Abstract
Gingival wound healing comprises a series of sequential responses that allow the closure of breaches in the masticatory mucosa. This process is of critical importance to prevent the invasion of microbes or other agents into tissues, avoiding the establishment of a chronic infection. Wound healing may also play an important role during cell and tissue reaction to long-term injury, as it may occur during inflammatory responses and cancer. Recent experimental data have shown that gingival wound healing is severely affected by the aging process. These defects may alter distinct phases of the wound-healing process, including epithelial migration, granulation tissue formation, and tissue remodeling. The cellular and molecular defects that may explain these deficiencies include several biological responses such as an increased inflammatory response, altered integrin signaling, reduced growth factor activity, decreased cell proliferation, diminished angiogenesis, reduced collagen synthesis, augmented collagen remodeling, and deterioration of the proliferative and differentiation potential of stem cells. In this review, we explore the cellular and molecular basis of these defects and their possible clinical implications.
Keywords: gingiva, gerontology, cell biology, myofibroblast, extracellular matrix, cytokines
Wound Healing
Aging is defined as a biological process characterized by a decrease in cell function derived by a gradual deficiency of the regenerative response of certain tissues (Sousounis et al. 2014). After tissue injury, distinct biological pathways immediately become activated and are synchronized to prevent infection and restore the damaged tissues. The cells recruited during wound healing include components of the immune system (neutrophils, monocytes, lymphocytes, and dendritic cells), endothelial cells, keratinocytes, and fibroblasts (Gurtner et al. 2008). These cells undergo massive fluctuations in gene expression (Iyer et al. 1999), commanding important changes in cell proliferation, differentiation, and migration (Gurtner et al. 2008). Unfortunately, aging is characterized by a dramatic change in the regulation of gene expression (Sousounis et al. 2014). The extracellular matrix (ECM) also plays an important role in wound healing (Greiling and Clark 1997). During tissue repair, cells must secrete and organize components of the ECM such as collagens, fibronectin, proteoglycans, and matricellular proteins, among others (Frantz et al. 2010). This response is critically important to fill the damaged tissues with new matrix components to allow the migration of cells and to remodel tissues after injury. Moreover, the ECM provides a physical support by assisting as a cell framework (Frantz et al. 2010). Tension derived from the ECM that is transmitted through collagen fibers into cells is also important for the modulation of gene expression, cell proliferation, and locomotion (Frantz et al. 2010). Therefore, it is possible to propose that proper and successful wound healing, and hopefully tissue regeneration, requires normally responding cells and a healthy ECM. Unfortunately, aging affects both the ability of cells to respond to injury and the physiology of the ECM.
The normal response to injury involves 3 overlapping however distinct stages: 1) inflammation, 2) new tissue formation, and 3) remodeling (Gurtner et al. 2008). As illustrated in Figure 1, distinct cell populations are involved in these phases of tissue repair. During these events, cells experience changes in gene expression, most of them driven by cell-matrix interactions and/or initiated soluble mediators. In the present review, we describe studies considering the involvement or disturbance of wound healing due to aging with a special focus on gingival tissues. As described in the Table, aging may affect several biological events that play a key role in the process of gingival or periodontal wound healing. In the present review, we analyze both classic and recent studies in the field of aging and wound healing with a special focus on the repair of the oral mucosa and the periodontium.
Figure 1.
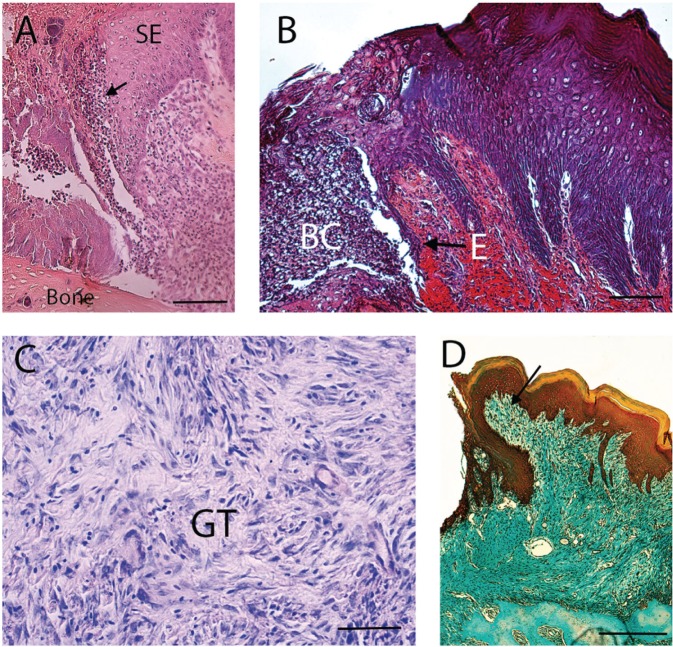
Cellular and histologic events involved in gingival wound healing. (A) Tissue section shows the inflammatory phase of gingival wound healing. Arrow indicates inflammatory cells. SE, sulcular epithelium. Tissue section was stained with eosin-hematoxylin. Bar = 250 µm. (B) Micrograph shows the migration of epithelial cells (E) during wound healing. BC, blood clot. Tissue section was stained with phosphotungstic acid hematoxylin. Bar = 250 µm. (C) A more advanced stage of wound healing shows the formation of granulation tissue (GT). Tissue staining was stained with Giemsa. Bar = 50 µm. (D) Section represents the tissue-remodeling phase. Wound has been replaced with connective tissue (arrow). This tissue has a high proportion of cells. The gingival epithelium has covered the wound. Section was stained with mallory trichrome. Bar = 250 µm.
