Abstract
Ephrin-A2–EphA2 and ephrin-B2–EphB4 interactions have been implicated in the regulation of bone remodeling. We previously demonstrated a potential role for members of the Eph-ephrin family of receptor tyrosine kinases for bone remodeling during orthodontic tooth movement: compression-dependent upregulation of ephrin-A2 in fibroblasts of the periodontal ligament (PDL) attenuated osteogenesis in osteoblasts of the alveolar bone. However, factors affecting the regulation of ephrin-A2 expression upon the application of compressive forces remained unclear. Here, we report a mechano-dependent pathway of ephrin-A2 induction in PDL fibroblasts (PDLFs) involving extracellular signal–regulated kinases (ERK) 1/2 and c-fos. PDLF subjected to compressive forces (30.3 g/cm2) upregulated c-fos and ephrin-A2 mRNA and protein expression and displayed increased ERK1/2 phosphorylation. Inhibition of the MAP kinase kinase (MEK)/ERK1/2 pathway using the specific MEK inhibitor U0126 significantly reduced ephrin-A2 messenger RNA upregulation upon compression. Silencing of c-fos using a small interfering RNA approach led to a significant inhibition of ephrin-A2 induction upon the application of compressive forces. Interestingly, ephrin-A2 stimulation of PDLF induced c-fos expression and led also to the induction of ephrin-A2 expression. Using a reporter gene construct in murine 3T3 cells, we found that ephrin-A2 was able to stimulate serum response element (SRE)–dependent luciferase activity. As the regulation of c-fos is SRE dependent, ephrin-A2 might induce c-fos via SRE activation. Taken together, we provide evidence for an ERK1/2- and c-fos–dependent regulation of ephrin-A2 in compressed PDLF and suggest a novel pathway for ephrin-A2 induction emanating from ephrin-A2 itself. We showed previously that ephrin-A2 at compression sites might contribute to tooth movement by inhibiting osteogenic differentiation. The regulatory pathway of ephrin-A2 induction during tooth movement identified in this study might be accessible for pharmacological interventions.
Keywords: Eph family receptors, tooth movement, cellular mechanotransduction, mitogen-activated protein kinase 1, periodontal ligament, serum response element
Introduction
Periodontal ligament (PDL) fibroblasts are among the first recipients of mechanical forces during tooth movement. Their ability to integrate mechanical stimuli and mediate bone remodeling processes could be of pivotal importance for the regulation of orthodontic tooth movement (OTM) (Kawarizadeh et al. 2005). However, the details of the molecular regulation of mechano-dependent bone remodeling and crosstalk between PDL fibroblasts (PDLFs) and osteoblasts of the alveolar bone during tooth movement are not fully elucidated. Recently, semaphorin 3A, ephrin-A2, and ephrin-B2, members of families of axon guidance molecules, were added to the increasing number of factors involved in bone remodeling (Zhao et al. 2006; Irie et al. 2009; Hayashi et al. 2012). The Eph-ephrin family shows a mechano-dependent regulation of function and expression at least in PDLF and endothelial cells (Korff et al. 2008; Obi et al. 2009; Diercke, Kohl, et al. 2011; Diercke, Sen, et al. 2011).
Previously, we showed that compression-dependent regulation of ephrin-A2 in PDLFs via the interaction with osteoblast EphA2-receptors resulted in an inhibition of osteoblastogenesis in osteoblasts and PDLFs and thereby might contribute to bone remodeling during OTM (Diercke, Sen, et al. 2011). Currently, the mechano-dependent regulation of ephrin-A2 in periodontal cells is not fully understood, and revealing data on putative pathways involved in the mechano-dependent regulation of ephrin-A2 remains a challenge.
During osteoclastogenesis of macrophage colony-stimulating factor (M-CSF)–dependent macrophages, a c-fos–dependent regulation of ephrin-A2 expression was reported (Irie et al. 2009). c-fos, together with c-jun, forms the heterodimeric transcription factor AP-1. AP-1 is known to be targeted by mechanically induced signaling events (Hipskind and Bilbe 1998). The role of AP-1 in the mechano-dependent induction of target genes was demonstrated in osteoblasts and PDLFs (Peake and El Haj 2003; Kook et al. 2009). A compressive force-dependent upregulation of c-fos messenger RNA (mRNA) and protein levels was shown in osteoblast-like cells (Glantschnig et al. 1996; Li et al. 2007). Mechano-dependent c-fos regulation in PDLFs and other mechano-inducible cell types, including chondrocytes, cardiac myocytes, and vascular smooth muscle cells, is dependent on the activation of mitogen-activated protein kinases (MAPkinases) (Li and Xu 2000; Peverali et al. 2001; Kletsas et al. 2002; Husse et al. 2007; Fitzgerald et al. 2008). We showed previously that PDLFs, upon the application of compressive forces, induced the activation of the ERK1/2 MAPkinases in a Ras GTPase-dependent manner (Diercke, Sen, et al. 2011). Hence, in the present study, we asked whether the compressive force-dependent regulation of ephrin-A2 in PDLFs might be dependent on a MAPkinase-dependent pathway leading to c-fos activation and thus AP-1–dependent induction of ephrin-A2.
Materials and Methods
Please see the Appendix for complete Materials and Methods.
