Abstract
Background. A number of studies suggested that the type of dialysis membrane is associated with differences in long-term outcome of patients undergoing haemodialysis, both in terms of morbidity and mortality. In the majority of dialysis units, synthetic membranes are being used. However, no studies are available so far for comparison between different biocompatible membranes. Therefore, we studied the influence of high- and low-flux polysulphone membranes (PS) in comparison with polymethylmethacrylate (PMMA) membranes on mortality and morbidity on the basis of various laboratory parameters.
Methods. In a cohort study, data of 260 consecutive haemodialysis patients entering our dialysis unit in the years 2003–07 were collected, comparing 435 PS patient-years and 85 PMMA patient-years. PMMA membranes (n = 33) were used for those patients who did not tolerate (e.g. for pruritus) PS membranes (n = 227). Low-flux dialysers (n = 233) were compared with high-flux (n = 37). Laboratory values were evaluated by unpaired t-test, and mortality was evaluated by log-rank test and Cox regression analysis adjusted for age, diabetes and laboratory parameters.
Results. Patients in our dialysis unit had a high cardiovascular risk as demonstrated by a proportion of 63% of peripheral arterial disease. Despite this, cumulative survival was almost 60% after 5 years on dialysis. It was slightly but not significantly higher in patients on PMMA (68%) compared with PS dialysers (54%) and on high-flux (61%) versus low-flux membranes (54%). After accounting for the confounding effect of age and diabetes in the multivariate Cox regression analysis, there was no impact of the membranes used (high- or low-flux, PMMA or PS) on survival. Only age at the onset of dialysis showed a significant influence on survival (P ≤ 0.001). Independent predictors of mortality in all patients in the multivariate Cox regression analysis were age, haemoglobin, leucocytes, C-reactive protein (CRP) and creatinine. Laboratory parameters between the high- and low- flux groups were not different. PS-treated patients showed significantly (P ≤ 0.05) higher values for leucocytes, thrombocytes, ferritin, and CRP and lower values for haemoglobin, transferrin, creatinine, uric acid, creatine kinase (CK), and sodium than PMMA-treated patients. Irrespective of the membrane used, in deceased patients, the following laboratory values were higher than for patients alive: leucocytes, thrombocytes, ferritin and CRP; the following were lower: haemoglobin, iron, total protein, urea, creatinine, uric acid and CK.
Conclusions. The data of 260 severely ill haemodialysis patients showed a slightly, but not significantly, reduced mortality in patients treated with PMMA membranes in comparison with PS and with high-flux membranes compared with low-flux. High- or low-flux membranes exhibited no difference in laboratory values. However, in PMMA patients, laboratory data with respect to inflammation, anaemia and nutrition were significantly improved compared with the PS group. A similarly positive laboratory pattern was seen in patients alive compared with patients deceased with both membrane types. The favourable effect of PMMA membranes may be explained by the reduced activation of catabolic components and inflammation, which, in turn, would result in an improved nutrition and better response to recombinant human erythropoietin.
Keywords: haemodialysis, laboratory parameters, PMMA membrane, polysulphone, survival
Introduction
In spite of many advances in the management of chronic haemodialysis patients, mortality remains high compared with the general population. Among the different factors that may contribute to this fact are a high number of co-morbidities, especially cardiovascular diseases, malnutrition, inflammation and anaemia. Therefore, it is obvious that there is a need to assess the effects of these variables that may likely be corrected and thereby may improve patient outcome. Many studies have focused on the role of dialysis membranes and its association with differences in long-term outcome of patients undergoing maintenance haemodialysis, both in terms of morbidity and mortality [1–6]. There are no prospective randomized studies suggesting that the use of biocompatible synthetic membranes in dialysis is associated with a lower morbidity and mortality when compared with dialysis using bioincompatible cellulose membranes. However, several non-randomized studies support this possibility [1–4]. Synthetic membranes are supposed to incite less of an immune response than cellulose membranes. In the majority of dialysis units, therefore, synthetic non-cellulose membranes are being used. No studies are available so far to compare between different biocompatible membranes. Moreover, membrane permeability may play a role: in observational studies, high-flux dialysis was accompanied by lower rates of amyloidosis [7] and death [2,8], whereas in randomized controlled studies, high-flux dialysis did not significantly affect all-cause mortality [5,6].
Therefore, in the following studies, we compared two different synthetic membranes—polysulphone and polymethylmethacrylate—with high or low permeability (high- or low-flux) on mortality and morbidity, based on various laboratory parameters with respect to the risk factors of dialysis anaemia, inflammation and nutrition in dialysis patients comprising a relatively high-risk population.
Materials and methods
Patients
In a cohort study, data of 260 haemodialysis patients treated in our dialysis unit from 2003–07 were collected. At the beginning of the study in January 2003, the group consisted of 92 patients being on dialysis already for several years. All consecutive new haemodialysis patients entering our hospital until the end of December 2007 were included; some of them replacing patients transferred to transplantation, sent to outpatient dialysis units or deceased. The mean age of all patients was 66.7 ± 0.92 (mean ± SEM); 130 patients were females, and 130 were males. In 227 patients, polysulphone membranes (PS) were used as these have been the standard used in our hospital since 1988. In 33 patients, polymethylmethacrylate (PMMA) membranes were applied; this has been the practice since 1989 for patients who did not tolerate polysulphone (Table 1). We compared 435 polysulphone patient-years and 85 PMMA patient-years.
Table 1.
Causes of changing from PS to PMMA membranes (n = 33)
| Total (n = 33) | |
|---|---|
| Pruritus | 22 |
| Cardiovascular instability | 3 |
| Nausea | 3 |
| Allergic symptoms | 2 |
| Others (headache, tightness, shortness of breath) | 3 |
High-flux dialysers of both membrane types have been used in our dialysis unit since 1989; they were prescribed to patients who stayed longer on haemodialysis with a higher risk of developing dialysis-related amyloidosis (n = 37) and low-flux membranes to patients with normal risk (n = 233).
Laboratory parameters
Pre-dialysis non-fasting blood samples were collected routinely every 6 weeks on the first dialysis session of the week. Laboratory values in the tables are expressed as mean values of yearly averaged value of each patient. The yearly averaged values for each patient were treated as independent data; therefore, all data of all patients in each group were used for calculation. Leucocytes, haemoglobin, thrombocytes, iron, ferritin, transferrin, total protein, C-reactive protein (CRP), creatinine, urea, uric acid, creatine kinase and sodium were determined by local laboratories using conventional auto-analysers.
