Abstract
Even though numerous studies have helped to better delineate abnormalities of either innate or adaptive immune system in end-stage renal disease (ESRD) patients, understanding immune dysfunctions in ESRD patients remains a very complex puzzle with missing pieces. In this context, we showed that the soluble form of CD40 (sCD40) is elevated in ESRD patients and is associated with a lack of response to hepatitis B vaccination. Interestingly, although most dialysis membranes are unable to clear sCD40, we demonstrated that polymethylmethacrylate (PMMA) BK-F membranes (Toray Medical Company, Japan) allow a dramatic diminution of the molecule. We took advantage of this observation to address the question of the potential usefulness of PMMA membrane (BK-F series) in the improvement of humoral immune response of ESRD patients. We, thus, present our recent data highlighting the potential role of BK-F membrane in the improvement of hepatitis B vaccination of ESRD patients who failed to mount a protective immune response despite one or more well-conducted anterior vaccination.
Taken as a whole, our findings reinforced the concept of seeing dialysis membranes not just as a simple diffusive device but as a tool to tailor dialysis procedure to improve the global quality of life of ESRD patients. This opens a wide area of investigation, notably for the management of immunological dysfunction of ESRD patients.
Keywords: haemodialysis, immune dysfunctions, polymethylmethacrylate (PMMA) membrane, hepatitis B vaccination, soluble CD40
Chronic renal failure and adaptive immune dysfunctions: central role of abnormal co-stimulatory signal
It is well established that end-stage renal disease (ESRD) is accompanied by deep disturbance of the immune system impacting both the innate and the adaptive arm (for extensive reviews, see [1,2]). As a result, ESRD patients display several features of immune response deficiency such as recurrent infections (bacterial or viral), high frequency of malignant tumours and poor response to thymo-dependent vaccination (see Table 1 and associated references). This immunodeficiency plays a key role in the increased morbidity and mortality of ESRD patients. In fact, sepsis-induced mortality is dramatically increased, and infections are the second leading cause of death in ESRD patients. However, immunodeficiency is not necessarily exclusively responsible as haemodialysis (HD) procedure by itself results in skin barrier breakage, which is also, although modestly, involved in high infection prevalence in ESRD patients. Interestingly, HD procedure can also accentuate the alteration of the immune system. In fact, as a result of metabolic disturbance linked to uraemic status, ESRD is accompanied by the accumulation of numerous toxic substances, so-called uraemic toxins, that are thought to increase the immunodeficiency of ESRD patients.
Table 1.
Clinical features of ERSD patients that might be associated with poor immunological functions
| Clinical features | Type | References |
|---|---|---|
| High cancer prevalence | Urinary and genital (uterine, kidney, bladder) | [24] |
| Myeloma, Hodgkin lymphoma | ||
| Hepatic | ||
| Pulmonary | ||
| Viral infections | EBV | [25] |
| HCV | [26] | |
| HBV | [27] | |
| Bacterial infections | Clostridium difficile | [28] |
| Tuberculosis | [29] | |
| Altered vaccinal response to thymo-dependent antigens | HBV | [30] |
| Influenza | [31] | |
| Tetanus | [32] | |
| Diphtheria | [33] |
A better understanding of factors associated with abnormal immune response is still a major goal towards ameliorating the management of ESRD patients—optimization of the HD procedure or development of new strategies of immune intervention. In this context, improvement of vaccination efficiency has been a long story. Even in reinforced vaccination protocols, 30–60% of ESRD patients fail to produce protective antibody titre against hepatitis B after vaccination (Table 2a and associated references). Moreover, even in responder patients, the level and the duration of the antibody-mediated protection are lower than those in normal population because of lower antibody levels after vaccination and a gradual loss of antibody titres [3]. Interestingly, among non-responder patients, only 30% are able to mount a proper response after a new vaccination, highlighting the deep alteration of humoral immune response in such patients (Table 2b and associated references).
Table 2a.
Seroconversion rate of ESRD patients after one anti-HBV vaccination
Table 2b.
Seroconversion rate of ESRD patients who did not respond to HBV vaccination after a second vaccination
The leading causes of this obvious humoral immune response impairment are not fully elucidated. In fact, although ESRD is associated with B-cell lymphopaenia, normal plasmatic levels of immunoglobulins are generally observed. However, in vivo and in vitro data clearly show a diminished antibody production to thymo-dependent antigen (relying on the co-stimulation of B cells by T-cell receptor-activated T lymphocytes) [4,5], whereas response to thymo-independent antigen (inducing the direct activation of B cells without the help of T lymphocytes) such as polysaccharides (i.e. pneumococcal vaccination) is conserved. Taken as a whole, the most likely explanation of such dysfunctions is an impaired co-stimulation of B cells by either antigen-presenting cells or T lymphocytes.
