Abstract
Pain is associated with anxiety in a dental setting. It has remained unclear how cognitive-affective factors modulate pain and anxiety in a stressful context, such as receiving dental procedures. We hypothesized that both the situational factor (unpredictability about painful stimuli) and the trait factor (pain catastrophizing, i.e., the tendency to interpret pain in negative orientation) account for dental pain. Fifteen healthy participants were recruited to perform an associative learning task. They were asked to learn the pairing between visual cues and the intensity of incoming painful stimuli delivered at the right upper central incisor. Brain activation associated with pain was recorded by functional magnetic resonance imaging (fMRI). The participants reported increased anxiety and pain in the stressful context, where stimuli intensity was not predicted by the preceding cue. The score of the Pain Catastrophizing Scale was positively correlated with the increased pain modulated by unpredictability. Brain activation at the right posterior hippocampus, a region critically related to associative learning of aversive stimuli and context, was correlated with the individual catastrophizing level. Our findings suggest that both the situational factor (unpredictability) and the trait factor (catastrophizing) influence dental pain, highlighting the role of cognitive-affective factors in pain control of dental patients.
Keywords: anxiety, dental care, functional MRI, hippocampus, pain catastrophizing, pain management
Introduction
Pain is modulated by complex cognitive and affective factors (Tracey and Mantyh, 2007). In a clinical setting, highly anxious patients anticipate and perceive worse pain when receiving stressful dental procedures (e.g., root canal treatment or extraction) (Klages et al., 2004; van Wijk and Hoogstraten, 2009), and dental fear leads to avoidance of treatment and poor oral health (Armfield et al., 2007). The etiology of dental pain could be misdiagnosed when patients were highly anxious (Eli, 1993), and patient satisfaction with treatment would decline due to unsuccessful relief of pain and anxiety (Ståhlnacke et al., 2007). Patients’ subjective experience of pain fluctuates in a stressful context of dental pain and dental procedures, yet its psychological and neurological mechanisms are largely unknown.
Feeling uncertain about an imminent threat is stressful (Asmundson et al., 2007). Receiving aversive stimuli with unpredictable occurrence or intensity would evoke sustained anxiety (Shankman et al., 2011). Nociceptive stimuli with unpredictable intensity would be perceived as more painful, even though the physical intensity of stimuli did not change (Ploghaus et al., 2001; Brown et al., 2008). The findings suggest that, on the one hand, the situational factor, such as unpredictability about pain, critically modulates our pain experience (Armfield, 2010). On the other hand, trait factors regarding the cognitive-affective processing of threat may contribute to individual vulnerability to develop anxiety and pain (Asmundson et al., 2007). A critical trait of this kind is pain catastrophizing, the tendency to anticipate pain and interpret experienced pain in a negative orientation (Quartana et al., 2009). This trait can be assessed by the pain catastrophizing scale (PCS) from 3 subcategories: rumination (regarding attentional engagement to pain-related experience), magnification (regarding the tendency to exaggerate pain-related threat), and helplessness (regarding the inability to cope with pain effectively) (Sullivan et al., 1995). People with a higher PCS score showed biased cognitive-affective processing regarding pain-related information (e.g., heightened attentional engagement with the cues predicting incoming pain) (Van Damme et al., 2002, 2004). In the dental setting, patients with higher PCS scores reported more dental anxiety and pain during dental procedures (Sullivan and Neish, 1998, 2000). Therefore, pain catastrophizing may contribute to individual pain experience in a stressful setting, in which the subjects need to learn the dynamic association between pain and the pain-related context. However, the underlying psychological and brain mechanisms remained unclear.