Table.
Key Biological Events Affected by Aging during Gingival and Periodontal Wound Healing.
| Age-Associated Defects That Affect Gingival and Periodontal Wound Healing | Study Model | Reference |
|---|---|---|
| Increased inflammatory response | Human periodontal ligament fibroblasts | Benatti et al. 2009 |
| Epithelial cell migration | Rat gingival wound healing | Cáceres et al. 2014 |
| Reduced cell proliferation | Periodontal ligament and gingival fibroblasts | Benatti et al. 2008; Cáceres et al. 2014 |
| Increased apoptosis | Gingival tissues | González et al. 2011 |
| Decreased collagen synthesis | Periodontal ligament | Ohi et al. 2006 |
| Increased production of matrix metalloproteinases | Human periodontal ligament fibroblasts | Benatti et al. 2008 |
| Increased collagen phagocytosis | Human gingival fibroblasts | Lee and McCulloch 1997 |
| Decreased stemness | Human periodontal ligament cells | Zhang et al. 2012 |
| Altered myofibroblastic differentiation | Gingival wound healing | Cáceres et al. 2014 |
The Inflammatory Phase of Wound Healing
The inflammatory response occurs immediately after injury, where coagulation cascade components, inflammatory pathways, and the immune system are activated to remove cell debris and prevent an infection (Dovi et al. 2003). Hemostasis is achieved by the formation of a platelet plug embedded in a fibrin-fibronectin matrix that prevents the loss of tissue fluids and becomes the scaffold for infiltrating cells. It has been assumed that platelets play a critical role in wound healing through the release of growth factors present in their granules, stimulating cell migration and proliferation. This was demonstrated by classic studies that evaluated skin wound healing in mice treated with an antiplatelet serum that induces thrombocytopenia (Szpaderska et al. 2003). Under these conditions, injuries were characterized by increased numbers of infiltrating inflammatory cells. Nevertheless, wounds healed normally, suggesting that platelets are mostly involved in hemostasia (Szpaderska et al. 2003). Neutrophils are recruited to the wound attracted by components of the complement system and products derived from platelets and/or invading bacteria (Kolaczkowska and Kubes 2013). Macrophages are also attracted to the wound and play important roles in tissue defense and in the initiation of granulation tissue formation and angiogenesis (Davies et al. 2013). Although it appears that inflammation is important to prevent wound colonization and infection, evidence has suggested that the presence of neutrophils and macrophages is not essential for tissue repair. For instance, skin wounds performed in mice depleted of neutrophils heal faster compared with control animals (Dovi et al. 2003). In addition, tissue repair studies performed in the PU.1 knockout mice, deficient in macrophages and neutrophils, have demonstrated an accelerated wound-healing response compared with their wild-type littermates (Martin et al. 2003). All of these studies suggest that infiltration by inflammatory cells seems to be critical for the prevention of an infection. However, a prolonged inflammatory response may delay wound healing and probably favor tissue fibrosis, reducing the chances of true regeneration (Pacios et al. 2012; Arancibia et al. 2013). It is important to consider that several studies have documented that aging cells and tissues show increased levels of inflammation (Chung et al. 2011).
Senescence-Associated Secretory Phenotype
Senescent cells are characterized by a proinflammatory trait referred to as a senescence-associated secretory phenotype (SASP) that includes the involvement of the nuclear factor–κB (NF-κB) and CCAAT/enhancer binding associated with the secretion of cytokines, chemokines, and proteolytic enzymes (Coppé et al. 2010). Classic studies using aging human dermal fibroblasts have reported higher levels of interleukin-1α (IL-1α) that stimulates plasminogen activator inhibitor-2 (PAI-2) (Kumar et al. 1992). Moreover, recent reports using human periodontal ligament fibroblasts from aged donors have identified that interleukin-6 (IL-6) and tumor necrosis factor–α (TNF-α) may be upregulated (Benatti et al. 2009). Since aging may prolong or stimulate the production of inflammatory cytokines, this altered response might affect the normal evolution of wound healing. Nevertheless, the precise role of inflammatory cytokines in wound repair remains unclear. Using soluble receptors that block these cytokines, Zhang et al. (2004) observed that inhibition of the function of these inflammatory mediators increased apoptosis in the healing tissues. Moreover, while short-term inhibition of these cytokines favored wound healing, a prolonged blockade was associated with adverse effects (Zhang et al. 2004). In conclusion, the evidence available suggests that a prolonged inflammatory phase may delay wound healing. Aging may promote the release of inflammatory mediators from fibroblasts. However, further studies are needed to characterize the impact of aging on the inflammatory response in gingival tissues.
