Abstract
Aim:
To investigate the effects of co-delivering IL-6 expressing plasmid pCI-IL-6 on the immunogenicity of the anti-caries DNA vaccine pCIA-P, which encodes the surface protein antigen PAc of Streptococcus mutans.
Methods:
Plasmid pCI-IL-6 was constructed by inserting the murine IL-6 gene into the pCI vector. Expression of IL-6 in vitro was assessed using Western blot analysis. BALB/c mice were intranasally co-immunized with pCIA-P plus pCI-IL-6 on d 0 and 14. Anti-PAc IgG and secretory IgA (sIgA) were assessed by ELISA. Splenocytes from the mice were re-stimulated with the PAc protein, and IFN-γ and IL-4 production was measured using ELISA. Splenocyte proliferation was analyzed with flow cytometry. Rats were similarly immunized, and dental caries scores were determined using the Keyes method.
Results:
Marked expression of IL-6 was found in COS-7 cells transfected with pCI-IL-6. In the pCI-IL-6 co-immunized mice, the specific IgG antibodies in serum and sIgA antibodies in saliva were significantly higher than those in the control mice at weeks 4 and 8. Moreover, the secretion of IFN-γ from splenocytes in response to re-stimulation with PAc protein was significantly higher in the pCI-IL-6 co-immunized mice than that in the control mice, whereas the secretion of IL-4 had no significant difference. The proliferation of splenocytes from the pCI-IL-6 co-immunized mice was significantly higher than that from the mice immunized with pCIA-P and pCI vector. In the rat caries model, the pCI-IL-6 co-immunization rats displayed lower caries scores than the control rats.
Conclusion:
Intranasal co-delivery of IL-6 gene significantly enhances the immunogenicity of the anti-caries DNA vaccine.
Keywords: DNA vaccine, vaccine adjuvant, dental caries, Streptococcus mutans, IL-6, cytokine, immunogenicity, stomatology
Introduction
Dental caries are one of the most common oral diseases worldwide, particularly in developing countries1. The World Health Organization (WHO) estimates that 5 of the 6.5 billion people in the world are affected by dental caries2. In the USA, caries are the most common chronic childhood disease and are 5 times more prevalent than asthma, afflicting 30% of children aged 2 to 5 years old3. An epidemiological survey in China showed that in the 5- to 6-year-old child group, the prevalence of caries was high (urban 78% and rural 86%) and the mean DMFT of the urban and rural children was 4.8 and 7.0, respectively. In the 12-year-old child group, the prevalence of caries was 41% (urban) and 42% (rural) and the mean DMFT score was 0.9 for both4. Therefore, it is necessary to identify effective public health strategies to prevent the incidence of dental caries. High numbers of cariogenic bacteria is a pathogenic factor, and Streptococcus mutans (S mutans) is a primary microorganismal etiologic agent of dental caries. The cell surface protein PAc is a S mutans virulence factor because it is involved in the initial adherence of the organism to tooth surfaces5,6. The A-region and P-region have been shown to be important to the function and immunogenicity of the PAc protein6,7.
A number of studies have shown that a DNA vaccination plasmid can endogenously produce a long-term and stable antigenic protein that can induce both the cellular and humoral immune responses8,9,10. In addition, this DNA vaccine has many potential advantages, including a simple engineering design modification and easy storage. In our previous studies, the DNA vaccine pCIA-P encoding the A- and P-regions of the S mutans pac gene was successfully constructed and induced systemic and mucosal antibody responses against S mutans in mice11,12. However, DNA vaccines have long been plagued by the problem of low immunogenicity in large animals and humans. Recently, studies have focused on the use of cytokines as intranasal vaccine adjuvants to enhance the immunogenicity of the DNA vaccine because of their potent effects on innate and adaptive immunity as well as functional diversity of immune responses. Studies have shown that cytokines, such as IL-1213,14, IL-215, IL-1516, type I interferon (IFN)17,18, IL-119, IL-620, and granulocyte-macrophage colony-stimulating factor (GM-CSF)21, enhanced antigen-specific immunity following delivery with different antigens.
IL-6 is a multifunctional Th2-associated cytokine produced by macrophages, dendritic cells, T cells, endothelial cells and hepatocytes and plays important roles in the terminal differentiation of B cells, the proliferation of lymphocytes and endothelial cells and the differentiation of cytotoxic T lymphocytes (CTL) responses22,23,24,25,26. IL-6 effectively functions as a mucosal adjuvant that significantly enhances the mucosal and systemic immune responses20,27. DNases present at mucosal surfaces can easily degrade the DNA vaccination plasmid used to stimulate mucosal immunity. However, the presence of IL-6 as a vaccine adjuvant can significantly elicit both the mucosal and systemic immune responses, which provides a method of stimulating mucosal immunity with the combined DNA plasmid and il-6 gene20,28. In vivo studies have shown that IL-6 plays a critical role in the development of mucosal IgA antibody responses, which acts as the first line of the host immune defense and is critical for the protection of mucosal tissues29. In this study, we determined whether co-immunization of the il-6 gene with pCIA-P induced higher levels of IgA in the saliva and IgG in the serum against PAc in intranasally immunized mice and provided better protection against dental caries in rats.
Materials and methods
Animals
Six-week-old female BALB/c mice were purchased from the Hubei Medical Laboratory Animal Center (Wuhan, China) and maintained under specified pathogen-free (SPF) conditions. The Review Board of Provincial Laboratory Animal Breeding and Research Center approved all protocols involving animal research. Groups of mice were used to detect the specific antibodies and levels of the cytokines.
