Abstract
Mutations in polycystin-1 (PC1) give rise to autosomal dominant polycystic kidney disease, an important and common cause of kidney failure. Despite its medical importance, the function of PC1 remains poorly understood. Here, we investigated the role of the intracellular polycystin-1, lipoxygenase, and α-toxin (PLAT) signature domain of PC1 using nuclear magnetic resonance, biochemical, cellular, and in vivo functional approaches. We found that the PLAT domain targets PC1 to the plasma membrane in polarized epithelial cells by a mechanism involving the selective binding of the PLAT domain to phosphatidylserine and l-α-phosphatidylinositol-4-phosphate (PI4P) enriched in the plasma membrane. This process is regulated by protein kinase A phosphorylation of the PLAT domain, which reduces PI4P binding and recruits β-arrestins and the clathrin adaptor AP2 to trigger PC1 internalization. Our results reveal a physiological role for the PC1-PLAT domain in renal epithelial cells and suggest that phosphorylation-dependent internalization of PC1 is closely linked to its function in renal development and homeostasis.
Keywords: autosomal dominant polycystic kidney disease, polycystic kidney disease, genetics and development
Autosomal dominant polycystic kidney disease (ADPKD) affects 1 in 400–1000 people worldwide.1 It leads to the spontaneous and continuous production of kidney cysts, which gradually enlarge to destroy normal renal parenchyma, leading to ESRD. In addition, ADPKD is associated with cysts in other organs as well as noncystic manifestations.1
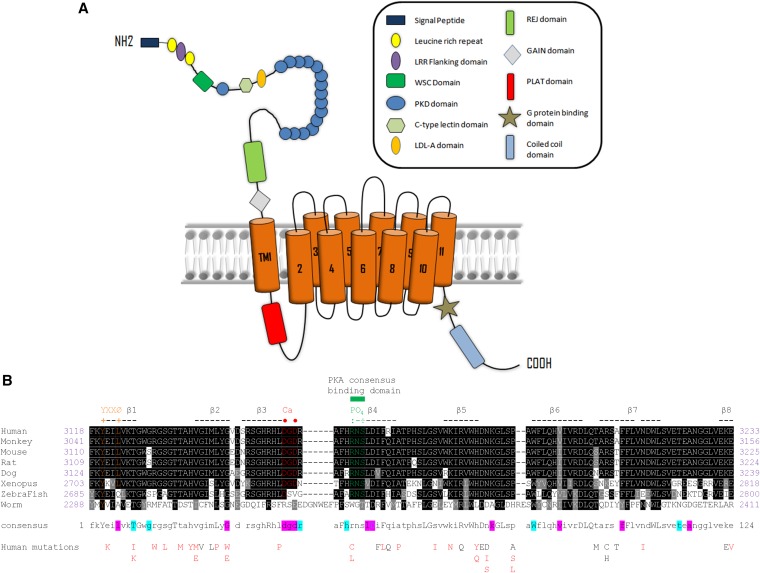
ADPKD arises from germ-line mutations in one of two genes, PKD1 and PKD2, which encode the proteins polycystin-1 (PC1) and PC2. PC2 (TRPP2) is a member of the TRP family of calcium channels, whereas PC1 is a 4303-residue transmembrane protein of uncertain function (Figure 1A). At least 85% of all ADPKD is caused by mutations in PKD1. PC1 has been shown to function as a mechanosensitive receptor in primary cilia, but it can also mediate cell adhesion at cell-cell or cell-matrix junctions.2–5 A primary function is to regulate PC2 trafficking, channel assembly, and activity.6,7 PC1 may also function as an atypical G protein–coupled receptor.8,9
Figure 1.
Predicted domain structure of PC1 and sequence conservation of PLAT. (A) Domain architecture of PC1. The name of each domain is labeled in the box on the right. The position of PLAT domain (red) is in the predicted first intracellular loop. (B) Amino acid sequence alignment of PLAT from different homologs. Conserved residues are labeled with a black background. Similar residues are in a gray background. There are 24 identically conserved residues (19.4% of the total of 116 residues), which are shown as capital letters in the consensus sequence, and 89 similarly conserved residues (71.8% of the total of 116 residues), which are represented as lowercase letters in the consensus line. Eight β-strands are indicated by hyphens and numbered sequentially. The calcium binding site (DGD) is colored red, with key residues marked by red dots. The phosphorylation site (RNS) is labeled green, and key residues are indicated by green colons. A tyrosine sorting signal (YEIL) is labeled orange. The residues involved in PI4P binding to PLAT are labeled with a light blue background in the consensus sequence, and those binding PS are shown with a magenta background. Accession numbers are human (P98161), monkey (G7NQU9), mouse (O08852), rat (F1MAD3), dog (Q7YQK5), Xenopus (Q4G444), zebrafish (U3JAI2), and worm (Q09624 and LOV-1). The 40 human mutations reported for PLAT so far (http://pkdb.mayo.edu/) are listed below: definitely, highly likely, or likely pathogenic mutations are in red, and indeterminate or likely neutral mutations are in black.
We investigated the polycystin-1, lipoxygenase, and α-toxin (PLAT) domain,10 a 116–amino acid region (amino acids 3118–3233) present in the first intracellular loop of PC1 (also termed the Lipoxygenase Homology 2 domain).11 Because of its high sequence conservation, PLAT is considered a signature domain of PC1 and PC1-like proteins. PLAT domains have been identified in over 1000 proteins. However, despite its likely importance, there has been no published information on the structure or function of the PLAT domain of PC1 (PC1-PLAT) since its first description.
Here, we report that PC1-PLAT binds to two distinct phospholipids as well as Ca2+ and identify the specific residues that mediate binding. PC1-PLAT acts as an independent basolateral targeting motif and when phosphorylated at S3164, mediates protein internalization in a β-arrestin– and AP2–dependent manner. Significantly, S3164 was critical for PLAT function in Xenopus pronephric development. These new findings implicate PLAT in regulating PC1 function through lipid, Ca2+, and protein interactions at sites distinct from those identified in other PLAT homologs and suggest that it plays a physiologic role in PC1 trafficking and function.
Results
Sequences and Mutations of PC1-PLAT
Comparison of the amino acid sequences of PC1-PLAT from human to worm revealed 24 identical residues (19% of 116) and 89 conserved residues (72%) (Figure 1B). Sequence comparisons suggest potential Ca2+ binding and phosphorylation sites, although neither is well conserved in the PC1-like proteins (Figure 1B, Supplemental Figure 1). Around 40 missense mutations in PLAT have been reported (Figure 1B), including likely pathogenic substitutions at R3162 (R3162C and R3162L) and a predicted internalization motif (YEIL3123; E3121K).12,13
PC1-PLAT Binds to Phospholipids and Calcium
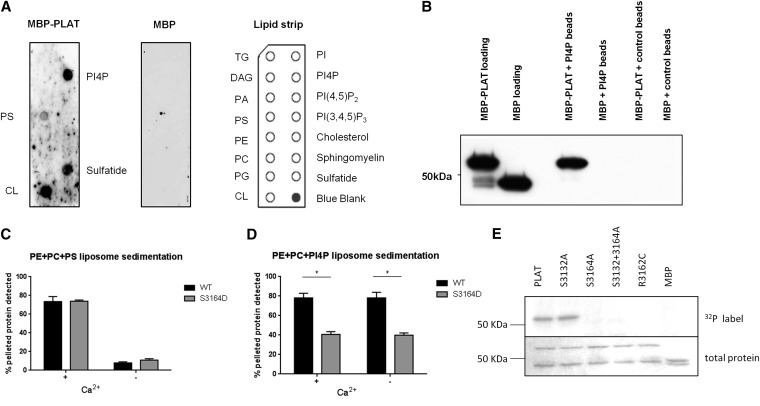
PC1-PLAT has been predicted to be involved in protein-lipid interaction10 and bind Ca2+ on the basis of the PLAT domain in α-toxin, which uses Ca2+ binding to regulate protein-membrane interactions.14 Therefore, the binding of MBP-PLAT to a range of membrane lipids was tested in vitro (Figure 2A). Binding was observed to immobilized l-α-phosphatidylinositol-4-phosphate (PI4P), phosphatidylserine (PS), sulfatide, and cardiolipin. Because of the likely localization of PC1 at the plasma membrane (PM), we focused on PI4P and PS, both of which are known to be enriched in the inner leaflet of the PM.15
Figure 2.
PC1-PLAT binds selectively to the anionic phospholipids PS and PI4P. (A) Protein-lipid overlay assay showing lipid spots bound by (left panel) MBP-PLAT protein and (center panel) negative control using MBP protein only. (Right panel) Lipids arrayed on strip are displayed. (B) Protein-lipid bead pulldown assay showing that MBP-PLAT is pulled down by PI4P–coated agarose beads, confirming an interaction between PLAT and PI4P. Control beads were uncoated with lipid. MBP did not bind to either control or PI4P-coated beads. (C and D) Cosedimentation assays of PS and PI4P-containing liposomes with wild–type or S3164D mutant MBP-PLAT in the presence or absence of Ca2+. The bars show the mean percentages of added total protein pelleted, and the error bars represent the SEM (n=3). *P<0.05 versus wild-type PLAT. (E, upper panel) Recombinant MBP-PLAT is phosphorylated by PKA on S3164 but not S3132 in an in vitro kinase assay. The pathogenic mutation R3162C prevents phosphorylation of PLAT by PKA. (E, lower panel) Equal loading is shown by Coomassie blue staining. CL, cardiolipin; WT, wild type.