Statistical analysis
Laboratory data were compared by unpaired t-test, given as mean ± SEM. Survival analysis data concerning patients who underwent transplantation, having a modification of treatment, or were lost to follow-up because they changed dialysis unit were censored at the date of modification or last available information.
The observed survival of patients treated since 1989 was analysed using the Kaplan–Meier method [9]. Log-rank test was used to compare survival curves. Multivariate Cox regression analysis was adjusted for independent prediction of survival [10]. Logistic regression analysis was used to investigate the association between risk of death and explanatory variables.
Results
The haemodialysis patients in our study had a high cardiovascular risk as demonstrated by a 63% proportion of vascular disease: 30% had a peripheral arterial disease with a decreased ankle brachial pressure index of 0.81 ± 0.04 (mean ± SEM), 33% had Mönckeberg's mediasclerosis with a false positive index of 1.62 ± 0.06 and only 37% were without a vascular disease, having a normal ankle brachial pressure index of 1.1 ± 0.05.
Despite this high cardiovascular risk, cumulative survival after 5 years on dialysis was 54% in PS-treated patients, 68% in PMMA-treated patients, 54% on low-flux dialysers and 61% on high-flux dialysers (Table 2).
Table 2.
Cumulative survival (%) on dialysis with regard to different membranes
| Years on dialysis |
n | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 5 | ||
| PS | 82 | 71 | 65 | 54 | 227 |
| PMMA | 97 | 79 | 68 | 68 | 33 |
| Low-flux | 83 | 70 | 64 | 54 | 223 |
| High-flux | 86 | 78 | 69 | 61 | 37 |
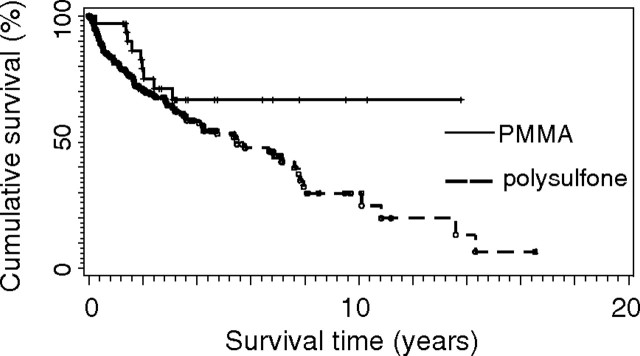
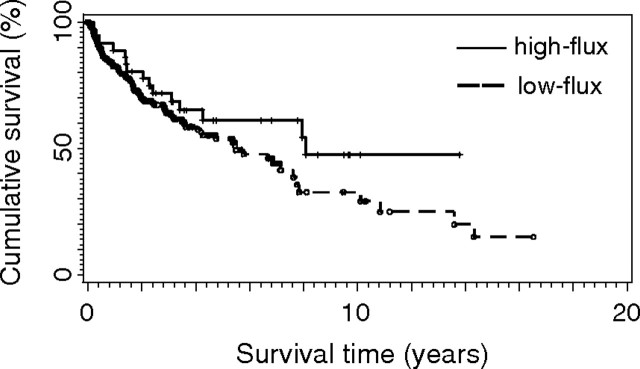
The Kaplan–Meier estimates of observed survival during follow-up with regard to all cause mortality are shown in Figure 1. In PMMA-treated patients, cumulative survival was slightly, but not significantly, higher than in patients on PS-membranes (P = 0.062). The cumulative survival in high-flux-treated patients compared with that in low-flux is shown in Figure 2. Although high-flux-treated patients survived at a slightly higher rate, there was no statistically significant difference (P = 0.114). After accounting for the confounding effect of age and diabetes by multivariate Cox regression analysis, there was no impact of the membranes used (high- or low-flux, PMMA or PS) on survival. Only age at the beginning of dialysis showed a significant influence on survival (P-value log-Wald test ≤ 0.001). Diabetes had no significant influence on survival in all groups.
Fig. 1.
Kaplan–Meier estimates of observed survival during follow-up with regard to all-cause mortality in haemodialysis patients treated by polysulphone (PS) or PMMA membranes. P = 0.062.
Fig. 2.
Kaplan–Meier estimates of observed survival during follow-up with regard to all-cause mortality in haemodialysis patients treated by high- or low-flux PS or PMMA membranes. P = 0.114.
In Table 3, laboratory parameters in high- and low-flux dialysis groups are summarized. There was no difference between either high- or low-flux PS-treated or between high- or low-flux PMMA-treated patients. In the multivariate Cox regression analysis, the independent predictors of mortality for all patients were leucocytes, CRP, haemoglobin and creatinine.
Table 3.
Comparison of biochemical data in dialysis patients treated with low- or high-flux PS and low- or high-flux PMMA membranes
| Polysulphone low | Polysulphone high | PMMA low | PMMA high | |
|---|---|---|---|---|
| Leucocytes (103/μl) | 8.90 ± 1.16 | 8.56 ± 0.41 | 7.90 ± 0.38 | 7.74 ± 0.36 |
| Thrombocytes (103/μl) | 250 ± 5.2 | 243 ± 12.0 | 204 ± 11.0 | 212 ± 14.0 |
| Ferritin (μg/l) | 485 ± 17.0 | 379 ± 38.0 | 354 ± 34.0 | 390 ± 41.0 |
| CRP (mg/dl) | 3.16 ± 0.26 | 3.02 ± 0.55 | 1.85 ± 0.31 | 2.38 ± 0.54 |
| Haemoglobin (g/dl) | 11.0 ± 0.08 | 11.2 ± 0.19 | 11.7 ± 0.22 | 11.6 ± 0.20 |
| Transferrin (mg/dl) | 147 ± 3.0 | 182 ± 45.0 | 173 ± 6.4 | 175 ± 8.1 |
| Total protein (g/dl) | 70.2 ± 0.37 | 71.1 ± 1.0 | 7.04 ± 1.0 | 6.85 ± 1.1 |
| Creatinine (mg/dl) | 6.95 ± 0.13 | 6.86 ± 0.43 | 7.46 ± 0.38 | 7.90 ± 0.45 |
| Uric acid (mg/dl) | 6.82 ± 0.10 | 6.98 ± 0.27 | 7.41 ± 0.24 | 7.43 ± 0.25 |
All values are mean ± SEM. No significant difference was found between low- and high-flux-treated patients with the same membrane.
