Abstract
Aim:
To investigate the anti-arthritis and immunomodulatory activities of ginsenoside compound K (C-K) in mice with collagen-induced arthritis (CIA).
Methods:
DBA/1 mice with CIA were treated with C-K (28, 56 or 112 mg·kg−1 ·d−1, ig) or the positive control methotrexate (2 mg/kg, ig, every 3 d) for 34 d. Splenic T and B lymphocytes were positively isolated using anti-CD3-coated magnetic beads or a pan B cell isolation kit. T lymphocyte subsets, and CD28, T cell receptor (TCR), cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) and programmed death-1 (PD-1) expression in purified splenic T lymphocytes were analyzed using flow cytometry, Western blotting and laser confocal microscopy.
Results:
C-K treatment significantly ameliorated the pathologic manifestations of CIA mice, remarkably inhibited T lymphocyte proliferation, and marginally inhibited the proliferation of B lymphocytes. C-K treatment significantly suppressed TNF-α and anti-CII antibody levels, and increased IFN-γ level in the joints of CIA mice, but did not alter IL-4 production. Treatment of CIA mice with C-K significantly decreased the percentages of activated T cells, co-stimulatory molecule-expressing T cells and effector memory T cells, and increased the frequencies of naive T cells and regulatory T cells. Furthermore, C-K treatment significantly decreased the expression of CD28 and TCR, whereas it increased the expression of CTLA-4 and PD-1 on T lymphocytes of CIA mice. Methotrexate treatment exerted comparable effects in all these experiments.
Conclusion:
C-K suppresses the progression of CIA through regulating TCR, CD28, CTLA-4 and PD-1 expression, thus inhibiting the abnormal activation and differentiation of T lymphocytes.
Keywords: ginseng, ginsenoside compound K, immunoregulator, arthritis, T lymphocyte, cytokine, CD28, cytotoxic T lymphocyte-associated antigen-4, programmed death-1
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease that causes irreversible destruction of cartilage, tendons, and bones and shortens the life expectancy of patients by affecting major organ systems. Despite recent advances in biologic therapies, more than 30% of RA patients fail to response to all therapies, and the majority cannot achieve remission1,2. RA is considered to be preferentially mediated by T cells and macrophages3,4. Much of our understanding of the pathogenic mechanisms involved in RA was derived from animal studies, and activation of T cells has been recognized as a crucial event in the pathogenesis of RA5,6.
The T cell response is shaped by the balance between co-stimulatory and co-inhibitory signals, and an expanding array of co-signaling molecules are now recognized as playing crucial roles in regulating T cell activation and tolerance7. The TCR, the co-stimulatory molecule CD28 and two inhibitory molecules, CTLA-4 and PD-1, are functionally important in the regulation of T cell activation and tolerance8,9. Simultaneous recognition of the cognate MHC-peptide complex by the TCR (signal 1) and CD80 or CD86 by CD28 (signal 2) results in T cell activation, proliferation, and differentiation10,11. In addition to cytokine production, PD-1 and CTLA-4 are inducibly expressed on T cells following a TCR signal, and subsequent binding of the TCR to one of these co-inhibitors results in cell cycle arrest and termination of T cell activation12,13,14.
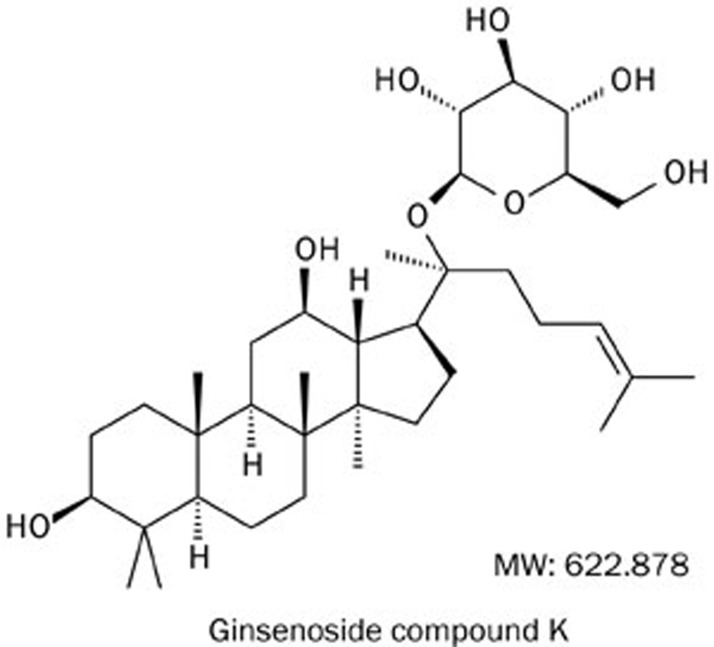
Ginseng (the root of Panax ginseng CA. Meyer, Family Araliaceae) is frequently used as a crude substance in Asian countries in food products and as a medicinal ingredient15,16. Ginsenosides, the major components of ginseng, exhibit various biological activities, including anti-inflammatory, anti-dementia, and anti-tumor effects17,18,19. The protopanaxadiol ginsenosides Rb1, Rb2, and Rc are metabolized to compound K (C-K; Figure 1) by intestinal bacteria in humans and rats20,21,22. C-K (20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol) exhibits various immunopharmacological properties, including anti-allergic and anti-carcinogenic effects23,24,25. However, the in vivo anti-arthritic effects of C-K have not been reported. Collagen-induced arthritis (CIA) is an established experimental model of polyarthritis with many histopathological features similar to human RA26,27. In the present study, we described a novel therapeutic anti-arthritic effect of C-K via the modulation of T lymphocyte activation.
Figure 1.
Chemical structure of ginsenoside compound K (C-K, C36H61O8, MW: 622.878).
Materials and methods
Experimental animals
Specific pathogen-free DBA/1 mice (male, 18±2 g) were obtained from Shanghai SLAC Laboratory Animal Co Ltd (production license No: SCXK [HU] 2012-0002). All mice were maintained in the SPF Animal Laboratory of Anhui Medical University. All experiments were approved by the Ethics Review Committee for Animal Experimentation of the Institute of Clinical Pharmacology, Anhui Medical University.
Reagents and drugs
Chicken type II collagen (CII), concanavalin A (ConA), and lipopolysaccharide (LPS) were purchased from Sigma Chemical Co (Milwaukee, WI, USA). Complete Freund's adjuvant (CFA) was obtained from Chondrex Inc (Redmond, WA, USA). [3H]-TdR was obtained from the Shanghai Institute of Applied Physics, Chinese Academy of Sciences. Mouse CD4-FITC, CD25-PE, CD154-PE, CD69-PE, CD62L-PE, CD3-APC, CD44-APC, TCR-APC, CD28-APC, CTLA-4-APC, PD-1-APC, and antibody for detecting mouse PD-1 were purchased from BioLegend Co, Ltd (San Diego, CA, USA), and the Foxp3-PE-Cy5 antibody was purchased from eBioscience Co, Ltd (San Diego, CA, USA). TNF-α, IFN-γ, anti-CII antibody and the IL-4 enzyme linked immunosorbent assay (ELISA) kit were purchased from R&D Systems (Minneapolis, MN, USA). A mouse pan T cell isolation kit was purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). Specific antibodies against the mouse TCR, CD28, and CTLA-4 were obtained from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA). C-K (molecular weight: 622.18) was a kind of gift from Dr Chang-liang DAI of Zhejiang Hisun Pharmaceutical Co, Ltd Methotrexate (MTX) was obtained from Shanghai Xinyi Pharmaceutical Co, Ltd (Shanghai, China). Before use, C-K and MTX were suspended in 0.5% sodium carboxymethylcellulose (CMC-Na).