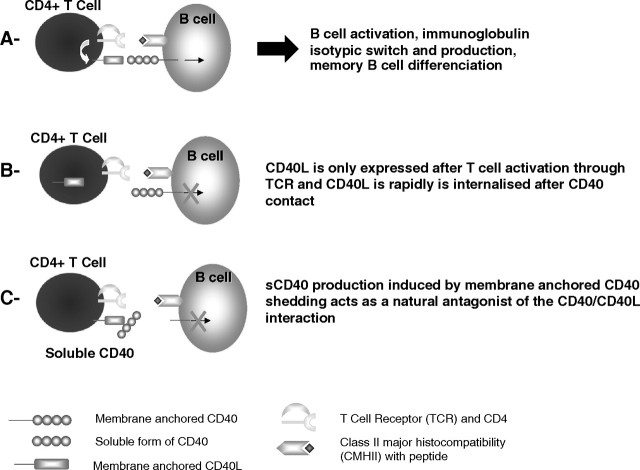
Among the co-stimulatory molecules involved in the development of a proper thymo-dependent B-cell response, the CD40/CD40L interaction plays a key role. CD40 is a 50-kDa glycoprotein that is expressed by a wide range of immune cells including B cells, dendritic cells, monocyte/macrophage cells or non-immune cells such as endothelial cells, epithelial cells (and notably renal epithelial tubular cells) or fibroblast. The expression of the ligand for CD40 or CD40L (CD154) is mainly restricted to activated CD4 T cells, but platelets, mastocytes or natural killer cells have also been shown to express this molecule. CD40 triggering by CD40L is pivotal for B-cell growth, differentiation and isotypic switch (the process leading to the production of IgG, IgA, IgE or IgD) as illustrated by the hyper-IgM syndrome in which mutation in the CD40L gene results in a normal or elevated IgM levels but no IgG, IgA, IgE or IgD. Moreover, CD40 activation on the surface of dendritic cells induces expression of the co-stimulatory molecules CD80 and CD86 and cytokine production necessary for a proper T-cell activation (for updated review, see [6]). Considering the pivotal role played by the CD40/CD40L interaction in the immunity and notably in the humoral immune response, it needs to be tightly regulated. This is achieved by two major mechanisms (see Figure 1) that are the transient expression of CD40L on the surface of activated CD4+ T cells [7] and the production, by proteolytic cleavage (so-called shedding), of a soluble form of CD40 (sCD40) consisting of the extracellular part of the molecule [8]. Natural soluble CD40 mostly co-exists as dimeric and even higher oligomerized forms of 50 and 150 kDa, respectively [9]. Those molecules act as natural antagonists of the CD40/CD40L contact [8–10] as demonstrated by in vitro experiments using purified natural sCD40. We showed that sCD40 is able to block immunoglobulin production by B cells cultured in the presence of CD40L-transfected cells [9].
Fig. 1.
Summary of the pivotal role of CD40/CD40L interaction in the humoral immune response (A) and mechanisms of its regulation (B and C).
Potential role of the soluble form of CD40 in hampered humoral immune response of ESRD patients
We and other authors showed that the level of sCD40 is dramatically increased in the serum of patients with chronic renal failure, with a marked elevation in patients with ESRD [9,11]. This augmentation is mainly due to the altered renal function because, in healthy subjects, sCD40 is normally eliminated in the urine. Although we did not retrieve augmented production of sCD40 in culture supernatant of peripheral blood mononuclear cells from ESRD patients (unpublished data), one can speculate that the uraemic milieu is able to promote in vivo an abnormal shedding of the molecule.
