Abstract
High-quality dialysis does not always mean high efficiency; dialysis should maintain nutritional balance and full biocompatibility. In undergoing treatment with polymethylmethacrylate (PMMA) and/or ethylene vinyl alcohol copolymer (EVAL) membrane dialysers, the body weight decrease caused by polysulfone membrane has been dramatically improved for those receiving predilution online haemodiafiltration (HDF) as well. These membranes are assumed to somewhat suppress the clearance of small molecular weight substances to maintain an exquisite balance of broadly removing low molecular weight proteins. This removal process is assumed to be extremely close to the balance maintained by the clearing characteristics of the kidneys. On the other hand, fluid purification is also one of the important factors in high-quality dialysis. Bacteriological contamination of dialysis fluid is one of the serious factors that deteriorate the biocompatibility of dialysis therapy.
From this therapeutic concept, the patient survival rate is excellent in our facilities: the 1-year survival rate is 91.1% and the 5-year survival rate is 76.6%, although the mean age of our patients is 69 years. We usually adjust the therapeutic modality based on patient complaints, and we call this concept ‘patient-oriented dialysis’ (POD). In the POD system, the prevalence of uraemic pruritus or sleep disturbances was lower than that of the DOPPS. The protein-leaking dialysis modalities with PMMA, EVAL or predilution online HDF form the key concept in the POD system.
Keywords: biocompatibility, nutrition, PMMA, POD system
Introduction
The number of chronic dialysis patients has risen to >280 000 in Japan. The growing concerns for dialysis treatment are increasing for the elderly, diabetics and long-term dialysis patients. The Japanese Society for Dialysis Therapy (JSDT) revealed that the mean age of dialysis patients was 65.3 years, the percentage of diabetic patients was 43.2% and 25.9% of patients had been receiving dialysis for >10 years [1]. Consequently, the goal of dialysis treatment has changed from saving the lives of patients with kidney failure in the 1960s and 1970s to the prevention of such complications as dialysis-related amyloidosis (DRA) in the 1980s and the 1990s. In the mid-1980s, beta-2 microglobulin (β2MG) was identified as the origin of DRA [2]. Since then, various synthetic membranes with high permeability (HPM) have been developed to effectively remove β2MG and to prevent DRA. HPM has been developed concomitantly with the purification of dialysis fluid, and both have actually delayed the onset of DRA [3,4]. In the last decade, inflammation caused by comorbidity and bioincompatibility of extracorporeal circulation are the most remarkable issues. This inflammation is now believed to be the cause of a specific variety of dialysis-related episodes such as malnutrition, inflammation and atherosclerosis (MIA) syndrome [5], resistance to erythropoiesis-stimulating agents and DRA [6]. Currently, the aim of chronic dialysis therapy is the prevention of MIA syndrome and increasing the quality of life for the patient.
Some patients in our facilities frequently complain of itching, irritability, depression, disturbed sleep and other discomforts. These symptoms are serious problems for the patient because they deteriorate the quality of life. Some of these symptoms are known as significant predictors of patient mortality [7,8]; however, no uraemic toxins have been identified as associated with these uncomfortable symptoms [9]. There is nothing written to tell us how to treat these symptoms. Many nephrologists do not regularly ask the patients about their feelings toward their dialysis, such as ‘How do you feel about this dialysis membrane?’ or ‘How have your feelings changed after switching from haemodialysis (HD) to haemodiafiltration (HDF)?’ If we ask them about their feelings, they would give us a variety of important suggestions. Many patients in our facilities favoured dialysers made of polymethylmethacrylate (PMMA), ethylene vinyl alcohol copolymer (EVAL), polyacrylonitrile (PAN; AN69) or predilution online HDF mode. Why do they love these therapeutic prescriptions? Is there any rationale as to why?
With this issue, we would like to consider the relationship between the patient’s preferences in relation to the characteristics of dialysis quality and, finally, propose a method to prevent MIA syndrome and improve the discomfort experienced by dialysis patients.
What is the golden target of dialysis?
The golden target for dialysis treatment should guarantee longer survival and a higher quality of life without dialysis-related complications. We believe that high-quality dialysis could achieve this golden target, but what kind of quality is important? There are several elements for evaluating the quality of dialysis, such as dialysis dose, efficacy of uraemic solute removal and biocompatibility. Some of these parameters are assessed by urea kinetic models, removal rates of a uraemic solute and other biochemical markers. However, we have not yet had a perfect answer to the question, ‘What is a high-quality dialysis?’ or ‘What is the most reliable parameter in evaluating the quality of dialysis?’
There have been many parameters for evaluating the adequacy of dialysis doses, such as Kt/V urea, normalized protein catabolic rate (nPCR), and serum level of β2MG. C-reactive protein, interleukin 6 and other inflammatory mediators have also been reported to predict patient mortality. All of these parameters are evaluated in relation to patient survival. Actually, there are no doubts that a good dialysis allows the patients to live longer; however, it is not easy to decide whether ongoing dialysis sessions are beneficial.