Induction and treatment of collagen-induced arthritis in mice
CII was dissolved in 0.1 mol/L acetic acid and emulsified with an equal volume of CFA at 3 mg/mL in an ice bath under sterile conditions and then incubated overnight at 4 °C. DBA/1 mice were injected intradermally twice with 0.1 mL of this emulsion (containing 100 mg of CII/mouse) in the back and the base of the tail. The day of the first immunization was defined as d 0, and the booster injection was administered into the back on d 21. After the onset of arthritis, animals were randomly divided into five groups, and each experimental group consisted of ten mice. Mice with CIA were intragastrically administered C-K (28, 56, or 112 mg/kg) once per day or MTX (2 mg/kg) once every 3 d from d 28 to d 51 after immunization. Normal and CIA mice were administered an equal volume of vehicle (CMC-Na) at the same time.
Arthritis assessment
Change in body weight is a sensitive general disease marker that accurately reflects episodes of disease worsening and improvement; body weight was evaluated every 6 d from d 0 to d 51. To quantitatively evaluate the severity of the arthritis, the arthritis index was evaluated every 4 d from d 24 to d 51. Inflammation of the four paws was graded from 0 to 4: 0, paws with no swelling and focal redness; 1, paws with swelling of the finger joints; grade 2, paws with mild swelling of the ankle or wrist joints; 3, paws with severe inflammation of the entire paw; 4, paws with deformity or ankylosis. Each paw was graded, and the four scores were summed so that the maximum possible score per mouse was 1628. The arthritis grade was scored by two independent observers.
Histopathological examination of the spleen and joints
Paraffin sections were stained with hematoxylin and eosin (H&E), and changes in the spleen and joints were evaluated histopathologically under blinded conditions. The severity of arthritis in the joints was graded from 0 to 4 according to the intensity of the lining layer hyperplasia, mononuclear cell infiltration, and pannus formation. Grade 0, normal ankle joint; grade 1, normal synovium with occasional mononuclear cells; grade 2, definite arthritis with a few layers of flat to rounded synovial lining cells containing scattered mononuclear cells; grade 3, clear hyperplasia of the synovium with three or more layers of loosely arranged lining cells and dense infiltration of mononuclear cells; grade 4, severe synovitis with erosions of the articular cartilage and pannus and subchondral bones29.
Five compartments were evaluated in the spleen: cellularity of the periarteriolar lymphoid sheath (PALS), lymphoid follicles, marginal zone, and red pulp and the total number of germinal centers (GCs) in each section. Histopathological changes in the spleen were graded from 0 to 3: 0, normal spleen; 1, mild proliferation of white pulp; 2, moderate proliferation of white pulp; 3, marked proliferation of white pulp and prominent germinal centers30.
Purification of T cells and B cells from mouse spleens
The spleens of each group were removed and dissociated. T cells were positively isolated with anti-CD3-coated magnetic beads, and B cells were negatively purified using a pan B cell isolation kit according to the manufacturer's instructions. Mononuclear spleen cell suspensions were incubated with beads in PBS and were then washed and purified using an automated magnetic cell sorter. More than 98% of CD3+ T cells and pan B cells were harvested, as determined by fluorescence-activated cell sorting (FACS)31.
T and B lymphocyte proliferation
Splenic T and B cells were suspended in DMEM at a concentration of 1×107 cells/mL. In 96-well culture plates, T cells were incubated with ConA (the final concentration was 3 mg/L) or CII (the final concentration was 100 mg/L), and B cells were treated with LPS (the final concentration was 4 mg/L). Triplicates were designed. The cultures were incubated for 48 h. Six hours before the end of the incubation, 20 μL of [3H]-TdR was added to each well. The radioactivity of [3H]-TdR was measured using a LS6500 liquid scintillation counter (Beckman Coulter, Fullerton, CA). The results are described as the average of triplicate counts per minute32.
Cytokine measurement
To determine cytokine levels in the joints, the knees were excised from each mouse by removing the skin and were placed immediately into 1 mL of cold PBS/200 mg of tissue. Joint extractions were performed using a tissue homogenizer. Homogenates were centrifuged at 2000×g for 20 min at 4 °C, and the supernatant concentrations of TNF-α, IFN-γ, anti-CII antibody and IL-4 were measured using ELISA according to the manufacturer's instructions. The concentration was calculated according to the absorbance (A) at 450 nm.
Percentages of T lymphocyte subsets
Spleens were removed and mechanically dissociated, and lymphocytes were separated using lymphocyte separation medium, as per the manufacturer's instructions. The splenic lymphocytes (100 μL) were transferred into a 12 mm×75 mm FACScan™ flow cytometer tube, and equal volumes of antibodies in combination, ie, CD3-APC/CD4-FITC, CD69-PE/CD4-FITC, CD154-PE/CD4-FITC, CD44-APC/CD62L-PE/CD4-FITC, or Foxp3-PE-Cy5/CD25-PE/CD4-FITC, were added to each tube. The sample was mixed gently, incubated for 30 min at 4 °C, and then analyzed using a flow cytometer (FC500, Beckman Coulter, Fullerton, CA, USA). Data analysis was performed using CellQuest™ analysis software33, and the percentages of T lymphocyte subsets were analyzed by gating on lymphocytes.
Western blot analysis
Mice were euthanized on d 51 after immunization, and spleens were homogenized with a tissue homogenizer in 5 volumes of homogenization buffer (25 mmol/L Tris-HCl, 2 mmol/L EGTA, 1 mmol/L benzamidine, 1 mmol/L PMSF, pH 7.4). Following centrifugation (3000×g for 20 min at 4 °C), the supernatant was collected. Samples were mixed with an equal volume of sample buffer, boiled, resolved by 12.5% sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA). Immunoblotting was performed with the indicated primary antibody followed by the appropriate horseradish peroxidase (HRP)-conjugated rabbit anti-goat or goat anti-rabbit IgG and immunodetection with enhanced chemiluminescence (ECL, Pierce, Rockford, IL, USA). Autoradiographs were scanned using a GS-700 Imaging Densitometer (Bio-Rad, Hercules, CA, USA). The relative levels of protein were determined by taking the ratio of the band intensity of the target protein against that of β-actin. All experiments reported in this study were performed three times, and the results were reproducible.
Laser confocal microscopy
Splenic T lymphocytes were fixed with 4% (w/v) paraformaldehyde. Cells were then treated with 0.1% Triton X-100 for 20 min and blocked in 1% BSA-PBS for 30 min prior to incubation with mouse anti-TCR, anti-CD28, anti-CTLA-4, or anti-PD-1 antibody overnight at 4 °C. The next day, cells were rinsed with PBS and cultured with the appropriate fluorescein-conjugated secondary antibody at 37 °C for 2 h. DAPI was used to stain the cell nuclei. Finally, the cells were washed briefly in PBS before being mounted on a slide. Digital images were processed by a Leica TCS SP5 laser-scanning confocal microscope (Leica Microsystems, Wetzlar, Germany).
Flow cytometry analysis
Splenic T lymphocytes were transferred into a 12 mm×75 mm FACS can flow cytometer tube, and equal volumes of antibodies in combination, including TCR-APC, CD28-APC, CTLA-4-APC, and PD-1-APC, were added to each tube. The sample was mixed gently, incubated for 30 min at 4 °C, and then analyzed using a flow cytometer. Data analysis was performed using CellQuest™ analysis software.