Wistar rats weaned at 18 d were maintained as described above. All rats were used in an experimental caries model to assess the protective efficacy of vaccination against dental caries.
Plasmids construction
The pCIA-P plasmid was constructed as previously described11. It encodes the A-P fragment of the S mutans MT8148 pac gene. The IL-6 expression plasmid was constructed as follows: the total RNA was separated from the mouse dendritic cell line DC2.4 and reversely transcribed into cDNA. The mouse IL-6 gene (Nucleotide ID: NM_031168.1) was amplified by polymerase chain reaction (PCR) using the cDNA as a template. The forward primer was 5′-CGGAATTCATGAAGTTCCTCTCTGCAAG-3′, with an EcoR I restriction site incorporated immediately at upstream of the transcription initiation codon, and the reverse primer was 5′-ACGCGTCGACCTAGGTTTGCCGAGTAGA-3′, with a Sal I restriction site incorporated immediately at downstream of the last codon. The PCR product was produced using the PCR expand system (Applied Biosystems, Foster City, CA, USA) for 35 cycles of 45 s at 98 °C, 60 s at 59 °C and 60 s at 72 °C. The PCR product was inserted into the pCI vector, which was digested with EcoR I and Sal I (NEB, Ipswich, MA, USA). The resulting plasmid was named pCI-IL-6.
IL-6 gene expression in vitro
To evaluate recombinant protein expression, COS-7 cells (CCTCC, Chinese Center for Type Culture Collection, Wuhan, China) were transfected with the pCI-IL-6 plasmid and the expression of IL-6 was detected by Western blot. Briefly, COS-7 cells were seeded (2×105/mL) in 24-well flat-bottomed cell culture plates (Corning Costar, Cambridge, MA, USA) and incubated at 37 °C in a CO2 incubator until the cells were 80% confluent. Then, 2 μg of pCI-IL-6 or pCI pre-mixed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was added to each well, according to the manufacturer's instructions. Two days after transfection, the cells were harvested, washed twice with ice-cold PBS, and lysed on ice for 10 min in radioimmunoprecipitation assay lysis buffer (Upstate Biotechnology, Lake Placid, NY, USA). The cell lysates were transferred to tubes, incubated on ice for an additional 20 min, and then centrifuged at 14 000×g for 15 min at 4 °C. The supernatants were collected and evaluated by Western blotting using a specific antibody against IL-6 (R&D systems, USA). Equal protein loading was monitored with the BCA protein assay kit (Thermo, Waltham, MA, USA). ECL Western blotting detection reagents were used to develop the blots according to the manufacturer's instructions (Amersham Bioscience, Piscataway, NJ, USA). β-Actin was the loading control.
Mouse immunization and sample collection
Sixteen mice were randomly divided into two groups (n=8). One group of mice was immunized with pCIA-P plus pCI-IL-6, and the other group was immunized with pCIA-P plus pCI vector on d 0 and 14. Briefly, a total of 25 μL of each plasmid (concentration 2 μg/μL) was administered into the nostrils of each mouse. Large-scale plasmid preparations were produced using the alkaline lysis method with the Endofree Qiagen Plasmid-Giga kits (Qiagen, Hilden, Germany), according to the manufacturer's instructions.
Mouse serum and saliva samples were collected prior to immunization (d 0) and at weeks 4, 8, 12, and 16 after the first immunization. Briefly, peripheral blood was collected from the angular vein with a 1 mL syringe needle and maintained at 4 °C for 1 h. The supernatant was obtained by centrifugation at 400×g for 10 min at room temperature. The saliva samples were collected after stimulation with intraperitoneal injection of 0.5 mL of 0.2% pilocarpine. All samples were stored at -70 °C until further use.
Antibody analysis
Enzyme linked immunosorbant assay (ELISA) was used to detect the specific antibody levels against PAc immunized mice samples. Microtiter plates (96-well) were coated with 10 μg/mL purified PAc protein dissolved in carbonate buffer (pH 9.6) at 4 °C overnight. In addition, several plates were coated with goat anti-mouse IgA antibody to measure the total IgA level in the saliva secretions. The wells were blocked with 3% BSA (bovine serum albumin)-PBST at 37 °C for 90 min and were washed five times. Next, a 1:50 dilution of mouse serum or 1:4 dilution of mouse saliva was added to the corresponding wells. A peroxidase-conjugated goat anti-mouse IgG (1:5000, Pierce, Rockford, IL, USA) or peroxidase-conjugated goat anti-mouse IgA (1:5000, Bethyl Laboratories, Montgomery, TX, USA) secondary antibody was added into each well and incubated at 37 °C for 2 h. The reaction was followed by adding the o-phenylenediamine substrate (Sigma, St Louis, USA) with 3% H2O2. After incubation at 37 °C for 30 min, the reaction was stopped with 2 mol/L H2SO4. Optical density (OD) values were recorded at 490 nm (OD490). The final result was counted by interpolation against standard curves using a mouse immunoglobulin reference serum (Bethyl Laboratories) and calculated by a computer program based on standard curves.
Effect of pCI-IL-6 co-immunization on T-cell cytokines and splenocyte proliferation
To determine the effect of IL-6 as a mucosal adjuvant on the differentiation of T cells, the production of IL-4 and IFN-γ by spleen cells from immunized mice was measured by ELISA. Two mice were immunized as described above. Seven days after the boost immunization, the mice were euthanized, the spleens were aseptically isolated, and single-cell suspensions were prepared and maintained in 96-well flat-bottomed plates containing RPMI-1640 complete medium at 37 °C in a 5% CO2 incubator. The cells were then stimulated with 20 μg/mL PAc. Twenty-four hours after stimulation, the cell supernatants were collected and centrifuged at 400×g for 10 min at room temperature. The levels of IFN-γ and IL-4 in the supernatants were measured using commercial cytokine ELISA kits (R&D Systems, Oxon, UK), according to the manufacturer's instructions.
