Abstract
Kidney transplantation is a cost-saving treatment that extends the lives of patients with ESRD. Unfortunately, the kidney transplant waiting list has ballooned to over 100,000 Americans. Across large areas of the United States, many kidney transplant candidates spend over 5 years waiting and often die before undergoing transplantation. However, more than 2500 kidneys (>17% of the total recovered from deceased donors) were discarded in 2013, despite evidence that many of these kidneys would provide a survival benefit to wait-listed patients. Transplant leaders have focused attention on transplant center report cards as a likely cause for this discard problem, although that focus is too narrow. In this review, we examine the risks associated with accepting various categories of donated kidneys, including discarded kidneys, compared with the risk of remaining on dialysis. With the goal of improving access to kidney transplant, we describe feasible proposals to increase acceptance of currently discarded organs.
Keywords: kidney transplantation, outcomes, epidemiology and outcomes
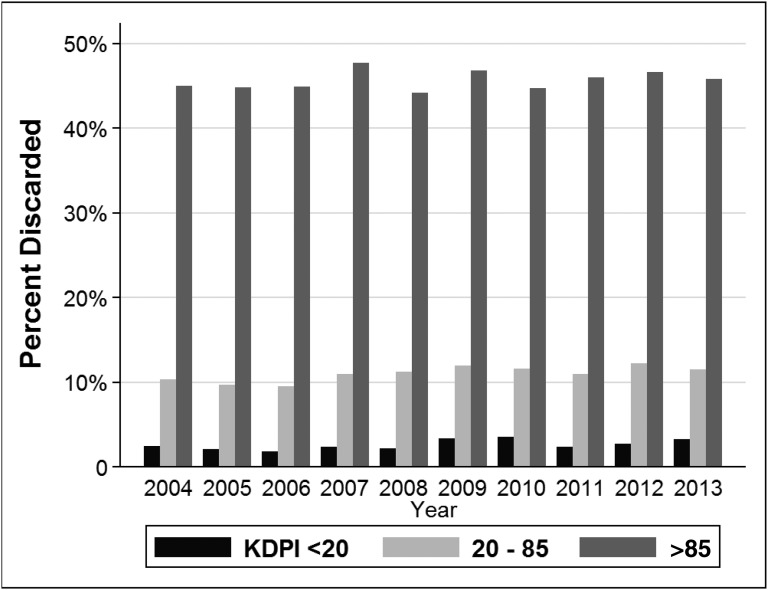
The lack of organs for kidney transplantation is a major public health problem due to its attributable mortality and excess cost to the United States health system. During the last decade, the waiting list for kidney transplantation in the United States has risen steadily to over 100,000 candidates. Unfortunately, the number of deceased donor kidney transplants has plateaued at around 11,000 per year and living kidney donation has also declined. As a result, the number of candidates added to the kidney transplant wait-list in 2013 was three times higher than the number of donated kidneys.1 Kidney transplant candidates commonly wait over 5 years for an organ in many areas, although individual waiting time depends on geography, blood type, and sensitization. Most patients receive chronic dialysis while wait-listed. Duration of dialysis is one of the strongest risk factors for death, even among patients ultimately transplanted.2 As a consequence of long waiting times, many candidates should be counseled that they have a greater probability of dying than they do of transplantation.3 For these reasons, the fact that >17% of procured kidneys were discarded in 2013 (Figure 1) requires scrutiny and new solutions.
Figure 1.
The kidney discard rate has remained high over time, especially for kidneys with KDPI >85. KDPI, kidney donor profile index.
Ever-lengthening waiting times for kidney transplantation have been mirrored by a rising percentage of patients with inactive status (referred to as status VII), meaning that they are ineligible for transplantation.4,5 One third of kidney transplant candidates are currently inactive. While the high percentage of inactive patients was driven by an Organ Procurement and Transplantation (OPTN) policy change in 2003 (allowing candidates to accrue wait-list priority whilst being classified as status VII), the rise in inactivity may also be driven by health deterioration during dialysis.6 Longer dialysis time leads to greater rates of cardiovascular events, infections, and other complications that render patients temporarily or permanently unable to undergo transplantation. In summary, as the disparity between supply and demand for transplantation has worsened, many patients never receive transplants, and some suffer considerably worsened health by the time transplantation occurs.
