Abstract
Little is known about circulation of influenza and other respiratory viruses in remote populations along the Thai-Cambodia border in western Cambodia. We screened 586 outpatients (median age 5, range 1–77) presenting with influenza-like-illness (ILI) at 4 sentinel sites in western Cambodia between May 2010 and December 2012. Real-time reverse transcriptase (rRT) PCR for influenza was performed on combined nasal and throat specimens followed by viral culture, antigenic analysis, antiviral susceptibility testing and full genome sequencing for phylogenetic analysis. ILI-specimens negative for influenza were cultured, followed by rRT-PCR for enterovirus and rhinovirus (EV/RV) and EV71. Influenza was found in 168 cases (29%) and occurred almost exclusively in the rainy season from June to November. Isolated influenza strains had close antigenic and phylogenetic relationships, matching vaccine and circulating strains found elsewhere in Cambodia. Influenza vaccination coverage was low (<20%). Western Cambodian H1N1(2009) isolate genomes were more closely related to 10 earlier Cambodia isolates (94.4% genome conservation) than to 13 Thai isolates (75.9% genome conservation), despite sharing the majority of the amino acid changes with the Thai references. Most genes showed signatures of purifying selection. Viral culture detected only adenovirus (5.7%) and parainfluenza virus (3.8%), while non-polio enteroviruses (10.3%) were detected among 164 culture-negative samples including coxsackievirus A4, A6, A8, A9, A12, B3, B4 and echovirus E6 and E9 using nested RT-PCR methods. A single specimen of EV71 was found. Despite proximity to Thailand, influenza epidemiology of these western Cambodian isolates followed patterns observed elsewhere in Cambodia, continuing to support current vaccine and treatment recommendations from the Cambodian National Influenza Center. Amino acid mutations at non-epitope sites, particularly hemagglutinin genes, require further investigation in light of an increasingly important role of permissive mutations in influenza virus evolution. Further research about the burden of adenovirus and non-polio enteroviruses as etiologic agents in acute respiratory infections in Cambodia is also needed.
Introduction
Acute respiratory infection (ARI) is the leading cause of morbidity and mortality in Cambodia [1]. Previous studies have attributed the etiology of acute viral respiratory infections in Cambodia to rhinovirus, respiratory syncytial virus (RSV), parainfluenza virus (PIV), influenza virus A and B, human metapneumovirus (HMPV), bocavirus, adenovirus, enterovirus, and coronavirus [2]. Influenza has been described to be one of the most important causes for hospitalization of children with an ARI [3]. It was suggested that some Southeast Asian countries, where influenza A infection is present year round, play an important role in the global spread of influenza that could trigger annual epidemics in temperate regions [4,5].
Until the establishment of the National Influenza Center (NIC) at Institut Pasteur du Cambodge (IPC) and a national influenza-like-illness (ILI)-sentinel surveillance system in 2006, little was known about influenza circulation in Cambodia. Since then, the sentinel surveillance system has, together with event-based surveillance, demonstrated evidence of seasonal, pandemic and avian influenza. Previously published data revealed that Thailand and Cambodia, which are positioned geographically in the Northern hemisphere, demonstrate differing influenza epidemiology with regard to seasonality, with Thailand exhibiting a northern hemisphere transmission pattern while transmission in Cambodia typically occurs from June to December, similar to the southern hemisphere [6]. The rainy season in Western Cambodia is June to November and the dry season is December to May. The more recent data suggests that influenza seasonality is more similar in Thailand and Cambodia than previously thought. [5].
Depending on the type of genes and the specific residues, phenotypic effects of random or neutral mutation of the virus can be positive, purifying or neutral [7]. Influenza virus efficiently escapes from host antibodies through an accumulation of mutations/single amino acid changes (antigenic drift) at the antigenic sites (epitopes) in surface glycoproteins of the hemagglutinin (HA) gene, and to a lesser extent, neuraminidase (NA) genes [8]. The antigenic sites are five somewhat overlapping regions (designated A to E for the H3 strains [9–11] and Ca1, Ca2, Cb, Sa, and Sb for the H1 strains [12]). Antigenic mapping by Smith et al. [13] showed that 11 antigenic clusters of viruses emerged during the 35-year period following the introduction of the A/H3N2 virus in humans in 1968 and that major jumps (or "cluster transitions") occur between antigenically distinct clusters of viral sequences roughly every 3 years (antigenic shift) [13].
Until 2009, the Cambodian national sentinel surveillance system only covered 8 out of 24 provinces based on a network of urban sentinel-sites at referral hospitals. No data existed about circulating influenza strains and other respiratory pathogens in Western-Cambodia along the Thai-Cambodia border, which is a poor and rural area with large volume of cross-border traffic. Between 2010 and 2012, the Armed Forces Research Institute of Medical Sciences (AFRIMS) established four additional influenza sentinel surveillance sites in four border provinces in western Cambodia, in collaboration with the Cambodian Communicable Disease Control (CCDC) Department and the IPC.
This study describes the circulation of influenza and non-influenza respiratory viruses and the genetic diversity of influenza viruses in western Cambodia along the Thai-Cambodia border which has provided information for treatment, prevention, and control strategies for these populations.
Materials and Methods
Ethics
The ILI and event-based surveillance systems are public health activities organized by the Ministry of Health in Cambodia and as such have a standing authorization from the National Ethics Committee. Samples were all anonymized for the purpose of this study. The study was approved by Scientific Review Committee at AFRIMS in Thailand, the Institutional Review Boards at the Walter Reed Army Institute of Research (WRAIR) in the United States and the National Ethics Committee for Health Research (NEHCR) in Cambodia. Written informed consent was obtained from volunteers or parents or legal guardians of children enrolled in the study.
Study design
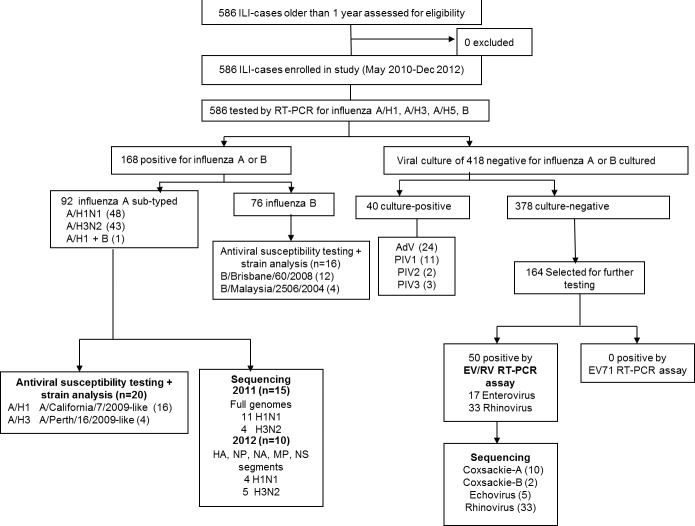
Between May 2010 and December 2012, we collected specimens and surveillance data for influenza and other viral respiratory pathogens from a subset of outpatients presenting with influenza-like-illness (ILI) at four sentinel sites-located in five health centers and hospitals in Battambang, Oddar Meanchey, Pailin and Banteay Meanchey provinces in Cambodia (Fig 1). Fig 1 was created using ArcView GIS version 3.1 (http://arcview.software.informer.com [14,15])
Fig 1. Locations of influenza-like-illness (ILI)-sentinel sites in Cambodia.
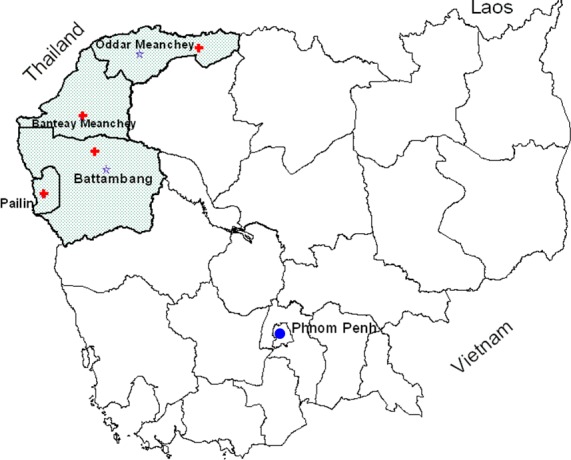
Study area is shaded; sentinel sites are indicated by a plus symbol in red and field laboratories are indicated by purple stars.
Sentinel health centers and hospitals were selected based on sufficient overall patient volume and patients with upper respiratory infections (URI), geographical representativeness and ease of specimen transport to the study laboratory. Sites were established sequentially: Battambang in 2010 (2 sites), Oddar Meanchey and Pailin in 2011, and Banteay Meanchey in 2012. Due to the cross-sectional design of our study in a region for which no previous baseline respiratory surveillance existed, no minimal sample size was calculated. Nevertheless, and in accordance with national and World Health Organization influenza surveillance guidelines [16], we aimed to collect a cross-section of data distributed over time and space. For every site -and pending on local epidemiology and health facility workload, 5 to 10 ILI specimens were collected each week (Monday to Friday) for 52 weeks per year and largely from the first two subjects meeting the predefined inclusion criteria.
Demographic, clinical data and respiratory specimens were collected from patients who met the case definition for ILI defined as persons of 1 year of age or older, arriving at the health center or hospital within 5 days of fever onset with a fever (axillary ≥38.1°C or oral ≥ 38.6°C) and cough or sore throat in the absence of other diagnoses. Patients younger than 1 year, who could not provide consent or those with nasal lesions or epistaxis were excluded. Demographic data included gender, occupation, presenting signs and symptoms, self-reported influenza vaccination history, medication use and travel within the last 7-days. Parents reported for children, which in our study are defined as all study volunteers younger than 18 years old.
Descriptive statistics of demographic and clinical data including frequencies and cross-tabulations were entered in a study database by double data-entry using Microsoft Excel 2013 and analyzed using IBM SPSS Statistics 21.0 (SPSS, IBM Inc.)