Binding to PI4P was confirmed by protein-lipid bead pulldown assays (Figure 2B). Furthermore, MBP-PLAT cosedimented with liposomes containing PS, but MBP-PLAT coprecipitated significantly more with PS liposomes with Ca2+ than without Ca2+ (Figure 2C, Supplemental Figure 2). MBP-PLAT also cosedimented with liposomes containing PI4P (Figure 2D). In this case, the presence or absence of Ca2+ had little effect, implying that the mechanisms of PLAT binding to PS and PI4P are distinct.
PC1-PLAT Is Phosphorylated at S3164 In Vitro and In Vivo
Two potential PKA phosphorylation sites were identified by Phosida16 within the PLAT domain at S3132 and S3164 (Figure 1B). A kinase assay showed that the PLAT domain could only be phosphorylated by PKA at S3164 in vitro (Figure 2E). Crucially, a pathogenic PKD1 mutation R3162C12 predicted to disrupt the consensus PKA binding site (RxS) at S3164 prevented phosphorylation of PLAT (Figure 2E). We confirmed these findings by LC-MS/MS in HEK293 cells transiently expressing YFP-PLAT, showing that S3164 is phosphorylated in vivo (Supplemental Figure 3). Of relevance, a phosphomimic MBP-PLAT (S3164D) showed a 50% reduction in binding affinity to PI4P liposomes but did not alter binding to PS liposomes (Figure 2, D and E). Similar results were obtained when MBP-PLAT was phosphorylated by PKA (Supplemental Figure 2C).
NMR Studies of PC1-PLAT Ligand Binding
The NMR spectrum of a Protein G-B1 domain (GB1)-PLAT fusion protein was assigned (88% complete)17 and used to create a refined model for the structure of PLAT on the basis of homologous structures (Supplemental Figure 4). The structure, as expected, is a β-sandwich, with four strands in each sheet.
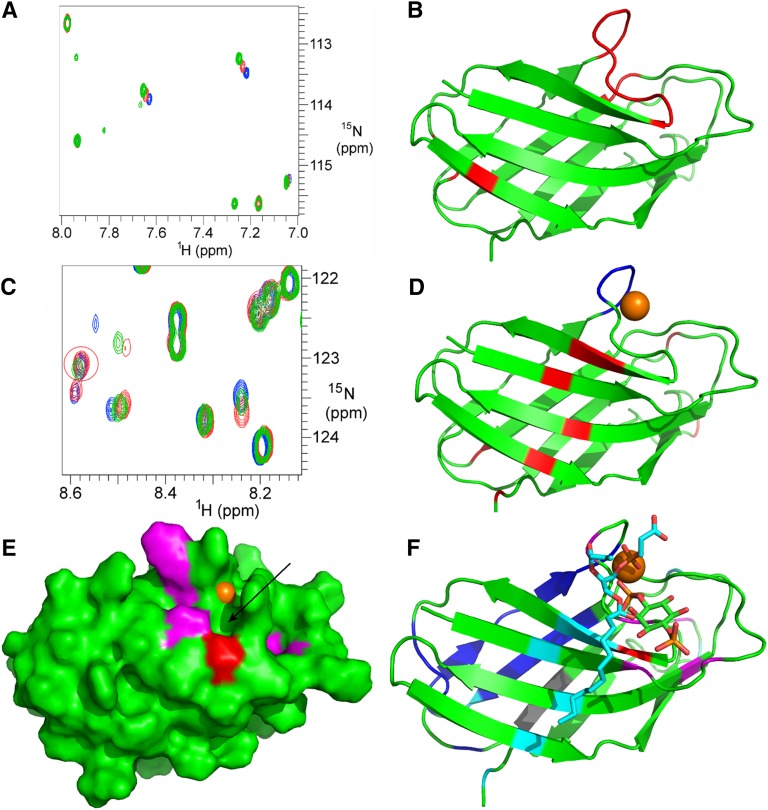
To determine the Ca2+ binding site, we titrated Ca2+ into PC1-PLAT. This produced chemical shift changes at a small number of residues (Figure 3A). Elimination of false positives (histidines) revealed the Ca2+ binding site to be the sequence DGD3157 with an affinity close to 1 nM (Figure 3B). Mutation of D3155 and D3157 to alanine abolished binding. We note that, although other PLAT domains bind to Ca2+, the location of the Ca2+ binding site is not conserved,18 and no other known PLAT domains bind Ca2+ at this position.
Figure 3.
NMR analysis reveals discrete binding residues in PC1-PLAT for Ca2+, DPPS and PI4P. (A) A small region of the 15N HSQC spectrum of GB1-PLAT showing chemical shift changes (of H3161 and H3174) on addition of Ca2+. Changes were observed for residues H3137, V3138, D3155, G3156, D3157, R3158, A3159, F3160, H3161, R3162, N3163, H3174, L3176, and S3210. (B) Cartoon representation of the structure of PC1-PLAT showing the locations of residues identified as titrating with Ca2+ (red). The calcium binding loop is identified by the cluster of residues at the top of the structure. (C) A region of the 15N HSQC spectrum of GB1-PLAT showing apo-PLAT (red), calcium-loaded PLAT (blue), and calcium-loaded PLAT plus DPPS (green). DPPS removes calcium from PLAT, meaning that, where chemical shift changes occur, the green peak is usually about 70% of the way from blue to red. However, specific shift changes caused by DPPS binding (e.g., F3212; ringed in red) show a different pattern. (D) The location of the binding site for PS. The calcium loop is shown in blue, with the approximate position of the Ca2+ ion as an orange sphere; other residues affected in DPPS titrations are in red. (E) The binding site on PC1-PLAT for PI4P. Residues that move on addition of PI4P are colored red for S3164 and magenta for the others. A potential phosphate pocket is indicated by the arrow. For reference, the Ca2+ ion is shown in orange. An NMR titration with PI(4,5)P2 showed no binding (data not shown). (F) Cartoon representation of the structure of PLAT showing the position of bound calcium (brown sphere), PS (cyan/red sticks; for clarity, only one lipid chain is shown), and PI4P (green/red and orange for the phosphates) plus residues affected by the binding of PS (cyan) and PI4P (magenta/red; like in E). Residues in dark blue are broadened (by self-association) in NMR, suggesting a protein interaction site, and it is significant that the KIR3183 sequence, identified as a potential interaction site, is adjacent to it and shown in gray.
Titration of PC1-PLAT with 1,2-dipalmitoyl-sn-glycero-3-phospho-l-serine (DPPS), a water–soluble PS analog, showed that DPPS reversibly removes bound Ca2+ from PLAT (Figure 3C) and binds adjacent to the Ca2+ site (Figure 3D). This result explains why the binding of PLAT to PS is Ca2+ dependent: PS will only bind to PLAT when PLAT has Ca2+ bound already. On titration of PC1-PLAT with PI4P, chemical shift changes were localized to a surface patch close to but different from the PS binding site (Figure 3E). It is noteworthy that one of the residues affected is S3164 (red in Figure 3E), hence providing a structural explanation for loss of PI4P binding on phosphorylation. There was no change in PI4P binding of an MBP-PLAT S3164A mutant (not shown). The ligand binding sites identified on PC1-PLAT are summarized in Figure 3F.
Phosphorylation of PC1-PLAT Changes Its Subcellular Localization
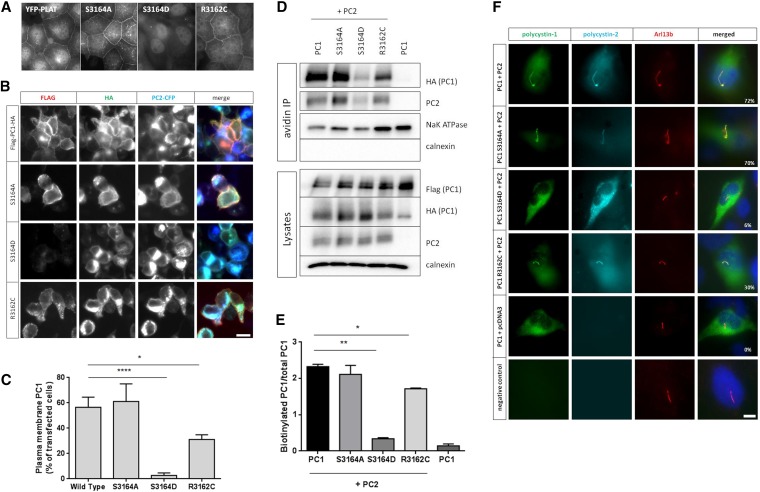
In mammalian cells, the isolated YFP-PLAT domain localized to the PM and in particular, the basolateral membrane in stably transfected polarized renal epithelial cells (Figure 4A, Supplemental Figure 4). Confocal microscopy confirmed that PLAT colocalized significantly with proteins expressed at the lateral junctions, such as E-cadherin, desmoplakin, and ZO-1, but not with gp135, an apical marker, or acetylated tubulin, which marks primary cilia (Supplemental Figure 4). The results indicate that PLAT contains an independent basolateral targeting or retention signal.
Figure 4.
