Abstract
Traditional histologic methods are limited in their ability to detect pathologic changes of CKD, of which cisplatin therapy is an important cause. In addition, poor reproducibility of available methods has limited analysis of the role of fibrosis in CKD. Highly labor-intensive serial sectioning studies have demonstrated that three-dimensional perspective can reveal useful morphologic information on cisplatin-induced CKD. By applying the new technique of multiphoton microscopy (MPM) with clearing to a new mouse model of cisplatin-induced CKD, we obtained detailed morphologic and collagen reconstructions of millimeter-thick renal sections that provided new insights into pathophysiology. Quantitative analysis revealed that a major long-term cisplatin effect is reduction in the number of cuboidal cells of the glomerular capsule, a change we term the "uncapped glomerulus lesion." Glomerulotubular disconnection was confirmed, but connection remnants between damaged tubules and atubular glomeruli were observed. Reductions in normal glomerular capsules corresponded to reductions in GFR. Mild increases in collagen were noted, but the fibrosis was not spatially correlated with atubular glomeruli. Glomerular volume and number remained unaltered with cisplatin exposure, but cortical tubulointerstitial mass decreased. In conclusion, new observations were made possible by using clearing MPM, demonstrating the utility of this technique for studies of renal disease. This technique should prove valuable for further characterizing the evolution of CKD with cisplatin therapy and of other conditions.
Keywords: chronic renal failure, cisplatin nephrotoxicity, histopathology, pathology, renal pathology, renal microscopy
The inability of traditional histologic methods to adequately visualize a range of important morphologic changes has hampered the study of the natural history, pathophysiology, and treatment of renal damage in CKD. Through labor-intensive and technically challenging detailed three-dimensional analysis, past studies have described the evolution in chronic renal failure of glomerular capsules that appear unconnected to proximal tubules, so-called atubular glomeruli.1–6 These and other investigations have also shown a characteristic and physiologically relevant organization to the overall nephron that is not possible to assess in single histology sections. Past studies have shown poor reproducibility of semi-quantitative fibrosis measures on single sections, partly because of heterogeneity.7–9 In addition, considerable investigation has addressed both the relevance and the challenges of measuring individual glomerular volumes in a variety of conditions.10–12 Unfortunately, these important observations have failed to bring about the routine use of millimeter-scale three-dimensional histologic assessment of renal specimens, largely because of the difficulties associated with processing using traditional histologic techniques.
Multiphoton microscopy (MPM) has improved our ability to detect these morphologic changes and has proven useful for renal assessment.13,14 However, a significant limitation of this and other optical sectioning methods remains the relatively shallow accessible depth. Formalin fixation increases light scatter and further reduces the usable depth for high-resolution analysis to <100 μm, even with low scattering excitation wavelengths of multiphoton lasers. Considering that mouse glomerular diameters are on the order of 50 μm, limited advantages are gained over traditional sectioning.
To overcome the depth limitations of sectioning microscopy, we have developed an approach to renal specimen processing that incorporates the use of clearing agents. Specifically, replacing water in tissue with a solution of benzyl alcohol/benzyl benzoate markedly reduces light scattering. With appropriate dyes, the preparation can extend the usable imaging depth of MPM by more than an order of magnitude, producing clear, high-resolution images at >1 mm of depth.15,16 In this article we describe the extension of this newly developed technique to the analysis of renal tissue in a mouse model of cisplatin-induced chronic renal failure.
Cisplatin is an effective chemotherapeutic agent whose nephrotoxicity restricts its utility. Administration of multiple cycles leads to permanent and irreversible loss of kidney function,17,18 but the cause of this progressive form of CKD is unknown. Prior studies suggest multifactorial mechanisms, including induction of apoptotic and cellular repair pathways, oxidative stress, and inflammatory responses.19,20 There is no known preventive therapy, and avoidance of additional doses limits the chances of successful treatment. Thus, there is considerable motivation to develop new tools for investigating cisplatin-induced CKD.
The potential effect of MPM with clearing for examination of renal failure with cisplatin use is highlighted by an in-depth morphologic analysis in rats presented by Marcussen.2 He showed that cisplatin-associated nephrotoxicity leads to several morphology changes that are difficult or impossible to visualize in single histologic sections. These include atrophic changes in the epithelium of the glomerular capsule, changes in glomerular volume, and the evolution of atubular glomeruli.
Development of CKD, defined as a fixed loss of glomerular filtration capacity that may be progressive, is also associated with loss of renal mass and the accumulation of fibrous tissue. A long-held theory proposes that progressive accumulation of fibrous tissue is directly causally linked to loss of nephron function,21 but the detailed mechanism remains elusive. Study of renal fibrosis has been helped by improved approaches for quantification using image analysis of histologic collagen stains, such as trichrome, anti-collagen III, and Sirius red.8,22 These stains have demonstrated improved precision and correlation with renal function measures compared with visual estimation, albeit to a limited degree.9
Further improvements in characterization of renal collagen fibrosis with the use of second harmonic generation (SHG) have more recently been proposed.23 Multiphoton excitation of collagen produces a nonfluorescent signal with high specificity. In this investigation, we have expanded the utility of SHG measurements by combining it with clearing, increasing significantly the volume that can be assessed for collagen fibrosis.
We have applied the combined MPM/SHG/clearing approach to the quantitative measurement of histomorphologic changes that develop in response to cisplatin in a newly developed chronic renal failure model. The goal was to better characterize the pathophysiologic lesion in cisplatin-induced chronic renal failure and to demonstrate the utility of MPM/clearing as a tool for morphologic investigation in renal disease.
