Abstract
The swine-origin A(H1N1) influenza virus that has emerged in humans in early 2009 has raised concerns about pandemic developments. In a ferret pathogenesis and transmission model, the 2009 A(H1N1) influenza virus was found to be more pathogenic than a seasonal A(H1N1) virus, with more extensive virus replication occurring in the respiratory tract. Replication of seasonal A(H1N1) virus was confined to the nasal cavity of ferrets, but the 2009 A(H1N1) influenza virus also replicated in the trachea, bronchi, and bronchioles. Virus shedding was more abundant from the upper respiratory tract for 2009 A(H1N1) influenza virus as compared with seasonal virus, and transmission via aerosol or respiratory droplets was equally efficient. These data suggest that the 2009 A(H1N1) influenza virus has the ability to persist in the human population, potentially with more severe clinical consequences.
In April 2009, swine-origin 2009 A(H1N1) influenza virus [2009 A(H1N1) influenza virus] was recognized as the causative agent of influenza-like illnesses in humans in North America (1–3). Since then, 76 countries have officially reported 35,928 cases of 2009 A(H1N1) influenza virus infection, including 163 deaths (4). The 2009 A(H1N1) influenza virus contains a previously unseen combination of gene segments from the North American and Eurasian swine influenza virus lineages (1, 5). The virus has apparently circulated in the swine population without detection and recently crossed the species barrier into humans. The most recent common ancestor of the human 2009 A(H1N1) influenza viruses was estimated to have emerged between November 2008 and March 2009. One of the unusual characteristics of the 2009 A(H1N1) influenza virus as compared with other recent zoonotic influenza viruses is sustained human-to-human transmission, with basic reproduction ratio (R0) estimates in the range of 1.2 to 1.6, which is higher than that reported for seasonal human influenza A viruses (1). In response to the available information on sustained human-to-human transmission in multiple parts of the world, the World Health Organization (WHO) raised the level of influenza pandemic alert to phase 6 on 11 June 2009 (6).
Currently available data indicate that the majority of laboratory-confirmed infections with the 2009 A(H1N1) influenza virus result in a self-limiting, uncomplicated influenza (2, 3, 7). Typical clinical symptoms include fever, rhinorrhea, cough, and sore throat, which are indistinguishable from the symptoms observed for seasonal A(H1N1) and A/H3N2 influenza virus infections. However, in addition to uncomplicated influenza, a variety of clinical symptoms unusual for seasonal influenza have been described, including vomiting and diarrhea in a relatively large proportion of cases. Moreover, some patients have required hospitalization because of severe pneumonia and respiratory failure, with a fatal outcome occurring in 0.5% of laboratory-confirmed cases. In contrast to seasonal influenza, a substantial proportion of the cases of severe illness and death have occurred among young and previously healthy adults. Severe illness and deaths have also been noted relatively frequently in adults with underlying disease and in pregnant women (2, 3, 7).
The distinct antigenic properties of the 2009 A(H1N1) influenza virus as compared with seasonal A(H1N1) virus suggests that population humoral immunity against pandemic 2009 A(H1N1) influenza virus is limited (5), although the age distribution of reported cases suggests some degree of protection in older age groups (1–3).
We used a ferret (Mustela putorius furo) model to study clinical signs, virus shedding, tissue distribution, pathology, and aerosol transmission of the pandemic 2009 A(H1N1) influenza virus as compared with a seasonal 2007 A(H1N1) virus. Ferrets are a suitable animal model for influenza A virus infections in humans because they are susceptible to natural infection and develop respiratory disease and lung pathology similar to humans when suffering from seasonal, avian, or pandemic influenza virus infections (8). Patterns of influenza virus attachment to cells in the trachea and lower respiratory tract are similar in ferrets and humans (9). The ferret model has also been used successfully for studies on virus transmission via direct contact and aerosols or respiratory droplets (10). Influenza virus A/Netherlands/602/2009 was isolated from the first case of laboratory-confirmed 2009 A(H1N1) influenza virus infection in the Netherlands. The patient was a 3-year-old child that traveled with the family from Mexico to the Netherlands and developed fever (>39.5°C) and respiratory symptoms upon return (11). The patient was treated with oseltamivir and recovered uneventfully. A nasopharyngeal swab was collected before the start of treatment, and the virus was isolated in 11-day-old embryonated chicken eggs then passaged once in Madin-Darby canine kidney (MDCK) cells. The virus differs in eight amino acid positions from influenza virus A/California/4/2009 (12). Seasonal influenza virus A/Netherlands/26/2007 was isolated in MDCK cells from a patient infected during the 2006–2007 influenza season.