New Tissue Formation
The formation of new tissue corresponds to the second stage of wound repair and occurs between 2 and 10 d after injury (Gurtner et al. 2008). As previously suggested, a persistent inflammatory response—which might occur in aged individuals—could alter the timing of these events. This phase involves the migration and proliferation of epithelial cells, the differentiation of myofibroblasts, and the ingrowth of new capillaries into the wound. In this section, we analyze these cell responses and their possible involvement during aging. As well, we discuss factors that may influence this phase, such as cell proliferation, serum-derived factors, and stem cells.
Epithelial Cell Response
One of the first steps observed in this stage is the migration of keratinocytes over the wound bead (Gurtner et al. 2008). Seminal studies identified that upon wounding, epithelial cells change their morphology from polarized to elongated migrating keratinocytes (Odland and Ross 1968). Basal epithelial cells adjacent to migrating cells start proliferating between 48 and 72 h after wounding, providing new cells for wound closure (Odland and Ross 1968). Cell-matrix interactions have also been identified as critically important for epithelial cell migration. In unwounded tissue, basal epithelial cells interact with several components of the intact basement membrane. Nevertheless, after tissue injury, keratinocytes are exposed to a diverse array of matrix components, including type I collagen, polymerized fibrin, and plasma fibronectin (Subdeck et al. 1997; Larjava et al. 2011). Activation of specific cell-membrane receptors, particularly collagen and fibronectin-binding integrins, drives cell migration and the expression of matrix metalloproteinases and plasminogen activators (Garlick et al. 1996; Netzel-Arnett et al. 2002). In a recent study performed by our group, we evaluated whether aging modifies gingival wound healing (Cáceres et al. 2014). To this end, a gingivectomy procedure was performed in the palatal aspect of the gingival tissue facing the upper first and second molars. Using morphometric analysis of histologic sections, we assessed the rate of wound closure at the epithelial level in young and middle-aged rats aged 2 and 18 mo, respectively. A significant delay in wound coverage by epithelial tissue was observed in middle-aged rats. As previously indicated, epithelial cell wound healing involves several cell responses that include cell proliferation of the keratinocytes residing close to migrating cells, activation of a migratory phenotype that involves modifications in the actin cytoskeleton, and the involvement of integrins that recognize matrix components present in the wound bead. Therefore, several biological responses deserve further studies to characterize the wound-healing defects associated with aging.
Myofibroblastic Differentiation
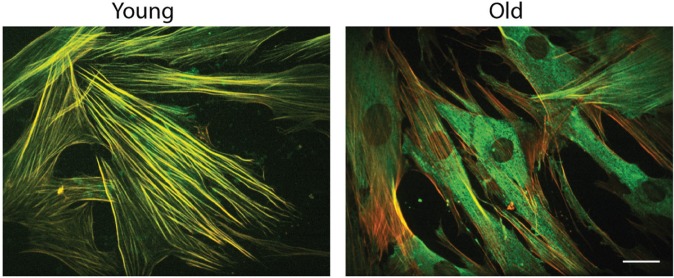
Different cell populations are attracted to migrate and invade the regenerating lesion to fill the wound and to populate the newly formed granulation tissue (Gurtner et al. 2008). Connective tissue cells surrounding the wound receive signals from ECM molecules and polypeptide growth factors released by platelets and macrophages present in the injured tissue (Gurtner et al. 2008). A specific circulating cell population known as fibrocytes may also derive from the bone marrow to the wounded tissues (Kao et al. 2011). However, the proportion of bone marrow cells contributing to soft tissue healing may vary considerably (Mackinnon and Forbes 2013). During wound healing, a specific phenotype of mesenchymal cells, known as myofibroblasts, is differentiated from several sources, including fibroblasts residing in the connective tissue surrounding the wound, circulating fibrocytes derived from the bone marrow, and cells that are transdifferentiated from blood vessels or epithelium (Hinz et al. 2012; Peng and Herzog 2012). Myofibroblasts play a critical role during wound healing by secreting matrix components and remodeling the newly formed tissues (Hinz et al. 2012). It is important to note that previous studies have characterized the role that aging has over myofibroblastic differentiation. A prominent deficiency in myofibroblastic differentiation has been identified in aged cardiac tissue (Cieslik et al. 2011) and in old skin fibroblasts (Simpson et al. 2010). To typify the effect of aging on gingival myofibroblastic differentiation, we have evaluated the expression and distribution of α–smooth muscle actin (α-SMA) in gingival fibroblasts derived from young and aged donors (Cáceres et al. 2014). To promote myofibroblastic differentiation, cells were stimulated with transforming growth factor–β1 (TGF-β1). As illustrated in Figure 2, both young and aged myofibroblasts demonstrated increased levels of α-SMA after TGF-β1 stimulation. However, only young fibroblasts established the development of actin stress fibers and the incorporation of α-SMA into these structures. In addition, we evaluated the ability of young and aged fibroblasts to remodel 3-dimensional collagen matrices. Compared with young cells, fibroblasts derived from aged individuals demonstrated a diminished capacity to remodel collagen gels (Cáceres et al. 2014). These results suggest that aging affects the dynamics of the actin cytoskeleton and collagen reorganization during wound healing, probably disturbing tissue homeostasis and function.