We hypothesized that individual variation in pain catastrophizing would account for participants’ exacerbated pain experience in the stressful context regarding dental pain (e.g., receiving painful pulpal stimulation). We adopted an associative learning paradigm to compare the self-reported pain intensity and pain-related anxiety in the condition where stimulating intensity was predictable vs. unpredictable (Ploghaus et al., 2001). We hypothesized that the PCS score predicts pain exacerbated by increased unpredictability, but not pain evoked by increased stimulating intensity. We reasoned that catastrophizers experience such unpredictabile-modulated pain because they tended to associate pain with the stressful context in negative orientation (e.g., through ruminating and magnifying pain-related information). We hypothesized that such a biased cognitive/affective processing of pain relates to functions of the hippocampus, a brain region critically related to acquisition of the association between aversive stimuli and the context (Fanselow and Dong, 2010; Goosens, 2011). To test the working hypothesis, we applied functional magnetic resonance imaging (fMRI) to investigate the correlation between hippocampal activation and the PCS score, when the participants received painful pulpal stimulation in a stressful context.
Materials & Methods
Participants
Sixteen healthy participants (seven men and nine women) were recruited from the public through an advertisement. One male subject was excluded because he did not follow experimental instructions, leaving 15 participants (mean age ± standard deviation [SD] = 26.3 ± 11.2 yrs). All participants were right-handed and had no history of neurological or psychiatric disease or chronic pain. Oral examination was performed to confirm that the stimulation site (the right upper central incisor) was intact. Written informed consent was obtained from participants. The study was approved by the Institutional Ethics Committee of Taipei Veterans General Hospital and conducted in accordance with the Declaration of Helsinki.
Psychological Assessment
Prior to the experiment, participants completed the Pain Catastrophizing Scale (PCS) (Sullivan et al., 1995), which consists of the subscale of rumination, magnification, and helplessness, and 3 assessments regarding the general and dental-specific traits about the cognitive-affective aspects of pain: Beck’s Depression Inventory (BDI; Beck et al., 1961), the Modified Dental Anxiety Scale (MDAS; Humphris et al., 1995), and the revised Dental Belief Survey (DBS; Milgrom et al., 1995). After the experiment, participants completed the short-form McGill pain questionnaire (MPQ; Melzack, 1987), a tool that quantified the pain they experienced during the experiment.
Experimental Design and Procedure
The experiment consisted of a behavioral task and fMRI scanning, which measures the blood-oxygen-level-dependent (BOLD) effect associated with the task-related brain activation. The behavioral task was modified from the associative learning paradigm (Ploghaus et al., 2001) (see Fig. 1A for detailed description of the paradigm). Briefly, the participants received electrical stimuli that evoked high and low levels of pain on their upper right central incisor (Brügger et al., 2012). The physical intensity of the stimuli (mA) was calibrated at the beginning of the experiment and fixed throughout the experiment. Details on the tooth stimulation paradigm can be found in the Appendix Methods under “Electrical Stimulation”. In a trial, a stimulus would be delivered in 1 of the 2 conditions (with different levels of stress): (i) a predictable condition, in which the participant would always receive a low-intensity stimulus; and (ii) an unpredictable condition, in which the participant would receive either a low- or a high-intensity stimulus.
Figure 1.