To analyze the proliferation of spleen cells re-stimulated with the same antigen in vitro, a bromodeoxyuridine (BrdU) method was used. Briefly, spleen cells were prepared and stimulated as above. The cells were then washed with FACS buffer (PBS supplemented with 2% bovine serum albumin and 0.1% sodium azide). For measuring BrdU incorporation, the cells were processed using a BD BrdU flow kit (BD Biosciences, CA, USA). The BrdU was added into the well and incubated with the cells for 5 to 6 h, according to the manufacturer's instructions. The cells were washed twice with FACS buffer, followed by staining with a fluorescent anti-BrdU-FITC monoclonal antibody and DNase for 20 min at room temperature. The cells were washed again and all samples were re-suspended in FACS buffer and assayed on a FACS Calibur instrument (BD Bioscience, CA, USA). The data were analyzed using the CellQuest software (BD Bioscience, CA, USA).
Rat immunization and caries score
Fourteen rats were immunized following the same protocol as the mice. After the boost immunization, all rats were fed the cariogenic diet 2000 until termination of the experiment. Antibiotics were added to the diet (ampicillin, chloramphenicol and carbenicillin, 1.0 g/kg diet) and drinking water (4000 U penicillin G per mL) during the first 4 d to suppress the oral flora and facilitate S mutans infection. Following antibiotic treatment, all rats were infected for 5 consecutive days with 2×109 CFU of S mutans Ingbritt by applying swabs presoaked with the bacterial suspension. Bacterial samples from the tooth surfaces of each rat were then examined to ensure complete infection. At the end of the experiment (2 months after infection), the rats were euthanized. The maxillary and mandibular bones were separated, cleaned with ammonium hydroxide and stained with 0.4% murexide for 24 h. Then, the teeth were hemisectioned, dried and stored in the dark. The rat teeth were observed under a stereomicroscope to determine the caries levels according to the Keyes dental caries scoring method30.
Statistical analysis
The SPSS 10.0 software (SPSS, Inc, Chicago, IL, USA) was used to perform the statistical analyses. The differences in the antibody levels, cytokine production and dental caries scores between groups were determined by Student's t-test. The differences were deemed significant at P<0.05 and highly significant at P<0.01.
Results
Construction and characterization of plasmid pCI-IL-6
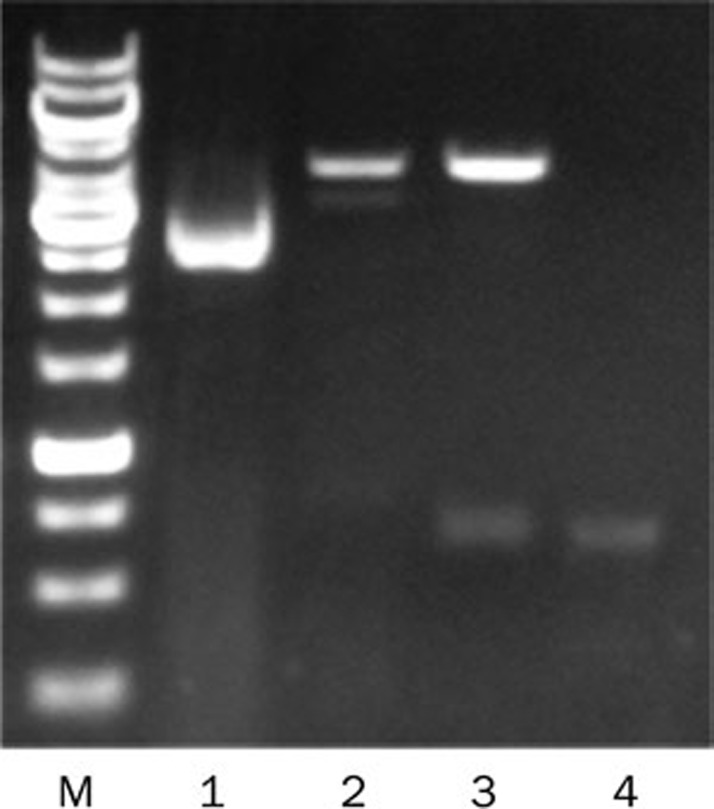
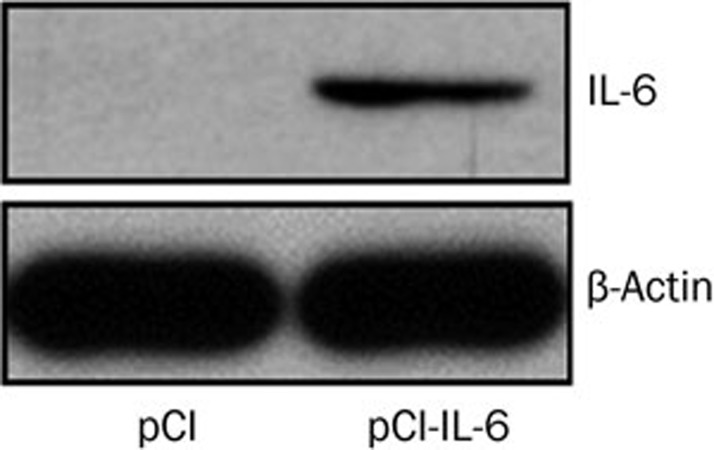
The pCI-IL-6 plasmid was constructed as described above and confirmed by PCR, enzyme digestion and sequence analysis. Electrophoresis after digestion showed that the il-6 gene was properly inserted into the vector plasmid pCI (Figure 1). Sequence analysis showed that the sequence of the cloned IL-6 gene was identical to the sequence in Genbank (Nucleotide ID: NM_031168.1). Plasmid expression was confirmed by Western blot, which showed that the expression of IL-6 in the pCI-IL-6 transfected group was higher than that in the pCI transfected group (Figure 2).
