Abstract
Wnt signaling plays an essential role in the dental epithelium and mesenchyme during tooth morphogenesis. However, it remains unclear if Wnt ligands, produced from dental mesenchyme, are necessary for odontoblast differentiation and dentin formation. Here, we show that odontoblast-specific disruption of Wntless (Wls), a chaperon protein that regulates Wnt sorting and secretion, leads to severe defects in dentin formation and root elongation. Dentin thickness decreased remarkably and pulp chambers enlarged in the mandibular molars of OC-Cre;WlsCO/CO mice. Although the initial odontoblast differentiation was normal in the mutant crown, odontoblasts became cuboidal and dentin thickness was reduced. In immunohistochemistry, Wnt10a, β-catenin, type I collagen, and dentin sialoprotein were significantly down-regulated in the odontoblasts of mutant crown. In addition, roots were short and root canals were widened. Cell proliferation was reduced in the developing root apex of mutant molars. Furthermore, Wnt10a and Axin2 expression was remarkably decreased in the odontoblasts of mutant roots. Deletion of the Wls gene in odontoblasts appears to reduce canonical Wnt activity, leading to inhibition of odontoblast maturation and root elongation.
Keywords: odontoblasts, mice, tooth development, Wnt signaling pathway, tooth roots, dentinogenesis
Introduction
Dentin is a major part of teeth produced by odontoblasts, which differentiate from dental papilla cells under the influence of the inner dental epithelium. Many signaling molecules, including Shh, Fgf, Bmp, and Wnt, have been associated with odontoblast differentiation, dentin formation, and tooth morphogenesis (Tummers and Thesleff 2009).
Wnt/β-catenin signaling plays an essential role in the morphogenesis and cellular differentiation of many tissues (Logan and Nusse 2004). During tooth development, Wnt/β-catenin signaling plays multiple roles in various stages of tooth morphogenesis (Liu and Millar 2010). Several genetic studies revealed that Wnt/β-catenin signaling is required for tooth formation in both dental epithelium and mesenchyme (Jarvinen et al. 2006; Liu et al. 2008; Chen et al. 2009). Although most Wnt family members are expressed in the dental epithelium during early tooth morphogenesis, some Wnts, such as Wnt5a and Wnt10a, as well as Wnt signaling mediators, Axin2 and Lef-1, are expressed in developing odontoblasts (Yamashiro et al. 2007; Lohi et al. 2010; Yokose and Naka 2010; Lin et al. 2011). Dkk1, an inhibitor of Wnt signaling, is strongly expressed in pre-odontoblasts but is decreased in secretory odontoblasts (Fjeld et al. 2005). These reports strongly suggest that modulation of Wnt signaling may play an important role in odontoblast differentiation and dentin formation. This idea is also supported by the tooth phenotypes observed in several transgenic mouse models. We recently found that tissue-specific inactivation or constitutive stabilization of β-catenin leads to disrupted odontoblast differentiation in roots or excessive dentin formation, respectively (Kim et al. 2011; Kim et al. 2013). In addition, tissue-specific overexpression of Dkk1 in odontoblasts leads to impaired dentin apposition and root elongation (Han et al. 2011). Based on previous reports, Wnt/β-catenin signaling may play a crucial role in the odontoblast differentiation and dentin formation. Wnt inhibitors, such as Dkk1, regulate the pathway to allow optimal differentiation, maturation, and matrix production.
The Wntless (Wls), a chaperon protein, is specifically required for the secretion of Wnt proteins in cells (Banziger et al. 2006). Since the secretion of Wnt proteins is blocked by inactivation of Wls (Bartscherer et al. 2006), conditional depletion of Wls has been extensively used to investigate the role of Wnt proteins in a variety of tissues, including brain, hair follicle, taste bud, and osteoblasts (Fu et al. 2011; Zhong et al. 2012; Myung et al. 2013; Wan et al. 2013; Zhu et al. 2014). Recently, Zhu et al. (2013) reported that tooth morphogenesis is arrested in tissue-specific ablation of Wls in the dental epithelium. Furthermore, disruption of Wls in osteocalcin (Ocn or bone gamma carboxyglutamate protein, Bglap) expressing cells leads to various tooth defects such as significant increase of dentin volume and density in upper incisors, idiopathic root resorption, and wider periodontal ligament space (Lim et al. 2014a, 2014b, 2014c). Although much evidence supports the possible roles of Wnt signaling in odontoblast differentiation and dentin formation, it remains unclear whether Wnt ligands secreted from odontoblasts are involved in odontoblast differentiation and matrix formation. We analyzed the role of Wls during odontoblast differentiation and dentin formation in conditionally deleted mice.
