight of 1000 Da. In contrast to dextran polysaccharides, isomaltoside 1000 is a short linear unbranched molecule, consisting of predominantly 3–5 glucose units and thereby having a theoretically reduced anaphylactogenic potential [18–21]. In addition, iron isomaltoside 1000 contains strongly bound iron within the iron–isomaltoside matrix formulation leading to the slow release of iron with potentially reduced risk of free iron toxicity [22]. These physiochemical properties obviate the requirement for a test dose and allow iron isomaltoside 1000 to be administered both in rapid high-dose single IV infusions or fractioned bolus injections. This offers high dosing flexibility with optimized administration convenience.
The development of Monofer® was enthusiastically acknowledged by both the authorities and clinicians, and in 2009, there has been fast approval of the drug in Europe via a decentralized registration procedure.
Pharmacological properties
Following IV administration, iron isomaltoside 1000 is rapidly taken up by the cells in the reticuloendothelial system (RES), particularly in the liver and spleen, from where iron is slowly released. The plasma half-life is 5 h for unbound circulating iron and 20 h for total iron (bound and circulating) [22].
Circulating iron is removed from the plasma by cells of the reticuloendothelial system which split the complex into its components of iron and isomaltoside 1000. The iron is immediately bound to the available protein moieties to form haemosiderin or ferritin, the physiological storage forms of iron, or to a lesser extent, to the transport molecule transferrin. This form of iron, which is subject to physiological control, replenishes haemoglobin and depleted iron stores [22].
Due to the size of the carbohydrate–iron complex, intact Monofer® is not eliminated via the kidneys. Small quantities of iron are eliminated in urine and faeces [22]. The carbohydrate component, isomaltoside 1000, is either metabolized or excreted [22].
Clinical trial experience in CKD and CHF
Basic study outlines
The main purpose of the published studies in CKD [23] and CHF [24] was to establish the safety profile of the product in relevant populations, efficacy being a secondary end point. Both pivotal studies were prospective, open-label, non-comparative studies. In both studies, a total of six visits were conducted during the study, and the treatment period was 8 weeks. The patients received Monofer® according to the investigator’s decision either as four repeated weekly IV boluses with 100–200 mg iron per dose or as a high single iron repletion dose at baseline (TDI). If the TDI exceeded 20 mg iron per kilogram, it was divided into two and given either side of a 1-week interval. Laboratory assessments (haematological) and biochemical (sodium, potassium, creatinine, albumin, urea, bilirubin and liver enzyme) analyses were performed at every visit. A complete physical examination was performed at screening and at the end of the study. Vital signs and biochemical monitoring of treatment effects were performed at each visit. In addition, patients in the CHF study filled in a linear analogue scale assessment for quality of life (QoL) questionnaire at baseline, and again after 4 and 8 weeks.
CKD study
The CKD study was conducted at 15 centres across three European countries. A total of 182 CKD patients (128 males, 54 females) either in the pre-dialysis phase (n = 21) or undergoing dialysis (n = 161) were included (mean age 63.3, range 21–90 years, mean baseline haemoglobin 112 g/L and mean baseline ferritin 350 μg/L). The vast majority of patients were receiving haemodialysis. The mean total administered dose during the trial was 529 mg and the largest single doses being as high as 1800 mg. In total, 584 treatments were given of which 540 were IV bolus (523 received 100 mg, 17 IV bolus 100–200 mg) and 44 were TDIs.
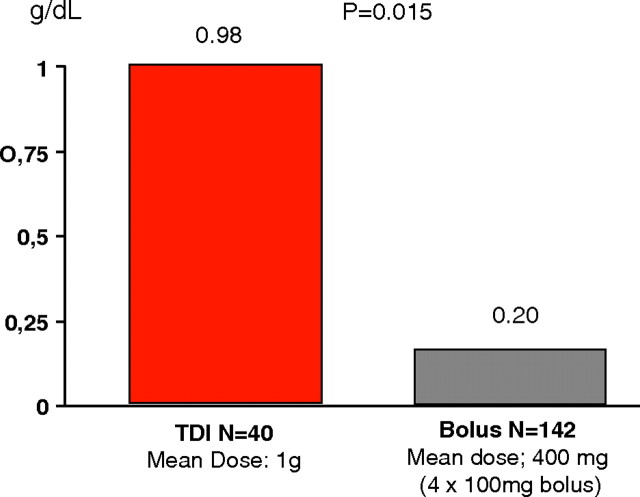
All efficacy parameters (haemoglobin, haematocrit, transferrin saturation, serum iron and serum ferritin) increased over time compared to baseline, and most of the changes were significant. Haemoglobin increased from 99.2 g/L (SD 9.0) at baseline to 111.2 g/L (SD 14.7) at week 8 in patients who were not currently being treated with parenteral iron at the time of the study (P < 0.001), and it increased slightly or stabilized in patients receiving IV iron maintenance therapy [23]. The haemoglobin rise was significantly greater in patients treated with TDI compared to those receiving bolus dosing (Figure 1). In the CKD population, the frequency of drug-related adverse events per treatment was low (3.3% for bolus injections and 2.3% for TDI) and of which most were mainly of a gastrointestinal nature (e.g. nausea, abdominal pain) [23]. No acute anaphylactic/anaphylactoid or delayed allergic reactions were reported.