In Table 4, laboratory parameters of PS- versus PMMA-treated patients and of patients alive versus deceased are listed.
Table 4.
Comparison of biochemical data in dialysis patients treated with PMMA versus PS membranes and in patients alive versus patients deceased
| PMMA | Polysulphone | Patients alive | Patients deceased | |
|---|---|---|---|---|
| 1Leucocytes (103/μ/l) | 7.82 ± 0.25 | 8.94 ± 0.16** | 8.44 ± 0.13 | 10.2 ± 0.46* |
| 2Thrombocytes (103/μ/l) | 208 ± 8.7 | 267 ± 4.9** | 253 ± 4.5 | 281 ± 12.9* |
| 3Ferritin (μg/l) | 372 ± 26 | 474 ± 19** | 433 ± 15 | 594 ± 49* |
| 4CRP (mg/dl) | 1.78 ± 0.74 | 4.09 ± 0.40** | 2.93 ± 0.34 | 6.27 ± 0.91* |
| 5Haemoglobin (g/dl) | 11.6 ± 0.15 | 11.0 ± 0.08** | 11.3 ± 0.08 | 10.5 ± 0.15* |
| 6Iron (μg/dl) | 54.2 ± 4.20 | 50.1 ± 1.56 | 52.6 ± 1.64 | 43.4 ± 3.40* |
| 7Transferrin (mg/dl) | 174 ± 5.20 | 150 ± 3.31** | 155 ± 3.28 | 148 ± 5.52 |
| 8Total protein (g/dl) | 69.5 ± 0.74 | 70.4 ± 0.36 | 70.9 ± 0.35 | 67.5 ± 0.74* |
| 9Urea (mg/dl) | 125 ± 4.30 | 121 ± 1.92 | 125 ± 1.86 | 110 ± 4.55* |
| 10Creatinine (mg/dl) | 7.69 ± 0.29 | 6.93 ± 0.13** | 7.29 ± 0.13 | 6.06 ± 0.22* |
| 11Uric acid (mg/dl) | 7.42 ± 0.17 | 6.84 ± 0.10** | 7.03 ± 0.10 | 6.51 ± 0.14* |
| 12CK (U/l) | 62.1 ± 6.80 | 48.7 ± 2.48** | 53.6 ± 2.66 | 38.5 ± 4.93* |
| 13Sodium (mmol/l) | 140 ± 0.35 | 138 ± 0.21** | 1.39 ± 0.20 | 138 ± 0.47 |
All values are mean ± SEM.
*P ≤ 0.05 patients alive versus deceased, **P ≤ 0.05 PMMA- versus PS-treated patients.
Inflammation
PS-treated patients showed significantly (P ≤ 0.05) higher values for parameters of inflammation (Table 4, 1–4): leucocytes, thrombocytes, ferritin and CRP than PMMA-treated patients.
Irrespective of the membrane used, deceased patients exhibited significantly higher values of these inflammatory parameters than patients alive. In general, PS-treated patients had changes in laboratory values similar to those of deceased patients, and PMMA-treated patients had values similar to those of patients alive. (Table 4, 1–4).
Anaemia
Haemoglobin was significantly decreased in PS-treated patients compared with PMMA-treated patients and in deceased patients compared with patients alive. Iron was significantly lower only in deceased patients, and transferrin was lower in PS-treated patients compared with the PMMA group (P ≤ 0.05) (Table 4, 5–7).
Malnutrition
Parameters directly or indirectly related to nutrition (Table 4, 8–13) were significantly lower in deceased patients. Creatine kinase was diminished in PS-treated patients compared with PMMA and in deceased patients compared with patients alive. Sodium was slightly but significantly attenuated in PS-treated patients compared with PMMA-treated ones. Creatinine was significantly lower in patients treated with PS than in those given PMMA and in deceased patients compared with patients alive. Urea was lower in deceased patients, and uric acid was significantly lower in PS-treated patients, compared with PMMA, and in deceased patients compared with patients alive.
Female patients exhibited lower values than males for haemoglobin, haematocrit, urea and creatinine in both dialyser groups. Mortality, however, was not statistically significantly different between males and females (data not shown).
Discussion
Our study in 260 chronic haemodialysis patients showed a slightly but statistically not significant improvement of survival in patients dialysed with PMMA membranes compared with PS and in high-flux versus low-flux dialysis. However, all laboratory parameters of inflammation, anaemia and malnutrition showed statistically significant improvement in the PMMA group in comparison with the PS group; membrane flux was without effect. In patients who switched to PMMA, intradialytic complaints, especially itching, were reduced. Pruritus is a major clinical problem in haemodialysis patients, affecting the quality of life [11–14]. PMMA membrane seems to be capable of reducing uraemic itching [15], maybe by directly or indirectly absorbing aetiological substances.
Membrane biocompatibility
There are no prospective randomized studies suggesting that the use of biocompatible membranes for chronic haemodialysis is associated with lower morbidity and mortality when compared with dialysis using bioincompatible membranes. However, several non-randomized studies support this possibility [1–4,16]. In one report, changing from cellulosic membrane to high-flux PS resulted in a lower annual mortality with the PS membrane (7% versus 20%) [2], and infection-related admissions were one-half in patients treated with PS. In two reports from the United States Renal Data System (USRDS) case mix adequacy study, cellulose (low-flux), modified cellulose or synthetic membranes (high-flux) were compared [3,4]. Dialysis with more compatible modified cellulose or synthetic membranes led to an adjusted relative risk of overall mortality of 0.75–0.80. In both studies, it remains unclear whether biocompatibility (cellulose versus polysulphone) or permeability (low- versus high-flux) was more important.
In a post hoc analysis of the 4D study, three membranes with similar permeability were included [17]. Compared with the low-flux synthetic membranes, there was a significantly greater risk of death in dialysis with low-flux cellulosic membranes [hazard ratio (HR) of 1.73] and low-flux semi-synthetic membranes (HR of 1.42).