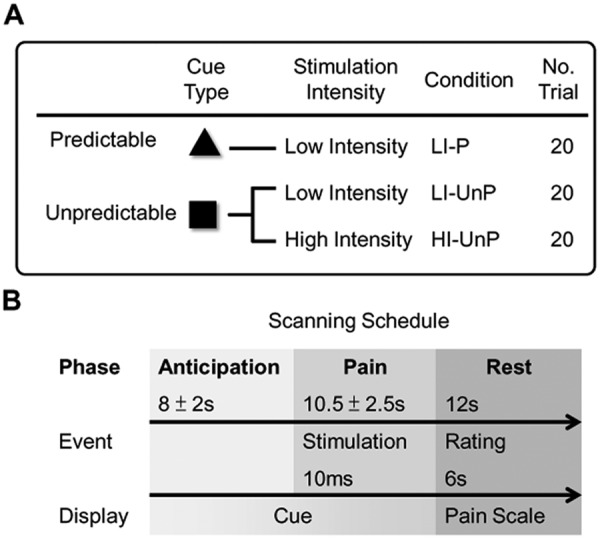
Experimental design and scanning protocol. (A) Experimental design. Each trial consisted of an anticipatory phase followed by a pain phase and ended with a rest phase. The anticipatory phase was initiated by 1 of 2 types of visual cues, which differed in predictability of the pain intensity of the impending stimulus and thus induced different levels of anticipatory anxiety. The high-predictability cue (P) was always followed by a single low-intensity painful stimulus (LI). The low-predictability cue (UnP) was followed by either a low-intensity (as above) or high-intensity (HI) painful stimulus. Overall, each of the cue-intensity events (LI-P, LI-UnP, and HI-UnP) was presented in equal numbers (20 trials) throughout the experiment. Participants were instructed that 2 types of cues would be displayed during functional scanning, and that attention should be paid to the cue-intensity association. Two sessions were performed, with 30 event-cycles per session and a five-minute rest between 2 sessions. Two fixed sequences were generated in which stimuli were randomly presented. The order of sequences with respect to sessions was counterbalanced across participants. (B) Scanning protocol. The visual cue was displayed throughout the anticipatory phase (mean duration = 8 sec) and pain phase (mean duration = 10.5 sec), with a 10-msec electrical pulpal stimulus. After the stimulus was delivered, the visual cue was replaced by a visual-analog scale (VAS) for the following rest phase (mean duration = 12 sec). In this phase, participants were required to rate the pain intensity of the stimulus just received via an online response box. The discrete 11-point pain intensity scale with verbal anchors used for psychophysical calibration and stimulus evaluation during functional scanning ranged from 0 (no pain) to 10 (intolerable pain). After scanning, participants rated pain-related anxiety based on a 0- to 5-point scale, in which ‘0’ represented ‘no anxiety about the impending pain’ and ‘5’ represented ‘extreme anxiety about the impending pain’.
In each trial, the stimulus was preceded by a visual cue, and the participants were asked to associate the cue and the following stimuli intensity (e.g., to differentiate if a cue was predictive or unpredictive to intensity). The participants thus encountered 1 of the 3 conditions in each trial: (1) low-intensity predictable (LI-P), (2) low-intensity unpredictable (LI-UnP), and (3) high-intensity unpredictable (HI-UnP). The participants received fMRI scanning concurrently when performing the behavioral task and rated the intensity of perceived stimulus, in each trial, after receiving the stimulus (Fig. 1A).
Statistical Analysis
Behavioral Data
To investigate our behavioral hypothesis regarding the role of pain catastrophizing, for each participant, we first quantified the degree of increased pain ratings (ΔP) modulated by (i) increased unpredictability (i.e., ΔPunpredictability) and (ii) increased nociceptive intensity (i.e., ΔPintensity), respectively:
Next, we performed stepwise multiple-regression analysis, across all participants, by taking ΔPunpredictability and ΔPintensity, respectively, as the dependent variable, with the participants’ age and the assessment scores from PCS, MDAS, DBI, MPQ, and BDI as the predictors. We expected the PCS score to be significantly positively correlated with ΔPunpredictability but not with ΔPintensity, with all the other variables being controlled.
Functional MRI Data
The acquired imaging data were pre-processed and analyzed with SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Details on imaging data processing can be found in the Appendix Methods under “Imaging Data Acquisition and Processing”. To test our imaging hypothesis regarding the role of the hippocampus, we performed region-of-interest (ROI) analysis, focusing on the hippocampal activation in the contrast image (the LI-UnP condition compared with the LI-P condition). We selected the anterior and posterior hippocampus as the ROIs and confined the regression analysis to only the voxels within the ROI (Poldrack, 2007). We also performed whole-brain exploratory analysis by searching in the whole brain for regions in which activation positively correlated with the PCS score. The whole-brain analysis was performed separately for the 3 baseline images and the contrast image. Details on the imaging statistical analysis can be found in the Appendix Methods under “Imaging Data Analysis” and “ROI Definition”.