Immunoprecipitation and Western Blotting
EphA2 receptor phosphorylation after stimulation with ephrin-A2–Fc was tested with an anti–phosphotyrosine antibody on immunoprecipitated EphA2. For immunoprecipitation, cell lysates (50 µg/mL) containing protease inhibitor cocktail and phosphatase inhibitor cocktail B (Roche, Mannheim, Germany; Santa Cruz Biotechnology, Heidelberg, Germany) were incubated overnight at 4°C with an anti–EphA2 antibody (#3874; Cell Signaling/New England Biolabs, Frankfurt, Germany). Then, 50 µL of immobilized protein A (Trisacryl GF-2000; Pierce, Bonn, Germany) was added and incubated for 2 h at room temperature. Precipitates were washed, subjected to lysis, and separated on 4% to 12% NuPAGE Gels (Invitrogen, Karlsruhe, Germany). Western blotting was performed as described below.
For Western blotting, protein lysates were obtained by lysis with ice-cold RIPA buffer supplemented with protease inhibitor cocktail (Roche). Then, 25 µg of protein was separated on NuPAGE Gels (Life Technologies, Karlsruhe, Germany). The separated proteins were transferred onto PVDF membranes (Life Technologies). Membranes were probed with antibodies against EphA2 (1:1000, #3874; Cell Signaling/New England Biolabs), ephrin-A2 (1:500; R&D Systems, Wiesbaden, Germany), c-fos (1:500, sc-52; Santa-Cruz Biotechnology), phosphotyrosine (1:2000, P-Tyr-100; Cell Signaling/New England Biolabs), and ERK1/2 MAP-kinase (clone 137F5) and p-ERK1/2 (Thr202/Tyr204) (1:2000 and 1:1000, both Cell Signaling/New England Biolabs). The Western Breeze chromogenic or chemiluminescent immunodetection systems (Life Technologies) were used for visualization. Before reprobing, membranes were stripped with Roti free stripping buffer (30 min, 70°C; Roth, Karlsruhe, Germany).
Results
Upregulation of Ephrin-A2 in PDLF by Compressive Forces Was Accompanied by ERK1/2 Activation and the Transcriptional Induction of c-fos
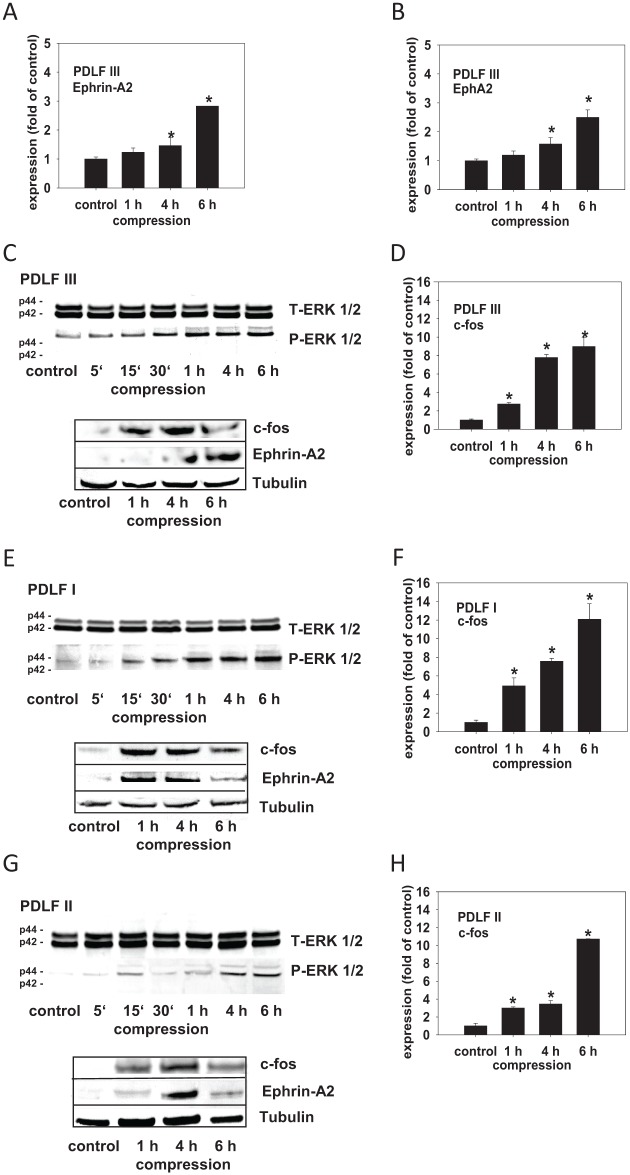
We previously showed a compressive force-dependent regulation of ephrin-A2 in 2 independent human PDLF populations (Diercke, Sen, et al. 2011). Using quantitative reverse transcription polymerase chain reaction (qRT-PCR), we confirmed compression-dependent upregulation of ephrin-A2 in another human primary PDL fibroblast population (PDLF III). After application of compressive forces (30.3 g/cm2, 1 h, 4 h, and 6 h), ephrin-A2 was significantly induced in PDLF III (Fig. 1A). Compression also significantly induced EphA2 (Fig. 1B). The signaling pathway leading to the upregulation of ephrin-A2 in PDLF upon the application of compressive forces is unclear. To gain insight into the regulation of ephrin-A2 expression, we tested first whether compressive forces are able to induce ERK1/2 (p44/p42) MAPkinase activation. Compressive forces (30.3 g/cm2, 5 min, 15 min, 30 min, 1 h, 4 h, 6 h) were applied and cell lysates were probed with antibodies against ERK1/2 and pERK1/2. ERK1/2 phosphorylation was induced 15 to 30 min after the application of compressive forces in PDLF I, II, and III (Fig. 1C, E, G, upper panels) and remained elevated during the course of the experiment.