Results
Murine Model of Cisplatin-Induced CKD
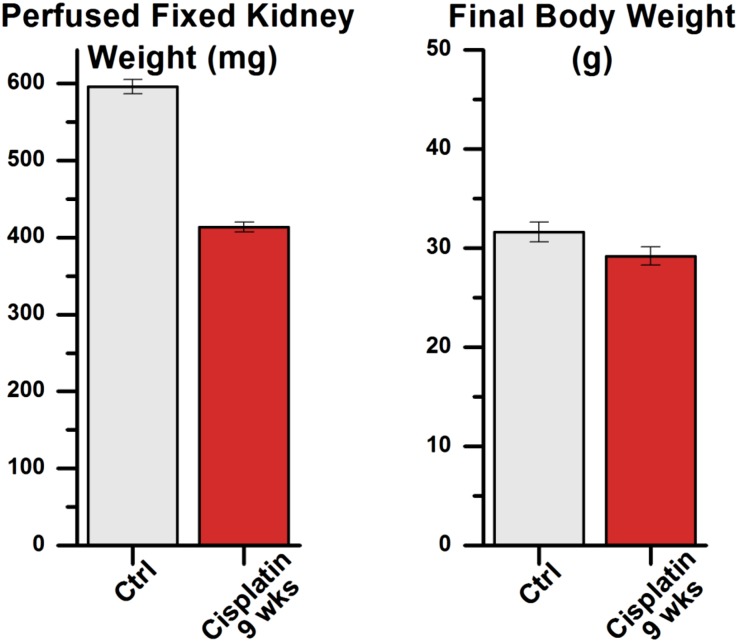
Two doses of cisplatin administered to mice 2 weeks apart resulted in physiologic changes characteristic of CKD. Reductions in GFR were noticeable after a single administration but progressed rapidly to a permanent decline after a second dose (Figure 1A), showing a persistent decline in GFR of approximately 50% at 9 weeks. Plasma creatinine was correspondingly elevated compared with controls, demonstrating a >3-fold increase at 9 weeks and progressing further at 16 weeks (Figure 1B). As was seen in other CKD studies in this strain of mice,24 the drop in GFR was not associated with a significant increase in albuminuria (data not shown). With the recognition that chronic renal failure was established at 9 weeks, this time-point was selected for detailed evaluation. At this 9-week time-point, treated mice showed decreases of 30% in formalin-perfused kidney weight relative to 9-week controls, while overall body weight was only minimally decreased (Figure 2).
Figure 1.
Cisplatin model shows physiologic evidence of chronic renal failure at 9 weeks. (A) Mice treated with two doses of cisplatin (n=4) showed significant (P<0.01) decreases in GFR starting at 4 weeks relative to controls (n=4). GFR remained low at 9 weeks after cisplatin (7 weeks after second dose). (B) Correspondingly, plasma creatinine remained markedly increased relative to controls (*P< 0.01) both at 9 weeks and up to 25 weeks after cisplatin administration, indicating chronic renal failure. Error bars, ±SEM.
Figure 2.
Cisplatin model shows gross evidence of specific renal damage at 9 weeks. Two-dose CP mice (n=4) had perfused kidney weights that were 413 mg, versus 596 mg for age-matched controls (n=4) (P<0.01), with only minimally decreased overall final body weight at week 9. Column values are mean±SEM.
The evolution of the pathologic lesion of chronic renal failure was confirmed using immunohistochemistry with Ki67 and Tunel. Specifically, there was evidence of significant cell turnover and increased apoptosis at 9 weeks after cisplatin administration (Figure 3). Endothelial rarefaction was also detectable; renal sections showed marked decreases in CD34 staining that persisted into the 25th week, characteristic of CKD (Figure 4A). In addition, an increase in myofibroblast proliferation was visible by smooth muscle actin stain at week 9, persisting into week 25, and macrophage activity increased transiently around week 9, normalizing by week 25, confirming a CKD pattern of injury (Figure 4, B and C).
Figure 3.
CP mouse kidneys showed immunohistochemical evidence of cell turnover at 9 weeks. There was a >3-fold increase in cellular proliferation rate by Ki67 immunohistochemistry (P<0.005) and a >10-fold increase in apoptosis by Tunel staining (P<0.05) in CP mouse kidneys (n=3) compared with controls (n=6). Box and whisker plot, #/hpf, number per high-power field.
Figure 4.
Immunohistochemistry showed long-term CKD-associated changes, including endothelial rarefaction, myofibroblast proliferation, and macrophage activity. (A) Endothelial cell immunostain (CD34) showed steady decreases in detectable endothelial cells over the course of 25 weeks. (B) Smooth muscle actin marker (SMA) showed evidence of myofibroblastic proliferation by week 9. (C) F4/80 stain (macrophages) showed transient activity of macrophages, corresponding to onset of myofibroblastic proliferation (Ctrl, control [n=6]; 9 wks, 9 weeks after cisplatin [n=3]; 25 wks, 25 weeks after cisplatin [n=6]). Percentages refer to grid areas of standard histology slide showing cells positive for given immunostain. Error bars, ±SEM.
Traditional histology of midcoronal kidney sections from cisplatin-treated (CP) mice with hematoxylin and eosin staining show the reduction in gross dimensions of the kidney after cisplatin exposure (Figure 5). There was apparent loss of interglomerular mass in CP kidneys, but there was no detectable glomerulosclerosis by histology. The normal appearance of the glomeruli at the light microscope level suggested that they may still be functioning; however, tubular connections could not be evaluated. Consistent differences in the morphology of tubules were also not readily discernible with traditional microscopy.