Figure 2.
Aging affects myofibroblastic differentiation. These images show human gingival myofibroblasts derived from young and aged donors stimulated with transforming growth factor–β1 (TGF-β1) for 72 h. In both cases, young and aged cells expressed the actin isoform α–smooth muscle actin (α-SMA) (green staining). However, α-SMA was incorporated into actin stress fibers (red) only in fibroblasts derived from young individuals. In aged cells, α-SMA (green) remained as a cytosolic staining, as shown in this image. These results suggest that aged myofibroblasts were not able to form mature actin fibers. These defects might affect tissue remodeling during wound healing. Bar = 10 µm. For details, see Cáceres et al. (2014). This figure is available in color online at http://jdr.sagepub.com.
Angiogenesis
New blood vessels form and capillaries associated with fibroblasts and macrophages replace the temporary fibrin matrix with granulation tissue during the tissue formation phase. Blood vessels develop rapidly after wounding by sprouting from preexisting capillaries through the incorporation of bone marrow–derived endothelial precursors and by the transdifferentiation of circulating monocytes into endothelial cells (Carmeliet 2003). Among the soluble mediators involved in tissue growth during granulation tissue formation, vascular endothelial growth factor (VEGF) and fibroblast growth factor-2 (FGF-2) play a crucial role in angiogenesis (Presta et al. 2005). Aging may affect angiogenesis at several levels, including changes in the production or signaling associated with VEGF and FGF-2 (Swift et al. 1999; Gunin et al. 2014). Interestingly, aging may differentially affect angiogenesis in a tissue-specific manner (Shimoda et al. 2014). Therefore, more studies are needed to evaluate if aging may affect angiogenesis during gingival or periodontal wound healing.
Cell Proliferation
A pivotal aspect of cellular senescence is the inability of cells to progress through the cell cycle. Nevertheless, aged cells may stop their growth, maintaining their metabolic activity (Campisi and d’Adda di Fagagna 2007). Furthermore, it has been proposed that aged skin fibroblasts cultured in 3-dimensional collagen matrices show a diminished proliferative capacity that correlates with a lower expression of insulin-like growth factor receptor (IGF-R) (Bentov et al. 2014). Telomere shortening and DNA damage response are considered important mechanisms that regulate the diminished cell proliferation described in aged cells. The function of telomeres is to act as a molecular clock that keeps a record of cell replication (Harley et al. 1990). Telomere loss through consecutive cell divisions results in critically short telomeres that derive in a phenomenon known as replicative senescence (Campisi and d’Adda di Fagagna 2007). The loss of telomeres is detected as a type of DNA damage, triggering the so-called DNA damage response. The main mediators of DNA damage response are the DNA damage kinases ATM, ATR, CHK1, and CHK2, which phosphorylate and activate several cell cycle proteins, including p53 (Campisi and d’Adda di Fagagna 2007). Moreover, phosphorylated p53 protein activates the expression of p21, which binds to and inhibits some CDK-cyclin complexes, particularly those involving CDK2, finally altering the proliferative activity of cells (Fumagalli et al. 2012). Both cell proliferation and differentiation are striking features of the new tissue formation phase, and several markers and morphologic features characterize senescent cells. These include the absence of the proliferative marker Ki-67, an increase in senescence-associated β-galactosidase activity, and the expression of tumor suppressors and cell cycle inhibitors (p16, ARF, p53, p21, p15, p27, and hypophosphorylated RB) (Muñoz-Espin and Serrano 2014). The biological purpose of senescence is, therefore, to eliminate unwanted cells that may have accumulated damage in their genome. Consequently, senescence and apoptosis are essential mechanisms that eliminate damaged cells (Muñoz-Espin and Serrano 2014). Although this strategy represents a key mechanism to avoid the perpetuation of genome alterations in daughter cells, it will definitively reduce the proliferation potential in aged tissues. Recent studies have evaluated the role of aging on the proliferative potential of periodontal cells. Concerning gingival and periodontal wound healing, Benatti et al. (2008) described that primary cultures of human periodontal ligament fibroblasts derived from aged donors displayed a reduction in cell proliferation compared with cells obtained from young individuals. These results were also confirmed in our recent study that evaluated cell proliferation in human gingival fibroblasts derived from young and aged donors (Cáceres et al. 2014). Future studies should evaluate which specific cell populations are affected by defects in cell proliferation in the oral masticatory mucosa and the periodontium.
Serum-Derived Factors in Wound Healing
Blood circulation slowly restores the oxygen supply to tissues through incremental angiogenesis during wound healing. Therefore, factors derived from circulation and serum may influence tissue repair. Conboy et al. (2005) studied the role of circulation in wound healing by performing a parabiosis experiment. They concluded that muscle tissues from aged mice experienced a rejuvenating process when they were exposed to a young circulatory system (Conboy et al. 2005). On the basis of these findings, we stimulated gingival fibroblasts with serum derived from young and middle-aged rats and observed that serum from young rats was able to stimulate cell migration (Cáceres et al. 2014). These findings strongly suggest that defects in gingival wound healing may reside not only on resident tissue cells but also in serum factors. These results are important when considering the use of autologous platelet-derived growth factors, particularly in the case of aged individuals.