Kt/V is one of the most frequently used parameters in determining the adequacy of dialysis because it is simple to calculate and gives some insight into the assessment of dialysis patient survival. Kt/V had consisted of a dialysis dose standardized by body size; however, Kt/V is still dependent on body mass. If we evaluate the adequacy of dialysis only by Kt/V, it would contradict the report that smaller-sized women or elderly patients are easily undertreated [10]. The Dialysis Outcomes and Practice Pattern Study (DOPPS) has not yet clarified the reason why patient survival in Japan has been so excellent, although the mean Kt/V is markedly lower [11]. These issues suggest that high Kt/V does not always lead to increased rates of patient survival and cannot be the golden target of high-quality dialysis.
β2MG is the most important and the only low molecular weight protein (LMWP) that has been proven a uraemic toxin, that is, leading to dialysis-related complications. In the past two decades, various types of dialysis membranes have been produced to effectively remove β2MG and prevent DRA. Some studies proposed that the serum level of β2MG was one of the effective predictors of patient survival and one of the parameters in assessing the quality of dialysis [12,13]. However, it remains controversial whether more β2MG removal could result in longer patient survival. The serum level of β2MG is affected not only by the efficacy of dialysis but also by residual renal function, dialysis vintage, and nutritional and inflammatory status [14]; so the serum level of β2MG cannot be the sole predictor of patient survival and the golden target of dialysis.
Body mass has been recognized as one of the most powerful predictors of patient survival in dialysis patients [15–17]. It is generally accepted because comorbidity and inflammatory complications cause patients to lose body mass, which then shortens survival. In these lines of evidence, maintaining body mass is a solo and indispensable parameter in assessing the quality of dialysis. If we could completely prevent muscle loss, we could ensure longer patient survival without complications.
As previously addressed, chronic dialysis patients have a variety of uncomfortable symptoms related to dialysis, such as pruritus, irritability, depression, insomnia and intradialytic hypotension. Although some of these symptoms have been clarified as a risk of death and deterioration in the quality of life, we do not yet have any parameters concerning the patient’s symptoms for evaluation of the quality of dialysis. Moreover, we have not had developed sufficient measures to improve all of these symptoms. In this article, we would like to propose that the patient’s feelings could be important parameters in evaluating the quality of dialysis. In other words, the dialysis prescription by which dialysis patients feel comfortable is the most reliable definition of high-quality dialysis.
Dialysis fluid quality in Japan
Bioincompatibility of dialysis therapy is supposed to cause chronic inflammatory responses inside dialysis patients and lead to dialysis-related complications like MIA syndrome and DRA. Bacteriological contamination of dialysis fluid is one of the important factors that reduce the biocompatibility of dialysis therapy [4]. Endotoxin fragments, peptidoglycan and bacterial DNA can easily pass through the dialysis membrane from dialysis fluid to blood and cause the inflammatory response. The more permeable the dialysis membrane is, the higher the risk of contamination would be. Many clinical effects of purified dialysis fluid have been reported, such as the retardation of the onset of DRA [18], the improvement of erythropoietin-resistant anaemia [19], and the improvement of inflammation and nutritional status [20]. The quality of purified dialysis fluid has become an indispensable factor in the prevention of MIA syndrome. We should purify the dialysis fluid when we use HPM. The JSDT surveyed the bacteriological quality of dialysis fluid and the management of quality control [21]. The JSDT standard for dialysis fluid is <0.050 EU/mL of endotoxin and a bacterial count <100 CFU/mL, which is the strictest in the world [22]. The standard was achieved in 89% of facilities for endotoxin and in 97% of facilities for bacterial count [22]. The survival rates were compared by level of endotoxin present in the dialysis fluid, and the survival rate was significantly higher for the group with <0.001 EU/mL compared with the one with 0.100–0.250 EU/mL [23]. As previously pointed out, although the dialysis dose as measured by Kt/V is lower in Japan, the survival rate for dialysis patients in Japan is excellent. The reason why the Japanese survival rate is so high compared with the rest of the world has not yet been clarified. The high bacteriological quality of dialysis fluid might be one important reason for such excellent results in Japan.
Solute removal pattern and nutritional status
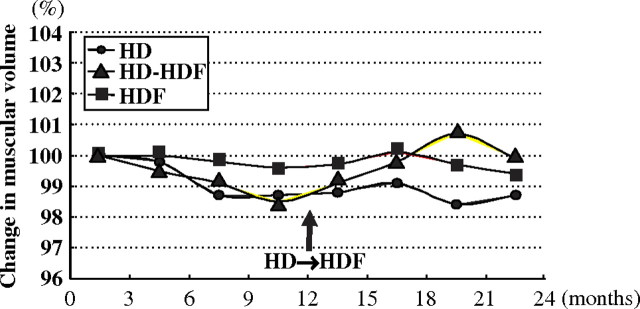
There are several reports stating that maintaining body mass had a beneficial effect on dialysis patient survival [15–17], but there have been only a few reports that clarified how to practically prevent the loss of body mass through dialysis. In the preliminary study, we proposed online HDF to maintain the muscle volume of dialysis patients [24] (Figure 1). The muscle volume calculated by bioelectrical impedance analysis gradually declined for 2 years in HD patients but was well preserved in online HDF patients. Patients who switched from HD to online HDF increased muscle volume just after the switch. Almost all of the online HDFs were performed with the predilution method. We compared the muscle volume change between predilution and postdilution, and the muscle volume was better preserved in predilution than in postdilution (data not shown).