Figure 5.
Traditional histology shows few morphologic changes other than reduced overall kidney size in response to two doses of cisplatin. Low-power coronal hematoxylin and eosin–stained kidney sections of normal control (A) and CP kidneys (C) show relative reduction in the overall diameter of the post-cisplatin kidney without overt architectural changes. Corresponding high-power images for normal (B) and CP (D) kidneys show no systematic histologic changes in glomeruli or tubules that can be readily identified. Scale bar=50 μm.
Three-Dimensional Morphologic Assessment by Multiphoton Microscopy
Multiphoton optical slices with 1 µm axial resolution and imaged in cleared tissue at 3-µm-deep intervals enabled analysis of millimeter-thick three-dimensional (3D) blocks of renal tissue and was of sufficient quality to identify all the morphologic features seen on traditional histology (Figure 6). Note that the orientation of the tubular connection does not affect its detection by this technique because tubules oriented perpendicular to the plane of virtual sectioning are readily identified when visualized in sequence, as can be visualized in the z-stack column (Figure 6, right).
Figure 6.
Multiphoton microscopy with clearing yields excellent morphologic detail at depth. A 1.3-mm-thick kidney coronal section from the midportion of the kidney could be reconstructed completely using multiphoton microscopy with benzyl alcohol/benzyl benzoate clearing (left). High-resolution images could be obtained throughout the entire thickness of the sample with excellent axial resolution (right).
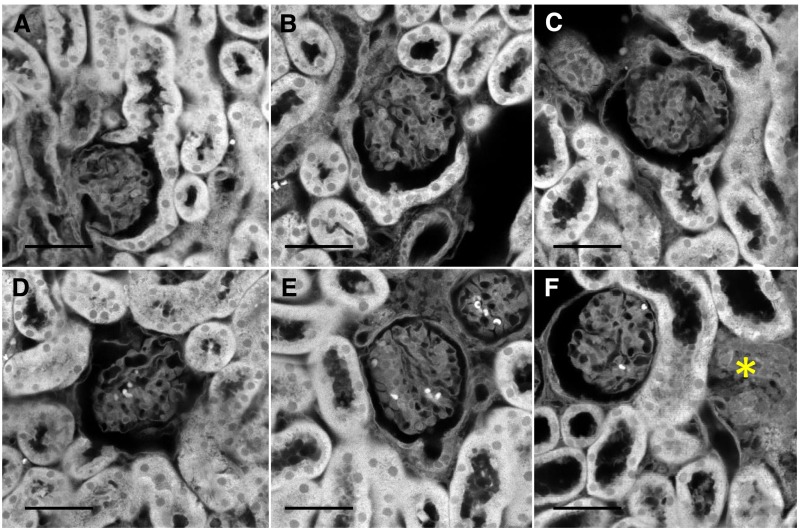
Systematic examination of tissue three-dimensional reconstructions revealed that a principal morphologic change associated with cisplatin exposure was loss of cuboidal cells that are contiguous with the flat parietal epithelium lining the Bowman capsule, a finding we describe as the "uncapped glomerular lesion." In control mice, approximately 50% (or more) of the surface of the capsule is covered by cells that are cuboidal in shape (Figure 7A); the remainder are flat parietal epithelial cells. In mice, the cuboidal cells appear to merge with the cells of the proximal tubule and have a similar appearance, but they have not been fully functionally characterized. In CP mice, many glomeruli showed loss or thinning of the cuboidal cells lining the capsule (Figure 7, B–D). In some cases, the cells of the proximal tubule at the junction with the Bowman capsule were decaying with irregular outlines and low protein staining or the proximal tubule was collapsed and could be traced to areas of cellularity without tubule definition (Figure 7, E and F). Cortical regions near most atubular glomeruli were devoid of tubules with only connective tissue, suggestive of complete tubular degeneration (Figure 8). These were most often found at the cortical surface but also sparsely in the remainder of the cortex, likely reflecting the usual nephron orientation with convolutions near the cortex. The degenerating tubules surrounding atubular glomeruli showed weak protein staining and often lacked identifiable lumens (see also Figure 13). Some damaged tubules were traceable back to an uncapped or atubular glomerulus (see below). In coronal kidney sections of CP mice, the degenerated areas made up only a small proportion (<1%) of the cortical area, explaining why they may be missed in traditional histologic sections.
Figure 7.
A range of abnormalities in cuboidal cells of the Bowman space were observed in kidneys from CP mice. (A) Cuboidal cells in a capsule from a control mouse most often show nearly complete surrounding of glomerulus. CP mice showed a range of changes, including (B) normal cuboidal cell component similar to that in the control mouse, (C) partial reduction in cuboidal cells with apparently intact proximal tubule, (D) complete loss of cuboidal cells with preserved proximal tubule morphology, (E) complete cuboidal cell loss with evidence of damage (based on reduced protein staining) in the proximal tubule cells, and (F) collapsed proximal tubule (*) that could be traced to an associated atubular but otherwise normal-appearing glomerulus. Scale bar=50 μm.
Figure 8.
Areas suggesting partial and complete tubular degeneration are present in CP mice and are often located near the cortical surface. Areas of architecturally disorganized connective tissue are demarcated by the enclosed regions. The cortical surface is visible near the top of these sections. The corresponding glomeruli/glomerular capsules associated with these two degenerated regions are both "atubular." A normal efferent arteriole is seen connecting to glomerulus on the left.