The Lost Opportunity: Many Available Kidneys are from Nonstandard Donors
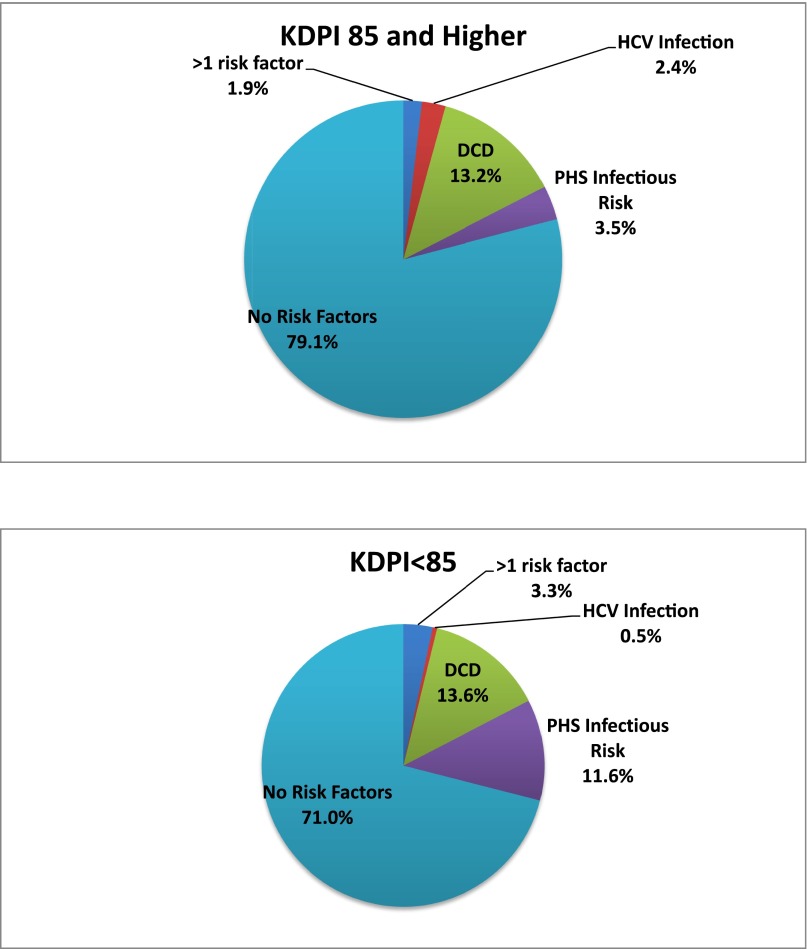
Expanding access to kidney transplantation will require centers and their patients to accept kidneys that present different types of risks. These risks can be placed into two categories: graft failure and transmissible disease. Figure 2 shows that a substantial percentage of kidneys transplanted in 2013 were not from standard criteria donors.
Figure 2.
A substantial percentage of kidneys transplanted in 2013 were not from standard criteria donors. Top panel: KDPI >85%; Lower panel: KDPI <85%. KDPI, kidney donor profile index; PHS, public health service; HCV, hepatitis C virus; DCDD, donation after circulatory determination of death.
Most patients probably understand that many choices, such as buying a car, require simultaneously considering several options with distinct risks and benefits. However, when a deceased donor organ is offered, the choice is to accept that transplant or continue waiting with uncertain information about the timing and quality of future organ offers.
Until recently, kidneys with a low risk of graft failure were categorized as “standard criteria.” Kidneys with elevated graft failure risk due to older donor age, hypertension, elevated terminal creatinine, or death by cerebrovascular accident were designated as expanded criteria donor organs.7 Organs from donors after circulatory determination of death (DCDD) are also considered nonstandard given elevated rates of delayed graft function, although graft survival has thus far proven to be nearly as good as for standard criteria donor kidneys.8,9 Although such favorable outcomes may, in part, reflect careful selection of DCDD organs, many currently discarded organs would almost certainly provide a favorable risk-benefit ratio to some wait-listed individuals.
With the new kidney allocation system, these binary categories have been largely supplanted by the kidney donor profile index (KDPI).10 The KDPI incorporates ten donor characteristics (including age, terminal creatinine, and hepatitis C virus [HCV] serostatus) into a score from 0% to 100%.11 For example, a KDPI of 85% implies that the kidney’s graft failure risk is higher than for 85% of the reference population of kidneys. Similar to earlier policy with expanded criteria donor kidneys, transplant candidates must provide additional consent to be offered kidneys with KDPI≥85%.
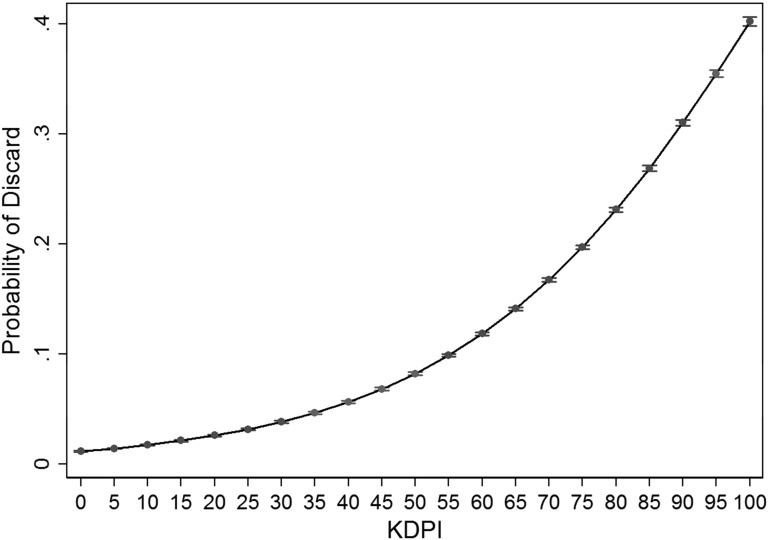
The rate of 2-year graft failure is about 9% for kidneys with the lowest (i.e., best) KDPI scores and rises linearly to about 29% for the highest KDPI donors. In contrast, the probability of kidney discard sharply increases as the KDPI nears 60%, as shown in Figure 3. However, the very high discard rates for kidneys with high KDPI scores may not be warranted.
