Introduction
Young women from inner city environments have high rates of allergy and asthma, and can experience significant psychological stress and consequent psychological dysfunction (e.g., depression due to a combination of factors related to poverty and violence. Chronic stress and stress correlates such as depression can affect immune function and potentially the risk of allergic diseases via multiple mechanisms, including alterations in glucocorticoids, catecholamines, endogenous opioids, hormones and cytokines.1 However, there is little information about how stress and depression influence proinflammatory and type-2 cytokine responses in young women.
Results from several previous cross-sectional studies suggest that both stress and depression are associated with chronic inflammation and an increased incidence of atopic disease, such as increased asthma symptoms in adolescents with depression and anxiety,2 increased hospitalizations for asthma in children exposed to community violence,3 and steroid resistance in peripheral blood mononuclear cells (PBMCs) in school-aged children experiencing decreased parental support.4 These effects may be more profound in individuals with low socioeconomic status which has been associated with activation of the innate immune system, as measured by IL-6 production.5 We previously reported that exposure to environmental stressors during the prenatal period was associated with changes in newborn cytokine responses that were pro-inflammatory and indicative of a Th2 bias (IL-13 responses to house dust mite and lower PHA-induced IFN-γ).6
Based on these findings, we hypothesized that environmental stressors, perceived stress and/or increased depressive symptoms in urban mothers would be associated with an increase in pro-inflammatory cytokine responses to innate stimuli involving tumor necrosis factor α (TNF-α) and interleukin-8 (IL-8) as well as type-2 cytokine skewing of responses (increased IL-13 and blunted IFN-γ) to adaptive immune stimuli. To test these hypotheses, we measured PBMC cytokine responses to innate and adaptive stimuli in mothers participating in a multicenter urban birth cohort study and tested for associations with concurrent measures of individual, household and neighborhood stress as well as depression.
Methods
Ethics statement
The protocol was approved by the human subjects committees at the University of Wisconsin as well as each of the clinical centers (Mt. Sinai School of Medicine, Columbia School of Medicine, Washington University, Boston University, and Johns Hopkins University), and written informed consent was obtained before enrollment.
Study population
The Urban Environment and Childhood Asthma (URECA) study is a prospective birth cohort that enrolled 557 families with at least one parent having a history of asthma or allergy and 49 with no history of asthma or allergy. The families were recruited from central low-income urban areas (census tracts with at least 20% of the population below the poverty line) in Baltimore, Boston, New York and Saint Louis. Infants had to be born at ≥ 34 weeks gestation.7
Assessment of external stressors, perceived stress and depression
Questionnaires to assess environmental stressors, perceived stress, and depression were administered at the same visit at which blood was obtained for the immunologic studies. External stressors were assessed across multiple realms including specific questionnaires to record difficult life circumstances,8–10 community violence,11,12 neighborhood/block conditions,12 and financial hardship and housing conditions,13,14 as described previously (see online data supplement for details). Latent variables were derived from the questionnaire data using factor analysis. Three stress domains were constructed by assigning items that had any factor loading ≥0.45 to the domain for which they had the greatest loading. One domain represented individual stressors (Interpersonal Problems) and two represented ecological-level strains (Housing Factor and Neighborhood Factor).6 The three-factor solution explained 75% of the total variance of the individual stress variables. Scores for each factor were created by multiplying the factor loadings by the values for the variables included in the factor and summing those values. Each factor score was then divided into tertiles and given values of 1=low, 2=medium, and 3=high exposure. An overall Composite Stressor score was derived by summing the tertile values across the three factors.6 For example, if a subject was in tertile 1 for individual-level stressors, tertile 2 for housing problems, and tertile 3 for neighborhood problems the composite score would equal 6 [range 3 (low on all domains) to 9 (high on all domains)]. In analyses, values of 3–5 represent low exposure to stressors, 6–7 represent moderate exposure, and 8–9 represent high overall stressors. Each factor or stressor domain was also considered separately in the analyses.
Our main analyses present findings from stressors measured at the time of the blood sample for immunologic testing. However, we also examined chronic exposure to stressors by averaging the values for the Composite Stressor score and separate domains (interpersonal problems, housing and neighborhood stress) across two time points, the blood collection visit and the prior visit (most often the prenatal visit). When only one time point was available, the data from the blood collection visit was used.
