Abstract
Surgery can be a highly effective treatment for medically refractory temporal lobe epilepsy (TLE). The emergence of minimally invasive resective and nonresective treatment options has led to interest in epilepsy surgery among patients and providers. Nevertheless, not all procedures are appropriate for all patients, and it is critical to consider seizure outcomes with each of these approaches, as seizure freedom is the greatest predictor of patient quality of life. Standard anterior temporal lobectomy (ATL) remains the gold standard in the treatment of TLE, with seizure freedom resulting in 60–80% of patients. It is currently the only resective epilepsy surgery supported by randomized controlled trials and offers the best protection against lateral temporal seizure onset. Selective amygdalohippocampectomy techniques preserve the lateral cortex and temporal stem to varying degrees and can result in favorable rates of seizure freedom but the risk of recurrent seizures appears slightly greater than with ATL, and it is not clear whether neuropsychological outcomes are improved with selective approaches. Stereotactic radiosurgery presents an opportunity to avoid surgery altogether, with seizure outcomes now under investigation. Stereotactic laser thermo-ablation allows destruction of the mesial temporal structures with low complication rates and minimal recovery time, and outcomes are also under study. Finally, while neuromodulatory devices such as responsive neurostimulation, vagus nerve stimulation, and deep brain stimulation have a role in the treatment of certain patients, these remain palliative procedures for those who are not candidates for resection or ablation, as complete seizure freedom rates are low. Further development and investigation of both established and novel strategies for the surgical treatment of TLE will be critical moving forward, given the significant burden of this disease.
Keywords: Gamma knife, Laser ablation, Responsive neurostimulation, Selective amygdalohippocampectomy, Temporal lobectomy
1. Introduction
Surgery can be a highly effective treatment for medically refractory epilepsy. In temporal lobe epilepsy (TLE), for example, anterior temporal lobectomy (ATL) has consistently been shown to produce excellent seizure outcomes, particularly in patients with mesial temporal sclerosis (MTS). This has been demonstrated in both randomized controlled trials as well as long-term longitudinal cohort studies [1–3]. There is a remarkable concordance across studies, with 60–80% of patients achieving seizure freedom at 1–2 years after surgery, and about 50% of individuals experience durable seizure freedom at 10 years [4–6]. Seizure freedom is the single best predictor of quality of life in epilepsy, as recurrent seizures lead to significant cumulative morbidity and increased mortality [7–10]. Overall, ATL is associated with a low risk of significant morbidity [11,12] and may result in improved life span [7,13], neuropsychological profile [14,15], and quality-adjusted life years compared to a patient's presurgical baseline.
To address the considerable variability in clinical practice patterns, leading professional associations including the American Academy of Neurology (AAN) and the American Association of Neurological Surgeons (AANS) created guidelines in 2001 recommending that patients with refractory epilepsy undergo comprehensive evaluation for surgery [16]. The guidelines were implemented in part because of clear evidence that epilepsy patients who fail to respond to just two AEDs are unlikely to respond completely to additional drug combinations [17,18]. Nevertheless, despite class I evidence and the introduction of clinical guidelines, epilepsy surgery remains dramatically underutilized [19–21].
While reasons for the underutilization of epilepsy surgery are likely multifactorial, one contributory factor is perceived risk and attendant fears about open brain surgery [19,21]. Surgery is typically treated as a last resort option, often long after irreversible neural injury has occurred in the setting of longstanding, repeated seizures [8,22,23]. More recently, however, new surgical approaches have been specifically developed to be less invasive treatment alternatives for intractable focal epilepsy. While ATL remains the most established and tested surgical therapy for TLE, patients and practitioners now have additional treatment options to consider, which have catalyzed a resurgence of interest in epilepsy surgery. In the present article, we review these minimally invasive surgical options for medically refractory TLE alongside the traditional ATL, including selective mesial temporal resection, stereotactic radiosurgery, laser thermo-ablation, and palliative device implantations such as vagus nerve stimulation (VNS), responsive neurostimulation (RNS), and deep brain stimulation (DBS). Table 1 offers a summary of the advantages and disadvantages related to the various surgical approaches discussed here.
Table 1.
Comparison of surgical treatments for temporal lobe epilepsy.
| Advantages | Disadvantages | |
|---|---|---|
| Anterior temporal lobectomy (ATL) | Supported by class I evidence; best seizure outcomes | Largest incision and craniotomy; questionable neuropsychological implications of lateral cortex resection |
| Selective amygdalohippocampectomy (SAH) | Preservation of lateral cortex; smaller incision and craniotomy | Slightly worse seizure outcomes than ATL; still requires open surgery |
| Transsylvian approach | Complete preservation of lateral cortex | Technically challenging; damage to temporal stem |
| Transcortical approach | Technically less challenging | Damage to lateral cortex |
| Subtemporal approach | Avoids both sylvian fissure and lateral cortex | Possible retraction damage to basal temporal lobe |
| Gamma knife radiosurgery (GKRS) | No invasive surgery | Antiseizure effects delayed by 12–24 months |
| Stereotactic laser thermo-ablation (STA) | Only burr hole required; preliminarily favorable neuropsychological outcomes | Higher risk of persistent seizures than resection; long-term outcomes require further study |
| Device implantation | No brain resection | Palliative; worse seizure outcomes than resection/ablation |
| Responsive neurostimulation (RNS) | Direct closed-loop therapy to EZ | EZ localization required; seizure freedom is rare |
| Vagus nerve stimulation (VNS) | EZ localization not required | Seizure freedom is rare |
| Deep brain stimulation (DBS) | EZ localization not required | Seizure freedom is rare |
EZ: epileptogenic zone.
2. Anterior temporal lobectomy
We begin by describing the standard temporal lobectomy, key anatomic landmarks, and outcomes and risks associated with the procedure to provide a basis of comparison and understanding for less invasive techniques. As the procedure depends critically on a thorough understanding of temporal lobe and nearby anatomical structures, Fig. 1 provides a brief anatomical review of this region. For a more thorough review of microsurgical anatomy relevant to this procedure, the reader is referred to a recent manuscript by Kucukyuruk and Rhoton [24].
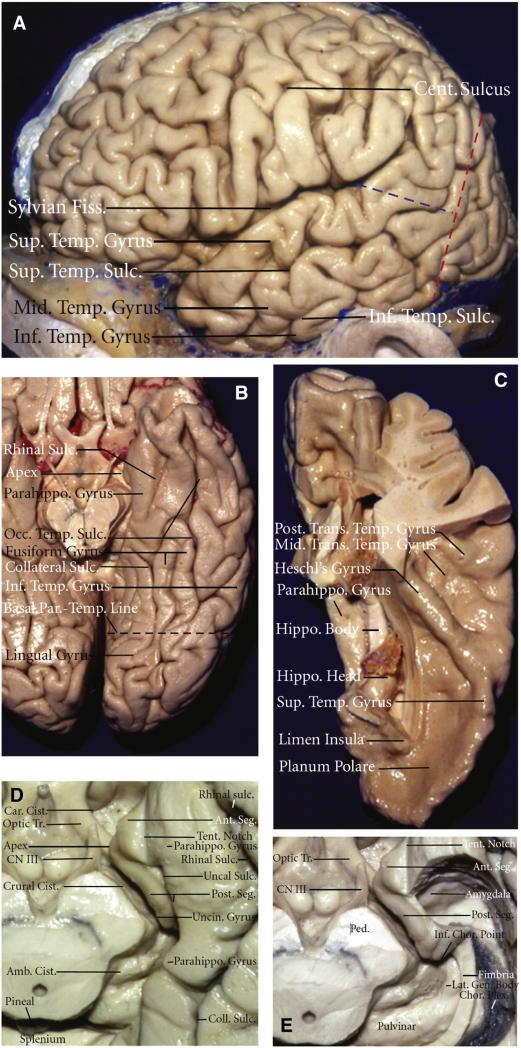
Fig. 1.