Figure 1.
The compression-dependent upregulation of ephrin-A2 in PDL is accompanied by ERK1/2 activation and the transcriptional induction of c-fos. We confirmed our previously reported data and showed compression-dependent upregulation of ephrin-A2 and EphA2 in a primary periodontal ligament (PDL) fibroblast population (PDLF III) (A, B). Primary human PDLFs (PDLF I, II, III) were subjected to compression (30.3 g/cm2) for 1, 4, 6 h. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis revealed a significant upregulation of c-fos in compressed PDLF I, II, and III (D, F, H). Significant upregulation of c-fos preceded the upregulation of ephrin-A2. The regulation of ephrin-A2 and c-fos, respectively, upon the application of compressive forces was confirmed on the protein level for PDLF I, II, and III (C, E, G, lower panels). For comparability, the Western blots were stripped and reprobed. Tubulin was used as a loading control. Compression experiments were performed in triplicates (n = 3). Static cells, time matched, served as controls. The qRT-PCR assays were performed in triplicates. Data are presented as mean ± SD. *P < 0.05 vs. control (one-way analysis of variance, Dunnett’s post hoc test).
A c-fos–dependent regulation of ephrin-A2 was reported (Irie et al. 2009). This finding and the fact that c-fos is known to be mechano-dependent prompted us to check if c-fos is regulated upon the application of compressive forces. qRT-PCR analysis revealed a significant upregulation of c-fos in compressed PDLF I, II, and III (Fig. 1D, F, H). The regulation of ephrin-A2 and c-fos upon the application of compressive forces was confirmed on the protein level for PDLF I, II, and III (Fig. 1C, E, G, lower panels). For comparability, the Western blots were stripped and reprobed. Tubulin was used a loading control. Taken together, we observed a coincidence of ERK1/2 phosphorylation and c-fos induction with ephrin-A2 upregulation in compressed PDLF.
Specific Inhibition of ERK 1/2 Activation or c-fos Transcriptional Silencing Attenuated the Compression-Dependent Upregulation of Ephrin-A2 in PDLFs
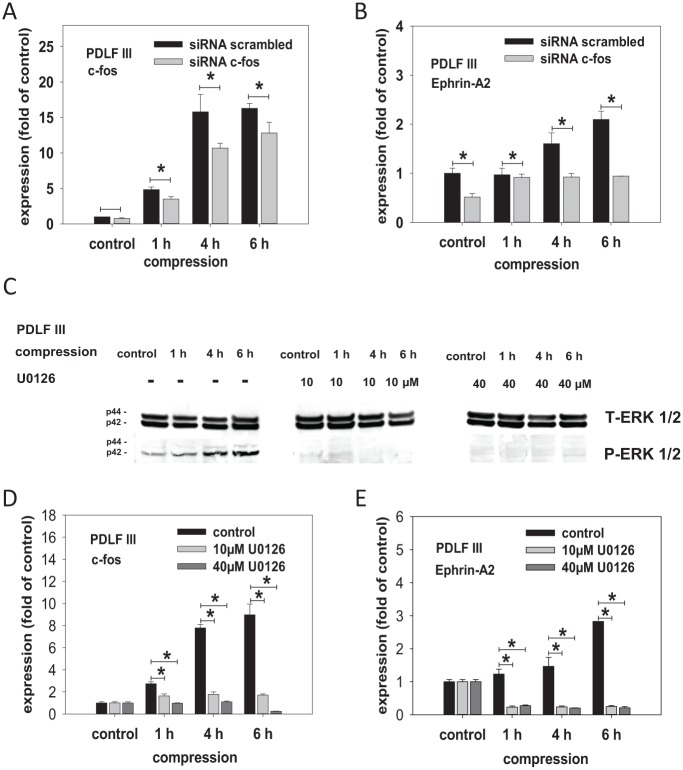
To test whether the activation of ERK1/2 and the induction of c-fos expression are causal for ephrin-A2 upregulation, we selectively blocked ERK1/2 activation or c-fos transcriptional induction in compressed PDLFs. ERK1/2 activation was blocked using the small-molecule MEK inhibitor U0126. Using a small interfering RNA (siRNA) approach, we inhibited c-fos at the transcriptional level. siRNA with a scrambled sequence served as a control. The compression-dependent induction of c-fos was significantly decreased by siRNA-mediated c-fos silencing (Fig. 2A). In addition, silencing of c-fos expression by siRNA led to a significant reduction of ephrin-A2 expression in compressed PDLFs (Fig. 2B). Inhibition of ERK1/2 phosphorylation in compressed PDLFs was obtained at U0126 concentrations of 10 µM and 40 µM (Fig. 2C). U0126 inhibition of ERK1/2 activation significantly reduced the compression-dependent induction of c-fos in PDLFs (Fig. 2D). U0126 administration also inhibited ephrin-A2 induction in compressed PDLFs (Fig. 2E).
Figure 2.
