Abstract
The plasticity of dendritic cells (DCs) permits phenotypic modulation ex vivo by gene expression or pharmacologic agents, and these modified DCs can exert therapeutic immunosuppressive effects in vivo through direct interactions with T cells, either inducing T regulatory cells (TREGs) or causing anergy. Sphingosine 1-phosphate (S1P) is a sphingolipid and the natural ligand for five G protein–coupled receptors (S1P1, S1P2, S1P3, S1P4, and S1P5), and S1PR agonists reduce kidney ischemia-reperfusion injury (IRI) in mice. S1pr3−/− mice are protected from kidney IRI, because DCs do not mature. We tested the therapeutic advantage of S1pr3−/− bone marrow–derived dendritic cell (BMDC) transfers in kidney IRI. IRI produced a rise in plasma creatinine (PCr) levels in mice receiving no cells (NCs) and mice pretreated with wild-type (WT) BMDCs. However, S1pr3−/− BMDC–pretreated mice were protected from kidney IRI. S1pr3−/− BMDC–pretreated mice had significantly higher numbers of splenic TREGs compared with NC and WT BMDC–pretreated mice. S1pr3−/− BMDCs did not attenuate IRI in splenectomized, Rag-1−/−, or CD11c+ DC–depleted mice. Additionally, S1pr3−/− BMDC–dependent protection required CD169+ marginal zone macrophages and the macrophage–derived chemokine CCL22 to increase splenic CD4+Foxp3+ TREGs. Pretreatment with S1pr3−/− BMDCs also induced TREG-dependent protection against IRI in an allogeneic mouse model. In summary, adoptively transferred S1pr3−/− BMDCs prevent kidney IRI through interactions within the spleen and expansion of splenic CD4+Foxp3+ TREGs. We conclude that genetically induced deficiency of S1pr3 in allogenic BMDCs could serve as a therapeutic approach to prevent IRI-induced AKI.
Keywords: acute renal failure, ischemia-reperfusion, immunosuppression, immunology
The pathogenesis of kidney ischemia-reperfusion injury (IRI) involves a complex interaction between altered microcirculatory hemodynamics, endothelial and epithelial cells, and infiltration of immune cells.1,2 Dendritic cells (DCs), the major subset of leukocytes in the kidney,3–5 contribute to innate and adaptive immunity of kidney IRI6 through activation of immune cells.7–9 Considerable data suggest that the immune system mediates AKI, and anti-inflammatory treatments significantly attenuate tissue injury and loss of function. However, the side effects of common anti–inflammatory therapies combined with the lack of clinical data, supporting the involvement of the immune system in AKI pathogenesis, have hindered the development of anti-inflammatory options. Therefore, dialysis remains the only treatment option available to patients with AKI.
Data from our laboratory10,11 and the laboratories of others12,13 show that modulation of sphingosine 1-phosphate receptors (S1PRs) significantly influences AKI development and progression in animal models. These receptors belong to a family of five G protein–coupled receptors (sphingosine 1-phosphate 1 [S1P1], S1P2, S1P3, S1P4, and S1P5) and modulate diverse physiologic responses, including cellular growth and proliferation, angiogenesis, apoptosis, and lymphocyte trafficking.14–17 The stimulation of S1PRs can have heterogeneous physiologic effects depending on the tissue, presence of receptor subtypes, and disease state. For example, activation of S1P3 protects hearts from IRI,18,19 but S1P3-deficient mice are protected from kidney IRI.10,20
Use of pharmacologic agents has limitations because of off–target and other associated adverse side effects. Cell–based therapeutic approaches have advantages; transferred cells are capable of sensing diverse signals, moving to specific sites in the body, making decisions, and executing complex responses. DCs are heterogeneous, professional antigen–presenting immune cells, and they are distributed throughout the lymphoid and nonlymphoid tissues.21 Previously, we found that adoptive transfer of bone marrow–derived dendritic cells (BMDCs)22 or T regulatory cells (TREGs) pretreated with an A2A receptor agonist23 significantly reduced renal IRI. In regards to S1PR expression, human and mouse leukocytes express similar levels of S1PRs.24–26 S1pr3−/− mice are protected from renal IRI through a mechanism involving BMDCs and their immune modulatory function.10 However, the phenotype of S1pr3−/− BMDCs and their site of action in vivo (intrarenal versus extrarenal) remain unclear.