Statistical analysis
All data are expressed as the mean±standard deviation (SD). Differences between groups were evaluated by Student's t-test. For multiple group comparisons, we used ANOVA with Tukey's post-test. P≤0.05 was considered significant.
Results
Effects of C-K on the body weight and arthritis scores of CIA mice
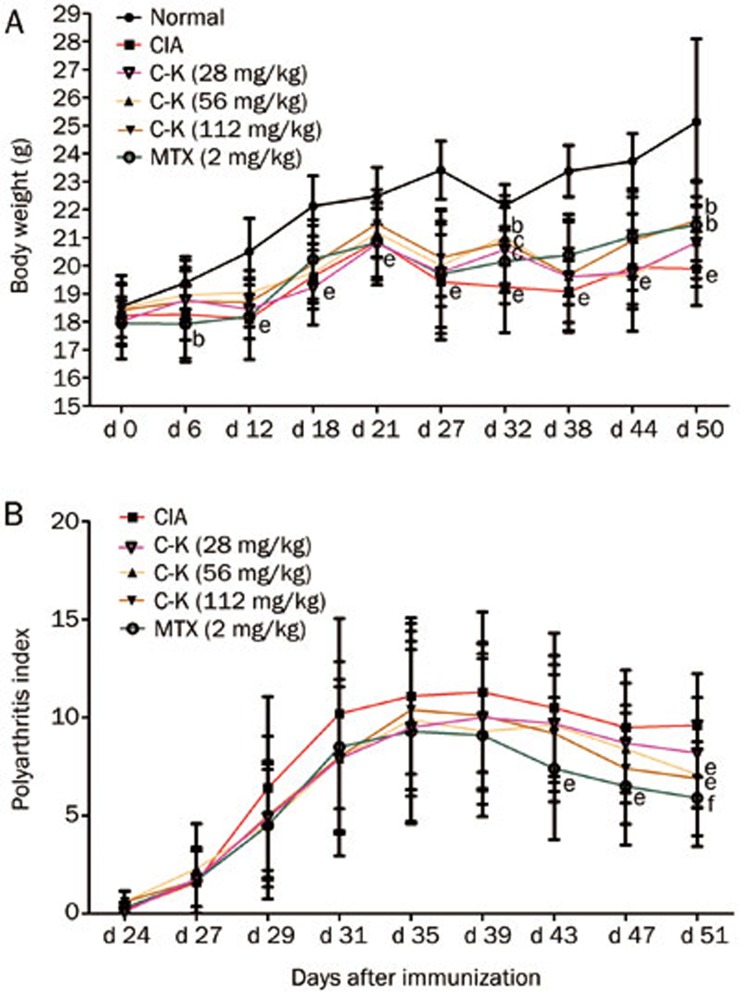
The weight of the CIA mice increased slowly and was significantly less than that of the normal DBA/1 mice beginning on d 3 after injection of the emulsion. The C-K (28, 56, and 112 mg/kg) mice recovered their weight by d 32 after the emulsion injection. C-K- (56 and 112 mg/kg) and MTX-treated (2 mg/kg) mice showed significantly increased body weight on d 50 as compared with CIA mice (Figure 2A).
Figure 2.
Effects of C-K on the body weight and arthritis scores of CIA mice. DBA/1 mice were immunized with CII and FCA on d 0 and d 21. The mice were then treated with C-K (28, 56, or 112 mg/kg, ig, qd, for 34 d) or methotrexate (MTX, 2 mg/kg, ig, every 3 d, for 34 d). Data are expressed as the mean±SD. n=10. bP<0.05, cP<0.01 vs normal mice. eP<0.05, fP<0.01 vs CIA mice.
Hind paw-swelling began on d 24 post-immunization. CIA mice were treated from d 28 to d 50. Arthritis scores were measured every 4 d beginning on d 24. The results showed that the swelling peaks appeared between d 35 and d 39. C-K (56 and 112 mg/kg) significantly reduced the arthritis scores of the mice on d 51. On d 43, MTX (2 mg/kg) had reduced the arthritis scores (Figure 2B).
Effects of C-K on the histopathology of the joints and spleens in CIA mice
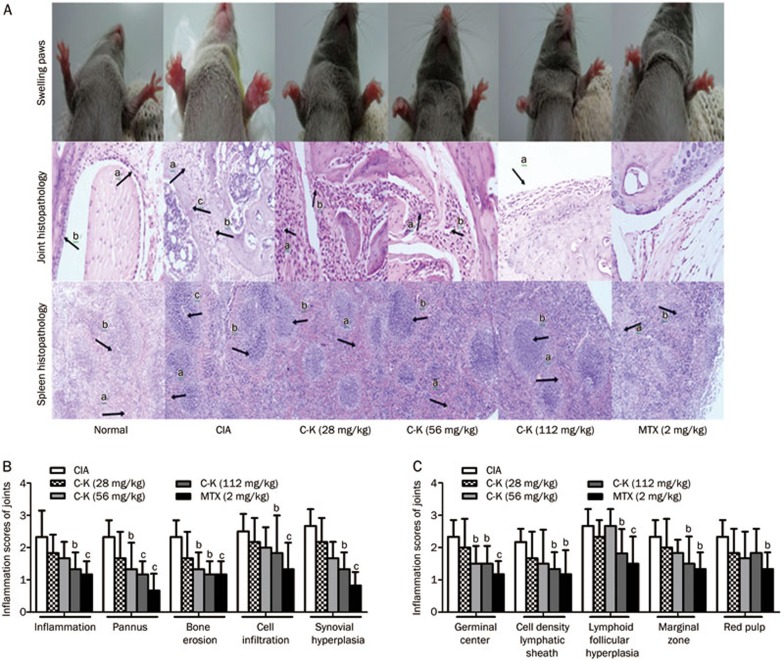
In CIA mice, synoviocytes proliferated to multiple layers, and the articular cartilage was destroyed and demonstrated inflammatory infiltration. The hyperplastic synovium in CIA mice included a large number of fibroblasts and new blood vessels. In mice treated with C-K (56 mg/kg), articular cartilage destruction and pannus formation were slightly inhibited. C-K (112 mg/kg) significantly reduced articular cartilage destruction, pannus formation, and synovial hyperplasia, although inflammatory cell infiltration was observed to a small extent (Figure 3A). The inflammatory scores of the joints in the model mice were increased in comparison with those of normal mice. C-K (56 and 112 mg/kg) and MTX (2 mg/kg) substantially rescued the increase in inflammatory scores observed in CIA mice (Figure 3B).
Figure 3.
Effects of C-K on the histopathology of the spleen and joints of CIA mice. A photomicrograph of joint histopathology showing synoviocyte hyperplasia (arrow a), blood vessels (arrow b), articular cartilage destruction and pannus (arrow c). A photomicrograph of spleen histopathology showing red pulp congestion (arrow a), white pulp proliferation (arrow b) and germinal center formation (arrow c) (A). Effects of C-K on joint histopathology (B). Effects of C-K on spleen histopathology (C). Mean±SD. n=10. bP<0.05, cP<0.01 vs CIA mice.
The normal spleen architecture included two major functional zones, the hematogenous red pulp and the lymphoid white pulp. The white pulp was composed of lymphoid follicles and periarteriolar lymphoid sheaths. In the model mice, the white pulp showed proliferation, GC development, and an increase in pathology scores. C-K (56, 112 mg/kg) and MTX (2 mg/kg) significantly reduced the increased pathology scores when compared with those observed in CIA mice (Figure 3C).