Figure 1.
Restriction pattern profiles of the digested plasmid. The pCI-IL-6 plasmid was enzymatically digested, and the digests were subjected to agarose gel electrophoresis. M lane, DNA Ladder; Lane 1, undigested plasmid pCI; Lane 2, EcoR I digested plasmid; Lane 3, EcoR I and Sal I digested plasmid; Lane 4, PCR product of target gene.
Figure 2.
IL-6 gene expression in vitro. COS-7 cells were transfected with either the pCI-IL-6 or pCI plasmid. After 48 h of culture, IL-6 expression in the cells was detected by Western blot.
IL-6 as an adjuvant increases the level of serum IgG
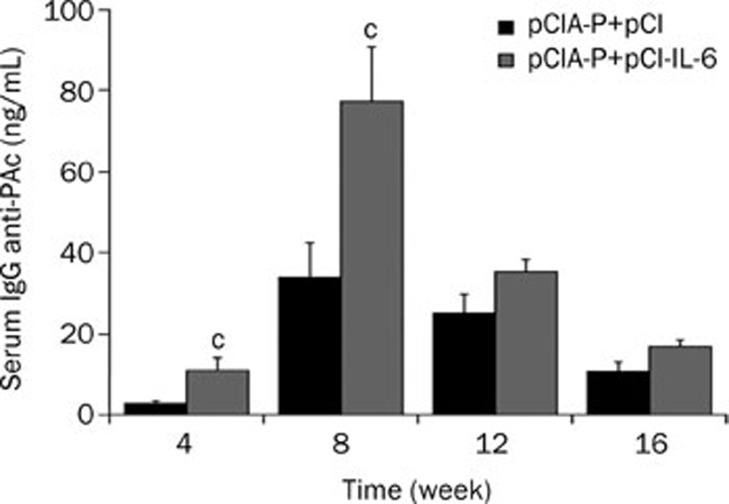
To determine the mucosal adjuvant potential of IL-6, the mice were intranasally immunized with pCIA-P plus pCI-IL-6 or pCIA-P plus pCI, and the serum was collected for ELISA detection of PAc specific IgG antibodies. The level of the serum specific IgG antibody was undetectable prior to immunization in both groups (data not shown). The mean serum specific IgG antibody levels of the pCIA-P plus pCI-IL-6 immunization group were significantly higher than those in the pCIA-P plus pCI immunization group at weeks 4 and 8 (P<0.01, Figure 3). Although the IgG levels of the IL-6 co-immunization group were higher than the control group at weeks 12 and 16, the statistical analysis showed no significant difference between the two groups (P>0.05). The highest level of serum specific IgG antibody appeared at week 8 and gradually decreased over time.
Figure 3.
Levels of serum specific IgG antibody. Blood was collected from mice immunized intranasally with pCIA-P plus pCI-IL-6 or pCIA-P plus pCI vector every 4 weeks and the serum was obtained for ELISA. Mean±SEM. n=8. cP<0.01 vs pCIA-P+pCI group.
IL-6 enhances the saliva IgA responses in mice
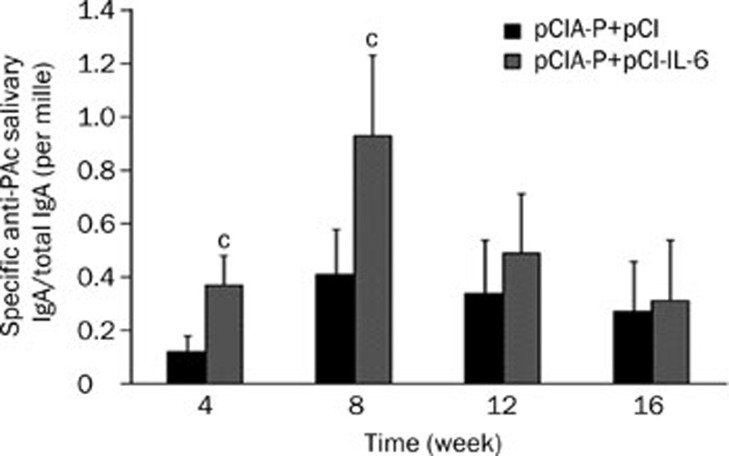
In this study, the ratio of specific anti-PAc salivary IgA to total IgA was used to represent the intensity of the salivary IgA responses. The saliva specific IgA antibody levels in the pCIA-P plus pCI-IL-6 immunization group were significantly higher than that in the pCIA-P plus pCI immunization group at weeks 4 and 8 (P<0.01) (Figure 4). However, there was no statistically significant difference (P>0.05) between the two groups at weeks 12 and 16. The highest level of saliva specific IgA antibody was also at week 8.
Figure 4.
