Abstract
Lactic acid bacteria (LAB) are the primary microorganisms used to ferment maize-, sorghum- or millet-based foods that are processed in West Africa. Fermentation contributes to desirable changes in taste, flavour, acidity, digestibility and texture in gruels (ogi, baca, dalaki), doughs (agidi, banku, komé) or steam-cooked granulated products (arraw, ciacry, dégué). Similar to other fermented cereal foods that are available in Africa, these products suffer from inconsistent quality. The use of LAB starter cultures during cereal dough fermentation is a subject of increasing interest in efforts to standardise this step and guaranty product uniformity. However, their use by small-scale processing units or small agro-food industrial enterprises is still limited. This review aims to illustrate and discuss major issues that influence the use of LAB starter cultures during the processing of fermented cereal foods in West Africa.
Keywords: Lactic Acid Bacteria, Starter Cultures, Cereals, Fermented Foods, West Africa
Abstract
Bakteria asid laktik (LAB) merupakan mikroorganisma primer yang digunakan untuk menapai makanan berasaskan jagung, sorgum atau sekoi yang diproses di Afrika Barat. Proses penapaian menyumbang kepada perubahan diingini dalam rasa, perisa, keasidan, pencernaan dan tekstur dalam bubur (ogi, baca, dalaki), doh (agidi, banku, komé) ataupun produk berbutir yang dikukus (arraw, ciacry, dégué). Sama dengan produk bijirin yang ditapai yang boleh didapati di Afrika, produk-produk ini terjejas dengan masalah kualiti yang tidak konsisten. Penggunaan kultur mula LAB semasa penapaian doh bijirin merupakan subjek yang semakin menarik perhatian dalam usaha memiawaikan proses ini dan menjamin keseragaman produk. Walau bagaimanapun, pengunaan oleh unit pemprosesan secara kecilan ataupun perusahaan agro-makanan masih terbatas. Ulasan ini bertujuan untuk menggambarkan dan membincangkan isu-isu utama yang memberi kesan terhadap penggunakan kultur mula LAB semasa proses penapaian makanan bijirin di Afrika Barat.
Keywords: Bakteria Asid Laktik, Kultur Mula, Bijirin, Makanan Fermentasi, Afrika Barat
INTRODUCTION
The traditional cereal-based foods that are consumed in West Africa are processed by the natural fermentation of maize, sorghum and/or millet and are particularly important as weaning foods for infants and as dietary staples for adults. In terms of texture, the fermented cereal foods are either liquid (porridge or gruel), stiff gels (solid) or dry (fried or steam-cooked granulated products). The fermentation process is often carried out on small or household scales and are characterised by the use of simple, non-sterile equipment, random or natural inoculums, unregulated conditions, sensory fluctuations, poor durability and unattractive packaging of the processed products (Olanrewaju et al. 2009).
West African countries are experiencing rapid changes in their social and economic environments, which are associated with changes in food consumption patterns. In response to increasing rates of urbanisation, efforts are now geared towards developing small-scale facilities for the processing of fermented cereal foods, thus ensuring the quality of the finished product (Trèche et al. 2002). The modern large-scale production of fermented cereal-based foods is almost entirely dependent on the use of defined strains of microorganisms, which could replace the undefined strain mixtures traditionally used for the manufacture of these products (Klaenhammer & Fitzgerald 1994). The development and improvement of inoculants containing high concentrations of live microorganisms, referred to as starter cultures, is a subject of increasing interest in efforts to standardise the fermentation step. Many studies have focused on the characterisation of the microorganisms that are commonly used in the processing of these products (Halm et al. 1993; Hounhouigan et al. 1993; Lei & Jakobsen 2004; Vieira-Dalodé et al. 2007; Nwachukwu et al. 2010; Sawadogo-Lingani et al. 2010; Songré-Ouattara et al. 2010; Oguntoyinbo et al. 2011; Turpin et al. 2011; Adimpong et al. 2012; Oguntoyinbo & Narbad 2012; Owusu-Kwarteng et al. 2012; Ekwem 2014; Obinna-Echem et al. 2014). Such research has demonstrated that fermentation was natural and involved mixed cultures of lactic acid bacteria (LAB), yeasts and fungi. The lactic acid bacteria species identified included Lactobacillus fermentum, Lactobacillus plantarum, Lactobacillus salivarius, Lactobacillus delbrueckii, Lactobacillus amylolyticus, Lactobacillus reuteri, Lactobacillus paraplantarum, Lactococcus lactis, Leuconostoc mesenteroides, Pediococcus acidilactici, Pediococcus pentosaceus, Streptococcus gallolyticus and Weissella confusa. Species identification was performed using phenotypic tests such as cell morphology, sugar fermentation patterns and gas production from glucose, as well as molecular typing techniques, including pulsed-field gel electrophoresis, PCR-based methods and DNA sequencing. The use of isolated strains during cereal dough fermentation was reported to (1) minimise dry matter loss, (2) enhance control over the fermentation step, (3) enhance acid production or reduction in pH levels, (4) contribute to aroma and taste formation, as well as to increase the overall acceptability of the product and (5) enhance the nutritional quality of the product through the formation of preservative compounds or a reduction in mycotoxins, such as aflatoxins and fumonisins (Hounhouigan et al. 1993; Halm et al. 1996; Annan et al. 2003; Lardinois et al. 2003; Fandohan et al. 2005; Teniola et al. 2005; Agarry et al. 2010; Songré-Ouattara et al. 2010; Enwa et al. 2011; Ekwem 2014). Despite these results, the use of LAB starter cultures by small-scale processing units or small-scale industrial agro-food enterprises continues to be limited.
The industrial use of LAB starter cultures in the food industry depends on the concentration and preservation technologies employed, which are required to permit long-term delivery of stable cultures in term of viability and functional activity (Carvalho et al. 2003). Commercial starter cultures are supplied in concentrated form by freeze-drying, vacuum-drying, spray-drying, drum-drying, fluidised bed-drying or air-drying. The use of dried starter cultures has been shown to be of great benefit by small-scale processing units in Senegal (Lardinois et al. 2003; Totté et al. 2003), where spray-dried L. plantarum starter cultures facilitated a greater degree of control over millet fermentation and standardisation of arraw, ciakri or cere processing. The aim of this paper is to highlight the current knowledge on the microbiology of fermented cereal-based foods in West Africa and to examine the major issues that influence the use of LAB starter cultures during cereal dough fermentation.
TRADITIONAL FERMENTED CEREAL-BASED FOODS IN WEST AFRICA
Traditional fermented foods prepared from millet, sorghum or maize are consumed in many West African countries. The majority of these products are consumed as beverages, or for breakfast or as snack foods, while a few are consumed as staples and used as child-weaning foods (Table 1). Generally, treatments such as drying, dehulling, washing, soaking, grinding and sieving are some of the steps applied during the processing of fermented gruels, whereas milling and sieving are required as pre-fermentation steps during the production of dry foods, such as bread. These cereal-based fermented foods can be classified based on either the raw cereal materials used or the texture of the fermented product.
Table 1:
Most common cereal-based fermented foods from West Africa.
Notes: Data with the same letter within a column are identical.
Raw material used: (a) maize, sorghum or millet, (b) maize, (c) millet, (d) sorghum.
Nature of use: (a) dough as staple, (b) porridge as staple, (c) basis for preparation of many dishes, (d) porridge as weaning food, (e) beverage for many adults, (f) fried cake as staple for breakfast and snack item.