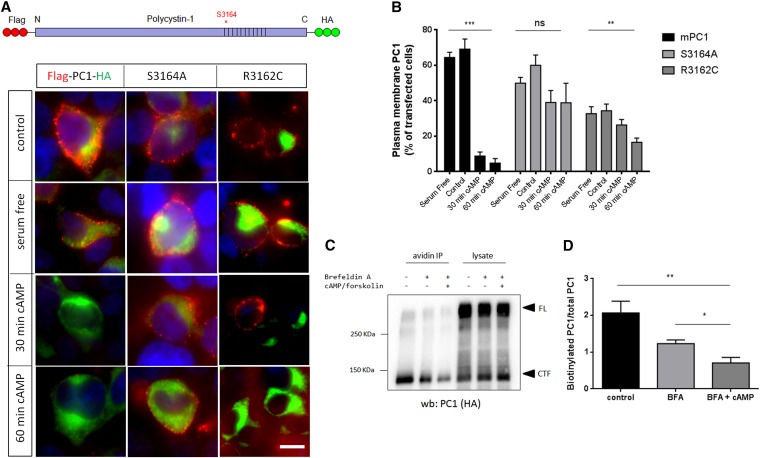
PC1–1 PLAT phosphorylation by PKA regulates its surface expression and cilia localization. (A) Fluorescence microscopy of stably expressed YFP-PLAT and YFP-PLAT mutant proteins in MDCK II cells. YFP-PLAT was consistently localized to the PM, except for the S3164D mutation, which resulted in complete loss of surface localization. (B and C) Live–cell surface labeling of PC1 in cells transfected with wild-type and S3164/R3162 FLAG-PC1-HA–tagged plasmids. Surface PC1 expression was visualized in nonpermeabilized cells by FLAG labeling; total number of PC1-expressing cells by HA staining after permeabilization are shown. PC2-CFP coexpression was visualized by epifluorescence. Cell surface labeling of PC1 was almost completely abolished in the S3164D mutant compared with the wild type or S3164A. The pathogenic mutation R3162C showed detectable but significantly reduced surface expression. Values shown are the means±SEM. Scale bar, 10 µm. (D and E) Surface biotinylation of PC1 and S3164/R3162 mutants. Wild-type PC1 was biotinylated only in the presence of coexpressed PC2 and significantly reduced in the S3164D mutant. Controls included the PM transporter Na+-K+-ATPase and the ER resident protein calnexin. Lysates show equivalent expression of PC1 and PC2 in different samples. The cleaved PC1-CTF (detectable by HA) was used for quantification of surface biotinylation as previously described, because the PC1-NTF is detached under the assay conditions used.19,53 The bars indicate the ratio of biotinylated PC1 to total PC1 (HA blot) from three experiments. (F) Cilia localization of PC1 and PC2 in a normal human kidney cell line (UCL93/3) expressing the wild type or S3164/R3162 FLAG-PC1-HA. Wild-type PC1 was only detected in primary cilia when PC2-CFP was coexpressed (72% ciliated cells). There was a significant reduction in cilia expression for S3164D (6%) and S3162C (30%) but not S3164A (70%), even in the presence of PC2-CFP. An Arl13b antibody was used to label primary cilia. Scale bar, 10 µm. *P<0.05; **P<0.01; ****P<0.0001.
Both S3164A and R3162C mutants showed identical surface localization to wild-type PLAT, although expression of R3162C was visibly reduced. In contrast, an S3164D mutant was not expressed at the surface, implying that phosphorylation at S3164 is critical for membrane localization (Figure 4A). To test whether PKA phosphorylation promotes PLAT internalization, MDCK cells stably expressing YFP-PLAT were stimulated with cAMP. We observed a time-dependent decrease in surface expression for wild-type PLAT but not the S3164A or R3162C mutants (Supplemental Figure 5G).
PKA Regulates the Internalization of Full-Length PC1
To investigate whether the PLAT domain was able to target full-length PC1 to the cell surface, live-cell staining of HEK293 cells transfected with double-tagged PC1 was performed. In agreement with previous reports,19 surface PC1 expression was dependent on coexpression of PC2 (Figure 4B, Supplemental Figure 6). There was a marked reduction in PM localization of a full–length S3164D PC1 mutant (Figure 4, B and C) as well as in primary cilia (Figure 4F), whereas the S3164A mutant was unchanged. Interestingly, the R3162C mutant also showed a significant decrease in PM and cilia localization (Figure 4, B and F), despite equivalent expression and GPS cleavage (Supplemental Figure 6B). These findings were confirmed in surface biotinylation experiments (Figure 4, D and E).
To confirm the role of PKA in mediating full–length PC1 internalization, HEK293 cells transiently expressing wild-type PC1 or S3164A-PC1 were stimulated with cAMP: a significant decrease in wild–type PC1 surface expression was observed by live-cell labeling and surface biotinylation (Figure 5), whereas the S3164A mutant did not respond to cAMP stimulation (Figure 5, A and B).
Figure 5.
Full-length PC1 undergoes cAMP-stimulated endocytosis dependent on phosphorylation at S3164. (A and B) Live–cell surface labeling of PC1 and S3164/R3162 mutants after cAMP stimulation. Unlike wild-type PC1, PC1-S3164A did not respond to cAMP. PC1-R3162C showed a significant reduction in baseline surface expression but also, a smaller response to cAMP at 60 minutes. **P<0.01; ***P<0.001 versus baseline (serum free; n=3). (C and D) Surface biotinylation of wild-type FLAG-PC1-HA after stimulation with dbcAMP and forskolin. Cells were pretreated for 30 minutes with brefeldin A (BFA) to block new protein export before cAMP stimulation. A significant decrease in surface PC1 (cleaved C–terminal fragment [CTF]) was observed after a 60-minute incubation with dbCAMP and forskolin. Arrowheads indicate full-length (FL) or CTF as detected by an HA blot. The blot shown is representative of three independent experiments. *P<0.05 BFA+cAMP versus BFA (n=3); **P<0.01 BFA+cAMP versus control.
PI4P Binding Is Insufficient for PM Targeting of the PLAT Domain
Because the S3164D mutant displayed reduced binding to PI4P (Figure 2D), we tested whether PI4P binding alone was sufficient to mediate PM targeting of the isolated PLAT domain using a chemical dimerization strategy on the basis of the rapamycin-inducible dimerization of FK506 binding protein 12 and fragment of mTOR that binds rapamycin domains to recruit inositol phosphatase enzymes acutely to the PM.20 Constructs encoding an enzymatic chimera of inositol polyphosphate 5-phosphatase E (INPP5E), which converts phosphatidyl-inositol-4,5-bisphosphate [PI(4,5)P2] to PI4P, and the Saccharomyces cerevisiae Sac1 phosphatase, which dephosphorylates PI4P, were used to deplete PI4P and PI(4,5)P2 singly or combined from the PM (Supplemental Figure 7). Rapamycin–induced surface localization of both INPP5E and Sac1 phosphatase (but not either alone) was required to induce YFP-PLAT internalization detectable within 15 minutes in HEK293 and CHO-K1 cells (Supplemental Figure 7, D and E). Our data confirm that PI4P binding alone is insufficient to mediate PLAT PM binding.
Knockdown of AP2M1 Subunit and ARRB1/2 Prevents cAMP-Mediated Endocytosis of PC1
To identify endocytic adaptors that could mediate cAMP–stimulated YFP-PLAT internalization, we conducted MS analysis of interacting proteins pulled down with PLAT from HEK293 cells and identified several members of the adaptor protein-2 (AP2) complex (Supplemental Figure 3). AP2 is a heterotetrameric protein complex comprising two large subunits (A1 or A2 and B2), one medium subunit (M1), and one small subunit (S2),21 and it is the major endocytic AP complex involved in clathrin-mediated endocytosis. We also tested the potential involvement of β-arrestins (ARRB), because these are known to act as endocytic adaptors for many activated G protein–coupled receptors by binding to phosphorylated residues and recruiting them to the AP2 complex, leading to their desensitization, endocytosis, and initiation of G protein–independent signaling.22,23
siRNA knockdown of AP2M1 or ARRB1 and ARRB2 in HEK293 cells significantly inhibited cAMP-stimulated endocytosis of YFP-PLAT compared with scrambled siRNA (Supplemental Figure 8, A–F). In fact, it dramatically inhibited internalization of transfected wild–type full–length PC1 (Figure 6, A and B). In these experiments, inhibition was greater in AP2 siRNA–transfected cells (Supplemental Figure 8, B and E). Similar results were found for endogenous PC1, which was detectable as a triplet high molecular weight band pattern in UCL93/3 cells (Supplemental Figure 9, A and B). Of interest, we found that only the highest molecular weight band, corresponding to the EndoH-resistant band (NTR), was consistently labeled (Supplemental Figure 9C). Two other EndoH–sensitive bands (NTS1 and NTS2) were also detected in this cell line by the PC1–specific mAb 7e12.24 A similar banding pattern for PC1 has been reported in a different human kidney cell line (RCTE) by a different group with 7e12.25 In these studies, it was proposed that NTS2 could be a nonspecific cross–reactive protein, because it was detected in a PKD1 null cystic line.
Figure 6.