Fig. 1.
Changes in muscular volume in HD and online HDF patients. The muscular volumes of HD patients gradually decreased for 2 years, but those of online HDF patients were well preserved. In switching from HD to online HDF, the muscular volumes of patients increased just after the switch.
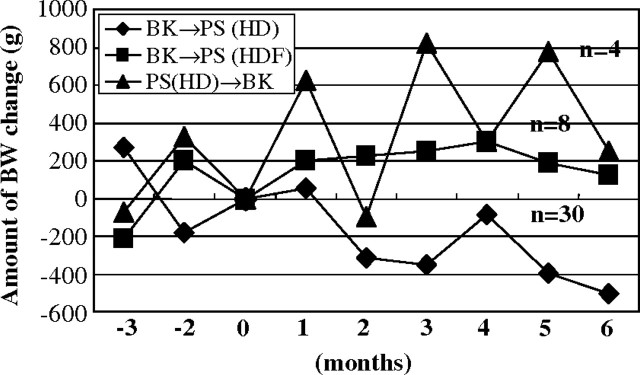
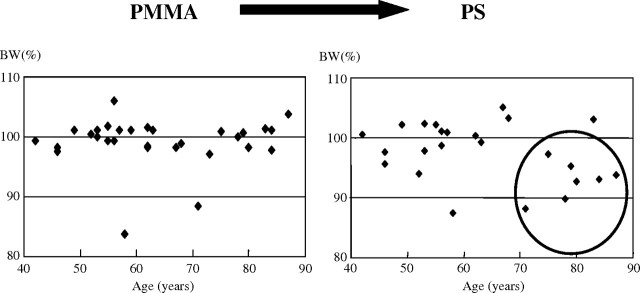
Muta et al. reported that body mass reduction observed in HD with PS membrane dramatically improved with the change in the dialysis membrane to EVAL in elderly dialysis patients [25]. Their hypothesis for the advantage of the EVAL membrane in maintaining muscle volume was that the loss of amino acids during a dialysis session was milder in HD with the EVAL membrane. Our similar study about body mass changes in PMMA HD revealed quite the same result [26]. The patients who switched from PMMA to PS membrane remarkably reduced body weight, but the patients who switched from PS to PMMA membrane or from PMMA HD to predilution online HDF with PS membrane maintained body weight (Figure 2). Body weight loss was more severe in elderly dialysis patients than in young patients (Figure 3). Similar results were reported in AN69 (personal communication). These interesting issues suggest that patients prefer some dialysis membranes or predilution online HDF that enable the patients to keep their body mass.
Fig. 2.
Amount of body weight change by switching dialysis membrane. By switching from PMMA to PS, the body weight of HD patients remarkably decreased. However, by switching from PS to PMMA or predilution online HDF, the patients gained body weight.
Fig. 3.
Changes in body weight of each patient for a year before switching from PMMA to PS (left) and after switching from PMMA to PS (right). By switching from PMMA to PS, body weight of elderly dialysis patients >70 years decreased.