Two groups of six female ferrets each were inoculated intranasally with 106 50% tissue culture infectious dose (TCID50) of virus. The animals were observed for clinical signs and weighed daily as an indicator of disease. Both viruses caused lethargy, sneezing, ruffled fur, decreased interest in food, and nasal discharge in the ferrets. The mean maximum weight loss was 10% for animals inoculated with the seasonal influenza virus and 12% for the 2009 A(H1N1) influenza virus–inoculated animals (Fig. 1A and fig. S1). Four days after inoculation onwards, the clinical condition of the animals infected with the seasonal influenza virus improved. In contrast, the recovery of the ferrets infected with the 2009 A(H1N1) influenza virus was delayed by ~2 days.
Fig. 1.
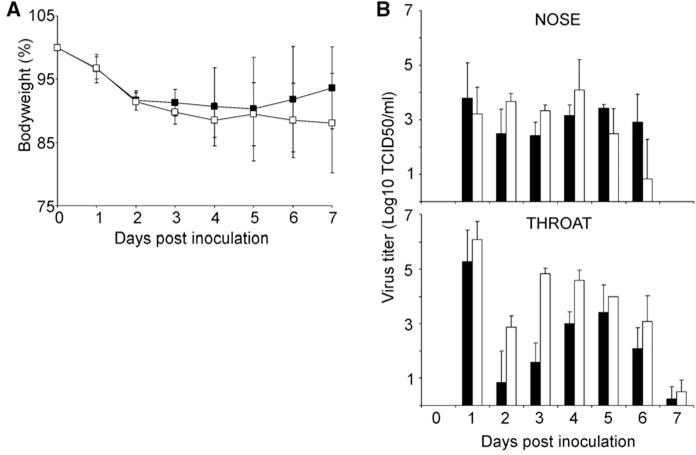
Weight loss and virus shedding in ferrets inoculated with seasonal and 2009 A(H1N1) influenza virus. Two groups of six ferrets each were inoculated intranasally with 106 TCID50 of virus. (A) Weight loss in inoculated animals is indicated as a percentage of body weight at the start of the experiment. Black squares indicate seasonal A(H1N1) and white squares indicate 2009 A(H1N1) influenza virus. (B) Virus shedding from the nose and throat of inoculated animals. Nose and throat swabs were collected daily, and virus titers in the swabs were determined by means of end-point titration in MDCK cells. Geometric mean titers are displayed; error bars indicate SD. Black bars indicate seasonal A(H1N1) and white bars indicate 2009 A(H1N1) influenza virus.
Nose and throat swabs were collected from inoculated animals daily, and virus titers were determined by means of end-point titration in MDCK cells. Virus shedding was observed to start at 1 day after inoculation for both viruses, with throat swabs generally containing higher virus titers than did nose swabs. Infectious virus shedding continued until 6 days after inoculation for the nose swabs and 7 days after inoculation for the throat swabs. On 2 and 3 days after inoculation, virus titers in both throat and nose swabs were significantly higher from animals inoculated with 2009 A(H1N1) influenza virus than from animals inoculated with seasonal influenza virus (Mann-Whitney test; 2 days after inoculation, nose P = 0.034, throat P = 0.022; 3 days after inoculation, nose P = 0.005, throat P = 0.004). The total virus shedding from the throat within the 7 days of the experiment (Fig. 1B) in animals inoculated with 2009 A(H1N1) influenza virus was ~1.5-fold higher as compared with shedding in animals inoculated with seasonal influenza virus (Mann Whitney test, P = 0.004). Virus shedding from the nose was comparable for both viruses (Fig. 1B).
At 3 and 7 days after inoculation, three animals from each group were euthanized and used for pathological and virological examination of the nasal turbinates, trachea, lungs, liver, spleen, kidney, and brain. At 3 days after inoculation, gross examination of the lungs revealed focal to multifocal mild consolidation in all ferrets from both groups. Upon gross examination of the lungs at 7 days after inoculation, 10, 20, and 40% of the lungs were affected in animals inoculated with seasonal influenza virus as compared with 20, 40, and 70% in animals inoculated with 2009 A(H1N1) influenza virus. Gross examination of the liver, spleen, kidney, and brain did not reveal lesions in the two ferret groups.