Levels of saliva specific IgA antibody. Saliva samples were collected from mice immunized intranasally with pCIA-P plus pCI-IL-6 or pCIA-P plus pCI vector every 4 weeks for ELISA. The results are shown as the ratios of specific anti-PAc salivary IgA to total IgA. Mean±SEM. n=8. cP<0.01 vs pCIA-P+pCI group.
IL-6 co-immunization increases IFN-γ production by splenocytes
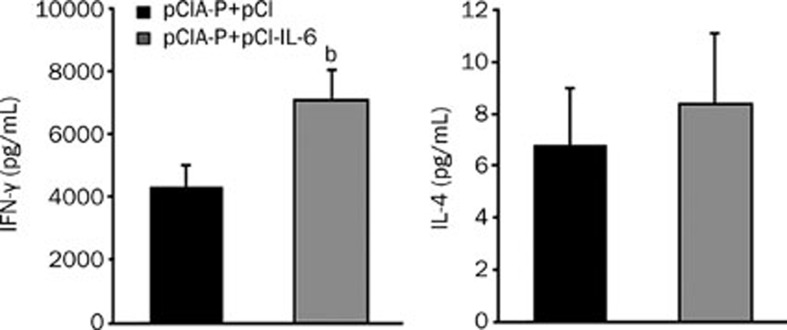
To analyze the effect of IL-6 co-immunization on the cytokine production by spleen cells, the mice were intranasally immunized twice, and splenocyte suspensions were prepared and re-stimulated with the PAc protein in vitro. After 24 h of culture, IFN-γ and IL-4 in the supernatants were analyzed by quantitative ELISA. Mice immunized with pCIA-P plus pCI-IL-6 showed a significantly higher IFN-γ production compared to the control group (P<0.05), whereas, there was no statistically significant difference in IL-4 production observed between the two groups (Figure 5).
Figure 5.
Analysis of cytokine production by antigen re-stimulated splenocytes. Two groups of mice were immunized with pCIA-P plus pCI-IL-6 or pCIA-P plus pCI vector twice biweekly. The splenocytes were isolated on d 7 after the second immunization and re-stimulated with 20 μg/mL PAc for 24 h. IFN-γ and IL-4 secretion were analyzed by quantitative ELISA. Mean±SEM. n=8. bP<0.05 vs pCIA-P+pCI.
IL-6 co-immunization enhances T cell proliferation
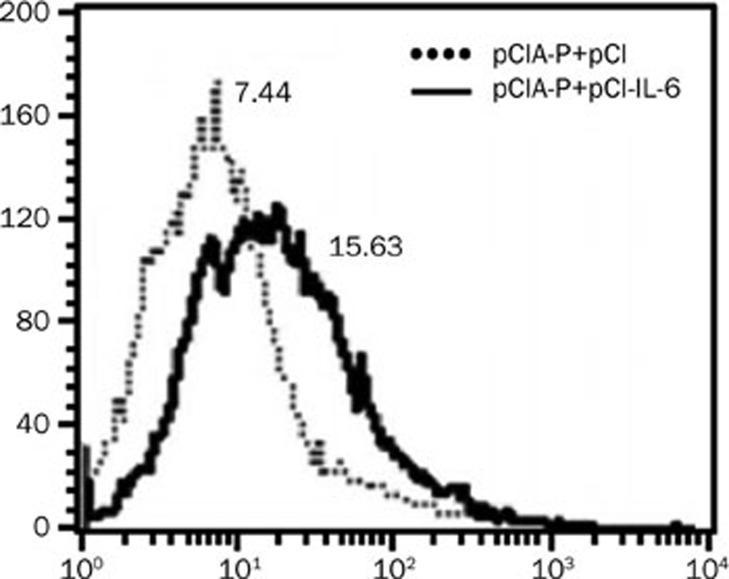
The mouse splenocytes were prepared and re-stimulated with Pac, as described above. After 24 h of culture, the splenocytes were harvested to determine the proliferation of T cells using BrdU and FACS. The proliferation of cells harvested from animals immunized with pCIA-P plus pCI-IL-6 was significantly higher than the control group (Figure 6).
Figure 6.
pCI-IL-6 co-immunization promotes the proliferation of spleen cells. The splenocytes were isolated on d 7 after the second immunization and re-stimulated with 20 μg/mL PAc for 24 h and then labeled with BrdU and incubated with fluorescence-conjugated anti-BrdU monoclonal antibody. The fluorescence intensities for T-cell proliferation were measured with a FACS Calibur instrument. The data were analyzed with the CellQuest software. The solid line represents the experimental group, and the dotted line represents the control group.
Protective efficacy in rat caries model
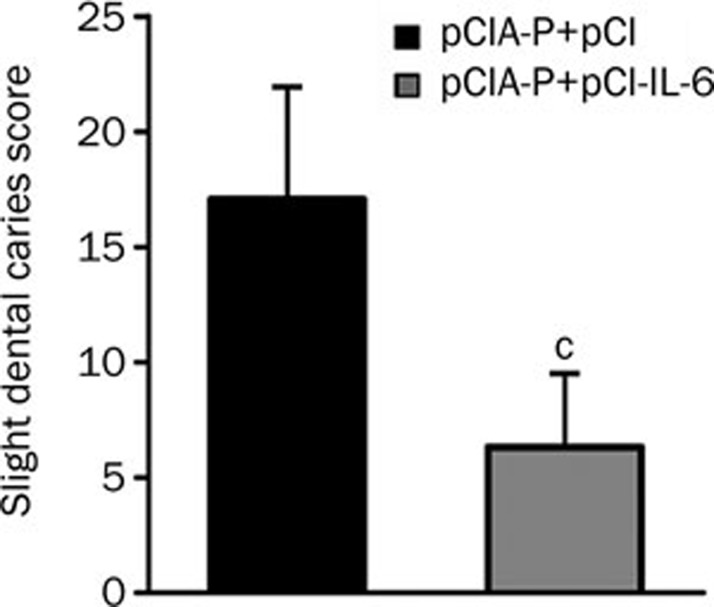
To assess the effect of IL-6 co-immunization on the protective efficacy of the anti-caries DNA vaccine pCIA-P, Wistar rats were immunized with pCIA-P plus pCI-IL-6 or pCIA-P plus pCI and then challenged with S mutans Ingbritt. At the end of the experiment, the dental caries scores were measured using the Keyes method30. The pCI-IL-6 co-immunization group displayed lower caries scores than the control group (P<0.01) (Figure 7).