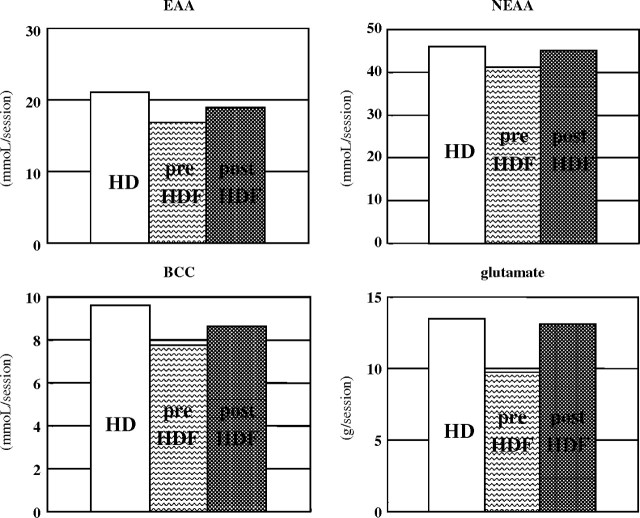
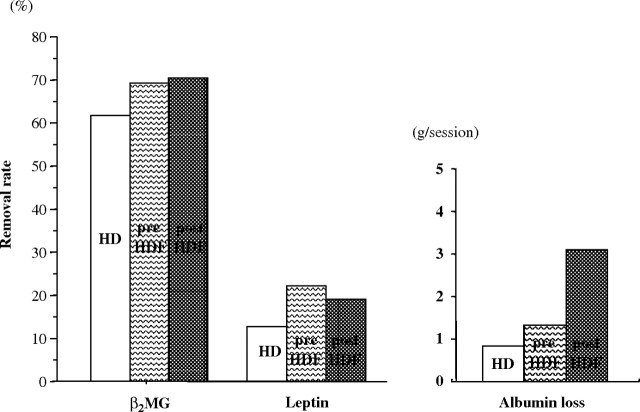
To clarify why predilution HDF has a nutritional advantage, we compared the solute removal pattern between HD, predilution online HDF and postdilution online HDF modes. All therapies were performed using PS membranes (TS-1.8UL, Toray Industries, Inc.) at a blood flow rate of 270–300 mL/min, and the total volume of substitution fluid per session was 48–72 L in predilution mode and 12–18 L in postdilution mode. In predilution online HDF mode, it has been widely assumed that small solute removal is lower than that in HD mode because of the decreased osmotic pressure gradient from the diluted plasma and the slowed dialysis fluid flow rate. In our study, small solute removal was reduced, but amino acids with similar molecular weights to creatinine were better preserved in predilution online HDF mode than in HD or postdilution HDF modes (Figure 4). On the other hand, LMWPs, such as β2MG (MW: 12 kDa) or leptin (MW: 16 kDa), were effectively removed in HDF mode, especially in predilution HDF mode in our therapeutic prescription. Albumin loss per session was 0.8 g in HD mode, 1.3 g in predilution HDF mode and 3.1 g in postdilution HDF mode (Figure 5). In proteomic analysis, peaks of protein around 16 kDa were well reduced after a session in predilution online HDF mode but were enhanced after a session in HD or postdilution HDF mode (data not shown). Leptin has been classified into both uraemic toxin groups: protein-bound solute toxins and middle molecules [9]. One of the most typical protein-bound solutes is p-cresol, and it was effectively removed by predilution online HDF, based advantageously on the dilution of serum in predilution [27]. The same mechanism was supposed to enhance greater removal of leptin in predilution HDF mode than HD or postdilution HDF mode. LMWPs and a little albumin were effectively removed by convection in HDF, large pore size in EVAL membranes, or protein adsorptive property in PMMA or PAN membranes. This broad removal pattern of the dialysis membrane or predilution online HDF mode might be similar to the native kidneys and has an advantage in maintaining body mass in dialysis patients.
Fig. 4.
Amino acid loss through one session. Amino acid loss was less in predilution HDF mode than in HD or postdilution HDF mode.
Fig. 5.
Removal rates of β2MG and leptin, and albumin loss. Predilution HDF mode has higher removability of β2MG or leptin than HD mode and less albumin loss than postdilution HDF mode.
With the native kidney, the clearance of urea is ∼60 mL/min, which is smaller than most dialysis membranes (almost 200 mL/min). Large amounts of nutrients, such as amino acids or carnitine, were filtered by glomeruli, but almost all were retrieved by the proximal tubules. The more efficiently we tried to remove small solutes, the more we lost the small solute nutrients. The small solute clearance of EVAL, PMMA, PAN membranes or predilution online HDF mode was lower than that of HD mode with PS. LMWP and partial albumin were filtered by glomeruli, reabsorbed and catabolized by the proximal renal tubules. The molecular weight of inflammatory cytokines related to MIA syndrome was ∼15–30 kDa, and that of leptin, which is recognized as a uraemic substance, was 16 kDa. Albumin was partially deteriorated in uraemic milieu because oxidative stress and uraemic toxins deteriorated the nature of albumin [28]. If renal failure progresses, inflammatory cytokines and deteriorated albumin would accumulate inside the body. The accumulation of inflammatory elements is the key concept behind MIA syndrome and chronic kidney disease. Large molecular weight uraemic toxins or protein-conjugated uraemic toxins were supposed to suppress erythropoiesis. Protein-permeable dialysis by EVAL and PMMA membranes reduced the resistance to erythropoiesis-stimulating agents [29,30]. Native kidneys act not only as a filter of small molecular weight substances but also play an important role as a metabolic organ for LMWPs or some albumin.