Microsurgical anatomy of the temporal lobe. A) Lateral view of the left hemisphere. The lateral surface of the temporal lobe consists of three parallel gyri: superior, middle, and inferior temporal gyri. These gyri are separated by the superior and inferior temporal sulci. The lateral parietotemporal line (red dashed line), an imaginary line connecting the preoccipital notch and parietooccipital sulcus, separates the temporal and occipital lobes, and the occipitotemporal line (blue dashed line), an imaginary line connecting the posterior margin of the sylvian fissure with lateral parietotemporal line, separates the temporal and parietal lobes. B) Inferior view of the left temporal lobe. The basal surface of the temporal lobe consists of, from lateral to medial, the inferior margin of the inferior temporal gyrus, the fusiform gyrus, and the parahippocampal gyrus. The fusiform gyrus is separated laterally from the inferior temporal gyrus by the occipitotemporal sulcus and medially from the parahippocampal gyrus by the collateral posteriorly and rhinal sulci anteriorly, which are not continuous in every case. The basal parietotemporal line connecting the preoccipital notch and inferior end of parietooccipital sulcus separates the temporal and occipital lobes at the basal surface. C) The superior view of the left temporal lobe. This surface facing the sylvian fissure is divided, from anterior to posterior, into three portions: the planum polare, the anterior transverse temporal gyrus, referred to as the Heschl's gyrus, and the planum temporale containing the middle and posterior transverse temporal gyri. D) Enlarged view of the anterior and middle segments of the medial temporal region. The anterior segment of the uncus faces the carotid cistern, and the posterior segment faces the crural cistern and the cerebral peduncle. The uncal apex is positioned lateral to the oculomotor nerve. The cortical component of the middle medial temporal region formed by the parahippocampal gyrus faces the midbrain across the ambient cistern. E) The medial temporal region with hippocampus and dentate gyrus having been removed while preserving the fimbria and the choroid plexus attached along the choroidal fissure. The amygdala forms the anterior wall of the temporal horn and fills most of the anterior segment of the uncus. The inferior choroidal point, located at the lower end of the attachment of the choroid plexus in the temporal horn, is positioned behind the head of the hippocampus, anterior to the lateral geniculate body, and lateral to the posterior edge of the cerebral peduncle. Amb.: ambient; Ant.: anterior; Calc.: calcarine; Car.: carotis; Cent., central; Chor.: choroidal; Cist: cistern; Coll.: collateral; CN III: oculomotor nerve; Fiss.: fissure; Gen.: geniculate; Hippo.: hippocampus; Inf.: inferior; Lat.: lateral; Mid.: middle; Occ.: occipital; Parahippo.: parahippocampal; Par.-Occip.: parietooccipital; Par.-Temp.: parietotemporal; Ped.: cerebral peduncle; Post.: posterior; Seg.: segment; Sulc.: sulcus; Sup.: superior; Temp.: temporal; Tent.: tentorial; Tr.: tract; Trans.: transverse; Uncin.: uncinate. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Figure and legend modified and reproduced with permission from Kucukyuruk et al. [24] distributed under the Creative Commons Attribution License.
It is first important to establish whether one is operating on the dominant or nondominant hemisphere, as this will impact how far posteriorly the resection can be made on the lateral cortical surface of the temporal lobe. The authors suggest that it is safe to resect 4–4.5 cm of temporal lobe on the dominant hemisphere to avoid injury to language areas and 5.5 cm on the nondominant side [5,25]. The vein of Labbé is another structure that can limit the extent of posterior resection and should also be visualized and protected throughout the procedure. Of note, extending the posterior resection margin on the temporal lobe can injure the geniculo-calcarine tracts causing homonymous hemianopsia and, therefore, should be avoided. Aside from language, memory is another important consideration when operating on the dominant hemisphere. In dominant hemisphere MTS in patients with normal memory, it is important to counsel patients that they are at risk for a decline in verbal memory, while in patients with diminished memory and dominant hemisphere MTS, surgical resection has not been shown to worsen memory and actually may offer some improvement over time if the seizures subside and the medications are decreased [5].
Once the appropriate distance has been measured posteriorly, corticectomy is performed a few millimeters below the sylvian fissure along the superior temporal gyrus and followed anteriorly towards the pole. Dura should be visible anterior to the temporal pole at this point. Subpial aspiration is continued over the insular pia and followed inferior until the inferior circular sulcus appears. Branches of the middle cerebral artery (MCA) can be visualized through the pia and care should be taken to avoid injury or excessive manipulation, which can lead to MCA vaso-spasm [26]. The temporal stem begins just under the inferior circular sulcus, and it is important not to resect deep to this structure, as injury to basal ganglia and brainstem structures may occur. The temporal stem contains white matter tracts important to cognition including the inferior frontooccipital fasciculus (IFOF) and the uncinate fasciculus. The posterior cut is then performed at the predetermined distance and may be extended posteriorly and inferiorly towards the middle cranial fossa floor. Subpial aspiration is utilized to extend the cut deeper within the temporal lobe. The collateral sulcus is an important structure that can assist with locating the temporal horn of the lateral ventricle by using gentle aspiration to follow its direction. Once this structure has been located, the lateral ventricular sulcus can be incised and followed anteriorly to assist with removal of the lateral temporal lobe.
At this point, the hippocampus and amygdala should be visible, along with some residual parahippocampal gyrus. This may be aspirated to allow for lateral rotation of the hippocampus and to assist with resection. The inferior choroidal point is an important landmark and, along with the roof of the temporal horn, serves as the superior border for removal of the mesial structures. Care must be taken to avoid injury to the anterior choroidal artery as this can lead to serious neurologic complications. One may follow the superior boundaries of the inferior choroidal point and roof of the ventricle to remove the amygdala and part of the uncus. The posterior boundary of the amygdala is the amygdala-hippocampal sulcus, and this is the last cut to be made prior to removal of the amygdala. Mesially, there is a layer of pia that will protect the underlying cisternal structures, and this should be respected. The hippocampus is then removed by subpial aspiration over the hippocampal sulcus and following this posteriorly until the fornix is visible. Tiny vessels within the hippocampal sulcus should be coagulated and sharply dissected, and care should be taken as these may retract into the perimesencephalic cisterns, causing subarachnoid bleeding which can be difficult to control. Posteriorly, the tail of the hippocampus may be resected as it extends around the brainstem.
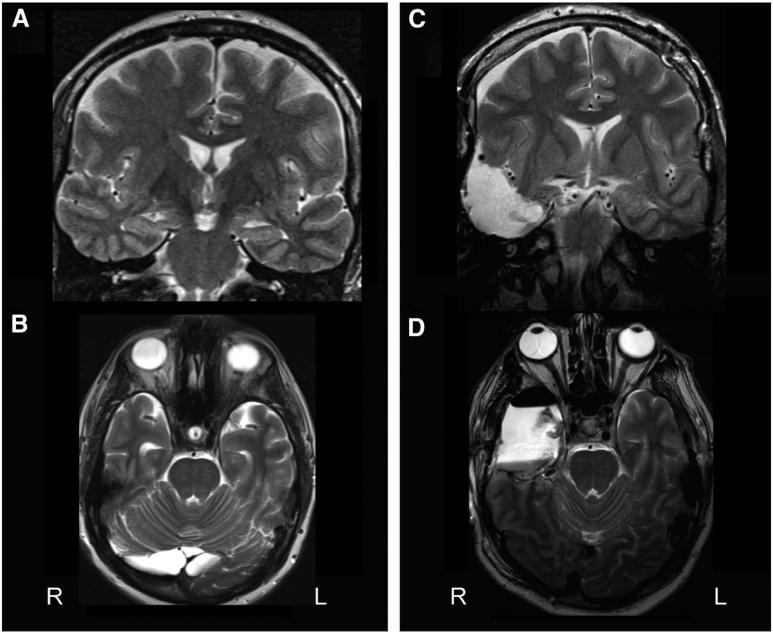
When the mesial structures have been completely removed, structures that should be visible through the mesial pia include the third cranial nerve, posterior cerebral artery, cerebral peduncles, and lateral geniculate nucleus. At this point, adequate hemostasis is performed, the craniotomy flap is replaced, and the wound is closed in layers. Fig. 2 shows an example MRI before and after ATL, delineating resection of the anterior hippocampus to the level of the tectal plate, the amygdala, and approximately a 4-cm resection of the anterolateral temporal neocortex, including the superior, middle, and inferior temporal gyri.