One has to consider that other factors in dialysis may mask or override reactions to cellulosic membranes: lower rate of co-morbidities, higher delivered dose of dialysis, increasing the time of dialysis or improving blood pressure control. The currently accepted ‘gold standard’ for patient survival on haemodialysis treatment was achieved by Charra and co-workers in Tassin, France using a long, slow dialysis on cuprophane membranes with excellent blood pressure control [18,19]. Nevertheless, most nephrologists now use biocompatible membranes as we did in our investigation. However, no studies are available so far that compare the effects of different synthetic membranes on clinical outcome [1], although there are a lot of variances in thickness, pore size, pore number, and structure or symmetry between dialyser types. Furthermore, the biological profile of the group of synthetic membranes also differs: whereas coagulation profile [20] and leucopenia [21–24] are similar between PS and PMMA, the activation of granulocyte elastase is higher with PMMA membranes than with PS [23,25]. The most striking difference is the absorption: PMMA has a much higher protein absorption capacity than PS [27–29] and removes more middle-molecular-weight proteins [30]. Furthermore, PMMA absorbs solutes, such as cytokines and some cationic compounds, and leads to a lower release of interleukin-6-soluble receptor [31]. In this regard, PMMA may work as a sorbent, which could be useful for reducing the inflammatory burden of patients on haemodialysis.
Membrane flux
Dialysis with more porous synthetic membranes (high-flux dialysis) is supposed to reduce intradialytic complications and may have long-term benefits, including enhanced beta2-microglobulin clearance and improved lipid abnormalities. Less stimulation of neutrophils and monocytes with lower activation of complement and cytokines may be the result of a better biocompatibility of the membrane and not the pore size. There are several non-randomized observations suggesting that high-flux dialysis may lead to a higher patient survival [2,3,8,32–34]. But there are only two large randomized studies on the effects of membrane flux on clinical outcomes [5,6], in which no benefit of the use of high-flux dialysers could be shown. The results of the Hemodialysis (HEMO) Study showed no difference in survival between patients treated with high- or low-flux membranes, except for relative risk of death from cardiac cause [5]. Secondary analysis suggested a beneficial effect of high-flux haemodialysis on survival only in patients treated by dialysis >3.7 years [35]. In the Membrane Permeability Outcome (MPO) Study, patients with serum albumin ≤4 g/dl had significantly higher survival rates in the high-flux group compared with the low-flux [6]. In addition, a secondary analysis revealed that high-flux membranes may significantly improve survival in patients with diabetes [6]. In our study, diabetes was without a significant influence on survival in all groups.
Our patients treated with high-flux dialysis showed a slightly but not significantly better survival than low-flux. Laboratory data showed no difference between low- and high-flux-treated patients with both membranes in all biochemical aspects of inflammation, anaemia or nutrition investigated.
Predictors of survival
After accounting for the confounding effect of age, diabetes, and the laboratory values of haemoglobin, leucocytes and CRP in the multivariate Cox regression analysis, there was no impact of the membranes used (high- or low-flux, PMMA or PS) on survival. Only age at the onset of dialysis showed a significant influence on survival (P < 0.001). The independent predictors of mortality in all patients in our study in the multivariate Cox regression analysis were age, haemoglobin, leucocytes and CRP. These results confirm the literature [36–38]. In addition, we found some more laboratory parameters being significantly different in deceased patients compared with patients alive. In deceased patients, laboratory parameters of anaemia (haemoglobin, iron) and nutrition (total protein, urea, creatinine, uric acid, CK, sodium) were lower, and parameters of inflammation (leucocytes, thrombocytes, ferritin, CRP) were higher than those in patients alive. These results serve as an internal reference when comparing the laboratory results of PS- and PMMA-treated patients.
Inflammation and infection
Elevated serum levels of acute-phase reactants such as serum CRP, ferritin or fibrinogen do not only indicate an acute episode of inflammation in uraemic patients but also signal a higher risk of cardiovascular complications; CRP in dialysis patients is a good marker of survival [39,40]. On the other hand, levels of negative acute-phase reactant such as albumin or transferrin [41,42] decrease in dialysis patients.
Infection is very common in uraemic patients. Enhanced production of pro-inflammatory cytokines by complement activation as well as inhibition of anti-inflammatory cytokine secretion may contribute to the cell-mediated immunosuppression seen in patients with end-stage renal disease. Uraemic patients have an enhanced susceptibility to infection, in part due to impaired neutrophil function [43,44]. In our study, laboratory parameters of acute-phase reactants were significantly lower in PMMA-treated patients than in PS-treated ones: leucocytes, thrombocytes, ferritin and CRP. Transferrin as a negative acute-phase reactant was increased.
The membrane used for haemodialysis may play a role in this high infection rate [45–48], especially in patients treated with cuprophane membranes. Most studies compare cellulose with synthetic membranes and not among different synthetic dialysers. Synthetic membranes generally induce markedly less complement activation and also reduce the upregulation of adhesion receptors compared with cuprophane membranes [49,50]. In cuprophane membranes, phagocyte function is suppressed together with complement activation. During haemodialysis with synthetic membranes, complement is only marginally elevated [44,51], whereas haemodialysis therapy with PMMA membranes results in only mild complement activation; degranulation of neutrophils is as pronounced as with cuprophane [52]. Patients with chronic renal failure display several features of a deficient immune response, e.g. a lack of response to vaccination. In this context, it is interesting to see that the soluble form of CD40, which is essential for B-cell growth and differentiation and immunoglobulin production, is enhanced in the serum of uraemic patients [53]. Most dialysis membranes are unable to clear sCD40, including PS, but PMMA membranes are capable of clearing the soluble form of CD40, thereby enhancing the response to hepatitis B virus (HBV) vaccination [53]. To date, it is impossible to determine the pathophysiology that links the membrane type to specific changes in laboratory parameters of infection, but it deserves further investigation.
Anaemia
Anaemia is a common complication of uraemia [54] and is associated with morbidity and mortality through vascular disease, malnutrition and inflammation [55]. Haemoglobin predicts long-term survival in dialysis patients [37]. Improvement in anaemia raising the haemoglobin level leads to partial correction of left ventricular hypertrophy and other cardiovascular complications in dialysis patients [56]. In our investigation, haemoglobin, iron and transferrin were higher in patients treated with PMMA membranes compared with those treated with PS. This is probably the result of a better response to recombinant human erythropoietin because the dose of erythropoietin was similar in both groups of patients. Previous studies have shown a positive effect of dialysis with PMMA membranes on anaemia, mainly by removing an inhibitor of erythropoiesis [57–61]. Our results show that this is down to the material of the membrane and not the pore size, since high-flux membranes did not improve anaemia. However, another non-randomized study [62] showed an improvement of anaemia with high-flux membranes.