Biocompatibility of dialysers
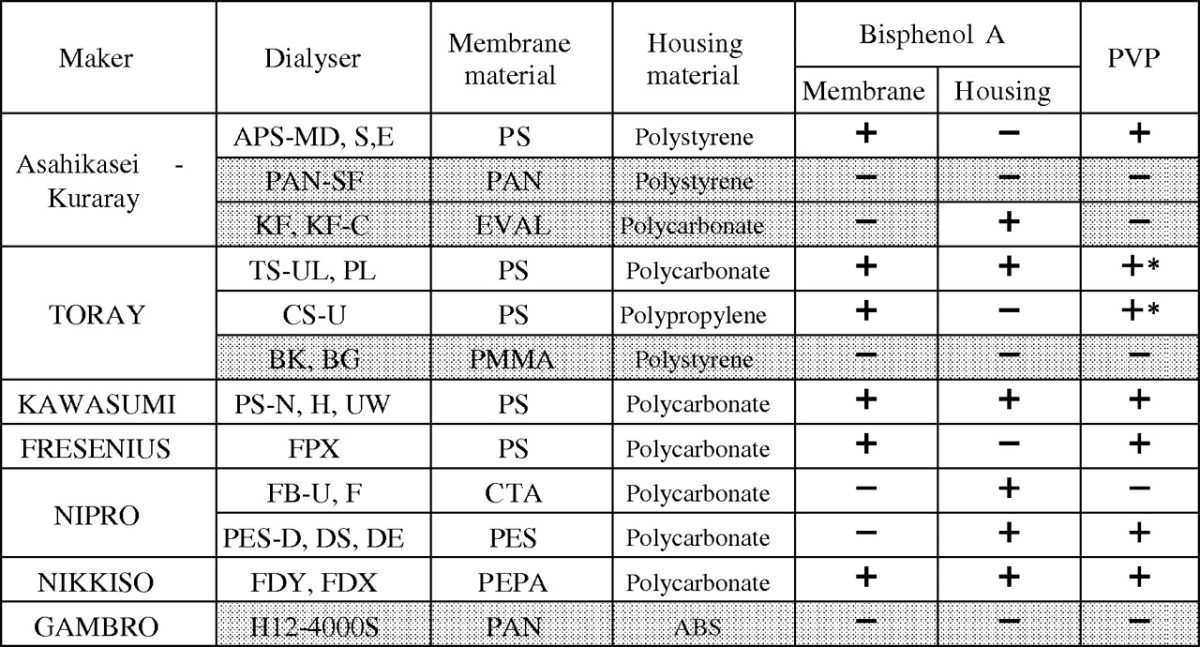
PMMA, EVAL, AN69 membranes and predilution online HDF mode have similar characteristics in solute removal patterns, which could contribute to the nutritional advantages of these therapies. One more common characteristic of these therapies is the biocompatibility based on the chemical property of the membrane. Polyvinylpyrrolidone (PVP) is a chemical agent that gives the hydrophilicity to hydrophobic products, so it is widely used to make many products such as beverages, soft contact lenses, povidone iodide (which is most frequently used as a bactericidal agent) and many synthetic dialysis membranes. PVP is an indispensable component in PS, polyethersulphone and many other synthetic membranes. Bisphenol-A is an essential element in the manufacture of plastics and polycarbonates, which are widely used for dialyser housing material. However, bisphenol-A is also well known as an environmental hormone or endocrine disrupter. There are many dialysis membranes that contain PVP or bisphenol-A, but some membranes do not (Table 1). PS is most widely used as a dialysis membrane material throughout the world; however, some recent studies have suggested that PS has some uncomfortable side effects, such as anaphylaxis, skin lesions and thrombocytopenia, which are supposedly caused by PVP [31,32]. Just after PS was changed to dialysis membranes that did not contain PVP or bisphenol-A, the symptoms disappeared, which was why PVP or bisphenol-A was believed to be related to these complications. In predilution HDF, the blood is more diluted before the dialyser, and a large amount of fluid is filtered from the blood side to the dialysis fluid side. If PVP or other chemical components from the dialyser were extracted, a large amount of fluid could wash these substances out to the dialysis fluid side. Much diluted blood in predilution HDF would reduce the close contact between blood cells and the dialysis membrane. We can say that the predilution online HDF is more biocompatible than HD and postdilution HDF. Surprisingly, our patients chose the therapies with PMMA, EVAL, AN69 membranes and predilution online HDF mode without any knowledge of the chemical components of dialysis membranes. These therapies are free from the influence of PVP or bisphenol-A.
Table 1.
Bisphenol-A and PVP included in dialysers in Japan. Some dialysers have neither bisphenol-A nor PVP. *Cross-linked PVP exists in Toray PS membranes
The results of patient-oriented dialysis system: POD system
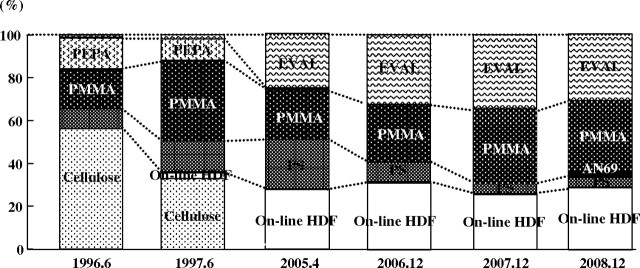
Dialysis membranes and HDF were nutritionally advantageous with good biocompatibility, which are common characteristics that patients preferred. From these findings, we established that the definition of a good dialysis was one free of uncomfortable dialysis-related symptoms and with no loss of body mass. If we want good dialysis, we have to consider the patient’s body mass, their feelings about dialysis and their daily lives. We named this therapeutic concept ‘the patient-oriented dialysis system: the POD system,’ and we have treated dialysis patients according to this concept since 2001. There are two basic tests that we perform twice a year in the POD system. The POD sheet has 36 questions about the quality of life and dialysis-related symptoms. The malnutrition inflammation score (MIS) sheet was originally composed by Kalanter-Zadeh as an assessment tool to screen nutritional status [16]. If any problems are indicated on the POD sheet and the MIS sheet, dialysis therapies and nutritional approaches will be reconsidered and changed. Under this therapeutic concept, the choice of dialysis membranes and online HDF mode are a major key to achieving good dialysis. More than 90% of our patients have been treated by EVAL, PMMA, AN69 membranes and predilution online HDF mode; in particular, EVAL was used for all new dialysis patients (Figure 6).