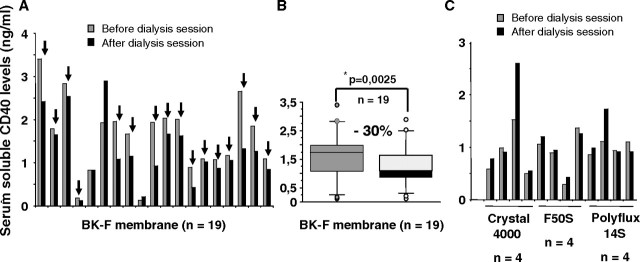
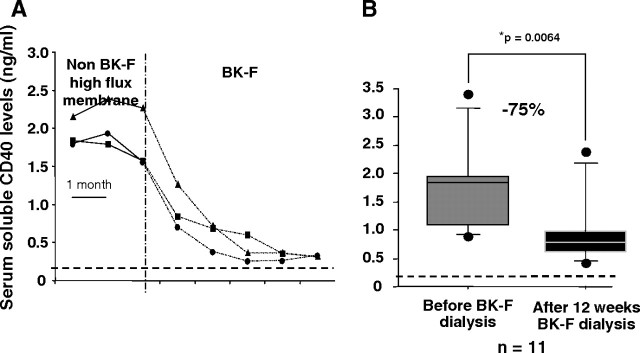
To compare the ability of different high-flux dialysis membranes to clear sCD40 from the patient’s sera, we measured sCD40 before and after dialysis session in the serum of 12 patients dialysed on high-flux membranes such as Arylane H4 (Hospal, France), Crystal 4000 (Hospal) or Polyflux 14S (Gambro, France) and 19 patients dialysed on polymethylmethacrylate (PMMA) membrane (BK-F, Toray Medical Company, Japan). As shown in Figures 2A and B, during the first session, dialysis with BK-F allows a mean of 30% epuration of sCD40 [median and range: 1.73 (0.13–3.40) ng/mL before dialysis vs 1.08 (0.13–2.90) ng/mL after dialysis, P = 0.0025 (non-parametric Wilcoxon test)], whereas other high-flux membranes were unable to do so [median and range: 1.05 (0.29–1.62) ng/mL before dialysis vs 0.97 (0.43–2.79) ng/mL after dialysis, P = 0.57] (Figure 2C). In a longitudinal follow-up of three patients, we demonstrated that sCD40 levels gradually dropped down reaching normal subjects’ values in an average of 3 months (see Figure 3A, adapted from [9]). This observation was further confirmed on a larger cohort of 11 patients (Figure 3B). sCD40 clearing could be linked to the high filtration and/or the high adsorptive capacities of the BK-F membrane. In fact, this membrane belongs to the high-permeability synthetic membrane with a high porosity and an increased hydraulic permeability, and those peculiar capacities allow the elimination of high molecular weight uraemic toxins such as β2 microglobulin [12,13], immunoglobulin free light chains [14], homocystein coupled to high molecular weight protein [15] or an inhibitor of erythropoiesis [16].
Fig. 2.
PMMA membrane (BK-F) is able to clear sCD40 from patient’s sera. Levels of serum sCD40 were measured by ELISA just before and after dialysis session in 19 patients for PMMA (BK-2.1F) membranes (A and B) or 12 patients for high-permeability non-PMMA membranes (C). *Non-parametric Wilcoxon U-test.
Fig. 3.
Longitudinal follow-up of sCD40 levels in patients dialysed on non-PMMA high-flux membranes or BK-2.1F membranes. (A) Soluble CD40 concentrations were measured monthly by ELISA in the serum of three patients dialysed on non-PMMA high-flux membrane and who were then switched to BK-2.1F membrane. Blood samples were taken after the dialysis session. Dotted line represents mean level of sCD40 in healthy subjects. (B) Levels of sCD40 in the serum of 11 patients before and after 12 weeks of dialysis on BK-2.1F membrane. *Non-parametric Wilcoxon U-test.
Thus, because the huge majority of dialysis membranes, including high-flux polysulfone, are not able to clear sCD40, it is thought that sCD40 accumulates gradually in the serum of ESRD patients. With regard to the physiological role of sCD40, accumulation of important amounts of the molecule could deeply alter the humoral immune response of ESRD patients. Interestingly, we found that the most elevated levels of sCD40 in ESRD patients were associated with a lack of response to HBV vaccination [9].
Collectively, these data highlight the potential role of sCD40 in the disturbance of humoral immune response in ESRD patients. We thus postulated that clearing sCD40 from ESRD patient’s sera by using dialysis on PMMA membrane would improve their humoral immune functions and notably their capacity to respond to HBV vaccination.
Dialysis with PMMA membrane improves the efficiency of HBV vaccination: patient’s description
We conducted this pilot study in 2007 in the Department of Nephrology of Pellegrin Hospital and the Center for Treatment of Kidney Diseases, Bordeaux, France. Fifteen patients with renal failure requiring dialysis participated in the study after giving their informed consent. All the patients were in stable condition and were dialysed for at least 3 months on non-PMMA membrane before inclusion. They were not immunized against hepatitis B (anti-HBs Ab <10 IU/L) despite anterior complete vaccination according to usual recommendations and were indeed considered as ‘immunologically refractive’ to vaccination. Patients with previous hepatitis B infection despite negative anti-HBs antibody but positive anti-HBC antibody and/or HBs antigenaemia were excluded. Patients with active neoplasia, plasma cell dyscrasia and lymphopaenia or those treated with immunosuppressive agents or corticosteroids were not included.