Malnutrition
Over the past decade, various nutritional parameters have emerged as powerful predictors of mortality in dialysis patients [63–65]. Malnutrition is highly prevalent and a major contributor of morbidity and mortality in these patients [66]. The impact of malnutrition in dialysis may lead to a paradoxical risk factor reversal: dialysis patients with a low body mass index or a low serum cholesterol have an increased mortality [67,68]. A number of factors can impair nutrition in dialysis patients: an insufficient dialysis dose delivery [69], loss of nutrients and amino acids into the dialysate [70–72], low food intake [73,74], and a catabolic situation by reduced protein synthesis [74,75]. The structure of the haemodialysis membrane may also play a role in malnutrition [76,77]. Bioincompatible membranes cause acute catabolic effects, triggering the activation of the complement system, while biocompatible membranes, independent of the pore size, have a more favourable effect [78,79]. In our study comparing two different synthetic membranes, urea, creatinine, uric acid and creatine kinase were significantly higher in patients treated with PMMA membranes in comparison with PS, probably an expression of more muscle mass and a higher food intake. Sodium was slightly but significantly diminished in PS-treated patients compared with PMMA-treated ones, an expression of volume overload; chronic volume overload may be directly associated with malnutrition, with improved fluid status increasing overall nutritional status [80–82]. Transferrin was decreased; it is a negative acute-phase reactant, but it may also be utilized as a nutritional marker, since serum levels are decreased with a decline in nutritional status. In a recent preliminary Japanese study [83], it has been demonstrated that body weight was decreased in dialysis patients with PS dialysers, and it improved in patients undergoing treatment with PMMA.
Conclusion
Although our cohort study was non-randomized and retrospective, it compared for the first time two different synthetic membranes. Our investigation provides some information on which membrane to choose for dialysis, especially in high-risk patients. According to our results, PMMA membranes have positive effects on laboratory parameters that are important for survival: inflammation, anaemia and malnutrition. All changes in laboratory parameters seen in dialysis patients treated with PMMA were in parallel with changes in patients alive, and changes in PS paralleled those in patients deceased.
The favourable effect of PMMA membranes may be explained by the higher absorption rate of middle-molecule substances with lower activation of catabolic compounds and inflammation. This would reduce risk factors, resulting in turn in an improved nutrition and body weight gain and a better response to recombinant human erythropoietin with improving anaemia.
It will be the task of future randomized controlled and prospective studies to confirm these data.
Acknowledgments
The authors are indebted to Dr T. Bruckner, Institute of Medical Biometry and Information, University of Heidelberg, for his statistical work.
Conflict of interest statement. None declared.
References
- 1.Grooteman MP, Nube MJ. Impact of the type of dialyser on the clinical outcome in chronic haemodialysis patients: does it really matter? Nephrol Dial Transplant. 2004;19:2965–2970. doi: 10.1093/ndt/gfh502. [DOI] [PubMed] [Google Scholar]
- 2.Hornberger JC, Chernew M, Petersen J, Garber AM. A multivariate analysis of mortality and hospital admissions with high-flux dialysis. J Am Soc Nephrol. 1992;3:1227–1237. doi: 10.1681/ASN.V361227. [DOI] [PubMed] [Google Scholar]
- 3.Hakim RM, Held PJ, Stannard DC, et al. Effect of the dialysis membrane on mortality of chronic hemodialysis patients. Kidney Int. 1996;50:566–570. doi: 10.1038/ki.1996.350. [DOI] [PubMed] [Google Scholar]
- 4.Bloembergen WE, Hakim RM, Stannard DC, et al. Relationship of dialysis membrane and cause-specific mortality. Am J Kidney Dis. 1999;33:1–10. doi: 10.1016/s0272-6386(99)70251-9. [DOI] [PubMed] [Google Scholar]
- 5.Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 6.Locatelli F, Martin-Malo A, Hannedouche T, et al. Effect of membrane permeability on survival of hemodialysis patients. J Am Soc Nephrol. 2009;20:645–654. doi: 10.1681/ASN.2008060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Ypersele de Strihou C, Jadoul M, Malghem J, Maldague B, Jamart J. Effect of dialysis membrane and patient's age on signs of dialysis-related amyloidosis. The Working Party on Dialysis Amyloidosis. Kidney Int. 1991;39:1012–1019. doi: 10.1038/ki.1991.128. [DOI] [PubMed] [Google Scholar]
- 8.Woods HF, Nandakumar M. Improved outcome for haemodialysis patients treated with high-flux membranes. Nephrol Dial Transplant. 2000;15:36–42. doi: 10.1093/oxfordjournals.ndt.a027962. [DOI] [PubMed] [Google Scholar]
- 9.Jager KJ, van Dijk PC, Zoccali C, Dekker FW. The analysis of survival data: the Kaplan–Meier method. Kidney Int. 2008;74:560–565. doi: 10.1038/ki.2008.217. [DOI] [PubMed] [Google Scholar]
- 10.van Dijk PC, Jager KJ, Zwinderman AH, Zoccali C, Dekker FW. The analysis of survival data in nephrology: basic concepts and methods of Cox regression. Kidney Int. 2008;74:705–709. doi: 10.1038/ki.2008.294. [DOI] [PubMed] [Google Scholar]