Fig. 1.
Change in haemoglobin from baseline in the CKD patients who received either TDI or bolus dosing.
CHF study
The study in CHF was conducted at nine centres in two European countries, and it included 20 CHF patients with mild anaemia (mean age 75, range 60–88 years, mean baseline haemoglobin 108 g/L and ferritin 180 μg/L). In contrast to giving repeated small bolus injections until iron repletion was achieved as seen in previous studies with IV iron in CHF [25], all patients received the total calculated iron requirement as a high single iron isomaltoside 1000 dose (TDI) with a mean infusion time of 59.8 min (range 50–67 min). The total dose given per patient in the 8-week period ranged from 650 to 1000 mg with a mean of 868 mg [24].
In this open-label study, there were increases in measures of haematologic response which were consistent with experience of iron supplementation in subjects with CHF and anaemia from previous studies. However, no significant change in haemoglobin was seen perhaps due to the small number of subjects. QoL was measured by LASA QoL. The LASA is a validated QoL assessment consisting of 100-mm linear analogue scales (0 worst) which measures the patient’s energy level, ability to do daily activities and overall QoL. All QoL assessments increased significantly 4 weeks after treatment. The empirical mean for energy level increased by 49%, ability to do daily activities increased by 38% and overall QoL increased by 23%. At the 8-week visit after treatment, energy level was significantly increased by 34%. Ability to do daily activities and overall QoL were increased by 20% and 13%, respectively, but the results were non-significant probably due to the sparse amount of data at visit 6 (8 weeks after treatment) [24]. In the CHF population, no adverse events were classified as possible or probable related to treatment by the investigator [24]. Given the open-label nature of the study, these results require validation in more robust study designs.
Conclusions
The new IV iron, iron isomaltoside 1000 Monofer®, which has recently become commercially available in several European countries, is composed of iron and chemically modified isomalto-oligosaccharides which have a mean molecular weight of 1000 Da and consist predominantly of 3–5 glucose units. In contrast to dextrans, the carbohydrate isomaltoside 1000 is a linear and unbranched structure with theoretically a low immunological potential. Hence, a test dose is not necessary according to the product labelling. Iron isomaltoside 1000 contains strongly bound iron within the iron–isomaltoside formulation, which enables a controlled, slow release of bioavailable iron to the iron-binding proteins, with potentially a reduced risk of free iron toxicity.
Collectively, the reviewed data in CKD and CHF demonstrate adequate efficacy and a good safety profile of iron isomaltoside 1000 in CKD and CHF patients even when administered without a test dose as a single rapid high dose. Hence, the recent introduction in Europe of this new IV iron preparation offers an effective, safe, flexible and time-efficient method of iron repletion. A substantial additional clinical programme has been initiated with ongoing and planned controlled comparative efficacy and safety studies in gastroenterology, nephrology, oncology and gynaecology.
Conflict of interest statement. P.A.K. is a consultant and speaker for Pharmacosmos, Amgen, Genzyme, Shire, Abbott, Novartis and Bristol Myers Squibb. He is a PI in multicentre studies designed and funded by Pharmacosmos, Shire, Genzyme, Abbott and Amgen.
