Abstract
Spironolactone and eplerenone are both mineralocorticoid-receptor antagonists. These compounds block both the epithelial and nonepithelial actions of aldosterone, with the latter assuming increasing clinical relevance. Spironolactone and eplerenone both affect reductions in blood pressure either as mono- or add-on therapy; moreover, they each afford survival benefits in diverse circumstances of heart failure and the probability of renal protection in proteinuric chronic kidney disease. However, as use of mineralocorticoid-blocking agents has expanded, the hazards inherent in taking such drugs have become more apparent. Whereas the endocrine side effects of spironolactone are in most cases little more than a cosmetic annoyance, the potassium-sparing effects of both spironolactone and eplerenone can prove disastrous, even fatal, if sufficient degrees of hyperkalemia emerge. For most patients, however, the risk of developing hyperkalemia in and of itself should not discourage the sensible clinician from bringing these compounds into play. Hyperkalemia should always be considered a possibility in patients receiving either of these medications; therefore, anticipatory steps should be taken to minimize the likelihood of its occurrence if long-term therapy of these agents is being considered.
Keywords: spironolactone, eplerenone, mineralocorticoid receptor antagonist, hyperkalemia, heart failure, resistant hypertension

D. A. Sica, M.D.
Introduction
Aldosterone is categorized as a mineralocorticoid hormone based on its ability to regulate sodium (Na+) and potassium (K+) transport in a variety of epithelial locations, including the distal convoluted/connecting tubules, salivary glands, and the gastrointestinal tract. Aldosterone has demonstrable epithelial and nonepithelial actions, and its blockade by an orally administered mineralocorticoid receptor antagonist (MRA) can both lower blood pressure (BP) as well as favorably influence altered structure/function relationships in the heart, kidney, and various vascular beds. Three compounds are commonly used MRAs: spironolactone, eplerenone, and canrenone. Of these, only spironolactone and eplerenone are available in the United States and will be the compounds discussed in this manuscript. Although each of these compounds is equally effective in a clinical setting, structure/function relationships as well as metabolite/half-life considerations distinguish these drugs relative to side-effect profiles. Of note, the frequency with which particular side effects occur with MRAs depends, to some degree, on the condition for which they are being used.1–3
Historical Perspective
When spironolactone was originally developed in the 1950s, scientific knowledge of the drug was limited to its effects on epithelial ion transport, and even such evaluations were in their nascent stages.4 As a result, spironolactone was originally classified solely as a diuretic with K+-sparing features. This classification, although technically correct, has proven unnecessarily restraining given the emerging nonepithelial role(s) for aldosterone in cardiovascular (CV) disease. Thus, the pharmacodynamic effects of MRAs now are viewed as encompassing epithelial ion transport, a spectrum of favorable nonepithelial cellular processes, and BP reduction, which may be influenced by both the epithelial and nonepithelial actions of MRA.5
Class Considerations
The first compound in a class of medications is generally well-defined mechanistically but oftentimes is administered in suboptimal doses and/or comes with troublesome side-effects. Thus, classes of medications generally evolve along the lines of more efficient delivery systems and/or increased mechanistic specificity to reduce the rate of side effects. The calcium channel blocker (CCB) class of medications illustrates how such progression occurs in the development of a drug class. For this particular drug class, sustained and/or delayed-release delivery systems have notably improved dosing flexibility compared to the immediate-release compounds that were originally on the market; in fact, more evolved CCBs with inherently reduced side-effect rates have become available. With the evolution of the MRA drug class, eplerenone has emerged as a more-specific receptor antagonist with reduced sexual side effects compared with the more burdensome progestogenic and antiandrogenic side effects that can occur with spironolactone.6
Natriuretic Response
Spironolactone and eplerenone are not potent natriuretic agents when acutely administered as monotherapy to either normotensive or hypertensive subjects. The efficacy of these agents is typically greatest when aldosterone levels are excessively elevated, as in the case of primary and, even more so, secondary hyperaldosteronism. Spironolactone, for example, is more efficacious when given to patients with cirrhosis and ascites or heart failure (HF), circumstances wherein clearly evident secondary hyperaldosteronism often exists. As such, a significant natriuretic response can occur particularly if a loop diuretic is coincidentally being given.7,8 Eplerenone has not been studied under similar circumstances of diuretic need.