Specimen collection
Up to three respiratory swabs per volunteer were collected by trained staff with standard procedures. One nasal swab was tested on-site with QuickVue Influenza A+B (Quidel, San Diego, Calif.) using monoclonal antibodies specific for influenza A and B virus. A combined nasal and throat specimen was placed in a universal transport medium (UTM, Copan Diagnostics, Corona, CA, USA), stored between 2–8°C or in liquid nitrogen and shipped to the AFRIMS-CNM (Cambodia National Malaria Center) laboratory at the Battambang Referral Hospital for testing for influenza virus.
Laboratory methods
All ILI-specimens were typed and subtyped for influenza A and B at the AFRIMS-CNM laboratory in Battambang. Influenza-positive specimens were forwarded to the Virology Unit at Institut Pasteur du Cambodge (IPC) in Phnom Penh and the WHO Collaborating Center (WHOCC) for Reference and Research on Influenza in Melbourne, Australia. Nucleotide sequence and phylogenetic analysis of influenza viruses was conducted at the Virology Branch at the Walter Reed Army Institute of Research (WRAIR), Silver Spring, Maryland, U.S. Influenza-negative specimens were sent to the Department of Virology at AFRIMS in Bangkok, Thailand for viral culture and further characterization of other respiratory viruses.
Testing for influenza virus
RNA was extracted from an aliquot of 140 μl of each combined nasal and throat specimen using Qiagen Viral RNA mini kits (Qiagen, Hilden, Germany). The influenza genome was amplified and detected using a standardized the real-time reverse transcription (rRT)-PCR assay on a SmartCycler® II platform (Cepheid, Sunnyvale, CA), to test for influenza A and/or B types. Samples positive for influenza A and/or B were further tested to confirm influenza A and/or B positivity and to determine influenza A subtypes.
The hemagglutinin-gene (HA) specific primers and probes were used for subtyping of human influenza A subtypes H1, H3, H5 and pandemic H1 (pH1, previously known as swH1). Nucleoprotein-gene (NP) specific primers and probe for universal swine influenza was also used to confirm influenza A subtype pH1 detection in some samples. The 25 μl reaction mixture consisted of 5 μl of RNA eluate and 20 μl of the master mixture which included 0.5 μl enzyme mixture (SuperScript III RT/Platinum® Taq DNA Polymerase-Life Technologies), 0.5 μl RNaseOUT™ Recombinant Ribonuclease Inhibitor (InVitrogen™, Carlsbad, CA), 12.5μl 2X Reaction Mix, 0.5μl of both primers and 0.5μl of probe, and 5μl RNase-free water (see S1 Table for primer sequences). Thermocycling reaction conditions for influenza typing were as follows: initial reverse transcription at 50°C for 30 min, followed by denaturation and PCR activation by Taq inhibitor activator at 95°C for 2 min and 45 cycles of PCR amplification (<95°C for 15 sec then 64°C for 30 sec). For influenza A subtyping: reverse transcription at 50°C for 30 min, PCR activation by Taq inhibitor activator at 95°C for 2 min and 45 cycles of PCR amplification (<95°C for 15 sec then 62°C for 30 sec). After influenza typing by rRT-PCR, two 500 μl aliquots of every influenza-positive specimen was transferred into cryogenic vials for transport in liquid nitrogen to IPC in Phnom Penh.
Definitive diagnostics for influenza
Specimens testing positive for influenza by rRT-PCR were inoculated onto Madin-Darby canine kidney (MDCK) cells for isolation of influenza strains at the Virology Unit of IPC. The influenza strains were analyzed by a hemagglutination inhibition test using reference antigens and anti-sera provided by the WHOCC for Reference and Research on Influenza in Melbourne, Australia. The NA-Star® kit (Life Technologies, Carlsbad, CA, USA), a chemiluminescent neuraminidase (NA) inhibition assay which utilizes a 1,2-dioxetane derivative of sialic acid as substrates, was used for NA inhibitor susceptibility testing on a subset of isolates, randomly selected across specimen collection sites and dates. The concentration of drug required to inhibit 50% of the NA activity (IC50) was calculated using the non-linear curve-fitting function in the Graphpad Prism 4 package (GraphPad Software, Inc., La Jolla, CA, USA). The average IC50 (nM) (± standard deviation) of two independent determinations was calculated for each virus. Outliers of more than 2 standard deviations from the overall mean were retested twice. A subset of isolates were forwarded to WHOCC in Melbourne for confirmation of strain analysis by HA inhibition test and NA inhibitor susceptibility using an NA enzyme inhibition assay with a fluorescent substrate MUNANA [2’-(4-Methylumbelliferyl)-α-D-N-acetylneuraminic acid sodium salt hydrate]. The typical range of IC50 was calculated as the mean IC50 ± 3 standard deviations using a panel of well-characterized reference strains kindly provided by the WHO Collaborative Center for Reference and Research on Influenza, Melbourne, Australia. Isolates with IC50 values within or close to the typical IC50 range were considered to be sensitive isolates. IC50 values outside of the typical range and between 50 and 200 nM were isolates with mildly reduced sensitivity and IC50 values well outside the typical range and greater than 200 nM were considered as isolates with highly reduced sensitivity.
Nucleotide sequence analysis and phylogenetics of influenza viruses
Viral RNA segments, extracted from MDCK supernatant were sequenced at IPC in Phnom Penh from 15 samples collected in 2011 and from 10 samples collected in 2012 for both pandemic influenza (pH1N1) and influenza H3N2 (H3N2), representing our four study sites with varying success (S2 Table). Cleaned nucleotide sequence data was sent in FASTA-format to the Bioinformatics Section at the Virology Branch of the WRAIR in Silver Spring, Maryland, USA.
Sequences for pH1N1 and H3N2 sequence datasets respectively were combined with references available from the Influenza Viral Resource at Genbank [17] and the GISAID EpiFlu database [18] representing global diversity of pH1N1 from 2009–2013. Sequences were aligned using MUSCLE version 3.8.31 [19] implemented in Seaview version 4.4.2 [20] and manually inspected for accuracy. Best models were determined from alignments using jmodeltest2 version 2.1.4 [21]. Maximum likelihood (ML) phylogenetic trees were generated using PhyML version 3.0 as implemented in Seaview version 4.4.2 [22]. See Table 1 for a detailed description of datasets and models used in analysis. ML phylogenies were annotated using MEGA version 5 and FigTree version 1.4.0 [23]. All influenza sequences used in analysis have been deposited into Genbank under accessions KU299790—KU299957.
Table 1. Dataset descriptions and models used in maximum likelihood phylogenetic analysis.
| Influenza | Segment | Full/ | No. of | Specimen collection by site and year | No. of References | Modelc | -lnL | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subtype | Partial | Samples | BTB | OM | PL | BMC | ||||||||
| 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | |||||||
| pH1N1 | HA | Partiala | 14 | 3 | 1 | 7 | 0 | 1 | 1 | 0 | 1 | 201 | GTR+G | 7486.9 |
| pH1N1 | NP | Full | 15 | 3 | 2 | 7 | 0 | 1 | 1 | 0 | 1 | 125 | GTR+G | 3852.9 |
| pH1N1 | NA | Partialb | 14 | 3 | 1 | 7 | 0 | 1 | 1 | 0 | 1 | 125 | GTR+G | 4081.0 |
| pH1N1 | MP | Full | 15 | 3 | 2 | 7 | 0 | 1 | 1 | 0 | 1 | 124 | GTR+I | 2212.4 |
| pH1N1 | NS | Full | 15 | 3 | 2 | 7 | 0 | 1 | 1 | 0 | 1 | 125 | GTR+I | 2292.2 |
| 5d | 7 d | 2 d | 1 d | |||||||||||
| H3N2 | HA | Full | 10 | 2 | 1 | 1 | 5 | 1 | 0 | 0 | 0 | 169 | GTR+G+I | 8028.8 |
| H3N2 | NA | Full | 10 | 2 | 1 | 1 | 5 | 1 | 0 | 0 | 0 | 103 | GTR+G | 4569.2 |
| H3N2 | MP | Full | 10 | 2 | 1 | 1 | 5 | 1 | 0 | 0 | 0 | 122 | GTR+G | 3156.4 |
| H3N2 | NS | Full | 10 | 2 | 1 | 1 | 5 | 1 | 0 | 0 | 0 | 110 | GTR+I | 3325.5 |
| 3 d | 6 d | 1 d | 0 d | |||||||||||
BTB: Battambang, OM: Oddar Meanchey, PL: Pailin, BMC: Banteay Meanchey.
a Covering nucleotides 22–1683 of the HA gene.
b Covering nucleotides 97–1396 of the NA gene.
c As determined by jmodeltest2 version 2.1.4 (http://code.google.com/p/jmodeltest2/).
d Total number of samples from site.
Sequence Statistics, Amino Acid Analysis and Selection Tests
Alignments were analyzed for diversity, percent nucleotide similarity, antigenic variation and selection using MEGA version 5 [24] and the HyPhy package [25] implemented through the DataMonkey webserver [26–28]. Annotation was conducted using Genbank’s annotation tool in the Influenza Viral Resource [17] and a literature review.
Testing for non-influenza respiratory viruses at AFRIMS, Bangkok
All specimens tested negative by influenza PCR were cultured for isolation of respiratory virus and detection by cytopathic effect (CPE) and/or with either immunofluorescence assay (IFA), or direct fluorescence assay (DFA). A subset of 164 culture-negative specimens (collected between May 2010 and April 2012), where we found a higher proportion (5.6%) of non-polio enteroviruses in children less than 5 years old as compared with previous studies (1%) in Cambodia [2], were tested for enterovirus and rhinovirus by two separate nested RT-PCR methods adapted from Coiras et al., 2004 and Singh et al., 2002 [29,30], one for simultaneous detection of pan-enteroviruses and rhinoviruses, and the other specific for enterovirus 71 (EV71). PCR-products positive for enterovirus or rhinovirus were sequenced for nucleotide analysis (Fig 2; S3 Table).