Parts of the nasal turbinates, trachea, lungs, liver, spleen, kidney, and brain were homogenized, and virus titers were determined by means of end-point titration in MDCK cells. Both the seasonal and 2009 A(H1N1) influenza viruses were detected in the nasal turbinates of inoculated ferrets at 3 days after inoculation, with 2009 A(H1N1) influenza virus yielding slightly higher virus titers (106.9 versus 106.3 TCID50/gram tissue and 95% confidence intervals of 6.34 to 7.47 and 5.63 to 7.04, respectively) (Table 1). The 2009 A(H1N1) influenza virus was also detected in the trachea and lungs (105.2 and 104.6 TCID50/gram tissue, respectively) of inoculated ferrets, whereas the seasonal influenza virus was not. At 7 days after inoculation, both viruses were detected at relatively low titers in the nasal turbinates and not detected in other parts of the respiratory tract. The 2009 A(H1N1) influenza virus was cleared from the lungs and trachea by this time. No virus was detected in the liver, spleen, kidney, or brain of animals inoculated with either virus at 3 or 7 days after inoculation.
Table 1.
Virus titers in the respiratory tract tissues of ferrets inoculated with seasonal and 2009 A(H1N1) influenza viruses. Three ferrets were euthanized at 3 and 7 days after inoculation. Virus titers in nasal turbinates, trachea, and lung were determined by means of end-point titration in MDCK cells. No virus was detected in liver, spleen, kidney, and brain tissue for either virus and were thus not included in the table. Geometric mean titer ± SD is indicated. ND, not detected. Cut-off value for trachea and lungs was <2 and <1.5 Log10 TCID50/gram tissue, respectively.
| Tissue | Virus titer (log10 TCID50/gram tissue)
|
|||
|---|---|---|---|---|
| Day 3 after inoculation
|
Day 7 after inoculation
|
|||
| Seasonal A(H1N1) | 2009 A(H1N1) | Seasonal A(H1N1) | 2009 A(H1N1) | |
| Nasal turbinates | 6.3 (±0.6) | 6.9 (±0.5) | 4.6 (±1.2) | 3.2 (±1.4) |
| Trachea | ND | 5.2 (±1.3) | ND | ND |
| Lung | ND | 4.6 (±1.5) | ND | ND |
Histopathological analyses revealed that ferrets inoculated with the 2009 A(H1N1) influenza virus had a multifocal mild or moderate necrotizing rhinitis, tracheitis, bronchitis, and bronchiolitis, whereas the ferrets inoculated with seasonal influenza virus displayed only multifocal moderate necrotizing rhinitis. We performed immunohistochemistry in order to assess the presence of influenza Avirus–infected cells in respiratory tissues obtained at 3 days after inoculation (Fig. 2). Infected cells were associated with superficial necrosis and inflammation. In ferrets inoculated with the 2009 A(H1N1) influenza virus, many infected cells were detected in the nasal cavity, trachea, bronchus, and bronchioles and rarely in the alveoli. In contrast, detection of virus-infected cells was limited to the nasal turbinates in ferrets inoculated with seasonal influenza virus. At 7 days after inoculation, most of the virus-infected cells were cleared from the respiratory tract of the animals in both groups, as determined with immunohistochemistry (fig. S2).
Fig. 2.
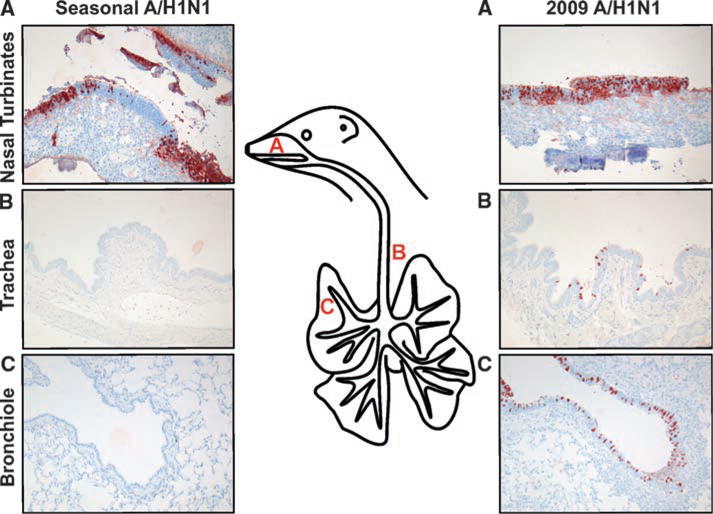
Immunohistochemical analysis of respiratory tract tissues of ferrets inoculated with seasonal or 2009 A(H1N1) influenza virus, collected at 3 days after inoculation. Tissue sections of the nasal turbinates (A), trachea (B), and bronchi (C) were stained with a monoclonal antibody against influenza A virus nucleoprotein, which is visible as a red-brown staining. In animals inoculated with seasonal influenza virus, only cells in the nasal turbinates stained positive for nucleoprotein, whereas in animals inoculated with 2009 A(H1N1) influenza virus, cells in the nasal turbinates, trachea, and bronchi stained positive. See fig. S2 for data taken at 7 days after inoculation.