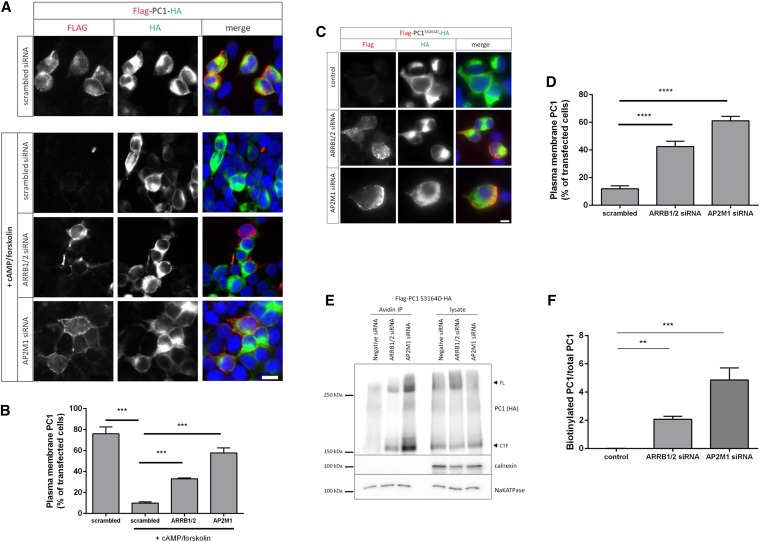
ARRB1/2 and AP2 mediate cAMP-mediated endocytosis of PC1. (A and B) Live–cell surface labeling of full-length PC1 in HEK293 cells cotransfected with scrambled-, AP2M1-, or ARRB1 1/2–specific siRNA. A significant inhibition of cAMP–stimulated PC1 endocytosis was observed with AP2M1 or ARRB1 siRNA–treated cells compared with scrambled siRNA–transfected cells. ***P<0.001. Scale bar, 10 µm. (C and D) Live–cell surface labeling of full–length PC1-S3164D in HEK293 cells cotransfected with scrambled-, AP2M1-, or ARRB1 1/2–specific siRNA. PC1-S3164D surface expression was significantly increased in cells transfected with AP2M1 or ARRB1/2 siRNA compared with scrambled siRNA–transfected cells. ****P<0.0001. Scale bar, 10 µm. (E and F) Surface biotinylation of PC1-S3164D in HEK293 cells showed that siRNA knockdown of AP2M1 or ARRB1/2 resulted in an increase in surface-labeled PC1 compared with scrambled siRNA controls. Arrowheads indicate full-length (FL) or cleaved C–terminal fragment (CTF) as detected by an HA blot. Controls included the PM transporter Na+-K+-ATPase and the ER resident protein calnexin. The bars show the ratio of biotinylated PC1 to total PC1 (CTF; HA blot) from three experiments. **P<0.01; ***P<0.001.
As predicted, siRNA knockdown of AP2M1 and ARRB1/2 increased surface expression of a PC1-S3164D mutant by both live-cell labeling and surface biotinylation of PC1 (Figure 6, C–F). The specificity of these depletion experiments was shown by the ability of cotransfected rat ARRB1/2 cDNA to rescue the increase in surface PC1-S3164D labeling seen (Supplemental Figure 10, A and B) and the parallel changes in the same cells of endogenous Na+-K+-ATPase (Supplemental Figure 10, A and D), a large transmembrane protein previously shown to be similarly regulated by ARRB1/2.26 Independently, we inhibited endocytosis using a dominant–negative dynamin mutant (K44A) and found that surface expression of S3164D-PC1 was significantly increased (Supplemental Figure 11). These results confirm that the reduction in S3164D-PC1 is because of increased internalization rather than reduced surface delivery.
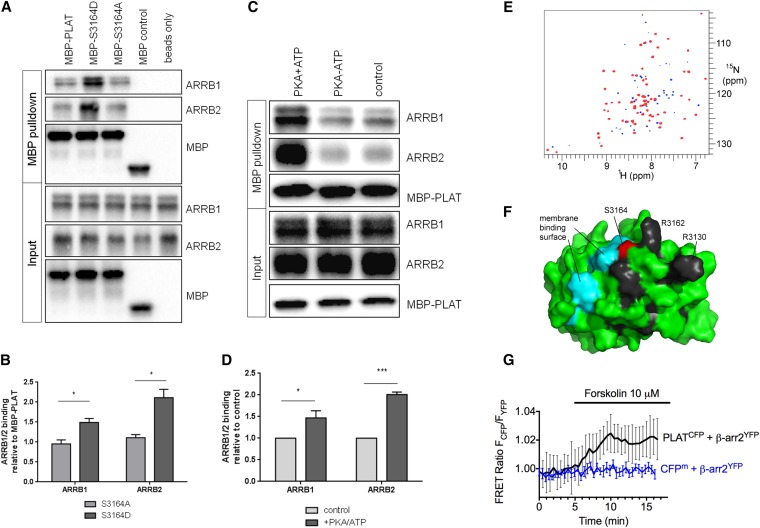
In vitro binding assays between recombinant MBP-PLAT and His-ARRB1 or His-ARRB2 confirmed increased binding of ARRB1 and ARRB2 to S3164D-PLAT compared with wild-type PLAT or S3164A (Figure 7, A and B). Similarly, PKA pretreatment increased binding of MBP-PLAT to ARRB1 and ARRB2 (Figure 7, C and D). To obtain direct biophysical evidence of an interaction between ARRB1 and PLAT, we used NMR. Full-length ARRB1 was titrated into 15N–labeled GB1-PLAT, producing selective changes in the NMR spectrum (Figure 7E), confirming direct binding, and identifying a surface-exposed patch immediately adjacent to S3164 (Figure 7F). Of interest, this includes two residues corresponding to PI4P binding sites (G3129 and H3161), and likely pathogenic mutations at two other residues (R3130 and R3162) have been reported (Figure 1B). These results are consistent with the data showing that phosphorylation of S3164 is required for internalization through ARRB1.
Figure 7.
PKA phosphorylation of PLAT increases its binding affinity to ARRB1/2. (A and B) MBP-PLAT pulldown assays show that PLAT can bind to ARRB1 and ARRB2, and this interaction was significantly increased by 2-fold for the S3164D-PLAT MBP fusion compared with the wild type or S3164A-PLAT. Molecular mass of MBP is 43kD, MBP-PLAT is 57kD, His-ARRB1 is 49kD, and His-ARRB2 is 46kD, respectively. *P<0.05 versus S3164A-PLAT (n=3). (C and D) MBP-PLAT pulldown assays after PKA phosphorylation of PLAT. PLAT binding to ARRB1 and ARRB2 was significantly increased by 1.5- to 2-fold after PKA pretreatment of MBP-PLAT. *P<0.05; ***P<0.001 versus control MBP-PLAT (n=3). (E) TROSY HSQC spectra of 200 μM GB1-PLAT alone (blue) and in the presence of 1.5 equivalents ARRB1 (red; superimposed). Peaks with significantly reduced intensity in the presence of ARRB1 are blue. Residues K3125, G3129, R3130, H3161, R3162, G3177, Q3198, D3217, S3220, and V3234 show a clear progressive loss of intensity throughout the titration. (F) Mapping of these residues onto the surface of PLAT. The most strikingly affected residues are shown in black. Also shown are S3164 (red), the site of phosphorylation, and the region shown to interact with phosphatidyl serine (cyan), indicating that ARRB1 binds adjacent to S3164 and the membrane binding site. (G) Averaged time course of the association between PLAT-CFP and ARRB2-YFP in response to forskolin. FRET recordings are shown as normalized ratios and represent mean values±SEM (n=15). Addition of forskolin to a single cell increased the FRET signal by approximately 2%, with a time constant of t1/2=1.14 minutes. A membrane-anchored CFP (CFPm) served as a negative control.
Finally, we investigated the interaction between PLAT and ARRB2 at the single-cell level using total internal reflection fluorescence (TIRF) microscopy in live HEK293 cells. We measured FRET between the PLAT domain tagged at its C terminus with CFP (PLAT-CFP) and ARRB2 tagged with YFP (ARRB2-YFP). TIRF microscopy was used to limit measurements to within approximately 100 nm of the PM.27 Addition of forskolin to a single cell increased the FRET signal by approximately 2% with a time constant of t1/2=1.14 minutes (Figure 7G). The small but significant change in FRET reflects the PLAT-ARRB interaction that is dependent on the cAMP/PKA signaling pathway.
Mutation of a Putative AP2 Binding Domain Inhibits Endocytosis of YFP-PLAT
We also identified a highly conserved putative AP2 binding site (YEIL3123) (Figure 1B) in PC1-PLAT, which is located 25 amino acids from the first transmembrane domain, consistent with a putative endocytic motif.22 A likely pathogenic mutation in this motif has been reported (E3121K).13 YXXØ consensus motifs are known to mediate rapid internalization in mammalian cells through binding to the M1 (µ2) subunit of AP2.28,29 Mutation of this sequence (Y3120N and L3123A) did not alter surface localization but reduced cAMP-induced endocytosis of YFP-PLAT in HEK293 cells (Supplemental Figure 8G). These results suggest that AP2 may also directly bind PLAT to mediate endocytosis.