The aim of this study was to explore the potential mechanism of S1pr3−/− BMDC therapy in preventing ischemic AKI. We found that adoptive transfer of S1pr3−/− BMDCs protected recipient mouse kidneys from IRI through mechanisms requiring the spleen, recipient lymphocytes (TREGs), CD11c+ DCs, and CD169+ macrophages. This protective effect is likely caused by increased numbers of Foxp3+ TREGs through the CCL22–CCR4 axis. Additional clinical relevance is on the basis of the observation that immature S1pr3−/− BMDCs reduce injury in an allogeneic IRI model, suggesting that this cell-based therapy would be efficacious in transplantation.
Results
S1pr3−/− BMDCs Have an Immature Phenotype
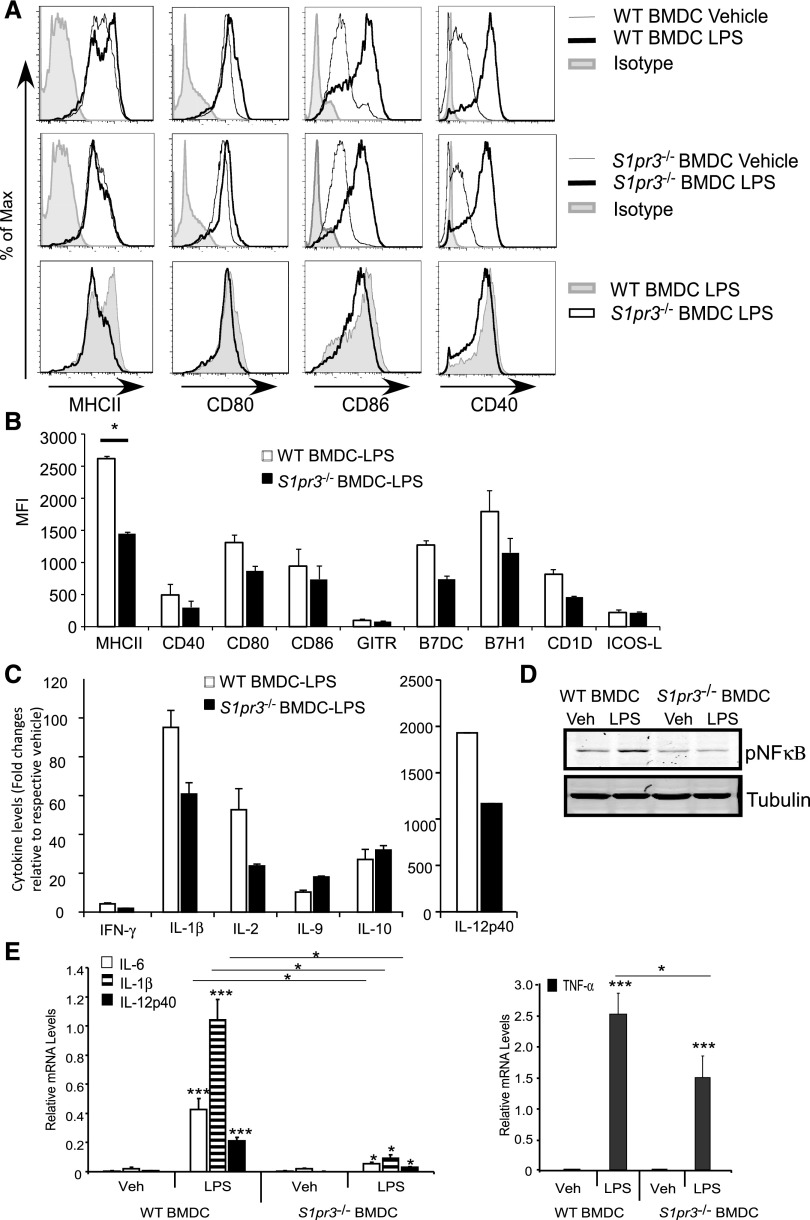
BMDCs were generated from wild-type (WT) or S1pr3−/− mice as described previously10 and treated with LPS for 24 hours. Compared with C57BL/6 BMDCs from WT mice, S1pr3−/− BMDCs had reduced surface expression of MHCII, CD40, CD80, B7H1, and CD1d and reduced protein expression of various cytokines after exposure to LPS (Figure 1, A–C). When stimulated with LPS, WT BMDCs (gated on CD11c) had a trend toward higher expression of CD8 and lower expression of CCR7 compared with S1pr3−/− BMDCs (data not shown). Phosphorylation of NFκB, a key step in leukocyte activation that drives the transcription of several proinflammatory cytokines, was also reduced in the S1pr3−/− BMDCs compared with the WT (Figure 1D), and this was accompanied by reduced mRNA expression of several proinflammatory cytokines: IL-6, IL-1β, IL-12p40, and TNF-α (Figure 1E).