Inhibition of ERK1/2 phosphorylation or small interfering (siRNA)–mediated silencing of c-fos reduced the compression-dependent upregulation of ephrin-A2 in periodontal ligament fibroblasts (PDLFs). To test whether the activation of ERK1/2 (p44/p42) MAPkinase and the induction of c-fos expression are causal for ephrin-A2 upregulation in PDLFs, we selectively blocked ERK1/2 activation or c-fos transcriptional induction in PDLFs subjected to compressive forces. For the inhibition experiments, only PDLF III were used. siRNA targeting c-fos was used to perturb c-fos expression at the transcriptional level. siRNA with a scrambled sequence served as a control. As revealed by quantitative reverse transcription polymerase chain reaction (qRT-PCR), the compression-dependent induction of c-fos (A) and ephrin-A2 (B) were significantly decreased by siRNA-mediated c-fos silencing. U0126, a selective small-molecule inhibitor of MEK, the upstream kinase of ERK1/2, was used to block ERK1/2 activation in PDLFs. (C) To prove the inhibitory effects of U0126 on ERK1/2 phosphorylation in compressed PDLFs, Western blotting was performed. Lysates of compressed PDLFs treated with U0126 (10 µM and 40 µM) were probed with antibodies against ERK1/2 and pERK1/2. U0126 at 10 µM led to a marked inhibition of ERK1/2 phosphorylation (C, middle panel). At 40 µM, ERK1/2 phosphorylation was undetectable (C, right panel). qRT-PCR for c-fos (D) and ephrin-A2 (E) in compressed PDLFs. U0126 at both concentrations significantly prevented the compression-dependent induction of c-fos and of ephrin-A2 in PDLFs. These data provide further evidence for a putative inductive pathway involving ERK1/2 and c-fos leading to compression-dependent ephrin-A2 regulation in PDLFs. Compression experiments were performed in triplicates (n = 3). Static cells, time matched, served as controls. The qRT-PCR assays were performed in triplicates. Data are presented as mean ± SD. *P < 0.05 vs. control (one-way analysis of variance, Dunnett’s post hoc test).
These data suggest a mechano-induced ERK1/2 and c-fos–dependent regulation of ephrin-A2 in PDLFs subjected to compressive forces.
Ephrin-A2 Contributes to Its Own Induction in Compressed PDLFs
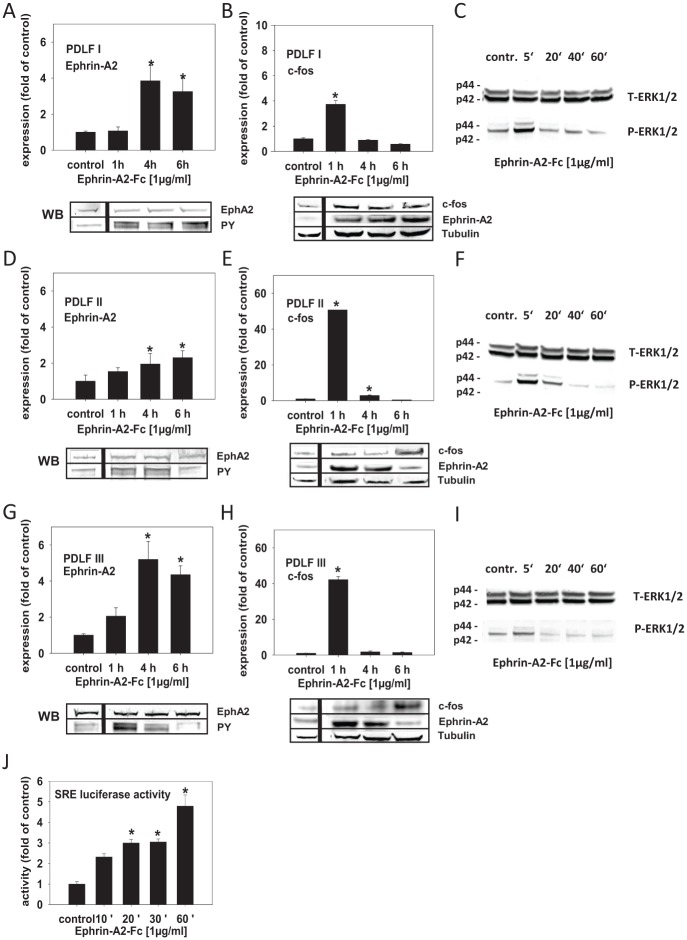
Ephrin-A stimulation of EphA2 was shown to promote the activation of ERKs, followed by an increase in the nuclear induction of Elk-1 (Pratt and Kinch 2002), a ternary complex transcription factor (TCF) that regulates transcription from the serum response element (Buchwalter et al. 2004). Serum response element (SRE) is involved in the regulation of c-fos transcription (Peake and El Haj 2003). Therefore, ephrin-A2 via ERK1/2 and the SRE might be involved in the regulation of c-fos, which could lead to its own transcriptional activation. To test this, we have stimulated PDLFs with preclustered ephrin-A2–Fc. Ephrin-A2–Fc significantly induced the transcription of ephrin-A2 in the 3 analyzed PDLF populations 4 h after stimulation, and transcription remained enhanced until 6 h after stimulation (Fig. 3A, D, G). The activation of ephrin-A2 transcription was preceded by a significant, transient induction of c-fos 1 h after stimulation with ephrin-A2–Fc (Fig. 3B, E, H). Immunoprecipitation of EphA2 and subsequent probing with an anti–phosphotyrosine antibody (PY) showed that ephrin-A2–Fc was able to phosphorylate the EphA2 receptor (Fig. 3A, D, G, lower panel). The regulation of ephrin-A2 and c-fos after ephrin-A2–Fc stimulation was confirmed on the protein level (Fig. 3B, E, H, lower panel). We observed a discrepancy between the kinetics of mRNA and protein levels upon stimulation with ephrin-A2–Fc. PDLF II and PDLF III (Fig. 3E, H) showed early induction (1 h) of ephrin-A2 on the protein level, while c-fos protein levels peaked after 6 h.