Materials and Methods
Mouse Strains
The Animal Welfare Committee of Chonbuk National University approved all experimental procedures. Wls-floxed allele mice (Carpenter et al. 2010) and R26R reporter mice (Soriano 1999) were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Osteocalcin-Cre (OC-Cre) mice were generated with 1.3-kb mouse osteocalcin promoter as described previously (Tan et al. 2007). This promoter is different from the OCN-Cre generated with human Osteocalcin promoter (10 kb; Zhang et al. 2002) used by Lim et al. (2014a, 2014b, 2014c). To generate OC-Cre;WlsCO/CO mice, OC-Cre;WlsCO/+ mice were crossed with WlsCO/CO mice. The offspring were genotyped by polymerase chain reaction (PCR) analysis using previously described primers (Tan et al. 2007; Carpenter et al. 2010). For analysis of Cre activity, the OC-Cre mice were crossed with R26R mice, and the mandibles of double transgenic mice were processed for X-gal staining as described previously (Kim et al. 2013). A total of 63 animals were used in this study.
Histology, Immunohistochemistry, and BrdU Labeling
For histologic analysis, tissues were fixed in 4% paraformaldehyde (PFA) and decalcified in 10% EDTA solutions for 2 to 4 wk at 4°C. Tissues were embedded in paraffin and sections used for hematoxylin and eosin (H&E) staining as well as immunohistochemistry.
For immunohistochemistry, sections were treated with 3% hydrogen peroxide and incubated with rabbit polyclonal antibodies against Wls (1:200; kindly provided by Dr. Zunyi Zhang), Wnt10a (1:50; Sigma-Aldrich, St. Louis, MO, USA), cytokeratin 14 (CK14, 1:900; Covance, Berkeley, CA, USA), β-catenin (1:250; Thermo Scientific, Fremont, CA, USA), pSmad1/5/8 (1:200; Cell Signaling Technology, Danvers, MA, USA), Osx (1:200; Abcam, Cambridge, UK), Axin2 (1:300; Abcam), Phex (1:50; Sigma-Aldrich), type I collagen (Col-1, 1:200; Abcam), decorin (Dcn, 1:800; kindly provided by Dr. Larry Fisher), Dsp (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and Cre recombinase (1:100; Covance). Histostain Plus rabbit primary (DAB) kit (Zymed Laboratories, San Francisco, CA, USA) was used according to the manufacturer’s instructions. To detect cell proliferation in developing roots, 5′-bromo-2′-deoxyuridine (BrdU) labeling reagent (45 µg/g body weight; Roche, Indianapolis, IN, USA) was injected intraperitoneally 2 h prior to euthanasia. The BrdU detection kit (Roche) was used as previously described (Kim et al. 2013). For statistical analysis, 3 independent littermates were used in each study.
Micro–Computed Tomography and Scanning Electron Microscopy
Mandibles from OC-Cre;WlsCO/+ (control) and OC-Cre; WlsCO/CO (mutant) were scanned using a desktop scanner (1076 Skyscan Micro-CT; Skyscan, Kontich, Belgium). They were subsequently reconstructed and analyzed with CTAn software (Skyscan).
To isolate molars, skin and muscles were clipped away from the dissected mandibles of 28-d-old (P28) mice. The mandibles were then incubated in 50 mM Tris-Cl (pH 8.0), 0.5% sodium dodecyl sulfate (SDS), and 0.2 mg proteinase K/mL at 55°C for 1 h. After a brief wash in water, molars were extracted and cleared in 1% KOH for 3 d. The specimen surfaces were sputter-coated with platinum after drying and examined with a scanning electron microscope (JSM-6400; JEOL, Tokyo, Japan) under 20-kV conditions.
Analysis of Dentin Apposition Rate by Double Labeling
To examine the dentin apposition rate, we performed double fluorescence labeling as described previously (Han et al. 2011). Briefly, a calcein label (5 mg/kg intraperitoneally [IP]; Sigma-Aldrich) was administered to P14 mice, followed by administration of calcein 14 d later. Mice were sacrificed 2 d after the second injection (P30). For statistical analysis, 3 independent littermates were used in each study.
Statistical Analysis
All data are presented as means ± SEM. All statistical analyses were done using GraphPad Prism software (GraphPad Software, La Jolla, CA, USA). Statistical differences were determined by a simple unpaired t test, and P < 0.05 was considered statistically significant.