Figure 1.
LPS–treated S1pr3−/− BMDCs produce fewer cytokines and costimulatory molecules compared with WT BMDCs. WT and S1pr3−/− BMDCs were treated with vehicle (PBS) or 100 ng/ml LPS for approximately 20 hours. (A) Flow cytometry histograms (overlays) illustrating percentage of maximum signal for MHCII, CD80, CD86, CD40, or control isotype IgG when gating on CD11c+ cells. (B) MFI calculated from FACS histograms. (C) Changes in protein expression levels of cytokines (mouse 30–plex Luminex) in supernatants collected from WT and S1pr3−/− BMDC cultures after incubation with PBS or LPS for 20 hours. (D) Western blot of phosphorylated NFκB (pNFκB) in PBS– (vehicle [Veh]) or LPS–treated WT or S1pr3−/− BMDCs. (E) Changes in mRNA expression of proinflammatory cytokines from WT and S1pr3−/− BMDCs treated with Veh (PBS) or 100 ng/ml LPS for 3 hours (values expressed relative to GAPDH). Values are mean±SEM (n=2–3 mice with three replicates for each experimental set). *P<0.05; ***P<0.001.
S1pr3−/− BMDCs Prevent Murine AKI in a Host Leukocyte–Dependent Manner
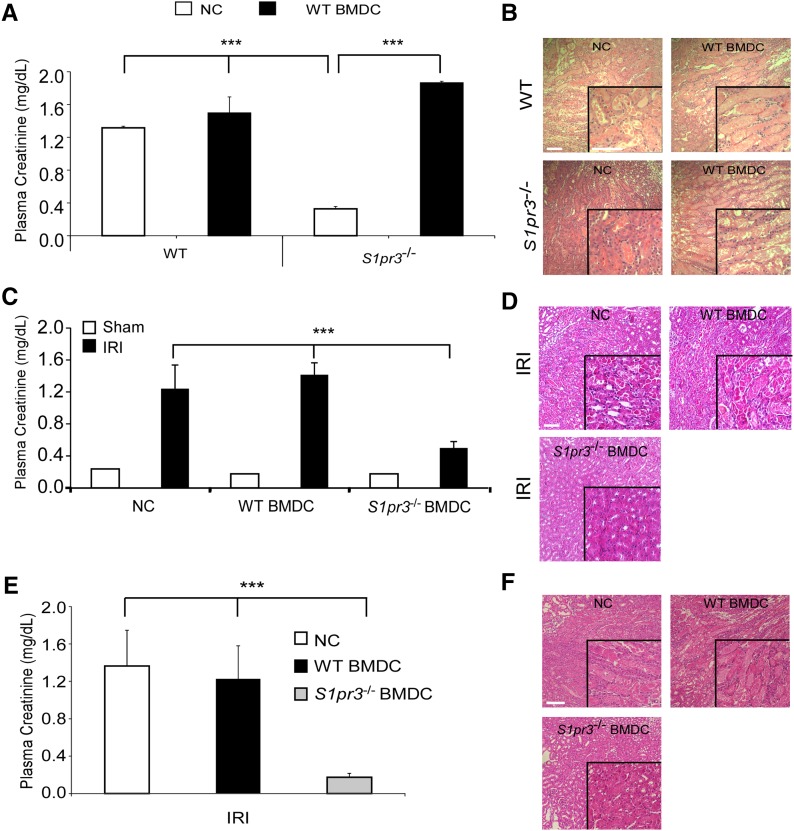
Next, we sought to determine the significance of BMDC S1pr3 on the development of AKI in vivo. BMDCs were isolated from WT or S1pr3−/− mice, activated with LPS, and injected into naïve WT or S1pr3−/− mice 24 hours before IRI. S1pr3−/− mice were protected from kidney IRI compared with WT mice10 as indicated by plasma creatinine (PCr) (Figure 2A) and acute tubular necrosis (ATN) (Figure 2B). However, administration of WT BMDCs abrogated this protection and resulted in elevated PCr and ATN 24 hours after IRI (Figure 2, A and B, Supplemental Table 1). Conversely, administration of S1pr3−/− BMDCs to WT mice 24 hours before IRI caused a significant reduction in AKI (PCr and ATN) after IRI (Figure 2, C and D, Table 1). This protective effect was dependent on viable cells, because UV irradiation of the S1pr3−/− BMDCs (resulting in 80% apoptotic BMDCs) prevented their protective effect (Supplemental Figure 1, A–C). The protection elicited by S1pr3−/− BMDCs was long lasting and observed with administration of S1pr3−/− BMDCs 7 days before IRI (Figure 2, E and F, Supplemental Table 2). However, administration of the S1pr3−/− BMDCs 1 or 4 hours after IRI did not significantly reduce PCr 24 hours after IRI (Supplemental Figure 1D).