Individual stress was measured using the 4-item Perceived Stress Scale (PSS). The PSS measures the degree to which respondents believe their lives were unpredictable, uncontrollable, and overwhelming in the preceding month (reliability, 0.85).15 These measures can all be considered to represent internal stress.
Depression was measured using the 10-item Edinburgh Postnatal Depression Scale (EPDS), a tool developed in England to assist primary care health professionals to detect mothers suffering from postnatal depression . Mothers reported on their emotions during the prior 7 days on a 0 to 3 Likert scale; scores ≥ 12 indicated a need for further mental health evaluation.16
Measurement of cytokine responses
A peripheral blood sample was obtained from 509 of the 606 mothers. Cytokine responses from PBMCs were assessed as a measure of systemic immune responses as previously described.7 The blood samples were processed at each of the four URECA sites (Baltimore, Boston, New York and St. Louis). After separation, PBMCs were incubated with a panel of specific stimuli selected to activate specific innate, adaptive, and polyclonal responses (Table 2). Cell separation from peripheral blood and cell stimulation were performed at each site, and then supernatant fluids from PBMC cultures were frozen in aliquots and sent to the central laboratory in Madison for cytokine analysis. Selected cytokines (Table 2) were measured by multiplex ELISA (Milliplex, Millipore). These assays were carefully standardized across the four clinical sites; technicians attended centralized training sessions, used the same equipment and centrally-prepared stimulants and reagents, and performance was assessed yearly by analysis of a blood sample that was split and analyzed at each of the sites.7 Adaptive immune response data were available from 507 of the 606 URECA mothers (84%), while innate immune response data were available from 506 (83%) mothers. 506 (83%) mothers have responses measured on both panels.
Table 2.
Stimulants used and cytokines measured in the mononuclear cell assays.
| Innate Stimuli | Cytokines | Adaptive and Mitogenic Stimuli | Cytokines |
|---|---|---|---|
|
IFN-α IFN-γ IL-10 IL-12p40 TNF-α IL-8 |
IFN-γ IL-10 IL-13 IL-4 IL-5† |
D. pteronyssinus
Stimulation not conducted on cells from the umbilical cord samples
Not measured in umbilical cord samples
The blood samples were also analyzed for total IgE levels as well as specific IgE (fluoroenzyme immunoassay [FEIA], Phadia) to several aeroallergens (birch or oak, ragweed, Timothy grass, Dermatophagoides farinae, Dermatophagoides pteronyssinus, dog epithelium, cat dander/epithelium, German cockroach, mouse urine protein, and Alternaria alternata) to screen for sensitization to allergens. Study participants with at least one positive test to an aeroallergen (≥0.35 kU/L) were classified as atopic.
Statistical analysis
Our final analysis population includes 469 mothers with data for both cytokine responses and stress variables. Univariate and multivariate analyses were performed using both Pearson and Spearman correlations in order to determine associations between stress factors and cytokine levels. All multivariate analyses were adjusted for potential confounding effects due to site, season of the mother’s blood draw, and mother’s smoking status in the first year of the child’s life. Spearman correlations were calculated as a conservative comparison and showed similar associations to the Pearson correlations; partial Pearson correlations are shown in the figures. Additionally, all immune responses have been log-transformed. Associations between scores for Composite Stressors, PSS and EPDS and cytokine responses were also stratified by allergy and asthma status. All statistical analyses were performed using SAS 9.2 and R 2.12.2.
Results
Study population
Mothers were predominantly young (mean age=24, range 13–42) minorities (73% Black, 18% Hispanic) of low socioeconomic status (70% with annual household income < $15,000) and without post-secondary education (75% with high school education or less, Table 1). For the majority of mothers, the child participating in URECA was not their first child; 61% had at least one other child. 40% of mothers smoked during the first year of their child’s life; 2 or more smokers were present in 36% of homes. Subjects also had a high prevalence of allergic disease: 46% of the mothers had previously been told they had asthma, 68% were atopic, and 64% reported hay fever or eczema at some point in their life.
Table 1.