Figure 3.
Ephrin-A2 is involved in its own induction in compressed periodontal ligament fibroblasts (PDLFs). EphA2 stimulation by different ephrin-As was shown to promote the activation of ERK kinases, which, via ternary complex transcription factor (TCFs), could lead to the induction of serum response element (SRE), the pivotal cis-element involved in the regulation of the transcription of immediate early genes, including c-fos. Therefore, ephrin-A2 via ERK1/2 SRE activation might be involved in the regulation of c-fos, which could lead to its own transcriptional activation. To test this, PDLFs were stimulated with preclustered ephrin-A2–Fc and incubated for 1, 4, and 6 h. (A, D, G) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) showed that ephrin-A2–Fc significantly induced the transcription of ephrin-A2 in the 3 analyzed PDLF populations (PDLF I, II, and III) 4 h after stimulation; transcription remained enhanced until 6 h after stimulation. (B, E, H) qRT-PCR demonstrated that the activation of ephrin-A2 transcription was preceded by a significant, transient induction of c-fos 1 h after stimulation with ephrin-A2–Fc. (C, F, I) Ephrin-A2–Fc stimulation might activate ephrin-A2 transcription via ERK1/2 phosphorylation. Western blotting for ERK1/2 and pERK1/2 showed that ERK1/2 phosphorylation was evident in PDLFs 5 min after stimulation, returning to background levels after 20 min. To test for EphA2 receptor phosphorylation after ephrin-A2–Fc stimulation, immunoprecipitation of EphA2 and subsequent probing with an anti–phosphotyrosine antibody (PY) was performed (A, D, G, lower panel). The regulation of ephrin-A2 and c-fos after ephrin-A2–Fc stimulation was confirmed on the protein level (B, E, H, lower panel). Tubulin served as a loading control. (J) To test whether ephrin-A2–Fc stimulation leading to ephrin-A2 transcriptional activation involves the activation of SRE, a reporter construct (pSRE-Luc) was transfected into murine 3T3 fibroblasts. 3T3 cells were stimulated with preclustered ephrin-A2–Fc for 10, 20, 30, and 60 min. Enhanced SRE-dependent luciferase activity was measured 10 min after stimulation, reached significance after 20 min, and remained significantly upregulated until the end of the observation period at 60 min. Stimulation experiments were performed in triplicates (n = 3). Cells treated with the anti–Fc antibody (0.1 µg/mL), time matched, served as controls. Luciferase reporter gene assays were performed twice in triplicates. The qRT-PCR assays were performed in triplicates. Data are presented as mean ± SD. *P < 0.05 vs. control (one-way analysis of variance, Dunnett’s post hoc test).
Ephrin-A2–Fc stimulation might activate TCFs via their ERK1/2-dependent phosphorylation. Indeed, ERK1/2 phosphorylation was evident in PDLFs 5 min after stimulation, returning to background levels 20 min after stimulation (Fig. 3C, F, I). A reporter construct (pSRE-Luc) was used to test for ephrin-A2–Fc–dependent SRE activation. Murine 3T3 fibroblasts were transfected with the reporter construct and stimulated with preclustered ephrin-A2–Fc. A significantly enhanced SRE-dependent luciferase activity was measured starting from 20 min after stimulation (Fig. 3J). These results suggest an ephrin-A2–Fc–dependent induction of ephrin-A2 transcription in PDLFs, which might be dependent on ERK1/2 activation and c-fos upregulation. It is possible that ephrin-A2–Fc activates c-fos expression via a pathway involving TCFs and the SRE. Via this putative pathway, ephrin-A2 would augment its own transcription in PDLFs.
Discussion
We showed previously that in PDLFs and osteoblasts of the alveolar bone, the expressions of specific members of the Eph-ephrin family are modulated by mechanical forces (Diercke, Kohl, et al. 2011; Diercke, Sen, et al. 2011). However, the regulation of ephrin-A2 expression in compressed PDLFs remained unclear. Therefore, we sought to elucidate the mechano-inductive pathway leading to ephrin-A2 transcriptional activation in compressed PDLFs.
Compression-Induced Ephrin-A2 and c-fos Expression in PDLFs
In this study, we confirmed the compression-dependent upregulation of ephrin-A2 in PDLFs (Diercke, Sen, et al. 2011). The signaling events leading to the mechano-dependent induction of ephrin-A2 are not fully elucidated. Mechanical forces in various cell types lead to the induction of immediate early genes, including c-fos and c-jun, which are components of the AP-1 heterodimeric transcription factor (Glantschnig et al. 1996; Li et al. 2007).
Evidence that ephrin-A2 regulation might be dependent on c-fos induction comes from results obtained on M-CSF–dependent macrophages. During RANKL (receptor activator of nuclear factor–κB ligand)–induced osteoclastogenesis, ephrin-A2 was induced in a c-fos–dependent manner (Irie et al. 2009).
In this study, we have analyzed compressed PDLFs and found a significant induction of c-fos, suggesting an AP-1–dependent regulation of ephrin-A2. The AP-1 transcription factor has previously been shown to be involved in the mechano-dependent regulation of different target genes in osteoblasts and PDLFs (Peake and El Haj 2003; Kook et al. 2009), and ephrin-A2 might be another mechano-inductive target of c-fos.