Figure 2.
Transfer of WT BMDCs to S1pr3−/− mice induces kidney injury after IRI. (A) WT BMDCs treated with 100 ng/ml LPS for approximately 20 hours (WT BMDCs) or no cells (NCs; PBS) were injected (iv) 1 day before sham surgery or IRI into naïve WT and S1pr3−/− mice. Recipient mouse kidneys were exposed to 26 minutes of ischemia (or sham surgery in some cases) followed by 24 hours of reperfusion, and samples were collected 24 hours later (n=3–4). (B) H&E staining of kidney sections from the same mice; insets show a ×2.5 magnified image. (C) Naïve WT mice were injected (iv) with NCs, WT BMDCs, or S1pr3−/− BMDCs 1 day before kidney IRI (n=5–6 for IRI and n=2–3 for sham-operated mice). (D) H&E staining of kidney sections from the same mice. (E) WT naïve mice were injected (iv) with NCs, WT BMDCs, or S1pr3−/− BMDCs 7 days before kidney IRI (n=3). (F) H&E staining of kidney sections from the same mice. Values are mean±SEM. Scale bars, 100 μm. ***P<0.001. H&E, hematoxylin and eosin.
Table 1.
ATN scores of WT (B6) mice treated with no cells, WT BMDCs, or S1pr3−/− BMDCs
| NCs | WT BMDCs | S1pr3−/− BMDCs |
|---|---|---|
| 3.2±0.01 | 3.5±0.02 | 1.5±0.01a |
Timing for IRI and BMDC injection is the same as in Figure 2, C and D. WT or S1pr3−/− BMDCs stimulated with LPS were injected into naïve WT mice 24 hours before IRI. Tissues were harvested 24 hours after kidney IRI. Values are mean±SEM (n=3–4 in each group). NC, no cell.
P<0.05 compared with NC (treated with PBS) and WT BMDC groups (n=4–6).
We next determined whether endogenous DCs were required for the protection elicited by S1pr3−/− BMDCs. S1pr3−/− BMDC–treated control (CD11c-DTR−[WT]→WT) mice are protected from kidney IRI. However, S1pr3−/− BMDC–dependent protection is abrogated in diphtheria toxin–treated CD11c-DTR+→WT mice (Supplemental Figure 2, A and B, Supplemental Table 3). These data suggest that endogenous CD11c+ cells are required for the protection elicited by S1pr3−/− BMDCs possibly through direct interaction or effects of locally released cytokines. Given the role of DCs in stimulating an adaptive (lymphocyte) response, we next determined