Figure 3.
Increasing Probability of Discard as the KDPI Rises, with 95% CI. KDPI, kidney donor profile index; 95% CI, 95% confidence interval.
Our Toolbox to Assess the Future Function of Donated Kidneys has Major Shortcomings
The C-statistic (a metric of predictive accuracy) of the KDPI for graft failure is approximately 0.6.12 A C-statistic in this low range demonstrates that the KDPI frequently generates incorrect predictions about post-transplant outcomes.11 There are multiple potential explanations for the KDPI’s modest ability to forecast graft failure.13 First, the formula relies heavily on donor age, although there is wide variation in renal function within individuals of the same age.14–16 Second, donor terminal creatinine may underestimate the quality of kidneys with acute injury. Third, the KDPI does not include HLA matching data. Additionally, because new HCV therapies have high cure rates, the KDPI may not accurately forecast outcomes for HCV-positive kidneys in future years.
Many centers also depend on donor histologic findings, such as percent fibrosis, to assess risk of graft failure.17 Table 1 shows that when kidneys are discarded, the OPTN frequently reports that the reason was biopsy findings. However, biopsies usually consist only of light microscopy that is interpreted under time pressure by pathologists with a range of renal expertise. Proposed histologic scoring systems such as the Maryland Aggregate Pathology Index, while promising, have been neither widely validated nor incorporated into OPTN organ offers.18,19 A similar lack of validation limits the utility of pumping parameters or perfusate biomarker concentrations to predict graft outcomes for kidneys that undergo machine perfusion.20
Table 1.
Primary reasons listed for kidney discard in 2013 by the OPTN
| Primary Reasonsa | KDPI<85 N=1206 | KDPI≥85 N=1440 | ||
|---|---|---|---|---|
| n | % | n | % | |
| Biopsy findings | 335 | 28 | 559 | 39 |
| No recipient located | 263 | 22 | 417 | 29 |
| Prior organ function | 122 | 10 | 110 | 8 |
| Anatomic abnormalities | 92 | 8 | 80 | 6 |
| Organ trauma | 68 | 6 | 15 | 1 |
| Diseased organ | 32 | 3 | 30 | 2 |
| Donor medical history | 4 | 0.3 | 11 | 0.8 |
| Donor infection | 15 | 1.2 | 13 | 0.9 |
| Organ too old | 23 | 2 | 9 | 0.6 |
| Donor social history | 7 | 0.6 | 0 | 0 |
| Recipient unsuitable | 7 | 0.6 | 6 | 0.4 |
| Long warm ischemia | 5 | 0.4 | 3 | 0.2 |
| Organ not as described | 7 | 0.6 | 1 | 0.1 |
| Otherb | 236 | 20 | 186 | 13 |
These categories are provided in OPTN dataset and involve subjective judgment.
Free text category; reasons included donor renal cell carcinoma, surgical trauma, and poor pump parameters.
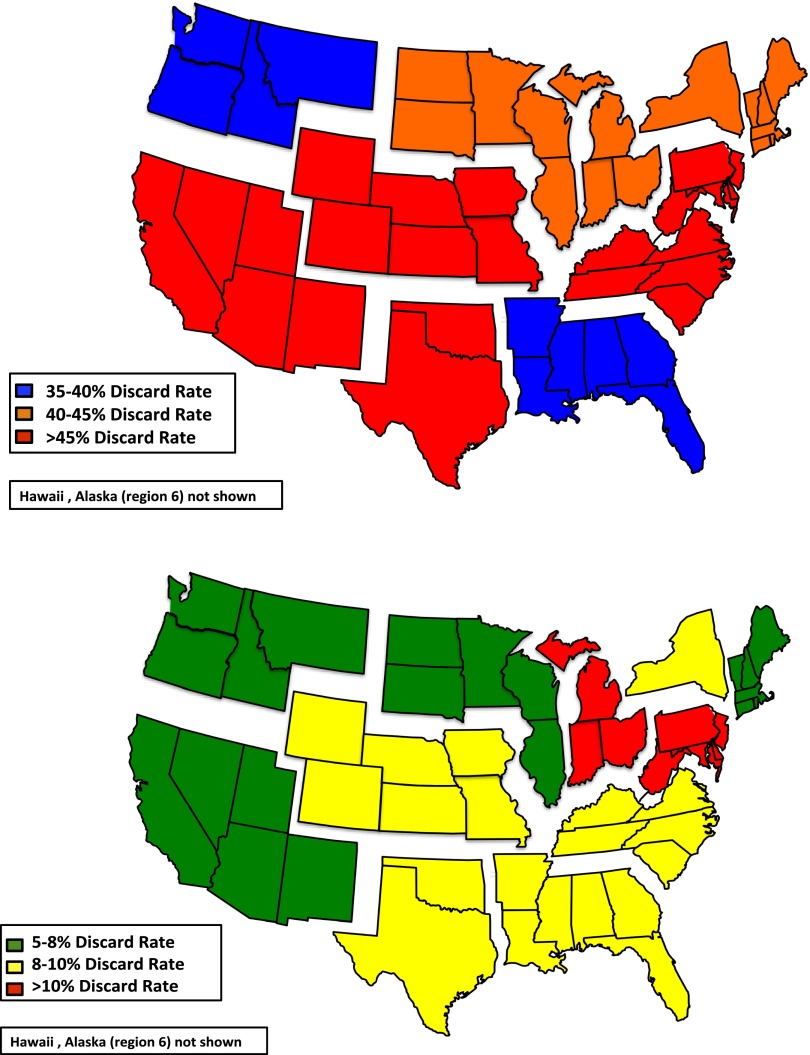
The lack of accurate methods to assess kidney quality probably explains some of the wide geographic variation in kidney discard rates (Figure 4). This variation suggests that organs considered too marginal in some regions would be transplanted in others. Studies are needed to explain this variation. Once differences in practice patterns are better understood, there may be important opportunities for programs that achieve good transplant outcomes using lower-quality organs to advise other programs about best practices.