Fig. 2.
Demonstration of anterior temporal lobectomy for mesial temporal lobe epilepsy. A, B) Preoperative T2-weighted coronal (A) and axial (B) MRI showing increased T2 signal, decreased size, and diminished cytoarchitecture of the left hippocampus, consistent with mesial temporal sclerosis. C, D) Preoperative T2-weighted coronal (C) and axial (D) MRI demonstrating the resection cavity after left anterior temporal lobectomy. The resection involves the anterior hippocampus to the level of the tectal plate, the amygdala, and an approximately 4-cm resection of the anterolateral temporal neocortex, including the middle and inferior temporal gyri.
Seizure outcomes with ATL are favorable, producing seizure freedom in 60–80% of patients with TLE across randomized controlled trials, retrospective cohort studies, and meta-analyses [1,3,12,27,28]. In contrast, less than 5% of patients who do not receive surgery (but continue aggressive medical management) achieve remission each year [2,17, 29–32]. Furthermore, ATL is associated with only 2% significant morbidity and 0.24% total surgical mortality [11,12,31], compared to a 0.5–1% annual mortality rate and a lifetime standardized mortality ratio of 2–3 times the general population with persistent intractable epilepsy [11,12,31]. Thus, while the risks of ATL must be carefully considered, they are relatively small compared to the lifetime risk of uncontrolled epilepsy.
3. Minimally invasive resective or ablative procedures
Less invasive approaches for resection or ablation of medial temporal structures in mesial TLE include selective amygdalohippocampectomy (SAH), gamma knife stereotactic radiosurgery (GKRS), and stereotactic laser thermo-ablation (SLA). These procedures typically allow greater preservation of tissue and permit a smaller incision and craniotomy (i.e., SAH), or small burr holes (i.e., SLA), or avoid open surgery altogether (i.e., GKRS). A critical distinction between these techniques and standard ATL is that the former involves preservation of the lateral temporal neocortex. Therefore, they are only indicated for patients with mesial temporal seizure onset and may confer a higher risk of persistent seizures in patients harboring a lateral temporal epileptogenic zone.
3.1. Selective amygdalohippocampectomy
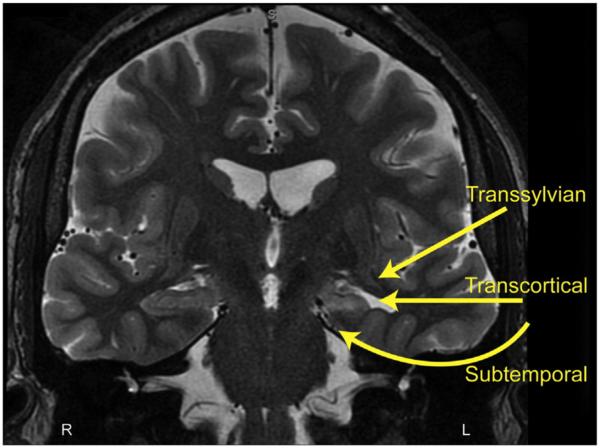
The three main surgical techniques that have been described for SAH for mesial TLE include the transsylvian, transcortical, and subtemporal approaches, as summarized in Fig. 3. Yasargil originally described a transsylvian approach to the mesial structures, involving microsurgical opening of the sylvian fissure to access the amygdala and hippocampus while avoiding resection of the lateral temporal lobe [33,34]. This technique requires partial transection of the temporal stem in order to access the body and tail of the hippocampus. To begin, a standard pterional craniotomy is performed and the proximal fissure is carefully dissected. The limen insulae is an important landmark, as it establishes the location of the MCA bifurcation. The pia is opened over the piriform cortex to expose the amygdala. This can be dissected out in its entirety. Then, the temporal horn is adequately visualized. The choroid plexus should be seen on the hippocampus. This can be retracted laterally and protected while the hippocampus resection is performed. The tail of the hippocampus is difficult to reach from this approach without significant retraction on the temporal stem, and for this reason, an opening is made in the temporal stem to gain access to the distal hippocampus. Alternatively, an ultrasonic tissue aspirator or suction may be used to re-sect the hippocampus in its entirety [34]. Compared to the other SAH approaches, the transsylvian technique is a more technically challenging procedure, which may put the MCA branches at greater risk [34].
Fig. 3.
Microsurgical approaches for selective amygdalohippocampectomy. Three main approaches are shown for selective resection of the mesial temporal structures while preserving lateral temporal neocortex. The transsylvian approach includes microsurgical splitting of the sylvian fissure and traversing a portion of the temporal stem, the transcortical approach proceeds through a limited lateral temporal corticectomy while the subtemporal approach involves gentle elevation of the temporal lobe to identify and enter the collateral sulcus. L: left; R: right.
Selective amygdalohippocampectomy can also be achieved with a transcortical approach, as described by Olivier. Unlikely the transsylvian approach, a middle cranial fossa craniotomy is performed. Neuronavigation is utilized for the transcortical portion of the surgery. The middle (or inferior) temporal gyrus is coagulated and opened parallel to the sulci and the white matter of the temporal lobe is gently aspirated to approach the mesial structures. The first structure encountered is the temporal horn, and utilization of neuronavigation is important to maintain the correct approach. Once the temporal horn has been entered, the amygdala, hippocampus, inferior choroidal point, and choroid plexus will be visualized. Resection of these structures is performed in the same manner that has been described previously [35,36]. Compared to the transsylvian procedure, the main benefit to a selective transcortical approach is that the arterial and venous structures are avoided, and minimal vessel sacrifice is required. The main criticism of the transcortical approach is that retraction injury to tissue around the corticectomy can be significant, and it may lead to some disconnection of the basal lateral temporal lobe.
Another variation of the SAH originally described by Hori utilizes a subtemporal approach to the mesial structures [37,38]. This technique was modified and updated using a small keyhole craniotomy approach, stereotactic guidance, and ultrasonic aspiration to approach the amygdala and hippocampus while minimizing damage to surrounding structures [39]. Both procedures are performed by centering the craniotomy around the root of the zygoma and positioning the patient so that the temporal lobe falls away from the middle cranial fossa floor. Again, the temporal horn is a critical landmark for demarcating the lateral boundary of the hippocampus, and it is entered in the subtemporal approach through the fusiform gyrus or collateral sulcus. Removal of the mesial structures is performed through this narrow working corridor [38,39]. This approach has the theoretical benefit of not damaging or disconnecting the remaining temporal lobe. The positioning of the patient can be difficult in this procedure, especially in obese individuals, and care must be taken to not retract the basal temporal lobe forcefully, as this can cause significant injury.
There has been significant interest in comparing both seizure and neuropsychological outcomes between SAH and standard ATL and among the various SAH approaches. With regard to postoperative seizure freedom, while conflicting results have been reported by smaller studies comparing SAH to ATL, a recent meta-analysis comparing these techniques across 11 studies of 1203 patients demonstrated significantly higher seizure freedom rates after standard ATL, with a summary risk difference of 8% [40]. Many proponents of SAH have argued that these techniques should improve postoperative neuropsychological outcomes because the lateral temporal lobe is not resected, but there is still a great deal of controversy in the literature about this [38, 41,42]. It has been hypothesized that the transection or retraction of a portion of the temporal stem may lead to the neurocognitive deficits experienced in these patients postoperatively and may explain why SAH has not been shown to be superior to resection of the lateral temporal lobe. Another important consideration is that ongoing epilepsy itself contributes to devastating cognitive and neuropsychological sequelae that are frequently suffered in this disorder [8,43,44]. Therefore, the authors suggest that if SAH has a lower rate of seizure freedom, improvement in neuropsychological testing may be mitigated by the cognitive decline associated with persistent seizures after SAH compared to ATL.