- 11.Wikström B. Itchy skin—a clinical problem for haemodialysis patients. Nephrol Dial Transplant. 2007;22:v3–v7. doi: 10.1093/ndt/gfm292. [DOI] [PubMed] [Google Scholar]
- 12.Mettang T, Pauli-Magnus C, Alscher DM. Uraemic pruritus—new perspectives and insights from recent trials. Nephrol Dial Transplant. 2002;17:1558–1563. doi: 10.1093/ndt/17.9.1558. [DOI] [PubMed] [Google Scholar]
- 13.Weisshaar E, Matterne U, Mettang T. How do nephrologists in haemodialysis units consider the symptom of itch? Results of a survey in Germany. Nephrol Dial Transplant. 2009;24:1328–1330. doi: 10.1093/ndt/gfn769. [DOI] [PubMed] [Google Scholar]
- 14.Merkus MP, Jager KJ, Dekker FW, et al. Physical symptoms and quality of life in patients on chronic dialysis: results of The Netherlands Cooperative Study on Adequacy of Dialysis (NECOSAD) Nephrol Dial Transplant. 1999;14:1163–1170. doi: 10.1093/ndt/14.5.1163. [DOI] [PubMed] [Google Scholar]
- 15.Aucella F, Vigilante M, Gesuete A, et al. Uraemic itching: do polymethylmethacrylate dialysis membranes play a role? Nephrol Dial Transplant. 2007;22:v8–v12. doi: 10.1093/ndt/gfm293. [DOI] [PubMed] [Google Scholar]
- 16.Koda Y, Nishi S, Miyazaki S, et al. Switch from conventional to high-flux membrane reduces the risk of carpal tunnel syndrome and mortality of hemodialysis patients. Kidney Int. 1997;52:1096–1101. doi: 10.1038/ki.1997.434. [DOI] [PubMed] [Google Scholar]
- 17.Krane V, Krieter DH, Olschewski M, et al. Dialyzer membrane characteristics and outcome of patients with type 2 diabetes on maintenance hemodialysis. Am J Kidney Dis. 2007;49:267–275. doi: 10.1053/j.ajkd.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 18.Charra B, Calemard E, Ruffet M, et al. Survival as an index of adequacy of dialysis. Kidney Int. 1992;41:1286–1291. doi: 10.1038/ki.1992.191. [DOI] [PubMed] [Google Scholar]
- 19.Charra B, Calemard M, Laurent G. Importance of treatment time and blood pressure control in achieving long-term survival on dialysis. Am J Nephrol. 1996;16:35–44. doi: 10.1159/000168968. [DOI] [PubMed] [Google Scholar]
- 20.Hildebrand U, Quellhorst E. Influence of various membranes on the coagulation system during dialysis. Contrib Nephrol. 1985;46:92–101. doi: 10.1159/000410771. [DOI] [PubMed] [Google Scholar]
- 21.Henderson LW, Chenoweth D. Biocompatibility of artificial organs: an overview. Blood Purif. 1987;5:100–111. doi: 10.1159/000169459. [DOI] [PubMed] [Google Scholar]
- 22.Aljama P, Martin-Malo A, Castillo D, et al. Anaphylatoxin C5a generation and dialysis-induced leukopenia with different hemodialyzer membranes. Blood Purif. 1986;4:88–92. doi: 10.1159/000169431. [DOI] [PubMed] [Google Scholar]
- 23.Schaefer RM, Heidland A, Hörl WH. Release of leukocyte elastase during hemodialysis. Effect of different dialysis membranes. Contrib Nephrol. 1985;46:109–117. [PubMed] [Google Scholar]
- 24.Fawcett S, Hoenich NA, Woffindin C, Ward MK. Influence of high permeability synthetic membranes on gas exchange and lung function during hemodialysis. Contrib Nephrol. 1985;46:83–91. doi: 10.1159/000410770. [DOI] [PubMed] [Google Scholar]
- 25.Hörl WH, Steinhauer HB, Schollmeyer P. Plasma levels of granulocyte elastase during hemodialysis: effects of different dialyzer membranes. Kidney Int. 1985;28:791–796. doi: 10.1038/ki.1985.199. [DOI] [PubMed] [Google Scholar]
- 26.Hörl WH, Steinhauer HB, Riegel W, et al. Effect of different dialyzer membranes on plasma levels of granulocyte elastase. Kidney Int. 1988;24:S90–S91. [PubMed] [Google Scholar]
- 27.Birk HW, Kistner A, Wizemann V, Schutterle G. Protein adsorption by artificial membrane materials under filtration conditions. Artif Organs. 1995;19:411–415. doi: 10.1111/j.1525-1594.1995.tb02351.x. [DOI] [PubMed] [Google Scholar]
- 28.Aoike I. Clinical significance of protein adsorbable membranes—long-term clinical effects and analysis using a proteomic technique. Nephrol Dial Transplant. 2007;22:v13–v19. doi: 10.1093/ndt/gfm295. [DOI] [PubMed] [Google Scholar]
- 29.Galli F. Protein damage and inflammation in uraemia and dialysis patients. Nephrol Dial Transplant. 2007;22:v20–v36. doi: 10.1093/ndt/gfm294. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa I, Chikazawa Y, Sato K, et al. Proteomic analysis of serum, outflow dialysate and adsorbed protein onto dialysis membranes (polysulfone and PMMA) during hemodialysis treatment using SELDI-TOF-MS. Am J Nephrol. 2006;26:372–380. doi: 10.1159/000094779. [DOI] [PubMed] [Google Scholar]
- 31.Memoli B, Postiglione L, Cianciaruso B, et al. Role of different dialysis membranes in the release of interleukin-6-soluble receptor in uremic patients. Kidney Int. 2000;58:417–424. doi: 10.1046/j.1523-1755.2000.00181.x. [DOI] [PubMed] [Google Scholar]
- 32.Dumler F, Stalla K, Mohini R, Zasuwa G, Levin NW. Clinical experience with short-time hemodialysis. Am J Kidney Dis. 1992;19:49–56. doi: 10.1016/s0272-6386(12)70202-0. [DOI] [PubMed] [Google Scholar]
- 33.Wolfe RA, Gaylin DS, Port FK, Held PJ, Wood CL. Using USRDS-generated mortality tables to compare local ESRD mortality rates to national rates. Kidney Int. 1992;42:991–996. doi: 10.1038/ki.1992.378. [DOI] [PubMed] [Google Scholar]