Essential Hypertension
Spironolactone has been used as monotherapy or in combination with a thiazide-type diuretic in the treatment of essential hypertension since the 1970s.9,10 Early studies found that spironolactone in doses of 200 to 400 mg/day did not bring about a greater antihypertensive effect than did 100 mg/day; thus, this compound demonstrated a relatively flat dose-response relationship in patients with essential hypertension beyond 100 mg/day.9 Other studies comparing single versus divided daily doses of 100-mg spironolactone showed that single-dose administration was generally as effective as a divided multiple daily-dose regimen.11 These data led to the recommendation that patients with essential hypertension who are treated with spironolactone monotherapy, with or without a thiazide diuretic, should receive the optimal dosage of between 50 and 100 mg.
Eplerenone also reduces BP when administered in the dose range of 50 to 200 mg/day, regardless of pretherapy plasma renin activity (PRA) values. Eplerenone reduces BP to a similar degree as an angiotensin-converting enzyme (ACE) inhibitor or a dihydropyridine CCB and has an additive effect when given to patients inadequately controlled with an ACE inhibitor or an angiotensin receptor blocker (ARB).12 It has also proven superior to monotherapy with the ARB losartan in African-American patients12; however, cost considerations, insurance coverage, and poor dissemination of information on the optimal frequency of dosing have limited the more routine use of this medication in the general practice of treating hypertension.
The BP-lowering effect of eplerenone is less than that of spironolactone on a milligram-for- milligram basis. For example, the degree to which BP is reduced with spironolactone 50 mg/twice daily is similar to that seen with eplerenone 200 mg/twice daily or 400 mg/once daily, and it is 1.3- to 2-times greater than that seen with eplerenone 50 mg/twice daily or 100 mg once daily.13 The efficiency of MRA can be assumed from treatment-related changes in PRA and plasma aldosterone levels. In one study, dose-dependent increases seen for PRA and active renin were smaller for doses of eplerenone < 400 mg/day than for spironolactone, all of which were significantly greater (P < 0.05) than what was observed with placebo.13
Resistant Hypertension
Single-drug treatment of hypertension with spironolactone or eplerenone is typically reserved for specific circumstances such as the presence of primary aldosteronism. However, spironolactone or eplerenone as add-on therapy in the poorly controlled hypertensive patient is increasingly viewed as an effective treatment option. This has been the case in two areas: end-stage renal disease (ESRD)14,15 and resistant hypertension.16–19 In this regard, functionally anuric ESRD patients with hypertension are increasingly being treated with an MRA (with or without other therapies), with the end result being significant BP reduction without the development of clinically relevant hyperkalemia.14,15
Mineralocorticoid receptor antagonist therapy with spironolactone has now become an accepted add-on therapy in the setting of resistant hypertension.16–19 This adjunctive effect with spironolactone occurs within a matter of weeks, persists for months, and is independent of ethnicity and the excretion rate of urinary aldosterone.16 When spironolactone (12.5 to 50 mg/day) was added to a regimen comprised of a diuretic, an ACE inhibitor, or an ARB, a mean BP decrease of 21 ± 20/10 ± 14 mm Hg was observed at 6 weeks, and the decrease persisted at 6 months of therapy (↓ 25 ± 20/12 ± 12 mm Hg).16 The general benefit of MRA therapy in patients with resistant hypertension suggests that either a relative or absolute aldosterone excess may be a more prominent factor in resistant hypertension than was originally believed; however, the addition of amiloride to a treatment regimen comprised of a diuretic and a CCB also incrementally reduces BP (↓ 9.8 ± 1.6/3.4 ± 1.0) for amiloride versus spironolactone (↓ 4.6 ± 1.6/1.8 ± 1.0).20 This suggests that amiloride and possibly triamterene may be suitable treatment alternatives in patients either intolerant of spironolactone or unable to access eplerenone because of its cost.