Fig. 6.
Changes in the selection of dialysis membranes (in HD mode) or HDF mode (with PS membrane) in our facilities. Recently, the majority has been PMMA, EVAL and online HDF.
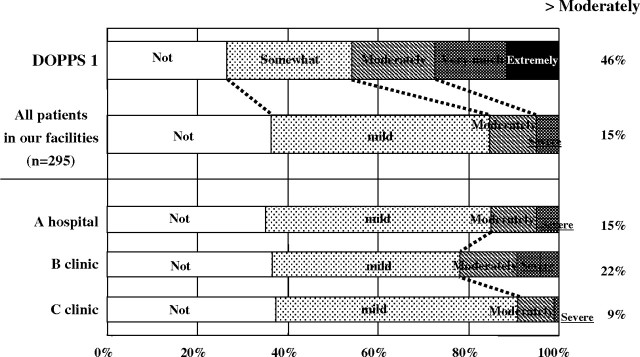
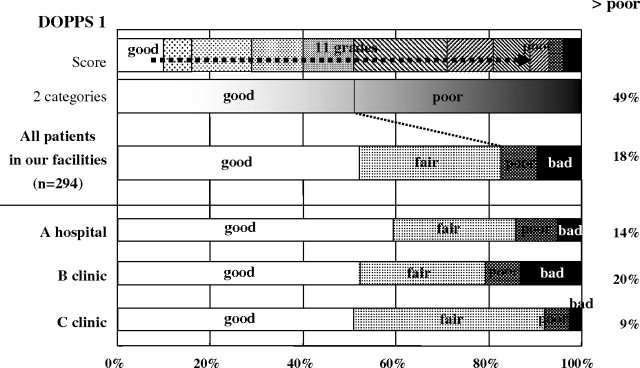
Uraemic pruritus was one of the most frequent symptoms and was recognized as associated with the risk of higher mortality and sleep disturbances. The prevalence of more than moderate itching was reported to be relatively high, 40–50% [7,33], but only 15% of patients complained about itching in our facilities (Figure 7). The prevalence of disturbed sleep reported as ‘poor’ or ‘bad’ was 18% and less frequent than DOPPS by one-third [34] (Figure 8).
Fig. 7.
Prevalence of pruritus in dialysis patients. Pruritus in our facilities was less than that of DOPPS I.
Fig. 8.
Prevalence of disturbed sleep in dialysis patients. Sleep disturbances in our facilities was also less than that of DOPPS I.
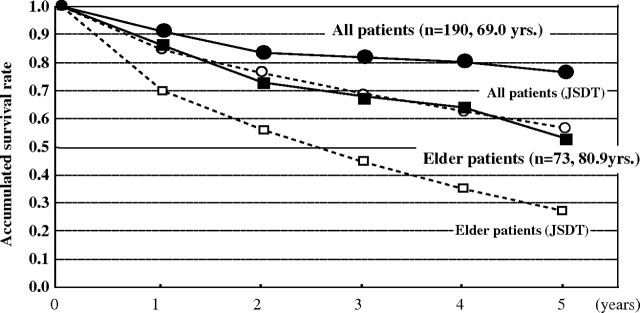
One hundred and ninety patients started chronic dialysis in our facilities from 2001 to 2007. The 5-year survival rate was calculated by the Kaplan–Meier method and compared with that of the JSDT. The accumulated 5-year survival rate was 77% in our facilities and 57% in the JSDT, although the mean age of patients was 69 years, which was 3 years older than of the mean age for the JSDT [35]. The 5-year survival rate for elderly patients was 52% in our facilities and 27% in the JSDT [35] (Figure 9). The POD system enables chronic dialysis patients to live longer without uncomfortable dialysis-related symptoms [36].
Fig. 9.
Survival rates of dialysis patients. Survival rates in our facilities were higher than that in the JSDT report.