Transmission of the 2009 A(H1N1) and seasonal influenza viruses via aerosol or respiratory droplets was tested in ferrets. We conducted transmission experiments in cages designed to prevent direct contact between animals but allow airflow from an inoculated ferret to a neighboring naïve ferret (fig. S3). Four ferrets were inoculated, and each animal was housed individually with a naïve ferret in a transmission cage so as to test the transmission of both virus isolates. Inoculated ferrets started to shed virus from the nose and throat at 1 or 2 days after inoculation, with virus titers up to 106 TCID50/ml (fig. S4). Both viruses were transmitted from the inoculated to naïve ferrets in four out of four transmission experiments (Table 2). Transmission was detected within the first 2 days after placing the naïve ferret in the cage adjacent to the inoculated ferret. Peak virus shedding in the naïve and inoculated ferrets was comparable in the 2009 A(H1N1) and seasonal influenza virus groups (fig. S4).
Table 2.
Transmission of seasonal and 2009 A(H1N1) influenza virus via aerosol or respiratory droplets. Four ferrets each were inoculated intranasally with 106 TCID50 of virus and placed in transmission cages. At 1 day after inoculation, one naïve ferret was added to each transmission cage. The transmission cages prevent direct contact of ferrets but allow airflow. Ferrets were observed daily for signs of disease, including sneezing. Nose and throat swabs were collected on 0, 1, 2, 3, 5, 7, and 9 days after inoculation (or days after exposure) to determine virus shedding by the inoculated animal and the contact ferret. Infection was confirmed with seroconversion in all inoculated and contact ferrets.
| Virus | Infected ferrets | Aerosol contacts | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Sneezing | Virus shedding | Onset of shedding (days after inoculation)* | Sneezing | Virus shedding | Onset of shedding (days after exposure)* | |
| Seasonal A(H1N1) | 4/4 | 4/4 | 1 | 3/4 | 4/4† | 1,2 |
| 2009 A(H1N1) | 4/4 | 4/4 | 1,2 | 4/4 | 4/4 | 1,2 |
Virus titers in nose and throat swabs are shown in fig. S4.
One of four aerosol contacts was positive as detected by means of reverse transcription polymerase chain reaction but not virus isolation.
Our results indicated that the 2009 A(H1N1) influenza virus replicates efficiently in the upper and lower respiratory tract of ferrets, is associated with mild or moderate clinical signs and pathological changes, and is transmitted efficiently between ferrets via aerosols or respiratory droplets. These results are in agreement with observations in humans, in which generally mild disease but relatively efficient human-to-human transmission has been observed (1–3, 5). Compared with other zoonotic influenza viruses such as highly pathogenic avian influenza viruses of the H5 and H7 subtypes and the 1918 Spanish H1N1 influenza virus, which are often fatal in ferret models (13–17), the 2009 A(H1N1) influenza virus is a relatively mild pathogen that did not replicate in the alveoli or tissues beyond the respiratory tract and did not cause any mortality in our ferret model. The 2009 A(H1N1) influenza virus may be a relatively mild pathogen in humans, but our data indicated that it is more pathogenic than seasonal influenza A(H1N1) virus in ferrets, with wider distribution of virus replication and associated lesions in the respiratory tract.
The 2009 A(H1N1) influenza virus used here was isolated from a mild case of disease. It is possible that other viruses could emerge that are intrinsically more pathogenic because of mutations in one or more of the viral gene segments. The ferret models described here will be useful to monitor such adaptive changes that affect pathogenicity or transmission of the circulating 2009 A(H1N1) influenza viruses.