Spironolactone and a Diuretic Effect in Resistant Hypertension
It has recently been found that patients with resistant hypertension often have occult volume expansion underlying their treatment resistance, and BP control was improved primarily through forced titration of diuretics or within diuretic class switches.21 It has not been routine for combination diuretic therapy to be used in the patient with difficult-to-treat hypertension except in the instance of concurrent renal insufficiency and/or with the use of potent Na+-retaining compounds, such as minoxidil.22 That being said, there is a lengthy history dating back to 1962 for the use of two diuretics together in the initial management of hypertension.23 Several studies have shown additive benefit for BP reduction when hydrochlorothiazide (HCTZ) or a thiazide-type diuretic was given together with spironolactone.24,25 These studies, among others, served as the experimental basis for the fixed-dose combination product Aldactone®, comprised of HCTZ and spironolactone. A likely explanation for the enhanced effect when spironolactone is added to HCTZ is an improved spironolactone response consequent to the volume reduction and neurohumoral changes produced by HCTZ.26
Hydrochlorothiazide remains a relatively short-acting thiazide diuretic of intermediate potency. Chlorthalidone is a more long-acting thiazide-type diuretic whose pharmacokinetic characteristics support a more long-acting natriuretic effect, and its use typically leads to a substantial net negative balance of Na+.27 Chlorthalidone is now more regularly used as the diuretic of choice in resistant forms of hypertension. The more frequent choice of this compound relates to its longer pharmacologic half-life and its more favorable cardiovascular outcomes data when compared to HCTZ.28 When spironolactone is given together with chlorthalidone, there appears to be a greater potential for diuretic additivity by sequential nephron blockade, although this concept has not been formally studied. The not-infrequent observation of rising serum creatinine levels when spironolactone is given together with chlorthalidone supports the possibility of sequential nephron blockade and a greater summed natriuretic effect.29 This additive response is such that clinicians must often consider a dose adjustment of the concomitant conventional diuretic therapy.30
End-Organ Protection
Head-to-head studies comparing end-organ effects and survival benefits of eplerenone and spironolactone are not available; moreover, no information is available for either compound concerning specific dose-ranging relationships with regard to cardiac and/or renal end-points. For example, in a study of the effect of eplerenone on either regression of left ventricular mass or reduction in urine protein excretion, patients were force dose-titrated eplerenone to 200 mg/day without any meaningful analysis of changes at intermediate dose levels.31,32 Also, in both the Randomized Aldactone Evaluation Study (RALES) and the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS), the doses of spironolactone and eplerenone were basically fixed at 25 and 50 mg, respectively.33,34 Thus, the threshold and/or optimal effect dose of an MRA is yet to be established in HF or proteinuric chronic kidney disease (CKD).
Left Ventricular Hypertrophy
Mineralocorticoid receptor antagonism effectively regresses left ventricular hypertrophy (LVH) and does so in a manner beyond what might be expected with BP reduction alone.31,35 In addition, MRA therapy decreases the microalbuminuria that can accompany LVH.31 The exact gain from regressing LVH with usual antihypertensive therapy remains unclear let alone with coadministration of an MRA. Since the risk of side effects with an MRA in a patient with LVH and normal renal function is minimal, this drug class can be considered if a patient with LVH and hypertension requires add-on therapy for additional BP reduction.