- 34.Chauveau P, Nguyen H, Combe C, et al. Dialyzer membrane permeability and survival in hemodialysis patients. Am J Kidney Dis. 2005;45:565–571. doi: 10.1053/j.ajkd.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Cheung AK, Levin NW, Greene T, et al. Effects of high-flux hemodialysis on clinical outcomes: results of the HEMO study. J Am Soc Nephrol. 2003;14:3251–3263. doi: 10.1097/01.asn.0000096373.13406.94. [DOI] [PubMed] [Google Scholar]
- 36.Honda H, Qureshi AR, Heimburger O, et al. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis. 2006;47:139–148. doi: 10.1053/j.ajkd.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 37.Avram MM, Blaustein D, Fein PA, et al. Hemoglobin predicts long-term survival in dialysis patients: a 15-year single-center longitudinal study and a correlation trend between prealbumin and hemoglobin. Kidney Int Suppl. 2003;87:S6–S11. doi: 10.1046/j.1523-1755.64.s87.3.x. [DOI] [PubMed] [Google Scholar]
- 38.Kovesdy CP, George SM, Anderson JE, Kalantar-Zadeh K. Outcome predictability of biomarkers of protein-energy wasting and inflammation in moderate and advanced chronic kidney disease. Am J Clin Nutr. 2009;90:407–414. doi: 10.3945/ajcn.2008.27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeun JY, Levine RA, Mantadilok V, Kaysen GA. C-reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2000;35:469–476. doi: 10.1016/s0272-6386(00)70200-9. [DOI] [PubMed] [Google Scholar]
- 40.Ducloux D, Bresson-Vautrin C, Kribs M, Abdelfatah A, Chalopin JM. C-reactive protein and cardiovascular disease in peritoneal dialysis patients. Kidney Int. 2002;62:1417–1422. doi: 10.1111/j.1523-1755.2002.kid562.x. [DOI] [PubMed] [Google Scholar]
- 41.Streetz KL, Wustefeld T, Klein C, Manns MP, Trautwein C. Mediators of inflammation and acute phase response in the liver. Cell Mol Biol (Noisy-le-grand) 2001;47:661–673. [PubMed] [Google Scholar]
- 42.Suffredini AF, Fantuzzi G, Badolato R, Oppenheim JJ, O'Graddy NP. New insights into the biology of the acute phase response. J Clin Immunol. 1999;19:203–214. doi: 10.1023/a:1020563913045. [DOI] [PubMed] [Google Scholar]
- 43.Vanholder R, Ringoir S. Polymorphonuclear cell function and infection in dialysis. Kidney Int Suppl. 1992;38:S91–S95. [PubMed] [Google Scholar]
- 44.Vanholder R, Ringoir S. Infectious morbidity and defects of phagocytic function in end-stage renal disease: a review. J Am Soc Nephrol. 1993;3:1541–1554. doi: 10.1681/ASN.V391541. [DOI] [PubMed] [Google Scholar]
- 45.Himmelfarb J, Zaoui P, Hakim R. Modulation of granulocyte LAM-1 and MAC-1 during dialysis—a prospective, randomized controlled trial. Kidney Int. 1992;41:388–395. doi: 10.1038/ki.1992.54. [DOI] [PubMed] [Google Scholar]
- 46.Zaoui P, Green W, Hakim RM. Hemodialysis with cuprophane membrane modulates interleukin-2 receptor expression. Kidney Int. 1991;39:1020–1026. doi: 10.1038/ki.1991.129. [DOI] [PubMed] [Google Scholar]
- 47.Himmelfarb J, Lazarus JM, Hakim R. Reactive oxygen species production by monocytes and polymorphonuclear leukocytes during dialysis. Am J Kidney Dis. 1991;17:271–276. doi: 10.1016/s0272-6386(12)80473-2. [DOI] [PubMed] [Google Scholar]
- 48.Vanholder R, Smet RD, Glorieux G, Dhondt A. Survival of hemodialysis patients and uremic toxin removal. Artif Organs. 2003;27:218–223. doi: 10.1046/j.1525-1594.2003.07212.x. [DOI] [PubMed] [Google Scholar]
- 49.Lundahl J, Skedinger M, Hed J, Johansson SG, Zetterstrom O. Lability in complement receptor mobilization of granulocytes in patients with bronchial hyperreactivity. Clin Exp Allergy. 1992;22:834–838. doi: 10.1111/j.1365-2222.1992.tb02828.x. [DOI] [PubMed] [Google Scholar]
- 50.Fujimori A, Naito H, Miyazaki T. Adsorption of complement, cytokines, and proteins by different dialysis membrane materials: evaluation by confocal laser scanning fluorescence microscopy. Artif Organs. 1998;22:1014–1017. doi: 10.1046/j.1525-1594.1998.06083.x. [DOI] [PubMed] [Google Scholar]
- 51.Ward RA. Phagocytic cell function as an index of biocompatibility. Nephrol Dial Transplant. 1994;9:46–56. [PubMed] [Google Scholar]
- 52.Hörl WH, Riegel W, Schollmeyer P, Rautenberg W, Neumann S. Different complement and granulocyte activation in patients dialyzed with PMMA dialyzers. Clin Nephrol. 1986;25:304–307. [PubMed] [Google Scholar]
- 53.Contin-Bordes C, Lacraz A, Précigout V. Potential role of the soluble form of CD40 in deficient immunological function of dialysis patients: new findings of its amelioration using polymethylmethacrylate (PMMA) membrane. Nephrol Dial Transplant. 2010 doi: 10.1093/ndtplus/sfq033. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eschbach JW, Adamson JW. Anemia of end-stage renal disease (ESRD) Kidney Int. 1985;28:1–5. doi: 10.1038/ki.1985.109. [DOI] [PubMed] [Google Scholar]
- 55.Foley RN, Parfrey PS, Harnett JD, et al. The impact of anemia on cardiomyopathy, morbidity, and mortality in end-stage renal disease. Am J Kidney Dis. 1996;28:53–61. doi: 10.1016/s0272-6386(96)90130-4. [DOI] [PubMed] [Google Scholar]
- 56.Silverberg DS, Wexler D, Blum M, et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol. 2000;35:1737–1744. doi: 10.1016/s0735-1097(00)00613-6. [DOI] [PubMed] [Google Scholar]
- 57.Yamada S, Kataoka H, Kobayashi H, et al. Identification of an erythropoietic inhibitor from the dialysate collected in the hemodialysis with PMMA membrane (BK-F) Contrib Nephrol. 1999;125:159–172. doi: 10.1159/000059957. [DOI] [PubMed] [Google Scholar]