Functional Analyses of PLAT in Xenopus Pronephric Development
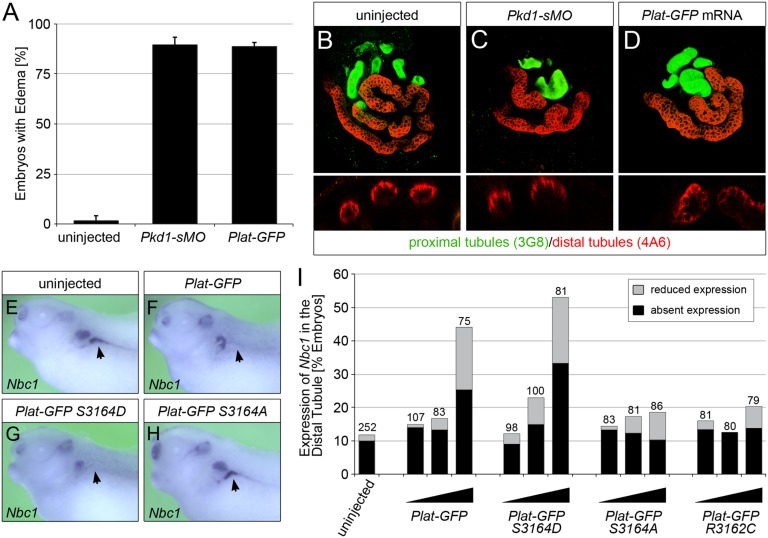
Finally, to examine the in vivo contribution of the PLAT domain to PC1 function, we turned to Xenopus as a model for PKD.30,31 Knockdown of Pkd1 using an antisense morpholino oligomer (MO) disrupting correct splicing of its mRNA causes a PKD phenotype; embryos develop edema as a sign of a dysfunctional kidney and dilated tubules and lose the DT1 distal tubular segment as visualized by whole–mount in situ hybridization for the sodium bicarbonate transporter Nbc1 (Figure 8, Supplemental Figure 12). Surprisingly, injection of an mRNA encoding the Plat domain only (Plat-GFP) completely mimicked this phenotype (Figure 8), suggesting that the Plat domain on its own is sufficient to antagonize endogenous PC1 function. On the basis of the findings above, we speculated that this is because of the regulation of PC1 localization. To address this, we injected increasing amounts of Plat-GFP mRNA as well as the three point mutations (S3164D, S3164A, and S3162C) unilaterally into Xenopus embryos at the four-cell stage and examined them for Nbc1 expression at stage 39 (when the kidney is fully patterned). As shown, the phosphomimic mutation S3164D mimicked wild-type Plat-GFP and even appeared slightly more active. Conversely, the S3164A or R3162C mutations did not affect the Nbc1 staining pattern. Together, these data implicate phosphorylation at S3164 as critical for PLAT function.
Figure 8.
Ectopic expression of PLAT-GFP causes PKD in Xenopus embryos. (A) Xenopus embryos injected radially with a total of 3.2 pmol Pkd1-sMO or 8 ng Plat-GFP mRNA as well as uninjected control embryos were analyzed at stage 42 by morphology. Bar diagram shows the percentage of embryos with edema as a sign of kidney dysfunction. Error bar corresponds to SD of multiple experiments. (B–D) Whole–mount immunofluorescence analysis with 3G8 (green) and 4A6 (red) of the embryos in A to visualize proximal tubules as well as distal tubules and duct, respectively. Upper panels show three-dimensional reconstruction of z stacks, whereas lower panels are cross-sections through individual proximal tubules. (E–I) Xenopus embryos were injected at the four-cell stage into a single blastomere with increasing amounts (0.5, 1.0, and 2.0 ng) of wild-type Plat-GFP, Plat-GFP(S3164D), Plat-GFP(S3164A), and Plat-GFP(R3162C). Expression of Nbc1 in the DT1 distal tubular segment was examined by whole–mount in situ hybridization comparing the injected and the contralateral noninjected pronephri at stage 39. E–H show representative embryos (Nbc1 staining in the DT1 segment is indicated by arrows). (I) Quantification of three independent experiments depicting the percentages of embryos with absent and reduced Nbc1 expressions. The total number of embryos analyzed is indicated above the bars.
Discussion
PLAT domains have been identified in >1000 proteins, of which several are important human proteins, such as PC1, hepatic, lipoprotein and triglycerol lipases,32 pancreatic lipase,33 and Rab6IP1, the effector of the small GTPase Rab6.34 Their functions are usually related to lipid binding,35 often in a Ca2+-dependent manner,14,36 but sometimes related to protein binding.33,34 They have also been linked to regulation of protein location. Here, we show that PC1-PLAT shares all of these functions, although the sites for binding Ca2+ and lipid membranes are different from those seen in other PLAT domains characterized to date.14,34,37
Surprisingly, little is known about the PC1-PLAT domain. Around 40 missense human mutations in PC1-PLAT have been reported (http://pkdb.mayo.edu) (Figure 1B). However, their pathologic significance has been difficult to assign in the absence of clear functional data. Two previous studies of the PLAT domain of LOV-1 (the worm homolog of PC1) have implicated it as a protein binding scaffold in regulating mating behavior.38,39 However, the overall domain structure and function of LOV-1 is very different to mammalian PC1, and the sequence identity of worm PLAT is low (Figure 1B). In addition, there is no conservation of the calcium binding (DGD3157), phosphorylation (RNS3164), or internalization (YEIL3123) motifs identified in this study.
Although PC1-PLAT had been predicted to bind lipids,10,11 no direct evidence has been forthcoming. Here, we show that PC1-PLAT binds to the anionic phospholipids, PS and PI4P, which are present in the inner leaflet of the PM.20,40 PC1-PLAT has distinct binding patterns to PS and PI4P, the former being highly Ca2+ dependent (Figure 2). This seems to be biologically relevant, because the most striking function of PLAT was in determining the subcellular localization of PC1. The isolated PLAT domain was able to localize to the PM and the basolateral membrane in fully polarized stably transfected MDCK cells (Supplemental Figure 4), although not to primary cilia.
The PLAT domain also regulates phosphorylation-dependent trafficking of PC1. Significantly, two pathogenic mutations at R3162, part of the PKA recognition sequence, have been reported (one mutation [R3162C] inherited in trans with a pathogenic HNF-1β mutation [Q311X] in a child with early-onset ADPKD born to normal parents].12,41 R3162C was predicted to be a hypomorphic mutation, because it was neutral in the affected parent but severe in the presence of the coinherited maternal HNF-1β mutation. Our results show the likely pathogenicity of this change with both loss of in vitro S3164 phosphorylation in the isolated PC1-PLAT (Figure 2E) and a reduction in surface delivery to both PM and primary cilia (Figure 4). Most likely, the R3162C mutant protein becomes misfolded, or the reactive cysteine anchors it to an intracellular compartment, thus impairing surface delivery by approximately 30%–50%.
By contrast, the phosphodeficient S3164A-PC1 showed no reduction in surface localization but unlike wild-type PC1, was not internalized in response to PKA stimulation. These results suggest that S3164 phosphorylation is dispensable for surface localization of PC1 but required for PLAT-mediated internalization. The mechanism seems to involve reduced affinity for PI4P but also, increased affinity to ARRB1/2. By NMR, ARRB1 was shown to bind to specific residues in PLAT adjacent to S3164, even in a nonphosphorylated form, and we show that S3164 phosphorylation further increases binding affinity (Figure 7).23 Of relevance, R3162 also bound ARRB1, and two residues (G3129 and H3161) share binding properties for both PI4P and ARRB1: potentially, S3164 phosphorylation could determine a switch in PLAT binding between PI4P and ARRB1/2. Finally, our results suggest that PKA modification of PC1-PLAT is relevant in vivo and tightly coupled to its function in a Xenopus model of PKD (Figure 8). This implies that endocytosed PC1 could itself be functional, and thus, disruption of this process by mutation could be of pathologic significance.42 Potentially, dysregulated PKA signaling caused by excessive cAMP levels could further reduce the threshold for cyst initiation by downregulating PC1 surface expression.
In summary, we have shown that PC1-PLAT has distinct lipid, Ca2+, and protein interactions at sites distinct from those identified in other PLAT homologs. In our refined model, PLAT is oriented to form a distinct membrane lipid binding surface adjacent to key endocytic signals for ARRB1/2 and AP2 (Figure 9). It is likely that PLAT also acts as a scaffold for other regulatory pathways modifying PC1 function and/or integrating downstream pathways, potentially including ARRB-dependent signaling. Our results begin to clarify the function of this conserved signature domain and provide the structural basis for future studies.
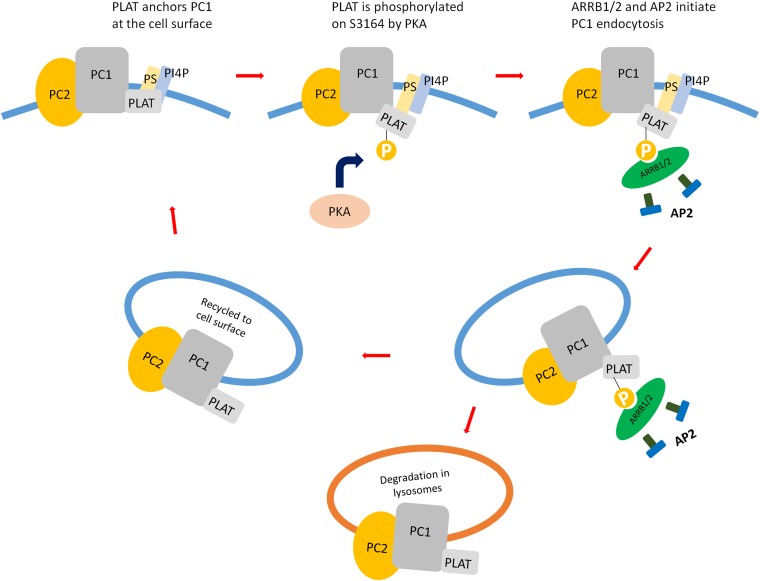
Figure 9.
Binding of phosphorylated PLAT to ARRB1/2 drives internalisation of a PC1/PC2 complex. PLAT binding to PI4P/PS at the inner leaflet of the PM is required for its surface localization and/or retention. As previously reported and confirmed in this study, binding to PC2 is required for PC1 trafficking to the cell surface. PKA phosphorylation at S3164 leads to a reduction in PI4P binding and the recruitment of ARRB1/2 and AP2. This results in the internalization of PC1 into endocytic vesicles from the PM and their likely trafficking into early endosomes for recycling and signaling or lysosomes for degradation.