To test whether c-fos was causal for the mechano-dependent induction of ephrin-A2, we used an siRNA approach to silence c-fos expression. c-fos silencing significantly reduced mechano-dependent ephrin-A2 induction in PDLFs. It is noteworthy that the siRNA directed against c-fos led only to a roughly 30% reduction of c-fos expression in compressed PDLFs. Nevertheless, ephrin-A2 expression was reduced by about 50% (4 h, 6 h; Fig. 2A, B), suggesting an important role for c-fos for the mechano-dependent regulation of ephrin-A2 in PDLFs.
Studies in various mechano-inducible cell types revealed the involvement of MAPkinases in the mechano-inductive pathway, leading to c-fos transcriptional activation and thereby AP-1 activation (Li and Xu 2000; Peverali et al. 2001; Kletsas et al. 2002; Husse et al. 2007; Fitzgerald et al. 2008). In compressed PDLFs, the administration of ERK1/2 inhibitors diminished AP-1 activity (Kook et al. 2009). We showed previously that ephrin-A2 induction by compressive forces in PDLFs is most likely dependent on the signaling pathway involving Ras and ERK1/2 (Diercke, Sen, et al. 2011). The present findings are in line with these observations. We found ERK1/2 phosphorylation in compressed PDLFs, which preceded the upregulation of c-fos and ephrin-A2.
To further test for the potential involvement of compression-dependent ERK1/2 activation, we used a selective MEK inhibitor (U0126) to attenuate ERK1/2 phosphorylation. Both c-fos and ephrin-A2 transcription were significantly reduced by U0126 in compressed PDLFs. The effects of inhibition of ERK1/2 phosphorylation on ephrin-A2 expression might be twofold: 1) the MAPkinase pathway, triggered by mechanical induction, is perturbed and the transcriptional activation of c-fos is disturbed (Kletsas et al. 2002; Yokoyama et al. 2013), and 2) the activity of the AP-1 transcription factor is diminished. c-jun and c-fos, as parts of the AP-1 transcription factors, are positively regulated by MAPkinases. Peverali and colleagues (2001) showed that extracts from mechanically stimulated PDLFs were able to phosphorylate c-fos and c-jun, and the activated c-fos/c-jun heterodimers were able to induce the expression of AP-1 target genes. Therefore, ERK1/2 inhibition could lead to diminished c-fos and c-jun phosphorylation and thereby to a reduced induction of AP-1–dependent gene expression.
Taken together, our data suggest that ephrin-A2 upregulation in compressed PDLFs might be regulated via an ERK1/2- and c-fos–dependent pathway. The potential involvement of this pathway sheds light on the previously unknown regulation of ephrin-A2 in compressed PDLFs.
Ephrin-A2 Is Involved in Its Own Transcriptional Regulation
Compression-dependent ephrin-A2 upregulation was shown to attenuate the osteogenic differentiation of alveolar osteoblasts and PDLFs (Diercke, Sen, et al. 2011). Ephrin-A2 stimulation of osteoblasts of the alveolar bone and PDLFs significantly reduced the expression of RUNX2, the pivotal transcription factor for osteogenic differentiation, and ALPL (alkaline phosphatase). Interestingly, the ephrin-A2–dependent reduction of RUNX and ALPL expression was more pronounced in PDLFs. We therefore hypothesized that ephrin-A2 in PDLFs might contribute to its own upregulation, thereby augmenting its antiosteogenic effects.
In this study, ephrin-A2–stimulated PDLFs showed a significantly increased ephrin-A2 expression. Ephrin-A2 induction in ephrin-A2–stimulated cells was accompanied by c-fos upregulation. It should be noted that in our experiments, the kinetics of the inductions of mRNA and protein expressions upon stimulation with ephrin-A2–Fc did not perfectly match. Various processes between transcription and translation events might influence the correlation of RNA and protein expressions (Vogel and Marcotte 2012). However, compared with baseline (control) levels, c-fos protein levels were increased upon the stimulation with ephrin-A2–Fc (Fig. 3B, E, H), and ephrin-A2 protein levels were likewise increased. Taken together, ephrin-A2 induction in ephrin-A2–stimulated cells was accompanied by ERK1/2 phosphorylation and c-fos upregulation, temporally preceding the upregulation of ephrin-A2, suggesting an ERK1/2- and c-fos–dependent pathway for ephrin-A2–dependent ephrin-A2 regulation in PDLFs.
This interpretation would be in line with findings from Pratt and Kinch (2002), who provided evidence that ephrin-A2–dependent EphA2 activation, via the Shc (Src homology 2 domain-containing) adaptor protein, which in turn recruits Grb2 (growth factor receptor–bound protein 2), promotes the phosphorylation and nuclear translocation of ERK1/2 followed by an increased induction of the transcription factor Elk-1 (Pratt and Kinch 2002). Elk-1 is one of the TCFs (Buchwalter et al. 2004) that regulates transcription from the SRE. SRE is the pivotal cis-element involved in the regulation of the transcription of immediate early genes, including c-fos (Peake and El Haj 2003).