Country of production: (a) Ghana, (b) Nigeria, (c) Benin, (d) Côte d’Ivoire, (e) Burkina-Faso, (f) Togo.
Classification based on the raw cereal materials:
maize-based foods, e.g., banku, kenkey, mawè, agidi;
millet-based foods, e.g., ben-saalga, dégué, arraw, dagnan;
sorghum-based foods, e.g., ogi, kunun-zaki, komé, gowé.
Classification based on the texture of the fermented product:
liquid (gruel and porridges), e.g., ogi, baca, ben-saalga, dalaki;
solid (dough and dumplings), e.g., kenkey, akidi, banku, komé;
dry (baked, fried and steam-cooked granulated products), e.g., arraw, dégué, masa, wômi.
Gruels and Porridges
The fermented cereal porridges consumed in West African countries vary greatly in consistency, from stiff and dry (e.g., mashed potatoes) to thin gruels. The differences in consistency can be attributed to differences in the solids content (the proportion of flour/meal to water used in preparation, 8%–10% total solids), their preparation and the degree to which the starch has been hydrolysed. They are eaten with bean cake or other protein-rich foods. Acidic stiff porridges, such as dalaki, are made in Nigeria by sourdough fermentation (Blandino et al. 2003). Ogi, koko, ben-saalga, baca and arraw, for example, are generally consumed as breakfast by adults and used as weaning foods in Benin, Nigeria, Ghana, Burkina-Faso, Côte d’Ivoire and Senegal (Broutin 2003; Lestienne 2004; Brou et al. 2008; Soro-Yao et al. 2013, 2014).
Gels/dough and Dumplings
Akidi, akamu, banku, dagnan, fura, kenkey and komé are popular types of dough and dumplings in West African countries and are produced mainly from millet, maize or sorghum grains blended with spices and water, with the resulting fermented slurry compressed into balls and cooked. The cooked dough balls can be broken up, made into porridge by mixing with yoghurt, fresh milk, sugar or water and consumed as a rather lumpy soup. In this mixture, milk is a source of protein, and the millet, maize or sorghum ball provide carbohydrates. The sour taste of the cooked dough balls is particularly suited for quenching thirst (Owusu-Kwarteng et al. 2012; Obinna-Echem et al. 2014).
Steam-cooked Granulated Products
These products result from the agglomeration or granulation of moistened flour or fermented dough and are sometimes steamed and then dried. Examples include dishes such as couscous, cere or cakry in Senegal, aklui in Benin and dégué in Burkina-Faso. Such foods are distinguished by the fineness of particle size, the existence and the length of a fermentation step and whether an initial steaming is performed. Couscous, the finest and most common granulated product, is often fermented in Senegal (Broutin 2003). Granulated products of average size, such as cacry in Senegal and dégué in Burkina Faso and Mali, are used to make, for example, porridge from curdled milk. Granulated products of larger sizes are not cooked before drying. Their processing may include a fermentation step that is more or less similar to that of couscous, if the cereal grains used are washed before they are ground into flour, which is often stored overnight and then granulated. The granulated product is not parboiled before drying, and fermentation which takes approximately 30 hours, can occur at the beginning of the drying step (Broutin 2003).
Baked or Fried Products
Millet or sorghum flour can be fermented to prepare many types of breads or cakes. Mixing their cereal flour with water makes a type of dough that can be fermented and then fried to make several types of cakes popular in West African countries (Nkama 1993). Massa and wômi are traditional fried cakes that are made from sorghum, maize or millet in Nigeria and Côte d’Ivoire, respectively (Lestienne 2004; Soro-Yao et al. 2013). These cakes are consumed in various forms by people of all ages. A crisp, brown edge and a mildly sour taste are the desired attributes of masa (Ayo et al. 2008). Neither sorghum nor millet contain gluten proteins, so small amounts of wheat flour are added to the mix to produce yeast-leavened breads. The amount of wheat flour substituted varies depending on the quality of the wheat flour, the baking procedure, the quality of sorghum or millet flour and the desired product.
IMPORTANCE OF FERMENTED CEREAL-BASED FOODS IN WEST AFRICA: AN UPDATE
Healthy and Safe Products
Lactic acid fermentation enhances the shelf life of the fermented product, most likely because the diverse array of antimicrobial metabolites produced during the fermentation process (Nout 1994). These metabolites include many organic acids, such as lactic, acetic and propionic acids produced as end products, which create an acidic environment unfavourable for the growth of many pathogenic and food-spoilage microorganisms (Caplice & Fitzgerald 1999). In addition to acids, LAB can produce a range of other antimicrobial metabolites, including ethanol generated via the heterofermentative pathway, H2O2 produced during aerobic growth, diacethyl formed from excess citrate-derived pyruvate and bacteriocins, which are ribosomally synthesised antimicrobial compounds (Diop et al. 2010). Lantibiotics, a special class of bacteriocins produced by LAB and other Gram-positives, have been widely studied. Nisin is a well-known lantibiotic produced by Lactococcus lactis and widely used as a food preservative. Its activity is based on the permeabilisation of the cytoplasmic membrane, leading to its depolarisation (Hyde et al. 2006). Plantaricin and pediocin are other bacteriocins distributed among L. plantarum and pediococci species (Diep et al. 2006; Wiedemann et al. 2006), respectively. An inhibition of Escherichia coli and Staphylococcus aureus species was observed during ogi fermentation with a bacteriocin-producing Lactobacillus (Olasupo et al. 1997) and after incubation with Lactobacillus species isolated from akamu (Ekwem 2014), respectively.
Probiotic and Prebiotic Potentials
There is growing awareness in West Africa of the health benefits associated with probiotic foods. The term ‘probiotic’ refers to a product that contains mono or mixed cultures of live microorganisms, which, when ingested, improves host health by enhancing microbial balance [Food and Agriculture Organisation/World Health Organisation (FAO/WHO 2001)]. Most probiotic organisms used in human food belong to the genera Lactobacillus or Bifidobacterium (Herbel et al. 2013). Escherichia coli Nissle 1917, L. lactis, S. thermophilus and Enterococcus faecium are also often used as probiotics in human and animal nutrition. Recent research has shown that some fermented cereal-based foods available in West Africa may have probiotic potential. Based on laboratory trials, Lei and Jacobsen (2004) found that LAB isolated from koko can withstand the physiological challenges posed by the gastrointestinal tract (GIT) and may be able to colonise the GIT. In controlled human trials, Lei et al. (2006) demonstrated that koko sour water (KSW) reduces diarrhoea in children. Faecal enteric bacteria, such as Salmonella, Shigella and E. coli, were found to be significantly less prevalent in children fed fermented maize gruel than in children who were not (Mensah et al. 1991; Tetteh et al. 2004). A Lactobacillus starter culture was used to produce an improved ogi called DogiK, which exhibited antimicrobial properties against some diarrhoeagenic bacteria (Olukoya et al. 1994). The use of cereal constituents in probiotic food formulation as fermentable substrates for LAB starter cultures, encapsulation material, and/or dietary fibre supplementation have been explored (Venter 2007; Arena et al. 2014). Water-soluble and insoluble β-glucan, arabinoxylans, oligosaccharides and resistant starch are cereal indigestible but fermentable dietary carbohydrates, which are used to grow probiotic LAB and could be used to realise the beneficial effect of both the probiotic and prebiotic effects (Lamsal & Faubion 2009).