Effects of C-K on T and B lymphocyte proliferation in CIA mice
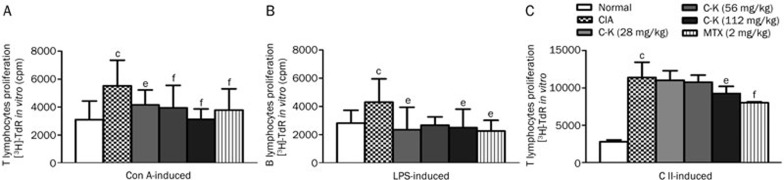
The results showed that the T and B lymphocyte proliferation induced by the different stimulators in CIA mice was increased compared with that in normal mice. C-K (28, 56, and 112 mg/kg) and MTX (2 mg/kg) clearly reduced ConA-induced T lymphocyte proliferation (Figure 4A). C-K (112 mg/kg) and MTX (2 mg/kg) mildly reduced LPS-induced B lymphocyte proliferation in CIA mice (Figure 4B). T lymphocyte proliferation induced by CII in CIA mice was increased compared with that in normal mice. Treatment with C-K (112 mg/kg) or MTX (2 mg/kg) reduced CII-induced T lymphocyte proliferation (Figure 4C). These data suggested that C-K primarily affects the function of T cells, but the characteristics and mechanism of C-K's effect need to be elucidated.
Figure 4.
Effects of C-K on T and B lymphocyte proliferations in CIA mice. The data were described as the average of triplicate counts per minute (cpm) or quadruplicate optical densities. ConA induced T lymphocyte proliferation (A). LPS induced B lymphocyte proliferation (B). CII induced T lymphocyte proliferation in vitro (C). Mean±SD. n=10. bP<0.05 vs normal mice. eP<0.05, fP<0.01 vs CIA mice.
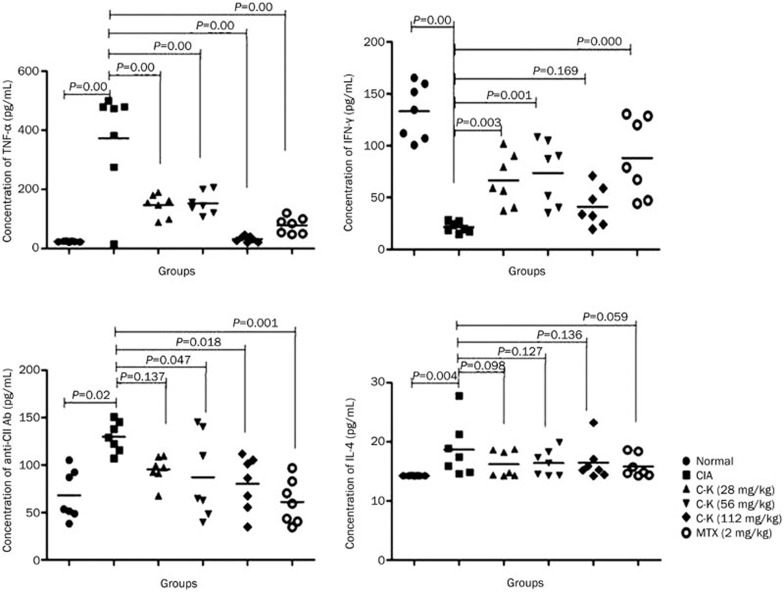
Effects of C-K on cytokine levels in the joints of CIA mice
The levels of TNF-α, IFN-γ, anti-CII antibody, and IL-4 in the joints were determined by ELISA. The results showed that the concentrations of TNF-α and IL-4 in the joints of CIA mice were increased, whereas the production of IFN-γ in the joints was decreased. C-K (28, 56, and 112 mg/kg) and MTX (2 mg/kg) significantly reduced the expression of TNF-α (Figure 5A). The concentration of IFN-γ in C-K-treated (28 and 56 mg/kg) mice was recovered (Figure 5B). Treatment with C-K (56 and 112 mg/kg) and MTX (2 mg/kg) significantly reduced the concentration of anti-CII antibody (Figure 5C). However, neither C-K nor MTX had a clear effect on the level of IL-4 in CIA mice (Figure 5D).
Figure 5.
The effect of C-K on cytokine levels in the joints of CIA mice. The levels of TNF-α, IFN-γ, anti-CII antibody and IL-4 were measured using ELISA according to the manufacturer's instructions. The concentration was calculated according to the absorbance (A) at 450 nm.
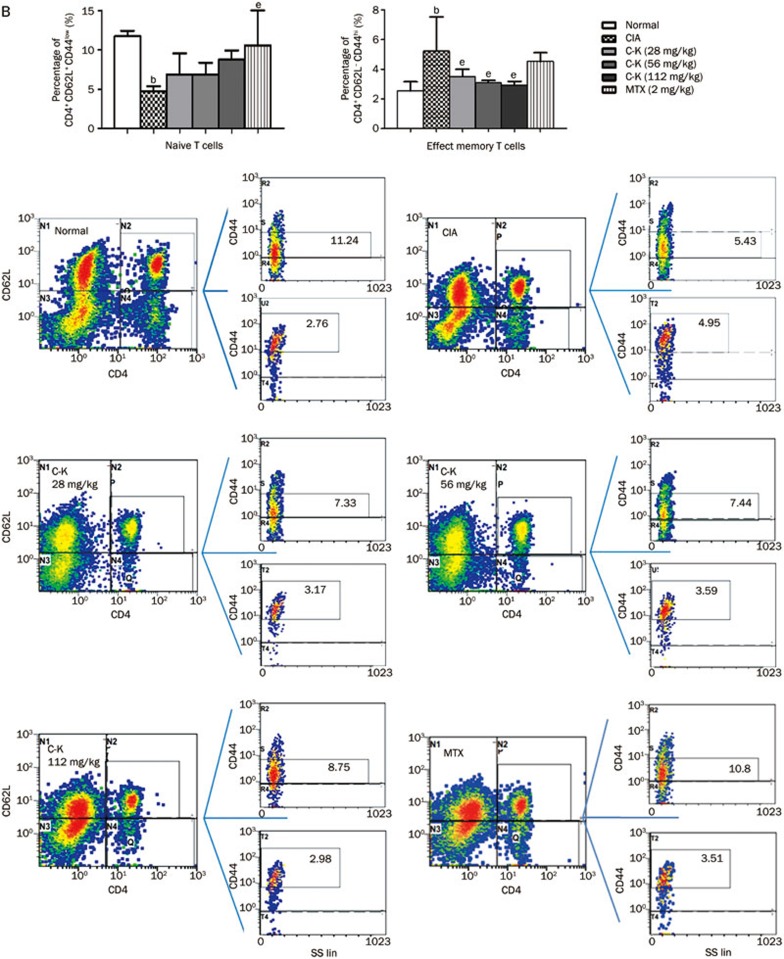
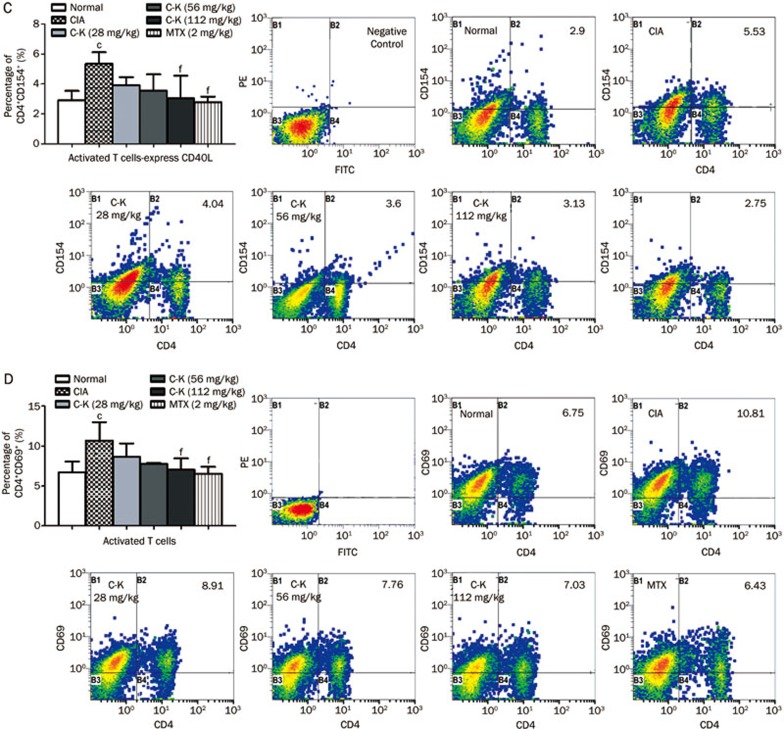
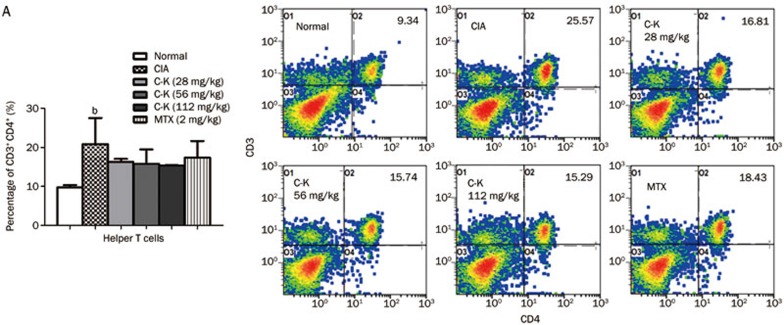
Effects of C-K on the subsets of T lymphocytes in CIA mice