Figure 4.
Geographic variation in discard rate by organ quality in 2013 (top panel: KDPI >85%; lower panel: KDPI <85%). KDPI, kidney donor profile index.
Organ Acceptance and Risks of Disease Transmission
Concerns about donor-derived infection or malignancy also affect organ acceptance. The KDPI and graft biopsies contain no information about these risks. While it is beyond this article’s scope to provide a comprehensive review of disease transmission in transplantation, the case of blood-borne viruses is instructive. Kidneys from donors with risk factors for blood-borne viral infections (such as injection drug use), but negative viral serologies, are referred to as Public Health Service (PHS) higher infectious-risk organs.21 These PHS-designated kidneys have a 33% higher odds of discard compared with other kidneys (OR 0.67 [95% CI 0.61–0.74]).22 Yet, for most patients, the absolute risk of donor disease transmission is much lower than the risk of continuing dialysis. Specifically, if nucleic acid testing is used to screen the donor for infection, the estimated risk of HCV transmission ranges from 0.027 (for a hemophiliac donor) to 32.4 (if the donor used injection drugs) per 10,000 transplants. For recipients of PHS infectious-risk kidneys, the estimated rates of HIV transmission are lower than those for HCV.23,24 By comparison, the annual death rate for wait-listed patients is approximately 4% and higher for subgroups with diabetes or over 60 years of age.25 Post-transplant patient and allograft survival appear to be similar between recipients of PHS infectious-risk versus standard criteria organs,26 which is not surprising given the tiny predicted rates of disease transmission. A study of willingness to consider PHS infectious-risk organs showed that most transplant candidates would accept these kidneys, particularly when otherwise faced with long wait-times.27
Evidence That Some Discarded Kidneys Would Provide a Survival Benefit If Transplanted
In 2013, 51% of kidneys procured with a KDPI>90 were discarded. However, even kidneys of the lowest quality (as manifested by the highest KDPI scores) may still confer benefits versus remaining wait-listed. Massie et al. examined the survival benefit of accepting a high KDPI kidney versus waiting for a better quality kidney. By 19.8 months post-transplantation, those candidates who accepted the highest KDPI kidneys (>90) had a lower cumulative death rate than those still waiting.28
Similarly, a simulation of survival outcomes by Chow et al. revealed that 82% of wait-listed candidates would live longer by adopting a strategy of accepting a PHS infectious-risk kidney. The survival benefit of accepting a PHS infectious-risk kidney was most evident for patients in regions with longer waiting times, older patients, patients with diabetes, and those with elevated panel reactive antibody.29 These simulations did not take into account quality of life, but most studies have demonstrated that kidney transplantation improves quality of life versus dialysis.30
Center Report Cards: A Disincentive to use Higher-Risk but Viable Kidneys?
The persistently elevated discard rates for high KDPI and PHS infectious-risk kidneys have drawn scrutiny. Some transplant professionals have assigned blame for discards on the pressure to optimize post-transplant outcomes.31
United States transplant centers receive report cards on quality twice a year. Every death and allograft failure after transplantation is analyzed for quality lapses and published on a publicly available website (www.srtr.org). Centers with poor risk-adjusted outcomes will face penalties from the OPTN. These centers often receive additional scrutiny from Medicare or private payers. These events create great stress and consume substantial resources because center staff must prepare extensive data to respond to audits.
Report cards should promote quality. However, nearly all report cards focus on effectiveness – that is, how patients survive and function after they receive care. Report cards commonly ignore other key elements of quality, such as whether a center promotes equitable access to transplantation by accepting a reasonable proportion of kidneys offered to them.