Stem Cells
Several studies have highlighted the role of adult stem cells in preserving tissue homeostasis and wound healing (Oh et al. 2014). Adult stem cells remain in a specialized microenvironment or niche, which provides essential signals for stem cell potential maintenance (Jung and Brack 2014). Stem cell functions decline with age, probably through alterations in self-renewal, differentiation potential, senescence-associated β-galactosidase expression, and the arrest of proliferation, leading to a reduced regenerative capacity of all tissues and organs. In recent years, research in stem cell aging has focused on the role of oxidative stress and impaired cellular antioxidant mechanisms, modifications in the systems that control the repair of damaged DNA, reduction of telomere length, and epigenetic changes induced by histone acetylation and methylation. All these events are affected by many different cell-intrinsic and cell-extrinsic pathways that influence not only stem cell function but also other cells present in the niche (Jung and Brack 2014; Oh et al. 2014). In oral tissues, studies performed by Zhang et al. (2012) explored the effects of aging on stem cells. These authors analyzed several cell responses in cell cultures derived from the periodontal ligament derived from donors of different ages and found that cell proliferation, migration, and pluripotency were significantly inhibited in stem cells obtained from aged tissues compared with young individuals (Zhang et al. 2012).
Tissue Remodeling
The timing of the initial wound remodeling stage is highly variable and will depend on the size of the wound and whether the injury healed through a primary or secondary intention. However, it has been assumed that this phase starts between 2 and 3 wk after injury and may last for 1 y or more (Gurtner et al. 2008). During this stage, all of the processes activated after injury wind down and conclude. Endothelial cells and myofibroblasts undergo apoptosis, leaving a new tissue that consists mostly of collagen and other ECM proteins. One of the striking changes observed during the tissue-remodeling phase is the substitution of the collagens present in the wound. Remarkably, type III collagen, which is the predominant matrix molecule in the granulation tissue, is degraded and replaced by fibrous type I collagen (Gurtner et al. 2008). In the aged lung and heart, type I collagen is increased, whereas type III collagen is diminished (Mays et al. 1988). However, the proportion of type I collagen is reduced during skin aging (Mays et al. 1988). Interestingly, in the aged periodontal ligament, collagen 1A1 gene expression is downregulated, probably due to hypermethylation of the promoter of this gene (Ohi et al. 2006). Collagen production is critically dependent on the activity of TGF-β1 (Brown et al. 1995). A recent study has shown that decreased TGF-β signaling and consequent reduced levels of connective tissue growth factor (CTGF) may be responsible for the progressive loss of collagen in the aged skin (Quan et al. 2010). Although TGF-β regulation may differ in skin versus gingival cells (Mah et al. 2014), it is plausible that gingival cells may experience changes in their response to TGF-β during aging. Collagen fibers are significantly modified and reorganized during the slow tissue-remodeling process in which molecules change their general orientation. Matrix metalloproteinases (MMPs) also have been involved in the remodeling of ECM components that are necessary to adjust the amount of newly formed tissue during gingival wound healing (Ravanti et al. 1999). Increased levels of MMPs and tissue inhibitors of MMPs (TIMPs) have been identified in aged periodontal ligament cells compared with cells derived from young donors (Benatti et al. 2008). Therefore, it is possible that tissue remodeling and maturation develop with an increased level of proteolytic enzymes in aged subjects, which might degrade and eventually weaken the newly formed tissue. Tissue remodeling is also dependent on the activity of the actin cytoskeleton and integrins involved in cell attachment to ECM components and cell signaling (Segal et al. 2001). All these changes involve the active role of integrins that participate in tissue remodeling. Integrin signaling may be altered during aging, as demonstrated in cardiac tissue, where aging modifies β1 integrin signaling, probably due to changes in the organization and mechanical properties of the ECM (Nishimura et al. 2014). More studies are needed to evaluate the involvement of integrins in aging gingival tissues.
In the final stages of the wound-healing process, activated fibroblasts, macrophages, and endothelial cells undergo apoptosis (Desmoulière et al. 1995; Zhang et al. 2004). Since aging modifies apoptotic signaling (González et al. 2011), it is likely that the normal removal of cells from the newly regenerated tissue is compromised in older individuals. Further studies are needed to explore this possibility.
Collagen is remodeled through phagocytosis during wound healing (Arlein et al. 1998). Collagen phagocytosis is a complex process that involves the initial degradation of collagen macromolecules by MMPs, the binding and internalization of collagen fragments by integrins, and their degradation in intracellular phagolysosomal domains (Segal et al. 2001). Using an in vitro cell culture model of fibroblast aging through consecutive cell culture passages, Lee and McCulloch (1997) demonstrated that cell aging was associated with an increase in collagen phagocytosis. It is tempting to speculate whether increased collagen phagocytosis may contribute to this altered phenotype as aged periodontal ligaments show a decrease in collagen content (Ohi et al. 2006). Moreover, in vivo studies are needed to further characterize involvement of collagen phagocytosis during tissue repair in aged tissues.