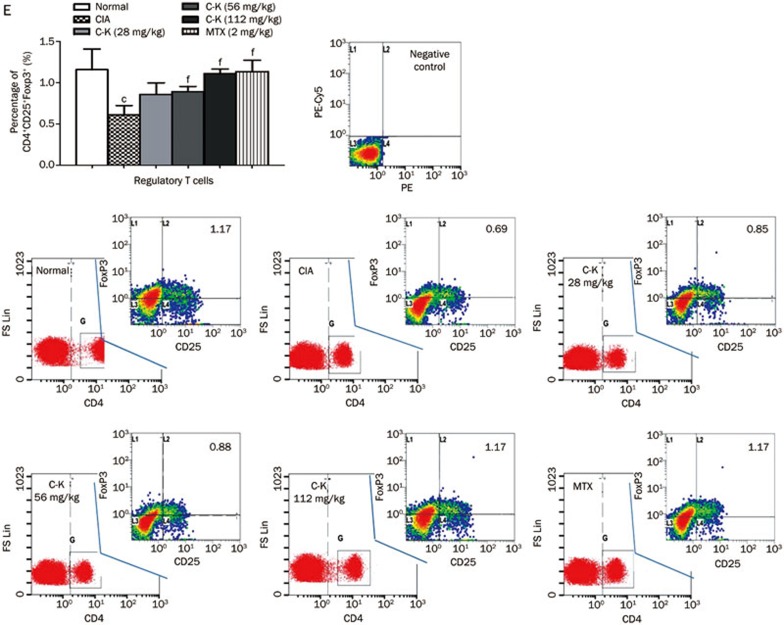
The subgroups of T lymphocytes were examined by flow cytometry. The results showed that the percentages of CD3+CD4+ cells (analogous to helper T cells, Th cells), CD4+CD154+ cells (Th cells expressing co-stimulatory molecules CD154), CD4+CD69+ cells (analogous to activated T cells) and CD4+CD62L−CD44hi cells (analogous to effector memory T cells, TEM) in the spleens of CIA mice were significantly higher compared with those in normal mice, whereas the percentages of CD4+CD62L+CD44low cells (analogous to naive cells) and CD4+CD25+ Foxp3+ cells (analogous to regulatory T cells, Tregs) were significantly lower. C-K treatment decreased the proportions of CD4+CD154+, CD4+CD69+, and CD4+CD62L−CD44hi cells (Figures 6B, 6C, and 6D) and increased the percentages of CD4+CD62L+CD44low and CD4+CD25+ Foxp3+ cells (Figures 6B and 6F). The proportion of CD3+CD4+Th cells was not affected by C-K or MTX administration (Figure 6A). The data we obtained indicated that C-K substantially modulated the activation and differentiation of T lymphocytes in CIA mice, which might be a novel mechanism underlying the effects of C-K in the treatment of RA.
Figure 6B.
Effects of C-K on the differentiation of CD4+CD62L+CD44low and CD4+CD62L-CD44hi T lymphocytes in CIA mice. The population size of different T lymphocytes subsets was determined by flow cytometry. The percentages of the T lymphocyte subsets were analyzed by gating on lymphocytes. The number in each plots represents the expression of the subset of T cells among lymphocytes. Mean±SD. n=10. bP<0.05 vs normal mice. eP<0.05 vs CIA mice.
Figure 6C–6D.
Effects of C-K on the differentiation of CD4+CD154+ and CD4+CD69+ T lymphocytes in CIA mice. The population size of different T lymphocyte subsets was determined by flow cytometry. The percentages of the T lymphocyte subsets were analyzed by gating on lymphocytes. The number in each plots represents the expression of that subset of T cells among lymphocytes. Mean±SD. n=10. cP<0.01 vs normal mice. fP<0.01 vs CIA mice.
Figure 6A.
Effects of C-K on the differentiation of CD3+CD4+ T lymphocytes in CIA mice. The population size of different T lymphocyte subsets was determined by flow cytometry. The percentages of the T lymphocyte subsets were analyzed by gating on lymphocytes. The number in each plots represents the expression of that subset of T cells among lymphocytes. Mean±SD. n=10. bP<0.05 vs normal mice.