Conclusion
Our aim was to find the golden target and whether patients' feelings about dialysis could be considered as a target in prescribing dialysis. Various parameters based on an analysis of patient survival were multifactorial and not based on a sole parameter for evaluating the quality of dialysis. In our facilities, the POD system was used to maintain body mass and to relieve uncomfortable dialysis-related symptoms. The prevalence of pruritus and disturbed sleep was significantly lower than in previous reports, and our survival rate was excellent. From these experiences, we concluded that the golden target of dialysis therapy is maintaining body mass and freedom from dialysis-related symptoms. To achieve this golden target, the choice of PMMA, EVAL, AN69 or predilution HDF is an important factor. It is easy to provide a good dialysis prescription for an individual patient purely by listening to their feelings about daily dialysis.
Conflict of interest statement. None declared.
References
- 1.Tsubakihara Y. An overview of regular dialysis treatment in Japan as of Dec. 31, 2006. Tokyo: Japanese Society for Dialysis Therapy; 2007. p. 3. [Google Scholar]
- 2.Gejyo F, Odani S, Yamada T, et al. Beta 2-microglobulin: a new form of amyloid protein associated with chronic hemodialysis. Kidney Int. 1986;30:385–390. doi: 10.1038/ki.1986.196. [DOI] [PubMed] [Google Scholar]
- 3.Koda Y, Nishi S, Miyazaki S, et al. Switch from conventional to high-flux membrane reduces the risk of carpal tunnel syndrome and mortality of hemodialysis patients. Kidney Int. 1997;52:1096–1101. doi: 10.1038/ki.1997.434. [DOI] [PubMed] [Google Scholar]
- 4.Masakane I. Review: Clinical usefulness of ultrapure dialysate—recent evidence and perspectives. Ther Apher Dial. 2006;10:348–354. doi: 10.1111/j.1744-9987.2006.00388.x. [DOI] [PubMed] [Google Scholar]
- 5.Stenvinkel P, Heimuburger O, Paultre F, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–1911. doi: 10.1046/j.1523-1755.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 6.Colton CK, Ward RA, Shaldon S. Scientific basis for assessment of biocompatibility in extracorporeal blood treatment. Nephrol Dial Transplant. 1994;9:11–17. [PubMed] [Google Scholar]
- 7.Pisoni R, Wirkstoem B, Akizawa T, et al. Pruritus in haemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2006;21:3495–3505. doi: 10.1093/ndt/gfl461. [DOI] [PubMed] [Google Scholar]
- 8.Lopes AA, Albert JM, Young EW, et al. Screening for depression in hemodialysis patients: associations with diagnosis, treatment, and outcomes in the DOPPS. Kidney Int. 2004;66:2047–2053. doi: 10.1111/j.1523-1755.2004.00977.x. [DOI] [PubMed] [Google Scholar]
- 9.Vanholder R, De Smet R, Glorieux G, et al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63:1934–1943. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- 10.Lowrie EG, Zhu X, Lew NL. Primary associates of mortality among dialysis patients: trends and reassessment of Kt/V and urea reduction ratio as outcome-based measures of dialysis dose. Am J Kidney Dis. 1998;32:S16–S31. doi: 10.1016/s0272-6386(98)70158-1. [DOI] [PubMed] [Google Scholar]
- 11.Goodkin DA, Bragg-Gresham JL, Koenig KG, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS) J Am Soc Nephrol. 2003;14:3270–3277. doi: 10.1097/01.asn.0000100127.54107.57. [DOI] [PubMed] [Google Scholar]
- 12.Cheung AK, Rocco MV, Yan G, et al. Serum beta-2 microglobulin levels predict mortality in dialysis patients: results of the HEMO study. J Am Soc Nephrol. 2006;17:546–555. doi: 10.1681/ASN.2005020132. [DOI] [PubMed] [Google Scholar]
- 13.Okuno S, Ishimura E, Kohno K, et al. Serum beta2-microglobulin level is a significant predictor of mortality in maintenance haemodialysis patients. Nephrol Dial Transplant. 2009;24:571–577. doi: 10.1093/ndt/gfn521. [DOI] [PubMed] [Google Scholar]
- 14.Vincent C, Revillard JP, Galland M, et al. Serum beta2-microglobulin in hemodialyzed patients. Nephron. 1978;21:260–268. doi: 10.1159/000181402. [DOI] [PubMed] [Google Scholar]
- 15.Kopple JD, Zhu X, Lew NL, Lowrie EG. Body weight-for-height relationships predict mortality in maintenance hemodialysis patients. Kidney Int. 1999;56:1136–1148. doi: 10.1046/j.1523-1755.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 16.Kalantar-Zadeh K, Kopple JD, Block G, et al. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38:1251–1263. doi: 10.1053/ajkd.2001.29222. [DOI] [PubMed] [Google Scholar]