Study design
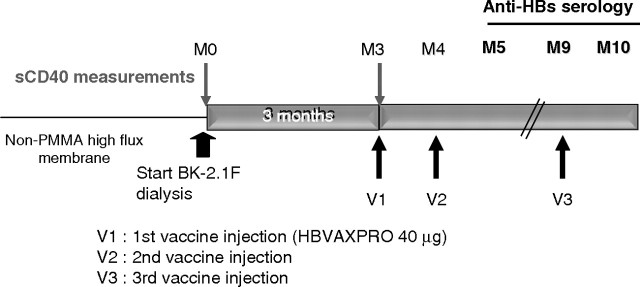
Briefly, patients were dialysed 3 months onto PMMA membrane (BK-F2.1, Toray Medical Company) to allow sCD40 clearing (see Figure 4); then, the vaccination protocol was started as follows: one injection of HBVAXPRO (40 μg, Sanofi Pasteur MSD, France) at months 3 (M3), M4 and M9. The patients were kept dialysed onto the BK-2.1F membrane until the completion of the study (i.e. M10). Serological control was assessed at M3 (to exclude patients developing HBV infection between the inclusion and the start of vaccination), M5, M9 and M10. Seroconversion was defined as level of anti-HBs antibody levels >10 IU/L. Serum sCD40 levels were measured at M0 and M3 by enzyme-linked immunosorbent assay (ELISA) as described elsewhere [9]. The major criterion was the percentage of patients able to develop a protective response to HBV vaccination according to the definition >1 month after the last injection (i.e. M10). Minor criteria consisted in the evaluation of the kinetic and the strength of the response and the correlation to sCD40 clearing.
Fig. 4.
Study design. Fifteen patients were enrolled in the study. All of them were never dialyzed on PMMA membrane before, and none of them developed a protective antibody level to HBV vaccination despite one or more well-conducted vaccinations. Patients were dialysed 3 months on BK-2.1F membranes before the start of the vaccination (three intramuscular injections of HBVAXPRO 40 μg at months 3, 4 and 9). Patients continue dialysis on BK-F membrane until the end of the study (i.e. month 10). Blood samples for sCD40 measurements were taken just before the start of BK-F dialysis (month 0, M0) and the first vaccine injection (month 3, M3). Anti-HBV serology was performed at M3, M5, M9 and M10.
Results
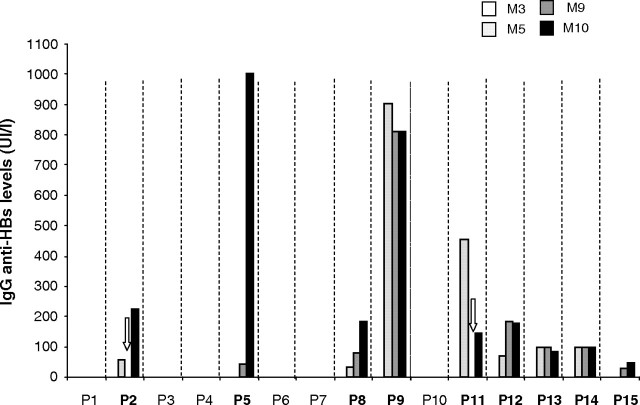
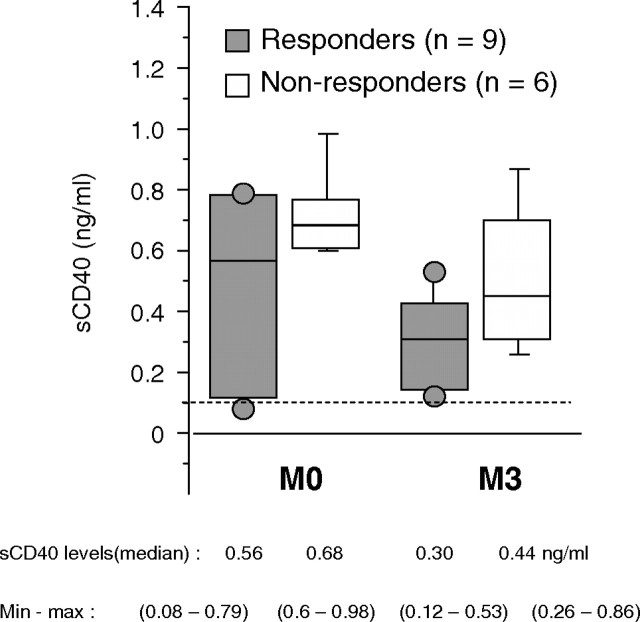
As depicted in Figure 5, nine (60%) of 15 patients were able to develop a protective immune response against HBV (anti-HBs antibody levels >10 IU/L) 1 month after the last vaccine injection. Of note, seven (78%) of nine patients exhibited anti-HBs antibody levels >100 IU/L, which is a crucial point because such levels allow a very good seroprotection against HBV. Moreover, for eight patients (89%), anti-HBV immunization is detectable as soon as M5, that is, 1 month after the second injection of vaccine. Among those patients, four already have levels >100 UI/L. For patients 5 and 9, no HBV infection (negative anti-HBc antibody and HBs antigen) was retrieved in spite of high anti-HBs antibody production confirming that it was indeed due to vaccination itself. We did not retrieve any difference neither in albuminaemia, C-reactive protein (CRP), parathyroid hormone (PTH) level, Kt/V nor total previous vaccine dose received between responder and non-responder patients (see Table 3). Results of the measurement of circulating sCD40 levels in the serum of responder and non-responder patients are shown in Figure 6. As seen, sCD40 levels drop down in both groups even if they did not reach normal ones; although non-statistically significant, there is a trend toward lower levels of the molecule in responder patients at M0 and M3 [median (range) M0 vs M3: 0.56 (0.08–0.79) vs 0.30 (0.12–0.53) for responders and 0.68 (0.6–0.98) vs 0.44 (0.26–0.86) for non-responders].