Figure 13.
High-resolution three-dimensional images reveal minimal tubular connections to damaged proximal tubules in “atubular” glomeruli. (A) An “atubular” glomerulus does not show overt abnormalities in a single section and requires serial sections to show lack of cuboidal cells and lack of visible tubular connection. A nearby tubule shows evidence of degradation with reduced nuclear staining and apparently collapsed lumen. (B) An angled plane cut through a 3D reconstruction of same glomerulus allows visualization of miniscule connection of glomerulus to associated damaged proximal tubule.
In general, glomeruli/nephrons could be grouped into several categories: (1) those with cuboidal cells surrounding >50% of a given glomerulus (and normal associated proximal tubules) (i.e., normal), (2) those with partial loss of capsular cuboidal cells, (3) those with complete absence of cuboidal cells, and (4) those without capsule cells and with no detectable connection to the proximal tubule (atubular glomeruli). Generally, those with a high cuboidal cell percentage had normal proximal tubules extending to medulla, while atubular glomeruli often showed nearby damaged tubules.
Three-dimensional reconstructions of the proximal nephron units best illustrate the range of these identified abnormalities in CP mice (Figure 9). Virtual “in situ” dissections show that some glomeruli had complete cuboidal capsule cell loss but patent proximal tubule connections, extending to the medulla (Figure 9B). Other nephrons showed cuboidal capsular cell loss and patent proximal tubules but with tubules that terminated blindly (Figure 9C). The reconstructions shown in Figure 9 also demonstrate the capability to trace connections and examine the thick ascending limb, the macula densa contact, distal tubule, and connecting tubule second feedback loop contact point with the afferent arteriole.
Figure 9.
Partial virtual nephron reconstructions are possible with clearing and 3D microscopy and illustrate most clearly the defect patterns in response to cisplatin. (A) Normal nephron showing glomerulus (green) with patent arterioles (red) and nearly complete coverage by capsular cuboidal cells (gray). (B) CP mouse nephron with normal-appearing glomerulus and arterioles, absent capsular cuboidal cells, but patent proximal tubule extending to medulla. (C) CP mouse nephron with absent capsular cuboidal cells and patent proximal tubule connection but a tubule that ends blindly before reaching medulla. The reduced brightness of tubules in parts B and C reflects reduced protein staining, suggesting cellular damage.
Age-matched control mice also showed a few nephrons with reductions in cuboidal capsular cells and even rare atubular glomeruli, but manual/visual classification showed CP mice have a marked reduction in intact/normal nephrons relative to controls (53.1% versus 89.5%; P<0.0001) (Figure 10). Cisplatin exposure was associated with increases in capsules with absent cuboidal cells and more modest increases in nephrons with reduced cuboidal cells and atubular glomeruli.
Figure 10.
Abnormalities of the glomerular capsule were visibly more frequent in CP mice than in control mice. The proportion of normal/reduced versus absent/atubular glomeruli significantly differed between control and CP groups at P<0.001 (chi-squared). Note that the percentage of normal glomerular capsules approximates the GFR differences observed with physiologic measurements (Figure 1). n=500 glomeruli/group; groups are four control mice at 9 weeks (Control) and four CP mice at 9 weeks (Cis Tx).
Quantitative 3D Morphologic Changes in CP Mice
To more accurately characterize the cuboidal cell loss in CP mice, we measured the volume of cuboidal cells covering glomerular capsules (Figure 11). Even after exclusion of atubular glomeruli, the data show a striking reduction in the volume of cuboidal cells lining the Bowman capsule in CKD (mean volume, 85,000 cells/μm3 versus 54,500 cells/μm3; P<1e-20). These changes prompted evaluation of the expression of the endocytic receptor megalin, which in mice is normally expressed in proximal tubule cells as well as capsular cuboidal cells; in this strain, parietal epithelial cells are expected to be megalin negative. Traditional section histology showed a significant decrease in megalin-expressing glomeruli in response to cisplatin exposure with 90 of 393 (22.9%) random glomerular sections lacking megalin-positive capsule cells versus 19 of 325 (5.8%) controls (P<0.01) (Figure 12).
Figure 11.
Cuboidal cells of the Bowman space are lost in CP mouse kidneys. An overlapping distribution of total capsular cell volume is present in normal and CP mice, but nearly all CP mouse capsular cell volumes are less than the mean in normal mice. Mean cuboidal cell volume is significantly lower in CP mice (P<0.001). n=200 glomeruli/group from 4 mice/group.
Figure 12.
As anticipated by 3D imaging data, cisplatin causes reduction in megalin expression in the glomerular capsule. Traditional sections with immunohistochemistry formegalin showed that glomerular capsules lacked megalin staining in 22.9% (90 of 393) of glomerular sections versus only 5.8% (19 of 325) of glomeruli in control sections (P<0.01).
As previously noted, the glomerular capsules that did not have a visually detectable connection to proximal tubules by 3D analysis were characterized as atubular. These capsules invariably lacked cuboidal cells. Interestingly, high resolution z-stacks revealed that nearly all glomerular capsules characterized as "atubular" contained minimal tubular connection remnants that could be traced to damaged or partly damaged proximal tubules (Figure 13), a finding that may be helpful in elucidating the pathophysiologic process.