Concluding Remarks
Aging provokes a clear detrimental effect on wound healing in several tissues, including the masticatory mucosa and the periodontium (Benatti et al. 2006; Cáceres et al. 2014). The 3 stages of tissue repair, including the inflammatory phase, new tissue formation, and tissue-remodeling phase, may be affected by aging. The tissue repair deficiencies associated with aging have been summarized and highlighted in Figure 3. A major objective of wound healing is the restoration of injured tissues preventing infection and chronic inflammation. Therefore, elderly patients may be at risk of bacterial colonization and inflammatory reactions. Although studies evaluating periodontal disease in older subjects have shown an increase in their prevalence, they have been unable to identify an increased risk of periodontal disease progression (Ship and Beck 1996; Renvert et al. 2013). This information is not necessarily contradictory since aging has been associated with the attenuation of several diseases, including renal and liver fibrosis, atherosclerosis, and cancer (reviewed by Muñoz-Espin and Serrano 2014). Gingival tissues play a key role in the protection of tooth structures and supporting periodontal tissues against trauma and/or infection. Therefore, more studies are needed to identify and characterize the role of aging in the response of gingival tissues upon microbial and/or physical injury.
Figure 3.
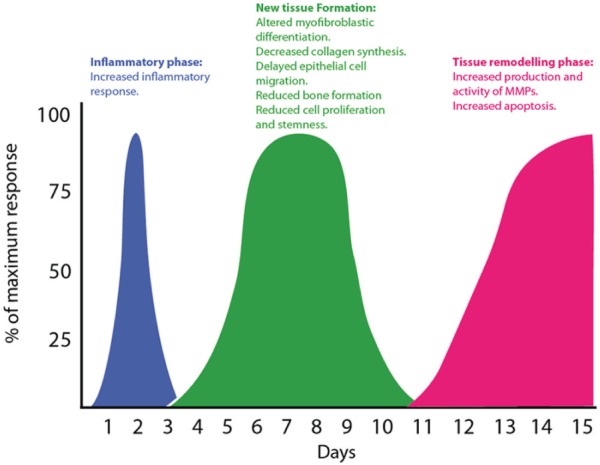
Phases of wound healing and tissue repair deficiencies associated with aging. The classically described stages of wound healing—inflammatory phase, new tissue formation, and tissue remodeling phase—are illustrated as a function of time. The main wound-healing defects associated with aging are indicated. MMP, matrix metalloproteinases.
Author Contributions
P.C. Smith, contributed to conception, design, and data analysis, drafted and critically revised the manuscript; M. Cáceres, contributed to conception and design, critically revised the manuscript; C. Martínez, A. Oyarzún, contributed to data analysis, critically revised the manuscript; J. Martínez, contributed to conception and design, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
The authors appreciate the funding provided by the National Fund for Science and Technology (FONDECYT) from Chile (grant nos. 1130618 and 3120041) that allowed the development of these studies.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Arancibia R, Oyarzún A, Silva D, Tobar N, Martínez J, Smith PC. 2013. Tumor necrosis factor–α inhibits transforming growth factor-β–stimulated myofibroblastic differentiation and extracellular matrix production in human gingival fibroblasts. J Periodontol. 84(5):683–693. [DOI] [PubMed] [Google Scholar]
- Arlein WJ, Shearer JD, Caldwell MD. 1998. Continuity between wound macrophage and fibroblast phenotype: analysis of wound fibroblast phagocytosis. Am J Physiol. 275(4 Pt 2):R1041–R1048. [DOI] [PubMed] [Google Scholar]
- Benatti BB, Neto JB, Casati MZ, Sallum EA, Sallum AW, Nociti FH., Jr. 2006. Periodontal healing may be affected by aging: a histologic study in rats. J Periodontal Res. 41(4):329–333. [DOI] [PubMed] [Google Scholar]
- Benatti BB, Silvério KG, Casati MZ, Sallum EA, Nociti FH., Jr. 2008. Influence of aging on biological properties of periodontal ligament cells. Connect Tissue Res. 49(6):401–408. [DOI] [PubMed] [Google Scholar]
- Benatti BB, Silvério KG, Casati MZ, Sallum EA, Nociti FH., Jr. 2009. Inflammatory and bone-related genes are modulated by aging in human periodontal ligament cells. Cytokine. 46(2):176–181. [DOI] [PubMed] [Google Scholar]
- Bentov I, Damodarasamy M., Plymate S, Reed MJ. 2014. Decreased proliferative capacity of aged dermal fibroblasts in a three dimensional matrix is associated with reduced IGF1R expression and activation. Biogerontology. 15(4):329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RL, Ormsby I, Doetschman TC, Greenhalgh DG. 1995. Wound healing in the transforming growth factor-beta–deficient mouse. Wound Repair Regen. 3(1):25–36. [DOI] [PubMed] [Google Scholar]
- Cáceres M, Oyarzun A, Smith PC. 2014. Defective wound-healing in aging gingival tissue. J Dent Res. 93(7):691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, d’Adda di Fagagna F. 2007. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 8(9):729–740. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. 2003. Angiogenesis in health and disease. Nat Med. 9(6):653–660. [DOI] [PubMed] [Google Scholar]
- Chung HY, Lee EK, Choi YJ, Kim JM, Kim DH, Zou Y, Kim CH, Lee J, Kim HS, Kim ND, et al. 2011. Molecular inflammation as an underlying mechanism of the aging process and age-related diseases. J Dent Res. 90(7):830–840. [DOI] [PubMed] [Google Scholar]