Fig 2. Laboratory testing flowchart for influenza-like-illness (ILI).
Cell culture of influenza-negative specimens
Patient specimens in UTM were inoculated into three cell lines for virus isolation: Madin-Darby canine kidney (MDCK), Human lung carcinoma (NCI-H292) and Rhesus monkey kidney (LLCMK2) cells. Upon the appearance of CPE, after 7–10 days of culture, or after three passages, the cells were spotted onto microscope slides. Cell suspensions were dried and fixed in chilled acetone for 15 minutes. IFA was performed to identify virus isolates. The Respiratory Virus Screening and Identification Kit (Light Diagnostics, Respiratory Panel 1, Viral Screening and Identification IFA, Millipore Corporation, MA, USA) was utilized for the identification of adenoviruses, influenza A, influenza B, PIV (types 1, 2, and 3), and RSV.
Enterovirus 71 (EV71) nested RT-PCR
Enterovirus EV71 detection included single-step first-round RT-PCR and semi-nested PCR (primers are in S3 Table). The EV71 type specific primers were modified from the previous study by Singh et al. 2002 [30]. The modifications were made by following the alignments of VP1 sequences of different EV71 strains collected during 2002–2011 from Thailand, Taiwan, Philippines, Vietnam, and China available in Genbank including the sequences with the accession No. JN191177-9, FJ969151, FJ969163, JQ621835, JQ621841, AM490141-63, JQ315092, and JX203305. Single-step first-round RT-PCR was performed in following mix: 5 μl RNA suspension, 5 μl of 10x PCR buffer II supplied with AmliTaqTM DNA polymerase (Applied Biosystems), 0.2 mM of each dNTP, 1.5 mM MgCl2, 0.25 pmol of each forward and reverse primer (EV71-VP1f and EV71-VP1r), 5 mM dithiothreitol (DTT), 1 U reverse transcriptase from avian myeloblastosis virus (AMV RT, Promega, Madison, WI), 1.25 U of AmpliTaqTM DNA polymerase (Applied Biosystems) in a final volume of 50 μl. The reverse transcription (RT) step was performed at 42°C for 60 min, followed by 35 cycles of thermocycling 94°C for 30 seconds, 50°C for 30 seconds, and 72°C for 30 seconds.
Pan-enteroviruses and rhinoviruses nested RT-PCR
Detection of pan-enteroviruses and rhinoviruses (pan-EV/RV nested RT-PCR) is similar to the procedure for EV71 including 2 rounds of PCR as reported before [29]. The primers for simultaneous detection of enteroviruses and rhinoviruses were designed in the polyprotein gene, between 5′ non-coding region (5′NCR) and VP4/VP2 regions that was previously described by Coiras et al. 2004 [29]. EV/RV-2n was modified from primer 2-EV/RV [29] for using in nested PCR reaction. Single-step first-round RT-PCR reaction was the same as described above except that 0.25 pmol of each forward and reverse primer (1-EV/RV and 2-EV/RV) was used (S3 Table). The RT step was performed at 42°C for 60 min, followed by 35 cycles of thermocycling 94°C for 30 seconds, 50°C for 1 min, and 72°C for 1 min. The reaction was further incubated at 72°C for 10 min. Five micro-liter of 1:20 diluted first round PCR products were added to the nested PCR reaction with the same reagents as in the first-round but without DTT and AMV-RT and the primers were 0.25 pmol of each forward and reverse primer, 3-EV/RV and EV/RV-2n. The reaction was incubated at 95°C for 5 min followed by 35 cycles of thermocycling 94°C for 30 seconds, 52°C for 1 min, and 72°C for 1 min. The PCR products (9 μl each) were subjected to electrophoresis in agarose gels, with 100-bp DNA ladder serving as a molecular marker. A negative control of RNA-free water and a positive control of cDNA template from the EV71 reference strain (ATCC® No. VR-1432TM) were included in each experiment.
Nucleotide sequence analysis of pan-enteroviruses and rhinoviruses
The PCR products from the positive samples were sequenced on both strands with the PCR primers (S3 Table). The specific bands from the first or second rounds PCR were purified using QIAquick Gel Extraction Kit (Qiagen, Germany) before sending for direct sequencing. Sequencing service was performed by AIT biotech (Singapore). The sequences from both strands were combined for analysis and edited with Sequencher (Gene Code Corp., USA). Homology searches were through nucleotide BLAST program [31] along with the percentage of sequence identity of the two given sequences.
Results
586 ILI-patients (median age 5 year, range 1–77) were enrolled from five sites from May 2010 to December 2012; among these, 168 (29%) tested positive for influenza by rRT-PCR (Table 2). Most frequent symptoms reported were fever (100%, inclusion criterion), cough (100%,), runny nose (90%), congestion (77%), sore throat (75%,) and headache (76%). Although gender distribution was similar in ILI-patients, more females were positive for enterovirus (71%) or rhinovirus (64%). Patients with influenza were slightly older (median age 7 years old) and presented more often with a sore throat (78%). Compared with other ILI-cases, patients testing positive for enterovirus reported body pain (20% vs. 44%) and headache (50% vs. 77%) less frequently, although chills (50% vs. 32%), vomiting (35% vs. 16%) and abdominal pain (29% vs. 19%) were more common. Dyspnea was uncommon, yet most often seen in patients positive for adenovirus (14%). Diarrhea was observed in ILI-patients (6%-12%) except those infected with enterovirus (0/17).
Table 2. General characteristics of the study population.
| ILI cases (n = 586) | Influenza (n = 168) | Enterovirus (n = 17) | Rhinovirus (n = 33) | Adenovirus (n = 24) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | ||
| Sex | Male | 281 | 48 | 93 | 55 | 5 | 30 | 12 | 36 | 14 | 59 |
| Female | 305 | 52 | 75 | 45 | 12 | 71 | 21 | 64 | 10 | 42 | |
| Age (yrs) | |||||||||||
| Mean, ± SD | 8.8±10.6 | 11.2±11.2 | 2.7±1.5 | 3.8±3.0 | 4.3±9.5 | ||||||
| Median, [range] | 5 [1–77] | 7 [1–72] | 2 [1–6] | 3 [1–15] | 2 [1–48] | ||||||
| 0–4 yrs* | 248 | 42 | 42 | 25 | 14 | 82 | 20 | 61 | 19 | 79 | |
| 5–17 yrs | 260 | 44 | 94 | 56 | 3 | 18 | 13 | 39 | 4 | 17 | |
| 18–49 yrs | 73 | 13 | 30 | 18 | 0 | 0 | 0 | 0 | 1 | 4 | |
| ≥ 50 yrs | 5 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Site | Thmor Koul **(BTB) | 107 | 18 | 37 | 22 | 2 | 12 | 5 | 15 | 2 | 8 |
| Tapoung **(BTB) | 184 | 31 | 47 | 28 | 12 | 71 | 14 | 42 | 5 | 21 | |
| Anlong Veng (OM) | 231 | 39 | 62 | 37 | 3 | 18 | 13 | 39 | 15 | 63 | |
| Pailin (PL) | 46 | 8 | 14 | 8 | 0 | 0 | 1 | 3.0 | 2 | 8 | |
| Preah Punlear (BM) | 18 | 3 | 8 | 5 | 0 | 0 | 0 | 0 | |||
| Occupation | |||||||||||
| Child/Student | 508 | 87 | 135 | 80 | 17 | 100 | 33 | 100 | 23 | 96 | |
| Farmer | 36 | 6 | 13 | 8 | 0 | 0 | 0 | 0 | 1 | 4 | |
| Housewife | 15 | 3 | 8 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Unemployed | 5 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Laborer | 11 | 2 | 6 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Government | 10 | 2 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Other | 1 | 0.2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Travel last 7 days | 36 | 6 | 12 | 7 | 2 | 12 | 2 | 6 | 2 | 8 | |
| Temperature (axillary-in °C) | |||||||||||
| Mean, ± SD | 38.7±0.5 | 38.8±0.5 | 38.7±0.5 | 38.6±0.4 | 38.7±0.5 | ||||||
| Median, [range] | 38.6 [38–41] | 38.6 [38–41] | 38.5 [38–40] | 38.5 [38–40] | 38.5 [38–40] | ||||||
| Signs and symptoms | |||||||||||
| Sore throat | 343/460 | 75 | 120/153 | 78 | 3/6 | 50 | 12/21 | 57 | 4/12 | 33 | |
| Cough | 583/586 | 99.5 | 168/168 | 100 | 17/17 | 100 | 33/33 | 100 | 24/24 | 100 | |
| Runny nose | 529/586 | 90 | 153/168 | 91 | 17/17 | 100 | 29/33 | 88 | 24/24 | 100 | |
| Congestion | 422/552 | 77 | 119/162 | 73 | 7/14 | 50 | 26/33 | 79 | 20/21 | 95 | |
| Difficulty breathing | 44/563 | 8 | 12/163 | 7 | 1/16 | 6.3 | 1/32 | 3 | 3/22 | 14 | |
| Body pain | 191/429 | 45 | 64/146 | 44 | 1/5 | 20 | 4/21 | 19 | 6/12 | 50 | |
| Chills | 169/526 | 32 | 52/159 | 33 | 6/12 | 50 | 5/31 | 16 | 4/20 | 20 | |
| Malaise | 150/464 | 32 | 45/147 | 31 | 3/10 | 30 | 5/26 | 19 | 3/14 | 21 | |
| Headache | 346/452 | 77 | 115/153 | 75 | 5/10 | 50 | 12/23 | 52 | 9/11 | 82 | |
| Signs and symptoms cont. | |||||||||||
| Vomiting | 96/586 | 16 | 17/168 | 10 | 6/17 | 35 | 2/33 | 6 | 4/24 | 17 | |
| Diarrhea | 39/586 | 7 | 10/168 | 6 | 0/17 | 0 | 4/33 | 12 | 2/24 | 8 | |
| Abdominal pain | 100/528 | 19 | 25/164 | 15 | 5/17 | 29 | 6/27 | 22 | 4/18 | 22 | |
| Ear pain | 24/552 | 4 | 6/166 | 4 | 0/17 | 0 | 0/31 | 0 | 0/21 | 0 | |
| Influenza vaccination history | |||||||||||
| Yes | 63 | 12 | 20 | 14 | 6 | 60 | 10 | 44 | 3 | 14 | |
| No | 520 | 148 | 10 | 23 | 21 | ||||||
| Previous treatment | |||||||||||
| Antipyretics | 384 | 66 | 113 | 67 | 11 | 65 | 22 | 67 | 15 | 63 | |
| Antibiotics | 133 | 23 | 34 | 20 | 6 | 35 | 7 | 21 | 5 | 21 | |
| Antivirals | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
* children <1 yr were excluded
** The Battambang sentinel site consists of 2 health centers (Thmor Koul and Tapoung) within close proximity and serve the same community.