Nutritional and Health Benefits
The products made from millet, maize or/and sorghum dough contribute to the protein requirements of West African peoples and are particularly important as weaning foods for children and as dietary staples for adults (Fig. 1, FAO 2012). Natural fermentation of cereals leads to a decrease in the level of carbohydrates, as well as some non-digestible poly- and oligosaccharides. Lactic acid fermentation also provides optimum pH conditions for enzymatic degradation of phytate, which is present in cereals in the form of complexes with polyvalent cations (such as iron, zinc, calcium, magnesium and proteins) (Coulibaly et al. 2011). The fermented gruel used as weaning food is usually inadequate in several nutrients, leading to widespread protein malnutrition during the weaning period (Ugwu 2009). Various attempts for nutrient restoration and supplementation of ogi, such as blending with fermented and unfermented legumes, adding pawpaw slurry at various levels of substitution (Otunola et al. 2006) and co-fermenting with cowpea (Oyarekua 2011), have been made. Additionally, the use of starter cultures to hydrolyse starch with the aim of increasing the energy value of cereal gruel was explored (Songré-Ouattara et al. 2009, 2010). Fermented cereal products have also been used as foods with enhanced heath properties. Probiotic bacteria are able to change the population of the gut microbiota by influencing the metabolic and nutritional functions of commensal bacteria (Ciorba 2012). Indirect and/or direct immune modulating capacities of probiotic bacteria through antigen production, modulation of sensory motor functions, an enhancement of mucosal barrier functions and/or production of anti-pathogenic effects have also been reported (Ciorba 2012). Lactobacillus pentosus, Lactobacillus brevis and other probiotic bacteria were able to bind aflatoxin B1, the most potent mycotoxin among various aflatoxins, which may be formed during storage of cereal grains (Hamidi et al. 2013; Zoghi et al. 2014).
Figure 1:
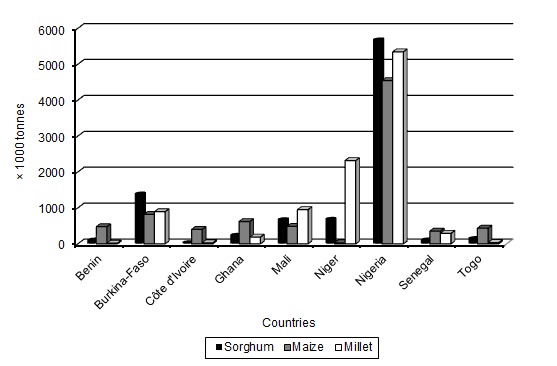
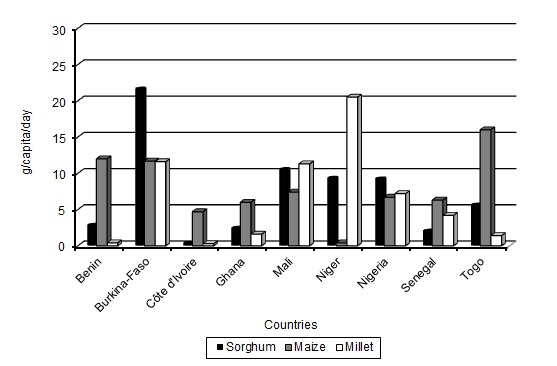
Millet, maize and sorghum’s (a) food supply quantity or (b) protein supply, in 2009.
USES OF LACTIC ACID BACTERIA STARTER CULTURES DURING CEREAL DOUGH FERMENTATION
Knowledge of the microbiology of fermenting cereal dough is essential for the development of starter cultures. The microbiology of many West African fermented cereal products – including the maize products mawè, ogi and koko sour water (Hounhouigan et al. 1993; Oguntoyinbo et al. 2011; Adimpong et al. 2012), the sorghum products gowè, kunun-zaki and ogi-baba (Odunfa & Adeyele 1985; Viera-Dalodé et al. 2007; Oguntoyinbo & Narbad 2012) and the millet products arraw, ben-saalga and akamu (Totté et al. 2003; Songré-Ouattara et al. 2008; Nwachukwu et al. 2010; Turpin et al. 2011) – have been examined. These analyses have shown that the fermentation process is natural and involves mixed cultures of LAB, yeasts, fungi and Bacillus species. LAB species of the genera Lactobacillus, Leuconostoc, Weissella, Streptococcus and Pediococcus, as well as yeast species of the genera Saccharomyces, Candida and Kluyeromyces, have been identified as the dominant microorganisms in the fermentation process of cereal-based foods (Table 2). The isolated LAB species have been used, often in combination with yeast species, as starter cultures in controlled laboratory trials (Table 3). Overall, higher lactic acid production, rapid acidification, superior product shelf life, improved organoleptic properties and a greater degree of control over the fermentation process have been achieved with the use of starter cultures compared to traditional processes (i.e., without the use a selected starter culture). Although many laboratory trials using LAB starter cultures have been undertaken, reports on fermented cereal-based foods produced with the identified and selected strains are extremely scarce. However, a collaborative project between Belgian cooperation and the Institute of Food Technology (ITA) in Dakar (Senegal) has been carried out in order to improve millet fermentation. In this research, spray-dried L. plantarum conditioned in millet flour was used as the starter culture during millet fermentation for arraw production. The project has led to the development of an improved processing of steam-cooked granulated products by small-scale urban processing units (Lardinois et al. 2003; Totté et al. 2003). Furthermore, this work showed that spray-drying was a cost-effective way of producing industrial-scale quantities of viable microorganisms and for long-term preservation of LAB starter cultures. The use of dried starter cultures could be of great benefit to the small-scale processing units in West Africa, considering the economic costs of and technical expertise required for production. A large majority of fermented cereal-based foods consumers in most West African countries are poor and disadvantaged, and as such, price, rather than food safety and quality, is their primary concern when purchasing food. In addition to economic considerations, the availability and the maintenance of the starter cultures could be suggested as another major factor; large quantities of starter cultures in active and pure forms are essential to the success of the fermentation stage of product manufacture. This can be achieved through the careful propagation of the inoculums. Propagation of starter cultures is time consuming, laborious, requires skilled personnel and is more prone to contamination, and necessitates significant investments in equipment (e.g., laboratory equipment, fermentors). Furthermore, contamination may occur during the process of culture production, which may result in poor growth of the LAB and defective products. Finally, dried starter cultures are easier to use and maintain, as they remain stable for up to two years (Brandt 2014).
Table 2:
Predominant fermenting microorganisms of some cereal-based fermented foods from West Africa.