Concise Methods
Protein Expression and Purification
Protein for NMR was expressed in BL21 DE3 RIPL (Stratagene) cells transformed with GEV-S2 plasmids using M9 minimal medium for uniform 15N, 13C, and 2H labeling with (15NH4)2SO4, 13C62H7 glucose (1 and 2 g/L, respectively), and 100% D2O as the sole nitrogen, carbon, and hydrogen sources, respectively. After induction with IPTG, cells were disrupted by sonication in the presence of sodium deoxycholate, and protein was purified using a 5-ml HisTrap HP Column (GE Healthcare). The protein buffer was exchanged to 50 mM Tris-HCl (pH 6.5), 500 mM NaCl, and 10 mM DTT using a Vivaspin 6 Centrifugal Filter Column (Sartorius). MBP-tagged protein was expressed in BL21 DE3 RIPL (Stratagene) cells transformed with pMAL-c2X plasmids using 2× YT medium and purified using amylose resin and eluting with 10 mM maltose.
Full–length rat ARRB1 and ARRB2 sequences were subcloned from pCDNA3-barr1HA and pCDNA3-barr2HA (Addgene) into pET-21a (Novagen) through 5′ NdeI and 3′ HindIII sites, transformed into Rosetta (DE3; Novagen) cells, and protein purified using a 5-ml HisTrap HP Column.
Generation of Stable Cell Lines
PKD1 PLAT cDNA was subcloned into mammalian expression vectors with fluorescent tags (KR45/47) by Topo cloning (Life Technologies) using the pENTR/D-TOPO entry vector and mutations introduced by PCR. Stable MDCKII clones expressing YFP-PLAT and YFP-PLAT mutants were generated by FACS from transfected cells, which were initially selected with 400 µg/ml G418 (Life Technologies) for 14 days.
Protein-Lipid Overlay Assay
Membrane/PIP lipid strips (Echelon Biosciences) were blocked with 1% nonfat dry milk in PBS and then incubated with 2 μg MBP or MBP-PLAT in 1% nonfat dry milk in PBS at room temperature for 1 hour, after which the strip was washed four times with PBS plus 0.2% Tween-20. Bound protein was detected with an MBP mAb (New England Biolabs) by standard Western blotting techniques.
Protein Lipid Bead Pulldown Assay
PI4P–coated, PS–coated, and uncoated agarose control beads (Echelon Biosciences) were washed two times, pelleted, and resuspended in binding buffer (10 mM Hepes [pH 7.4], 0.25% NP-40, and 150 mM NaCl); 1–10 µg protein was added, incubated at room temperature for 1 hour with rotation, centrifuged, and washed five times with 1 ml binding buffer. Two times Laemmli sample buffer was added to the pelleted beads, which were then boiled at 95°C for 5 minutes followed by centrifugation at 13,000 rpm for 1 minute. The supernatant was analyzed by Western blotting.
Liposome Cosedimentation Assay
Lipids (57% wt/vol 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine, 28% wt/vol 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine, and 15% wt/vol PI4P or DPPS; Avanti) were dissolved in chloroform, dried, redissolved in diethyl ether, dried again, suspended in liposome buffer (20 mM Hepes [pH 7.2], 100 mM KCl, 2 mM MgCl2, and 1 mM DTT), and incubated in a water bath at 60°C for 10–30 minutes. The protein buffer was exchanged to liposome buffer and then spun (90,000 rpm at 4°C for 15 minutes). The cosedimentation reaction was set up by gently mixing liposome, protein, 2 mM CaCl2 (or 2 mM EGTA), 10 mM MgCl2, 5 mM DTT (final concentrations), and liposome buffer in high–speed centrifuge tubes (Beckman) and incubating for 30 minutes at room temperature. The tubes were then centrifuged at 80,000 rpm at 4°C for 15 minutes, and the supernatant analyzed by SDS-PAGE. Gels were scanned using a Bio-Rad Model GS-690 Imaging Densitometer and quantitated by Bio-Rad Multi-Analyst, version 1.1.
NMR
All NMR measurements were carried out on a construct containing GB1 fused at the N terminus of residues 3112–3245 of PLAT with the short intervening linker sequence LVPRGS.43 Residue Trp3128 was mutated to alanine to improve solubility, and a hexahistidine tag was added at the N terminus to aid purification of the fusion protein. All experiments used 200- to 350-μM uniformly 2H–, 13C–, and 15N–labeled protein. All solutions were in 50 mM Tris, 500 mM NaCl, 10 mM DTT, and 10% D2O (pH 6.5). Unless intended for Ca2+ titration, solutions also contained 10 mM EDTA. NMR measurements were done at 25°C on Bruker Avance 600 and 800 spectrometers as well as on a Bruker 900 at the European NMR Large-Scale Facility at Utrecht.
Assignments were made using the standard triple–resonance backbone experiments run as TROSY versions. Data were processed using FELIX (Felix NMR Inc., San Diego, CA) and assigned using Asstools.44 A structural model of PLAT was made using Swiss-Model.45 Structure calculations were done using XPLOR-NIH46 using dihedral restraints obtained from TALOS-N47 and hydrogen bond restraints obtained from amide proton exchange rates. The structure calculations used the structural model as a starting point and were carried out using torsional dynamics at 500 K followed by slow cooling and energy minimization.
In Vitro Kinase Labeling
In vitro kinase assays were performed on 2 μg recombinant protein as previously described.48 Proteins were incubated for 10 minutes at 30°C in enzyme buffer with 1 µM ATP in the presence of recombinant PKA (NEB) and 32P. Reactions were terminated by the addition of SDS–PAGE loading buffer, separated, and labeled MBP-PLAT detected by autoradiography. Equal loading was confirmed by Coomassie staining. Nonradioactive PKA treatment of MBP-PLAT was also performed in the same manner without 32P. Two control reactions were prepared in the same way, except that one reaction excluded ATP and the other did not contain ATP or PKA.
LC-MS/MS Analyses
Immunoprecipitation was carried out using a rabbit N–terminal GFP antibody conjugated to Dynabeads for 1 hour at 4°C followed by extensive washing. Interacting proteins were eluted with sample buffer and electrophoresed. Relevant bands were excised and digested with trypsin; resultant peptides were identified, and phosphorylation site analysis was undertaken using a dissociation–enabled (ETD) Thermo Fisher Scientific Orbitrap Elite using Mascot and PhosphoRS3.1 software essentially as described.49 Proteins required a minimum of two peptides with a 95% confidence interval or above to be reported.
Live–Cell Surface Labeling of PC1
Live–cell surface labeling was carried out using a modification of the method by Chapin et al.19 In brief, HEK293 cells plated onto coated glass coverslips were cotransfected with Flag-PC1-HA and/or PKD2-Pk or PKD2-CFP. After 24 hours, live cells were incubated with primary antibody directed to the (extracellular) Flag tag at 4°C in a blocking buffer of 0.1% BSA in PBS with 100 nM CaCl2 and 1 mM MgCl2 (PBS++). After 60 minutes, labeled cells were washed with PBS++, fixed for 20 minutes in 4% paraformaldehyde, permeabilized in PBS++ with 0.3% Triton X-100 and 0.1% BSA, and blocked for 30 minutes in goat serum dilution buffer (GSDB; 16% goat serum, 120 mM sodium phosphate, 0.3% Triton X-100, and 450 mM NaCl). After another 1 hour of incubation with monoclonal anti–HA primary antibody diluted in GSDB, they were washed and then incubated for 1 hour with the AlexaFluor–conjugated secondary antibodies diluted in GSDB.
To quantify the surface expression of PC1, the threshold intensity for background Flag staining was first determined on the basis of the fluorescence intensity of untransfected controls. Of the transfected cells, the number of cells that were positive for surface PC1 staining in each field (above the background threshold intensity) was counted and expressed as a percentage over the total number of transfected HA–positive cells (n>150 cells). Images were captured from at least 10 random fields in triplicate wells using an Imaging Systems Inverted IX71 Microscope (Olympus, Tokyo, Japan) configured for multifluorescence image capture. Images were acquired using SimplePCI Imaging Software (Compix, Hamamatsu, Sewickley, PA). Data are presented as means±SEM and representative of at least three separate experiments.
To inhibit endocytosis, HEK293 cells were transfected with a dominant–negative dynamin plasmid (K44A). Cells were washed with ice–cold live–cell imaging solution containing HBSS, 1% BSA, and 20 mM glucose and incubated for 30 minutes at 37°C with Transferrin-594 (25 µg/ml in ice–cold live–cell imaging solution) to visualize endocytic uptake.
Cell Surface Biotinylation
HEK293 or UCL93/3 kidney epithelial cells50 were cultured to 90% confluency in six-well dishes, washed three times with PBS, and incubated for 40 minutes with 1 mg/ml sulpho-NHS-SC-Biotin in PBS (pH 8.0) at 4°C. In some experiments, cells were pretreated for 30 minutes with 5 µg/ml brefeldin A before incubation with 100 µM dbcAMP and 0.1 mM forskolin for 60 minutes. UCL93/3 cells were transfected with control siRNA (Ambion) or siRNA targeting ARRB1/2 (MWG Operon)51 or AP2M1 (Smartpool; Dharmacon)52 for 48 hours before surface biotinylation.