Some donated kidneys truly cannot help potential recipients, and ought to be discarded. For example, some kidneys have damaged vessels or present infection or cancer transmission risks that compromise quality in ways not reflected in the KDPI. It is also uncertain which currently discarded kidneys would have similar outcomes to accepted kidneys with the same KDPI. However, report cards exacerbate the existing scarcity of usable organs because centers perceive that more liberal acceptance of organs or sicker patients will put their outcomes at risk.32,33 A bad report card threatens a center’s financial viability, because payers cancel contracts and patients leave.31 Additionally, recipients of lower-quality kidneys have more complications and cost the center more to transplant.
The focus on post-transplant survival in these reports may also deter innovation, because risk-adjustment models struggle to keep pace with scientific advances. For example, if a center pursues innovative treatments like desensitizing patients to antigens present on kidney allografts, the center’s outcomes are not adjusted for that procedure.34 Yet, the use of desensitization actually serves as a marker for broader acceptance of donors or recipients, because centers that do not perform this procedure are less likely to successfully transplant some patients with very elevated panel reactive antibodies.
Potential Remedies to Improve Organ Acceptance Rates
While report cards may cause centers to be excessively cautious, this “stick” may be a smaller problem than the lack of “carrots” to motivate centers to accept more organs. If the risk-benefit ratio of accepting kidneys were more favorable, many more patients could be spared years of dialysis (Table 2). Higher-risk kidneys would be rescued from discard.
Table 2.
Proposed policies to incentivize transplant centers to accept kidneys with a high KDPI or designated by the PHS as increased risk for blood-borne viral infection
| Current Barrier | New Incentives | Challenges to Successful Implementation |
|---|---|---|
| Acceptance of high KDPI kidneys imposes additional costs for centers, such as perioperative complications requiring treatment and longer length of stay. | Medicare and other payers should reimburse at higher rates for higher-risk kidneys. | Payers have not historically embraced risk-adjusted payment structures. |
| Kidney allocation rules should be changed so that centers that achieve good outcomes with higher-risk kidneys subsequently get priority for higher-risk kidneys. | This approach would require new changes to the national allocation system. | |
| Kidney allocation rules should be changed across all organ procurement organizations so that when a donor is identified whose kidneys are at highest risk for discard, centers can use the pair of kidneys for one recipient. | If kidneys at risk for discard are not accurately identified, one patient may get two kidneys where two patients might have instead benefitted. | |
| Transplant centers are penalized for worse-than-expected patient and allograft survival after transplantation. | Calculation of center survival outcomes should exclude patients who received the lowest-quality kidneys. | Potentially valuable information about center performance would not be included in the survival statistics. |
| Report cards should include an “organ acceptance metric” which would reward centers for accepting higher-risk organs. | The organ acceptance metric would require careful risk adjustment for organ quality and the center population. | |
| Each year, regulatory organizations should recognize and/or reward centers that aggressively accepted organs. | The organ acceptance metric might put pressure on centers to accept lower-quality kidneys for patients that would be better served by waiting longer for a higher-quality organ. | |
| It is unknown how to optimally design this recognition or reward to change center behavior. | ||
| High KDPI and PHS infectious risk kidneys are often turned down by many patients, leading to prolonged cold storage and eventual discard. | Novel methods for prospective informed consent should be developed, such that patients are consented on the waiting list for lower-quality organs and minimal additional consent is required at the organ offer; kidneys are accepted quickly. | Transplant clinicians may feel that obtaining a second consent at the time that an organ is offered is necessary to protect them from subsequent liability. |
Note that higher KDPI kidneys have an elevated estimated rate of graft failure. PHS infectious-risk kidneys, such as a kidney donated by an individual with an injection drug-use history, pose slightly higher risks of viral transmission (such as HIV) compared with other kidneys).
The initial group of remedies would reduce the cost to centers of accepting a lower-quality kidney. First, payers should develop tiered payments according to organ quality. Any transplant center should achieve good outcomes by accepting only kidneys from 20-year-old donors, but a center that accepts a 68-year-old kidney will likely need to expend more resources to achieve that same outcome for its recipient.9 Therefore, a center that transplants a high KDPI kidney should be rewarded with higher reimbursement. Alternatively, a center that accepted a high KDPI kidney could be offered additional priority when the next kidney is offered. Another approach would be to encourage centers to transplant two lower-quality kidneys into one recipient; these recipients usually enjoy additional years of good kidney function.35 Because we can predict which kidneys are at high risk of discard,11 the OPTN could change allocation so that more high KDPI kidneys are rapidly offered as a pair to single recipients. Currently, only 2% of donated kidneys are transplanted as dual organs.36 This proposal might only generate a small number of additional transplants, but would increase survival for patients who received them.