Results
Thin Dentin, Widening Pulp Chamber, and Short Roots in Mandibular Molars of OC-Cre;WlsCO/CO Mice
To determine the roles of Wnts produced in odontoblasts, a conditional Wls gene inactivation mouse was generated using OC-Cre mice. We confirmed that Cre recombination from the OC-Cre promoter was active in odontoblasts of postnatal molars of OC-Cre;R26R double transgenic mice. No LacZ and Cre expression was observed in the developing tooth germ prior to P0 (Appendix Fig. 1A–C). At P4 and P6, OC-Cre was specifically expressed in crown odontoblasts (Appendix Fig. 1D–F). At P10, LacZ expression was especially strong in the odontoblasts of developing roots (Appendix Fig. 1G). We focused our analysis on mice between 8 and 28 d, by which time roots on the first molars are fully formed.
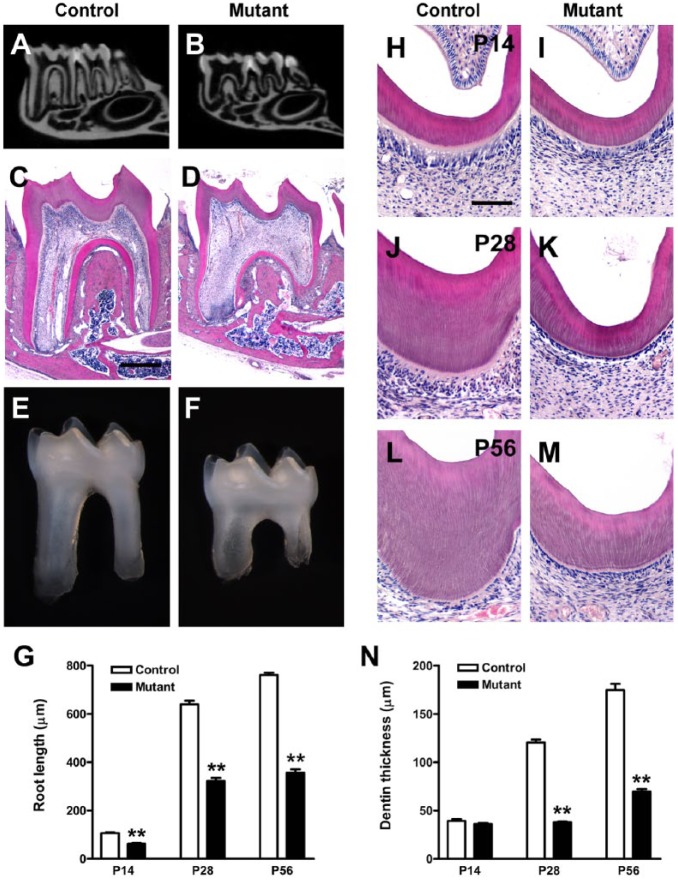
At P28, mandibular molars in OC-Cre;WlsCO/CO mice exhibited thin dentin, enlarged pulp chamber, and short roots. Micro–computed tomography revealed that dentin thickness decreased remarkably in both crown and root regions with enlarged pulp chambers and root canals (Fig. 1A, B). These findings were also clearly observed in the H&E-stained midsagittal sections of the mandibular first molars (Fig. 1C, D). In isolated mandibular first molars, mutant roots were shorter than control roots, whereas there was no difference in crown height (Fig. 1E, F). In controls, root length rapidly increased with age, whereas mutant root length increased at about half this rate (P < 0.01; Fig. 1G).
Figure 1.
Tooth phenotypes in OC-Cre;WlsCO/CO mice. Mandibular molars of mutant mice exhibited thin dentin, enlarged pulp chambers, and short roots. (A, B) Micro–computed tomographic view of mandibles from control and mutant mice at P28. (C–F) Histologic features and stereoscopic appearance of the mandibular first molar of control and mutant mice at P28. (G) Quantification of root length of the mandibular first molar at P14, P28, and P56 (n = 6, in each genotype for stages, respectively). (H–M) Hematoxylin and eosin–stained sections of crown dentin at P14, P28, and P56. (N) Differences in dentin thickness of controls and mutants at P14, P28, and P56 (n = 6, in each genotype for stages, respectively). **P < 0.01. Scale bars: 400 µm (C, D), 100 µm (H–M).
There was no remarkable difference in dentin thickness between the control and mutant groups at P14 (P > 0.05; Fig. 1H, I, N). However, dentin thickness increased slightly in the mutant group at P28 and P56 in contrast to dramatically increased dentin thickness in controls (P < 0.01; Fig. 1J–N). In double fluorescence labeling, the dentin apposition rate was significantly reduced in both crown and cervix of mutant molars (P < 0.05; Appendix Fig. 2).