- Cieslik KA, Trial J, Entman ML. 2011. Defective myofibroblast formation from mesenchymal stem cells in the aging murine heart rescue by activation of the AMPK pathway. Am J Pathol. 179(4):1792–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. 2005. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 433(7027):760–764. [DOI] [PubMed] [Google Scholar]
- Coppé JP, Desprez PY, Krtolica A, Campisi J. 2010. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 5:99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies LC, Jenkins SJ, Allen JE, Taylor PR. 2013. Tissue-resident macrophages. Nat Immunol. 14(10):986–995.24048120 [Google Scholar]
- Desmoulière A, Redard M, Darby I, Gabbiani G. 1995. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 146(1):56–66. [PMC free article] [PubMed] [Google Scholar]
- Dovi JV, He LK, DiPietro LA. 2003. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol. 73(4):448–455. [DOI] [PubMed] [Google Scholar]
- Fumagalli M, Rossiello F, Clerici M, Barozzi S, Cittaro D, Kaplunov JM, Bucci G, Dobreva M, Matti V, Beausejour CM, et al. 2012. Telomeric DNA damage is irreparable and causes persistent DNA-damage response activation. Nature Cell Biol. 14(4):355–365. Erratum in: Nat Cell Biol. 2012;14(5):555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz C, Stewart KM, Weaver VM. 2010. The extracellular matrix at a glance. J Cell Sci. 123(Pt 24):4195–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick JA, Parks WC, Welgus HG, Taichman LB. 1996. Re-epithelialization of human oral keratinocytes in vitro. J Dent Res. 75(3):912–918. [DOI] [PubMed] [Google Scholar]
- González OA, Stromberg AJ, Huggins PM, Gonzalez-Martinez J, Novak MJ, Ebersole JL. 2011. Apoptotic genes are differentially expressed in aged gingival tissue. J Dent Res. 90(7):880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiling D, Clark RA. 1997. Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci. 110(Pt 7):861–870. [DOI] [PubMed] [Google Scholar]
- Gunin AG, Petrov VV, Golubtzova NN, Vasilieva OV, Kornilova NK. 2014. Age-related changes in angiogenesis in human dermis. Exp Gerontol. 55:143–151. [DOI] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. 2008. Wound repair and regeneration. Nature. 453(7193):314–321. [DOI] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, Greider CW. 1990. Telomeres shorten during ageing of human fibroblasts. Nature. 345(6274):458–460. [DOI] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, De Wever O, Mareel M, Gabbiani G. 2012. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 180(4):1340–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer VR, Eisen MB, Ross DT, Schuler G, Moore T, Lee JC, Trent JM, Staudt LM, Hudson J, Jr, Boguski MS, et al. 1999. The transcriptional program in the response of human fibroblasts to serum. Science. 283(5398):83–87. [DOI] [PubMed] [Google Scholar]
- Jung Y, Brack A. 2014. Cellular mechanisms of somatic stem cell aging. Curr Top Dev Biol. 107:405–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao HK, Chen B, Murphy GF, Li Q, Orgill DP, Guo L. 2011. Peripheral blood fibrocytes: enhancement of wound healing by cell proliferation, re-epithelialization, contraction, and angiogenesis. Ann Surg. 254(6):1066–1074. [DOI] [PubMed] [Google Scholar]
- Kolaczkowska E, Kubes P. 2013. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 13(3):159–175. [DOI] [PubMed] [Google Scholar]
- Kumar S, Millis AJ, Baglioni C. 1992. Expression of interleukin 1–inducible genes and production of interleukin 1 by aging human fibroblasts. Proc Natl Acad Sci USA. 89(10):4683–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larjava H, Koivisto L, Häkkinen L, Heino J. 2011. Epithelial integrins with special reference to oral epithelia. J Dent Res. 90(12):1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, McCulloch CA. 1997. Deregulation of collagen phagocytosis in aging human fibroblasts: effects of integrin expression and cell cycle. Exp Cell Res. 237(2):383–393. [DOI] [PubMed] [Google Scholar]
- Mackinnon A, Forbes S. 2013. Bone marrow contributions to fibrosis. Biochim Biophys Acta. 1832(7):955–961. [DOI] [PubMed] [Google Scholar]
- Mah W, Jiang G, Olver D, Cheung G, Kim B, Larjava H, Häkkinen L. 2014. Human gingival fibroblasts display a non-fibrotic phenotype distinct from skin fibroblasts in three-dimensional cultures. PLoS One. 9(3):e90715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, D’Souza D, Martin J, Grose R, Cooper L, Maki R, McKercher SR. 2003. Wound healing in the PU.1 null mouse—tissue repair is not dependent on inflammatory cells. Curr Biol. 13(13):1122–1128. [DOI] [PubMed] [Google Scholar]
- Mays PK, Bishop JE, Laurent GJ. 1988. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 45(3):203–212. [DOI] [PubMed] [Google Scholar]