| Lactic acid bacteria and other microorganisms | Source of isolation | Raw material used | References |
|---|---|---|---|
| L. fermentum, L. reuteri and Candida spp., Saccharomyces spp. | Kenkey or koko | a | Halm et al. 1993 |
| L. fermentum, L. reuteri, L. brevis | Mawè | a | Hounhouigan et al. 1993 |
| P. acidilactici, W. confusa, L. fermentum, L. reuteri, L. salivarius, L. paraplantarum | Fura | b | Owusu-Kharteng et al. 2012 |
| W confusa, L fermentum, L. salivarius, Pediococcus spp. | Koko sour water | c |
Lei and Jakobsen 2004; Adimpong et al. 2012 |
| L. plantarum | Arraw, cere or cakry | b | Totté et al. 2003 |
| L. plantarum, L. pentosus, L. celibiosus, P. pentosaceus, L. mesenteroides | Akamu | c | Nwachukwu et al. 2010 |
| L. acidophilus, L. delbrueckii, L, lactis, L. casei, L. fermentum, L. bulgaricus, L. plantarum | Ekwem 2014 | ||
| L. plantarum 4.4, L. plantarum 6.1, L. fermentum 11.11.2, L. fermentum 3.7, L. fermentum 7.4 | Ben-saalga | b |
Songré-Ouattara et al. 2008; Turpin et al. 2011 |
| L. plantarum and B. subtilis L. fermentum, L. plantarum, S. gallolyticus subsp, macedonicus, P. pentosaceus W. confusa | Kunun-zaki | Sorghum or millet | Inyang and Dabot 1997 ; Oguntoyinbo et al. 2011; Oguntoyinbo and Narbad 2012 |
| L. fermentum, P. acidilactici, W. confusa | Malted sorghum grains | d | Sawadogo-Lingani et al. 2010 |
| L. fermentum, W. confusa, L. mucosae, P. acidilactici and Kluyveromyces marxianus, Pichia anomala | Gowé | d | Viera-Dalodé et al. 2007 |
| Lactococcus raffinolactis, Pediococcus sp., P. pentosaceus, L. plantarum, L. suebicus, L. brevis | Ogi | c | Teniola et al. 2005 |
| L. plantarum, L. brevis, P. pentosaceus and B.subtilis, Candida valida, Candida krusei, Geotrichum candidum | Teniola and Odunfa 2002 | ||
| L. plantarum, L. pantheris, L. vaccinostercus | Ogi-baba | d | Oguntoyinbo et al. 2011 |
| L. plantarum, S. lactis and C. krusei | Odunfa and Adeyele 1985 |
Notes: Data with the same letter within a column are identical.
Raw material used: (a) maize, (b) millet (c) millet, maize or sorghum (d) sorghum.
FACTORS ENABLING THE DEVELOPMENT OF DRIED LACTIC ACID STARTER CULTURES FOR CEREAL DOUGH FERMENTATION
Fermented cereal-based foods provide West African consumers with an affordable source of food and contribute to their food and nutritional security. Growing incomes and improved levels of education in urban centres across some West African countries are leading to changes in dietary habits and a wider variety of foods being consumed. As a result, fermented foods are no longer the main staples in many areas, but are still consumed as side dishes or condiments. Fermented foods offer many opportunities for diversification as a result of their global popularity; demand for many fermented cereal-based products is increasing worldwide, as they suit social and cultural culinary traditions in many parts of the world. Rural or urban dwellers are reassured that the product may be safer to consume than other food and beverages, as a result of fermentation. Increasing international travel due to globalisation has changed the eating habits of consumers across the globe. Moreover, export markets have expanded the potential consumer base for fermented foods beyond simply meeting the needs of developing countries. Markets catering to the growing international demand for niche and ethnic products are opening up as well. The need to assure the safety and quality of fermented food products for international consumers and the demand of West African consumers for safe, high-quality food have been driving forces for the development of agro-food processing companies in Africa. Several reviews have focused on the rapid evolution of the food-processing sector in West Africa (Trèche et al. 2002; Aworth 2008; Broutin & Subsol 2011). Small Agro-Food Industrial (SAFI) enterprises are positioned between the diffuse sector of the informal food processing micro-enterprises and the large-scale food industries. West African SAFI have evolved in tandem with the increasing urbanisation that is occurring in 15 West African countries [Economic Community Of West African States (ECOWAS 2014)] by their marginal food security and nutritional status. SAFI enterprises have two major functions, (1) to meet an increasing consumer demand for safe food products and (2) to improve the supply of nutritious food products that will aid in reducing public health problems, such as infant malnutrition, micronutrient deficiency and food safety. An increasing number of successful initiatives have been carried out in West Africa to improve the processing of fermented products. For example, in Senegal, Benin and Nigeria, millet, maize or sorghum requires short processing times, and products such as arraw, cacry, cere, kunun-zaki, mawé and ogi are now found in most markets and supermarkets in the form of vacuum-sealed packets (Trèche et al. 2002).
SELECTION CRITERIA OF DRIED LACTIC ACID BACTERIA STARTER CULTURES FOR CEREAL DOUGH FERMENTATION
Technological effectiveness must be considered when selecting LAB strains for cereal fermentation. Selection criteria for LAB depend on the desired characteristics of the final product, the desired metabolic activities, the characteristics of the raw materials and the applied technology.
Ability to Realise Fast Acidification and Production of Antimicrobial Compounds
Food preservation by lactic fermentation relies on the removal of fermentable carbohydrates, the consumption of oxygen, the formation of organic acids and a concomitant decrease in pH. The immediate and rapid production of sufficient quantities of organic acids to reduce pH below 4.0 within 24 h of fermentation is an essential requirement of fermented cereal-based foods (Hounhouigan et al. 1993, 1999; Annan et al. 2003; Viéira-Dalodé et al. 2008). Lactic acid bacteria microbial antagonism could be attributed to the production of organic acids, ethanol, diacetyl, hydrogen peroxide or carbon dioxide, alone or in combination, and could further result from the production of bacteriocins (De Vuyst & Vandamme 1994). The rapid production of these compounds may contribute to the inhibition of pathogenic or spoilage flora and thus enhance the shelf life and microbial safety of the fermented product (Omemu & Faniran 2011; Okerere et al. 2012; Ekwem 2014).
Ability to Dominate the Indigenous Microbiota
The ability of LAB to dominate the indigenous population during cereal dough fermentation is another important characteristic of a starter culture. The dominance of the starter culture would be exerted by its fast and predominant growth under fermentation conditions and/or its ability to produce antagonistic substances, such as bacteriocins. Huch et al. (2008) reported the use of molecular fingerprinting techniques e.g., Random Amplified Polymorphic DNA with Polymerase Chain Reaction (RAPD-PCR) and Pulsed-field Gel Electrophoresis (PFGE), to amplify the growth of a selected freeze-dried LAB starter culture during cassava fermentation for gari production.
Ability to Exert Probiotic Effects
Human trials to test the tangible physiological/health benefits of LAB present a difficult challenge. In vitro studies could be used to examine potential probiotic characteristics, such as antimicrobial properties and survival in acids and bile, as well as lactic acid, hydrogen peroxide, biosurfactant and bacteriocin production (Bayane et al. 2006; Anukam & Reid 2009). In addition to these characteristics, potential probiotic lactic acid bacteria should adhere to the epithelial tissue, colonise the GIT, stimulate a host immune response, influence metabolic activities such as vitamin production and compete with pathogenic microorganisms, thereby preventing their survival in the GIT (Kalui et al. 2010). Probiotic bacteria prevent growth of pathogenic microorganisms through competition, exclusion and the production of organic acid and antimicrobial compounds. Acid and bile tolerance are two fundamental properties that demonstrate the ability of probiotic microorganism to survive passage through the upper gastrointestinal tract.
Ability to Improve the Nutritional Quality of the Fermented Food
Millet, sorghum and maize have significant amounts of inositol hexaphosphates (IP6), referred to as phytic acid or phytates. Phytates have been recognised as antinutritional factors that affect the bioavailability of both major minerals, such as calcium and phosphorus, and trace minerals, such as zinc, iron, copper and manganese. In West African countries, the low bioavailability of minerals (e.g., iron and zinc) in cereal-based foods is a crucial problem for child nutrition (Camara & Amaro 2003). Other antinutrients of importance in cereal grains are tannins and α-galacto-oligo-saccharides (α-GOS) e.g., stachyose and raffinose. Decreasing the amount of phytic acid or tannins and metabolising stachyose or raffinose will be very helpful, due to their influence on the nutritional quality of cereal grains; thus, a phytase, α-galactosidase or tannase producing LAB will be useful during cereal dough fermentation. In addition to these characteristics, the ability of LAB strains to bind mycotoxin such as aflatoxin, which may form during the storage of cereal grains, could be explored.