Because the 2009 A(H1N1) influenza virus shares the predominant site of virus replication—the upper respiratory tract—with seasonal A(H1N1) and A/H3N2 influenza viruses in the ferret model, and displays comparable receptor-binding properties (fig. S6), the possibility of reassortment of 2009 A(H1N1) influenza virus with seasonal influenza viruses in humans is a serious concern (18, 19). Because 2009 A(H1N1) influenza virus also replicates deeper down the airways, reassortment with A/H5N1 viruses could be an additional concern. Reassortment can facilitate the rapid emergence of viruses that are better adapted to humans (20) or are resistant to neuraminidase inhibitors after acquiring the N1 gene segment from the oseltamivir-resistant seasonal H1N1 viruses that have emerged recently (21).
The shedding and transmission data suggested that the 2009 A(H1N1) is sufficiently fit to compete with the seasonal A(H1N1) virus. Because serological cross-reactivity between 2009 A(H1N1) and seasonal H1N1 viruses was found to be negligible (5), and different antigenic variants of influenza B viruses have coexisted for extended periods of time (22), the 2009 A(H1N1) influenza virus should be considered capable of continued cocirculation in the human population with the existing seasonal influenza viruses.
Supplementary Material
Acknowledgments
We thank M. Schutten, J. Guldenmeester, G. Arron, S. Chutinimitkul, L. Leijten, P. van Run, R. Diaz-D’Ullois, and G. van Amerongen for technical assistance. V.M., E.d.W, and R.F. were financed through National Institute of Allergy and Infectious Diseases–NIH contract HHSN266200700010C.
Footnotes
References and Notes
- 1.Fraser C, et al. Science. 2009;324:1557. doi: 10.1126/science.1176062. published online 11 May 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shinde V, et al. N Engl J Med. 2009;7 doi: 10.1056/NEJMoa0903812. [DOI] [Google Scholar]
- 3.A. V. I. T. Novel Swine-Origin Influenza. N Engl J Med. 2009;360:2605. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 4.WHO. 2009 www.who.int/csr/don/2009_05_29/en/index.html.
- 5.Garten RJ, et al. Science. 2009;22 doi: 10.1126/science.1176225. [DOI] [Google Scholar]
- 6.WHO. 2009 www.who.int/csr/disease/swineflu/en.
- 7.WHO. Wkly Epidemiol Rec. 2009;21:185. [Google Scholar]
- 8.Maher JA, DeStefano J. Lab Anim NY) 2004;33:50. doi: 10.1038/laban1004-50. [DOI] [PubMed] [Google Scholar]
- 9.van Riel D, et al. Am J Pathol. 2007;171:1215. doi: 10.2353/ajpath.2007.070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorrell EM, Wan H, Araya Y, Song H, Perez DR. Proc Natl Acad Sci USA. 2009;106:7565. doi: 10.1073/pnas.0900877106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ProMed. 2009 www.promedmail.org/pls/otn/f?p=2400:1202:6682247203355318::NO::F2400_P1202_CHECK_DISPLAY,F2400_P1202_PUB_MAIL_ID:X,77295.
- 12.Materials and methods are available as supporting material on Science Online.
- 13.Tumpey TM, et al. Science. 2007;315:655. doi: 10.1126/science.1136212. [DOI] [PubMed] [Google Scholar]
- 14.Belser JA, et al. J Virol. 2007;81:11139. doi: 10.1128/JVI.01235-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maines TR, et al. J Virol. 2005;79:11788. doi: 10.1128/JVI.79.18.11788-11800.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zitzow LA, et al. J Virol. 2002;76:4420. doi: 10.1128/JVI.76.9.4420-4429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govorkova EA, et al. J Virol. 2005;79:2191. doi: 10.1128/JVI.79.4.2191-2198.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson MI, et al. PLoS Pathog. 2008;4:e1000012. doi: 10.1371/journal.ppat.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rambaut A, et al. Nature. 2008;453:615. doi: 10.1038/nature06945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belshe RB. N Engl J Med. 2005;353:2209. doi: 10.1056/NEJMp058281. [DOI] [PubMed] [Google Scholar]
- 21.Lackenby A, et al. Euro Surveill. 2008;13:113. doi: 10.2807/ese.13.05.08026-en. [DOI] [PubMed] [Google Scholar]
- 22.Mizuta K, et al. Epidemiol Infect. 2004;132:721. doi: 10.1017/s0950268804002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