Influenza vaccination coverage in 2010 and 2011 approximated 20% in response to the influenza A/pH1N1(2009) pandemic yet decreased to zero in 2012 by subjects’ reports. Five hundred and seventeen ILI cases were taking medication at the time of presentation: 384 (66%) antipyretics (paracetamol) and 133 (23%) antibiotics. Antibiotic use was higher than average (35%) in patients positive for enterovirus. No use of antivirals was reported.
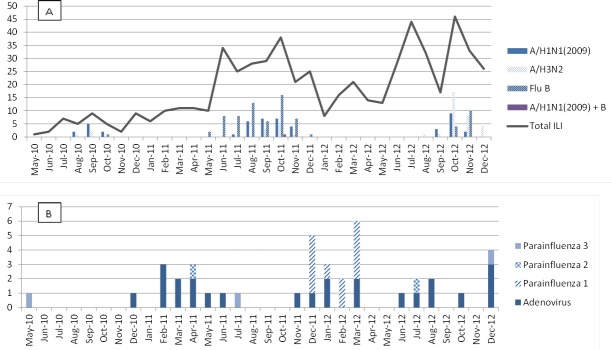
Between 2010 and 2012, influenza cases in Western Cambodia were almost exclusively seen in the rainy season (June to November), with almost no influenza detected in the dry seasons (December to May). Dominant influenza subtypes were A/pH1N1(2009) in 2010, influenza B in 2011 and influenza A/H3N2 in 2012. No human influenza A/H5N1 cases were detected. Antigenic analysis showed that all A/pH1N1 influenza strains found in 2011 (n = 13) belonged to the A/California/7/2009-like group and matched the reference strains included in the southern hemisphere vaccine for 2011. The four A/H3N2 influenza strains in 2011 all belonged to the A/Perth/16/2009-like strain. The B influenza strains belonged to either the B/Brisbane/60/2008-like group (n = 20) or the B/Malaysia/2506/2004-like group (n = 5) but all were within the Victoria lineage of influenza B, showing only a partial matching with the reference strain in the 2011 vaccine (Table 3 and Figs 2 and 3A). Vaccination coverage during the entire study period was 11% (63/583). The probability of developing influenza among ILI-patients who had been vaccinated (20/63) and who had not (148/520) was similar and not significantly different (0.32 vs. 0.28, p = 0.28). All IC50-values of influenza A and B isolates tested from 2011 were within the susceptible range for both oseltamivir and zanamivir (Table 3).
Table 3. IC50-values (nM) of influenza A and B isolates from 2011 to oseltamivir and zanamivir [Range(s) = Range for sensitive control isolates; Range(r) = Range for resistant control isolates).
| Drug | Strain | IC50 (nM)-NA-Star | IC50 (nM)-MUNANA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Range | Mean ± SD | Range (s)a | Range (r)b | n | Range | Mean ± SD | Range (s) | ||
| Oseltamivir | A/California/7/2009 | 7 | 0.35–0.86 | 0.58 ± 0.18 | 0.2–10.0 | 101.9–195.9 | ||||
| Oseltamivir | B/Brisbane/60/2008 | 8 | 0.87–8.13 | 4.74 ± 2.28 | 0.2–11.6 | 5 | 5.89–19.8 | 12.7 ± 5.39 | 0.1–40.3 | |
| Oseltamivir | B/Malaysia/2506/2004 | 4 | 1.43–17.3 | 9.18 ± 6.99 | ||||||
| Zanamivir | A/California/7/2009 | 7 | 0.56–1.52 | 0.78 ± 0.33 | 0.1–13.5 | 1.5–1.9 | ||||
| Zanamivir | B/Brisbane/60/2008 | 8 | 0.61–14.4 | 10.14 ± 4.33 | 0.4–17.9 | 5 | 1.16–2.67 | 1.80 ± 0.58 | 0.1–4.1 | |
| Zanamivir | B/Malaysia/2506/2004 | 4 | 1.67–3.00 | 2.41 ± 0.57 | ||||||
a IC50 range (nM)for sensitive wild type influenza A/H1N1 and influenza B isolates.
b IC50 range (nM) for resistant control A/H1N1 isolate A/Mississippi/274Y
Fig 3. Influenza and other respiratory viruses detected between 2010–2012.
(A) Number of ILI and influenza subtypes, May 2010-Dec 2012. (B) Number of other respiratory viruses among influenza-negative ILI specimens May 2010-Dec 2012.
Other respiratory viruses
Overall, at least 1 respiratory virus was detected in 258 out of 586 (44%) ILI-specimens collected between May 2010 and December 2012 (Fig 2); of which most were influenza (168, 29% of ILI-cases), followed by rhinovirus (33, 6%), adenovirus (24, 4%), non-polio enterovirus (17, 3%) and parainfluenza virus (PIV) (16, 3%). All of the 418 ILI specimens that tested negative for influenza A or B by rRT-PCR were sent for viral culture. Forty (10%) were culture positive; 24 adenovirus, 11 PIV1, 2 PIV2, 3 PIV3 were isolated (Figs 2 and 3B). No RSV, HMPV or coronaviruses were identified.
Pan-EV/RV and EV71 nested RT-PCR (S4 Table) detected 17 samples positive for enterovirus, which included coxsackievirus A (n = 10, 1 A4, 3 A6, 1 A8, 1 A9, 4 A12), echovirus (n = 5, 2 E6, 3 E9) and coxsackievirus B (n = 2, B3, B4). A single specimen was found to be negative during the first round of RT-PCR but positive by second round pan-EV/RV and EV71 nested RT-PCR. The 662 base pairs PCR product fragment obtained from pan-EV/RV nested RT-PCR was sequenced using the RV/EV inner primers. The 528 bases obtained from sequencing were found to be 99% identical to the Enterovirus A71/Homo sapiens/VNM/208/2011 strain (Accession: KJ686294). When the 227 bp PCR product obtained from EV71 nested RT-PCR was sequenced using the EV71 inner primers, 131 bases was obtained. This sequence was found to be 99% identical to Enterovirus A71/Cambodia: Banteay Meanchey 2012 strains (Accessions: KP308459, KP308453, KP308450, KP308448, KP308430, KP308427, KP308410, KP308406). Out of the 33 specimens positive with rhinoviruses, only 18 could be serotyped. Of these 18, most were rhinovirus A (n = 11), followed by rhinovirus B (n = 4) and rhinovirus C (n = 3) (Fig 4B). Amplicons were sequenced and sequences were analyzed using Genbank’s BLAST tool to identify the virus species.
Fig 4. Enteroviruses and rhinoviruses detected by viral culture and sequencing between May 2010-April 2012.
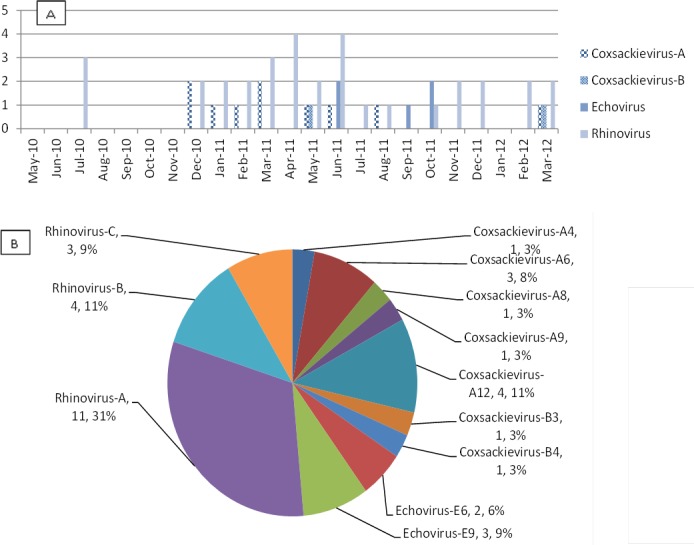
(A) Number of enteroviruses and rhinoviruses detected by EV/RV assay in 164 culture negative specimens, May 2010-April 2012. (B) Number and percentage of enterovirus and rhinovirus serotypes among a total of 50 (17 EV and 33 RV) positive samples.