Figure 7.
Dental caries scores of rats immunized with pCIA-P plus pCI-IL-6 or pCIA-P plus pCI vector. The rats were immunized as described above. After 2 months, all rats were euthanized and the teeth were collected to determine the dental caries scores. The scores of caries activities were determined using the Keyes method. Mean±SEM. n=7. cP<0.01 vs pCIA-P+pCI.
Discussion
S mutans is the principal etiological agent of dental caries. Therefore, the development of vaccines against S mutans may be an effective strategy to control the disease. PAc, a S mutans surface fibrillar protein, mediates the initial sucrose-independent attachment of bacteria to the acquired pellicles on tooth surfaces and is one of the most important virulence factors of S mutans. Many studies have shown that PAc is a rational candidate antigen for the development of anti-caries vaccines. One of our previous studies showed that the DNA vaccine pCIA-P, which encodes the A-P fragment of Pac, induces PAc-specific antibodies in the saliva and serum and provides protection against dental caries in rodent models.
Although a DNA vaccine has achieved great success in small animals, poor immunogenicity in primates and humans hinders the development of DNA vaccines. Different methods have been adopted to enhance the immunogenicity of DNA vaccines, including cytokine adjuvants. The co-delivery of plasmids encoding a cytokine is advantageous because the cytokine is expressed and acts at the site of antigen expression14,31,32,33. In the present study, we focused on IL-6 as a natural immune stimulator. IL-6 is a pleiotropic Th2 cytokine secreted by many different cell types, including macrophages, dendritic cells, T cells, endothelial cells, and hepatocytes. It functions in the terminal differentiation of B cells into plasma cells, the proliferation of lymphocytes and the regulation of IL-2 receptor expression22,23,24,34. Furthermore, IL-6 plays a critical role in the development of local IgA antibody responses and vector-directed cytokine gene therapy in vivo29.
We determined whether IL-6 is a potential mucosal adjuvant for an anti-caries DNA vaccine. Therefore, the IL-6 expressing plasmid pCI-IL-6 was constructed and co-immunized with pCIA-P intranasally in mice and rats. It was found that the levels of antigen-specific salivary IgA and serum IgG antibodies to PAc were significantly higher in the co-immunized mice compared to the pCIA-P immunized mice at weeks 4 and 8. In addition, the rats receiving pCIA-P and pCI-IL-6 displayed lower caries activity than the pCIA-P immunized rats. These findings suggest that the IL-6 plasmid as a mucosal adjuvant effectively improved both the mucosal and systemic immune responses, thereby inhibiting the severity of S mutans-induced dental caries. Furthermore, our study showed that the pCI-IL-6 co-immunized mice developed more potent cell-mediated immune responses, as evidenced by the higher levels of splenocyte IFN-γ production when re-stimulated with PAc. IFN-γ and IL-4 are Th1-type and Th2-type cytokines, respectively, and the former represents the cell-mediated immune response35, whereas the latter represents the humoral immune response36. Although the reason for Th1 biased T-cell induction rather than Th2 biased T-cell stimulation remains unknown, previous studies may partially explain the cause of T-cell differentiation after DNA vaccination. First, DNA vaccination itself tends to induce a Th1-biased response37,38. Furthermore, Th1-type cytokines are hypothesized to suppress the production of Th2-type cytokines, such as IL-439. In the present study, a minimal level of IL-4 was induced in both groups compared to IFN-γ. At such a low level, the difference in IL-4 between the two groups is hard to detect. Second, the specific immunogenicity of specific antigens may cause this effect. Our previous studies found that animals immunized with the anti-caries DNA vaccine pCIA-P or pGJA-P/VAX, containing an antigen similar to pCIA-P, tended to augment the levels of IFN-γ secreted by CD4+ Th cells; however, no significant increase in IL-4 production was observed40,41. In addition, when spleen cells were re-stimulated with PAc, the proliferation of co-immunized mouse spleen cells was significantly increased compared to the control mice.
The WHO is interested in needle-free methods of immunization because of disease transmission and phobia42,43,44. Our previous studies have shown that the nasal route of immunization is an attractive method for caries vaccines based on the protective effect against cariogenic bacterial infection. Because many cytokines, including IL-12, IL-2, IL-15, GM-CSF, and type I IFN, have been used intranasally to enhance the immunogenicity of vaccines, here, we focused on IL-6 as an immunomodulatory molecule to enhance the efficacy of the DNA vaccine following nasal delivery. Our results showed that IL-6 as a mucosal adjuvant enhanced both the mucosal and systemic immune responses in mice. Furthermore, the pCI-IL-6 plasmid cause significant T-cell development into functional Th1-type cells. In addition, the proliferation of spleen cells was improved by il-6 gene co-immunization. Importantly, the rats receiving pCIA-P and pCI-IL-6 displayed less caries activity than the pCIA-P and pCI immunized rats. These results suggest that intranasal delivery of IL-6 is an effective method of augmenting the immunogenicity of an anti-caries DNA vaccine and that IL-6 may also be a promising mucosal adjuvant for other DNA vaccines.