Demographics
| Participants (N=469) % (n) | |
|---|---|
| Blood draw visit | |
| 12-month | 89% (420) |
| 24-month | 8% (36) |
| 36-month | 3% (13) |
| Age at screening (mean ± standard deviation) | 24.4 ± 5.9 |
| Education level | |
| Less than high school | 40% (187) |
| High school | 35% (163) |
| More than high school | 25% (118) |
| Race or ethnicity | |
| African American | 73% (343) |
| Hispanic | 18% (84) |
| Other | 9% (41) |
| Site | |
| Baltimore | 29% (134) |
| Boston | 24% (115) |
| New York | 17% (78) |
| St. Louis | 30% (142) |
| Annual income < $15,000 | 70% (328) |
| Parity (mean ± standard deviation) | 1.3 ± 1.5 |
| Primiparous (first child) | 39% (184) |
| BMI at screening (mean ± standard deviation) | 31.6 ± 8.7 |
| Smoke exposure | |
| Has ever smoked | 45% (210) |
| Smoked during first year of child’s life | 40% (186) |
| Number of smokers in the home during first year | |
| 0 | 33% (156) |
| 1 | 31% (143) |
| 2 or more | 36% (169) |
| Positive allergen-specific IgE (n=318) | |
| Any positive IgE | 68% (318) |
The mean Composite Stressor score for the mothers was 6.1 ± 1.8, with a maximum score of 9.0. The mean PSS score was 4.6 ± 2.8 and the maximum score was 13.8. Depression scores (EPDS) had a mean of 6.3 ± 6.4 and a maximum of 26; 22% (104 of 468) of the mothers had scores above the clinical cut-off for further evaluation (EPDS ≥ 12). There were moderate positive correlations between these measures (Composite Stressors and PSS R=0.41, Composite Stressors and EPDS R=0.34, PSS and EPDS R=0.68,; all p<0.0001).
Relationship of external stressors to cytokine responses
There were no statistically significant associations between the Composite Stressor score and blood cell cytokine responses (Figure 1). When stressor scores from more than one time point were averaged to model chronic stress, there were no important changes in the findings.
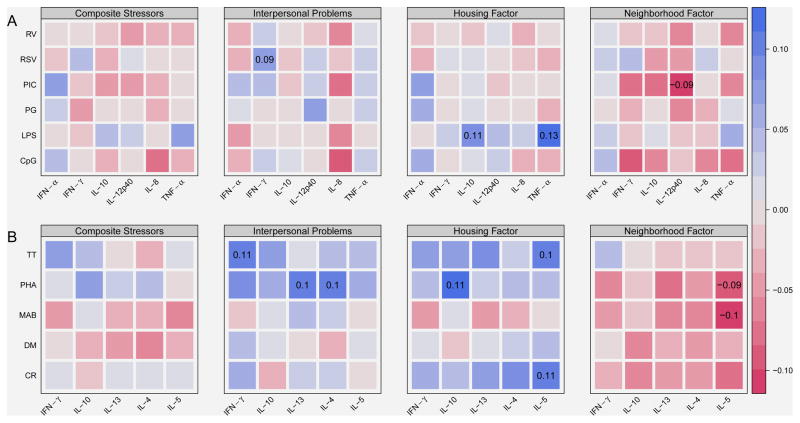
Figure 1.
Associations between composite stressors and individual stress domains with cytokine responses to innate (A) and adaptive (B) stimuli. Color-coded Pearson partial correlations are adjusted for site, season of blood draw, and mother’s smoking status. Red colors indicate negative associations, while blue colors are indicative of positive associations. The x-axis contains cytokine responses. The y-axis contains stimulants. Abbreviations for the stimulants and cytokines are listed in Table 2. Correlation coefficients are shown for correlations significant at p<0.05.
We next tested the exploratory hypothesis that the individual stressor domains might have distinct effects on immune responses. For Interpersonal Problems and Housing Factor domains, most associations with cytokine responses were in the positive direction (Figure 1). For example, Interpersonal Problems were positively associated with PHA-stimulated IL-13 (ρ = 0.10, p = 0.04), IL-4 (ρ = 0.10, p = 0.02), and TT-induced IFN-γ (ρ = 0.11, p = 0.03), as well as with RSV-stimulated IFN-γ (ρ = 0.09, p = 0.05). Similarly, the Housing Factors domain was positively associated with several innate cytokine responses (LPS-induced TNF-α [ρ = 0.13, p = 006] and LPS-induced IL-10 [ρ = 0.11, p = 0.02), adaptive responses (CR- and TT-induced IL-5 [ρ = 0.11, p = 0.02 and ρ = 0.10, p= 0.03]), and PHA-induced IL-10 (ρ = 0.11, p = 0.02). By contrast, the Neighborhood Factor domain had a negative association with PIC-induced IL-12p40 (ρ = −0.09, p = 0.04), PHA and MAB induced IL-5 (ρ = −0.09, p = 0.05 and ρ = −0.10, p = 0.04) and trends for negative associations with several other cytokine responses.