More detailed analysis of glomerular features revealed that direct volume measurements of glomeruli (excluding atubular glomeruli) showed no statistically significant difference between normal controls and CP mice (109×103 glomeruli/µm3 versus 112×103 glomeruli/µm3; P=0.40) (Figure 14A). However, in CP mice, "atubular" glomeruli were significantly smaller than other glomeruli (66×103 glomeruli/µm3 versus 112×103 cells/µm3; P<0.001) (Figure 15), possibly as a result of the increased capsular space pressure from blocked urinary flow at some point in their evolution. Notably, calculated glomerular volume derived from maximum diameter resulted in results nearly identical to that from complete 3D measurements (data not shown).
Figure 14.
Glomerular volume and size are unaffected by cisplatin renal damage. (A) The distribution of non-atubular glomerular volumes was not significantly different between control and cisplatin-treated mice (histogram, n=200/group, 4 mice/group; P=0.40). Solid lines show Gaussian fit of data sets. (B) Similarly, the maximal glomerular diameter of non-atubular glomeruli remained relatively unchanged with cisplatin treatment (box and whisker plot, n=400/group, 4 mice/group; P=0.06).
Figure 15.
Glomeruli associated with an absent or nearly absent proximal tubule connection in cisplatin therapy are significantly smaller than non-atubular glomeruli. Nearly half of the atubular glomeruli (below horizontal line of box on right in this box and whisker plot) are smaller than the smallest non-atubular glomerulus (bottom whisker on left). CP glomeruli, n=150; atubular CP glomeruli, n=30.
Quantitative measures of glomerular number showed significantly increased density of glomeruli in the renal cortex in CP mice (210 versus 145 glomeruli/mm3; P<0.001 (Figure 16A), providing indirect evidence of loss of nonglomerular cortical components. This finding was further supported by a manual counting estimation of total glomeruli per kidney on two mice that did not show any reduction in the total number of glomeruli in response to cisplatin (control mouse, 22,240 glomeruli/kidney; CP mouse, 23,820 glomeruli/kidney). Furthermore, coronal section area analysis showed more directly that there was a significant reduction in cortical tubulointerstitial cross-sectional area in CP mice (8.55±0.2 versus 6.88±0.5×106 μm2/section; P<0.001) (Figure 16B). In this same analysis there was no significant change in glomerular area (controls versus CP mice, 249±25 versus 262±34×103 μm2/section; P=0.12), corresponding to the direct volume and diameter measurements.
Figure 16.
Glomeruli are preserved in CP mice. (A) Box and whisker plot shows that the density of glomeruli per unit volume of kidney cortex was significantly increased in CP mice (n=3) versus control mice (n=3) (P<0.001). (B) The average cortical non–glomerular cell cross-sectional area in midcoronal optical sections was significantly decreased in CP mice (n=28/group from 4 mice/group; P<0.001). (C) In contrast, the glomerular cross-sectional area in same sections was not significantly different between groups (P=0.12). Error bars in (B) and (C), ±SEM.
Use of large-scale 3D reconstructions of second harmonic generation signal yielded additional information about the cisplatin-associated chronic kidney lesion. Traditional Sirius red staining did not demonstrate any appreciable differences in degree or distribution of collagen between control and CP mice (Figure 17). Similarly, two-dimensional images of collagen distribution by SHG demonstrated some possible increases in peritubular collagen but did not clearly show marked differences between groups (Figure 18). However, visual inspection of large-scale 3D reconstructions of SHG suggests mild to moderate increases in the amount of collagen in CP mice, manifesting mainly as thickened portions of the normally distributed collagen, which is primarily perivascular (Figure 19). Analysis of the stacks revealed an interslice variation approaching 2-fold in the collagen component of complete midcoronal kidney sections (e.g., 1.4%–2.5%), suggesting that single complete cross-section collagen analysis would not be able to reliably detect differences less than two times normal. Notably, no spatial correlation was found between areas of increased collagen fibrosis and defective nephrons, including no association of collagen with atubular glomeruli (Figures 18 and 20). Also of interest, this analysis demonstrates that atubular glomeruli were found generally scattered throughout the cortex but with occasional clusters (Figure 20).
Figure 17.
Sirius red staining did not reveal overt differences in the collagen fibrosis in response to cisplatin. (A) Low-power polarization image of control kidney section highlighting collagen staining with Sirius red (original magnification, ×4; scale bar=1 cm). (B) High-power image of part A showing nearly absent interstitial collagen (original magnification, ×50; scale bar=100 µm). (C) Low-power polarization image of 9-week cisplatin kidney with similar degree of Sirius red staining as control (original magnification, ×4). (D) Corresponding high-power image of part C without visible increase in interstitial collagen compared with control (original magnification, ×50).
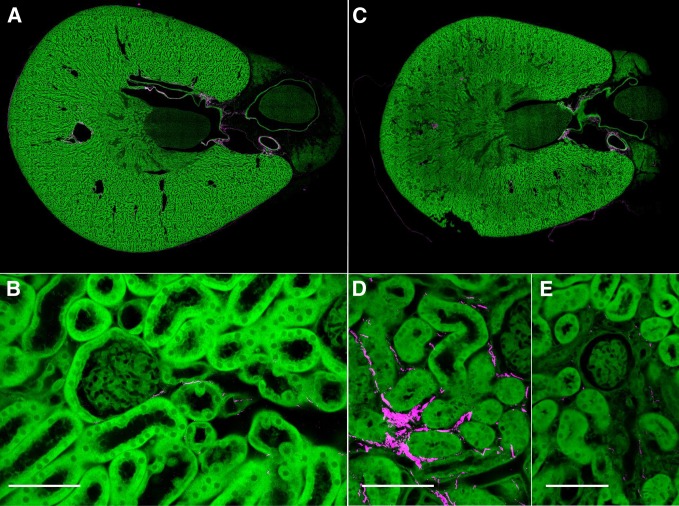
Figure 18.