Deletion of Wls in Odontoblasts Inhibits Polarization and Secretion of Dentin Matrix
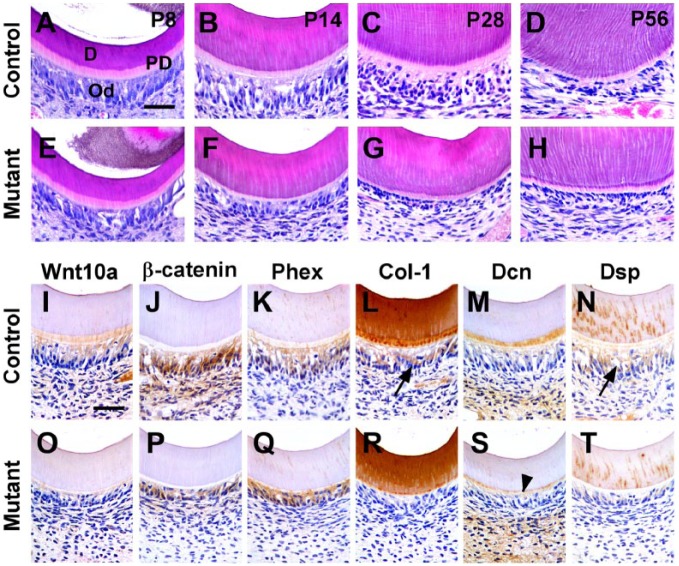
Since dentin thickness was decreased abruptly following ablation of Wls in odontoblasts, histologic differences in the odontoblasts of mandibular first molars were compared for mutant and control mice. At P8, odontoblasts were well differentiated with a tall columnar shape in both control and mutant mice with no difference in dentin thickness (Fig. 2A, E). From P14 to P56, mutant odontoblasts lost most of their cytoplasm and changed to a cuboidal shape in contrast to the control odontoblasts, which had a columnar shape and abundant cytoplasm (Fig. 2B–D, F–H).
Figure 2.
Morphologic and molecular changes in crown odontoblasts of OC-Cre;WlsCO/CO mice during dentin formation. (A–H) Histologic features of odontoblasts in the crowns of control and mutant mice at P8, P14, P28, and P56. (I–T) Localization of Wnt10a, β-catenin, Phex, Col-1, Dcn, and Dsp on the crown odontoblasts of the mandibular first molar at P14. Black arrowhead indicates predentin. D, dentin; Od, odontoblasts; PD, predentin. Scale bars: 40 µm (A–H), 100 µm (I–T).
Because a cellular change appeared from P14, immunohistochemistry was performed to assess the molecular changes. In controls, Wnt10a was localized in odontoblasts and predentin matrix, but in the mutants, Wnt10a could not be detected (Fig. 2I, O). A key intracellular mediator of Wnt/β-catenin signaling, β-catenin is normally expressed in wild-type odontoblasts and pulp cells, but in mutants, β-catenin expression was decreased (Fig. 2J, P). Note it was not possible using immunohistochemical staining methods to resolve whether β-catenin protein was primarily nuclear (active) or cytoplasmic (inactive). Nevertheless, the qualitative staining differences suggest an overall decrease in β-catenin, which is the major signal transduction molecule in the canonical Wnt pathway.
There were also differences in the quality of the dentin matrix in the coronal part of the mutant molars. In both controls and mutants, Phex (a phosphate-regulating endopeptidase; Kim et al. 2013) was localized to odontoblasts and dentin matrix (Fig. 2K, Q). Col-1 was expressed in control odontoblasts but was absent in mutant odontoblasts (Fig. 2L, R). In controls, Dcn (a proteoglycan, decorin) was specifically localized in the predentin and pulp core, but it was significantly downregulated in mutant predentin (Fig. 2M, S). Dsp, an important mineralization regulator of dentin, was localized in control odontoblasts and dentinal tubules. Dsp expression was restricted to mutant dentinal tubules (Fig. 2N, T). We next investigated in detail the root tissues to determine if the same decrease in canonical Wnt signaling had taken place.