After biotinylation, cells were rinsed two times initially with PBS containing 100 mM glycine, and excess biotin was quenched by additional incubation for 20 minutes with PBS containing 100 mM glycine and 0.1% BSA. After additional PBS washes, they were lysed by the addition of 500 µl lysis buffer (50 mM Tris, 500 mM NaCl, 5 mM EDTA, and 1% TX-100 [pH 7.5]) for 1 hour at 4°C. Equal amounts of cell lysate were incubated with 100 µl streptavidin beads overnight at 4°C. Beads were washed three times each with lysis buffer, high-salt buffer (50 mM Tris, 500 mM NaCl, and 0.1% TX-100), and nonsalt buffer (10 mM Tris [pH 7.5]) before being resuspended in 40 µl 2× SDS sample buffer. Samples were analyzed by SDS-PAGE and Western blotting. For quantification of transfected PC1, we used the PC1-CTF (detected by HA blot) as the biotinylation protocol can lead to detachment of the PC1-NTF under these assay conditions as previously reported.19,53
Deglycosylation Analyses of PC1
Enzymatic deglycosylation analysis of endogenous PC1 in the human cell line UCL93/3 was performed on freshly prepared cell lysates using PNGaseF and EndoH as previously described.54
MBP Pulldown Protocol
In total, 2 µg His-ARRB1 or His-ARRB2 proteins were added to 2 µg MBP-PLAT wild-type, S3164D, or S3164A proteins in 300 µl binding buffer (5 mM Hepes [pH 7.9], 150 mM NaCl, 1.5 mM MgCl2, 0.1 mM EDTA, and 0.1% NP-40) and incubated at room temperature for 3 hours with rotation. Binding between MBP-CT1 and His-CT2 proteins was used as a positive control.7 Negative controls included were MBP and Amylose Resin alone; 60 µl 50% Amylose Resin (New England Biolabs) in binding buffer was added to each mixture and incubated at room temperature for an additional 1 hour. The resin in each tube was collected by centrifugation at 9000 rpm for 2 minutes, washed with 1 ml Column buffer (20 mM Tris-HCl [pH 7.4], 200 mM NaCl, and 1 mM EDTA), and then boiled in 50 µl 1× SDS gel loading buffer for 5 minutes. After centrifugation, the samples were analyzed by Western blotting.
Depletion of PM PI4P and PI(4,5)P2
Depletion of PM PI4P and PI(4,5)P2 was carried out as described by Hammond et al.20 Briefly, HEK293 and CHO-K1 cells were transfected with an RFP–labeled enzymatic chimera (PJ) of INPP5E, which converts PI(4,5)P2 to PI4P, and the S. cerevisiae Sac1 phosphatase, which dephosphorylates PI4P, INPP5E inactivated (PJ-Sac), Sac inactivated (PJ-INPP5E), or INPP5E/Sac inactivated (PJ-Dead). Cells were cotransfected with CFP-Lyn11-fragment of mTOR that binds rapamycin, which facilitates rapamycin-inducible recruitment of enzymes to the PM and YFP-PLAT. Recruitment of enzymes to the PM was initiated by addition of 1 µM rapamycin after 20 seconds. Images were captured every 10 seconds for up to 90 seconds to monitor enzyme translocation to the cell surface. Cells showing surface expression of YFP-PLAT in 10 random fields were counted and expressed as a percentage over the total number of YFP-PLAT–expressing cells (n>150 cells). Data are presented as means±SEM from at least three separate experiments.
TIRF Microscopy
PLAT-CFP and ARBB2-YFP plasmids were used for FRET experiments. A membrane-anchored CFP was used as a negative control. Cells plated on poly-d-lysine–coated glass coverslips and maintained in FRET buffer were imaged using a Nikon Ti-E Inverted Microscope equipped with an oil immersion 60× NA 1.49 plan-apo TIRF objective and a TIRF excitation arm with motorized critical angle control (Nikon). Critical angles for CFP and YFP excitation were determined in advance, and identical angles were used in every experiment. Inline neutral density filters were used to minimize bleaching. Excitation light came from a 442-nm solid–state laser (CFP; Melles Griot) and the 514-nm line of an argon gas laser (YFP; Melles Griot) using appropriate dichroic filters. YFP was detected using 500±20- (ex) and 535±30-nm (em) filters, CFP was detected using 436±20- (ex) and 480±40-nm (em) filters, and FRET was detected using 436±20- (ex) and 535±30-nm (em) filters.
Xenopus Embryo Manipulations
Xenopus experiments were approved by the Institutional Animal Care and Use Committee and adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Embryos obtained by in vitro fertilization were maintained in 0.1× modified Barth medium55 and staged following the work by Nieuwkoop and Faber.56 Knockdown of Pkd1 was performed using an antisense MO targeting 3′–splice donor sites (Pkd1-sMO, 5′-TCC TTA TGG TCC GAG TTA CCT TGG G-3′; GeneTools). Microinjection assays, RT-PCR, and sequencing of the products showed the efficacy of the Pkd1-sMO to prevent proper splicing (Supplemental Figure 11). To generate synthetic mRNA, pCS2-Plat-GFP and its derivatives carrying the corresponding point mutations were linearized with NotI and transcribed with SP6 RNA polymerase using the mMessage mMachine (Ambion).
Whole–mount in situ hybridization embryos at stage 39 were processed as described.30 For whole-mount immunofluorescence, embryos were fixed in Dent’s fixative (4:1 methanol:DMSO) at stage 42, incubated sequentially with the 3G8 and 4A6 antibodies,57 and developed using AlexaFluor-448– and AlexaFluor-555–labeled α–mouse secondary antibodies (Life Technologies). Embryos were analyzed by confocal microscopy.
Statistical Analyses
Data are presented as mean values±SEM; t test was used for statistical analysis, with a P value of <0.05 indicating statistical significance. All analyses were carried out using Prism (Graphpad).
Disclosures
None.
Supplementary Material
Acknowledgments
We dedicate this paper to the memory of the late Peter J. Artymiuk (P.J.A.), a good friend and colleague who died before completion of the project. We thank Dr. Hans Wienk and the European NMR Large Scale Facility Utrecht (SONNMRLSF) for access to the 900-MHz spectrometer; Svetlana Sedelnikova, Isobel Woodman, and Richard Beniston for technical assistance; Yiqiang Cai, Stefan Somlo, Robert Lefkowitz, and Robin Irvine for gifts of reagents; and Ellen Allwood, Kathryn Ayscough, and Elizabeth Smythe for helpful discussion and comments on the manuscript.
This project was funded by Kidney Research United Kingdom Grant RP19/2010 (to P.J.A., M.P.W., and A.C.M.O.) and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health Grants R01-DK087688 (to J.-P.V.), R01-DK102495 (to J.-P.V.), and R01-DK080745-05 (to O.W.). A.J.N. was supported by a Medical Research Council Studentship. Mass spectrometry analysis was undertaken in biOMICS, the University of Sheffield Mass Spectrometry Facility, which is supported by funding from Yorkshire Cancer Research Grant SHEND01, the European Structural Fund Programme, and the University of Sheffield Alumni Fund.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014111074/-/DCSupplemental.