Second harmonic generation sections showed similar patterns to Sirius red with possible mild increases in interstitial collagen in CP mice. (A and B) Control mouse MPM/SHG of kidney showing minimal collagen (magenta), almost exclusively in perivascular orientation. (C) Low-power view CP-treated kidney does not show marked differences compared with control. (D) High power view shows some interstitial peritubular collagen (magenta), but the collagen was not associated with abnormal glomeruli or "atubular" glomeruli as in part E. Scale bar=50 μm.
Figure 19.
Large-scale 3D reconstructions allow visualization of collagen increase with cisplatin. (A) Control mouse kidney. The collagen fibrosis appears to be primarily perivascular. (B) There is apparent mild increase in overall collagen in CP mouse kidney, still oriented perivascularly. Scale bar=500 μm.
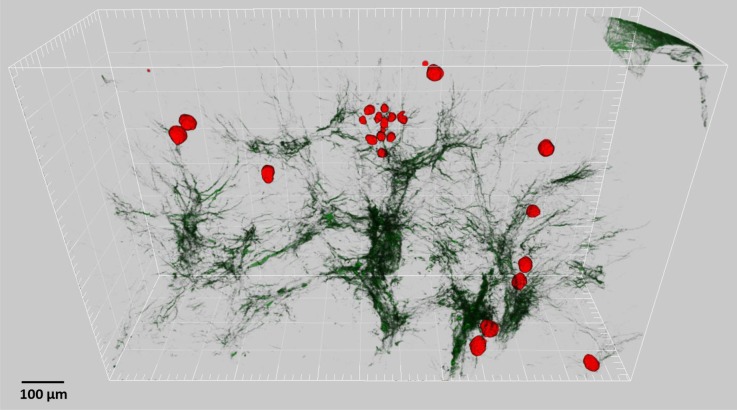
Figure 20.
The collagen fibrosis in CP kidneys is not co-localized with atubular glomeruli. Atubular glomeruli from this CP mouse kidney are pseudocolored in red. Second harmonic collagen signal is dark green. The atubular glomeruli are distributed throughout the kidney section and span cortex to medulla. A small cluster of small atubular glomeruli is evident in center.
Discussion
Physiologic and immunohistochemical measurements show that a newly developed two-dose cisplatin administration model in mice faithfully reproduces the CKD pattern seen in humans. Fixed renal insufficiency is present in treated animals and is accompanied by evolution toward a shrunken, damaged kidney, characterized by increases in activation of fibroblasts and endothelial rarefaction. The model meets criteria of chronic tubulointerstitial disease, mimicking the syndrome seen in humans after repeated exposure to cisplatin.
Despite the marked gross changes and secondary immunohistochemical evidence of repair, traditional hematoxylin and eosin and collagen stains did not elucidate the pathologic lesion. By combining the use of multiphoton microscopy with clearing, we expanded the morphologic analysis and adequately characterized changes that are directly associated with the evolution of CKD but not readily apparent on single histologic sections.
More than 1000 glomeruli and their associated proximal tubules were systematically analyzed in 3D detail for each kidney. Glomeruli remained largely intact, with some clusters of small atubular glomeruli detected. Degenerating tubules were found associated with atubular glomeruli, and tubulointerstitial mass loss appears predominantly responsible for the renal mass loss. A major finding was loss of cuboidal cells in the glomerular capsule in CKD. Additionally, large-scale 3D SHG imaging demonstrated moderate increases in overall collagen of the CP mice and significant heterogeneity. Examining a large portion of renal tissue sample at high resolution with multiphoton microscopy in cleared samples improved our ability to evaluate and investigate renal histology and fibrosis.
This study’s findings complement previously reported observations in a rat model of cisplatin-induced renal failure in which meticulous, labor intensive, and time-consuming serial sectioning was applied to identify atubular glomeruli.2,4 More recently, Forbes et al. applied similar techniques to find proximal tubule injury and atubular glomeruli in a chronic obstruction model.25 Our findings demonstrate conclusively that atubular glomeruli develop in cisplatin-induced chronic renal failure. However, our analysis suggests additional interpretations pertaining to cisplatin-induced CKD, including: (1) on the basis of the small proportion of atubular glomeruli, the decreased GFR is not solely dependent on their evolution, (2) loss of cuboidal cells lining the Bowman space is associated with decreased renal function, (3) there is little histologic evidence of glomerular damage or loss other than reductions in size of atubular glomeruli, (4) the reduction in kidney mass is primarily cortical tubulointerstitium, (5) "atubular" glomeruli are associated with damaged proximal tubules, and (6) collagen fibrosis does not play an obvious role in the evolution of atubular glomeruli.
It is also notable that the percentage of normal-appearing glomerular capsules in control and CP mice corresponded almost exactly to the relative GFR. It remains undetermined whether capsular cuboidal cells represent modified parietal cells or extensions of the proximal tubule cells they simulate, but the morphology and megalin staining point to the latter. The physical connection between uncapped glomeruli and damaged tubules, associations of atubular glomeruli with degenerating tubules, and area analysis showing preferential loss of nonglomerular tubulointerstitium suggest that the “uncapped glomerular lesion” may be a sign of nonfunctional or poorly functioning nephron units. Correlative studies are needed to better characterize the pattern of progression of the morphologic findings during CKD evolution for a better understanding of the pathophysiology. Given that prior studies have shown morphologic similarities between cisplatin-induced injury and obstructive CKD, the inferences drawn from the current study may be more broadly applicable.