Author contribution
Ming-wen FAN and Qing-an XU designed the study and revised the manuscript; Ling-kai SU, Fei YU, Zhao-fei LI, and Chang ZENG performed the experiments; and Ling-kai SU wrote the manuscript.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No 81000435 and 81271129).
References
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet 2007; 9555: 51–9. [DOI] [PubMed] [Google Scholar]
- World Health Organization: changing levels of dental caries experience (DMFT) among 12-year-olds in developed and developing countries. Geneva: WHO; 2001.
- Dye BA, Tan S, Smith V, Lewis BG, Barker LK, Thornton-Evans G, et al. Trends in oral health status: United States, 1988–1994 and 1999–2004. Vital Health Stat 11 2007; 248: 1–92. [PubMed] [Google Scholar]
- Wong MC, Lo EC, Schwarz E, Zhang HG. Oral health status and oral health behaviors in Chinese children. J Dent Res 2001; 5: 1459–65. [DOI] [PubMed] [Google Scholar]
- Russell MW, Mansson-Rahemtulla B. Interaction between surface protein antigens of Streptococcus mutans and human salivary components. Oral Microbiol Immunol 1989; 2: 106–11. [DOI] [PubMed] [Google Scholar]
- Okahashi N, Sasakawa C, Yoshikawa M, Hamada S, Koga T. Molecular characterization of a surface protein antigen gene from serotype c Streptococcus mutans, implicated in dental caries. Mol Microbiol 1989; 5: 673–8. [DOI] [PubMed] [Google Scholar]
- Brady LJ, Cvitkovitch DG, Geric CM, Addison MN, Joyce JC, Crowley PJ, et al. Deletion of the central proline-rich repeat domain results in altered antigenicity and lack of surface expression of the Streptococcus mutans P1 adhesin molecule. Infect Immun 1998; 9: 4274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Doolan DL, Le TP, Hedstrom RC, Coonan KM, Charoenvit Y, et al. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 1998; 5388: 476–80. [DOI] [PubMed] [Google Scholar]
- Xiang ZQ, Spitalnik SL, Cheng J, Erikson J, Wojczyk B, Ertl HC. Immune responses to nucleic acid vaccines to rabies virus. Virology 1995; 2: 569–79. [DOI] [PubMed] [Google Scholar]
- Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol 1997; 15: 617–48. [DOI] [PubMed] [Google Scholar]
- Fan MW, Bian Z, Peng ZX, Zhong Y, Chen Z, Peng B, et al. A DNA vaccine encoding a cell-surface protein antigen of Streptococcus mutans protects gnotobiotic rats from caries. J Dent Res 2002; 11: 784–7. [DOI] [PubMed] [Google Scholar]
- Li Y, Jin J, Yang Y, Bian Z, Chen Z, Fan M. Enhanced immunogenicity of an anti-caries vaccine encoding a cell-surface protein antigen of Streptococcus mutans by intranasal DNA prime-protein boost immunization. J Gene Med 2009; 11: 1039–47. [DOI] [PubMed] [Google Scholar]
- Bradney CP, Sempowski GD, Liao HX, Haynes BF, Staats HF. Cytokines as adjuvants for the induction of anti-human immunodeficiency virus peptide immunoglobulin G (IgG) and IgA antibodies in serum and mucosal secretions after nasal immunization. J Virol 2002; 2: 517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka H, Hoyt T, Yang X, Golden S, Bosio CM, Crist K, et al. A nasal interleukin-12 DNA vaccine coexpressing Yersinia pestis F1-V fusion protein confers protection against pneumonic plague. Infect Immun 2008; 10: 4564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferko B, Kittel C, Romanova J, Sereinig S, Katinger H, Egorov A. Live attenuated influenza virus expressing human interleukin-2 reveals increased immunogenic potential in young and aged hosts. J Virol 2006; 23: 11621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toka FN, Rouse BT. Mucosal application of plasmid-encoded IL-15 sustains a highly protective anti-Herpes simplex virus immunity. J Leukoc Biol 2005; 1: 178–86. [DOI] [PubMed] [Google Scholar]
- Bracci L, Canini I, Puzelli S, Sestili P, Venditti M, Spada M, et al. Type I IFN is a powerful mucosal adjuvant for a selective intranasal vaccination against influenza virus in mice and affects antigen capture at mucosal level. Vaccine 2005; 23: 2994–3004. [DOI] [PubMed] [Google Scholar]
- Proietti E, Bracci L, Puzelli S, Di Pucchio T, Sestili P, De Vincenzi E, et al. Type I IFN as a natural adjuvant for a protective immune response: lessons from the influenza vaccine model. J Immunol 2002; 1: 375–83. [DOI] [PubMed] [Google Scholar]
- Staats HF, Ennis FA Jr. IL-1 is an effective adjuvant for mucosal and systemic immune responses when coadministered with protein immunogens. J Immunol 1999; 10: 6141–7. [PubMed] [Google Scholar]