Using a reporter gene construct, we found in murine 3T3 fibroblasts that ephrin-A2–Fc was able to stimulate SRE-dependent luciferase activity significantly. The findings in PDLF—elevated ERK1/2 phosphorylation and induction of c-fos and ephrin-A2 after ephrin-A2–Fc stimulation—together with the SRE-dependent induction of luciferase activity in murine fibroblasts suggest the involvement of the ephrin-A2–dependent inductive pathway proposed by Pratt and Kinch (2002) in our PDLFs. However, as we were not able to successfully transfect primary human PDLFs with the reporter gene construct, we cannot rule out a different pathway excluding SRE activation for ephrin-A2–dependent ephrin-A2 induction in PDLFs. Moreover, conflicting results concerning the activation or inhibition (Miao et al. 2001) of MAPkinases downstream of activated EphA2 make a conclusive interpretation of EphA2-MAPK crosstalk difficult.
The evidence provided within this study suggests an EphA2 activation-dependent induction of ERK1/2. The transcriptional activation of c-fos in turn is mediated by SRE-binding transcription factors. c-fos and c-jun heterodimerize to form the AP-1 transcription factor and induce ephrin-A2 expression. Ephrin-A2 thereby augments its own expression; the elevated levels of ephrin-A2 in the PDLF population might explain the pronounced antiosteogenic effects we observed in PDLFs after ephrin-A2 stimulation (Diercke, Sen, et al. 2011).
In this and our previous studies on ephrin-Eph interactions during tooth movement, we observed different molecular outcomes after ephrin-A2 stimulation in PDLFs and osteoblasts. This controversy can be explained by the different nature and state of differentiation of the cells and, most important, by the complex dynamic protein kinase network consisting of several levels of phosphorelay modules that are involved in the reception, integration, and transduction of signals or stresses via the ERK1/2-pathway. Specificity of signal integration and specific functional response is most likely mediated by different MAPK kinase kinases (MKKKs). MKKKs might be regarded as hubs that integrate responses, which lead to specific MAPK activation and downstream responses. The MKKK for the canonical Ras-Raf-MEK-ERK1/2 pathway is Raf. Raf can interact with scaffold proteins, and these Raf scaffold signaling complexes can integrate signals from diverse stimuli for the spatiotemporal control of ERK1/2, thus regulating specific ERK1/2 functions (reviewed in Cuevas et al. 2007). We believe that the controversial findings in PDLFs can be explained by the complex network and integrating hub function of the ERK1/2 pathway, which in different cell types and after specific stimulation might have different or even opposing outcomes. For example, EphA2 receptor activation might lead to either ERK1/2 activation (Pratt and Kinch 2002) or inactivation (Miao et al. 2001).
The compression model we are using does not allow for the prolonged application of compressive forces. Therefore, we do not know whether the compression-dependent ephrin-A2 regulation in PDLFs continues over time or is an acute, transient response to early compressive forces. Clearly, we will have to conduct experiments using prolonged compression to fully elucidate its effects on ephrin-A2 expression. The nature and the integration of mechanical stresses are different from ligand-dependent receptor activation; therefore, it is possible that compression could lead to a sustained effect, and ephrin-A2 might be induced as long as the compressive forces reach a certain threshold. During tooth movement, compression should decrease, and a threshold concept for compression-dependent ephrin-A2 regulation might be in line with the temporal regulation of bone remodeling during tooth movement.
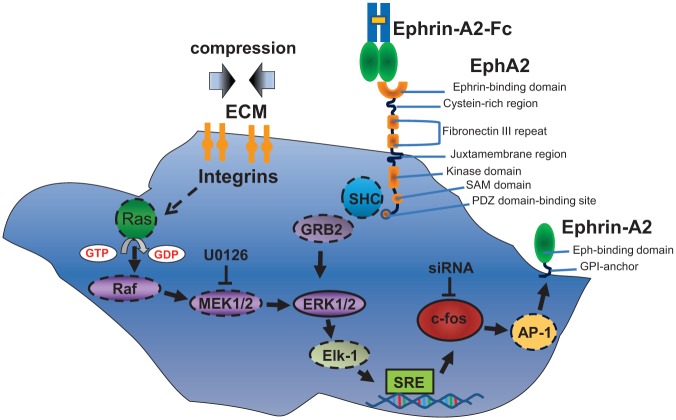
Taken together, we provided evidence for an ERK1/2- and c-fos–dependent regulation of ephrin-A2 in compressed PDLFs and suggest a novel pathway for ephrin-A2 induction emanating from ephrin-A2 itself (these putative pathways are depicted in Fig. 4). We showed previously that ephrin-A2 at compression sites might contribute to tooth movement by inhibiting osteogenic differentiation. In this study, we suggest a complex regulatory pathway of ephrin-A2 induction during tooth movement that might be accessible for pharmacological interventions.
Figure 4.
Putative signaling pathways involved in the regulation of ephrin-A2 in periodontal ligament fibroblasts (PDLFs). Please see Discussion section for details. Solid lines: Involvement was shown in this study. Dashed lines: Involvement was shown or suggested elsewhere.