the contribution of lymphocytes in S1pr3−/− BMDC therapy. Rag-1−/− mice were treated with PBS, WT BMDCs, or S1pr3−/− BMDCs 1 day before kidney IRI. WT or S1pr3−/− BMDCs did not protect Rag-1−/− mice from kidney IRI (Supplemental Figure 2, C and D), suggesting that S1pr3−/− BMDC–mediated protection from IRI is through interactions that require recipient lymphocytes.
S1pr3−/− BMDC Therapy Is Mediated by the Spleen
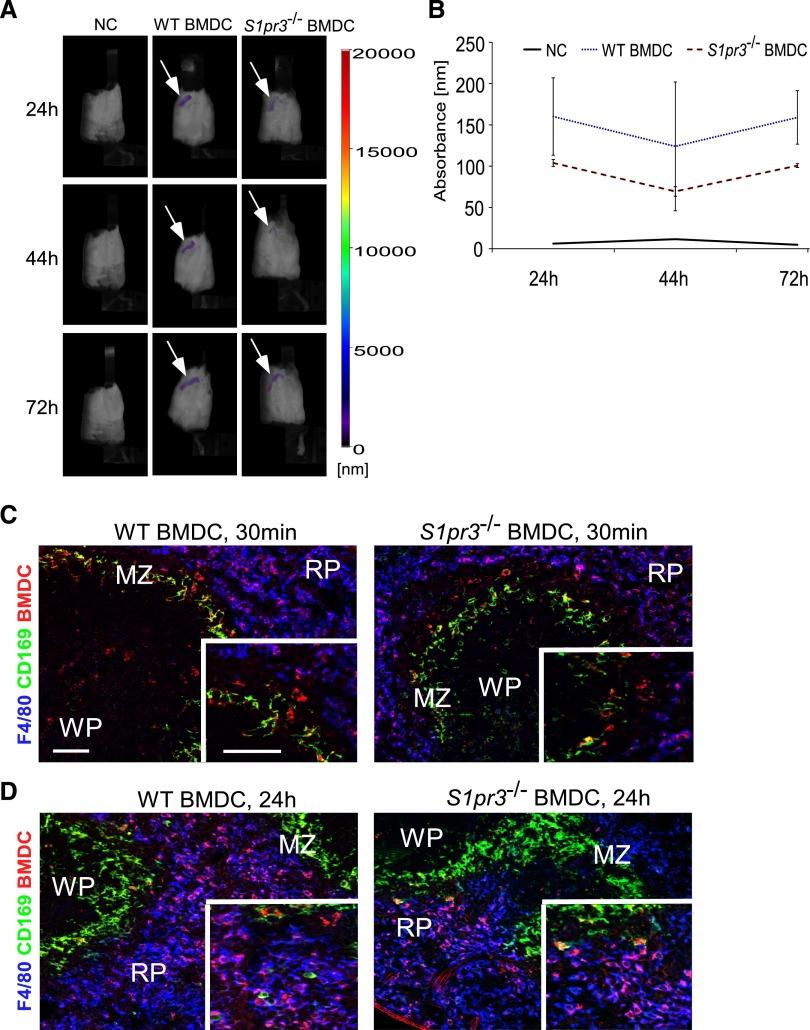
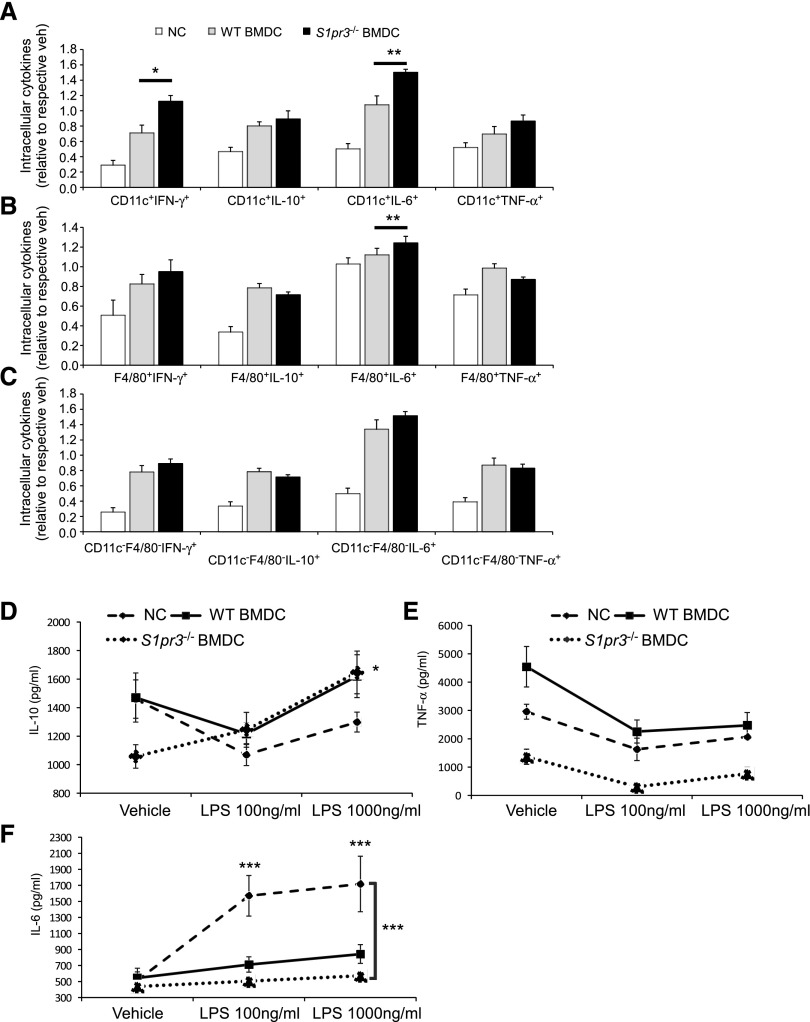
We next set out to determine whether BMDCs influence IRI through direct interactions with the kidneys or modulate the inflammatory response in an extrarenal manner. BMDCs were labeled with VivoTrack-680 (VT-680) for FMT or PKH26 for immunofluorescence to monitor their trafficking in vivo. We observed that activated WT or S1pr3−/− BMDCs primarily localize to the spleen as early as 30 minutes after injection and that this signal persisted up to 72 hours after injection (Figure 3). Immunofluorescence staining showed that labeled BMDCs interact with splenic marginal zone (MZ) macrophages as early as 30 minutes after injection and then migrate or are cleared by red pulp F4/80+ macrophages at 24 hours (Figure 3, C and D). No difference in trafficking was observed between the activated WT or S1pr3−/− BMDCs (Figure 3B). Furthermore, no difference was observed in total populations of splenic CD11c+, CD11c+CD103+, CD11c+CCR7+, CD11c+CD86+, CD11c+CD40+, CD11c+MHCII+, F4/80+, CD1dtetTCRβ, NK1.1+, or B220+ cells from mice treated with either WT or S1pr3−/− BMDCs. To determine if S1pr3−/− BMDCs administration influences splenocytes function, we isolated splenocytes 24 hours after injection and stimulated them with LPS. We measured key cytokines known to influence AKI.27 Splenocytes from mice treated with S1pr3−/− BMDCs had significantly higher intracellular IFN-γ and IL-6 in CD11c+ and F4/80+ cells compared with WT BMDC–treated mice (Figure 4, A–C). However, S1pr3−/− BMDC–treated mice had lower levels of secreted IL-6 and TNF-α and higher levels of IL-10 compared with splenocytes from control mice (Figure 4, D–F).