Ability to Hydrolyse Starch
The use of amylolytic LAB to hydrolyse starch with the aim of increasing the energy density of cereal gruels could be explored (Songré-Ouattara et al. 2009). Amylolytic LAB may decrease the viscosity of bulk, starchy weaning gruel, which may improve nutrient density while maintaining an acceptable thickness for feeding young children (FAO/WHO 1995).
Ability to Have Good Stability during Production and Storage
The stability of dried LAB refers to its viability and metabolic activity (acidifying activity). The suitability of LAB starter cultures for large-scale production and their stability during drying and long-term storage are important criteria for starter culture selection (Yao et al. 2009a). Cultures must be able to withstand large-scale fermentation, drying and long-term storage in the dried form. Proper packaging for long-term storage of dried cultures is important. The dried culture should contain more than 95% dry matter and be stored at low temperature (4°C), vacuum-sealed, and protected from light and moisture to avoid loss of viability and metabolic activity (Yao et al. 2008, 2009b; Coulibaly et al. 2009). To achieve health benefits, probiotic bacteria should be viable in the range of approximately 106–107 cfu/g of product during consumption (Lamsal & Faubion 2009). Important factors such as pH, post-acidification, production of hydrogen peroxide, oxygen level, temperature, food matrix and interaction with the starter organisms affect LAB viability during refrigerated storage of fermented products (Dave & Shah 1997).
CONCLUSION AND FUTURE PROSPECTS
Lactic acid fermentation contributes to the safety, health, organoleptic, technological and nutritional properties of fermented cereal-based foods of West Africa. The challenges here relate to the stability, reproducibility and productivity of fermentations. L. fermentum, L. plantarum, L. salivarius, L. delbrueckii, L. amylolyticus, L. reuteri, L. paraplantarum, Lact. lactis, Leuc. mesenteroides, P. acidilactici, P. pentosaceus, Str. gallolyticus and W. confusa species have been identified in efforts to standardise the fermentation step. The use of LAB starter cultures has largely been restricted to laboratory applications and has not been transferred to industrial applications. Dried LAB starter cultures are easier to use and offer excellent possibilities for greater control over the fermentation process. The question “which starter culture technology for which production cost?” summarises the future directions for the application of dried starter cultures in West Africa. Careful selection of dried LAB starter cultures for cereal fermentation should take into account their ability to (1) realise fast acidification and produce antimicrobial compounds, (2) dominate the indigenous microbiota, (3) exert probiotic effects, (4) improve the nutritional quality of the fermented product, (5) hydrolyse starch, and (6) be stable during production and storage. To date, only a handful of studies have examined the health-related properties of West African fermented cereal-based foods and their potential use as probiotic foods. Fermented cereal-based foods offer opportunities to include probiotics, prebiotics and fibres into the diets of West African consumers (Lamsal & Faubion 2009). Millet, maize and sorghum grain represent examples of such opportunities. Cereal grain and cereal components could be used as probiotic carriers with the added advantage of providing healthful bioactive components and fibres. Furthermore, commitments to probiotics research and in vivo or in vitro trials of both identified and potential probiotic foods must be made and pursued, respectively.
Acknowledgments
We thank the International Foundation for Science (IFS, grant number E/4953-1/2) and The World Academy of Sciences (TWAS, grant FR:3240262723) for literature acquisition.
REFERENCES
- Aboua F, Nemlin J, Kossa A, Kamenan A. Transformation traditionnelle de quelques céréales cultivées en Côte d’Ivoire. Colloque International de Technologie: Céréales en régions chaudes.; Centre universitaire de N’gaoundéré N’gaoundéré, Cameroon. 22–26 February 1988; France: John Libbey Eurotext; 1989. [Google Scholar]
- Adimpong B, Nielsen DS, Sørensen KI, Derkx PMF, Jespersen L. Genotypic characterization and safety assessment of lactic acid bacteria from indigenous African fermented products. BMC Microbiology. 2012;12:75–89. doi: 10.1186/1471-2180-12-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarry OO, Nkama I, Akoma O. Production of kunun-zaki (a Nigerian fermented cereal beverage) using starter culture. International Research Journal of Microbiology. 2010;1(2):18–25. [Google Scholar]
- Ali KL, Djalé A. Substitution partielle de la farine de blé ou autres farines par la farine de sorgho en boulangerie, biscuiterie et produits locaux (‘Ablo’, ‘kome’). In: Akintayo I, Sedgo J, editors. Proceedings of a Technical Workshop of the West and Central Africa Research Network: Towards Sustainable Sorghum Production, Utilization, and Commercialization in West Africa; International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Lomé, Togo. 19–22 April 1999; Andhra Pradesh, India: ICRISAT; 2001. [Google Scholar]
- Annan NT, Poll L, Sefa-Dedeh S, Plahar WA, Jakobsen M. Volatile compounds produced by Lactobacillus fermentum, Saccharomyces cerevisiae and Candida krusei in single starter culture fermentations of Ghanaian maize dough. Journal of Applied Microbiology. 2003;94(3):462–474. doi: 10.1046/j.1365-2672.2003.01852.x. [DOI] [PubMed] [Google Scholar]
- Annan-Prah A, Agyeman JA. Nutrient content and survival of selected pathogenic bacteria in kenkey used as a weaning food in Ghana. Acta Tropica. 1997;65(1):33–42. doi: 10.1016/s0001-706x(97)00650-5. [DOI] [PubMed] [Google Scholar]
- Anukam KC, Reid G. African traditional foods and probiotics. Journal of Medicinal Food. 2009;12(6):1177–1184. doi: 10.1089/jmf.2008.0163. [DOI] [PubMed] [Google Scholar]
- Arena MP, Caggianiello G, Fiocco D, Russo P, Torelli M, Spano G, Capozzi V. Barley β-glucans-containing food enhances probiotic performances of beneficial bacteria. International Journal of Molecular Sciences. 2014;15(2):3025–3039. doi: 10.3390/ijms15023025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aworth OC. The role of traditional food processing technologies in national development: the West African experience. Oakville, Ontario, Canada: International Union of Food Science and Technology; 2008. http://www.iufost.org/publications/books/documents/Revd.pdf (accessed on 18 April 2014) [Google Scholar]
- Ayo JA, Agu H, Famoriyo OF. Effect of different cereals on the quality of Masa. Pakistan Journal of Nutrition. 2008;7(4):530–533. [Google Scholar]
- Bayane A, Roblain D, Dauphin RD, Destain J, Diawara B, Thonart P. Assessment of the physiological and biochemical characterization of a Lactic acid bacterium isolated from chicken faeces in sahelian region. African Journal of Biotechnology. 2006;5(8):629–634. [Google Scholar]