Heart Failure
Diuretic combinations can be used in HF patients otherwise refractory to loop diuretics alone. Because structural adaptations occur in the distal nephron with prolonged loop diuretic therapy, the combination of a distal-acting diuretic and a loop diuretic is particularly effective in such patients.8 The combination of bumetanide and metolazone (a thiazide-type diuretic) produces an additive, if not synergistic, diuretic effect.8 During prolonged furosemide therapy, the responsiveness to a thiazide is considerably augmented. Numerous reports have demonstrated a profound diuresis (several liters daily) accompanied by clinical improvement when adding metolazone to a loop diuretic (usually furosemide) in HF patients previously resistant to loop diuretic therapy alone.7,8 Metolazone is particularly effective because its duration of action is prolonged, it is lipophilic, and it remains effective in states of renal impairment.36 Spironolactone also has been used in combination with loop diuretics and has been followed by an improvement in the overall diuretic response in HF patients.37 Above and beyond the known diuretic properties of spironolactone, it has recently been demonstrated that, as an MRA, it blocks a wide range of damaging tissue-based effects attributable to aldosterone, including processes such as enhanced vascular and myocardial fibrosis.38,39
It has recently been recognized that aldosterone levels are often elevated (“aldosterone escape”) in HF patients notwithstanding the use of ACE inhibitors.40 This observation at least in part provided a basis for the study of MRA therapy in various forms of HF; as a result, spironolactone, and more recently eplerenone, is being increasingly advocated as adjunctive therapy in HF.41,42 This therapeutic recommendation is a byproduct of RALES and EPHESUS.33,34 In the RALES trial, spironolactone (25 mg/day) was shown to reduce the risk of all-cause mortality by roughly 30% in NYHA Class IV HF patients already being treated with a standard ACE inhibitor and diuretic during an average follow-up of 2 years.33 It has also been shown that the use of spironolactone in patients with severe HF does not obviate the need for additional treatment that interferes with the adverse cardiac effects of sympathetic activation, as is the case with b-blockade.43 In EPHESUS, eplerenone (50 mg/day) added to optimal medical therapy substantially reduced morbidity and mortality among patients with an acute myocardial infarction complicated by left ventricular dysfunction and HF, with the observed benefit(s) evident within 30 days of randomization.34 To date, the BP-independent benefit(s) in HF patients who are given an MRA have been limited to those with a reduced ejection fraction. In the instance of HF with a preserved ejection fraction, add-on treatment with spironolactone has not been shown to significantly reduce major CV end-points44; thus, guidelines developed for the treatment of HF patients with an MRA are limited to those with reduced fractions.45
Side Effects
Both eplerenone and spironolactone are associated with a dose-related increase in serum K+ levels, which is by far the most worrisome side-effect with these medications.33,34 There are certain patient variables that govern the incidence of MRA-related hyperkalemia, with the level of renal function being the single most important determinant of risk among several variables that influence serum K+ values (Table 1). Thus, patients with CKD and, in particular, with CKD and diabetes and/or conditions with a high frequency of accompanying renal failure—as is the case with HF—are the ones most prone to an increase in serum K+ values with MRA therapy.33,34,43
Table 1.
Risk factors for hyperkalemia * with mineralocorticoid receptor antagonist therapy.
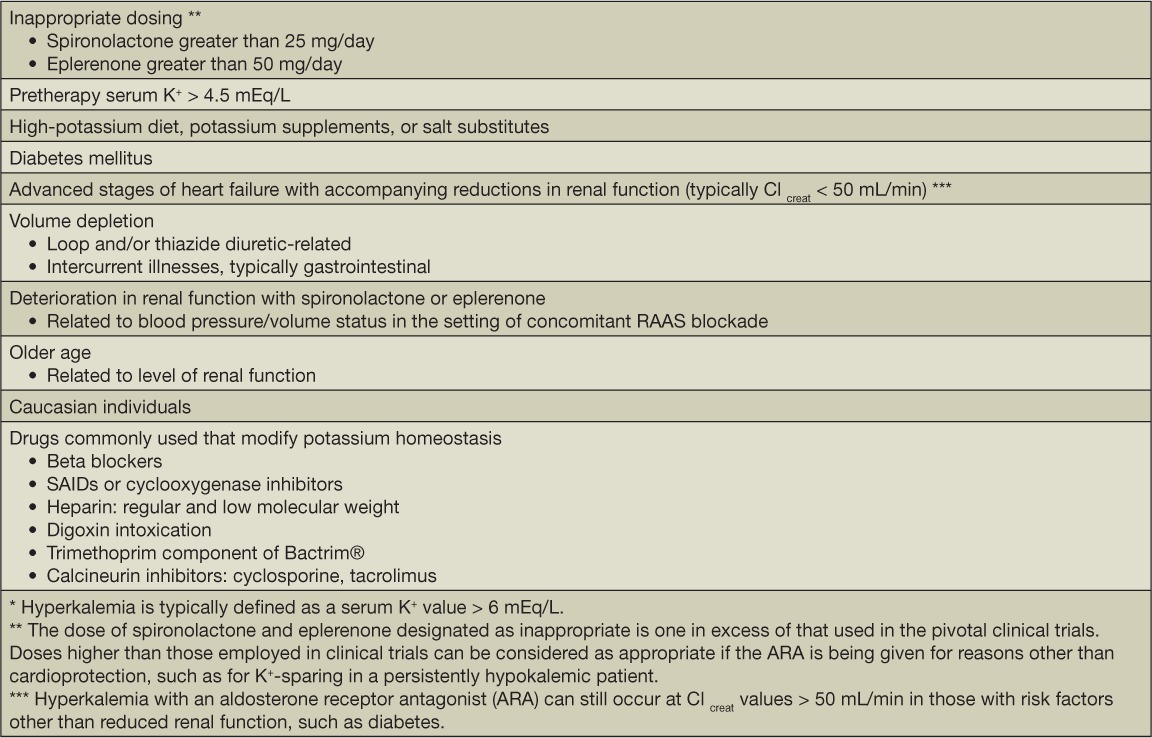
The pattern of serum K+ change in HF, as was observed in RALES, provides a good basis for understanding the risk-to-benefit ratio for MRA therapy.33 RALES was preceded by a short, dose-finding study in which hyperkalemia (serum K+ 3; 5.5 mEq/L) occurred in 13%, 20%, and 24% of patients treated with spironolactone at doses of 25, 50, and 75 mg/day, respectively.46 These incidence numbers were a strong consideration in the selection of the 25.0 mg spironolactone dose used in RALES.