3.2. Stereotactic radiosurgery
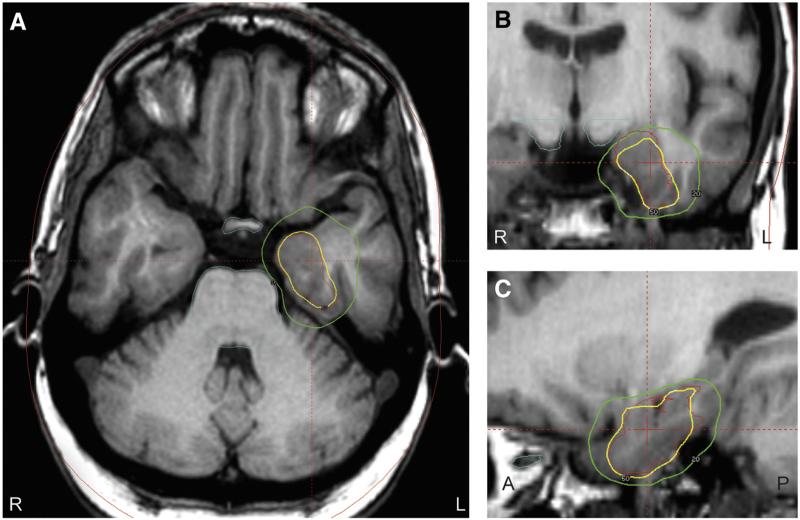
As previously discussed, methods to treat the mesial temporal structures (amygdala and hippocampus) without injury to the surrounding neocortical regions have the theoretical benefit of minimizing cognitive decline in patients with MTS. For this reason, great interest has developed for SRS, which utilizes stereotactic radioactive cobalt to deliver 192 beams of radiation to a focal region in the brain while leaving the remaining brain tissue intact [45,46]. Fig. 4 demonstrates an example of the stereotactic radiation dose planning for treatment of the mesial temporal structures. Stereotactic radiosurgery may also be an excellent option for patients who refuse invasive open surgical treatment for the treatment of MTS. Several studies, including a pilot multicenter prospective trial, have been performed utilizing gamma knife SRS to treat patients with MTS [45,47,48]. These have shown seizure outcomes similar to ATL, especially in patients undergoing high dose therapy (24 vs 20 Gy; targets to amygdala, head of the hippocampus, and parahippocampal gyrus). In these investigations, side effects were minimal and included temporary steroid requirements, headaches, and visual deficits. One patient in the multicenter trial was found to have malignant edema after treatment, which included severe headaches, papilledema and visual field deficits not responsive to steroids, eventually requiring temporal lobectomy [48]. Furthermore, neurocognitive decline was not observed as postoperative neuropsychological outcomes were stable from baseline [49]. This pilot study confirmed SRS as a potential treatment option in patients with MTS and led to a prospective randomized trial of SRS versus open ATL currently underway (Radiosurgery or Open Surgery for Epilepsy [ROSE] trial).
Fig. 4.
Gamma knife stereotactic radiosurgery planning in a patient with mesial temporal lobe epilepsy. On axial (A), coronal (B), and sagittal (C) T1-weighted MRI images, treatment is planned at the mesial temporal structures, with 50% isodose line shown in yellow, 20% isodose line displayed in green, and crosshairs approximating the center of the target. Care is taken to limit the radiation dose to the optic apparatus and brainstem. A: anterior; L: left; P: posterior; R: right. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Importantly, the effects of SRS on seizure control may take up to 12–24 months after treatment to appear compared to immediate postoperative seizure control with resective strategies. Chang et al. described that MRI characteristics within one year following SRS may serve as a predictor of seizure freedom at three years after the treatment [50]. T2 hyperintensity volumes at nine months were found to be highly related to seizure remission at three years. Spectroscopy was consistent with a radiation necrosis mechanism of action, suggesting that the treatment resulted in tissue destruction. Fig. 5 summarizes the development of these radiographic changes over time after SRS. Long-term studies will need to be performed to evaluate seizure and cognitive outcomes, specifically relating to safety and efficacy compared with ATL.
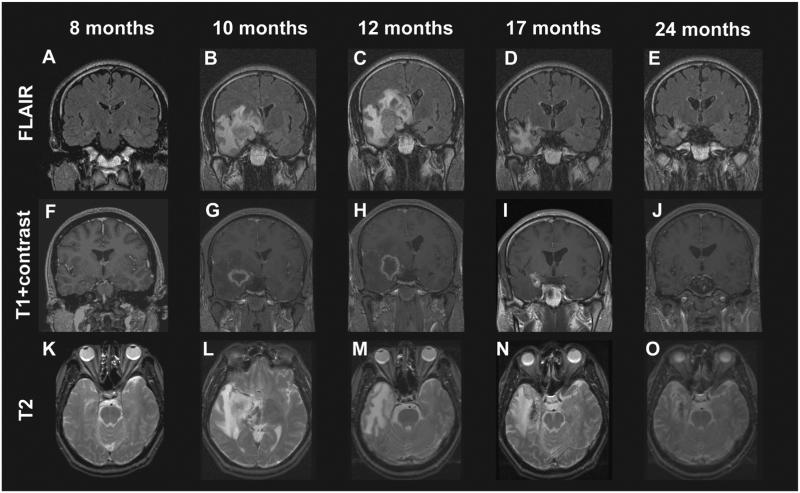
Fig. 5.
Development of radiologic changes in a patient with mesial temporal lobe epilepsy treated with a 24-Gy dose gamma knife stereotactic radiosurgery. FLAIR (A–E) and T2 (K–O) hyperintensity appeared within the medial temporal lobe beginning by the 10th postoperative month and peaked in intensity at 12 months, corresponding to a decline in the proportion of patients experiencing complex partial seizures. Contrast enhancement (F–J) followed a similar time course, except that it preceded T2 changes and diminished quickly after months 10–12. Enhancement was typically ring-enhancing and centered over the target region.
Figure and legend reproduced with license and permission from Chang et al. [73].
3.3. Stereotactic laser thermo-ablation
Radiofrequency ablation has been utilized to create lesions for the treatment of Parkinson's disease but difficulty controlling and monitoring the ablation limited its use in treating larger targets [74]. A renewed interest in laser interstitial thermal therapy has emerged as improvements in real-time MRI thermometry have made this a safe and precise method to perform lesioning techniques. Specifically, STA techniques, such as those from Visualase (Medtronic) and NeuroBlate (Monteris), have been advocated as a minimally invasive technique to create lesions in the mesial temporal lobe structures with minimal damage to surrounding tissues [51,52]. In fact, when the hippocampus is accessed from its longitudinal axis, the inferior horn of the lateral ventricle and the cisterns medially act as a heat sink, thereby preventing thermal injury from extending outside of the mesial structures [75]. The scalp incision may be as small as 1 cm and closed simply with one or two staples. An example of laser thermal ablation for mesial TLE is depicted in (Fig. 6).