- Blandino A, Al-Aseeri ME, Pandiella SS, Cantero D, Webb C. Cereal-based fermented foods and beverages. Food Research International. 2003;36(6):527–543. [Google Scholar]
- Brandt MJ. Starter cultures for cereal based foods. Food Microbiology. 2014;36(6):527–543. doi: 10.1016/j.fm.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Brou K, Gbogouri A, Ocho AL, Djéni NT, Kone Y, Gnakri D. Assessment of some chemical and nutritional properties of maize, rice and millet grains and their weaning mushes. Pakistan Journal of nutrition. 2008;7(6):721–725. [Google Scholar]
- Broutin C. Produits et procédés: Les produits roulés. In: Professionals for Fair Development (GRET), editor. Transformer les céréales pour les nouveaux marchés urbains: Opportunités pour les petites entreprises en Afrique. Paris: GRET; 2003. pp. 89–98. [Google Scholar]
- Broutin C, Subsol S. Pour une politique de services aux transformatrices et transformateurs de céréales locales. Grain de sel. 2011;54–56:53–55. [Google Scholar]
- Camara E, Amaro MA. Nutritional aspect of zinc availability. International Journal of Food Sciences and Nutrition. 2003;54(2):143–151. doi: 10.1080/0963748031000084098. [DOI] [PubMed] [Google Scholar]
- Caplice E, Fitzgerald GF. Food fermentations: Role of microorganisms in food production and preservation. International Journal of Food Microbiology. 1999;50(1):131–149. doi: 10.1016/s0168-1605(99)00082-3. [DOI] [PubMed] [Google Scholar]
- Carvalho AS, Silva JH, Teixera P, Malcata FX, Gibbs P. Impedimetric method for estimating the residual activity of freeze-dried Lactobacillus delbrueckii ssp. bulgaricus. International Dairy Journal. 2003;13(6):463–468. [Google Scholar]
- Ciorba MA. A gastroenterologist’s guide to probiotics. Clinical Gastroenterology and Hepatology. 2012;10(9):960–968. doi: 10.1016/j.cgh.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coda R, Di Cagno R, Rizzello CG, Nionelli L, Edema MO, Gobbetti M. Utilization of African grains for sourdough bread making. Journal of Food Science. 2011;76(6):M329–M335. doi: 10.1111/j.1750-3841.2011.02240.x. [DOI] [PubMed] [Google Scholar]
- Coulibaly A, Kouakou B, Chien J. Phytic acid in cereal grains: Structure, healthy or harmful ways to reduce phytic acid in cereal and their effects on nutritional quality. American Journal of Plant Nutrition and Fertilization Technology. 2011;1(1):1–22. [Google Scholar]
- Coulibaly I, Yao AA, Lognay G, Destain J, Fauconnier ML, Thonart P. Survival of freeze-dried Leuconostoc mesenteroides and Lactobacillus plantarum related to their cellular fatty acids composition during storage. Applied Biochemistry and Biotechnology. 2009;157(1):70–84. doi: 10.1007/s12010-008-8240-1. [DOI] [PubMed] [Google Scholar]
- Dave RI, Shah NP. Viability of yoghurt and probiotic bacteria in yoghurts made from commercial starter culture. International Dairy Journal. 1997;7(1):31–41. [Google Scholar]
- De Vuyst L, Vandamme EJ. Antimicrobial potential of lactic acid bacteria. In: Leroy AF, De Vuyst L, editors. Bacteriocins of lactic acid bacteria. London: Blackie Academic and Professional; 1994. pp. 91–142. [Google Scholar]
- Diep DB, Godager L, Nes DF. Data mining and characterization of a novel pediocin-like bacteriocin system from the genome of Pediococcus pentosaceus ATCC 25745. Microbiology. 2006;152(6):1649–1659. doi: 10.1099/mic.0.28794-0. [DOI] [PubMed] [Google Scholar]
- Diop MB, Destain J, Tine E, Thonart P. Les produits de la mer au Sénégal et le potentiel des bactéries lactiques et des bactériocines pour la conservation. Biotechnologie Agronomie Société et Environnement. 2010;14(2):341–350. [Google Scholar]
- Economic Community of West African States (ECOWAS) ECOWAS member states. 2014. http://ecowas.int/ (accessed on 13 November 2014)
- Ekwem OH. Isolation of antimicrobial producing lactobacilli from akamu (a Nigerian fermented cereal gruel) African Journal of Microbiology Research. 2014;8(7):718–720. [Google Scholar]
- Enwa FO, Beal J, Arhewoh MI. Effect of maize and bacteria starter culture on maize fermentation process. International Journal of Biomedical Research. 2011;2(11):561–567. [Google Scholar]
- Fandohan P, Zoumenou D, Hounhouigan DJ, Marasas WF, Wingfield MJ, Hell K. Fate of aflatoxins and fumonisins during the processing of maize into food products in Benin. International Journal of Food Microbiology. 2005;98(3):249–259. doi: 10.1016/j.ijfoodmicro.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organisation (FAO) Food and Agriculture Organisation statistical data (FOSTAT) Rome: FAO; 2012. http://faostat3.fao.org/home/index.html#VISUALIZE_BY_DOMAIN (accessed on 18 April 2014) [Google Scholar]
- Food and Agriculture Organisation (FAO) Maïs. In: FAO, editor. Utilisation des aliments tropicaux: Céréales. Rome: FAO; 1990. pp. 87–114. [Google Scholar]
- Food and Agriculture Organisation/World Health Organisation (FAO/WHO) Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Geneva: WHO; 2001. http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf (accessed on 18 April 2014) [Google Scholar]
- Food and Agriculture Organisation/World Health Organisation (FAO/WHO) Fermentation, assessment of benefits and risks. In: FAO/WHO, editor. Proceedings of the Joint FAO/WHO Workshop on Assessment of Fermentation as a Household Technology for Improving Food Safety; Pretoria, South Africa. 11–15 December 1995; Geneva: WHO; 1995. [Google Scholar]
- Guyot JP, Mouquet C, Tou EH, Counil E, Traore AS, Trèche S. Study of the processing of pearl millet (Pennisetum glaucum) into ben-saalga, a fermented gruel from Burkina-Faso. In: International Relief and Development (IRD), editor. Proceedings of the 2nd International Workshop in Food-based Approaches for a Healthy Nutrition; Ouagadougou, Burkina Faso. 23–28 November 2003; Arlington, US: IRD; 2004. [Google Scholar]
- Halm M, Osei-Yaw A, Hayford A, Kpodo KKA, Amoa-Awua WKA. Experiences with the use of a starter culture in the fermentation of maize for ‘kenkey’ production in Ghana. World Journal of Microbiology and Biotechnology. 1996;12(5):531–536. doi: 10.1007/BF00419468. [DOI] [PubMed] [Google Scholar]
- Halm M, Lillie A, Sorensen AK, Jakobsen M. Microbiological and aromatic characteristics of fermented maize doughs for kenkey production in Ghana. International Journal of Food Microbiology. 1993;19(2):135–143. doi: 10.1016/0168-1605(93)90179-k. [DOI] [PubMed] [Google Scholar]
- Hama F, Savadogo A, Ouattara CAT, Traore A. Biochemical, microbial and processing study of Dèguè a fermented food (from pearl millet dough) from Burkina Faso. Pakistan Journal of Nutrition. 2009;8(6):759–764. [Google Scholar]