In addition, the potential for other informative findings from the clearing/MPM data are apparent from the partial virtual nephron dissections. It is possible to systematically identify and trace the entire nephron. More detailed examination of complete individual nephrons at different points after exposure to toxins could help characterize the natural history of morphologic changes and pinpoint key anatomic locations.
Concise Methods
Detailed methods are provided in the Supplemental Data.
Murine Model of Cisplatin-Induced CKD
Ten-week-old C57BL/6 mice (Harlan-Sprague-Dawley) were administered two doses of cisplatin (Aldrich 479306; 15 mg/kg in sterile saline delivered intraperitoneally) 2 weeks apart under brief isoflurane anesthesia. In an initial study, kidneys were harvested at 9 and 25 weeks. In a subsequent study, GFR was measured by FITC-inulin clearance up to 9 weeks after the first injection of cisplatin. Kidneys were perfusion fixed with 4% formaldehyde at euthanasia to preserve dilation of fluid-filled spaces and facilitate multiphoton microscope analysis; perfusion pressure was not normalized. Kidneys were divided midcoronally and sections were split for paraffin embedding with routine tissue histologic analysis and MPM.
Immunohistochemistry/Immunofluorescence
Immunohistochemical and immunofluorescent staining and scoring were performed for Ki-67, Tunel, α-smooth muscle actin, F4/80, and CD34 as previously described, with blinded scoring performed by an expert renal pathologist using a square grid technique.26–28
Tissue Clearing and Multiphoton Microscopy
Formalin-fixed mouse kidneys were cut midcoronally into roughly 1-mm tissue sections with a razor blade. The sections were transferred into a methanol solution containing 0.5% volume eosin (final concentration approximately 1 mM), then stored overnight heated at 42°C. Subsequently the samples were transferred to a 100% solution of benzyl alcohol/benzyl benzoate in 1:2 ratio for approximately 2 hours before being imaged on a custom-built multiphoton microscope based on a Ti-sapphire laser.
Image Processing and Analysis
Collected image stacks were processed and visualized using macros programmed on the FIJI implementation of ImageJ,29 including the Grid/Collection stitching plugin.30 Downsampling to 25% of original resolution was performed for the low-power visualization of the largest stacks as well as for SHG analysis. The final SHG 3D reconstructions were created using Imaris (Bitplane AG, Zurich, Switzerland).
Glomeruli and tubular connections in an image stack representing a fixed volume of kidney were individually identified and tagged using Fiji. Glomerular tuft diameters were determined by identifying the largest cross-sectional profile in the image stack and recording the average of the maximum and minimum diameters. Glomerular volume distribution was determined by manual tracing of glomerular outline through all sections, extraction of area, multiplication by z-step size, and addition of volumes. Single glomerulus/tubule 3D reconstructions were achieved by tracing corresponding sections in consecutive stack slices and composing the tracings into volumes using ImageJ’s built-in 3D volume plugin.
Quantitative Calculations and Statistical Analyses
Glomerular density was determined by manually counting the number of glomeruli in measured volumes. Coronal slices were divided into 60-micron-thick sections and 1.2-mm-thick slices from center coronal sections were evaluated from CP mice at 9 weeks after initial dose compared with control mice of same age. Statistical comparisons for physiologic tests and distributions of glomerular volume, cuboidal cell volume, and tubulointerstitial area were based on two-tailed t test. Categorical tests of capsular integrity and immunohistochemical square grid data were compared using Pearson chi-squared test. Total glomeruli per kidney were estimated by multiplying the total number of glomeruli in the coronal slices by the fractional weight of the coronal slice relative the total kidney weight with approximately 10% of each kidney imaged and analyzed. Fractional surface area measurements were performed by manually isolating cortical section, extraction of glomeruli, and using a pixel threshold that excluded tubular and vascular spaces. Thus, tubule-interstitial area corresponds to cellular mass only, reducing impact of perfusion pressure variations.
Disclosures
R.T. and M.J.L. have an ownership interest in Applikate Technologies LLC, a company that develops technology for 3D histology.
Supplementary Material
Acknowledgments
The study was supported by the George M O'Brien Kidney Center at Yale, National Institutes of Health grant P30-DK079310 and Yale Mouse Phenotyping Core grant U24-DK059635. R.T. is supported with startup funds from Department of Laboratory Medicine of Yale School of Medicine. G.V.D. acknowledges grant support from National Cancer Institute 1R41CA189537-01 of the National Institutes of Health.
Reprints will not be available from the authors.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015010079/-/DCSupplemental.