Fig. 5.
Longitudinal follow-up of anti-HBs antibody levels. The graph represents the results of anti-HBs antibody measurements (IU/L) at month 3 (M3), M5, M9 and M10 in the sera of the 15 patients (P1–P15) enrolled in the study. All patients’ serological tests were negative at M3 excluding a recent HBV infection. For patients 2 and 11 (P2 and P11), anti-HBV serology was not performed (white arrowheads). For the patients with levels of anti-HBs ≥1000 IU/L (P5 and P9), HBV detection was conducted by PCR and was negative. Responder patients according to the abovementioned criteria appear in bold (9/15).
Table 3.
Comparison between responder and non-responder groups
| Responders |
Non-responders |
Pa | |||
|---|---|---|---|---|---|
| Median | Min–max | Median | Min–max | ||
| Age | 69 | 49–85 | 75 | 62–86 | ns |
| Sex ratio (women/men) | 1.3 | 2 | ns | ||
| Length of dialysis (months) | 18 | 10–48 | 35.5 | 17–108 | ns |
| Weekly length of dialysis | 12 | 9–12.5 | 12 | 12–12 | ns |
| CRP | 7.5 | 3–38.3 | 9.15 | 1.8–38 | ns |
| Albumin | 36,6 | 25.2–39 | 32 | 23.1–36.4 | ns |
| PTH | 224 | 99–547 | 234.5 | 35–553 | ns |
| Kt/V | 1.365 | 1.17–1.63 | 1.355 | 1.09–1.56 | ns |
| Total vaccine dose received (μg) | 120 | 120–240 | 160 | 120–200 | ns |
Non-parametric Mann–Whitney U-test; ns, non-significant.
Fig. 6.
sCD40 levels before and 3 months after dialysis on BK-F membrane. Soluble CD40 levels were measured before the dialysis session at M0 and M3 by ELISA. The graph shows sCD40 levels in the responder group (dark grey) and the non-responder group (light grey) of patients. Median levels and range of sCD40 in each group are noted underneath.
Taken as a whole, these results emphasized the potential role of PMMA membrane in the improvement of humoral immune response of ESRD patients through its unique capacity to clear some uraemic toxins including sCD40.
Discussion
ESRD is characterized by a profound innate and acquired immunity deficiency. The leading cause is multifactorial based on uraemia and associated accumulating uraemic toxins, complications of chronic renal failure and the haemodialysis procedure. Better understanding of the molecules associated with altered immune response is crucial and could lead to therapeutical intervention and/or adaptation of the dialysis procedure itself to improve quality of life of ESRD patients.
It is now acknowledged that uraemia decreases antigen presentation abilities of dendritic cells and macrophages. Indeed, monocytes/macrophages exhibit a defective CD86 expression that hampers their co-stimulatory function toward T cells [17]. Impaired dendritic cells and macrophage co-stimulatory functions might be related to abnormal Toll-like receptor (TLR) stimulation notably through TLR4 whose expression is diminished in ESRD patients [18]. Moreover, high production of IL-12 by antigen-presenting cells and notably monocytes are involved in a Th1 bias with high IFN gamma production that is implicated in the activation of the cellular arm of the immune response detrimental to the humoral one classically linked to the Th2 polarization (IL4 and IL10) [19,20]. Interestingly, uraemic toxin such as PTH has been involved in the alteration of immunoglobulin production by B cells. As demonstrated by the Massry group, PTH inhibited IgG production by cultured B cells from HD patients, and the potential role of PTH hormone in altered humoral immune response to specific antigen was then confirmed with a model of influenza vaccination of parathyroidectomized rat [4,21].