References
- 1.Ong AC, Harris PC: Molecular pathogenesis of ADPKD: The polycystin complex gets complex. Kidney Int 67: 1234–1247, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J: Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Streets AJ, Wagner BE, Harris PC, Ward CJ, Ong AC: Homophilic and heterophilic polycystin 1 interactions regulate E-cadherin recruitment and junction assembly in MDCK cells. J Cell Sci 122: 1410–1417, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibraghimov-Beskrovnaya O, Bukanov NO, Donohue LC, Dackowski WR, Klinger KW, Landes GM: Strong homophilic interactions of the Ig-like domains of polycystin-1, the protein product of an autosomal dominant polycystic kidney disease gene, PKD1. Hum Mol Genet 9: 1641–1649, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Streets AJ, Newby LJ, O’Hare MJ, Bukanov NO, Ibraghimov-Beskrovnaya O, Ong AC: Functional analysis of PKD1 transgenic lines reveals a direct role for polycystin-1 in mediating cell-cell adhesion. J Am Soc Nephrol 14: 1804–1815, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG: Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408: 990–994, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Giamarchi A, Feng S, Rodat-Despoix L, Xu Y, Bubenshchikova E, Newby LJ, Hao J, Gaudioso C, Crest M, Lupas AN, Honoré E, Williamson MP, Obara T, Ong AC, Delmas P: A polycystin-2 (TRPP2) dimerization domain essential for the function of heteromeric polycystin complexes. EMBO J 29: 1176–1191, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parnell SC, Magenheimer BS, Maser RL, Rankin CA, Smine A, Okamoto T, Calvet JP: The polycystic kidney disease-1 protein, polycystin-1, binds and activates heterotrimeric G-proteins in vitro. Biochem Biophys Res Commun 251: 625–631, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Delmas P, Nauli SM, Li X, Coste B, Osorio N, Crest M, Brown DA, Zhou J: Gating of the polycystin ion channel signaling complex in neurons and kidney cells. FASEB J 18: 740–742, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Bateman A, Sandford R: The PLAT domain: A new piece in the PKD1 puzzle. Curr Biol 9: R588–R590, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Ponting CP, Hofmann K, Bork P: A latrophilin/CL-1-like GPS domain in polycystin-1. Curr Biol 9: R585–R588, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Bergmann C, von Bothmer J, Ortiz Brüchle N, Venghaus A, Frank V, Fehrenbach H, Hampel T, Pape L, Buske A, Jonsson J, Sarioglu N, Santos A, Ferreira JC, Becker JU, Cremer R, Hoefele J, Benz MR, Weber LT, Buettner R, Zerres K: Mutations in multiple PKD genes may explain early and severe polycystic kidney disease. J Am Soc Nephrol 22: 2047–2056, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornec-Le Gall E, Audrézet MP, Chen JM, Hourmant M, Morin MP, Perrichot R, Charasse C, Whebe B, Renaudineau E, Jousset P, Guillodo MP, Grall-Jezequel A, Saliou P, Férec C, Le Meur Y: Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol 24: 1006–1013, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naylor CE, Eaton JT, Howells A, Justin N, Moss DS, Titball RW, Basak AK: Structure of the key toxin in gas gangrene. Nat Struct Biol 5: 738–746, 1998 [DOI] [PubMed] [Google Scholar]
- 15.van Meer G, Voelker DR, Feigenson GW: Membrane lipids: Where they are and how they behave. Nat Rev Mol Cell Biol 9: 112–124, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gnad F, Gunawardena J, Mann M: PHOSIDA 2011: The posttranslational modification database. Nucleic Acids Res 39: D253–D260, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y, Ong AC, Williamson MP, Hounslow AM: Backbone assignment and secondary structure of the PLAT domain of human polycystin-1 [published online ahead of print May 6, 2015]. Biomol NMR Assign [DOI] [PubMed] [Google Scholar]
- 18.Eek P, Järving R, Järving I, Gilbert NC, Newcomer ME, Samel N: Structure of a calcium-dependent 11R-lipoxygenase suggests a mechanism for Ca2+ regulation. J Biol Chem 287: 22377–22386, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapin HC, Rajendran V, Caplan MJ: Polycystin-1 surface localization is stimulated by polycystin-2 and cleavage at the G protein-coupled receptor proteolytic site. Mol Biol Cell 21: 4338–4348, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammond GR, Fischer MJ, Anderson KE, Holdich J, Koteci A, Balla T, Irvine RF: PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science 337: 727–730, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boehm M, Bonifacino JS: Adaptins: The final recount. Mol Biol Cell 12: 2907–2920, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonifacino JS, Traub LM: Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem 72: 395–447, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Shukla AK, Xiao K, Lefkowitz RJ: Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci 36: 457–469, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ong AC, Harris PC, Davies DR, Pritchard L, Rossetti S, Biddolph S, Vaux DJ, Migone N, Ward CJ: Polycystin-1 expression in PKD1, early-onset PKD1, and TSC2/PKD1 cystic tissue. Kidney Int 56: 1324–1333, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Gainullin VG, Hopp K, Ward CJ, Hommerding CJ, Harris PC: Polycystin-1 maturation requires polycystin-2 in a dose-dependent manner. J Clin Invest 125: 607–620, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura T, Allen PB, Nairn AC, Caplan MJ: Arrestins and spinophilin competitively regulate Na+,K+-ATPase trafficking through association with a large cytoplasmic loop of the Na+,K+-ATPase. Mol Biol Cell 18: 4508–4518, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feinstein TN, Yui N, Webber MJ, Wehbi VL, Stevenson HP, King JD, Jr., Hallows KR, Brown D, Bouley R, Vilardaga JP: Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin. J Biol Chem 288: 27849–27860, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonifacino JS, Dell’Angelica EC: Molecular bases for the recognition of tyrosine-based sorting signals. J Cell Biol 145: 923–926, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owen DJ, Luzio JP: Structural insights into clathrin-mediated endocytosis. Curr Opin Cell Biol 12: 467–474, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Tran U, Pickney LM, Ozpolat BD, Wessely O: Xenopus Bicaudal-C is required for the differentiation of the amphibian pronephros. Dev Biol 307: 152–164, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran U, Zakin L, Schweickert A, Agrawal R, Döger R, Blum M, De Robertis EM, Wessely O: The RNA-binding protein bicaudal C regulates polycystin 2 in the kidney by antagonizing miR-17 activity. Development 137: 1107–1116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hegele RA, Tu L, Connelly PW: Human hepatic lipase mutations and polymorphisms. Hum Mutat 1: 320–324, 1992 [DOI] [PubMed] [Google Scholar]
- 33.van Tilbeurgh H, Sarda L, Verger R, Cambillau C: Structure of the pancreatic lipase-procolipase complex. Nature 359: 159–162, 1992 [DOI] [PubMed] [Google Scholar]
- 34.Recacha R, Boulet A, Jollivet F, Monier S, Houdusse A, Goud B, Khan AR: Structural basis for recruitment of Rab6-interacting protein 1 to Golgi via a RUN domain. Structure 17: 21–30, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Aleem AM, Jankun J, Dignam JD, Walther M, Kühn H, Svergun DI, Skrzypczak-Jankun E: Human platelet 12-lipoxygenase, new findings about its activity, membrane binding and low-resolution structure. J Mol Biol 376: 193–209, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Guillouard I, Alzari PM, Saliou B, Cole ST: The carboxy-terminal C2-like domain of the alpha-toxin from Clostridium perfringens mediates calcium-dependent membrane recognition. Mol Microbiol 26: 867–876, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Gillmor SA, Villaseñor A, Fletterick R, Sigal E, Browner MF: The structure of mammalian 15-lipoxygenase reveals similarity to the lipases and the determinants of substrate specificity. Nat Struct Biol 4: 1003–1009, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Hu J, Barr MM: ATP-2 interacts with the PLAT domain of LOV-1 and is involved in Caenorhabditis elegans polycystin signaling. Mol Biol Cell 16: 458–469, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu J, Bae YK, Knobel KM, Barr MM: Casein kinase II and calcineurin modulate TRPP function and ciliary localization. Mol Biol Cell 17: 2200–2211, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S: Membrane phosphatidylserine regulates surface charge and protein localization. Science 319: 210–213, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Audrézet MP, Cornec-Le Gall E, Chen JM, Redon S, Quéré I, Creff J, Bénech C, Maestri S, Le Meur Y, Férec C: Autosomal dominant polycystic kidney disease: Comprehensive mutation analysis of PKD1 and PKD2 in 700 unrelated patients. Hum Mutat 33: 1239–1250, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Torres VE, Harris PC: Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J Am Soc Nephrol 25: 18–32, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koenig BW, Rogowski M, Louis JM: A rapid method to attain isotope labeled small soluble peptides for NMR studies. J Biomol NMR 26: 193–202, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Reed MAC, Hounslow AM, Sze KH, Barsukov IG, Hosszu LLP, Clarke AR, Craven CJ, Waltho JP: Effects of domain dissection on the folding and stability of the 43 kDa protein PGK probed by NMR. J Mol Biol 330: 1189–1201, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Kiefer F, Arnold K, Künzli M, Bordoli L, Schwede T: The SWISS-MODEL Repository and associated resources. Nucleic Acids Res 37: D387–D392, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwieters CD, Kuszewski JJ, Tjandra N, Clore GM: The Xplor-NIH NMR molecular structure determination package. J Magn Reson 160: 65–73, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Shen Y, Bax A: Protein backbone and sidechain torsion angles predicted from NMR chemical shifts using artificial neural networks. J Biomol NMR 56: 227–241, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Streets AJ, Wessely O, Peters DJ, Ong AC: Hyperphosphorylation of polycystin-2 at a critical residue in disease reveals an essential role for polycystin-1-regulated dephosphorylation. Hum Mol Genet 22: 1924–1939, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taus T, Köcher T, Pichler P, Paschke C, Schmidt A, Henrich C, Mechtler K: Universal and confident phosphorylation site localization using phosphoRS. J Proteome Res 10: 5354–5362, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Parker E, Newby LJ, Sharpe CC, Rossetti S, Streets AJ, Harris PC, O’Hare MJ, Ong AC: Hyperproliferation of PKD1 cystic cells is induced by insulin-like growth factor-1 activation of the Ras/Raf signalling system. Kidney Int 72: 157–165, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahn S, Nelson CD, Garrison TR, Miller WE, Lefkowitz RJ: Desensitization, internalization, and signaling functions of beta-arrestins demonstrated by RNA interference. Proc Natl Acad Sci U S A 100: 1740–1744, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Motley A, Bright NA, Seaman MN, Robinson MS: Clathrin-mediated endocytosis in AP-2-depleted cells. J Cell Biol 162: 909–918, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cai Y, Fedeles SV, Dong K, Anyatonwu G, Onoe T, Mitobe M, Gao JD, Okuhara D, Tian X, Gallagher AR, Tang Z, Xie X, Lalioti MD, Lee AH, Ehrlich BE, Somlo S: Altered trafficking and stability of polycystins underlie polycystic kidney disease. J Clin Invest 124: 5129–5144, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Newby LJ, Streets AJ, Zhao Y, Harris PC, Ward CJ, Ong AC: Identification, characterization, and localization of a novel kidney polycystin-1-polycystin-2 complex. J Biol Chem 277: 20763–20773, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Sive HL, Grainger RM, Harland RM: Early Development of Xenopus Laevis: A Laboratory Manual, Cold Spring Harbor, New York, Cold Spring Harbor Laboratory Press, 2000 [Google Scholar]
- 56.Nieuwkoop PD, Faber J: Normal Table of Xenopus Laevis, New York, Garland Publishing, 1994 [Google Scholar]
- 57.Vize PD, Jones EA, Pfister R: Development of the Xenopus pronephric system. Dev Biol 171: 531–540, 1995 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.