Similar findings were obtained when the individual stressors were considered as averages of two separate measurements in successive years (data not shown).
Perceived stress and cytokine responses
We next tested whether stress perception was associated with pro-inflammatory or Th2 cytokine responses (Figure 2). There were no significant associations between PSS and TNFα, IFNγ or IL-13 responses, and a negative relationship with a single IL-8 response (CpG induced IL-8; ρ = −0.10, p < 0.05). In addition, there were negative associations between the PSS and RV induced IL-12p40 (ρ = −0.10, p < 0.05), and CR induced IL-10 (ρ = −0.13, p < 0.05).
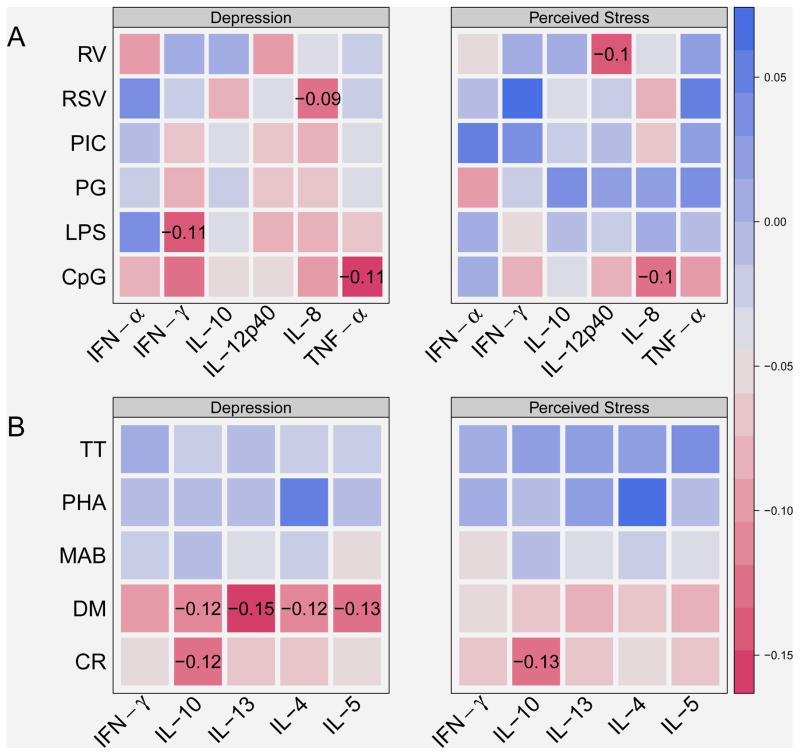
Figure 2.
Associations between depression/perceived stress and cytokine responses to innate (A) and adaptive (B) stimuli. Color-coded Pearson partial correlations are adjusted for site, season of blood draw, and mother’s smoking status. Red colors indicate negative associations, while blue colors are indicative of positive associations. The x-axis contains cytokine responses. The y-axis contains stimulants. Abbreviations for the stimulants and cytokines are listed in Table 2. Correlation coefficients are shown for correlations significant at p<0.05.
Depression and cytokine responses
Depression was inversely associated with several innate immune responses (Figure 2A), including CpG-induced TNF-α (ρ = −0.11, p = 0.02), RSV induced IL-8 (ρ = −0.09, p < 0.05), and LPS induced IFN-γ (ρ = −0.11, p = 0.02). Similarly, there were inverse relationships between depression and several adaptive responses (Figure 2B), including several DM-induced cytokines (IL-4, IL-5, IL-10, and IL-13; ρ = −0.12–−0.15, p < 0.05), and CR-induced IL-10 (ρ = −0.12, p = 0.01).
Stratification by allergy and asthma status
We considered the hypothesis that stressors, perceived stress or depression might enhance Th2 or pro-inflammatory (IL-8 and TNFα) responses primarily in mothers with allergic sensitization (positive test for allergen-specific IgE) or asthma. To test this hypothesis, stratified analyses were conducted based on asthma status (eFigure 1) and atopy status (eFigure 2). The results showed that stress, stressors or depression were not positively associated with type 2 or pro-inflammatory responses in mothers with asthma or atopy. On the contrary, type-2 cytokine responses to dust mite were inversely related to depression and perceived stress in mothers without asthma or atopy (eFIgures 1 and 2).