The second set of remedies would change report cards so that they do not disincentivize aggressive acceptance of kidneys. For instance, post-transplant outcomes could exclude data from patients who received the highest KDPI kidneys. One criticism of this remedy is that, due to thorough risk adjustment, outcomes for patients receiving high KDPI kidneys do not usually cause bad center report cards.37 However, this criticism rests on the assumption that current risk adjustment – based on outcomes for kidneys accepted in the past – are sufficient to predict outcomes for currently discarded kidneys. Additionally, even if center reluctance to use high KDPI kidneys is based on a flawed understanding of risk adjustment, removing some high KDPI kidneys from report cards might still encourage greater utilization of these kidneys.
An alternative approach is for the OPTN to adopt a complementary metric of a center’s organ acceptance rate. The organ acceptance rate is calculated by dividing the number of organs each center accepts by the number offered to that center’s patients that are ultimately accepted somewhere. This metric would be adjusted for kidney quality and patient comorbidities. Centers that transplanted greater proportions of patients might therefore avoid penalties despite worse than expected survival rates. This dual-reporting approach may be particularly valuable at centers with virtual monopolies on the local ESRD population. Currently, these centers can afford to refuse all but the highest-quality organs.33 The OPTN has developed a proposal for the organ acceptance rate metric, which is out for public comment.38 Unfortunately, this proposal (entitled the “Composite Pre-transplant Metric”) has paired the organ acceptance rate with a second measure – the center’s transplant rate. The problem is that the transplant rate will be inversely proportional to the proportion of potential transplant candidates that a center chooses to list. If graded on transplant rates, centers may not add vulnerable patients to the wait-list, thereby making their numbers look better but reducing access to transplantation. For this reason, the organ acceptance metric should be implemented alone.
The third approach would focus on fast-tracking high KDPI and PHS infectious-risk kidneys to centers that are likely to accept them. For example, the OPTN could identify centers with a record of achieving good outcomes with these organs. In the following year, these centers would get enhanced access to these organs through prioritized offers, reducing cold ischemia time. A complementary strategy would be to leverage the competitive nature of transplant professionals with public recognition of centers who accepted a high rate of lower-quality organs. This recognition could be an elite status that is reported on public websites, perhaps accompanied by supplemental Medicare funds. Notably, the new kidney allocation system promotes regional sharing of high KDPI kidneys, but early analyses of this system suggest that discard rates have not decreased.39 This finding lends support to the idea that wider sharing of kidneys is not enough to increase organ acceptance; instead, allocation must direct organs to centers likely to accept them.
A final remedy is to change the informed consent process. With the development of thoughtful approaches, patients could undergo informed consent while on the wait-list about accepting some kidneys that are at risk for discard. These kidneys could be rapidly offered with limited requirements for repeat consent at transplantation. This approach might be paired with the fast-tracking of high KDPI kidneys described above. This new approach would avoid the current problem that high KDPI and PHS infectious-risk kidneys are damaged by cold ischemia while centers across the country repeat the full informed consent process with patients and ultimately refuse the organs.40
The Role of Referring Nephrologists in Reducing Kidney Discard
General nephrologists have a fundamental role to play in facilitating access to transplantation by promoting the acceptance of donated kidneys. First, they can counsel patients that all treatment options for ESRD, including dialysis, involve risk. Transplantation with high KDPI or PHS infectious-risk kidneys often reduces mortality risk compared with continuing dialysis. Second, when communicating with transplant centers, referring nephrologists can help to identify which wait-listed patients are willing to consider nonstandard kidneys and likely to benefit. In some cases, they may also request information about whether the center turned down any kidneys offered to these patients. General nephrologists should develop expertise with managing complications such as delayed graft function that commonly accompany the use of high KDPI and DCDD kidneys.
Conclusions
A total of 17% of procured kidneys from deceased donors are discarded, despite evidence that some of these kidneys could benefit wait-listed patients. This review outlines a range of proposals to increase organ acceptance. These proposals pose some challenges. Although the use of lower-quality organs serves a public health purpose by promoting transplant access, some patients may experience complications that would not have occurred had they instead waited for a standard organ. Outcome measures such as the organ acceptance rate will require statistical refinement. Ongoing review will be needed to ensure that an undue proportion of lower-quality kidneys are not allocated to vulnerable groups, such as less-educated patients. Changing consent procedures would also require rethinking the basis for ethical informed consent in transplantation. However, the added value of a second consent may be very limited under the time-pressured situation where organ offers are considered.
Compared with the current situation, where many donated kidneys are discarded, these risks seem like a reasonable tradeoff to achieve transplant’s benefits. By embracing effective incentives, the field of transplantation can recalibrate its approach to risk and bring kidney transplant to more people who need it.
Disclosures
None.
Acknowledgments
Dr. Reese is Chair of the Ethics Committee for the United Network of Organ Sharing. His efforts were supported by the Greenwall Foundation.
Dr. Abt is Co-Chair of the Ethics Committee of the American Society of Transplant Surgeons.
Dr. Halpern is a member of the Technical Advisory Committee to the Scientific Registry of Transplant Recipients, and of the federal Advisory Committee on Blood and Tissue Safety and Availability.
Dr. Harhay’s efforts were supported by a K23 grant from the National Institute of Diabetes and Digestive and Kidney Diseases (grant no. DK105207-01).