Impaired Root Elongation in OC-Cre;WlsCO/CO Mice
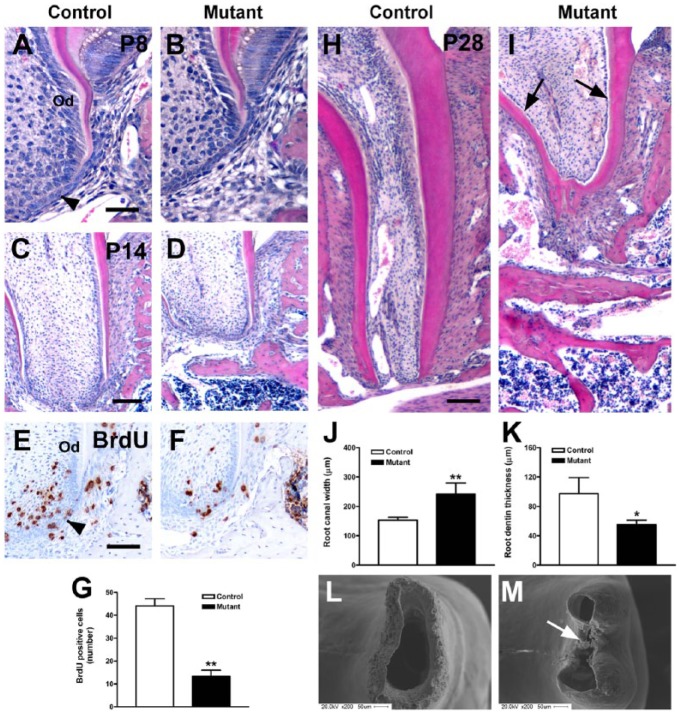
OC-Cre;WlsCO/CO mice had short molar roots with enlarged root canals. Histologic differences were observed in the developing roots of mandibular first molars of mutant and control mice from P8 to P28. In the cervical region of control mice at P8, odontoblasts were differentiated and already produced root dentin. In contrast, odontoblast differentiation and dentin formation was delayed in mutants (Fig. 3A, B and Appendix Fig. 3). In addition, there were some differences in Hertwig’s epithelial root sheath (HERS). In controls at P8, the coronal part of HERS had fragmented adjacent to newly formed root dentin. In mutants, the HERS was not fragmented (Appendix Fig. 3A, B). There appears delayed odontoblast differentiation in the cervical region in the mutants. At P14, molar root length was significantly decreased in mutants compared with controls (P < 0.01; Fig. 1G, Fig. 3C, D); therefore, extension of HERS was delayed. The mechanisms for the delay appear to be a lack of proliferation in the dental mesenchyme of the mutants (P < 0.01; Fig. 3E–G). At P28, normal molar roots were almost their final length, but the root apex had not closed. Root dentin was formed by well-differentiated odontoblasts (Fig. 3H). In contrast, mutant molar roots were short, about half the length of controls (P < 0.01; Fig. 1G, Fig. 3I). Mutant root canals were widened and surrounded by thin radicular dentin (P < 0.01; Fig. 3I–K). Root odontoblasts were a flattened cuboidal shape and aligned inside the root dentin. Some parts of the root apex were prematurely closed (Fig. 3I). In scanning electron microscopic examination, control root apexes were still open at P28. In mutants, root dentin was sharp in the apex and was partly obstructed (Fig. 3L, M).
Figure 3.
Impaired root elongation in the mandibular first molar of OC-Cre;WlsCO/CO mice. (A–D, H–I) Hematoxylin and eosin–stained sections of the mandibular first molar in control and mutant mice at P8, P14, and P28. (E–G) In the developing root apex of controls at P14, BrdU-labeled proliferating cells were abundant. However, few cells were positively labeled with BrdU in mutant mice. (J–K) Root canal width and radicular dentin thickness in control and mutant mice at P28. (L–M) Basal view of distal root apex of the mandibular first molar at P28. *P < 0.05 and **P < 0.01. Black arrowhead indicates Hertwig’s epithelial root sheath. Od, odontoblasts. Scale bars: 40 µm (A, B), 80 µm (E, F), and 100 µm (C, D, G, H).
We then went back to examine the effect of Wls deletion on root dentin at the time when deposition is taking place, P14. In controls, Phex (a phosphate-regulating endopeptidase; Kim et al. 2013) was localized in mature odontoblasts but not in differentiating odontoblasts (Fig. 4A). Phex localization in mutants was similar to that of controls (Fig. 4E). In controls, Dcn (a proteoglycan, decorin) was localized in the predentin between dentin and odontoblasts as well as in the periodontium (Fig. 4B). In mutants, Dcn was also observed in predentin along developing roots, but it was very thin compared with controls (Fig. 4F). Col-1 and Dsp were localized in dentin in both controls and mutants (Fig. 4C, D, G, H).
Figure 4.