Results
Behavioral Results
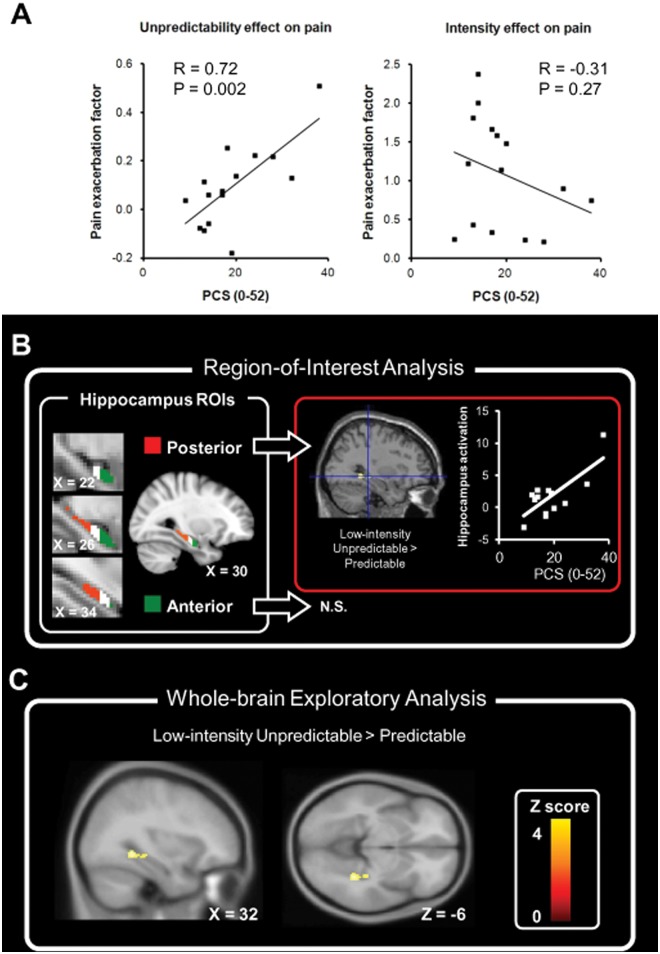
The participants rated increased pain and pain-related anxiety in the unpredictable condition compared with the predictable condition. Multiple-regression analysis revealed the PCS total score as the only variable significantly correlated with ΔPunpredictability (t = 3.75, p = 0.002, zero-order r = 0.72) (Fig. 2A). In contrast, the PCS total score was not correlated with ΔPintensity (Fig. 2A) (see Appendix Table for results of psychological assessment). Notably, the score of each of the 3 PCS subscales (rumination, magnification, and helplessness) was positively correlated with ΔPunpredictability. The finding confirmed our behavioral hypothesis that pain catastrophizing predicted the increased pain modulated by unpredictability; in contrast, it did not predict the increased pain modulated by increased nociceptive intensity. Individual variations in increased anxiety did not significantly correlate with the increased pain (p = 0.37) or the PCS total score (p = 0.98). The findings suggest that the changing anxiety per se did not account for the changing pain experience. Details on the behavioral results can be found in the Appendix Results under “Behavioral Results”.
Figure 2.
Summary of main results. (A) Behavioral results. The score of the Pain Catastrophizing Scale predicted the exacerbated pain modulated by increased unpredictability) (left panel) but not that modulated by increased nociceptive intensity (right panel). (B) ROI analysis. The posterior (red) and anterior (green) hippocampus each occupied 1/3 of the hippocampus ROI (see Appendix for ROI definition), and ROI analysis was performed for the anterior and posterior hippocampus (left panel). The PCS score was positively correlated with brain activation in the contrast image in the posterior hippocampus (framed with a red border), but not the anterior hippocampus (N.S.), of the right hemisphere (right panel). (C) Whole-brain analysis. Brain regions with activation positively correlated with PCS were found at the right hippocampus in the contrast (LI-UnP > LI-P), consistent with the finding from the above ROI analysis. The color bar indicates the Z score from a voxel-wise comparison of brain activation between the 2 conditions.