Phylogenetics
Pandemic influenza A (pH1N1)
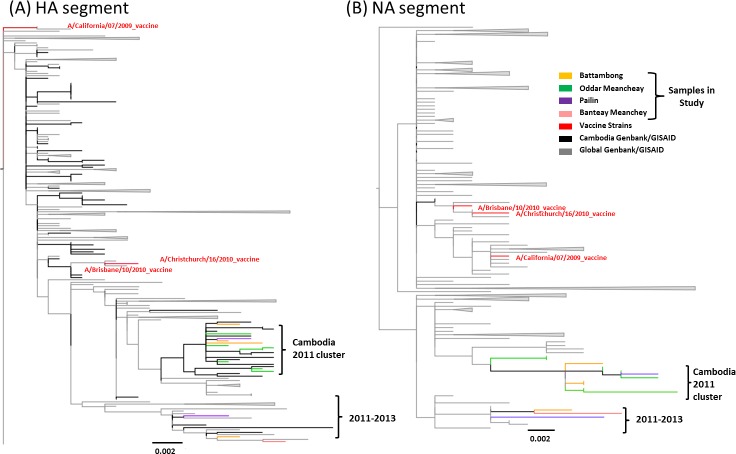
All sequences were highly related with no more than 1.67% divergence between the strains detected in the study and respective seed viruses included in the vaccine composition (A/California/7/2009, A/Brisbane/10/2010, and A/Christchurch/16/2010; S5 Table, Fig 5) for any of the segments analyzed. When compared to the A/California/7/2009 vaccine strain, all Cambodian viruses on average had 11 amino acid changes within the HA segment. Amino acid changes found in the HA gene can be found in Table 4. No evidence for amino acid substitutions leading to changes in glycosylation was seen in the HA dataset; however, several changes among sequenced samples occurred in antigenic sites, polymorphic sites or had a recorded effect in in vitro analyses per the literature (S6 Table). For the NA gene, several amino acid substitutions resulted in addition/loss of glycosylation sites, or were found in polymorphic or antigenic sites (epitopes) (S6 Table). For the MP gene, all samples contained the S31N mutation that may confer amantadine resistance as determined by the Genbank Influenza Viral Resource Annotation tool [32]. No features or changes of interest were seen within the NP or NS segment. The HA and NA segments contained the most amino acid substitutions when compared to the reference (A/California/7/2009) as well as to the yearly defined groups (2011 and 2012). The MP and NP segments showed the fewest substitutions (Table 4). There were no samples within the HA amino acid analysis that had unique substitutions relative to the global sequences. However, the NA gene had unique amino acid substitutions not found with the global references; the function of these substitutions is unknown (S7 Table). Selection pressure was determined using the dN/dS statistic. Values of dN/dS > 1 are indicative of positive or adaptive selection, values <1 are indicative of purifying or negative selection and a value = 1 indicates neutral or no selection ongoing in the dataset. All samples were under purifying selection for all segments with exception to the NS segment of the 2011 Cambodian samples, which had a dN/dS value of 1.0 suggesting neutral selection (S8 Table).
Fig 5.
pH1N1 maximum likelihood phylogenetic trees (aLRT support >70 for all vaccine clades and major nodes) for the (A) HA and (B) NA segments for 14 samples from Cambodia and references in Genbank (grey) collected in 2011 and 2012. Vaccine strains are highlighted in red. Samples are colored coded by sampling site.
Table 4. pH1N1 and H3N2 amino acid changes a specific to sample sets for the HA and NA genes.
| Influenza Subtype | Segment | Sample sets (n) and AA changes found | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (vaccine compared to) | All d | 2011d | 2012d | Individual samples (n) | ||||||||
| BTB | OM | PL | BMC | |||||||||
| 2011 (5) | 2012 (3) | 2011 (8) | 2012 (5) | 2011 (2) | 2012 (1) | 2011 (0) | 2012 (1) | |||||
| pH1N1 (A/California/7/2009) n = 15 | HA | E391K | N277D/G | H155R | G172E (1) | G172E (1) | N173D (1) | G172E (1) | G172E(1), S200P (1) | |||
| pH1N1 (A/California/7/2009) n = 15 | NA | N248D | N44S | D451G | N63S(1) | I396V(1) | ||||||
| H3N2 (A/Victoria/361/2011) n = 10 | HA | Q172H, V202G, Y235S | N161S | N24D (2) | N61S (1) I64T (1) D69S (1) A154S (1) S225R (1) | I156M (1) N161S (1) | N61S (4) I64T (4) D69S (4) A154S (1) T147K (1) | N24D (1) | ||||
| H3N2 (A/Victoria/361/2011) n = 10 | NA | K258E, T329N | G401S (1) | D151N (1), D402N (1) | ||||||||
BTB: Battambang, OM: Oddar Meanchey, PL: Pailin, BMC: Banteay Meanchey
a All substitutions were also found within global sequences. Only those substitutions noted in previous studies are included.
For pH1N1 there was no evidence of geographic clustering of sequences within sampling site (colored branches in Fig 5); nor was there evidence of reassortment among the sequences sampled. Sequences (references and samples) from Cambodia 2011 distributed throughout the trees; however, the majority of 2011 sequences, including those corresponding to strains detected in this study appeared within a 2011 specific Cambodian clade. However, this clade was only evident in HA and NA phylogenies and did not appear in NP, MP or NS phylogenies (S1–S5 Figs).
Influenza A H3N2 (H3N2)
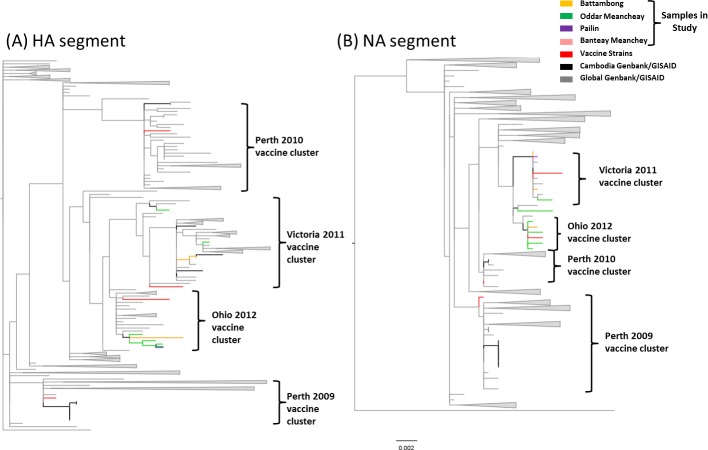
All sequences were highly related with no more than 1.77% divergence between the samples and the furthest related respective vaccine (S9 Table, Fig 6) for any of the segment. The majority of segments were more closely related to the A/Victoria/361/2011 vaccine strain; therefore all subsequent analyses were compared to this strain. For the HA gene, when compared to the A/Victoria/361/2011 vaccine strain, all samples on average had 9 amino acid changes within the HA segment (S10 Table). There were several amino acid changes among the sequenced samples occurring in antigenic sites, polymorphic sites or which resulted in a change of glycosylation (Table 5). For the NA gene, several amino acid substitutions resulted in addition/loss of glycosylation sites, or were found in polymorphic or antigenic/catalytic sites (epitopes) (Table 5). For the MP gene, it was noted that all samples contained the S31N mutation that may confer adamantane resistance as determined by the Genbank Influenza Viral Resource Annotation tool [32]. No features or changes of interest were seen within the NS segment for any sample.
Fig 6.
H3N2 maximum likelihood phylogenetic trees (aLRT support >70 for all vaccine clades and major nodes) for the (A) HA and (B) NA segments for 10 samples from Cambodia and references in Genbank (grey) collected in 2011 and 2012. Vaccine strains are highlighted in red. Samples are colored coded by sampling site.
Table 5. Changes in glycosylation, antigenic and polymorphic sites linked to amino acid (AA) substitutions and sub-antigenic/catalytic sites (epitopes) where AA substitutions were found for hemagglutinin (HA) and neuraminidase (NA) segments of H3N2.
| Segment | AA Substitutiona | Notation |
|---|---|---|
| HAb | N161S | Antigenic region A [33–35] |
| HAb | Q172H | Antigenic region B [33–35] |
| HAb | V202G | Antigenic region B [33,34] |
| HAb | Y235S | Antigenic region D [33–35] |
| HAb | N61S | Loss of glycosylation site (NSS to SSS); Antigenic region C [33,34] |
| HAb | I64T | Antigenic region C [33,34] |
| HAb | D69S | Antigenic region C [33,34] |
| HAb | A154S | Antigenic region A [33,34] |
| HAb | S225R | Antigenic region D [33–35] |
| HAb | T147K | Antigenic region A [33,34] |
| HAb | N24D | Loss of glycosylation site (NST to DST) |
| HAb | I156M | Antigenic region A [33–35] |
| NAc | K258E | Unknown |
| NAc | T329N | Addition of glycosylation site (TDS to NDS) |
| NAc | D151N | Catalytic site [35] |
| NAc | G401S | Antigenic site A |
| NAc | D402N | Addition of glycosylation site (DRS to NRS) |
AA: amino acid
a Amino acid of reference on left, sample substitution on right of amino acid position number.
b HA numbering starts from Methionine as position 1.
c NA numbering starts from Methionine as position 1.
The HA and NA segments contained the most amino acid substitutions when compared to the reference (A/Victoria/361/2011) as well as to the yearly defined groups (2011 and 2012). The MP and NS segments showed the fewest substitutions (S10 Table). There were several amino acid substitution within the HA amino acid analysis that were unique relative to the global sequences (and vaccine references); two of which occurred in antigenic regions (Table 5) and five additional changes of unknown effect or location significance (S11 Table). One amino acid substitution in the NA gene was found to be unique in antigenic site A (Table 5) and 7 unique substitutions of unknown effect or location significance were also found (S12 Table). All ‘unique’ AA substitutions were not found with the global sequences or vaccine references. All samples were under purifying selection for all segments with the exception to the NS segment. When samples were separated based on the respective years of collection, the NS gene showed evidence of neutral selection (dN/dS ~ 1). However, when all samples were combined to increase sample size, it showed overall evidence of purifying selection (dN/dS < 1; S10 Table).