In this line, we have evidenced a role for sCD40 in the alteration of humoral immune response in ESRD patients and notably in their impaired capacity of response to HBV vaccination [9]. This molecule is not eliminated by classical dialysis procedure, even on high-permeability polysulfone membranes, but on PMMA membrane (BK-2.1F). The mechanism responsible for sCD40 clearing was not demonstrated in our study, but one can speculate that it might be linked to its high adsorptive capacity of middle and high molecular weight molecules as recently demonstrated by a proteomic approach [22]. Of interest, our data emphasized that dialysis on BK-F membrane contributed to the amelioration of the seroconversion rate to HBV vaccination in ESRD patients who failed to mount a protective immune response despite numerous well-conducted vaccinations. However, the direct link between sCD40 clearing and seroconversion improvement was not formally demonstrated, in part because of the low number of patients included and the lack of control membrane unable to clear sCD40. That is why we are now starting a multicentric randomized clinical study in which seroconversion to HBV vaccination and measurements of sCD40 levels will be assessed in two groups of patients who did not respond to anterior HBV vaccination: one group (50 patients) will be dialysed on PMMA BK-2.1F membrane and the other (50 patients) on high-flux polysulfone membrane unable to clear sCD40.
Yet, dialysis procedure cannot be only seen as a replacement therapy. Because of the specific properties of some membranes, and notably those with high adsorptive capacities, one can speculate that they could contribute to the amelioration of adverse long-term clinical outcomes. In fact, use of PMMA membranes ameliorates pruritus because of the adsorption of a 160 000-kDa-molecular-weight molecule with stimulatory effect on mast cells [23]. Importantly, dialysis on PMMA membrane also improves carpal tunnel syndrome or total joint pain score compared with cellulosic membrane. As described by Yamada et al. [16], patients dialysed with PMMA membrane have lower need for erythropoietin that might be due to the elimination of an inhibitor of erythropoiesis retrieved in the dialysate.
Conclusions
Taken as a whole, these data associated to the already published papers on peculiar capacities of PMMA membranes reinforce the concept of seeing dialysis membranes not just as a simple diffusive device but a tool to tailor dialysis procedure to improve the global quality of life of ESRD patients. This opens a wide area of investigation, notably for the management of immunological dysfunction of ESRD patients.
Acknowledgments
We thank all the patients who accepted to participate in the study. We are indebted to Dr A. Pommereau for his contribution to the study and his helpful discussions.
Conflict of interest statement. None declared.
References
- 1.Eleftheriadis T, Antoniadi G, Liakopoulos V, Kartsios C, Stefanidis I. Disturbances of acquired immunity in hemodialysis patients. Semin Dial. 2007;20:440–451. doi: 10.1111/j.1525-139X.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 2.Kato S, Chmielewski M, Honda H, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3:1526–1533. doi: 10.2215/CJN.00950208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohler H, Arnold W, Renschin G, Dormeyer HH, Meyer zum Buschenfelde KH. Active hepatitis B vaccination of dialysis patients and medical staff. Kidney Int. 1984;25:124–128. doi: 10.1038/ki.1984.18. [DOI] [PubMed] [Google Scholar]
- 4.Gaciong Z, Alexiewicz JM, Linker-Israeli M, et al. Inhibition of immunoglobulin production by parathyroid hormone. Implications in chronic renal failure. Kidney Int. 1991;40:96–106. doi: 10.1038/ki.1991.186. [DOI] [PubMed] [Google Scholar]
- 5.Raskova J, Ghobrial I, Czerwinski DK, et al. B-cell activation and immunoregulation in end-stage renal disease patients receiving hemodialysis. Arch Intern Med. 1987;147:89–93. [PubMed] [Google Scholar]
- 6.Elgueta R, Benson MJ, de Vries VC, et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy M, Waldschmidt T, Aruffo A, Ledbetter JA, Noelle RJ. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J Immunol. 1993;151:2497–2510. [PubMed] [Google Scholar]