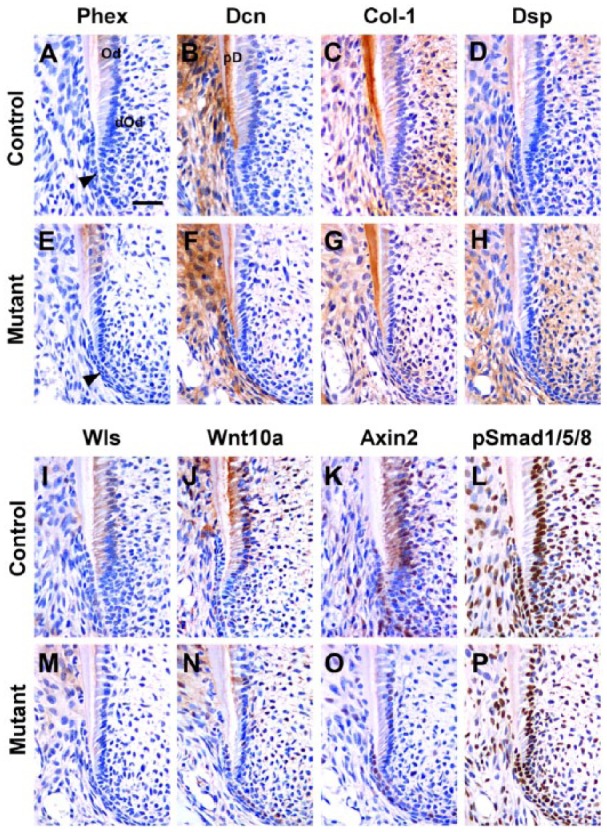
Inhibition of odontoblast maturation during root elongation in developing molar roots of OC-Cre;WlsCO/CO mice. (A, E) Both in controls and mutants, Phex was localized in mature odontoblasts but not in differentiating odontoblasts. (B, F) In controls, Dcn specifically marked predentin, which was thinner in mutants. (C, D, G, H) Col-1 and Dsp were localized in dentin in both controls and mutants. (I, M) Wls was localized in mature odontoblasts and differentiating odontoblasts in control but was not found in mutant odontoblasts. (J, K, N, O) Wnt10a and Axin2 were widely expressed in control odontoblasts, differentiating odontoblasts, and Hertwig’s epithelial root sheath (HERS) cells, but their overall expression was decreased in mutants. (L, P) pSmad1/5/8 was abundantly expressed in both control and mutant odontoblasts, HERS, and pulp cells. Black arrowhead indicates differentiating odontoblasts. Od, odontoblasts; dOd, differentiating odontoblasts; PD, predentin. Scale bars: 40 µm (A–P).
Next we examined Wnt pathway markers. Wls was localized in mature odontoblasts and differentiating odontoblasts in controls but was not found in mutant odontoblasts (Fig. 4I, M). In controls, Wnt10a and Axin2 were localized in mature and differentiating odontoblasts and HERS cells (Fig. 4J, K). In mutants, Wnt10a and Axin2 expressions in odontoblasts were decreased but remained in HERS cells (Fig. 4N, O). Thus, there is a possible decrease in Wnt activity in the odontoblasts. Since the Wnt pathway has crosstalk with the Bmp (bone morphogenic protein) pathway, we also examined readout of active Bmp signaling, pSmad1/5/8. No differences in expression were detected between controls and mutants (Fig. 4L, P). Therefore, the Bmp pathway is not affected by the decrease in Wnt signaling.
Discussion
This study investigated the roles of Wnt signaling in odontoblast differentiation and dentin formation. OC-Cre–mediated Wls conditional knockout mice exhibited mandibular molar phenotypes characterized by thin dentin, enlarged pulp chamber, and short roots. This abnormality was closely associated with delayed odontoblast maturation, failure of HERS extension, and reduced dentin apposition.
It has been suggested that signaling molecules produced in the inner enamel epithelium may induce odontoblasts (Tummers and Thesleff 2009). Since numerous Wnt family members such as Wnt3, Wnt4, Wnt6, and Wnt10b are expressed in the dental epithelium and Wnt readouts are active in the dental mesenchyme, Wnt ligands may be inductive signals for odontoblast differentiation (Chen et al. 2009). However, Zhu et al. (2013) recently reported that the dental mesenchyme retains its own odontogenic program despite epithelial-specific ablation of Wls. In this experiment, all secreted Wnts from the inner enamel epithelium do not get synthesized, and thus paracrine signaling by Wnts is not required for odontoblast differentiation. To understand more clearly the autocrine roles of Wnt signaling, we deleted Wls in odontoblasts after they had begun to polarize in the crown but were still cuboidal in the root. In the mandibular molars of OC-Cre;WlsCO/CO mice, odontoblasts abruptly changed a cuboidal shape, and dentin thickness decreased after mantle dentin formation. There was an indirect effect of the loss of Wls on the protein levels of the ligand Wnt10a and 2 Wnt pathway molecules, Axin2 and β-catenin, in the crown and root. The decrease in 2 important signal transduction molecules suggests that there would be less canonical activity, which in turn inhibits odontoblast maturation and dentin apposition.