Discussion
In this large multicenter study involving nearly 500 young mothers, we measured stressors, perceived stress and depression using multiple validated questionnaires and compared these data to detailed measurements of blood cell cytokine responses to innate, adaptive, and mitogenic immune stimuli using a carefully standardized assay. We hypothesized that measures of stress would positively correlate with proinflammatory responses, and a bias towards type-2 cytokine responses that are associated with allergic diseases and asthma. Contrary to this hypothesis, the Composite Stressor score was not significantly related to PBMC cytokine responses. The depression scale was inversely associated with some responses, but except for lower responses to house dust mite, no consistent patterns were noted. These findings do not support our hypothesis that stress has substantial effects on mononuclear cell cytokine responses that are associated with allergy in this large population of urban women. A stratified analysis demonstrated that these findings were generally the same among women with vs. without allergies or asthma.
Much of the previous research on stress and cytokine responses focuses on acute stressful events, which have some consistent effects on cytokine responses.17,18 A meta-analysis of acute psychological stress found that acute stress was associated with a low-grade inflammatory response (increased IL-1β, TNFα and C-reactive protein [CRP]) measured in serum or plasma within 2 hours of the inciting event. Some studies also included stimulation of PBMC, and found similar effects on IL-1β responses. Acute stressors may also affect the balance of Th1/Th2 cytokine responses. For example, examination-induced stress reduced blood cell mitogen-induced IFN-γ responses or IFN-γ/IL-10 ratios.19,20 These effects were interpreted as possibly increasing susceptibility to viral illnesses or promoting allergy. In studies of participants with asthma, acute stressful events were associated with alterations in circulating cell counts, cell proliferative responses to mitogens, and release of cytokines involved in the TH2 response.21 These short-term effects of acute stressful events on cytokine responses could be mediated by changes in circulating glucocorticoids or other stress hormones.17,22
In contract to acute stressful events, urban environments characterized by high rates of poverty include chronic exposure to environmental stressors, and our analysis identified contributing factors related to interpersonal relationships, neighborhood factors and poor housing conditions. Studies of effects of chronic stressors have reported more variable relationships with cytokine responses, perhaps related to the specific stressor.18 Stress of chronic caregiving was associated with increased LPS-induced IL-10 but no changes in mitogen-induced IFN-γ.23 Psychological stress can be associated with small changes in circulating cytokines such as IL-1 and IL-6, that can originate from either immune cells, or non-immune cells such as endothelial cells and adipocytes.24 There is paucity of data on the effects of major life events on Th1/Th2 cytokine responses in humans.
While exposure to composite stressors was not significantly related to cytokine responses in our study, exploratory analyses were performed to test the possibility that specific types of chronic stressors might have distinct effects on immune responses, and some unique associations were present. For example, Interpersonal Problems was associated with increased IFN-γ responses to tetanus toxoid and increased Th2 responses to polyclonal stimulation (PHA). Housing Stress was positively associated with LPS-induced TNF-α and IL-10, which are elements of the acute inflammatory response,25 and enhanced Th2 cytokine responses to both cockroach and tetanus proteins, which could promote allergy.26 By contrast, Neighborhood Factors were negatively associated with CpG-stimulated IFN-γ. Due to the large number of comparisons and modest correlation coefficients, these results need to be interpreted with caution, but raise the possibility that specific stressors might have distinct effects on PBMC cytokine responses.
The psychological effects of external stressors on an individual can vary considerably. As an indicator of individual stress perceptions to the various external stressors, we tested for associations between the Perceived Stress Score (PSS) and cytokine responses. There were a few negative associations between the PSS and specific cytokine responses, but these did not involve TNFα, IFNγ or IL-13. Thus, our results do not suggest that either exposure to chronic environmental stressors nor individual perceptions of stress enhance pro-inflammatory or Type 2 cytokine responses.
There were several inverse associations between measures of depression and specific innate and adaptive cytokine responses. For example, our results provide evidence for a potential link between depression and reduced responses to several microbial stimuli. We also observed an inverse relationship between depression and reactivity to dust mite, and this relationship does not support the hypothesis that depression contributes to dust mite allergy. Previous studies have provided evidence that depression affects the HPA axis and can induce increases in serum cortisol,27 which may blunt various immune responses including cytokine responses.28 Incidentally, there were weak inverse relationships between depression and a few of the innate immune responses measured in this study.