Figure 3.
Labeled WT or S1pr3−/− BMDCs are detected in recipient spleen after adoptive transfer. (A) C57BL/6 WT mice were injected with VT-680–labeled WT and S1pr3−/− BMDCs, and in vivo imaging was performed 24, 44, and 72 hours after injection. The majority of the injected BMDCs went to the recipient spleen (white arrows), with little to no measureable signal found in lungs, liver, kidneys, or lymph nodes. Compared with no cells (NCs; mice injected with PBS), no obvious differences in BMDC intensity were observed in mice with WT or S1pr3−/− BMDCs. Fluorescent intensity is indicated by the color-coded bar. (B) Graph shows quantified differences in absorbance between NCs, WT BMDCs, and S1pr3−/− BMDCs in mice at 24, 44, and 72 hours after injection (n=3). (C and D) Injected BMDCs are phagocytized by F4/80 red pulp [RP] macrophages at 24 hours. Mice were injected with PKH26GL-labeled BMDCs from WT or S1pr3−/− mice. Spleens were harvested (C) 30 minutes or (D) 24 hours after BMDC injection, and sections were labeled with CD169 (clone MOMA-1; MZ macrophages) and F4/80 (RP macrophages). Red dye–labeled BMDCs and possibly free dye are shown in all panels. WP, white pulp. Scale bar, 100 μm.
Figure 4.
Splenocytes from S1pr3−/− BMDCs treated mice produce less inflammatory cytokines. Characterization of splenocytes from mice injected with no cells (NCs), WT BMDCs, or S1pr3−/− BMDCs. Splenocytes were isolated 24 hours after BMDC injection; 1×105 splenocytes per well were treated with 100 or 1000 ng/ml LPS for an additional 24 hours. (A–C) Splenocytes were restimulated with PMA (10 ng/ml), ionomycin (2 μg/ml), and brefeldin A (5 μg/ml) for an additional 5 hours; intracellular cytokines (IFN-γ, IL-10, IL-6, and TNF-α) were measured by flow cytometry in (A) CD11c+ cells, (B) F4/80+ cells, and (C) CD11c−F4/80− (double negative) cells. Values are mean±SEM (n=3). *P<0.05; **P<0.01. (D–F) Secreted levels of cytokines were measured by ELISA at 24 hours after vehicle or LPS (100 or 1000 ng/ml) in supernatants. (D) IL-10 (values are mean±SEM; n=3). *P<0.05, S1pr3−/− BMDC (vehicle compared with 1000 ng/ml LPS). (E) TNF-α and (F) IL-6 (values are mean±SEM; n=3). ***P<0.001, NCs (vehicle compared with both LPS doses); S1pr3−/− BMDC–treated splenocytes with LPS compared with NC LPS–treated splenocytes at both LPS doses.