Abnormalities in dentin apposition have been reported in several animal models with disrupted Wnt signaling, but not all of these gave similar phenotypes to ours. One of these studies (Lim et al. 2014a) used a similar strategy to ours and deleted Wls in odontoblasts, using a different osteocalcin-Cre (Zhang et al. 2002). In their study, dentin thickness increased and pulp volume decreased in the incisors (Lim et al. 2014a). However, in our experiments, the opposite phenotypes were observed, including thin dentin with enlarged pulp chambers and short roots. The differences are likely due to timing of expression of the osteocalcin-Cre recombinase. Our Cre promoter drove expression postnatally, whereas the other line may have included prenatal expression. Other studies support our findings. Tissue-specific overexpression of the Wnt antagonist, Dkk1, in odontoblasts led to short roots and impaired dentin apposition in mandibular molars (Han et al. 2011). One of our previous studies used the same OC-Cre line to conditionally delete the gene coding for β-catenin (Kim et al. 2013). Molars without roots emerged into the oral cavity in OC-Cre;Ctnnb1CO/CO mice. There was less of an effect on coronal dentin than in the present study. This suggests that Wnt ligands were possibly acting via other pathways in the crown compared with the root. A final piece of evidence supporting cell-autonomous roles for mesenchymal Wnt signaling in dentin formation comes from a gain-of-function experiment. Constitutive stabilization of β-catenin in dental mesenchyme led to increased dentin thickness (Kim et al. 2011).
Taken together, the results suggest that Wnt/β-catenin signaling in odontoblasts is finely regulated for differentiation and matrix production. Wnt ligands secreted from odontoblasts are required for odontoblast maturation, dentin apposition, and root elongation during development.
Author Contributions
C.H. Bae, T.H. Kim, E.S. Cho, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; S.O. Ko, contributed to conception, data analysis, and interpretation, critically revised the manuscript; J.C. Lee, contributed to conception, data acquisition, and analysis, critically revised the manuscript; X. Yang, contributed to design and data interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
We thank Dr. Larry Fisher and Dr. Zunyi Zhang for providing antibody of decorin and Wls, respectively.
Footnotes
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (no. 2013R1A 2A1A01007642) and Global Ph.D. Fellowship Program (no. 2013034141 to CB).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Banziger C, Soldini D, Schütt C, Zipperlen P, Hausmann G, Basler K. 2006. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 125(3):509–522. [DOI] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M. 2006. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 125(3):523–533. [DOI] [PubMed] [Google Scholar]
- Carpenter AC, Rao S, Wells JM, Campbell K, Lang RA. 2010. Generation of mice with a conditional null allele for Wntless. Genesis. 48(9):554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lan Y, Baek JA, Gao Y, Jiang R. 2009. Wnt/beta-catenin signaling plays an essential role in activation of odontogenic mesenchyme during early tooth development. Dev Biol. 334(1):174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjeld K, Kettunen P, Furmanek T, Kvinnsland IH, Luukko K. 2005. Dynamic expression of Wnt signaling-related Dickkopf1, -2, and -3 mRNAs in the developing mouse tooth. Dev Dyn. 233(1):161–166. [DOI] [PubMed] [Google Scholar]
- Fu J, Ivy Yu HM, Maruyama T, Mirando AJ, Hsu W. 2011. Gpr177/mouse Wntless is essential for Wnt-mediated craniofacial and brain development. Dev Dyn. 240(2):365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han XL, Liu M, Voisey A, Ren YS, Kurimoto P, Gao T, Tefera L, Dechow P, Ke HZ, Feng JQ. 2011. Post-natal effect of overexpressed DKK1 on mandibular molar formation. J Dent Res. 90(11):1312–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I. 2006. Continuous tooth generation in mouse is induced by activated epithelial Wnt/β-catenin signaling. Proc Acad Natl Sci USA. 103(49):18627–18632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Bae CH, Lee JC, Ko SO, Yang X, Jiang R, Cho ES. 2013. β-Catenin is required in odontoblasts for tooth root formation. J Dent Res. 92(3):215–221. [DOI] [PubMed] [Google Scholar]