- 17.Port FK, Ashby VB, Dhingra RK, Rogs EC, Wolfe RA. Dialysis dose and body mass index are strongly associated with survival in hemodialysis patients. J Am Soc Nephrol. 2002;13:1061–1066. doi: 10.1681/ASN.V1341061. [DOI] [PubMed] [Google Scholar]
- 18.Baz M, Durand C, Ragon A, et al. Using ultrapure water in hemodialysis delays carpal tunnel syndrome. Int J Artif Organs. 1991;14:681–685. [PubMed] [Google Scholar]
- 19.Schiffl H, Lang SM, Bergner A. Ultrapure dialysate reduces dose of recombinant human erythropoietin. Nephron. 1999;83:278–279. doi: 10.1159/000045525. [DOI] [PubMed] [Google Scholar]
- 20.Schiffl H, Lang SM, Stratakis D, et al. Effects of ultrapure dialysate fluid on nutritional status and inflammatory parameters. Nephrol Dial Transplant. 2001;16:1863–1869. doi: 10.1093/ndt/16.9.1863. [DOI] [PubMed] [Google Scholar]
- 21.Masakane I, Tsubakihara Y, Akiba T, et al. Bacteriological qualities of dialysis fluid in Japan as of 31 December 2006. Ther Apher Dial. 2008;12:457–463. doi: 10.1111/j.1744-9987.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- 22.Kawanisi H, Akiba T, Masakane I, et al. The standard on bacterial management of fluids for hemodialysis and related therapies from Japanese Society for Dialysis Therapy (JSDT) 2008. Ther Apher Dial. 2009;13:161–166. doi: 10.1111/j.1744-9987.2009.00674.x. [DOI] [PubMed] [Google Scholar]
- 23.Masakane I, Nakai S, Akiba T, et al. Bacteriological water quality has a strong impact on the outcome of chronic dialyzed patients. J Am Soc Nephrol. 2008;19:693A. [Google Scholar]
- 24.Masakane I, Seki H, Atsumi R, et al. On-line hemodiafiltration is effective to keep muscle volume in CKD patients. Blood Purif. 2005;23:404. [Google Scholar]
- 25.Muta T, Fujimoto T, Harada Y, et al. Are there any differences on amino acid loss during dialysis session by the dialysis membrane material? Kidney and Dial 59 (suppl) High Performance Membrane ’05. 2005:241–244. (in Japanese) [Google Scholar]
- 26.Masakane I, Kaneda H, Esashi S. PMMA is more desirable for elder dialysis patients than PS. Kidney and Dial 63 (suppl) High Performance Membrane ’07. 2007:202–203. (in Japanese) [Google Scholar]
- 27.Bamments B, Evnepoel P, Verbeke K, et al. Removal of the protein-bound solute p-cresol by convective transport: a randomized crossover study. Am J Kidney Dis. 2004;44:278–285. doi: 10.1053/j.ajkd.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 28.Himmelfarb J, McMonagle E. Albumin is the major plasma protein target of oxidant stress in uremia. Kidney Int. 2001;60:358–363. doi: 10.1046/j.1523-1755.2001.00807.x. [DOI] [PubMed] [Google Scholar]
- 29.Saito A, Suzuki L, Chung TG, et al. Separation of an inhibitor of erythropoiesis in “Middle molecules” from hemodialysis from patients with chronic renal failure. Clin Chem. 1986;32:1938–1941. [PubMed] [Google Scholar]
- 30.Yamada S, Kataoka H, Kobayashi H, Ono T, Minakuchi J, Kawano Y. Identification of erythropoietic inhibitor from the dialysate collected in the hemodialysis with PMMA membrane (BK-F) and its clinical effects. Contrib Nephrol. 1998;125:159–172. doi: 10.1159/000059957. [DOI] [PubMed] [Google Scholar]
- 31.Ohashi N, Yonemura K, Goto T, et al. A case of anaphylactoid shock induced by the BS polysulfone hemodialyzer but not by the F8-HPS polysulfone hemodialyzer. Clin Nephrol. 2003;60:214–217. doi: 10.5414/cnp60214. [DOI] [PubMed] [Google Scholar]
- 32.Shin S, Nishioka M, Shinko S, et al. Side effects caused by PS membrane. Kidney and Dial 63 (suppl) High Performance Membrane ’07. 2007:275–279. (in Japanese) [Google Scholar]
- 33.Narita I, Alchi B, Omori K, et al. Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int. 2006;69:1626–1632. doi: 10.1038/sj.ki.5000251. [DOI] [PubMed] [Google Scholar]
- 34.Elder SJ, Pisoni RL, Akizawa T, et al. Sleep quality predicts quality of life and mortality risk in haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2008;23:998–1004. doi: 10.1093/ndt/gfm630. [DOI] [PubMed] [Google Scholar]
- 35.Tsubakihara Y. An overview of regular dialysis treatment in Japan as of Dec. 31, 2007 (CD ROM) Tokyo. Japanese Society for Dialysis Therapy. 2007 Table 207. [Google Scholar]
- 36.Masakane I, Ito S. What is the most important factor to improve the survival rate of elder dialysis patients? J Am Soc Nephrol. 2009;20:678A. [Google Scholar]