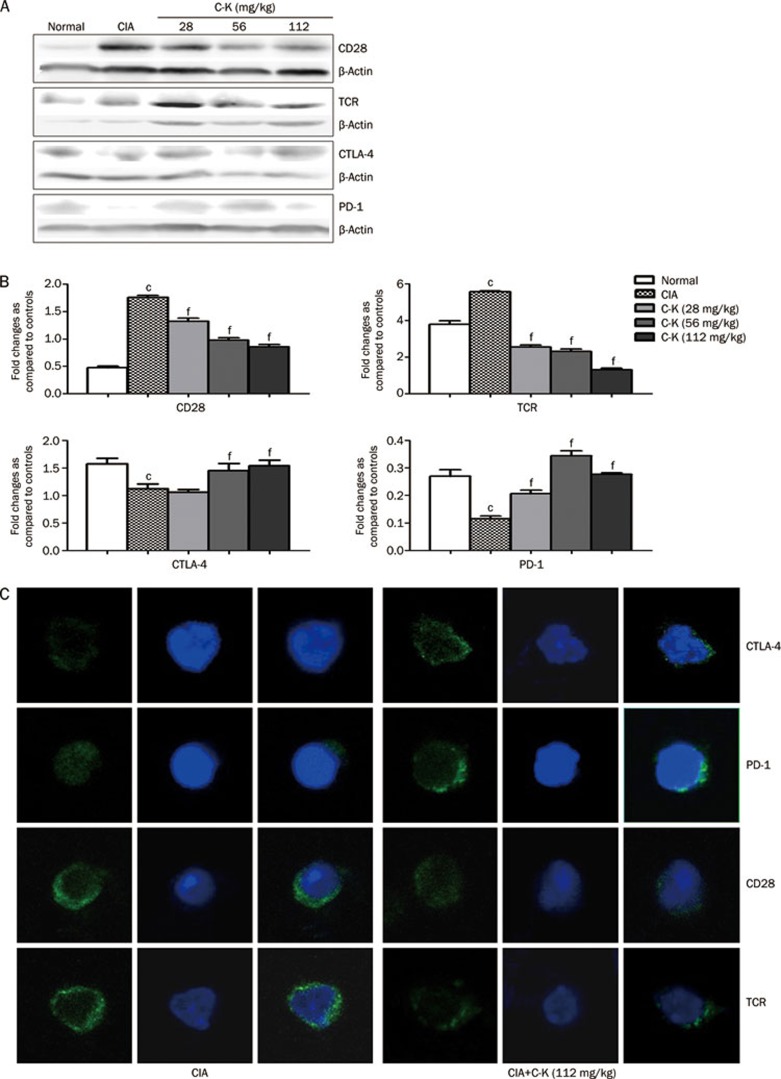
Effects of C-K on the expression of CD28, TCR, CTLA-4, and PD-1 in the T lymphocytes of CIA mice
The activation and differentiation of T lymphocytes are controlled by the expression of the TCR, CD28, CTLA-4, and PD-1. In this study, the expression of CD28 and the TCR in the T lymphocytes of CIA mice increased, whereas the levels of CTLA-4 and PD-1 in T lymphocytes was significantly decreased (Figure 7A). Compared with the CIA mice, C-K greatly decreased the expression of CD28 and the TCR, whereas it significantly increased the expression of CTLA-4 and PD-1 (Figure 7B). To verify this result, we used a laser confocal microscope to determine the expression of these important signaling molecules, which are involved in the activation of T lymphocytes. In accordance with the Western blot analysis, both the CD28 and TCR expression levels were increased on T cells isolated from CIA mice, whereas CTLA-4 and PD-1 expression decreased. Compared with T lymphocytes from CIA mice, those from C-K-treated mice (112 mg/kg) showed decreased expression of CD28 and the TCR, whereas CTLA-4 and PD-1 expression was significantly increased (Figure 7C).
Figure 7A–7C.
Effects of C-K on the expression of CD28, TCR, CTLA-4, and PD-1 on the T lymphocytes of CIA mice. T lymphocytes were isolated using magnetic beads. The result was representative of those from three experiments (A and B). Laser confocal microscopy demonstrated changes in CD28, TCR, CTLA-4, and PD-1 expression similar to Western blotting (C). Mean±SD. n=10. cP<0.01 vs normal mice. fP<0.01 vs CIA mice.
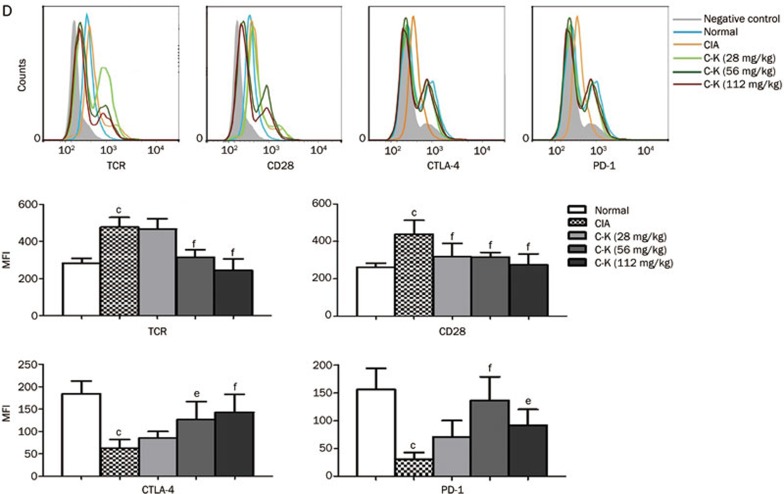
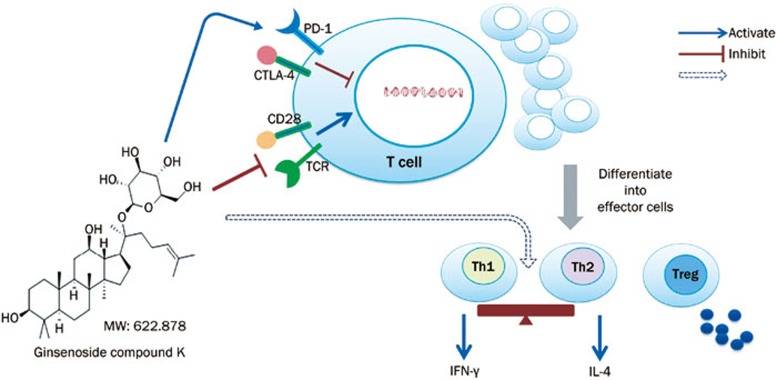
We also used flow cytometry to determine the expression levels of CD28, the TCR, CTLA-4, and PD-1. In agreement with the changes observed via Western blotting, the expression of both CD28 and the TCR was increased on T cells isolated from CIA mice, whereas the expression of CTLA-4 and PD-1 was decreased. Compared with T lymphocytes from CIA mice, those from mice treated with C-K (56 and 112 mg/kg) showed decreased expression of CD28 and TCR and significantly increased expression of CTLA-4 and PD-1 (Figure 7D). These results showed that C-K modulated the activation and differentiation of T lymphocytes, primarily through the simultaneous inhibition of stimulatory signals and upregulation of inhibitory signals (Figure 8).
Figure 7D.
The expression of CD28, TCR, CTLA-4, and PD-1 (D) detected by flow cytometry. Mean±SD. n=10. cP<0.01 vs normal mice. eP<0.05, fP<0.01 vs CIA mice.
Figure 8.
C-K inhibits the abnormal activation of T lymphocytes in CIA mice.
Discussion
RA is a chronic, autoimmune disease that primarily affects the joints, and without proper treatment, RA results in their progressive destruction. However, the exact pathogenesis is still unclear. This disease is associated with various immunological abnormalities, such as increased numbers of activated T lymphocytes and aberrant expression of inflammatory cytokines34. CIA resembles RA in pathological, histological, and immunological profiles; it is therefore accepted as a disease model for RA and is among the most widely used models for studies of RA pathogenesis and for screening new drugs35.
In the present study, we found a marked secondary inflammatory response in the CIA model that was accompanied by paw swelling, increased arthritis scores and histopathological changes in the joints and spleen; however, these symptoms were restored to normal by treatment with C-K. Furthermore, the findings of our study showed that C-K could reduce the proliferation of T and B lymphocytes obtained from the spleen of CIA mice. However, the effect of C-K on T lymphocytes was much stronger than that on B lymphocytes. These findings suggest that C-K might exert its immunoregulatory effects by regulating T lymphocyte-mediated immune responses in CIA mice. The immunogenetics of RA suggest a key role for aberrant pathways of T lymphocyte activation in the initiation and/or perpetuation of disease36. The importance of T lymphocytes can be observed following the transfer of CD4+ T lymphocytes from an arthritic animal to a healthy animal, which induces tissue damage in the recipient37. Therefore, C-K may be a promising new drug for the treatment of RA.