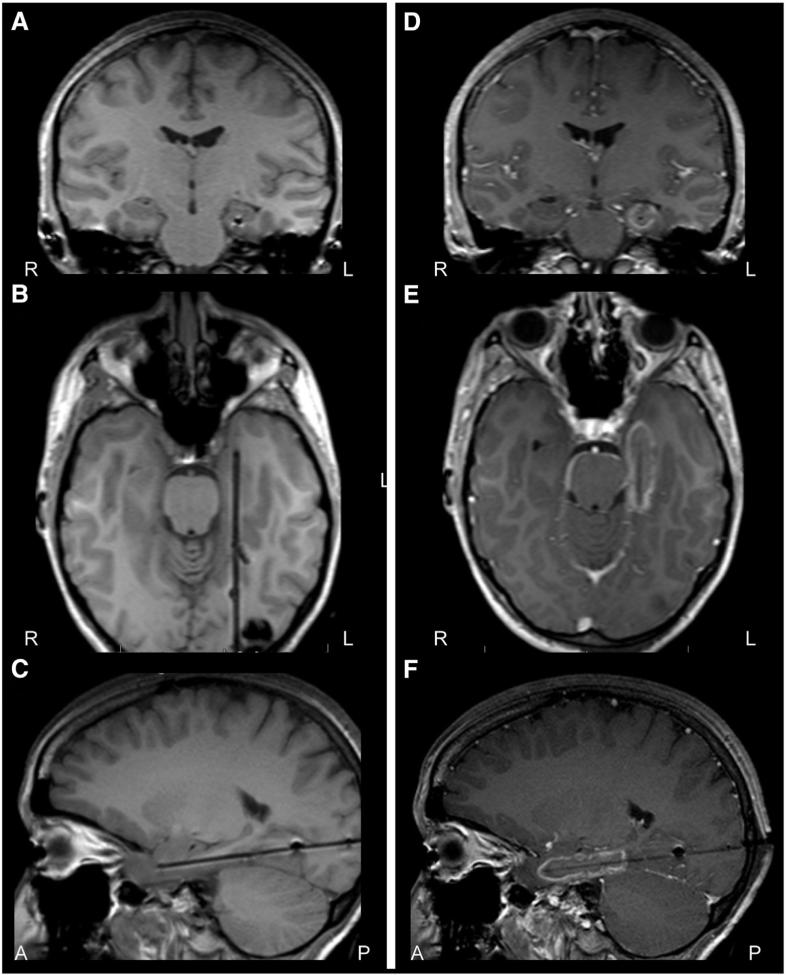
Fig. 6.
Stereotactic laser thermo-ablation in mesial temporal lobe epilepsy. A–C) T1-weighted periprocedural MRI coronal (A), axial (B), and sagittal (C) images showing laser probe placement along the axis of the left hippocampus, prior to thermo-ablation in a patient with mesial temporal lobe epilepsy. D–F) Contrast-enhanced T1-weighted MRI coronal (D), axial (E), and sagittal (F) images after thermo-ablation of mesial temporal lobe structures, with contrast enhancement observed in the region of ablation. A: anterior; L: left; P: posterior; R: right.
In one recent series of 13 adult patients with intractable mesial TLE with or without mesial temporal sclerosis, a mean 60% volume of the amygdalohippocampal complex was ablated, and median hospitalization was 1 day [52]. With follow-up ranging from 5 to 26 months (median, 14 months), 54% were free of disabling seizures. One significant complication included a visual field defect, resulting from deviated insertion of a stereotactic aligning rod, which was corrected before ablation [52]. Based on preliminary data, a few authors have suggested improved neuropsychological profiles with STA compared to SAH, with favorable naming and recognition outcomes [51,53].
Overall, early data suggest relatively favorable seizure outcomes with STA for MTS, although seizure freedom is likely less common compared with resection. Nevertheless, the minimally invasive aspect of the procedure may be quite desirable for some patients who wish to avoid open surgery. Given the early stage of this novel treatment, significantly more data are required to ascertain long-term seizure and cognitive outcomes with SLA compared to ATL and SAH.
4. Minimally invasive nonresective palliative procedures
In most cases, the goal of epilepsy surgery is to excise or ablate an abnormal region (or regions) of the brain harboring an epileptogenic zone and free the patient of seizures. However, many patients with pharmacoresistant epilepsy are not candidates for resection, such as those with generalized epilepsy syndromes and those with a seizure focus that is poorly localized, multifocal, or positioned in the eloquent brain. In TLE, seizure origination from bilateral mesial temporal lobes is an example in which resection or ablation is not an option. For these individuals, palliative surgical options are considered to reduce the frequency and severity of seizures. These include device implantation for stimulation-based therapy using vagus nerve stimulation (VNS), deep brain stimulation (DBS), or responsive neurostimulation (RNS). Each of these three stimulation devices has been evaluated in randomized, controlled trials.
4.1. Responsive neurostimulation
Unlike open-loop stimulation paradigms which utilize uninterrupted electrical pulses, such as VNS and DBS, RNS uses a closed-loop stimulation system. Implanted subdural and depth electrodes continuously record and analyze regional electrocorticographic signals, and stimulation is triggered by electrographic activity concerning for seizure initiation, with the hope of terminating the discharge before it becomes clinically apparent [54]. In February 2013, RNS received US FDA approval for the treatment of intractable partial epilepsy in adults [55]. In patients with bilateral mesial TLE, bilateral electrode placement in the hippocampi can be performed [56].
Responsive neurostimulation was evaluated in a recent multicenter, double-blind, randomized, controlled trial termed the RNS System Pivotal Trial [54]. In the study, 191 adults with pharmacoresistant partial epilepsy were implanted with the RNS system and randomized to receive responsive stimulation or seizure detection alone during a 12-week blinded period. Patients receiving stimulation reported a decrease in seizure frequency of 38% versus 17% in the sham-treated group, and 29% of stimulated patients reported ≥50% reduction in seizures, though this outcome was also reported in 27% of control subjects. After 3 months, patients in both groups were assigned to receive therapeutic stimulation during the open-label period. The median percent reduction in seizures during the open-label period was 44% at 1 year and 53% at 2 years, representing a progressive improvement with time [57]. Overall adverse events included device site infection (5.2%), headache (10.5%), dysesthesia (6.3%), increase in complex-partial (5.8%) or generalized (4.7%) seizures, and other less common complications [54].
Overall, RNS appears safe, and many patients do see clinical improvement after 1–2 years of treatment. Tailored responsive stimulation therapy for epilepsy is an important area of investigation, given potential benefits over closed-loop stimulation therapies, including decreased side effects and increased device battery life [54,58]. Broader clinical use of RNS will be dependent on further study and continued improvements in this technology.
4.2. Vagus nerve stimulation
Vagus nerve stimulation was approved by the United States Food and Drug Administration (US FDA) in 1997 as an adjunctive treatment for pharmacoresistant epilepsy, and over 100,000 stimulators have been implanted worldwide. Vagus nerve stimulation is often considered in patients with intractable epilepsy who are poor candidates for, or have failed, resective surgery. In TLE, this may include individuals with persistent debilitating seizures after maximal unilateral temporal lobe resection or those who refuse resective therapy. The efficacy of VNS for intractable seizures has been evaluated by 3 blinded, randomized controlled trials. In the first trial, Ben-Menachem and colleagues randomized 114 patients with focal epilepsy to receive therapeutic or sham stimulation after VNS implantation, and reported a significantly greater reduction in seizure frequency with therapeutic stimulation after 3 months of treatment (25% versus 6%) [59]. Similar results were reported in 2 subsequent blinded randomized, controlled trials [60,61] and 2 nonblinded controlled studies [62,63].
In a large meta-analysis including 3321 patients treated with VNS from 77 reports, 51% of patients treated with VNS achieved ≥50% reduction in seizure frequency from baseline, after a mean follow-up of 10 months. Longer duration of therapy had a significant positive influence on seizure control rates, although few (5–10%) patients achieved complete seizure freedom, and one-quarter of individuals reported no measurable benefit from stimulation. Similar outcomes have been found with analysis of the device manufacturer's patient database. Interestingly, patients with a history of posttraumatic epilepsy or Lennox–Gastaut syndrome may have improved response to treatment [64–68]. Adverse events associated with treatment include hoarseness (37–62%), cough (7–21%), pain (6–17%), and infection (4–6%), and rare incidences of asystole have been reported [59,61,65,69]. Given relatively similar rates of seizure reduction, a prospective comparison of RNS to VNS in patients with TLE who are not candidates for resection or ablation is warranted.