References
- 1.Hsu CY, McCulloch CE, Curhan GC. Iron status and hemoglobin level in chronic renal insufficiency. J Am Soc Nephrol. 2002;13:2783–2786. doi: 10.1097/01.asn.0000034200.82278.dc. [DOI] [PubMed] [Google Scholar]
- 2.Ma JZ, Ebben J, Xia H, et al. Hematocrit level and associated mortality in hemodialysis patients. J Am Soc Nephrol. 1999;10:610–619. doi: 10.1681/ASN.V103610. [DOI] [PubMed] [Google Scholar]
- 3.Collins AJ, Li S, St Peter W, et al. Death, hospitalization, and economic associations among incident hemodialysis patients with hematocrit values of 36 to 39% J Am Soc Nephrol. 2001;12:2465–2473. doi: 10.1681/ASN.V12112465. [DOI] [PubMed] [Google Scholar]
- 4.Ofsthun N, Labrecque J, Lacson E, et al. The effects of higher hemoglobin levels on mortality and hospitalization in hemodialysis patients. Kidney Int. 2003;63:1908–1914. doi: 10.1046/j.1523-1755.2003.00937.x. [DOI] [PubMed] [Google Scholar]
- 5.Fusaro M, Munaretto G, Spinello M, et al. Piccoli Soluble transferrin receptors and reticulocyte hemoglobin concentration in the assessment of iron deficiency in hemodialysis patients. J Nephrol. 2005;18:72–79. [PubMed] [Google Scholar]
- 6.National Collaborating Centre for Chronic Conditions . Anemia management in chronic kidney disease: national clinical guideline for management in adults and children. London: Royal College of Physicians; 2006. Available at: http://guidance.nice.org.uk/cg39. [PubMed] [Google Scholar]
- 7.Fishbane S, Maesaka JK. Iron management in end stage renal disease. Am J Kidney Dis. 1997;29:319–333. doi: 10.1016/s0272-6386(97)90192-x. [DOI] [PubMed] [Google Scholar]
- 8.Pfeffer MA, Burdmann EA, Chen CY, et al. TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 9.Drüeke TB, Locatelli F, Clyne N, et al. CREATE Investigators: Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 10.Singh AK, Szczech L, Tang KL, et al. CHOIR Investigators: Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 11.Gotloib L, Silverberg D, Fudin R, et al. Iron deficiency is a common cause of anemia in chronic kidney disease and can often be corrected with intravenous iron. J Nephrol. 2006;19:161–167. [PubMed] [Google Scholar]
- 12.Macdougall IC. Evolution of IV iron compounds over the last century. J Ren Care. 2009;35:8–13. doi: 10.1111/j.1755-6686.2009.00127.x. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher F, London E. Intravenous iron. Br Med J. 1954;1:984. [Google Scholar]
- 14.London E. The molecular formula and proposed structure of the iron-dextran complex, imferon. J Pharm Sci. 2004;93:1838–1846. doi: 10.1002/jps.20093. [DOI] [PubMed] [Google Scholar]
- 15.Chertow GM, Mason PD, Vaage-Nilsen O, et al. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant. 2006;21:378–382. doi: 10.1093/ndt/gfi253. [DOI] [PubMed] [Google Scholar]
- 16. SmPC venofer. [Google Scholar]
- 17. SmPC ferrlecit. [Google Scholar]
- 18.Ljungstrom KG. Pretreatment with dextran 1 makes dextran 40 therapy safer. J Vasc Surg. 2006;43:1070–1072. doi: 10.1016/j.jvs.2005.11.056. [DOI] [PubMed] [Google Scholar]
- 19.Ljungstrom KG. Safety of dextran in relation to other colloids-ten years experience with hapten inhibition. Infusionsther Transfusionsmed. 1993;20:206–210. doi: 10.1159/000222845. [DOI] [PubMed] [Google Scholar]
- 20.Altman LC, Petersen PE. Successful prevention of an anaphylactoid reaction to iron dextran. Ann Intern Med. 1988;109:346–347. doi: 10.7326/0003-4819-109-4-346. [DOI] [PubMed] [Google Scholar]
- 21.Richter W. Hapten inhibition of passive antidextran dextran anaphylaxis in guinea pigs. Role molecular size anaphylactogenicity precipitability dextran fractions. Int Arch Allergy Appl Immunol. 1971;41:826–844. doi: 10.1159/000230575. [DOI] [PubMed] [Google Scholar]
- 22. Monofer SPC. [Google Scholar]
- 23.Wikström B, Bhandari S, Barany P, et al. J Nephrol. Iron isomaltoside 1000 a novel intravenous iron for treating Iron deficiency in chronic kidney disease. In press. [DOI] [PubMed] [Google Scholar]
- 24.Hildebrandt P, Bruun NE, Nielsen OW Pantev E, et al. TATM. 2010. Effects of administration of iron isomaltoside 1000 in patients with chronic heart failure. A pilot study. doi: 10.1111/j 1778-428x.2010.01145.x. [Google Scholar]
- 25.Anker SD, Comin Colet J, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]