The overexpression of TNF-α induces leukocyte and endothelial cell activation, synovial fibroblast activation and survival, pain receptor sensitization, and angiogenesis, which together represent key pathological features of RA38. Therapeutic blockade of TNF-α yields a clinical response in approximately 70% of patients with established RA, and it results in the suppression of leukocyte migration, endothelial cell deactivation and the recovery of regulatory T cell function and phenotype39. IFN-γ is a well-known pro-inflammatory cytokine produced by Th1 cells. Previous studies have shown that GC-rich tissues produce significantly more IFN-γ and that treatment with C-K results in GC destruction and marked inhibition of IFN-γ transcription40,41. IL-4 is a major anti-inflammatory cytokine that can mimic Th2 cell activity, thus inhibiting Th1 cell activity and inflammation in RA and restoring the disturbed Th1/Th2 balance in this disease. Previous work in our laboratory has also revealed that the Th1/Th2 imbalance plays an important role in the pathogenesis of adjuvant arthritis (AA) in rats42. Here, we demonstrated that TNF-α was significantly increased in the joints of CIA mice, whereas it was reduced in the treated mice. These findings suggest that C-K may have therapeutic effects on RA via inhibiting the production of TNF-α, which may be one of the mechanisms underlying the effects of C-K in anti-CIA treatment as well as its anti-inflammatory and immunosuppressive effects. Our study also demonstrated that C-K regulated Th cell-mediated immune responses by modulating the balance of Th1/Th2 cells in the joints of CIA mice. Further studies are required to assess whether C-K affects the development, maturation, and differentiation of Th1 and Th2 cells.
Th cells are a subgroup of lymphocytes that play an important role in establishing and maximizing the capabilities of the immune system. CD4+ T cells play a key role in the initiation and perpetuation of CIA by producing IFN-γ, a potent inducer of the inflammatory response27. The results showed that C-K downregulated the expression of the early T cell activation marker CD69, the costimulatory molecule CD154 and the effector memory T cell marker CD62L−CD44hi on CD4+ cells in the spleens of CIA mice. Moreover, it upregulated the T cell inactivation marker CD62L+CD44low and the Treg marker CD25+ Foxp3+ expressed on CD4+ cells in the spleens of CIA mice, although it had no obvious effect on the percentage of Th cells. These results demonstrated that C-K regulated the T cell response by inhibiting the differentiation and activation of CD4+ T cells instead of reducing the number of CD4+T cells. Tregs control the responses of APCs and effector T cells in the periphery through direct interaction with these cells or through anti-inflammatory cytokine production, and they play an important role in limiting inflammation and regulating adaptive immunity43,44. C-K restored the percentage of Tregs, which may be one of the mechanisms underlying the anti-CIA effects of C-K as well as its anti-inflammatory and immunosuppressive effects.
T lymphocytes have been proposed to play a central role in the disease process45. T cell activation requires the interaction of the TCR, MHC, and antigen in the presence of additional co-stimulatory signals that are provided through co-stimulatory molecules. The TCR is the central signaling receptor regulating T cell biology. TCR signaling in naive T cells drives their activation and expansion. In effector or memory T cells, TCR signaling drives the expansion and triggering of effector functions, such as cytokine production and cytotoxicity. A number of additional molecules also act as positive or negative regulators of TCR signaling8,46,47. Foremost among these is the costimulatory receptor CD2848. In the absence of CD28 co-stimulation, TCR ligation induces sub-optimal signaling, which can result in the induction of an anergic state. In contrast, the inhibitory receptor CTLA-4 (which competes for the same ligands as CD28) antagonizes TCR signaling49. Similarly, an inhibitory signal is conducted through PD-1 when ligated simultaneously with the TCR. In CIA mice, our data showed that C-K could greatly decrease the expression of the co-stimulatory molecule CD28 and the TCR and increase the expression of CTLA-4 and PD-1 in T lymphocytes. These findings suggest that C-K treatment can modulate the T cell response by altering the balance between co-stimulatory and co-inhibitory signals.
In conclusion, this study suggests that the abnormal activity of T lymphocytes plays a crucial role in the pathogenesis of CIA and that C-K has therapeutic effects in CIA mice, as indicated by the decreased arthritis index and the improved joint and spleen histopathology. The therapeutic effects might be related to the ability of C-K to regulate the Th1/Th2 immune balance and to inhibit the activation and differentiation of T cells by modulating co-stimulatory and co-inhibitory signals. This may aid our understanding of how C-K regulates Th cell development, maturation and differentiation and how it induces T lymphocyte tolerance, which will aid the development of new therapies regulating the balance between immune tolerance and the immune response in autoimmune diseases.
Author contribution
Wei WEI designed the study; Kang-kang LIU, Qing-tong WANG, Jing-yu CHEN, Hua-xun WU, and Si-min YANG performed the research; Kang-kang LIU and Qing-tong WANG analyzed the data; Kang-kang LIU and Qing-tong WANG wrote the paper; Wei WEI revised the manuscript.
Figure 6E.
Effects of C-K on the differentiation of CD4+CD25+Foxp3+ T lymphocytes in CIA mice. The population size of different T lymphocyte subsets was determined by flow cytometry. The percentages of the T lymphocyte subsets were analyzed by gating on lymphocytes. The number in each plots represents the expression of that subset of T cells among lymphocytes. Mean±SD. n=10. cP<0.01 vs normal mice. fP<0.01 vs CIA mice.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (30973543, 81173075, 81202541, and 81330081), the Anhui Provincial Natural Science Foundation (1208085QH146), the Grants for Scientific Research of BSKY (XJ201213), the Foundation for Young Academic Backbone of Anhui Medical University, and the Grants for Young Talents of Anhui Medical University (2013).