Strengths of this study include a much larger sample size than most previous studies, and comprehensive evaluation of multiple cytokine responses to well-defined innate and adaptive immune stimuli. In addition, the cytokine assays were performed with extensive attention to standardization and quality control.29 These immunologic assays have been validated in previous studies conducted by the same group of investigators in which relationships were demonstrated between cytokine responses and predictors (e.g. ethnicity, perinatal history, birthweight)30 and clinical outcomes (e.g wheezing illnesses, atopic dermatitis, allergic sensitization and food allergy).31 The questionnaires were comprehensive and utilized validated instruments to measure stressors, perceived stress and depression. Stratified analyses were included to determine whether stress, stressors or depression had distinct effects related to allergy or asthma status.
This study also had some limitations that need to be considered in interpreting the results. For example, these analyses involved multiple comparisons, which increases the possibility of type I error. In comparing individual stress domains with effects on cytokine responses, which would be unlikely to occur by chance. Even so, these are exploratory analyses that should be interpreted with caution. In addition, characterizing stress and depression is challenging. Although study participants reported experiencing discrete stressors, the degree of psychological distress experienced by individuals (i.e., stress appraisal) can vary even when faced with similar life stressors. However, the analysis of perceived stress did not show significant positive associations with cytokines associated with pro-inflammatory or Th2 responses. Furthermore, this study used a questionnaire designed to measure postnatal depression, and it is possible that a more global assessment of depression may have yielded different results. Finally, this study was performed in an inner-city cohort with high rates of asthma and environmental allergies, and the results may not be generalizable to other demographic groups.
In summary, we conducted one of the largest and most detailed studies to date examining effects of neighborhood, housing and personal stressors on innate, adaptive, and polyclonal systemic cytokine responses. The study results indicate that scores for Composite Stressors, perceived stress or depression were not positively associated with pro-inflammatory or Th2 cytokine responses. These findings do not support the hypothesis that external stressors, perceived stress or depression in poor urban neighborhoods augment cytokine responses that promote or accentuate allergy and asthma. It may be that interactions between stress and correlates such as depression and the immune system may be more complex and that different types of stress may have distinct and subtle effects. Moreover, psychological stress and altered psychological functioning influences the immune system through disruption of other key pathways (e.g., hypothalamic pituitary adrenal axis, autonomic disruption), thus studies may need to consider an integrated approach that accounts for interactions across these stress- response system to elucidate immune pathways.
Supplementary Material
Acknowledgments
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract numbers NO1-AI-25496, NO1-AI-25482, HHSN272200900052C and HHSN272201000052I. Additional support was provided by the National Center for Research Resources, National Institutes of Health, under grants RR00052, M01RR00533, UL1RR025771, M01RR00071, 1UL1RR024156, and 5UL1RR024992-02.
The Urban Environment and Childhood Asthma Study is a collaboration of the following institutions and investigators (principal investigators are indicated by an asterisk; protocol chair is indicated by double asterisks):
Johns Hopkins University, Baltimore, MD – R Wood*, E Matsui, H Lederman, F Witter, J Logan, S Leimenstoll, D Scott, L Daniels, L. Miles, D. Sellers, A Swift; Boston University School of Medicine, Boston, MA – G O’Connor*, W Cruikshank, M Sandel, A Lee-Parritz, C Jordan, E Gjerasi, P. Price-Johnson, B. Caldwell, M Tuzova; Harvard Medical School, Boston, MA – D Gold, R Wright; Columbia University, New York, NY – M Kattan*, C Lamm, N Whitney, P Yaniv, C Sanabia, A Valones; Mount Sinai School of Medicine, New York, NY – H Sampson, R Sperling, N Rivers; Washington University School of Medicine, St Louis, MO – G Bloomberg*, L Bacharier, Y Sadovsky, E Tesson, C Koerkenmeier, R Sharp, K Ray, J Durrange, I Bauer; Statistical and Clinical Coordinating Center - Rho, Inc, Chapel Hill, NC – H Mitchell*, P Zook, C Visness, M Walter, R Bailey, K. Jaffee, W Taylor, R Budrevich; Scientific Coordination and Administrative Center – University of Wisconsin, Madison, WI – W Busse*, J Gern**, P Heinritz, C Sorkness, M Burger, K Grindle, A Dresen; National Institute of Allergy and Infectious Diseases, Bethesda, MD – P Gergen, A Togias, E Smartt.