Author Contributions
S. Sen, R. Erber, contributed to conception, design, and data acquisition, drafted and critically revised the manuscript; K. Diercke, S. Zingler, contributed to conception, design, and data acquisition, critically revised the manuscript; C. J. Lux, contributed to data acquisition, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Footnotes
This investigation was supported by a grant from the Dres. Majic-Schlez-Foundation to S.S. and by a grant from the “FRONTIER Innovationsfonds” of the University of Heidelberg. We thank Annette Kohl for expert technical assistance.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Buchwalter G, Gross C, Wasylyk B. 2004. Ets ternary complex transcription factors. Gene. 324:1–14. [DOI] [PubMed] [Google Scholar]
- Cuevas BD, Abell AN, Johnson GL. 2007. Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene. 26(22):3159–3171. [DOI] [PubMed] [Google Scholar]
- Diercke K, Kohl A, Lux CJ, Erber R. 2011. Strain-dependent up-regulation of ephrin-B2 protein in periodontal ligament fibroblasts contributes to osteogenesis during tooth movement. J Biol Chem. 286(43):37651–37664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diercke K, Sen S, Kohl A, Lux CJ, Erber R. 2011. Compression-dependent up-regulation of ephrin-A2 in PDL fibroblasts attenuates osteogenesis. J Dent Res. 90(9):1108–1115. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JB, Jin M, Chai DH, Siparsky P, Fanning P, Grodzinsky AJ. 2008. Shear- and compression-induced chondrocyte transcription requires MAPK activation in cartilage explants. J Biol Chem. 283(11):6735–6743. [DOI] [PubMed] [Google Scholar]
- Glantschnig H, Varga F, Rumpler M, Klaushofer K. 1996. Prostacyclin (PGI2): a potential mediator of c-fos expression induced by hydrostatic pressure in osteoblastic cells. Eur J Clin Invest. 26(7):544–548. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H. 2012. Osteoprotection by semaphorin 3A. Nature. 485(7396):69–74. [DOI] [PubMed] [Google Scholar]
- Hipskind RA, Bilbe G. 1998. MAP kinase signaling cascades and gene expression in osteoblasts. Front Biosci. 3:d804–d816. [DOI] [PubMed] [Google Scholar]
- Husse B, Briest W, Homagk L, Isenberg G, Gekle M. 2007. Cyclical mechanical stretch modulates expression of collagen I and collagen III by PKC and tyrosine kinase in cardiac fibroblasts. Am J Physiol Regul Integr Comp Physiol. 293(5):R1898–R1907. [DOI] [PubMed] [Google Scholar]
- Irie N, Takada Y, Watanabe Y, Matsuzaki Y, Naruse C, Asano M, Iwakura Y, Suda T, Matsuo K. 2009. Bidirectional signaling through ephrinA2-EphA2 enhances osteoclastogenesis and suppresses osteoblastogenesis. J Biol Chem. 284(21):14637–14644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawarizadeh A, Bourauel C, Gotz W, Jager A. 2005. Early responses of periodontal ligament cells to mechanical stimulus in vivo. J Dent Res. 84(10):902–906. [DOI] [PubMed] [Google Scholar]
- Kletsas D, Basdra EK, Papavassiliou AG. 2002. Effect of protein kinase inhibitors on the stretch-elicited c-Fos and c-Jun up-regulation in human PDL osteoblast-like cells. J Cell Physiol. 190(3):313–321. [DOI] [PubMed] [Google Scholar]
- Kook SH, Hwang JM, Park JS, Kim EM, Heo JS, Jeon YM, Lee JC. 2009. Mechanical force induces type I collagen expression in human periodontal ligament fibroblasts through activation of ERK/JNK and AP-1. J Cell Biochem. 106(6):1060–1067. [DOI] [PubMed] [Google Scholar]
- Korff T, Braun J, Pfaff D, Augustin HG, Hecker M. 2008. Role of ephrinB2 expression in endothelial cells during arteriogenesis: impact on smooth muscle cell migration and monocyte recruitment. Blood. 112(1):73–81. [DOI] [PubMed] [Google Scholar]
- Li C, Xu Q. 2000. Mechanical stress–initiated signal transductions in vascular smooth muscle cells. Cell Signal. 12(7):435–445. [DOI] [PubMed] [Google Scholar]
- Li J, Chen G, Zheng L, Luo S, Zhao Z. 2007. Osteoblast cytoskeletal modulation in response to compressive stress at physiological levels. Mol Cell Biochem. 304(1–2):45–52. [DOI] [PubMed] [Google Scholar]
- Miao H, Wei BR, Peehl DM, Li Q, Alexandrou T, Schelling JR, Rhim JS, Sedor JR, Burnett E, Wang B. 2001. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat Cell Biol. 3(5):527–530. [DOI] [PubMed] [Google Scholar]
- Obi S, Yamamoto K, Shimizu N, Kumagaya S, Masumura T, Sokabe T, Asahara T, Ando J. 2009. Fluid shear stress induces arterial differentiation of endothelial progenitor cells. J Appl Physiol. 106(1):203–211. [DOI] [PubMed] [Google Scholar]
- Peake MA, El Haj AJ. 2003. Preliminary characterisation of mechanoresponsive regions of the c-fos promoter in bone cells. FEBS Lett. 537(1–3):117–120. [DOI] [PubMed] [Google Scholar]
- Peverali FA, Basdra EK, Papavassiliou AG. 2001. Stretch-mediated activation of selective MAPK subtypes and potentiation of AP-1 binding in human osteoblastic cells. Mol Med. 7(1):68–78. [PMC free article] [PubMed] [Google Scholar]
- Pratt RL, Kinch MS. 2002. Activation of the EphA2 tyrosine kinase stimulates the MAP/ERK kinase signaling cascade. Oncogene. 21(50):7690–7699. [DOI] [PubMed] [Google Scholar]
- Vogel C, Marcotte EM. 2012. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 13(4):227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K, Hiyama A, Arai F, Nukaga T, Sakai D, Mochida J. 2013. C-Fos regulation by the MAPK and PKC pathways in intervertebral disc cells. PLoS One. 8(9):e73210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K. 2006. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 4(2):111–121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.