After 1 year of therapy with 25 mg/day of spironolactone in the RALES study, the median K+ concentration rose by 0.3 mmol/L, which was statistically significant. There were 10 and 14 cases of serious hyperkalemia in the placebo and spironolactone treatment groups, respectively. Importantly, this study excluded patients with a serum creatinine level > 2.5 mg/dL (221 μmol/L) and/or a baseline serum K+ level > 5.0 mmol/L. Spironolactone is now more broadly used without attention to the HF class and/or ejection fraction and without optimization of background treatment with ACE inhibitors and β-blockers; thus, the incidence and prevalence of hyperkalemia is likely to be higher and very situationally specific. In these cases, there may be occasional small changes in renal function when an aldosterone receptor antagonist is given at a dose of 0.1 to 0.2 mg/dL. The degree of renal function change has several determinants, including baseline level of renal function, extent of any ongoing diuresis/volume contraction, concomitant use of an ACE inhibitor or an ARB, beta-blocker use, and/or presence of HF.47–49 The change in serum creatinine values with spironolactone can be obscured by increases that fall within a normal range of values, therefore the change may not be perceived as “abnormal” per se.50
Conclusion
Spironolactone and eplerenone are MRAs commonly used to manage treatment-resistant forms of hypertension as well as HF characterized by a reduced ejection fraction. Spironolactone is a commonly used add-on diuretic that provides incremental benefit for salt-and-water excretion above and beyond what may be seen with a loop diuretic and/or a thiazide-type diuretic. The dose-response relationships for natriuresis/CV protection with spironolactone have not been completely explored. The adverse electrolyte and renal function side effects with MRAs are not uncommon in at-risk patients, such as those with CKD or HF; therefore, dosing should take into account the propensity for these drugs to cause clinically relevant hyperkalemia.