References
- Smolen JS, Aletaha D. Developments in the clinical understanding of rheumatoid arthritis. Arthritis Res Ther 2009; 11: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet 2007; 370: 1861–74. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol 2007; 7: 429–42. [DOI] [PubMed] [Google Scholar]
- Schulze-Koops H, Kalden JR. The balance of Th1/Th2 cytokines in rheumatoid arthritis. Best Pract Res Clin Rheumatol 2001; 15: 677–91. [DOI] [PubMed] [Google Scholar]
- Leah E. Rheumatoid arthritis: Metabolic reprogramming of T cells in RA. Nat Rev Rheumatol 2013; 9: 635. [DOI] [PubMed] [Google Scholar]
- Boissier MC, Semerano L, Challal S, Saidenberg-Kermanac'h N, Falgarone G. Rheumatoid arthritis: from autoimmunity to synovitis and joint destruction. J Autoimmun 2012; 39: 222–8. [DOI] [PubMed] [Google Scholar]
- Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J Immunol 2009; 182: 5891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 2013; 13: 227–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol 2004; 4: 336–47. [DOI] [PubMed] [Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol 2005; 23: 515–48. [DOI] [PubMed] [Google Scholar]
- Brownlie RJ, Zamoyska R. T cell receptor signalling networks: branched, diversified and bounded. Nat Rev Immunol 2013; 13: 257–69. [DOI] [PubMed] [Google Scholar]
- Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol 2006; 24: 65–97. [DOI] [PubMed] [Google Scholar]
- Snauwaert S, Verstichel G, Bonte S, Goetgeluk G, Vanhee S, Van Caeneghem Y, et al. In vitro generation of mature, naive antigen-specific CD8+ T cells with single T cell receptor by agonist selection. Leukemia 2013 Oct 4. doi: 10.1038/leu.2013.285. [DOI] [PubMed]
- Lan KH, Liu YC, Shih YS, Tsaid CL, Yen SH, Lan KL. A DNA vaccine against cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) prevents tumor growth. Biochem Biophys Res Commun 2013; 440: 222–8. [DOI] [PubMed] [Google Scholar]
- Im DS, Nah SY. Yin and Yang of ginseng pharmacology: ginsenosides vs gintonin. Acta Pharmacol Sin 2013; 34: 136773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J, Koo J, Kim S, Park TY, Kim MY. Ginsenoside Rp1 exerts anti-inflammatory effects via activation of dendritic cells and regulatory T cells. J Ginseng Res 2012; 36: 375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Xue W, Zhao WJ, Li KX. Pharmacokinetics and dopamine/acetylcholine releasing effects of ginsenoside Re in hippocampus and mPFC of freely moving rats. Acta Pharmacol Sin 2013; 34: 214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki M, Yoo YC, Matsuzawa K, Sato K, Saiki I, Tono-oka S, Samukawa K, et al. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3 of red ginseng. Biol Pharm Bull 1995; 18: 1197–202. [DOI] [PubMed] [Google Scholar]
- Sun J, Song X, Hu S. Ginsenoside Rg1 and aluminum hydroxide synergistically promote immune responses to ovalbumin in BALB/c mice. Clin Vaccine Immunol 2008; 15: 303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Kim Y, Han SH, Jeon JY, Hwang M, Im YJ, et al. Development and validation of an LC-MS/MS method for determination of compound K in human plasma and clinical application. J Ginseng Res 2013; 37: 135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan LH, Piao JY, Min JW, Yang DU, Lee HN, Yang DC. Bioconversion of ginsenoside Rb1 into compound K by Leuconostoccitreum LH1 isolated from kimchi. Braz J Microbiol 2011; 42: 1227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Chung KS, Choi JH, Kim DH, Lee KT. Compound K, a metabolite of ginseng saponin, induces apoptosis via caspase-8-dependent pathway in HL-60 human leukemia cells. BMC Cancer 2009; 9: 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin 2008; 29: 1109–18. [DOI] [PubMed] [Google Scholar]
- Choo MK, Sakurai H, Kim DH, Saiki I. A ginseng saponin metabolite suppresses tumor necrosis factor-α-promoted metastasis by suppressing nuclear factor-κ B signaling in murine colon cancer cells. Oncol Rep 2008; 19: 595–600. [PubMed] [Google Scholar]
- Kim DY, Yuan HD, Chung IK, Chung SH. Compound K, intestinal metabolite of ginsenoside, attenuates hepatic lipid accumulation via AMPK activation in human hepatoma cells. J Agric Food Chem 2009; 57: 1532–7. [DOI] [PubMed] [Google Scholar]
- Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med 1977; 146: 857–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand DD, Kang AH, Rosloniec EF. Immunopathogenesis of collagen arthritis. Springer Semin Immunopathol 2003; 25: 3–18. [DOI] [PubMed] [Google Scholar]
- Huang B, Wang QT, Song SS, Wu YJ, Ma YK, Zhang LL, et al. Combined use of etanercept and MTX restores CD4+/CD8+ ratio and Tregs in spleen and thymus in collagen-induced arthritis. Inflamm Res 2012; 61: 1229–39. [DOI] [PubMed] [Google Scholar]
- Tong T, Zhao W, Wu YQ, Chang Y, Wang QT, Zhang LL, et al. Chicken type II collagen induced immune balance of main subtype of helper T cells in mesenteric lymph node lymphocytes in rats with collagen-induced arthritis. Inflamm Res 2010; 59: 369–77. [DOI] [PubMed] [Google Scholar]
- Wang QT, Wu YJ, Huang B, Ma YK, Song SS, Zhang LL, et al. Etanercept attenuates collagen-induced arthritis by modulating the association between BAFFR expression and the production of splenic memory B cells. Pharmacol Res 2013; 68: 38–45. [DOI] [PubMed] [Google Scholar]
- Dibra D, Cutrera JJ, Li S. Coordination between TLR9 signaling in macrophages and CD3 signaling in T cells induces robust expression of IL30. J Immunol 2012; 188: 3709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HX, Chen JY, Wang QT, Sun WY, Liu LH, Zhang LL, et al. Expression and function of β-arrestin 2 stimulated by IL-1β in human fibroblast-like synoviocytes and the effect of paeoniflorin. Int Immunopharmacol 2012; 12: 701–6. [DOI] [PubMed] [Google Scholar]
- Wang QT, Ma YK, Huang B, Liu DD, Wei W. Effect of rhTACI-Ig fusion protein on antigen-specific T cell responses from keyhole limpet haemocyanin challenged mice. Mol Immunol 2011; 49: 380–6. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Benham H, Cope AP, Thomas R. T-cell receptor signaling and the pathogenesis of autoimmune arthritis: insights from mouse and man. Immunol Cell Biol 2012; 90: 277–87. [DOI] [PubMed] [Google Scholar]
- Wang QT, Zhang LL, Wu HX, Wei W. The expression change of β-arrestins in fibroblast-like synoviocytes from rats with collagen-induced arthritis and the effect of total glucosides of paeony. J Ethnopharmacol 2011; 133: 511–6. [DOI] [PubMed] [Google Scholar]
- Cope AP, Schulze-Koops H, Aringer M. The central role of T cells in rheumatoid arthritis. Clin Exp Rheumatol 2007; 25: S4–11. [PubMed] [Google Scholar]
- Skapenko A, Leipe J, Lipsky PE, Schulze-Koops H. The role of the T cell in autoimmune inflammation. Arthritis Res Ther 2005; 2: S4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol 2007; 7: 429–42. [DOI] [PubMed] [Google Scholar]
- Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J Exp Med 2004; 200: 277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Ko E, Lee J, Pho S, Ko S, Shin MK, et al. Ginsenoside Rg1 enhances CD4+ T-cell activities and modulates Th1/Th2 differentiation. Int Immunopharmacol 2004; 4: 235–44. [DOI] [PubMed] [Google Scholar]
- Shin YW, Bae EA, Kim SS, Lee YC, Kim DH. Effect of ginsenoside Rb1 and compound K in chronic oxazolone-induced mouse dermatitis. Int Immunopharmacol 2005; 5: 1183–91. [DOI] [PubMed] [Google Scholar]
- Wu H, Wei W, Song L, Zhang L, Chen Y, Hu X. Paeoniflorin induced immune tolerance of mesenteric lymph node lymphocytes via enhancing beta 2-adrenergic receptor desensitization in rats with adjuvant arthritis. Int Immunopharmacol 2007; 7: 662–73. [DOI] [PubMed] [Google Scholar]
- Li Q, Shen HH. Neonatal bacillus Calmette-Guérin vaccination inhibits de novo allergic inflammatory response in mice via alteration of CD4+CD25+ T-regulatory cells. Acta Pharmacol Sin 2009; 30: 125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetta R, Gambineri E, Roncarolo MG. Role of regulatory T cells and FOXP3 in human diseases. J Allergy Clin Immunol 2007; 120: 227–35. [DOI] [PubMed] [Google Scholar]
- Klimiuk PA, Yang H, Goronzy JJ, Weyand CM. Production of cytokines and metalloproteinases in rheumatoid synovitis is T cell dependent. Clin Immunol 1999; 90: 65–78. [DOI] [PubMed] [Google Scholar]
- Brownlie RJ, Zamoyska R. T cell receptor signalling networks: branched, diversified and bounded. Nat Rev Immunol 2013; 13: 257–69. [DOI] [PubMed] [Google Scholar]
- Jerome KR. Viral modulation of T-cell receptor signaling. J Virol 2008; 82: 4194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JL, June CH. The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood 2005; 105: 13–21. [DOI] [PubMed] [Google Scholar]
- Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol 2006; 24: 65–97. [DOI] [PubMed] [Google Scholar]