- Hamidi A, Mirnejad R, Yahaghi E, Behnod V, Mirhosseini A, Amani S, Sattari S, Darian EK. The aflatoxin B1 isolating potential of two lactic acid bacteria. Asian Pacific Journal of Tropical Biomedicine. 2013;3(9):732–736. doi: 10.1016/S2221-1691(13)60147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbel SR, Vahjen W, Wieler LH, Guenther S. Timely approaches to identify probiotic species of the genus Lactobacillus. Gut Pathogens. 2013;5:27–40. doi: 10.1186/1757-4749-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde AJ, Parisot J, McNichol A, Bonev BB. Nisin-induced changes in Bacillus morphology suggest a paradigm of antibiotic action. Proceedings of the National Academy of Sciences. 2006;103(52):19896–19901. doi: 10.1073/pnas.0608373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounhouigan DJ, Nout MJR, Nago CM, Houben JH, Rombouts FM. Use of starter cultures of lactobacilli and yeast in the fermentation of mawè, an African maize product. Tropical Science. 1999;39(4):220–226. [Google Scholar]
- Hounhouigan DJ, Nout MJR, Nago CM, Houben JH, Rombouts FM. Composition and microbiological and physical attributes of mawè, a fermented maize dough from Benin. International Journal of Food Science and Technology. 1993;28(5):513–517. [Google Scholar]
- Huch M, Hanak A, Specht I, Dortu C, Thonart P, Mbugua S, Holzapfel WH, Hertel C, Franz C. Use of Lactobacillus strains to start cassava fermentation for Gari production. International Journal of Food Microbiology. 2008;128(2):258–267. doi: 10.1016/j.ijfoodmicro.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Inyang CU, Dabot YA. Storability and portability of pasteurized and sterilised kunun-zaki: A fermented sorghum beverage. Journal of Food Processing and Preservation. 1997;21(1):1–7. [Google Scholar]
- Kalui CM, Mathara JM, Kutina PM. Probiotic potential of spontaneously fermented cereal based foods: A review. African Journal of Biotechnology. 2010;9(17):2490–2498. [Google Scholar]
- Klaenhammer TR, Fitzgerald GF. Bacteriophages and bacteriophage resistance. In: Gasson MJ, de Vos W, editors. Genetics and biotechnology of lactic acid bacteria. London: Backie and Son; 1994. pp. 106–168. [Google Scholar]
- Lamsal BP, Faubion JM. The beneficial use of cereal components in probiotic foods. Food Reviews International. 2009;25(2):103–114. [Google Scholar]
- Lardinois M, Totté A, Tounkara L, Mbaye CT, Beye C, Thonart P, Ngom EHA, et al. Conservation de produits locaux à travers un transfert de technologie de séchage: Cas de l’atomisation au Sénégal. In: International Relief and Development (IRD), editor. Proceedings of the 2nd International workshop in Food-based Approaches for a Healthy Nutrition; Ouagadougou, Burkina Faso. 23–28 November 2003; Arlington, US: IRD; 2003. [Google Scholar]
- Lei V, Friis H, Michaelsen KF. Spontaneously fermented millet product as a natural probiotic treatment for diarrhoea in young children, an intervention study in Northern Ghana. International Journal of Food Microbiology. 2006;110(3):246–253. doi: 10.1016/j.ijfoodmicro.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Lei V, Jakobsen M. Microbiological characterization and probiotic potential of koko and koko sour water, African spontaneously fermented millet porridge and drink. Journal of Applied Microbiology. 2004;96(2):384–397. doi: 10.1046/j.1365-2672.2004.02162.x. [DOI] [PubMed] [Google Scholar]
- Lestienne I. Contribution à l’étude de la biodisponibilité du fer et du zinc dans le grain de mil et conditions d’amélioration dans les aliments de complément. Université de Monpellier II; 2004. PhD diss. [Google Scholar]
- Mensah P, Tomkins AM, Drasa BS, Harrison TJ. Antimicrobial effect of fermented Ghanaian maize dough. Journal of Applied Bacteriology. 1991;70(3):203–210. doi: 10.1111/j.1365-2672.1991.tb02925.x. [DOI] [PubMed] [Google Scholar]
- Mestres C, Hounhouigan JD, Nago MC, Barro C. L’aklui sec: Un nouveau produit de petit déjeuner prêt à l’emploi: Expérience d’une production artisanale au Bénin. Agriculture et développement. 1999;23:108–117. [Google Scholar]
- Ndiaye C, Xu SY, Ngom PM, Ndoye AS. Malting germination effect on rheological properties and cooking time of millet (P. typhoïdes) and sorghum (S. bicolor) flours and rolled flour products (arraw) American Journal of Food Technology. 2008;3(6):373–383. [Google Scholar]
- Nkama I. Studies on improving the nutritional quality of masa Traditional Nigerian fermented-cereal based food. Mysore: Central Food Technological Research Institute (CFTRI), United Nations University; 1993. [Google Scholar]
- Nout MJR. Fermented foods and food safety. Food Research International. 1994;27(3):291–298. [Google Scholar]
- Nwachukwu E, Achi OK, Ijeoma IO. Lactic acid bacteria in fermentation of cereals for the production of indigenous Nigerian foods. African Journal of Food Science and Technology. 2010;1(2):21–26. [Google Scholar]
- Obinna-Echem PC, Kuri V, Beal J. Evaluation of the microbial community, acidity and proximate composition of akamu, a fermented maize food. Journal of the Science of Food and Agriculture. 2014;94(2):331–40. doi: 10.1002/jsfa.6264. [DOI] [PubMed] [Google Scholar]
- Odunfa SA, Adeyele S. Microbiological changes during the traditional production of ogi-baba, a West African fermented Sorghum Gruel. Journal of Cereal Science. 1985;3(2):173–180. [Google Scholar]
- Oguntoyinbo FA, Narbad A. Molecular characterization of lactic acid bacteria and in situ amylase expression during traditional fermentation of cereal foods. Food Microbiology. 2012;31(2):254–262. doi: 10.1016/j.fm.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Oguntoyinbo FA, Tourlomousis P, Gasson MJ, Narbad A. Analysis of bacterial communities of traditional fermented West African cereal foods using culture independent methods. International Journal of Food Microbiology. 2011;145(1):205–210. doi: 10.1016/j.ijfoodmicro.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Okereke HC, Achi OK, Ekwenye UN, Orji FA. Antimicrobial properties of probiotic bacteria from various sources. African Journal of Biotechnology. 2012;11(39):9416–9421. [Google Scholar]
- Olanrewaju OO, Victor OO, Titilayo TA. Safety of small-scale food fermentations in developing countries. Internet Journal of Food Safety. 2009;11:29–34. [Google Scholar]
- Olasupo NA, Olukoya DK, Odunfa SA. Assessment of a bacteriocin-producing Lactobacillus strain in the control of spoilage of a cereal-based African fermented food. Folia Microbiology. 1997;42(1):31–34. doi: 10.1007/BF02898642. [DOI] [PubMed] [Google Scholar]
- Olasupo NA, Olukoya DK, Odunfa SA. Studies on local strains of amylolytic Lactobacillus from Nigerian fermented foods. Narhung. 1996;40(1):44–46. doi: 10.1002/food.19960400113. [DOI] [PubMed] [Google Scholar]
- Olasupo NA, Osikoya AF, Odunfa SA, Kuboye AO, Olatunji O. An investigation on the preservation of kunun-zaki, an African Fermented Cereal-Based Food drink. Acta Alimentaria. 2000;29(4):385–392. [Google Scholar]
- Omemu AM, Faniran OW. Assessment of the antimicrobial activity of lactic acid bacteria isolated from two fermented maize products – ogi and kunnu-zaki. Malaysian Journal of Microbiology. 2011;7(3):124–128. [Google Scholar]