Acknowledgments
Conflict of Interest Disclosure: The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
References
- 1.Frishman WH, Stier CT., Jr. Aldosterone and aldosterone antagonism in systemic hypertension. Curr Hypertens Rep. 2004 Jun;6(3):195–200. doi: 10.1007/s11906-004-0069-6. [DOI] [PubMed] [Google Scholar]
- 2.Stier CT, Jr, Koenig S, Lee DY, Chawla M, Frishman WH. Aldosterone and aldosterone antagonism in cardiovascular disease focus on eplerenone (Inspra) Heart Dis. 2003 Mar–Apr;5(2):102–18. doi: 10.1097/01.hdx.0000061698.20666.aa. [DOI] [PubMed] [Google Scholar]
- 3.Mantero F, Lucarelli G. Aldosterone antagonists in hypertension and heart failure. Ann Endocrinol (Paris) 2000 Feb;61(1):52–60. [PubMed] [Google Scholar]
- 4.Garthwaite SM, McMahon EG. The evolution of aldosterone antagonists. Mol Cell Endocrinol. 2004 Mar 31;217(1–2):27–31. doi: 10.1016/j.mce.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Sica DA. Pharmacokinetics and pharmacodynamics of mineralocorticoid blocking agents and their effects on potassium homeostasis. Heart Fail Rev. 2005 Jan;10(1):23–9. doi: 10.1007/s10741-005-2345-1. [DOI] [PubMed] [Google Scholar]
- 6.Ménard J. The 45-year story of the development of an anti-aldosterone more specific than spironolactone. Mol Cell Endocrinol. 2004 Mar 31;217(1–2):45–52. doi: 10.1016/j.mce.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Sica DA. Aldosterone and volume management in hypertensive heart disease. Semin Nephrol. 2014 Mar;34(3):323–32. doi: 10.1016/j.semnephrol.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Sica DA, Gehr TW. Diuretic combinations in refractory oedema states: pharmacokinetic-pharmacodynamic relationships. Clin Pharmacokinet. 1996 Mar;30(3):229–49. doi: 10.2165/00003088-199630030-00004. [DOI] [PubMed] [Google Scholar]
- 9.Schrijver G, Weinberger MH. Hydrochlorothiazide and spironolactone in hypertension. Clin Pharmacol Ther. 1979 Jan;25(1):33–42. doi: 10.1002/cpt197925133. [DOI] [PubMed] [Google Scholar]
- 10.Ramsay LE, Hettiarachchi J, Fraser R, Morton JJ. Amiloride, spironolactone, and potassium chloride in thiazide-treated hypertensive patients. Clin Pharmacol Ther. 1980 Apr;27(4):533–43. doi: 10.1038/clpt.1980.75. [DOI] [PubMed] [Google Scholar]
- 11.Henningsen NC. Single versus divided dose bioavailability of spironolactone in hypertensive patients. In: Addison GM, Wirenfeldt Asmussen N, Corvol P., Kloppenborg PWC, Norman N, Schroder R, Robertson JIS, editors. Aldosterone antagonists in clinical medicine. Amsterdam: Excerpta Medica; 1978. p. 227. p. [Google Scholar]
- 12.Weinberger MH, White WB, Ruilope LM et al. Effects of eplerenone versus losartan in patients with low-renin hypertension. Am Heart J. 2005 Sep;150(3):426–33. doi: 10.1016/j.ahj.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Weinberger MH, Roniker B, Krause SL, Weiss RJ. Eplerenone, a selective aldosterone blocker, in mild-to-moderate hypertension. Am J Hypertens. 2002 Aug;15(8):709–16. doi: 10.1016/s0895-7061(02)02957-6. [DOI] [PubMed] [Google Scholar]
- 14.Shavit L, Neykin D, Lifshitz M, Slotki I. Effect of eplerenone on blood pressure and the renin-angiotensin-aldosterone system in oligo-anuric chronic hemodialysis patients – a pilot study. Clin Nephrol. 2011 Nov;76(5):388–95. doi: 10.5414/cn106973. [DOI] [PubMed] [Google Scholar]
- 15.Ni X, Zhang J, Zhang P et al. Effect of spironolactone on dialysis patients with refractory hypertension: a randomized controlled study. J Clin Hypertens (Greenwich) 2014 Sep;16(9):658–63. doi: 10.1111/jch.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003 Nov;16(11 Pt 1):925–30. doi: 10.1016/s0895-7061(03)01032-x. [DOI] [PubMed] [Google Scholar]
- 17.Ouzan J, Pérault C, Lincoff AM, Carré E, Mertes M. The role of spironolactone in the treatment of patients with refractory hypertension. Am J Hypertens. 2002 Apr;15(4 Pt 1):333–9. doi: 10.1016/s0895-7061(01)02342-1. [DOI] [PubMed] [Google Scholar]