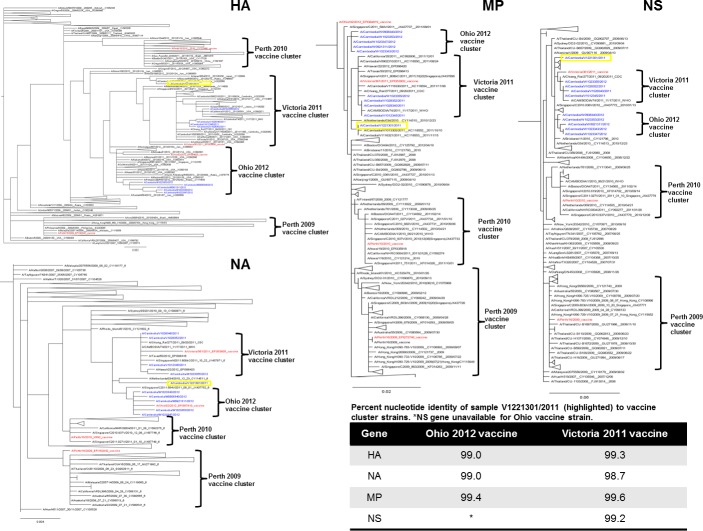
Overall, for H3N2, there was no evidence of geographic clustering of sequences within the same sampling site except samples from Oddar Meanchey in which all but one sample clustered into the Ohio 2012 vaccine cluster (green branches in Fig 6). This clustering was visual only, not statistically significant due to small sample size, though the cluster was well supported (cluster node value 81, value obtained using associated likelihood ratio test (aLRT); analogous to bootstrap analysis). There was no evidence of reassortment among the samples analyzed and all samples were consistently grouping with either the A/Ohio/02/2012 or A/Victoria/361/2011 vaccines. There was one exception; sample A/Cambodia/V1221301/2011 consistently fell outside of both the Ohio 2012 and Victoria 2011 vaccine clusters (Fig 7) for all segments analyzed.
Fig 7. H3N2 maximum likelihood phylogenetic trees (aLRT support >70 for all vaccine clades and major nodes) for all segments analyzed in this study for 10 samples from Cambodia (colored in blue) and references in Genbank collected in 2011 and 2012.
Vaccine strains are highlighted in red and clades defined by brackets. Unique sample that did not fall within vaccine clades highlighted in yellow with percent nucleotide identity indicated in bottom right table.
Discussion
This is to the best of our knowledge the first influenza surveillance study in remote border areas in western Cambodia. Over the time course of 31 months, spanning 3 influenza seasons including the last few months of pandemic A/pH1N1(2009) influenza circulation, seasonal influenza virus was the most commonly detected respiratory virus in this predominantly pediatric population of subjects who presented with ILI (Fig 3).
Despite detection of human highly pathogenic avian influenza (HPAI) H5N1 virus in Western Cambodia since early 2011 [36–38], H5N1, typically detected in south-central Cambodia, was not found at our sentinel sites. Influenza subtypes varied by year with A/pH1N1(2009) being predominate in 2010, influenza B in 2011, and influenza A/H3N2 in 2012. We did not expect to detect any H5N1 in our laboratory surveillance system as most suspected H5N1 cases would have been screened out by the health centers based on reported exposure to dead or diseased poultry. The specimen of one patient with reported H5N1 exposure in 2011 was investigated in our laboratory and tested negative for H5N1.
No national policies have been established in Cambodia for seasonal influenza vaccination to date. History of vaccination was low in this population. Available influenza vaccines during the study period were the A(H1N1)2009pdm monovalent vaccine in the public sector and the trivalent seasonal vaccine in 2009, 2010 and 2011 in the private sector [39]. Our data revealed that the influenza B did not match well with strains included in vaccine composition of trivalent vaccines; therefore, if vaccination is to be implemented, the quadrivalent influenza vaccine that contains the two influenza B lineages, rather than the trivalent vaccine, may be more suitable. Aside from a limited quantity of oseltamivir, stockpiled by the government for management of severe disease by pandemic or avian influenza [40], use of antivirals for influenza is very low in Cambodia [40]. As a result, all strains we detected were susceptible to neuraminidase inhibitors (NIs) such as oseltamivir and zanamivir, which can be partially explained by the absence of these medications in Cambodia. Due to widespread adamantane resistance, we did not conduct susceptibility testing on this class of antiviral drugs; however, we did find the S31N mutation conferring adamantane resistance in all of our influenza isolates tested.
Phylogenetically, all our samples clustered together within each of the two subtypes (A/pH1N1 or A/H3N2), which is not unusual given the location and specimen collection dates compared with the reference sequences used. Our western Cambodian pH1N1(2009) isolates were more closely related, based on full genome analysis, to 10 earlier isolates from Cambodia (94.4% genome conservation) than to the 13 Thai isolates (75.9% genome conservation) or the California 2009-vaccine reference. However, it was also noted that amino acid changes were shared with the Thai references suggesting a possibility of mixing between Thai and Cambodian influenza as expected. Isolates from Battambang, being western Cambodia's major transportation hub, showed the highest diversity of amino acid changes.
Analysis of sequence data from our influenza patients revealed a random or neutral mutation of the influenza genomes as expected. Koel et al. [41] discovered that periodic major antigenic change in influenza A/H3N2 virus was caused mainly by single amino acid substitutions, which occurred at only seven out of 131 possible amino acid positions in HA at antigenic sites immediately adjacent to the receptor binding site (RBS). These substitutions were located in antigenic sites A (position 145) and B (positions 155, 156, 158, 159, 189, and 193), with none in sites C, D, or E. We did not see these changes within antigenic sites A or B in our dataset.
The HA and NA genes in our H1 and H3 isolates showed signatures of purifying selection meaning that the virus keeps these genes conserved by removing random mutations as they occur. These single amino acid mutations were found at non-epitope sites of HA [41] that have not been associated with major antigenic changes on their own. However, it is possible that some of these mutations may constitute permissive or compensatory mutations that would be important in enabling co-mutations that could affect viral fitness, and as such, have an incentive to remain fixed under purifying selection. Permissive mutations have been described in the spread of oseltamivir resistant influenza A/H1N1 virus that carry the H274Y mutation and are increasingly being recognized as a major force in evolution [42,43].
Glycosylation (addition of oligosaccharide chain to the surface protein) is another common form of protein modification. Alteration of glycosylation sites can affect folding and conformation changes in the surface glycoprotein, hereby impacting virus survival and transmissibility. In addition, glycosylation can affect interaction with receptors and cause a virus to be more [or less] recognizable by the innate host immune system and antibodies [44]. None of the changes in glycosylation in our isolates have been reported in the literature as having an effect on viral structure or function; but more data may be needed. Mapping sites of mutation and glycosylation on epitopes provides a better understanding of antigenic drift and is important for improving vaccine strategies [45].
Overall, and despite adequate storage and transportation of our specimens, culture yield was low in our dataset (~10%) with isolation of only adenovirus and parainfluenza. This may be due to an exclusion of children younger than 1 year, the outpatient setting with less severe disease and possibly a later presentation to the health facility (which was possible up to 5 days after fever onset) resulting in a lower viremia. Previous work among hospitalized patients has shown presence of human coronavirus, human bocavirus, HMPV and RSV in Cambodia [2] but these were not detected by our culture techniques. Therefore, the number of non-influenza viruses in our population is possibly underestimated. Additional testing is ongoing at AFRIMS in order to elucidate true disease burden of non-influenza viruses on the population.
In comparison with previous work in Cambodia [46], we found a relatively higher proportion (9.3% versus 1.3%) of ILI-patients testing positive for adenovirus over 2 dry seasons in 2011 and 2012 which may be explained by our younger study population who may be disproportionately affected by this virus. PIV-3 (0.9%), which in previous dry seasons was described as the most common parainfluenza-virus in Cambodia [2], was the least common type during our surveillance period, whereas PIV-1 (5.6%) was most common, particularly during November 2011 to March 2012. This is not consistent with previous reports for Cambodia [46]. The lack of presence of PIV-3 in our study population may be due to an outbreak of PIV-1 during the surveillance time frame and the fact that we did not include children under 1 year old, an age when 40% of PIV-3 is usually detected [47].
By employing a highly sensitive and specific nested RT-PCR assay for enteroviruses and rhinoviruses, we found a higher proportion (5.6%) of non-polio enteroviruses in children less than 5 years old compared with previous studies (1%) in Cambodia [2] which could be partially explained by the predominantly outpatient population in our study sample. This is likely an underestimation of the true prevalence in this population since we excluded children less than one year of age, an age group more likely infected by enteroviruses at rates exceeding those of older children and adults by several fold [48–50]. The most common enteroviruses found among children under 5 years old in our population were coxsackieviruses (7.3%) and echoviruses (3%). Although we did not find coxsackievirus A16, the most common etiologic agent for hand-foot-mouth-disease (HFMD), we found other coxsackieviruses that can cause HFMD such as coxsackievirus A6 (n = 3) and A12 (n = 4). Coxsackievirus A6 is known to cause either an atypical rash resembling eczema herpeticum or chickenpox (United States 2011–2012 and in Europe since 2009) or nail loss one to two months after onset of symptoms (Finland 2009, Taiwan 2010, Japan 2011). Coxsackievirus A12 was one of the enteroviruses implicated in HFMD outbreaks in China in 2008 and 2009 [51]. While these patients reported symptoms of fever (38.1–39°C), runny nose, chills, cough, congestion and abdominal pain, they did not report symptoms specific to HFMD such as herpangina and skin rash.
We did not detect in our study EV71 virus genotype C4, which was the principal etiologic agent causing the 2012 HFMD outbreak across Southeast-Asia that manifested as a life-threatening neuro-respiratory syndrome in 78 children under 3 years old from 14 provinces in Cambodia [52]. Although we did not have EV71 test results from specimens collected during the peak of the outbreak, our data suggests that EV71 virus was not present yet in the border areas of Western Cambodia before April 2012, the time that the first cases were found elsewhere in Cambodia.