References
- 1.Chevalier RL, Forbes MS: Generation and evolution of atubular glomeruli in the progression of renal disorders. J Am Soc Nephrol 19: 197–206, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Marcussen N: Atubular glomeruli in cisplatin-induced chronic interstitial nephropathy. An experimental stereological investigation. APMIS 98: 1087–1097, 1990 [DOI] [PubMed] [Google Scholar]
- 3.Marcussen N: Atubular glomeruli and the structural basis for chronic renal failure. Lab Invest 66: 265–284, 1992 [PubMed] [Google Scholar]
- 4.Marcussen N, Jacobsen NO: The progression of cisplatin-induced tubulointerstitial nephropathy in rats. APMIS 100: 256–268, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Thornhill BA, Forbes MS, Marcinko ES, Chevalier RL: Glomerulotubular disconnection in neonatal mice after relief of partial ureteral obstruction. Kidney Int 72: 1103–1112, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Najafian B, Kim Y, Crosson JT, Mauer M: Atubular glomeruli and glomerulotubular junction abnormalities in diabetic nephropathy. J Am Soc Nephrol 14: 908–917, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Masseroli M, O’Valle F, Andújar M, Ramírez C, Gómez-Morales M, de Dios Luna J, Aguilar M, Aguilar D, Rodríguez-Puyol M, Del Moral RG: Design and validation of a new image analysis method for automatic quantification of interstitial fibrosis and glomerular morphometry. Lab Invest 78: 511–522, 1998 [PubMed] [Google Scholar]
- 8.Grimm PC, Nickerson P, Gough J, McKenna R, Stern E, Jeffery J, Rush DN: Computerized image analysis of Sirius Red-stained renal allograft biopsies as a surrogate marker to predict long-term allograft function. J Am Soc Nephrol 14: 1662–1668, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Farris AB, Adams CD, Brousaides N, Della Pelle PA, Collins AB, Moradi E, Smith RN, Grimm PC, Colvin RB: Morphometric and visual evaluation of fibrosis in renal biopsies. J Am Soc Nephrol 22: 176–186, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olson JL, Heptinstall RH: Nonimmunologic mechanisms of glomerular injury. Lab Invest 59: 564–578, 1988 [PubMed] [Google Scholar]
- 11.Lane PH, Steffes MW, Mauer SM: Estimation of glomerular volume: A comparison of four methods. Kidney Int 41: 1085–1089, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Hoy WE, Douglas-Denton RN, Hughson MD, Cass A, Johnson K, Bertram JF: A stereological study of glomerular number and volume: Preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int Suppl 63: S31–S37, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Peti-Peterdi J, Burford JL, Hackl MJ: The first decade of using multiphoton microscopy for high-power kidney imaging. Am J Physiol Renal Physiol 302: F227–233, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sipos A, Toma I, Kang JJ, Rosivall L, Peti-Peterdi J: Advances in renal (patho)physiology using multiphoton microscopy. Kidney Int 72: 1188–1191, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres R, Vesuna S, Levene MJ: High-resolution, 2- and 3-dimensional imaging of uncut, unembedded tissue biopsy samples. Arch Pathol Lab Med 138: 395–402, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Vesuna S, Torres R, Levene MJ: Multiphoton fluorescence, second harmonic generation, and fluorescence lifetime imaging of whole cleared mouse organs. J Biomed Opt 16: 106009–, 106009–106006., 2011 [DOI] [PubMed] [Google Scholar]
- 17.Kollmannsberger C, Kuzcyk M, Mayer F, Hartmann JT, Kanz L, Bokemeyer C: Late toxicity following curative treatment of testicular cancer. Semin Surg Oncol 17: 275–281, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Fosså SD, Aass N, Winderen M, Börmer OP, Olsen DR: Long-term renal function after treatment for malignant germ-cell tumours. Ann Oncol 13: 222–228, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Arany I, Safirstein RL: Cisplatin nephrotoxicity. Semin Nephrol 23: 460–464, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Pabla N, Dong Z: Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int 73: 994–1007, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Zeisberg M, Neilson EG: Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol 21: 1819–1834, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Servais A, Meas-Yedid V, Buchler M, Morelon E, Olivo-Marin J-C, Lebranchu Y, Legendre C, Thervet E: Quantification of interstitial fibrosis by image analysis on routine renal biopsy in patients receiving cyclosporine. Transplantation 84: 1595–1601, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Strupler M, Hernest M, Fligny C, Martin J-L, Tharaux P-L, Schanne-Klein M-C: Second harmonic microscopy to quantify renal interstitial fibrosis and arterial remodeling. J Biomed Opt 13: 054041, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Leelahavanichkul A, Yan Q, Hu X, Eisner C, Huang Y, Chen R, Mizel D, Zhou H, Wright EC, Kopp JB, Schnermann J, Yuen PS, Star RA: Angiotensin II overcomes strain-dependent resistance of rapid CKD progression in a new remnant kidney mouse model. Kidney Int 78: 1136–1153, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forbes MS, Thornhill BA, Chevalier RL: Proximal tubular injury and rapid formation of atubular glomeruli in mice with unilateral ureteral obstruction: A new look at an old model. Am J Physiol Renal Physiol 301: F110–F117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mason S, Hader C, Marlier A, Moeckel G, Cantley LG: Met activation is required for early cytoprotection after ischemic kidney injury. J Am Soc Nephrol 25: 329–337, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soda K, Balkin DM, Ferguson SM, Paradise S, Milosevic I, Giovedi S, Volpicelli-Daley L, Tian X, Wu Y, Ma H, Son SH, Zheng R, Moeckel G, Cremona O, Holzman LB, De Camilli P, Ishibe S: Role of dynamin, synaptojanin, and endophilin in podocyte foot processes. J Clin Invest 122: 4401–4411, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huen SC, Moeckel GW, Cantley LG: Macrophage-specific deletion of transforming growth factor-β1 does not prevent renal fibrosis after severe ischemia-reperfusion or obstructive injury. Am J Physiol Renal Physiol 305: F477–F484, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A: Fiji: An open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Preibisch S, Saalfeld S, Tomancak P: Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 25: 1463–1465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.