Footnotes
David A. Gruenberg participated in conception and design of the study, analysis and interpretation of the data and preparation of the manuscript, Rosalind J. Wright, participated in conception and design of the study, analysis and interpretation of the data and preparation of the manuscript, Cynthia M. Visness participated in conception and design of the study, analysis and interpretation of the data and preparation of the manuscript, Katy F. Jaffee participated in conception and design of the study, analysis and interpretation of the data and preparation of the manuscript, Gordon R. Bloomberg participated in conception and design of the study, data generation and critical revision of the manuscript, William W. Cruikshank participated in conception and design of the study, data generation and critical revision of the manuscript, Meyer Kattan participated in conception and design of the study, data generation and critical revision of the manuscript, Megan T. Sandel participated in conception and design of the study, data generation and critical revision of the manuscript, Robert A. Wood participated in conception and design of the study, data generation and critical revision of the manuscript, James E. Gern participated in conception and design of the study, analysis and interpretation of the data and preparation of the manuscript. All authors approve this final version of the manuscript.
ClinicalTrials.gov Identifier: NCT00114881
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dantzer R, Kelley KW. Stress and immunity: an integrated view of relationships between the brain and the immune system. Life sciences. 1989;44(26):1995–2008. doi: 10.1016/0024-3205(89)90345-7. [DOI] [PubMed] [Google Scholar]
- 2.Richardson LP, Lozano P, Russo J, McCauley E, Bush T, Katon W. Asthma symptom burden: relationship to asthma severity and anxiety and depression symptoms. Pediatrics. 2006 Sep;118(3):1042–1051. doi: 10.1542/peds.2006-0249. [DOI] [PubMed] [Google Scholar]
- 3.Apter AJ, Garcia LA, Boyd RC, Wang X, Bogen DK, Ten Have T. Exposure to community violence is associated with asthma hospitalizations and emergency department visits. The Journal of allergy and clinical immunology. 2010 Sep;126(3):552–557. doi: 10.1016/j.jaci.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller GE, Gaudin A, Zysk E, Chen E. Parental support and cytokine activity in childhood asthma: the role of glucocorticoid sensitivity. The Journal of allergy and clinical immunology. 2009 Apr;123(4):824–830. doi: 10.1016/j.jaci.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Azad MB, Lissitsyn Y, Miller GE, Becker AB, HayGlass KT, Kozyrskyj AL. Influence of socioeconomic status trajectories on innate immune responsiveness in children. PloS one. 2012;7(6):e38669. doi: 10.1371/journal.pone.0038669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright RJ, Visness CM, Calatroni A, et al. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. American journal of respiratory and critical care medicine. 2010 Jul 1;182(1):25–33. doi: 10.1164/rccm.200904-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gern JE, Visness CM, Gergen PJ, et al. The Urban Environment and Childhood Asthma (URECA) birth cohort study: design, methods, and study population. BMC pulmonary medicine. 2009;9:17. doi: 10.1186/1471-2466-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Booth CL, Mitchell SK, Barnard KE, Spieker SJ. Development of maternal social skills in multiproblem families: Effects on the mother-child relationship. Developmental Psychology. 1989;25:403–412. [Google Scholar]
- 9.Pascoe JM, Kokotailo PK, Broekhuizen FF. Correlates of multigravida women's binge drinking during pregnancy. A longitudinal study. Archives of pediatrics & adolescent medicine. 1995 Dec;149(12):1325–1329. doi: 10.1001/archpedi.1995.02170250031004. [DOI] [PubMed] [Google Scholar]
- 10.Ritter C, Hobfoll SE, Lavin J, Cameron RP, Hulsizer MR. Stress, psychosocial resources, and depressive symptomatology during pregnancy in low-income, inner-city women. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2000 Nov;19(6):576–585. doi: 10.1037//0278-6133.19.6.576. [DOI] [PubMed] [Google Scholar]