4.3. Deep brain stimulation
In the early and mid-20th century, intraparenchymal electrical stimulation of the cerebellum was first examined as a potential treatment for intractable epilepsy [70,71]. Since then, several other brain structures have been proposed as potential stimulation targets, including the hippocampus, subthalamic nucleus, caudate nucleus, and centromedian nucleus. More recently, focus has turned to the anterior nucleus of the thalamus, a structure intimately involved in limbic circuitry and with widespread neocortical projections. Thalamic stimulation has received approval as an adjunctive treatment for pharmacoresistant epilepsy in Europe and Canada, but it is not approved by the US FDA.
In 2010, the effectiveness of thalamic DBS was studied in 100 adults with pharmacoresistant partial epilepsy in the double-blinded, randomized Stimulation of the Anterior Nucleus of Thalamus for Epilepsy (SANTE) trial [72]. In the initial 3-month blinded phase of the SANTE trial, patients receiving stimulation had a significantly larger decrease in seizure frequency (40%) than those in the control group (14.5%). After patients were unblinded and all were treated with stimulation for 2 years, median seizure frequency was reduced by 56%, with 54% of individuals achieving seizure reduction of ≥50%. There was a trend towards better seizure control with longer periods of stimulation, resembling a similar relationship between treatment duration and efficacy observed with VNS. Adverse events in the first year of thalamic DBS included paresthesias in 18% of patients, surgical site pain in 11%, site infections in 9%, and lead replacement in 8%, though these rates declined in the second treatment year [72]. There were no differences in cognition or mood between treated and untreated patients, but depression was more commonly reported with stimulation. In patients with bilateral TLE, studies directly comparing continuous open-loop thalamic stimulation with DBS versus periodic closed-loop hippocampal stimulation with RNS have not yet been reported.
5. Conclusions
Despite class I evidence and practice guidelines supporting surgical evaluation for patients with medically refractory focal epilepsy, surgical treatment of TLE remains significantly underutilized. The emergence of several less invasive resective and nonresective treatment options has led to a renewed interest in epilepsy surgery among patients and providers. Furthermore, many of these treatments offer reduced surgical risk, fewer cosmetic concerns, and shorter recovery periods. Nevertheless, not all procedures are appropriate for all patients, and it is critical to consider seizure outcomes with each of these approaches, as seizure freedom remains the greatest predictor of patient quality of life.
Standard ATL remains the gold standard in the treatment of TLE, with seizure freedom resulting in 60–80% of patients. It is currently the only resective epilepsy surgery supported by randomized controlled trials and offers the best protection against lateral temporal neocortical seizure onset. Selective amygdalohippocampectomy techniques preserve the lateral cortex and temporal stem to varying degrees and result in favorable rates of seizure freedom, but the risk of recurrent seizures appears slightly greater than with ATL. Furthermore, data supporting improved neuropsychological outcomes with SAH compared to ATL have not been particularly convincing.
Stereotactic radiosurgery presents the opportunity to avoid invasive surgery altogether, with seizure outcomes based on studies to date resembling resection, with the important disadvantage of antiseizure effects lagging 1–2 years after the procedure. Stereotactic laser thermo-ablation allows destruction of the mesial temporal structures with low complication rates and minimal recovery time, but seizure freedom rates appear lower compared with open resection, and long-term outcomes remain under investigation. Finally, while neuromodulatory devices such as RNS, VNS, and DBS have an important role in the treatment of certain patients, these remain palliative procedures for individuals who are not candidates for resection or ablation, as complete seizure freedom rates are low. Further development and investigation of both established and novel strategies for the surgical treatment of TLE will be critical moving forward, given the significant burden presented by this disease.
Footnotes
Conflict of interest
The authors have no conflicts of interest to disclose.
References
- 1.Englot DJ, Lee AT, Tsai C, Halabi C, Barbaro NM, Auguste KI, et al. Seizure types and frequency in patients who “fail” temporal lobectomy for intractable epilepsy. Neurosurgery. 2013;73:838–44. doi: 10.1227/NEU.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 2.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–8. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 3.Engel J, Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. 2012;307:922–30. doi: 10.1001/jama.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeha LE, Najm IM, Bingaman WE, Khandwala F, Widdess-Walsh P, Morris HH, et al. Predictors of outcome after temporal lobectomy for the treatment of intractable epilepsy. Neurology. 2006;66:1938–40. doi: 10.1212/01.wnl.0000219810.71010.9b. [DOI] [PubMed] [Google Scholar]
- 5.Vadera S, Kshettry VR, Klaas P, Bingaman W. Seizure-free and neuropsychological outcomes after temporal lobectomy with amygdalohippocampectomy in pediatric patients with hippocampal sclerosis. J Neurosurg Pediatr. 2012;10:103–7. doi: 10.3171/2012.4.PEDS1233. [DOI] [PubMed] [Google Scholar]
- 6.Englot DJ, Chang EF. Rates and predictors of seizure freedom in resective epilepsy surgery: an update. Neurosurg Rev. 2014;37:389–404. doi: 10.1007/s10143-014-0527-9. discussion 404–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi H, Sell RL, Lenert L, Muennig P, Goodman RR, Gilliam FG, et al. Epilepsy surgery for pharmacoresistant temporal lobe epilepsy: a decision analysis. JAMA. 2008;300:2497–505. doi: 10.1001/jama.2008.771. [DOI] [PubMed] [Google Scholar]
- 8.Helmstaedter C, Kockelmann E. Cognitive outcomes in patients with chronic temporal lobe epilepsy. Epilepsia. 2006;47(Suppl. 2):96–8. doi: 10.1111/j.1528-1167.2006.00702.x. [DOI] [PubMed] [Google Scholar]
- 9.Elliott I, Kadis DS, Lach L, Olds J, McCleary L, Whiting S, et al. Quality of life in young adults who underwent resective surgery for epilepsy in childhood. Epilepsia. 2012;53:1577–86. doi: 10.1111/j.1528-1167.2012.03594.x. [DOI] [PubMed] [Google Scholar]
- 10.Macrodimitris S, Sherman EM, Williams TS, Bigras C, Wiebe S. Measuring patient satisfaction following epilepsy surgery. Epilepsia. 2011;52:1409–17. doi: 10.1111/j.1528-1167.2011.03160.x. [DOI] [PubMed] [Google Scholar]
- 11.Wiebe S. Effectiveness and safety of epilepsy surgery: what is the evidence? CNS Spectr. 2004;9:120–2. doi: 10.1017/s1092852900008488. [DOI] [PubMed] [Google Scholar]
- 12.Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7:525–37. doi: 10.1016/S1474-4422(08)70109-1. [DOI] [PubMed] [Google Scholar]