Functional Mri Results
ROI Analysis
For the contrast image (LI-UnP > LI-P), within the pre-defined ROIs, we found brain activation positively correlated with PCS scores at the right posterior hippocampus ([x, y, z] = [32, -32, -6], Z = 2.83, SVC-corrected p = 0.048, corrected for family-wise error) (Fig. 2B). The results confirmed our imaging hypothesis that the hippocampal activation is associated with catastrophizing in the stressful context modulated by unpredictability.
Whole-brain Analysis
For the contrast image, within the whole brain, we found brain activation positively correlated with PCS scores only at the posterior hippocampus (Table, A; Fig. 2C). For images of the respective baseline conditions, the PCS-related activation was found in the hippocampus for the conditions LI-UnP and HI-UnP (Table, C and D), but not in the condition LI-P (Table, B). The findings suggested that the coupling between hippocampal activation and PCS scores was specific to the stressful context.
Table.
Summary of Imaging Findings
| (A) Significant Brain Activation in the Contrast Image | ||||||||
|---|---|---|---|---|---|---|---|---|
| Low-intensity Unpredictable > Predictable (LI-UnP > LI-P) Condition | ||||||||
| MNI Coordinates |
||||||||
| Brain Region | BA | Side | Cluster Size | Z Score | p value | x | y | z |
| Hippocampus | 37 | R | 58 | 3.01 | 0.001 | 34 | −46 | −6 |
| Hippocampus | 37 | R | 2.83 | 0.002 | 32 | −32 | −6 | |
| (B) Significant Brain Activation in the Low-intensity Predictable (LI-P) Condition | ||||||||
| MNI Coordinates |
||||||||
| Brain Region | BA | Side | Cluster Size | Z Score | p value | x | y | z |
| S2/posterior insula | 13 | L | 267 | 4.33 | < 0.001 | −36 | −28 | 18 |
| Superior temporal gyrus | 22 | L | 386 | 3.79 | < 0.001 | −48 | −8 | −6 |
| Temporal pole | 21 | L | 3.72 | < 0.001 | −46 | 8 | −18 | |
| Putamen | L | 2.97 | 0.001 | −26 | 12 | −10 | ||
| Anterior insula | 13 | R | 102 | 3.48 | < 0.001 | 34 | 12 | −14 |
| Anterior cingulate cortex | 32 | R | 49 | 3.36 | < 0.001 | 2 | 46 | 6 |
| Precuneus | 7 | L | 39 | 3.21 | 0.001 | −14 | −72 | 32 |
| M1 | 4 | L | 26 | 3.03 | 0.001 | −4 | −30 | 50 |
| (C) Significant Brain Activation in the Low-intensity Unpredictable (LI-UnP) Condition | ||||||||
| MNI Coordinates |
||||||||
| Brain Region | BA | Side | Cluster Size | Z Score | p value | x | y | z |
| Hippocampus | 37 | R | 372 | 2.88 | 0.002 | 32 | −42 | −2 |
| (D) Significant Brain Activation in the High-intensity Unpredictable (HI-UnP) Condition | ||||||||
| MNI Coordinates |
||||||||
| Brain Region | BA | Side | Cluster Size | Z Score | p value | x | y | z |
| Hippocampus | 37 | R | 54 | 4.02 | < 0.001 | 32 | −40 | −2 |
BA, Brodmann area; S2, Secondary somatosensory cortex; M1, primary motor cortex.
All p values reported are uncorrected for multiple comparison. Cluster size is measured by the number of voxels.