- 18.Sharabi Y, Adler E, Shamis A, Nussinovitch N, Markovitz A, Grossman E. Efficacy of add-on aldosterone receptor blocker in uncontrolled hypertension. Am J Hypertens. 2006 Jul;19(7):750–5. doi: 10.1016/j.amjhyper.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Dahal K, Kunwar S, Rijal J et al. The effects of aldosterone antagonists in patients with resistant hypertension: a meta-analysis of randomized and nonrandomized studies. Am J Hypertens. 2015 Mar 23 doi: 10.1093/ajh/hpv031. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Saha C, Eckert GJ, Ambrosius WT et al. Improvement in blood pressure with inhibition of the epithelial sodium channel in blacks with hypertension. Hypertension. 2005 Sep;46(3):481–7. doi: 10.1161/01.HYP.0000179582.42830.1d. [DOI] [PubMed] [Google Scholar]
- 21.Taler SJ, Textor SC, Augustine JE. Resistant hypertension: comparing hemodynamic management to specialist care. Hypertension. 2002 May;39(5):982–8. doi: 10.1161/01.hyp.0000016176.16042.2f. [DOI] [PubMed] [Google Scholar]
- 22.Ram CVS, Reichgott MJ. Treatment of loop-diuretic resistant edema by the addition of metolazone. Curr Ther Res. 1977;22:686–91. [Google Scholar]
- 23.Cranston WI, Juel-Jemsen BE. The effects of spironolactone and chlorthalidone on arterial pressure. Lancet. 1962 Jun 2;1(7240):1161–4. doi: 10.1016/s0140-6736(62)92199-2. [DOI] [PubMed] [Google Scholar]
- 24.Winer BM, Lubbe WF, Colton T. Antihypertensive actions of diuretics. Comparative study of an aldosterone antagonist and a thiazide, alone and together. JAMA. 1968 May 27;204(9):775–9. doi: 10.1001/jama.204.9.775. [DOI] [PubMed] [Google Scholar]
- 25.Akbar FA, Boston PF, Chapman J, Pearce MY. Spironolactone and hydroflumethiazide in the treatment of hypertension. Br J Clin Prac. 1981 Sep;35(9):317–21. [PubMed] [Google Scholar]
- 26.Sica DA. Rationale for fixed-dose combinations in the treatment of hypertension: the cycle repeats. Drugs. 2002;62(3):443–62. doi: 10.2165/00003495-200262030-00003. [DOI] [PubMed] [Google Scholar]
- 27.Sica DA. Chlorthalidone: has it always been the best thiazide-type diuretic. Hypertension. 2006 Mar;47(3):321–2. doi: 10.1161/01.HYP.0000203147.75714.ba. [DOI] [PubMed] [Google Scholar]
- 28.Roush GC, Kaur R, Ernst ME. Diuretics: a review and update. J Cardiovasc Pharmacol Ther. 2014 Jan;19(1):5–13. doi: 10.1177/1074248413497257. [DOI] [PubMed] [Google Scholar]
- 29.Bobrie G, Frank M, Azizi M et al. Sequential nephron blockade versus sequential renin-angiotensin system blockade in resistant hypertension: a prospective, randomized, open blinded endpoint study. J Hypertens. 2012 Aug;30(8):1656–64. doi: 10.1097/HJH.0b013e3283551e98. [DOI] [PubMed] [Google Scholar]
- 30.Svensson M, Gustafsson F, Galatius S, Hildebrandt PR, Atar D. How prevalent is hyperkalemia and renal dysfunction during treatment with spironolactone is patients with congestive heart failure? J Card Fail. 2004 Aug;10(4):297–303. doi: 10.1016/j.cardfail.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Pitt B, Reichek N, Willenbrock R et al. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation. 2003 Oct 14;108(15):1831–8. doi: 10.1161/01.CIR.0000091405.00772.6E. [DOI] [PubMed] [Google Scholar]
- 32.Epstein M, Buckalew V, Martinez F et al. Antiproteinuric efficacy of eplerenone, enalapril, and eplerenone/enalapril combination in diabetic hypertensives with microalbuminuria. Am J Hypertens. 2002;15(Suppl 1):A24. [Google Scholar]
- 33.Pitt B, Zannad F, Remme WJ et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999 Sep 2;341(10):709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 34.Pitt B, Remme W, Zannad F et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003 Apr 3;348(14):1309–21. doi: 10.1056/NEJMoa030207. Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. [DOI] [PubMed] [Google Scholar]