- 8.Contin C, Pitard V, Itai T, et al. Membrane-anchored CD40 is processed by the tumor necrosis factor-alpha-converting enzyme. Implications for CD40 signaling. J Biol Chem. 2003;278:32801–32809. doi: 10.1074/jbc.M209993200. [DOI] [PubMed] [Google Scholar]
- 9.Contin C, Pitard V, Delmas Y, et al. Potential role of soluble CD40 in the humoral immune response impairment of uraemic patients. Immunology. 2003;110:131–140. doi: 10.1046/j.1365-2567.2003.01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Kooten C, Gaillard C, Galizzi JP, et al. B cells regulate expression of CD40 ligand on activated T cells by lowering the mRNA level and through the release of soluble CD40. Eur J Immunol. 1994;24:787–792. doi: 10.1002/eji.1830240402. [DOI] [PubMed] [Google Scholar]
- 11.Schwabe RF, Engelmann H, Hess S, Fricke H. Soluble CD40 in the serum of healthy donors, patients with chronic renal failure, haemodialysis and chronic ambulatory peritoneal dialysis (CAPD) patients. Clin Exp Immunol. 1999;117:153–158. doi: 10.1046/j.1365-2249.1999.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aoike I, Gejyo F, Arakawa M. Learning from the Japanese Registry: how will we prevent long-term complications? Niigata Research Programme for beta 2-M Removal Membrane. Nephrol Dial Transplant. 1995;10:7–15. doi: 10.1093/ndt/10.supp7.7. [DOI] [PubMed] [Google Scholar]
- 13.Bonomini M, Fiederling B, Bucciarelli T, et al. A new polymethylmethacrylate membrane for hemodialysis. Int J Artif Organs. 1996;19:232–239. [PubMed] [Google Scholar]
- 14.Cohen G, Rudnicki M, Schmaldienst S, Hörl WH. Effect of dialysis on serum/plasma levels of free immunoglobulin light chains in end-stage renal disease patients. Nephrol Dial Transplant. 2002;17:879–883. doi: 10.1093/ndt/17.5.879. [DOI] [PubMed] [Google Scholar]
- 15.Galli F, Benedetti S, Buoncristiani U, et al. The effect of PMMA-based protein-leaking dialyzers on plasma homocysteine levels. Kidney Int. 2003;64:748–755. doi: 10.1046/j.1523-1755.2003.00134.x. [DOI] [PubMed] [Google Scholar]
- 16.Yamada S, Kataoka H, Kobayashi H, et al. Identification of an erythropoietic inhibitor from the dialysate collected in the hemodialysis with PMMA membrane (BK-F) Contrib Nephrol. 1999;125:159–172. doi: 10.1159/000059957. [DOI] [PubMed] [Google Scholar]
- 17.Girndt M, Sester M, Sester U, Kaul H, Kohler H. Defective expression of B7-2 (CD86) on monocytes of dialysis patients correlates to the uremia-associated immune defect. Kidney Int. 2001;59:1382–1389. doi: 10.1046/j.1523-1755.2001.0590041382.x. [DOI] [PubMed] [Google Scholar]
- 18.Kuroki Y, Tsuchida K, Go I, et al. A study of innate immunity in patients with end-stage renal disease: special reference to toll-like receptor-2 and -4 expression in peripheral blood monocytes of hemodialysis patients. Int J Mol Med. 2007;19:783–790. [PubMed] [Google Scholar]
- 19.Daichou Y, Kurashige S, Hashimoto S, Suzuki S. Characteristic cytokine products of Th1 and Th2 cells in hemodialysis patients. Nephron. 1999;83:237–245. doi: 10.1159/000045516. [DOI] [PubMed] [Google Scholar]
- 20.Sester U, Sester M, Hauk M, et al. T-cell activation follows Th1 rather than Th2 pattern in haemodialysis patients. Nephrol Dial Transplant. 2000;15:1217–1223. doi: 10.1093/ndt/15.8.1217. [DOI] [PubMed] [Google Scholar]
- 21.Gaciong Z, Alexiewicz JM, Massry SG. Impaired in vivo antibody production in CRF rats: role of secondary hyperparathyroidism. Kidney Int. 1991;40:862–867. doi: 10.1038/ki.1991.286. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa I, Chikazawa Y, Sato K, et al. Proteomic analysis of serum, outflow dialysate and adsorbed protein onto dialysis membranes (polysulfone and PMMA) during hemodialysis treatment using SELDI-TOF-MS. Am J Nephrol. 2006;26:372–380. doi: 10.1159/000094779. [DOI] [PubMed] [Google Scholar]
- 23.Aoike I. Clinical significance of protein adsorbable membranes—long-term clinical effects and analysis using a proteomic technique. Nephrol Dial Transplant. 2007;22:v13–19. doi: 10.1093/ndt/gfm295. [DOI] [PubMed] [Google Scholar]
- 24.Maisonneuve P, Agodoa L, Gellert R, et al. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet. 1999;354:93–99. doi: 10.1016/s0140-6736(99)06154-1. [DOI] [PubMed] [Google Scholar]