Discussion
Increased Pain is Modulated by Both Context and Trait Factors
Exaggeration or magnification of pain of dental patients has been widely documented (Klages et al., 2004; van Wijk and Hoogstraten, 2009). Yet the psychological mechanisms of such a phenomenon remained unexplored. The trait view focused on the influence of personality factors, such as dental anxiety, depression, or neuroticism, on the worst pain experience in a dental setting. The situational (or contextual) view, in contrast, focused on the situational factors specific to the context regarding dental pain and its treatment, such as uncontrollability, unpredictability, or dangerousness (Armfield, 2006). It is noteworthy that these 2 lines of theoretical views are not mutually exclusive: The cognitive-affective factors may create a threatening or stressful context where individuals are prone to feel about pain, and their predisposing traits predict how bad the pain would be. In this integrative model, both situational factors and trait factors shape our pain experience (Fig. 3).
Figure 3.
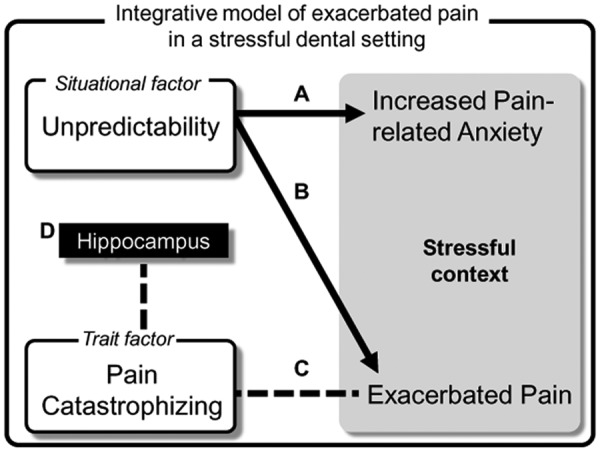
A proposed cognitive-affective model regarding exacerbated pain in a stressful context. Increased unpredictability, a situational factor, evokes both heightened pain-related anxiety (A) and increased pain (B), resulting in a stressful context (shaded by gray). The individual variation in the degree of magnified pain is predicted by the trait factor, pain catastrophizing (C). Note that the change of anxiety per se does not predict the change of pain. Participants with higher pain catastrophizing showed increased hippocampal activation, which is associated with memory and learning of aversive stimuli (D). The current model highlights that both the stressful context (modulated by unpredictability) and the catastrophizing trait are critical to pain experience in a stressful dental setting.
Our findings support such an integrative model regarding pain experience exacerbated in a stressful dental setting. We have demonstrated that PCS scores predicted the increased pain in the unpredictable vs. predictable context, where the participants also felt increased anxiety. Notably, PCS scores did not predict the increased pain modulated by stimuli intensity. The finding suggested that pain catastrophizing modulates pain experience via modulating the cognitive-affective aspects, rather than the sensory aspects, regarding stimuli. Our findings are in line with the cognitive model of dental fear (Armfield, 2006; Armfield et al., 2007), which highlights the role of contextual features, such as unpredictability, in shaping anxiety. Because the heightened pain-related stress was induced by an associative learning paradigm, the finding highlights the cognitive theory of pain catastrophizing, which proposed that pain catastrophizing can be characterized by biased information-processing regarding threat (e.g., increased attention biases to pain information) (Van Damme et al., 2002, 2004; Quartana et al., 2009).