- 58.Buoncristiani U, Galli F, Benedetti S, et al. Quantitative and qualitative assessment and clinical meaning of molecules removed with BK membranes. Contrib Nephrol. 1999;125:133–158. doi: 10.1159/000059956. [DOI] [PubMed] [Google Scholar]
- 59.Manzoni C, Locatelli F. Biocompatibility of PMMA membranes in acute and chronic patients with renal failure. Contrib Nephrol. 1999;125:65–75. doi: 10.1159/000059950. [DOI] [PubMed] [Google Scholar]
- 60.Bonomini M, Fiederling B, Bucciarelli T, et al. A new polymethylmethacrylate membrane for hemodialysis. Int J Artif Organs. 1996;19:232–239. [PubMed] [Google Scholar]
- 61.Locatelli F, Andrulli S, Del Vecchio L. Anemia of hemodialysis patients: evaluation of the effect of BK-F polymethylmethacrylate membrane. Contrib Nephrol. 1999;125:173–181. doi: 10.1159/000059958. [DOI] [PubMed] [Google Scholar]
- 62.Ayli D, Ayli M, Azak A, et al. The effect of high-flux hemodialysis on renal anemia. J Nephrol. 2004;17:701–706. [PubMed] [Google Scholar]
- 63.Avram MM, Mittman N, Bonomini L, et al. Markers for survival in dialysis: a seven-year prospective study. Am J Kidney Dis. 1995;26:209–219. doi: 10.1016/0272-6386(95)90176-0. [DOI] [PubMed] [Google Scholar]
- 64.Avram MM, Bonomini LV, Sreedhara R, Mittmann N. Predictive value of nutritional markers (albumin, creatinine, cholesterol, and hematocrit) for patients on dialysis for up to 30 years. Am J Kidney Dis. 1996;28:910–917. doi: 10.1016/s0272-6386(96)90394-7. [DOI] [PubMed] [Google Scholar]
- 65.Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15:458–482. doi: 10.1016/s0272-6386(12)70364-5. [DOI] [PubMed] [Google Scholar]
- 66.Acchiardo SR, Moore LW, Latour PA. Malnutrition as the main factor in morbidity and mortality of hemodialysis patients. Kidney Int Suppl. 1983;16:S199–S203. [PubMed] [Google Scholar]
- 67.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 68.Kalantar-Zadeh K, Abbott KC, Kronenberg F, et al. Epidemiology of dialysis patients and heart failure patients. Semin Nephrol. 2006;26:118–133. doi: 10.1016/j.semnephrol.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 69.Lindsay RM, Spanner E, Heidenheim RP, et al. Which comes first, Kt/V or PCR—chicken or egg? Kidney Int Suppl. 1992;38:S32–S36. [PubMed] [Google Scholar]
- 70.Blumenkrantz MJ, Gahl GM, Kopple JD, et al. Protein losses during peritoneal dialysis. Kidney Int. 1981;19:593–602. doi: 10.1038/ki.1981.57. [DOI] [PubMed] [Google Scholar]
- 71.Ikizler TA, Flakoll PJ, Parker RA, Hakim RM. Amino acid and albumin losses during hemodialysis. Kidney Int. 1994;46:830–837. doi: 10.1038/ki.1994.339. [DOI] [PubMed] [Google Scholar]
- 72.Navarro JF, Marcen R, Teruel JL, et al. Effect of different membranes on amino-acid losses during haemodialysis. Nephrol Dial Transplant. 1998;13:113–117. doi: 10.1093/ndt/13.1.113. [DOI] [PubMed] [Google Scholar]
- 73.Sherman RA, Cody RP, Rogers ME, Solanchick JC. Interdialytic weight gain and nutritional parameters in chronic hemodialysis patients. Am J Kidney Dis. 1995;25:579–583. doi: 10.1016/0272-6386(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 74.Lim VS, Ikizler TA, Raj DS, Flanigan MJ. Does hemodialysis increase protein breakdown? Dissociation between whole-body amino acid turnover and regional muscle kinetics. J Am Soc Nephrol. 2005;16:862–868. doi: 10.1681/ASN.2004080624. [DOI] [PubMed] [Google Scholar]
- 75.Gutierrez A, Alvestrand A, Wahren J, Bergstrom J. Effect of in vivo contact between blood and dialysis membranes on protein catabolism in humans. Kidney Int. 1990;38:487–494. doi: 10.1038/ki.1990.230. [DOI] [PubMed] [Google Scholar]
- 76.Qureshi AR, Alvestrand A, Divino-Filho JC, et al. Inflammation, malnutrition, and cardiac disease as predictors of mortality in hemodialysis patients. J Am Soc Nephrol. 2002;13:S28–36. [PubMed] [Google Scholar]
- 77.Kaysen GA, Dubin JA, Muller HG, et al. Relationships among inflammation nutrition and physiologic mechanisms establishing albumin levels in hemodialysis patients. Kidney Int. 2002;61:2240–2249. doi: 10.1046/j.1523-1755.2002.00076.x. [DOI] [PubMed] [Google Scholar]
- 78.Lindsay RM, Spanner E, Heidenheim P, Kortas C, Blake PG. PCR, Kt/V and membrane. Kidney Int Suppl. 1993;41:S268–S273. [PubMed] [Google Scholar]
- 79.Parker TF, 3rd, Wingard RL, Husni L, et al. Effect of the membrane biocompatibility on nutritional parameters in chronic hemodialysis patients. Kidney Int. 1996;49:551–556. doi: 10.1038/ki.1996.78. [DOI] [PubMed] [Google Scholar]
- 80.Cheng LT, Tang W, Wang T. Strong association between volume status and nutritional status in peritoneal dialysis patients. Am J Kidney Dis. 2005;45:891–902. doi: 10.1053/j.ajkd.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 81.Jones CH, Wells L, Stoves J, Farquhar F, Woodrow G. Can a reduction in extracellular fluid volume result in increased serum albumin in peritoneal dialysis patients? Am J Kidney Dis. 2002;39:872–875. doi: 10.1053/ajkd.2002.32010. [DOI] [PubMed] [Google Scholar]
- 82.Dumler F. Hypoalbuminemia is a marker of overhydration in chronic maintenance patients on dialysis. ASAIO J. 2003;49:282–286. doi: 10.1097/01.mat.0000065465.52748.bb. [DOI] [PubMed] [Google Scholar]
- 83.Masakane I. High quality dialysis: lessons from the Japanese experience. Nephrol Dial Transplant. 2010;3([Suppl 1]):i28–i35. doi: 10.1093/ndtplus/sfq034. [DOI] [PMC free article] [PubMed] [Google Scholar]