The views expressed in this essay are the authors’ own, and do not necessarily reflect the views of these organizations or the US Government.
The authors would also like to acknowledge the advice of John Friedewald, Richard Formica, and Darren Stewart in providing data and feedback related to the effects of the new kidney allocation policy on organ discard. The authors also thank Vishnu Potluri for help with analyses.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Based on OPTN data as of August 21, 2015. URL:http://optn.transplant.hrsa.gov/converge/latestData/rptData.asp
- 2.Wolfe RA, McCullough KP, Schaubel DE, Kalbfleisch JD, Murray S, Stegall MD, Leichtman AB: Calculating life years from transplant (LYFT): methods for kidney and kidney-pancreas candidates. Am J Transplant 8: 997–1011, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Schold J, Srinivas TR, Sehgal AR, Meier-Kriesche HU: Half of kidney transplant candidates who are older than 60 years now placed on the waiting list will die before receiving a deceased-donor transplant. Clin J Am Soc Nephrol 4: 1239–1245, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grams ME, Massie AB, Schold JD, Chen BP, Segev DL: Trends in the inactive kidney transplant waitlist and implications for candidate survival. Am J Transplant 13: 1012–1018, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delmonico FL, McBride MA: Analysis of the wait list and deaths among candidates waiting for a kidney transplant. Transplantation 86: 1678–1683, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Shafi S, Zimmerman B, Kalil R: Temporary inactive status on renal transplant waiting list: causes, risk factors, and outcomes. Transplant Proc 44: 1236–1240, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Rao PS, Ojo A: The alphabet soup of kidney transplantation: SCD, DCD, ECD – fundamentals for the practicing nephrologist. Clin J Am Soc Nephrol 4: 1827–1831, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Locke JE, Segev DL, Warren DS, Dominici F, Simpkins CE, Montgomery RA: Outcomes of kidneys from donors after cardiac death: implications for allocation and preservation. Am J Transplant 7: 1797–1807, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Saidi RF, Elias N, Kawai T, Hertl M, Farrell ML, Goes N, Wong W, Hartono C, Fishman JA, Kotton CN, Tolkoff-Rubin N, Delmonico FL, Cosimi AB, Ko DS: Outcome of kidney transplantation using expanded criteria donors and donation after cardiac death kidneys: realities and costs. Am J Transplant 7: 2769–2774, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Israni AK, Salkowski N, Gustafson S, Snyder JJ, Friedewald JJ, Formica RN, Wang X, Shteyn E, Cherikh W, Stewart D, Samana CJ, Chung A, Hart A, Kasiske BL: New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes. J Am Soc Nephrol 25: 1842–1848, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, Port FK, Sung RS: A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation 88: 231–236, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Organ Procurement and Transplantation Network: Guide to Calculating and Interpreting the KDPI. Available at: http://optn.transplant.hrsa.gov/ContentDocuments/Guide_to_Calculating_Interpreting_KDPI.pdf. Accessed December 31, 2014
- 13.Lee AP, Abramowicz D: Is the Kidney Donor Risk Index a step forward in the assessment of deceased donor kidney quality? Nephrol Dial Transplant 30: 1285–1290, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Epstein M: Aging and the kidney. J Am Soc Nephrol 7: 1106–1122, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Fliser D, Franek E, Ritz E: Renal function in the elderly – is the dogma of an inexorable decline of renal function correct? Nephrol Dial Transplant 12: 1553–1555, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Sung RS, Christensen LL, Leichtman AB, Greenstein SM, Distant DA, Wynn JJ, Stegall MD, Delmonico FL, Port FK: Determinants of discard of expanded criteria donor kidneys: impact of biopsy and machine perfusion. Am J Transplant 8: 783–792, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Munivenkatappa RB, Schweitzer EJ, Papadimitriou JC, Drachenberg CB, Thom KA, Perencevich EN, Haririan A, Rasetto F, Cooper M, Campos L, Barth RN, Bartlett ST, Philosophe B: The Maryland aggregate pathology index: a deceased donor kidney biopsy scoring system for predicting graft failure. Am J Transplant 8: 2316–2324, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Cockfield SM, Moore RB, Todd G, Solez K, Gourishankar S: The prognostic utility of deceased donor implantation biopsy in determining function and graft survival after kidney transplantation. Transplantation 89: 559–566, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Bhangoo RS, Hall IE, Reese PP, Parikh CR: Deceased-donor kidney perfusate and urine biomarkers for kidney allograft outcomes: a systematic review. Nephrol Dial Transplant 27: 3305–3314, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seem DL, Lee I, Umscheid CA, Kuehnert MJ, United States Public Health Service : PHS guideline for reducing human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission through organ transplantation. Public Health Rep 128: 