Hippocampal Activation Reflects Individual Variations of Pain Catastrophizing in a Stressful Context
We found that the hippocampus activation was associated with increased PCS scores. The coupling between hippocampal activation and PCS was found only in the stressful context when pain was unpredictable. An interesting finding in our results was that only the posterior part of the hippocampus showed significant correlation with PCS scores. The hippocampus is a functionally heterogeneous region. The anterior part is predominantly associated with anxiety and fear, whereas the posterior hippocampus plays a key role in context-modulated fear conditioning (i.e., associating an aversive stimulus with the context of stimulation) (Fanselow and Dong, 2010; Goosens, 2011). Therefore, while the anterior hippocampus reflects the degree of anxiety induced by a threat (McHugh et al., 2004), the posterior hippocampus reflects how an individual acquires the threat-context association. Its activation may therefore indicate biased information-processing about a threat in a stressful context, and reflect the vulnerability in which one perceives pain-related anxiety (when anticipating the threat to come). In line with our finding, a recent study has revealed a functional segregation regarding anxiety in the hippocampus. While anterior hippocampal activation is associated with state anxiety (i.e., the anxiety about a particular situation or activity), posterior hippocampal activation is associated with trait anxiety (i.e., the proneness to experience anxiety across various contexts and events) (Satpute et al., 2012). Further discussion on the low-intensity predictable conditions can be found in the Appendix Discussion.
Clinical Significance
Our behavioral and neuro-imaging findings highlight the role of cognitive-affective factors for pain control in dental patients. Until now, a systematic approach for managing highly anxious dental patients and alleviating their pain has not been fully established. Based on our behavioral and neurological findings, we suggest that:
(1) Manipulating the predictability of pain is crucial to the relief of pain-related anxiety. This issue can be particularly important during complicated dental treatment (e.g., implant surgery or root canal treatment), when the patients feel uncertain about the effect of a dental procedure.
(2) It is crucial to assess trait pain catastrophizing for highly anxious dental patients, especially when they are scheduled to undergo very stressful procedures. For dentists, to understand such a pain-related ‘cognitive profile’ of patients would contribute to a customized strategy for pain control.
(3) Dental patients would not just passively receive a painful stimulus, but would also actively associate their experienced pain with the context wherein they received the stimulus. The patients with a higher pain catastrophizing score may be more prone to learn an association between the context and pain from a negative orientation and form traumatic memory regarding pain. The neurobiological evidence related to pain catastrophizing during dental pain would highlight the influence of biopsychosocial factors on oral health (Marcenes et al., 1993).
Supplementary Material
Acknowledgments
C.-S.L. and D.M.N. conceived and designed the study, performed the experiments, and analyzed data. C.-S.L., D.M.N., M.-L.H., and J.-C.H. interpreted the data. C.-S.L. and D.M.N. wrote the paper.
Footnotes
This study was funded by the National Science Council of Taiwan (NSC 97-2314-B-010-050-MY2; NSC 96-2752-B-010-008-PAE) and ‘Aim for the Top University Plan from Ministry of Education’.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Armfield JM. (2006). Cognitive vulnerability: a model of the etiology of fear. Clin Psychol Rev 26:746-768. [DOI] [PubMed] [Google Scholar]
- Armfield JM. (2010). Towards a better understanding of dental anxiety and fear: cognitions vs. experiences. Eur J Oral Sci 118:259-264. [DOI] [PubMed] [Google Scholar]
- Armfield JM, Stewart JF, Spencer AJ. (2007). The vicious cycle of dental fear: exploring the interplay between oral health, service utilization and dental fear. BMC Oral Health 7:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmundson GJ, Norton PJ, Vlaeyen JWS. (2007). Fear-avoidance models of chronic pain: an overview. In: Understanding and treating fear of pain. Asmundson GJ, Vlaeyen JW, Crombez G, editors. New York, NY: Oxford University Press. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. (1961). An inventory for measuring depression. Arch Gen Psychiatry 4:561-571. [DOI] [PubMed] [Google Scholar]