- Olukoya DK, Ebigwei SI, Olasupo NA, Ogunjimi AA. Production of DogiK, an improved ogi (Nigerian fermented weaning food) with potentials for use in diarrhoea control. Journal of Tropical Pediatrics. 1994;40(2):108–113. doi: 10.1093/tropej/40.2.108. [DOI] [PubMed] [Google Scholar]
- Otunola ET, Ogunsola O, Abioye VF. Effect of tempeh on some properties of ‘agidi’, a West African fermented maize gel. International Journal of Food Agricultural Research. 2006;3(1):119–128. [Google Scholar]
- Owusu-Kwarteng J, Akabanda F, Nielsen DS, Tano-Debrah K, Glover RLK, Jespersen L. Identification of lactic acid bacteria isolated during traditional fura processing in Ghana. Food Microbiology. 2012;32(1):72–78. doi: 10.1016/j.fm.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Oyarekua MA. Evaluation of the nutritional and microbiological status of co-fermented cereals/cowpea ‘OGI’. Agriculture and Biology Journal of North America. 2011;2(1):61–73. [Google Scholar]
- Sawadogo-Lingani H, Diawara B, Glover RK, Tano-Debrah K, Traoré AS, Jakobsen M. Predominant lactic acid bacteria associated with the traditional malting of sorghum grains. African Journal of Microbiology Research. 2010;4(3):169–179. [Google Scholar]
- Songré-Ouattara LT, Mouquet-Rivier C, Humblot C, Rochette I, Diawara B, Guyot JP. Ability of selected lactic acid bacteria to ferment a pearl millet-soybean slurry to produce gruels for complementary foods for young children. Journal of Food Science. 2010;75(5):261–269. doi: 10.1111/j.1750-3841.2010.01640.x. [DOI] [PubMed] [Google Scholar]
- Songré-Ouattara LT, Mouquet-Rivier C, Icard-Verniere C, Rochette I, Diawara B, Guyot JP. Potential of amylolytic lactic acid bacteria to replace the use of malt for partial starch hydrolysis to produce African fermented pearl millet gruel fortified with groundnut. International Journal of Food Microbiology. 2009;130(3):258–264. doi: 10.1016/j.ijfoodmicro.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Songré-Ouattara LT, Mouquet-Rivier C, Verniere C, Humblot C, Diawara B, Guyot JP. Enzyme activities of lactic acid bacteria from a pearl millet fermented gruel (ben-saalga) of functional interest in nutrition. International Journal of Food Microbiology. 2008;128(2):395–400. doi: 10.1016/j.ijfoodmicro.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Soro-Yao AA, Brou K, Koussémon M, Djè KM. Proximate composition and microbiological quality of millet gruels sold in Abidjan (Côte d’Ivoire) International Journal of Agriculture Innovations and Research. 2014;2(4):472–479. [Google Scholar]
- Soro-Yao AA, Brou K, Koffi-Nevry R, Djè KM. Microbiology of Ivorian fermented products: A review. Asian Journal of Agriculture and Food Science. 2013;1(2):37–47. [Google Scholar]
- Teniola OD, Holzapfel WH, Odunfa SA. Comparative assessment of fermentation techniques useful in the processing of ogi. World Journal of Microbiology and Biotechnology. 2005;21(1):39–43. [Google Scholar]
- Teniola OD, Odunfa SA. Microbial assessment and quality evaluation of ogi during spoilage. World Journal of Microbiology and Biotechnology. 2002;18(8):731–737. [Google Scholar]
- Teniola OD, Odunfa SA. The effects of processing methods on the levels of lysine, methionine and the general acceptability of ogi processed using starter cultures. International Journal of Food Microbiology. 2001;63(1–2):1–9. doi: 10.1016/s0168-1605(00)00321-4. [DOI] [PubMed] [Google Scholar]
- Tetteh GL, Sefa-Dedeh SK, Phillips RD, Beuchat LR. Survival and growth of acid-adapted and unadapted Shigella flexneri in a traditional fermented Ghanaian weaning food as affected by fortification with cowpea. International Journal of Food Microbiology. 2004;90(2):189–195. doi: 10.1016/s0168-1605(03)00301-5. [DOI] [PubMed] [Google Scholar]
- Totté A, Tine E, Seye N, Mathiam JM, Roblain D, Thonard P. Innovation et transfert de technologie: Cas du contrôle de la fermentation du mil par l’utilisation d’un starter lactique. In: International Relief and Development (IRD), editor. Proceedings of the 2nd International workshop in Food-based Approaches for a Healthy Nutrition; Ouagadougou, Burkina Faso. 23–28 November 2003; Arlington, US: IRD; 2003. [Google Scholar]
- Trèche S, Den Hartog AP, Nout RMJ, Traore A. Les petites industries agroalimentaires en Afrique de l’Ouest: Situation actuelle et perspectives pour une alimentation saine. Cahiers d’études et de recherches francophones/Agricultures. 2002;5(11):343–348. [Google Scholar]
- Turpin W, Humblot C, Guyot JP. Genetic screening of functional properties of lactic acid bacteria in a fermented pearl millet slurry and in the metagenome of fermented starchy foods. Applied and Environmental Microbiology. 2011;77(24):8722–8734. doi: 10.1128/AEM.05988-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugwu FM. The potentials of roots and tubers as weaning foods. Pakistan Journal of Nutrition. 2009;8(10):1701–1705. [Google Scholar]
- Venter CS. Prebiotics: An update. Journal of Family Ecology and Consumer Sciences. 2007;35:17–25. [Google Scholar]
- Vieira-Dalode G, Madode YE, Hounhouigan DJ, Jespersen L, Jakobsen M. Use of starter cultures of lactic acid bacteria and yeasts as inoculum enrichment for the production of gowé, a sour beverage from Benin. African Journal of Microbiology Research. 2008;2(7):179–186. [Google Scholar]
- Vieira-Dalodé G, Jespersen L, Hounhouigan DJ, Moller PL, Nago CM, Jakobsen M. Lactic acid bacteria and yeasts associated with gowé production from sorghum in Bénin. Journal of Applied Microbiology. 2007;103(2):342–349. doi: 10.1111/j.1365-2672.2006.03252.x. [DOI] [PubMed] [Google Scholar]
- Wiedemann I, Bottiger T, Bonelli RR, Schneider T, Sahl HG, Martinez B. Lipid II-based antimicrobial activity of the lantibiotic plantaricin C. Applied and Environmental Microbiology. 2006;72(4):2809–2814. doi: 10.1128/AEM.72.4.2809-2814.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao AA, Dortu C, Egounlety M, Pinto C, Vinodh AE, Huch (Née Kostinek) M, Franz CMAP, Holzapfel W, Mbugua S, Mengu M, et al. Production of freeze-dried lactic acid bacteria starter culture for cassava fermentation into gari. African Journal of Biotechnology. 2009;8(19):4996–5004. [Google Scholar]
- Yao AA, Wathelet B, Thonart P. Effect of protective compounds on the survival, electrolyte leakage and lipid degradation of freeze-dried Weissella paramesenteroides LC11 during storage. Journal of Microbiology and Biotechnology. 2009;19(8):810–817. [PubMed] [Google Scholar]
- Yao AA, Coulibaly I, Lognay G, Fauconnier ML, Thonart P. Impact of polyunsaturated fatty acid degradation on survival and acidification activity of freeze-dried Weissella paramesenteroides LC11 during storage. Applied Microbiology and Biotechnology. 2008;79(6):1045–1052. doi: 10.1007/s00253-008-1497-z. [DOI] [PubMed] [Google Scholar]
- Zoghi A, Khosravi-Darani K, Sohrabvandi S. Surface binding of toxins and heavy metals by probiotics. Mini-reviews in Medicinal Chemistry. 2014;14(1):84–98. doi: 10.2174/1389557513666131211105554. [DOI] [PubMed] [Google Scholar]