- 11.Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science. 1997 Aug 15;277(5328):918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- 12.Wright RJ, Mitchell H, Visness CM, et al. Community violence and asthma morbidity: the Inner-City Asthma Study. American journal of public health. 2004 Apr;94(4):625–632. doi: 10.2105/ajph.94.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn JR. Housing and inequalities in health: a study of socioeconomic dimensions of housing and self reported health from a survey of Vancouver residents. Journal of epidemiology and community health. 2002 Sep;56(9):671–681. doi: 10.1136/jech.56.9.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans J, Hyndman S, Stewart-Brown S, Smith D, Petersen S. An epidemiological study of the relative importance of damp housing in relation to adult health. Journal of epidemiology and community health. 2000 Sep;54(9):677–686. doi: 10.1136/jech.54.9.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of health and social behavior. 1983 Dec;24(4):385–396. [PubMed] [Google Scholar]
- 16.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. The British journal of psychiatry : the journal of mental science. 1987 Jun;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 17.Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16(5):300–317. doi: 10.1186/1710-1492-4-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott GR, Eisdorfer C. Stress and human health: An analysis and implications of research. A study by the Institute of Medicine, National Academy of Sciences. New York: Springer Publishing; 1982. [Google Scholar]
- 19.Glaser R, Rice J, Speicher CE, Stout JC, Kiecolt-Glaser JK. Stress depresses interferon production by leukocytes concomitant with a decrease in natural killer cell activity. Behavioral neuroscience. 1986 Oct;100(5):675–678. doi: 10.1037//0735-7044.100.5.675. [DOI] [PubMed] [Google Scholar]
- 20.Marshall GD, Jr, Agarwal SK, Lloyd C, Cohen L, Henninger EM, Morris GJ. Cytokine dysregulation associated with exam stress in healthy medical students. Brain, behavior, and immunity. 1998 Dec;12(4):297–307. doi: 10.1006/brbi.1998.0537. [DOI] [PubMed] [Google Scholar]
- 21.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychological bulletin. 2004 Jul;130(4):601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gadek-Michalska A, Tadeusz J, Rachwalska P, Bugajski J. Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacological reports : PR. 2013;65(6):1655–1662. doi: 10.1016/s1734-1140(13)71527-5. [DOI] [PubMed] [Google Scholar]
- 23.Glaser R, MacCallum RC, Laskowski BF, Malarkey WB, Sheridan JF, Kiecolt-Glaser JK. Evidence for a shift in the Th-1 to Th-2 cytokine response associated with chronic stress and aging. The journals of gerontology. Series A, Biological sciences and medical sciences. 2001 Aug;56(8):M477–482. doi: 10.1093/gerona/56.8.m477. [DOI] [PubMed] [Google Scholar]
- 24.Rohleder N. Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosomatic medicine. 2014 Apr;76(3):181–189. doi: 10.1097/PSY.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 25.Kay AB, Klion AD. Anti-interleukin-5 therapy for asthma and hypereosinophilic syndrome. Immunology and allergy clinics of North America. 2004 Nov;24(4):645–666. vii. doi: 10.1016/j.iac.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Erzurum SC. Inhibition of tumor necrosis factor alpha for refractory asthma. The New England journal of medicine. 2006 Feb 16;354(7):754–758. doi: 10.1056/NEJMe058266. [DOI] [PubMed] [Google Scholar]
- 27.Ising M, Horstmann S, Kloiber S, et al. Combined dexamethasone/corticotropin releasing hormone test predicts treatment response in major depression - a potential biomarker? Biological psychiatry. 2007 Jul 1;62(1):47–54. doi: 10.1016/j.biopsych.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 28.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine reviews. 2000 Feb;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 29.Shreffler WG, Visness CM, Burger M, et al. Standardization and performance evaluation of mononuclear cell cytokine secretion assays in a multicenter study. BMC immunology. 2006;7:29. doi: 10.1186/1471-2172-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gold DR, Bloomberg GR, Cruikshank WW, et al. Parental characteristics, somatic fetal growth, and season of birth influence innate and adaptive cord blood cytokine responses. The Journal of allergy and clinical immunology. 2009 Nov;124(5):1078–1087. doi: 10.1016/j.jaci.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wood RA, Bloomberg GR, Kattan M, et al. Relationships among environmental exposures, cord blood cytokine responses, allergy, and wheeze at 1 year of age in an inner-city birth cohort (Urban Environment and Childhood Asthma study) The Journal of allergy and clinical immunology. 2011 Apr;127(4):913–919. e911–916. doi: 10.1016/j.jaci.2010.12.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.