- 13.Sperling MR, Feldman H, Kinman J, Liporace JD, O'Connor MJ. Seizure control and mortality in epilepsy. Ann Neurol. 1999;46:45–50. doi: 10.1002/1531-8249(199907)46:1<45::aid-ana8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 14.Wachi M, Tomikawa M, Fukuda M, Kameyama S, Kasahara K, Sasagawa M, et al. Neuropsychological changes after surgical treatment for temporal lobe epilepsy. Epilepsia. 2001;42(Suppl. 6):4–8. [PubMed] [Google Scholar]
- 15.Westerveld M, Sass KJ, Chelune GJ, Hermann BP, Barr WB, Loring DW, et al. Temporal lobectomy in children: cognitive outcome. J Neurosurg. 2000;92:24–30. doi: 10.3171/jns.2000.92.1.0024. [DOI] [PubMed] [Google Scholar]
- 16.Engel J, Jr, Wiebe S, French J, Sperling M, Williamson P, Spencer D, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60:538–47. doi: 10.1212/01.wnl.0000055086.35806.2d. [DOI] [PubMed] [Google Scholar]
- 17.Kwan P, Sperling MR. Refractory seizures: try additional antiepileptic drugs (after two have failed) or go directly to early surgery evaluation? Epilepsia. 2009;50(Suppl. 8):57–62. doi: 10.1111/j.1528-1167.2009.02237.x. [DOI] [PubMed] [Google Scholar]
- 18.Haneef Z, Stern J, Dewar S, Engel J., Jr Referral pattern for epilepsy surgery after evidence-based recommendations: a retrospective study. Neurology. 2010;75:699–704. doi: 10.1212/WNL.0b013e3181eee457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Englot DJ, Ouyang D, Garcia PA, Barbaro NM, Chang EF. Epilepsy surgery trends in the United States, 1990–2008. Neurology. 2012;78:1200–6. doi: 10.1212/WNL.0b013e318250d7ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Englot DJ, Ouyang D, Wang DD, Rolston JD, Garcia PA, Chang EF. Relationship between hospital surgical volume, lobectomy rates, and adverse perioperative events at US epilepsy centers. J Neurosurg. 2013;118:169–74. doi: 10.3171/2012.9.JNS12776. [DOI] [PubMed] [Google Scholar]
- 21.Engel J., Jr Why is there still doubt to cut it out? Epilepsy Curr. 2013;13:198–204. doi: 10.5698/1535-7597-13.5.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonilha L, Rorden C, Appenzeller S, Coan AC, Cendes F, Li LM. Gray matter atrophy associated with duration of temporal lobe epilepsy. Neuroimage. 2006;32:1070–9. doi: 10.1016/j.neuroimage.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 23.Diehl B, LaPresto E, Najm I, Raja S, Rona S, Babb T, et al. Neocortical temporal FDG-PET hypometabolism correlates with temporal lobe atrophy in hippocampal sclerosis associated with microscopic cortical dysplasia. Epilepsia. 2003;44:559–64. doi: 10.1046/j.1528-1157.2003.36202.x. [DOI] [PubMed] [Google Scholar]
- 24.Kucukyuruk B, Richardson RM, Wen HT, Fernandez-Miranda JC, Rhoton AL., Jr Micro-surgical anatomy of the temporal lobe and its implications on temporal lobe epilepsy surgery. Epilepsy Res Treat. 2012;2012:769825. doi: 10.1155/2012/769825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spencer DD, Spencer SS. Surgery for epilepsy. Neurol Clin. 1985;3:313–30. [PubMed] [Google Scholar]
- 26.Lackner P, Koppelstaetter F, Ploner P, Sojer M, Dobesberger J, Walser G, et al. Cerebral vasospasm following temporal lobe epilepsy surgery. Neurology. 2012;78:1215–20. doi: 10.1212/WNL.0b013e318250d7d6. [DOI] [PubMed] [Google Scholar]
- 27.Englot DJ, Rolston JD, Wang DD, Sun PP, Chang EF, Auguste KI. Seizure outcomes after temporal lobectomy in pediatric patients. J Neurosurg Pediatr. 2013;12:134–41. doi: 10.3171/2013.5.PEDS12526. [DOI] [PubMed] [Google Scholar]
- 28.Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128:1188–98. doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- 29.Choi H, Heiman GA, Munger Clary H, Etienne M, Resor SR, Hauser WA. Seizure remission in adults with long-standing intractable epilepsy: an extended follow-up. Epilepsy Res. 2011;93:115–9. doi: 10.1016/j.eplepsyres.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gazzola DM, Balcer LJ, French JA. Seizure-free outcome in randomized add-on trials of the new antiepileptic drugs. Epilepsia. 2007;48:1303–7. doi: 10.1111/j.1528-1167.2007.01136.x. [DOI] [PubMed] [Google Scholar]
- 31.Thom M, Mathern GW, Cross JH, Bertram EH. Mesial temporal lobe epilepsy: how do we improve surgical outcome? Ann Neurol. 2010;68:424–34. doi: 10.1002/ana.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–9. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 33.Wieser HG, Yasargil MG. Selective amygdalohippocampectomy as a surgical treatment of mesiobasal limbic epilepsy. Surg Neurol. 1982;17:445–57. doi: 10.1016/s0090-3019(82)80016-5. [DOI] [PubMed] [Google Scholar]
- 34.Yasargil MG, Krayenbuhl N, Roth P, Hsu SP, Yasargil DC. The selective amygdalohippocampectomy for intractable temporal limbic seizures. J Neurosurg. 2010;112:168–85. doi: 10.3171/2008.12.JNS081112. [DOI] [PubMed] [Google Scholar]
- 35.Olivier A. Transcortical selective amygdalohippocampectomy in temporal lobe epilepsy. Can J Neurol Sci. 2000;27(Suppl. 1):S68–76. doi: 10.1017/s031716710000069x. discussion S92-6. [DOI] [PubMed] [Google Scholar]
- 36.Tanriverdi T, Olivier A. Cognitive changes after unilateral cortico-amygdalohippocampectomy unilateral selective-amygdalohippocampectomy mesial temporal lobe epilepsy. Turk Neurosurg. 2007;17:91–9. [PubMed] [Google Scholar]
- 37.Hori T, Kondo S, Takenobu A, Hirao J, Kohaya N, Takeuchi H, et al. Retrolabyrinthine presigmoid transpetrosal approach for selective subtemporal amygdalohippocampectomy. Neurol Med Chir (Tokyo) 1999;39:214–24. doi: 10.2176/nmc.39.214. discussion 224–5. [DOI] [PubMed] [Google Scholar]
- 38.Hori T, Yamane F, Ochiai T, Kondo S, Shimizu S, Ishii K, et al. Selective subtemporal amygdalohippocampectomy for refractory temporal lobe epilepsy: operative and neuropsychological outcomes. J Neurosurg. 2007;106:134–41. doi: 10.3171/jns.2007.106.1.134. [DOI] [PubMed] [Google Scholar]
- 39.Little AS, Smith KA, Kirlin K, Baxter LC, Chung S, Maganti R, et al. Modifications to the subtemporal selective amygdalohippocampectomy using a minimal-access technique: seizure and neuropsychological outcomes. J Neurosurg. 2009;111:1263–74. doi: 10.3171/2008.10.17673. [DOI] [PubMed] [Google Scholar]
- 40.Josephson CB, Dykeman J, Fiest KM, Liu X, Sadler RM, Jette N, et al. Systematic review and meta-analysis of standard vs selective temporal lobe epilepsy surgery. Neurology. 2013;80:1669–76. doi: 10.1212/WNL.0b013e3182904f82. [DOI] [PubMed] [Google Scholar]
- 41.Renowden SA, Matkovic Z, Adams CB, Carpenter K, Oxbury S, Molyneux AJ, et al. Selective amygdalohippocampectomy for hippocampal sclerosis: postoperative MR appearance. AJNR Am J Neuroradiol. 1995;16:1855–61. [PMC free article] [PubMed] [Google Scholar]
- 42.Gleissner U, Helmstaedter C, Schramm J, Elger CE. Memory outcome after selective amygdalohippocampectomy: a study in 140 patients with temporal lobe epilepsy. Epilepsia. 2002;43:87–95. doi: 10.1046/j.1528-1157.2002.24101.x. [DOI] [PubMed] [Google Scholar]
- 43.Laurent A, Arzimanoglou A. Cognitive impairments in children with nonidiopathic temporal lobe epilepsy. Epilepsia. 2006;47(Suppl. 2):99–102. doi: 10.1111/j.1528-1167.2006.00703.x. [DOI] [PubMed] [Google Scholar]
- 44.Hermann BP, Seidenberg M, Schoenfeld J, Davies K. Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Arch Neurol. 1997;54:369–76. doi: 10.1001/archneur.1997.00550160019010. [DOI] [PubMed] [Google Scholar]