- 25.Winkelspecht B, Mueller-Lantzsch N, Kohler H. Serological evidence for reactivation of EBV infection due to uraemic immunodeficiency. Nephrol Dial Transplant. 1997;12:2099–2104. doi: 10.1093/ndt/12.10.2099. [DOI] [PubMed] [Google Scholar]
- 26.Fabrizi F, Poordad FF, Martin P. Hepatitis C infection and the patient with end-stage renal disease. Hepatology. 2002;36:3–10. doi: 10.1053/jhep.2002.34613. [DOI] [PubMed] [Google Scholar]
- 27.Girndt M, Kohler H. Hepatitis B virus infection in hemodialysis patients. Semin Nephrol. 2002;22:340–350. [PubMed] [Google Scholar]
- 28.Cunney RJ, Magee C, McNamara E, Smyth EG, Walshe J. Clostridium difficile colitis associated with chronic renal failure. Nephrol Dial Transplant. 1998;13:2842–2846. doi: 10.1093/ndt/13.11.2842. [DOI] [PubMed] [Google Scholar]
- 29.Chia S, Karim M, Elwood RK, FitzGerald JM. Risk of tuberculosis in dialysis patients: a population-based study. Int J Tuberc Lung Dis. 1998;2:989–991. [PubMed] [Google Scholar]
- 30.Crosnier J, Jungers P, Courouce AM, et al. Randomised placebo-controlled trial of hepatitis B surface antigen vaccine in French haemodialysis units: II. haemodialysis patients. Lancet. 1981;1:797–800. doi: 10.1016/s0140-6736(81)92679-9. [DOI] [PubMed] [Google Scholar]
- 31.Cappel R, Van Beers D, Liesnard C, Dratwa M. Impaired humoral and cell-mediated immune responses in dialyzed patients after influenza vaccination. Nephron. 1983;33:21–25. doi: 10.1159/000182898. [DOI] [PubMed] [Google Scholar]
- 32.Girndt M, Pietsch M, Kohler H. Tetanus immunization and its association to hepatitis B vaccination in patients with chronic renal failure. Am J Kidney Dis. 1995;26:454–460. doi: 10.1016/0272-6386(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 33.Kreft B, Klouche M, Kreft R, Kirchner H, Sack K. Low efficiency of active immunization against diphtheria in chronic hemodialysis patients. Kidney Int. 1997;52:212–216. doi: 10.1038/ki.1997.322. [DOI] [PubMed] [Google Scholar]
- 34.Docci D, Cipolloni PA, Baldrati L, et al. Immune response to a recombinant hepatitis B vaccine in hemodialysis patients. Int J Artif Organs. 1990;13:451–453. [PubMed] [Google Scholar]
- 35.Smit-Leijs MB, Kramer P, Heijtink RA, Hop WC, Schalm SW. Hepatitis B vaccination of haemodialysis patients: randomized controlled trial comparing plasma-derived vaccine with and without pre-S2 antigen. Eur J Clin Invest. 1990;20:540–545. doi: 10.1111/j.1365-2362.1990.tb01899.x. [DOI] [PubMed] [Google Scholar]
- 36.Fleming SJ, Moran DM, Cooksley WG, Faoagali JL. Poor response to a recombinant hepatitis B vaccine in dialysis patients. J Infect. 1991;22:251–257. doi: 10.1016/s0163-4453(05)80007-6. [DOI] [PubMed] [Google Scholar]
- 37.Allegra V, Vasile A, Maschio M, Mengozzi G. Immune response after vaccination with recombinant hepatitis surface antigen in maintenance hemodialysis patients and healthy controls. Nephron. 1992;61:339–340. doi: 10.1159/000186932. [DOI] [PubMed] [Google Scholar]
- 38.Carletti P, Bibiano L, Boggi R, et al. HBV infection in hemodialysis patients: monitoring and prevention. Nephron. 1992;61:269–270. doi: 10.1159/000186901. [DOI] [PubMed] [Google Scholar]
- 39.Fabrizi F, Di Filippo S, Marcelli D, et al. Recombinant hepatitis B vaccine use in chronic hemodialysis patients. Long-term evaluation and cost-effectiveness analysis. Nephron. 1996;72:536–543. doi: 10.1159/000188935. [DOI] [PubMed] [Google Scholar]
- 40.Elwell RJ, Neumann M, Bailie GR. Factors associated with long-term antibody production induced by hepatitis B vaccine in patients undergoing hemodialysis: a retrospective cohort study. Pharmacotherapy. 2003;23:1558–1563. doi: 10.1592/phco.23.15.1558.31954. [DOI] [PubMed] [Google Scholar]
- 41.Donati D, Gastaldi L. Controlled trial of thymopentin in hemodialysis patients who fail to respond to hepatitis B vaccination. Nephron. 1988;50:133–136. doi: 10.1159/000185143. [DOI] [PubMed] [Google Scholar]
- 42.Fabrizi F, Andrulli S, Bacchini G, Corti M, Locatelli F. Intradermal versus intramuscular hepatitis b re-vaccination in non-responsive chronic dialysis patients: a prospective randomized study with cost-effectiveness evaluation. Nephrol Dial Transplant. 1997;12:1204–1211. doi: 10.1093/ndt/12.6.1204. [DOI] [PubMed] [Google Scholar]