- Kim TH, Lee JY, Baek JA, Lee JC, Yang X, Taketo MM, Jiang R, Cho ES. 2011. Constitutive stabilization of β-catenin in the dental mesenchyme leads to excessive dentin and cementum formation. Biochem Biophys Res Commun. 412(4):549–555. [DOI] [PubMed] [Google Scholar]
- Lim WH, Liu B, Cheng D, Hunter DJ, Zhong Z, Ramos DM, Williams BO, Sharpe PT, Bardet C, Mah SJ, et al. 2014a. Wnt signaling regulates pulp volume and dentin thickness. J Bone Miner Res. 29(4):892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim WH, Liu B, Cheng D, Williams BO, Mah SJ, Helms JA. 2014b. Wnt signaling regulates homeostasis of the periodontal ligament. J Periodontal Res. 49(6):751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim WH, Liu B, Hunter DJ, Cheng D, Mah SJ, Helms JA. 2014c. Downregulation of Wnt causes root resorption. Am J Orthod Dentofacial Orthop. 146(3):337–345. [DOI] [PubMed] [Google Scholar]
- Lin M, Li L, Liu C, Liu H, He F, Yan F, Zhang Y, Chen Y. 2011. Wnt5a regulates growth, patterning, and odontoblast differentiation of developing mouse tooth. Dev Dyn. 240(2):432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, Yang SH, Lu MM, Piccolo S, Schmidt-Ullrich R, et al. 2008. Wnt/β-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol. 313(1):210–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Millar SE. 2010. Wnt/β-catenin signaling in oral tissue development and disease. J Dent Res. 89(4):318–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. 2004. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 20:781–810. [DOI] [PubMed] [Google Scholar]
- Lohi M, Tucker AS, Sharpe PT. 2010. Expression of Axin2 indicates a role for canonical Wnt signaling in development of the crown and root during pre- and postnatal tooth development. Dev Dyn. 239(1):160–167. [DOI] [PubMed] [Google Scholar]
- Myung PS, Takeo M, Ito M, Atit RP. 2013. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J Invest Dermatol. 133(1):31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 21(1):70–71. [DOI] [PubMed] [Google Scholar]
- Tan X, Weng T, Zhang J, Wang J, Li W, Wan H, Lan Y, Cheng X, Hou N, Liu H, et al. 2007. Smad4 is required for maintaining normal murine postnatal bone homeostasis. J Cell Sci. 120(Pt 13):2162–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummers M, Thesleff I. 2009. The importance of signal pathway modulation in all aspects of tooth development. J Exp Zool B Mol Dev Evol. 312B(4):309–319. [DOI] [PubMed] [Google Scholar]
- Wan Y, Lu C, Cao J, Zhou R, Yao Y, Yu J, Zhang L, Zhao H, Li H, Zhao J, et al. 2013. Osteoblastic Wnts differentially regulate bone remodeling and the maintenance of bone marrow mesenchymal stem cells. Bone. 55(1):258–267. [DOI] [PubMed] [Google Scholar]
- Yamashiro T, Zheng L, Shitaku Y, Saito M, Tsubakimoto T, Takada K, Takano-Yamamoto T, Thesleff I. 2007. Wnt10a regulates dentin sialophosphoprotein mRNA expression and possibly links odontoblast differentiation and tooth morphogenesis. Differentiation. 75(5):452–462. [DOI] [PubMed] [Google Scholar]
- Yokose S, Naka T. 2010. Lymphocyte enhancer-binding factor 1: an essential factor in odontoblastic differentiation of dental pulp cells enzymatically isolated from rat incisors. J Bone Miner Metab. 28(6):650–658. [DOI] [PubMed] [Google Scholar]
- Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, et al. 2002. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 177(46):44005–44012. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Zylstra-Diegel CR, Schumacher CA, Baker JJ, Carpenter AC, Rao S, Yao W, Guan M, Helms JA, Lane NE, et al. 2012. Wntless functions in mature osteoblasts to regulate bone mass. Proc Natl Acad Sci USA. 109(33):E2197–E2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Liu Y, Zhao P, Dai Z, Yang X, Li Y, Qiu M, Zhang Z. 2014. Gpr177-mediated Wnt signaling is required for fungiform placode initiation. J Dent Res. 93(6):582–588. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhao P, Liu Y, Zhang X, Fu J, Ivy Yu HM, Qiu M, Chen Y, Hsu W, Zhang Z. 2013. Intra-epithelial requirement of canonical Wnt signaling for tooth morphogenesis. J Biol Chem. 288(17):12080–12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.