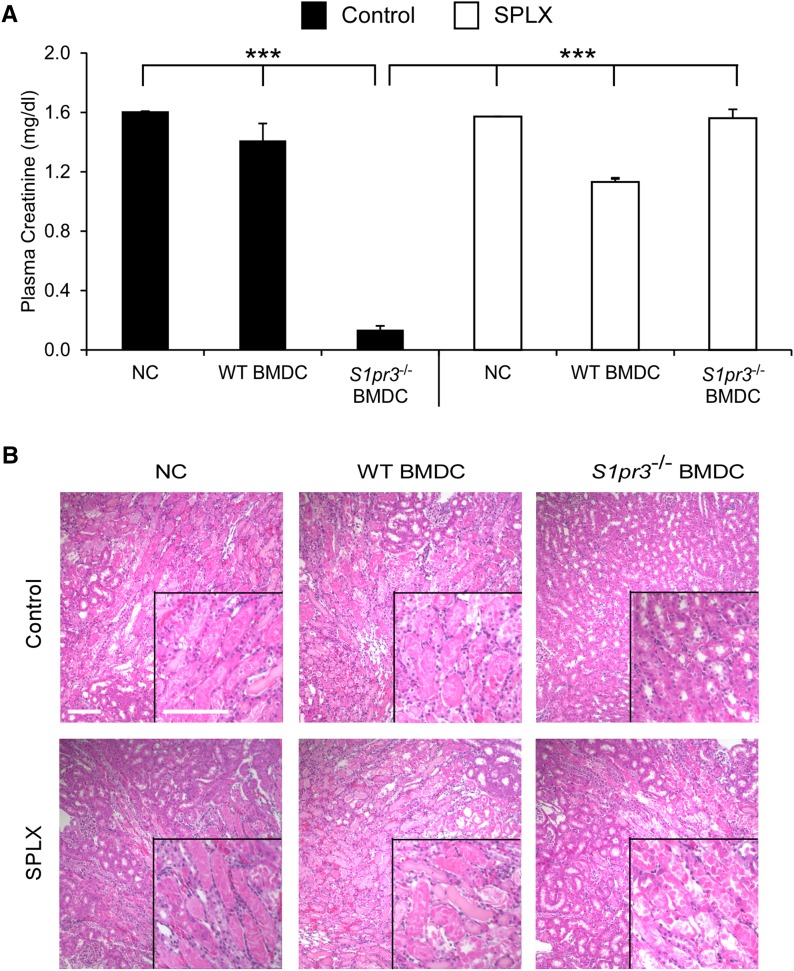
We28 and others29 have shown that effective prophylactic treatments for IRI require the spleen. As shown in Figure 3, injected BMDCs were found predominantly in the spleen. Next, we tested if S1pr3−/− BMDCs required recipient spleen for their therapeutic effect. WT and S1pr3−/− mice underwent splenectomy (SPLX) 7 days before kidney IRI. SPLX had no significant effect on IRI in WT mice but abolished the protection observed in S1pr3−/− mice (Supplemental Figure 3A). SPLX exacerbated ATN in S1pr3−/− mice (Supplemental Figure 3B, Supplemental Table 4). Similar to the S1pr3−/− mice, the protective effect of adoptively transferred S1pr3−/− BMDCs into WT mice was lost in splenectomized mice (Figure 5, Table 2).
Figure 5.
SPLX blocks the protective effect of S1pr3−/− BMDC transfer in WT mice exposed to IRI. Mice were subjected to sham surgery (control) or SPLX 7 days before kidney IRI surgery. Mice were injected with no cells (NCs), WT BMDCs, or S1pr3−/− BMDCs 1 day before IRI. (A) PCr 24 hours after kidney IRI (n=4–5). ***P<0.001. (B) H&E staining of kidney sections from the same mice. Values are mean±SEM. Scale bar, 100 μm. H&E, hematoxylin and eosin.
Table 2.
ATN scores after IRI in kidneys of control and SPLX C57BL/6 WT mice treated with no cells, WT BMDCs, or S1pr3−/− BMDCs
| Control | SPLX | ||||
|---|---|---|---|---|---|
| NCs | WT BMDCs | S1pr3−/− BMDCs | NC | WT BMDCs | S1pr3−/− BMDCs |
| 4.0±0.02 | 3.5±0.01 | 1.25±0.14a | 3.5±0.02 | 3.0±0.03 | 3.75±0.04 |
Timing for IRI and SPLX is the same as in Figure 5. Tissues were collected 24 hours after kidney IRI. Values are mean±SEM (n=3–4 in each group). NC, no cell (treated with PBS).
P<0.05 compared with control (NC) and SPLX (NC).