Regarding testing for non-influenza viruses, the use of the less sensitive viral culture as the first-line test supplemented by IFA on only a subset of influenza-negative ILI-specimens underestimates overall extent of transmission as well as co-infections. Exclusion of children under 1 year of age may also contribute to underestimation. Our data was derived from a passive surveillance system with convenience sampling. While we attempted to select surveillance sites representative of the nearby geographic locations, we had no accurate figures of the catchment populations or denominators at our respective sentinel sites. Our staggered method of adding sentinel sites suggests that not all sentinel sites enrolled patients evenly over 3 influenza seasons. Similarly, with a small sample size and limited number of sampling locations, no inferences could be made with regard to temporal or spatial flow of influenza strains in our phylogenetic analysis. Now with a more established system in place, our data may provide a useful baseline for future molecular evolution studies of influenza in Cambodia and in the region.
Conclusions
Despite proximity to Thailand, influenza activity, seasonality, antigenicity and anti-viral susceptibility in western Cambodian isolates followed patterns observed elsewhere in Cambodia rather than Thailand. This supports earlier recommendations from the Cambodian NIC to use the northern hemisphere influenza vaccine on a southern hemisphere vaccination schedule. Additionally, use of the quadrivalent versus the trivalent vaccine should improve coverage of influenza B strains circulating in Cambodia. Neuraminidase inhibitors may still be used for treatment and chemoprophylaxis for seasonal influenza given little evidence for resistance. The amino acid mutations at non-epitope sites, in particular of HA, require further investigation in light of the increasingly important role of permissive mutations in the evolution of influenza virus. Further research to clarify the burden of adenovirus and non-polio enteroviruses as etiologic agents in acute respiratory infections in Cambodia is needed.
Supporting Information
Vaccine strains are highlighted in red. Node support was calculated with aLRT and was >0.70 for all major nodes. The 2011 samples fell within the same clade and 2012 samples fell within a different clade, all samples clustered with other sequences isolated from Cambodia.
(TIF)
Vaccine strains are highlighted in red. Node support was calculated with aLRT and was >0.70 for all major nodes. All samples fell within the same major clade that included other sequences isolated from Cambodia.
(TIF)
Vaccine strains are highlighted in red. Node support was calculated with aLRT and was >0.70 for all major nodes. The 2011 samples fell within the same clade and 2012 samples fell within a different clade.
(TIF)
Vaccine strains are highlighted in red. Node support was calculated with aLRT and was >0.70 for all major nodes. The 2011 samples fell within the same clade and 2012 samples fell within a different clade.
(TIF)
Vaccine strains are highlighted in red. Node support was calculated with aLRT and was >0.70 for all major nodes. The 2011 samples fell within the same clade and 2012 samples fell within a different clade.
(TIF)
(DOCX)
(DOCX)
The modifications were made by following the alignments of VP1 sequences of different EV71 strains collected during 2002–2011 from Thailand, Taiwan, Philippines, Vietnam, and China available in Genbank including the sequences with the accession No. JN191177-9, FJ969151, FJ969163, JQ 621835, JQ621841, AM490141-63, JQ315092, and JX203305. The primers for simultaneous detection of enteroviruses and rhinoviruses were designed in the polyprotein gene, between 5′ non-coding region (5′NCR) and VP4/VP2 regions that was previously described by Coiras et al. 2004 [29]. EV/RV-2n was modified from primer 2-EV/RV [29] for using in nested PCR reaction.
(DOCX)
(DOCX)
(DOCX)
AA substitution nomenclature is as follows; reference amino acid (A/California/7/2009), amino acid site, sample amino acid. Amino acids are numbered from the start codon of the segment (ATG:Methionine).
(DOCX)
AA substitution nomenclature is as follows; reference amino acid (A/California/7/2009), amino acid site, sample amino acid. Amino acids are numbered from the start codon of the segment (ATG:Methionine).
(DOCX)
(DOCX)
(DOCX)
(DOCX)
AA substitution nomenclature is as follows; reference amino acid (A/Victoria/361/2011), amino acid site, sample amino acid. Amino acids are numbered from the start codon of the segment (ATG:Methionine).
(DOCX)
AA substitution nomenclature is as follows; reference amino acid (A/Victoria/361/2011), amino acid site, sample amino acid. Amino acids are numbered from the start codon of the segment (ATG:Methionine).
(DOCX)
Sequencing results of the EV71 isolates based on (A) pan rhinovirus/enterovirus inner primer and (B) Sequencing results of the EV71 isolates based on an enterovirus 71 inner primer.
(DOCX)
Acknowledgments
We gratefully thank all the volunteers for their study participation and study site and Provincial Health Department staff in Battambang, Oddar Meanchey, Banteay Meanchey and Pailin for collecting the data and specimens. We would like to acknowledge the Thai-Cambodian AFRIMS field team for their dedication and commitment: Mr. Buth Sam El, Mr. Soy Pros, Mr. Sirasri Sittidech, Mr. Montri Arsanok, Ms. Saowaluk Wongarunkochakorn, Ms. Monticha Kongthaisong, Mr. Thanawat Assawariyathiphat, Mr. Worachet Kuntawunginn, Ms. Kingkan Pidtana, Dr. Sea Darapiseth, Ms. Soklyda Chan, Ms. Taing Davy, Ms. Doungngoen Khaminthakul, Mr. Theera Wimonwattrawatee. Mr. Sareth Rith, Dr. Mardy Sek for their support. Drs. Douglas Walsh, Robert Gibbons, In-Kyu Yoon, Carl Mason, Mitchell Meyers and Sam Yingst supported this project in their capacity as AFRIMS Department Chiefs. We are grateful for the strain and NAI susceptibility analysis conducted by Prof. Anne Kelso and Dr. Ian Barr and their team from the WHO CC in Melbourne, Australia.
Data Availability
All sequences are available from the NCBI Genbank database (accession number(s) KU299790-KU299957).
Funding Statement
Funding was provided by The Armed Forces Health Surveillance Center’s Global Emerging Infections and Response System. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization (2012) Cambodia Health Service Delivery Profile, 2012.
- 2.Guerrier G, Goyet S, Cheng ET, Rammaert B, Borand L, Te V, et al. (2013) Acute viral lower respiratory tract infections in Cambodian children: clinical and epidemiologic characteristics. Pediatr Infect Dis J 32: e8–13. 10.1097/INF.0b013e31826fd40d [DOI] [PubMed] [Google Scholar]
- 3.Neuzil KM, Zhu Y, Griffin MR, Edwards KM, Thompson JM, Tollefson SJ, et al. (2002) Burden of interpandemic influenza in children younger than 5 years: a 25-year prospective study. J Infect Dis 185: 147–152. [DOI] [PubMed] [Google Scholar]
- 4.Rambaut A, Pybus OG, Nelson MI, Viboud C, Taubenberger JK, Holmes EC (2008) The genomic and epidemiological dynamics of human influenza A virus. Nature 453: 615–619. 10.1038/nature06945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saha S, Chadha M, Al Mamun A, Rahman M, Sturm-Ramirez K, Chittaganpitch M, et al. (2014) Influenza seasonality and vaccination timing in tropical and subtropical areas of southern and south-eastern Asia. Bulletin of the World Health Organization 92: 318–330. 10.2471/BLT.13.124412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mardy S, Ly S, Heng S, Vong S, Huch C, Nora C, et al. (2009) Influenza activity in Cambodia during 2006–2008. BMC Infect Dis 9: 168 10.1186/1471-2334-9-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plotkin JB, Dushoff J (2003) Codon bias and frequency-dependent selection on the hemagglutinin epitopes of influenza A virus. Proc Natl Acad Sci U S A 100: 7152–7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wrigley NG (1979) Electron microscopy of influenza virus. Br Med Bull 35: 35–38. [DOI] [PubMed] [Google Scholar]
- 9.Wiley DC, Wilson IA, Skehel JJ (1981) Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature 289: 373–378. [DOI] [PubMed] [Google Scholar]
- 10.Wiley DC, Wilson IA, Skehel JJ (1984) The hemagglutinin membrane glycoprotein of influenza virus. Biological Macromolecules and Assemblies, ed McPherson A 1: 299–336. [Google Scholar]
- 11.Wiley DC, Skehel JJ (1987) The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem 56: 365–394. [DOI] [PubMed] [Google Scholar]
- 12.Caton AJ, Raymond FL, Brownlee GG, Yewdell JW, Gerhard W (1983) Antigenic variation in influenza virus. Biochem Soc Trans 11: 435–441. [DOI] [PubMed] [Google Scholar]
- 13.Smith DJ, Lapedes AS, de Jong JC, Bestebroer TM, Rimmelzwaan GF, Osterhaus AD, et al. (2004) Mapping the antigenic and genetic evolution of influenza virus. Science 305: 371–376. [DOI] [PubMed] [Google Scholar]
- 14.Dempsey C (2008) Why ArcView 3.x is till in use GIS Lounge: GIS Lounge. [Google Scholar]
- 15.Shayya WH (2013) An introduction to ArcView GIS. Morrisville State College: Morrisville State College. [Google Scholar]
- 16.World Health Organization (2008) A practical guide to harmonizing virological and epidemiological infuenza surveillance In: Organization WH, editor. World Health Organization: World Health Organization. [Google Scholar]
- 17.Bao Y, Bolotov P, Dernovoy D, Kiryutin B, Zaslavsky L, Tatusova T, et al. (2008) The influenza virus resource at the national center for biotechnology information. Journal of Virology 82: 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogner P, Capua I, Lipman DJ, Cox NJ (2006) A global initiative on sharing avian flu data. Nature 442. [Google Scholar]
- 19.Edgar RC (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5: 113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gouy M, Guindon S, Gascuel O (2010) SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27: 221–224. 10.1093/molbev/msp259 [DOI] [PubMed] [Google Scholar]
- 21.Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darriba D, Taboada GL, Doallo R, Posada D. (2012) jModelTest 2: more models, new heuristicsSeaView version 4: A multiplatform graphical user interface for sequence alignment and parallel computingphylogenetic tree building. Nat MethodsMol Biol Evol 927: 772221–772224. [Google Scholar]
- 23.Rambaut A (2009) FigTree v1. 3.1: Tree figure drawing tool. Available: http://treebioedacuk/software/figtree/.