- Brown CA, Seymour B, Boyle Y, El-Deredy W, Jones AK. (2008). Modulation of pain ratings by expectation and uncertainty: Behavioral characteristics and anticipatory neural correlates. Pain 135:240-250. [DOI] [PubMed] [Google Scholar]
- Brügger M, Lutz K, Bronnimann B, Meier ML, Luechinger R, Barlow A, et al. (2012). Tracing toothache intensity in the brain. J Dent Res 91:156-160. [DOI] [PubMed] [Google Scholar]
- Eli I. (1993). Dental anxiety: a cause for possible misdiagnosis of tooth vitality. Int Endod J 26:251-253. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. (2010). Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65:7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA. (2011). Hippocampal regulation of aversive memories. Curr Opin Neurobiol 21:460-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphris GM, Morrison T, Lindsay SJ. (1995). The Modified Dental Anxiety Scale: validation and United Kingdom norms. Community Dent Health 12:143-150. [PubMed] [Google Scholar]
- Klages U, Ulusoy O, Kianifard S, Wehrbein H. (2004). Dental trait anxiety and pain sensitivity as predictors of expected and experienced pain in stressful dental procedures. Eur J Oral Sci 112:477-483. [DOI] [PubMed] [Google Scholar]
- Marcenes WS, Croucher R, Shelham A, Marmot M. (1993). The relationship between self-reported oral symptoms and life-events. Psychology & Health 8:123-134. [Google Scholar]
- McHugh SB, Deacon RM, Rawlins JN, Bannerman DM. (2004). Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci 118:63-78. [DOI] [PubMed] [Google Scholar]
- Melzack R. (1987). The short-form McGill Pain Questionnaire. Pain 30:191-197. [DOI] [PubMed] [Google Scholar]
- Milgrom P, Weinstein P, Kleinknecht R, Getz T. (1995). Treating fearful dental patients: a patient management book. 2nd ed. Seattle, WA: University of Washington Continuing Dental Education. [Google Scholar]
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, et al. (2001). Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci 21:9896-9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. (2007). Region of interest analysis for fMRI. Soc Cogn Affect Neurosci 2:67-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartana PJ, Campbell CM, Edwards RR. (2009). Pain catastrophizing: a critical review. Expert Rev Neurother 9:745-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpute AB, Mumford JA, Naliboff BD, Poldrack RA. (2012). Human anterior and posterior hippocampus respond distinctly to state and trait anxiety. Emotion 12:58-68. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Robison-Andrew EJ, Nelson BD, Altman SE, Campbell ML. (2011). Effects of predictability of shock timing and intensity on aversive responses. Int J Psychophysiol 80:112-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståhlnacke K, Söderfeldt B, Unell L, Halling A, Axtelius B. (2007). Patient satisfaction with dental care in one Swedish age cohort. Part II—What affects satisfaction. Swed Dent J 31:137-146. [PubMed] [Google Scholar]
- Sullivan MJ, Neish NR. (1998). Catastrophizing, anxiety and pain during dental hygiene treatment. Community Dent Oral Epidemiol 26:344-349. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Neish N. (2000). Catastrophic thinking and the experience of pain during dental procedures. J Indiana Dent Assoc 79:16-19. [PubMed] [Google Scholar]
- Sullivan MJ, Bishop S, Pivik J. (1995). The pain catastrophizing scale: development and validation. Psychol Assess 7:524: 532. [Google Scholar]
- Tracey I, Mantyh PW. (2007). The cerebral signature for pain perception and its modulation. Neuron 55:377-391. [DOI] [PubMed] [Google Scholar]
- Van Damme S, Crombez G, Eccleston C. (2002). Retarded disengagement from pain cues: the effects of pain catastrophizing and pain expectancy. Pain 100:111-118. [DOI] [PubMed] [Google Scholar]
- Van Damme S, Crombez G, Eccleston C. (2004). Disengagement from pain: the role of catastrophic thinking about pain. Pain 107:70-76. [DOI] [PubMed] [Google Scholar]
- van Wijk AJ, Hoogstraten J. (2009). Anxiety and pain during dental injections. J Dent 37:700-704. [DOI] [PubMed] [Google Scholar]
- Wiech K, Lin CS, Brodersen KH, Bingel U, Ploner M, Tracey I. (2010). Anterior insula integrates information about salience into perceptual decisions about pain. J Neurosci 30:16324-16331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.