- Muñoz-Espín D, Serrano M. 2014. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 15(7):482–496. [DOI] [PubMed] [Google Scholar]
- Netzel-Arnett S, Mitola DJ, Yamada SS, Chrysovergis K, Holmbeck K, Birkedal-Hansen H, Bugge TH. 2002. Collagen dissolution by keratinocytes requires cell surface plasminogen activation and matrix metalloproteinase activity. J Biol Chem. 277(47):45154–45161. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Kumsta C, Kaushik G, Diop SB, Ding Y, Bisharat-Kernizan J, Catan H, Cammarato A, Ross RS, Engler AJ, et al. 2014. A dual role for integrin-linked kinase and β1-integrin in modulating cardiac aging. Aging Cell. 13(3):431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odland G, Ross R. 1968. Human wound repair, I: epidermal regeneration. J Cell Biol. 39(1):135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Lee YD, Wagers A. 2014. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med. 20(8):870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi T, Uehara Y, Takatsu M, Watanabe M, Ono T. 2006. Hypermethylation of CpGs in the promoter of the COL1A1 gene in the aged periodontal ligament. J Dent Res. 85(3):245–250. [DOI] [PubMed] [Google Scholar]
- Pacios S, Kang J, Galicia J, Gluck K, Patel H, Ovaydi-Mandel A, Petrov S, Alawi F, Graves DT. 2012. Diabetes aggravates periodontitis by limiting repair through enhanced inflammation. FASEB J. 26(4):1423–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Herzog EL. 2012. Fibrocytes: emerging effector cells in chronic inflammation. Curr Opin Pharmacol. 12(4):491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presta M, Dell’Era P, Mitola S, Moroni E, Ronca R, Rusnati M. 2005. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 16(2):159–178. [DOI] [PubMed] [Google Scholar]
- Quan T, Shao Y, He T, Voorhees JJ, Fisher GJ. 2010. Reduced expression of connective tissue growth factor (CTGF/CCN2) mediates collagen loss in chronologically aged human skin. J Invest Dermatol. 130(2):415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanti L, Häkkinen L, Larjava H, Saarialho-Kere U, Foschi M, Han J, Kähäri VM. 1999. Transforming growth factor–beta induces collagenase-3 expression by human gingival fibroblasts via p38 mitogen-activated protein kinase. J Biol Chem. 274(52):37292–37330. [DOI] [PubMed] [Google Scholar]
- Renvert S, Persson RE, Persson GR. 2013. Tooth loss and periodontitis in older individuals: results from the Swedish National Study on Aging and Care. J Periodontol. 84(8):1134–1144. [DOI] [PubMed] [Google Scholar]
- Segal G, Lee W, Arora PD, McKee M, Downey G, McCulloch CA. 2001. Involvement of actin filaments and integrins in the binding step in collagen phagocytosis by human fibroblasts. J Cell Sci. 114(Pt 1):119–129. [DOI] [PubMed] [Google Scholar]
- Shimoda Y, Matsuo K, Ono K, Soma Y, Ueyama T, Matoba S, Yamada H, Ikeda K. 2014. Aging differentially alters the expression of angiogenic genes in a tissue-dependent manner. Biochem Biophys Res Commun. 446(4):1243–1249. [DOI] [PubMed] [Google Scholar]
- Ship JA, Beck JD. 1996. Ten-year longitudinal study of periodontal attachment loss in healthy adults. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 81(3):281–290. [DOI] [PubMed] [Google Scholar]
- Simpson RM, Wells A, Thomas D, Stephens P, Steadman R, Phillips A. 2010. Aging fibroblasts resist phenotypic maturation because of impaired hyaluronan-dependent CD44/epidermal growth factor receptor signaling. Am J Pathol. 176(3):1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousounis K, Baddour JA, Tsonis PA. 2014. Aging and regeneration in vertebrates. Curr Top Dev Biol. 108:217–246. [DOI] [PubMed] [Google Scholar]
- Sudbeck BD, Pilcher BK, Welgus HG, Parks WC. 1997. Induction and repression of collagenase-1 by keratinocytes is controlled by distinct components of different extracellular matrix compartments. J Biol Chem. 272(35):22103–22110. [DOI] [PubMed] [Google Scholar]
- Swift ME, Kleinman HK, DiPietro LA. 1999. Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest. 79(12):1479–1487. [PubMed] [Google Scholar]
- Szpaderska AM, Egozi EI, Gamelli RL, DiPietro LA. 2003. The effect of thrombocytopenia on dermal wound healing. J Invest Dermatol. 120(6):1130–1137. [DOI] [PubMed] [Google Scholar]
- Zhang J, An Y, Gao LN, Zhang YJ, Jin Y, Chen FM. 2012. The effect of aging on the pluripotential capacity and regenerative potential of human periodontal ligament stem cells. Biomaterials. 33(29):6974–6986. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kohli M, Zhou Q, Graves DT, Amar S. 2004. Short- and long-term effects of IL-1 and TNF antagonists on periodontal wound healing. J Immunol. 173(5):3514–3523. [DOI] [PubMed] [Google Scholar]