- Wang G, Pan L, Zhang Y, Wang Y, Zhang Z, Lu J, et al. Intranasal delivery of cationic PLGA nano/microparticles-loaded FMDV DNA vaccine encoding IL-6 elicited protective immunity against FMDV challenge. PLoS One 2011; 11: e27605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsburg E, Publicover J, Buonocore L, Poholek A, Robek M, Palin A, et al. A vesicular stomatitis virus recombinant expressing granulocyte-macrophage colony-stimulating factor induces enhanced T-cell responses and is highly attenuated for replication in animals. J Virol 2005; 24: 15043–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol 1993; 1–78. [DOI] [PubMed]
- Hirano T, Taga T, Nakano N, Yasukawa K, Kashiwamura S, Shimizu K, et al. Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFp-2). Proc Natl Acad Sci U S A 1985; 16: 5490–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttenhove C, Coulie PG, Van Snick J. T cell growth and differentiation induced by interleukin-HP1/IL-6, the murine hybridoma/plasmacytoma growth factor. J Exp Med 1988; 4: 1417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudo E, Arese M, Toniatti C, Strasly M, Primo L, Mantovani A, et al. IL-6 is an in vitro and in vivo autocrine growth factor for middle T antigen-transformed endothelial cells. J Immunol 1996; 6: 2618–23. [PubMed] [Google Scholar]
- Takai Y, Wong GG, Clark SC, Burakoff SJ, Herrmann SH. B cell stimulatory factor-2 is involved in the differentiation of cytotoxic T lymphocytes. J Immunol 1988; 2: 508–12. [PubMed] [Google Scholar]
- Jiang W, Jin N, Cui S, Li Z, Zhang L, Wang H, et al. Enhancing immune responses against HIV-1 DNA vaccine by coinoculating IL-6 expression vector. J Virol Methods 2006; 1–2: 1–7. [DOI] [PubMed] [Google Scholar]
- Ramsay AJ, Leong KH, Boyle D, Ruby J, Ramshaw IA. Enhancement of mucosal IgA responses by interleukins 5 and 6 encoded in recombinant vaccine vectors. Reprod Fertil Dev 1994; 3: 389–92. [DOI] [PubMed] [Google Scholar]
- Ramsay AJ, Husband AJ, Ramshaw IA, Bao S, Matthaei KI, Koehler G, et al. The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science 1994; 5158: 561–3. [DOI] [PubMed] [Google Scholar]
- Keyes PH. Dental caries in the molar teeth of rats. II. A method for diagnosing and scoring several types of lesions simultaneously. J Dent Res 1958; 6: 1088–99. [DOI] [PubMed] [Google Scholar]
- Barouch DH, Santra S, Steenbeke TD, Zheng XX, Perry HC, Davies ME, et al. Augmentation and suppression of immune responses to an HIV-1 DNA vaccine by plasmid cytokine/Ig administration. J Immunol 1998; 4: 1875–82. [PubMed] [Google Scholar]
- Li W, Li S, Hu Y, Tang B, Cui L, He W. Efficient augmentation of a long-lasting immune responses in HIV-1 gag DNA vaccination by IL-15 plasmid boosting. Vaccine 2008; 26: 3282–90. [DOI] [PubMed] [Google Scholar]
- Calarota SA, Dai A, Trocio JN, Weiner DB, Lori F, Lisziewicz J. IL-15 as memory T-cell adjuvant for topical HIV-1 DermaVir vaccine. Vaccine 2008; 40: 5188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Flamme AC, Pearce EJ. The absence of IL-6 does not affect Th2 cell development in vivo, but does lead to impaired proliferation, IL-2 receptor expression, and B cell responses. J Immunol 1999; 10: 5829–37. [PubMed] [Google Scholar]
- Saade F, Petrovsky N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev Vaccines 2012; 2: 189–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. The Th1/Th2 paradigm. Immunol Today 1997; 6: 263–6. [DOI] [PubMed] [Google Scholar]
- Navia JM. Animal models in dental research. Tuscaloosa: University of Alabama Press; 1977. p280.
- Wang S, Heilman D, Liu F, Giehl T, Joshi S, Huang X, et al. A DNA vaccine producing LcrV antigen in oligomers is effective in protecting mice from lethal mucosal challenge of plague. Vaccine 2004; 22: 3348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweon MN, Fujihashi K, Vancott JL, Higuchi K, Yamamoto M, McGhee JR, et al. Lack of orally induced systemic unresponsiveness in IFN-γ knockout mice. J Immunol 1998; 160: 1687–93. [PubMed] [Google Scholar]
- Liu GX, Xu QA, Jin J, Li YH, Jia R, Guo JH, et al. Mucosal and systemic immunization with targeted fusion anti-caries DNA plasmid in young rats. Vaccine 2009; 27: 2940–7. [DOI] [PubMed] [Google Scholar]
- Li YH, Huang S, Du M, Bian Z, Chen Z, Fan MW. Immunogenic characterization and protection against Streptococcus mutans infection induced by intranasal DNA prime-protein boost immunization. Vaccine 2010; 28: 5370–6. [DOI] [PubMed] [Google Scholar]
- Jodar L, Duclos P, Milstien JB, Griffiths E, Aguado MT, Clements CJ. Ensuring vaccine safety in immunization programmes — a WHO perspective. Vaccine 2001; 13–14: 1594–605. [DOI] [PubMed] [Google Scholar]
- Kermode M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot Int 2004; 1: 95–103. [DOI] [PubMed] [Google Scholar]
- Nir Y, Paz A, Sabo E, Potasman I. Fear of injections in young adults: prevalence and associations. Am J Trop Med Hyg 2003; 3: 341–4. [PubMed] [Google Scholar]