247–343, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan KI, Englesbe MJ, Volk ML: Centers for Disease Control ‘high-risk’ donors and kidney utilization. Am J Transplant 10: 416–420, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Kucirka LM, Sarathy H, Govindan P, Wolf JH, Ellison TA, Hart LJ, Montgomery RA, Ros RL, Segev DL: Risk of window period hepatitis-C infection in high infectious risk donors: systematic review and meta-analysis. Am J Transplant 11: 1188–1200, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kucirka LM, Sarathy H, Govindan P, Wolf JH, Ellison TA, Hart LJ, Montgomery RA, Ros RL, Segev DL: Risk of window period HIV infection in high infectious risk donors: systematic review and meta-analysis. Am J Transplant 11: 1176–1187, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Stewart DE, Cherikh WS, Wainright JL, Boyle G, Snyder JJ, Israni AK, Kasiske BL: OPTN/SRTR 2013 Annual Data Report: kidney. Am J Transplant 15[Suppl 2]: 1–34, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Reese PP, Feldman HI, Asch DA, Halpern SD, Blumberg EA, Thomasson A, Shults J, Bloom RD: Transplantation of kidneys from donors at increased risk for blood-borne viral infection: recipient outcomes and patterns of organ use. Am J Transplant 9: 2338–2345, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reese PP, Tehrani T, Lim MA, Asch DA, Blumberg EA, Simon MK, Bloom RD, Halpern SD: Determinants of the decision to accept a kidney from a donor at increased risk for blood-borne viral infection. Clin J Am Soc Nephrol 5: 917–923, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massie AB, Luo X, Chow EK, Alejo JL, Desai NM, Segev DL: Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am J Transplant 14: 2310–2316, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Chow EK, Massie AB, Muzaale AD, Singer AL, Kucirka LM, Montgomery RA, Lehmann HP, Segev DL: Identifying appropriate recipients for CDC infectious risk donor kidneys. Am J Transplant 13: 1227–1234, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Dew MA, Switzer GE, Goycoolea JM, Allen AS, DiMartini A, Kormos RL, Griffith BP: Does transplantation produce quality of life benefits? A quantitative analysis of the literature. Transplantation 64: 1261–1273, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Schold JD, Buccini LD, Srinivas TR, Srinivas RT, Poggio ED, Flechner SM, Soria C, Segev DL, Fung J, Goldfarb DA: The association of center performance evaluations and kidney transplant volume in the United States. Am J Transplant 13: 67–75, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Kasiske BL, McBride MA, Cornell DL, Gaston RS, Henry ML, Irwin FD, Israni AK, Metzler NW, Murphy KW, Reed AI, Roberts JP, Salkowski N, Snyder JJ, Sweet SC: Report of a consensus conference on transplant program quality and surveillance. Am J Transplant 12: 1988–1996, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Garonzik-Wang JM, James NT, Weatherspoon KC, Deshpande NA, Berger JA, Hall EC, Montgomery RA, Segev DL: The aggressive phenotype: center-level patterns in the utilization of suboptimal kidneys. Am J Transplant 12: 400–408, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Scientific Registry of Transplant Recipients: Guide to the Program-Specific Reports v 13.5, 2013. Available at: http://www.srtr.org/csr/archives/201106/all_csr_documentation.pdf. Accessed September 1, 2015
- 35.Remuzzi G, Grinyò J, Ruggenenti P, Beatini M, Cole EH, Milford EL, Brenner BM, Double Kidney Transplant Group (DKG) : Early experience with dual kidney transplantation in adults using expanded donor criteria. J Am Soc Nephrol 10: 2591–2598, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Tanriover B, Mohan S, Cohen DJ, Radhakrishnan J, Nickolas TL, Stone PW, Tsapepas DS, Crew RJ, Dube GK, Sandoval PR, Samstein B, Dogan E, Gaston RS, Tanriover JN, Ratner LE, Hardy MA: Kidneys at higher risk of discard: expanding the role of dual kidney transplantation. Am J Transplant 14: 404–415, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snyder J, Salkowski N, Zaun D, Schold J, Israni A, Kasiske B: The Effects of High-Risk Donors and Recipients on Scientific Registry of Transplant Recipients Program-Specific Outcomes [abstract]. Am J Transplant 15 (Suppl 3), 2015. Available at: http://www.atcmeetingabstracts.com/abstract/the-effects-of-high-risk-donors-and-recipients-on-scientific-registry-of-transplant-recipients-program-specific-outcomes/. Accessed May 12, 2015
- 38.Organ Procurement and Transplantation Network. Proposal to Implement Pre-Transplant Performance Review by the Membership and Professional Standards Committee. Available at: http://optn.transplant.hrsa.gov/media/1124/11_mpsc_pre_transplant_performance.pdf. Accessed November 15, 2014
- 39.Organ Procurement and Transplantation Network: Kidney Allocation System (KAS) “Out-of-the-gate” monitoring report, April 23, 2015. Available at: http://optn.transplant.hrsa.gov/media/1168/kas_report_04-2015.pdf. Accessed May 12, 2015
- 40.Halpern SD, Shaked A, Hasz RD, Caplan AL: Informing candidates for solid-organ transplantation about donor risk factors. N Engl J Med 358: 2832–2837, 2008 [DOI] [PubMed] [Google Scholar]