- 24.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pond SL, Frost SD, Muse SV (2005) HyPhy: hypothesis testing using phylogenies. Bioinformatics 21: 676–679. [DOI] [PubMed] [Google Scholar]
- 26.Delport W, Poon AF, Frost SD, Kosakovsky Pond SL (2010) Datamonkey 2010: a suite of phylogenetic analysis tools for evolutionary biology. Bioinformatics 26: 2455–2457. 10.1093/bioinformatics/btq429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pond SL, Frost SD (2005) Datamonkey: rapid detection of selective pressure on individual sites of codon alignments. Bioinformatics 21: 2531–2533. [DOI] [PubMed] [Google Scholar]
- 28.Poon AF, Frost SD, Pond SL (2009) Detecting signatures of selection from DNA sequences using Datamonkey. Methods Mol Biol 537: 163–183. 10.1007/978-1-59745-251-9_8 [DOI] [PubMed] [Google Scholar]
- 29.Coiras MT, Aguilar JC, Garcia ML, Casas I, Perez-Brena P (2004) Simultaneous detection of fourteen respiratory viruses in clinical specimens by two multiplex reverse transcription nested-PCR assays. J Med Virol 72: 484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh S, Chow VT, Phoon MC, Chan KP, Poh CL (2002) Direct detection of enterovirus 71 (EV71) in clinical specimens from a hand, foot, and mouth disease outbreak in Singapore by reverse transcription-PCR with universal enterovirus and EV71-specific primers. J Clin Microbiol 40: 2823–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. Journal of Molecular Biology 215: 403–410. [DOI] [PubMed] [Google Scholar]
- 32.Bao Y, Bolotov P, Dernovoy D, Kiryutin B, Tatusova T (2007) FLAN: a web server for influenza virus genome annotation. Nucleic Acids Res 35: W280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi Y, Suzuki Y (2012) Evidence for N-glycan shielding of antigenic sites during evolution of human influenza A virus hemagglutinin. J Virol 86: 3446–3451. 10.1128/JVI.06147-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee MS, Chen JS (2004) Predicting antigenic variants of influenza A/H3N2 viruses. Emerg Infect Dis 10: 1385–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chutinimitkul S, Chieochansin T, Payungporn S, Samransamruajkit R, Hiranras T, Theamboonlers A, et al. (2008) Molecular characterization and phylogenetic analysis of H1N1 and H3N2 human influenza A viruses among infants and children in Thailand. Virus Res 132: 122–131. [DOI] [PubMed] [Google Scholar]
- 36.Chea N, Yi SD, Rith S, Seng H, Ieng V, Penh C, et al. (2014) Two clustered cases of confirmed influenza A(H5N1) virus infection, Cambodia, 2011. Euro Surveill 19. [DOI] [PubMed] [Google Scholar]
- 37.Admin (2014) Avian Influenza. Cambodian Ministry of Health. [Google Scholar]
- 38.World Health Organization (2011) Avian Influenza—situation in Cambodia—update.
- 39.Gupta V, Dawood FS, Muangchana C, Lan PT, Xeuatvongsa A, Sovann L, et al. (2012) Influenza vaccination guidelines and vaccine sales in southeast Asia: 2008–2011. PLoS One 7: e52842 10.1371/journal.pone.0052842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Sa J, Mounier-Jack S, Darapheak C, Narann LK, Phetsouvanh R, Chanthakoummane N, et al. (2010) Responding to pandemic influenza in Cambodia and Lao PDR: challenges in moving from strategy to operation. Southeast Asian J Trop Med Public Health 41: 1104–1115. [PubMed] [Google Scholar]
- 41.Koel BF, Burke DF, Bestebroer TM, van der Vliet S, Zondag GC, Vervaet G, et al. (2013) Substitutions near the receptor binding site determine major antigenic change during influenza virus evolution. Science 342: 976–979. 10.1126/science.1244730 [DOI] [PubMed] [Google Scholar]
- 42.Butler J, Hooper KA, Petrie S, Lee R, Maurer-Stroh S, Reh L, et al. (2014) Estimating the fitness advantage conferred by permissive neuraminidase mutations in recent oseltamivir-resistant A(H1N1)pdm09 influenza viruses. PLoS Pathog 10: e1004065 10.1371/journal.ppat.1004065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bloom JD, Gong LI, Baltimore D (2010) Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science 328: 1272–1275. 10.1126/science.1187816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vigerust DJ, Shepherd VL (2007) Virus glycosylation: role in virulence and immune interactions. Trends Microbiol 15: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bush RM, Bender CA, Subbarao K, Cox NJ, Fitch WM (1999) Predicting the evolution of human influenza A. Science 286: 1921–1925. [DOI] [PubMed] [Google Scholar]
- 46.Buecher C, Mardy S, Wang W, Duong V, Vong S, Naughtin M, et al. (2010) Use of a multiplex PCR/RT-PCR approach to assess the viral causes of influenza-like illnesses in Cambodia during three consecutive dry seasons. J Med Virol 82: 1762–1772. 10.1002/jmv.21891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henrickson KJ (2003) Parainfluenza viruses. Clin Microbiol Rev 16: 242–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gelfand HM, Holguin AH, Marchetti GE, Feorino PM (1963) A Continuing Surveillance of Enterovirus Infections in Healthy Children in Six United States Cities. I. Viruses Isolated during 1960 and 1961. Am J Hyg 78: 358–375. [DOI] [PubMed] [Google Scholar]
- 49.Marier R, Rodriguez W, Chloupek RJ, Brandt CD, Kim HW, Baltimore RS, et al. (1975) Coxsackievirus B5 infection and aseptic meningitis in neonates and children. Am J Dis Child 129: 321–325. [DOI] [PubMed] [Google Scholar]
- 50.Wilfert CM, Lauer BA, Cohen M, Costenbader ML, Myers E (1975) An epidemic of echovirus 18 meningitis. J Infect Dis 131: 75–78. [DOI] [PubMed] [Google Scholar]
- 51.Zhu B, Zhong JY, Xia HM, Gong ST, Xiao MS, Xie JH, et al. (2010) [Etiology of hand, foot and mouth disease in Guangzhou in 2008]. Zhonghua Er Ke Za Zhi 48: 127–130. [PubMed] [Google Scholar]
- 52.Ledermann JP, Guillaumot L, Yug L, Saweyog SC, Tided M, Machieng P, et al. (2014) Aedes hensilli as a potential vector of Chikungunya and Zika viruses. PLoS Negl Trop Dis 8: e3188 10.1371/journal.pntd.0003188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh S, Poh CL, Chow VT (2002) Complete sequence analyses of enterovirus 71 strains from fatal and non-fatal cases of the hand, foot and mouth disease outbreak in Singapore (2000). Microbiol Immunol 46: 801–808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Vaccine strains are highlighted in red. Node support was calculated with aLRT and was >0.70 for all major nodes. The 2011 samples fell within the same clade and 2012 samples fell within a different clade, all samples clustered with other sequences isolated from Cambodia.
(TIF)
Vaccine strains are highlighted in red. Node support was calculated with aLRT and was >0.70 for all major nodes. All samples fell within the same major clade that included other sequences isolated from Cambodia.
(TIF)
Vaccine strains are highlighted in red. Node support was calculated with aLRT and was >0.70 for all major nodes. The 2011 samples fell within the same clade and 2012 samples fell within a different clade.
(TIF)
Vaccine strains are highlighted in red. Node support was calculated with aLRT and was >0.70 for all major nodes. The 2011 samples fell within the same clade and 2012 samples fell within a different clade.
(TIF)
Vaccine strains are highlighted in red. Node support was calculated with aLRT and was >0.70 for all major nodes. The 2011 samples fell within the same clade and 2012 samples fell within a different clade.
(TIF)
(DOCX)
(DOCX)
The modifications were made by following the alignments of VP1 sequences of different EV71 strains collected during 2002–2011 from Thailand, Taiwan, Philippines, Vietnam, and China available in Genbank including the sequences with the accession No. JN191177-9, FJ969151, FJ969163, JQ 621835, JQ621841, AM490141-63, JQ315092, and JX203305. The primers for simultaneous detection of enteroviruses and rhinoviruses were designed in the polyprotein gene, between 5′ non-coding region (5′NCR) and VP4/VP2 regions that was previously described by Coiras et al. 2004 [29]. EV/RV-2n was modified from primer 2-EV/RV [29] for using in nested PCR reaction.
(DOCX)
(DOCX)
(DOCX)
AA substitution nomenclature is as follows; reference amino acid (A/California/7/2009), amino acid site, sample amino acid. Amino acids are numbered from the start codon of the segment (ATG:Methionine).
(DOCX)
AA substitution nomenclature is as follows; reference amino acid (A/California/7/2009), amino acid site, sample amino acid. Amino acids are numbered from the start codon of the segment (ATG:Methionine).
(DOCX)
(DOCX)
(DOCX)
(DOCX)
AA substitution nomenclature is as follows; reference amino acid (A/Victoria/361/2011), amino acid site, sample amino acid. Amino acids are numbered from the start codon of the segment (ATG:Methionine).
(DOCX)
AA substitution nomenclature is as follows; reference amino acid (A/Victoria/361/2011), amino acid site, sample amino acid. Amino acids are numbered from the start codon of the segment (ATG:Methionine).
(DOCX)
Sequencing results of the EV71 isolates based on (A) pan rhinovirus/enterovirus inner primer and (B) Sequencing results of the EV71 isolates based on an enterovirus 71 inner primer.
(DOCX)
Data Availability Statement
All sequences are available from the NCBI Genbank database (accession number(s) KU299790-KU299957).