- 45.Regis J, Bartolomei F, Rey M, Hayashi M, Chauvel P, Peragut JC. Gamma knife surgery for mesial temporal lobe epilepsy. J Neurosurg. 2000;93(Suppl. 3):141–6. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 46.Regis J, Bartolomei F, Hayashi M, Roberts D, Chauvel P, Peragut JC. The role of gamma knife surgery in the treatment of severe epilepsies. Epileptic Disord. 2000;2:113–22. [PubMed] [Google Scholar]
- 47.Regis J, Rey M, Bartolomei F, Vladyka V, Liscak R, Schrottner O, et al. Gamma knife surgery in mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia. 2004;45:504–15. doi: 10.1111/j.0013-9580.2004.07903.x. [DOI] [PubMed] [Google Scholar]
- 48.Barbaro NM, Quigg M, Broshek DK, Ward MM, Lamborn KR, Laxer KD, et al. A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Ann Neurol. 2009;65:167–75. doi: 10.1002/ana.21558. [DOI] [PubMed] [Google Scholar]
- 49.Quigg M, Broshek DK, Barbaro NM, Ward MM, Laxer KD, Yan G, et al. Neuropsycho-logical outcomes after gamma knife radiosurgery for mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia. 2011;52:909–16. doi: 10.1111/j.1528-1167.2011.02987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang EF, Quigg M, Oh MC, Dillon WP, Ward MM, Laxer KD, et al. Predictors of efficacy after stereotactic radiosurgery for medial temporal lobe epilepsy. Neurology. 2010;74:165–72. doi: 10.1212/WNL.0b013e3181c9185d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hawasli AH, Bandt SK, Hogan RE, Werner N, Leuthardt EC. Laser ablation as treatment strategy for medically refractory dominant insular epilepsy: therapeutic and functional considerations. Stereotact Funct Neurosurg. 2014;92:397–404. doi: 10.1159/000366001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Willie JT, Laxpati NG, Drane DL, Gowda A, Appin C, Hao C, et al. Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Neurosurgery. 2014;74:569–84. doi: 10.1227/NEU.0000000000000343. discussion 584–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drane DL, Loring DW, Voets NL, Price M, Ojemann JG, Willie JT, et al. Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia. 2015;56:101–13. doi: 10.1111/epi.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morrell MJ, Group RNSSiES Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 55.FDA. Device approvals and clearances. 2013 http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/default.htm.
- 56.Spencer D, Gwinn R, Salinsky M, O'Malley JP. Laterality and temporal distribution of seizures in patients with bitemporal independent seizures during a trial of responsive neurostimulation. Epilepsy Res. 2011;93:221–5. doi: 10.1016/j.eplepsyres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 57.Heck CN, King-Stephens D, Massey AD, Nair DR, Jobst BC, Barkley GL, et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. 2014;55:432–41. doi: 10.1111/epi.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fisher RS. Therapeutic devices for epilepsy. Ann Neurol. 2012;71:157–68. doi: 10.1002/ana.22621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ben-Menachem E, Manon-Espaillat R, Ristanovic R, Wilder BJ, Stefan H, Mirza W, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35:616–26. doi: 10.1111/j.1528-1157.1994.tb02482.x. [DOI] [PubMed] [Google Scholar]
- 60.Amar AP, Heck CN, Levy ML, Smith T, DeGiorgio CM, Oviedo S, et al. An institutional experience with cervical vagus nerve trunk stimulation for medically refractory epilepsy: rationale, technique, and outcome. Neurosurgery. 1998;43:1265–76. doi: 10.1097/00006123-199812000-00001. discussion 1276–80. [DOI] [PubMed] [Google Scholar]
- 61.Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, et al. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 1998;51:48–55. doi: 10.1212/wnl.51.1.48. [DOI] [PubMed] [Google Scholar]
- 62.DeGiorgio C, Heck C, Bunch S, Britton J, Green P, Lancman M, et al. Vagus nerve stimulation for epilepsy: randomized comparison of three stimulation paradigms. Neurology. 2005;65:317–9. doi: 10.1212/01.wnl.0000168899.11598.00. [DOI] [PubMed] [Google Scholar]
- 63.Scherrmann J, Hoppe C, Kral T, Schramm J, Elger CE. Vagus nerve stimulation: clinical experience in a large patient series. J Clin Neurophysiol. 2001;18:408–14. doi: 10.1097/00004691-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 64.Englot DJ, Chang EF, Auguste KI. Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. J Neurosurg. 2011;115:1248–55. doi: 10.3171/2011.7.JNS11977. [DOI] [PubMed] [Google Scholar]
- 65.Englot DJ, Chang EF, Auguste KI. Efficacy of vagus nerve stimulation for epilepsy by patient age, epilepsy duration, and seizure type. Neurosurg Clin N Am. 2011;22:443–8. doi: 10.1016/j.nec.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 66.Englot DJ, Rolston JD, Wang DD, Hassnain KH, Gordon CM, Chang EF. Efficacy of vagus nerve stimulation in posttraumatic versus nontraumatic epilepsy. J Neurosurg. 2012;117:970–7. doi: 10.3171/2012.8.JNS122. [DOI] [PubMed] [Google Scholar]
- 67.Frost M, Gates J, Helmers SL, Wheless JW, Levisohn P, Tardo C, et al. Vagus nerve stimulation in children with refractory seizures associated with Lennox–Gastaut syndrome. Epilepsia. 2001;42:1148–52. doi: 10.1046/j.1528-1157.2001.23900.x. [DOI] [PubMed] [Google Scholar]
- 68.Kostov K, Kostov H, Tauboll E. Long-term vagus nerve stimulation in the treatment of Lennox–Gastaut syndrome. Epilepsy Behav. 2009;16:321–4. doi: 10.1016/j.yebeh.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 69.DeGiorgio CM, Schachter SC, Handforth A, Salinsky M, Thompson J, Uthman B, et al. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 2000;41:1195–200. doi: 10.1111/j.1528-1157.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 70.Moruzzi G. Effects at different frequencies of cerebellar stimulation upon postural tonus and myotatic reflexes. Electroencephalogr Clin Neurophysiol. 1950;2:463–9. doi: 10.1016/0013-4694(50)90083-6. [DOI] [PubMed] [Google Scholar]
- 71.Cooke PM, Snider RS. Some cerebellar influences on electrically induced cerebral seizures. Epilepsia. 1955;4:19–28. doi: 10.1111/j.1528-1157.1955.tb03170.x. [DOI] [PubMed] [Google Scholar]
- 72.Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 73.Chang EF, Quigg M, Oh MC, Dillon WP, Ward MM, Laxer KD, et al. Predictors of efficacy after stereotactic radiosurgery for medial temporal lobe epilepsy. Neurology. 2010;74:165–72. doi: 10.1212/WNL.0b013e3181c9185d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmitt FC, Voges J, Buentjen L, Woermann F, Pannek HW, Skalej M, Heinze HJ, Ebner A. Radiofrequency lesioning for epileptogenic periventricular nodular heterotopia: a rational approach. Epilepsia. 2011;52:e101–5. doi: 10.1111/j.1528-1167.2011.03116.x. [DOI] [PubMed] [Google Scholar]
- 75.Gonzalez-Martinez J, Vadera S, Mullin J, Enatsu R, Alexopoulos AV, Patwardhan R, Bingaman W, Najm I. Robot-assisted stereotactic laser ablation in medically intractable epilepsy: operative technique. Neurosurgery. 2014;(Suppl 2):167–72. doi: 10.1227/NEU.0000000000000286. discussion 172–173. [DOI] [PubMed] [Google Scholar]