- 35.Sato A, Hayashi M, Saruta T. Relative long-term effects of spironolactone in conjunction with an angiotensin-converting enzyme inhibitor on left ventricular mass and diastolic function in patients with essential hypertension. Hypertens Res. 2002 Nov;25(6):837–42. doi: 10.1291/hypres.25.837. [DOI] [PubMed] [Google Scholar]
- 36.Sica DA. Metolazone and its role in edema management. Congest Heart Fail. 2003 Mar–Apr;9(2):100–5. doi: 10.1111/j.1527-5299.2003.01907.x. [DOI] [PubMed] [Google Scholar]
- 37.van Vliet AA, Donker AJ, Nauta JJ, Verheugt FW. Spironolactone in congestive heart failure refractory to high-dose loop diuretic and low-dose angiotensin-converting enzyme inhibitor. Am J Cardiol. 1993 Jan 21;71(3):21A–28A. doi: 10.1016/0002-9149(93)90241-4. [DOI] [PubMed] [Google Scholar]
- 38.Farquharson CA, Struthers AD. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation. 2000 Feb 15;101(6):594–7. doi: 10.1161/01.cir.101.6.594. [DOI] [PubMed] [Google Scholar]
- 39.Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001 Dec 6;345(23):1689–97. doi: 10.1056/NEJMra000050. [DOI] [PubMed] [Google Scholar]
- 40.Jorde UP, Vittorio T, Katz SD, Colombo PC, Latif F, Le Jemtel TH. Elevated plasma aldosterone levels despite complete inhibition of the vascular angiotensin-converting enzyme in chronic heart failure. Circulation. 2002 Aug 27;106(9):1055–7. doi: 10.1161/01.cir.0000030935.89559.04. [DOI] [PubMed] [Google Scholar]
- 41.Rocha R, Williams GH. Rationale for the use of aldosterone antagonists in congestive heart failure. Drugs. 2002;62(5):723–31. doi: 10.2165/00003495-200262050-00001. [DOI] [PubMed] [Google Scholar]
- 42.Bauersachs J, Heck M, Fraccarollo D et al. Addition of spironolactone to angiotensin-converting enzyme inhibition in heart failure improves endothelial vasomotor dysfunction: role of vascular superoxide anion formation and endothelial nitric oxide synthase expression. J Am Coll Cardiol. 2002 Jan;39:351–8. doi: 10.1016/s0735-1097(01)01729-6. [DOI] [PubMed] [Google Scholar]
- 43.Pitt B, Pfeffer MA, Assmann SF et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014 Apr 10;370(15):1383–92. doi: 10.1056/NEJMoa1313731. TOPCAT Investigators. [DOI] [PubMed] [Google Scholar]
- 44.2013 ACCF/AHA Practice Guideline for the Management of Heart Failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013 Oct 15;128(16):e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 45.Sato A, Hayashi K, Saruta T. Antiproteinuric effects of mineralocorticoid receptor blockade in patients with chronic renal disease. Am J Hypertens. 2005 Jan;18(1):44–9. doi: 10.1016/j.amjhyper.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 46.Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]) Am J Cardiol. 1996 Oct 15;78(8):902–7. doi: 10.1016/s0002-9149(96)00465-1. [DOI] [PubMed] [Google Scholar]
- 47.Svensson M, Gustafsson F, Galatius S, Hildebrandt PR, Atar D. How prevalent is hyperkalemia and renal dysfunction during treatment with spironolactone is patients with congestive heart failure? J Card Fail. 2004 Aug;10(4):297–303. doi: 10.1016/j.cardfail.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 48.Wei L, Struthers AD, Fahey T, Watson AD, MacDonald TM. Spironolactone use and renal toxicity: population-based longitudinal analysis. BMJ. 2010 May 18;340:c1768. doi: 10.1136/bmj.c1768. [DOI] [PubMed] [Google Scholar]
- 49.Tamirisa KP, Aaronson KD, Koelling TM. Spironolactone-induced renal insufficiency and hyperkalemia in patients with heart failure. Am Heart J. 2004 Dec;148(6):971–8. doi: 10.1016/j.ahj.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 50.Scherstén B, Thulin T, Kuylenstierna J et al. Clinical and biochemical effects of spironolactone administered once daily in primary hypertension. Hypertension. 1980 Sep–Oct;2(5):672–9. doi: 10.1161/01.hyp.2.5.672. [DOI] [PubMed] [Google Scholar]