Transferred S1pr3−/− BMDCs Increase Splenic TREGs through CCL22 Signaling
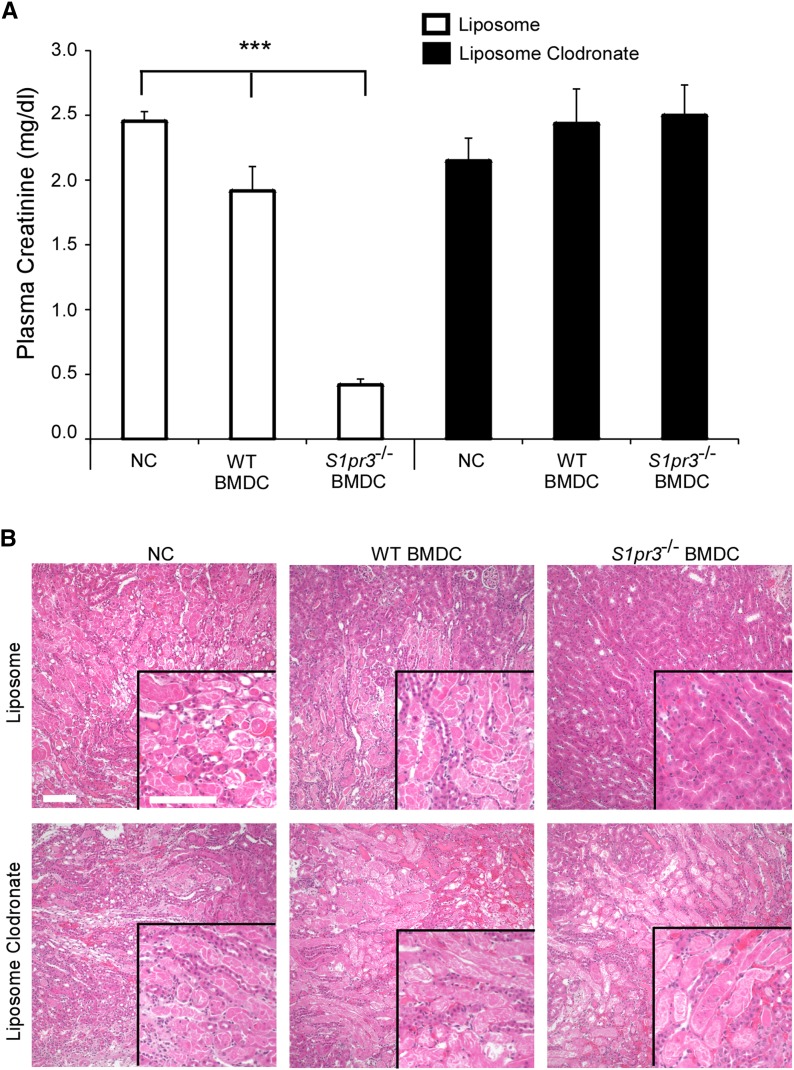
We next set out to determine how the spleen modulates S1pr3−/− BMDC–mediated protection from IRI. To determine the significance of the interaction of S1pr3−/− BMDCs with MZ macrophages, low–dose liposomal clodronate (LC; 167 µg) was administered to specifically deplete CD169+ MZ macrophages before WT or S1pr3−/− BMDC therapy (Supplemental Figure 4).30 No changes in splenic CD103+ DCs were observed in LC-treated mice compared with control liposome treatment (data not shown). We did not determine if other MZ cell populations (CD1d+ B cells or CD209b/DC-SIGN-R1+ macrophages) were affected in LC-treated mice. The administration of low-dose LC or liposomes alone did not significantly affect IRI. However, depletion of MZ CD169+ macrophages abolished the protective effect of activated S1pr3−/− BMDCs (Figure 6, Table 3).
Figure 6.
Depletion of CD169 macrophages prevents S1pr3−/− BMDC–dependent protection in kidney IRI. Mice were injected (ip) with liposomes or LC (167 μg/kg) 1 day before injection of BMDCs, and IRI was performed 24 hours later. (A) PCr 24 hours after kidney IRI. ***P<0.001. (B) H&E staining of kidney sections from the same mice. Values are mean±SEM (n=4–5 mice). NC, no cell. Scale bar, 100 μm. H&E, hematoxylin and eosin.
Table 3.
ATN scores in kidneys of liposome- or LC-pretreated mice subsequently treated with no cells, WT BMDCs, and S1pr3−/− BMDCs
| Liposome | LC | ||||
|---|---|---|---|---|---|
| NCs | WT BMDCs | S1pr3−/− BMDCs | NCs | WT BMDCs | S1pr3−/− BMDCs |
| 4.0±0.01 | 3.2±0.17 | 1.6±0.2a | 4.0±0.3 | 4.5±0.3 | 3.8±0.3 |
Timing for IRI, liposome, LC, and BMDC injections is the same as in Figure 6. Tissues were collected 24 hours after kidney IRI. Values are mean±SEM (n=3–4 in each group). NC, no cell.
P<0.05 compared with NCs and WT BMDCs in liposome-treated mice.
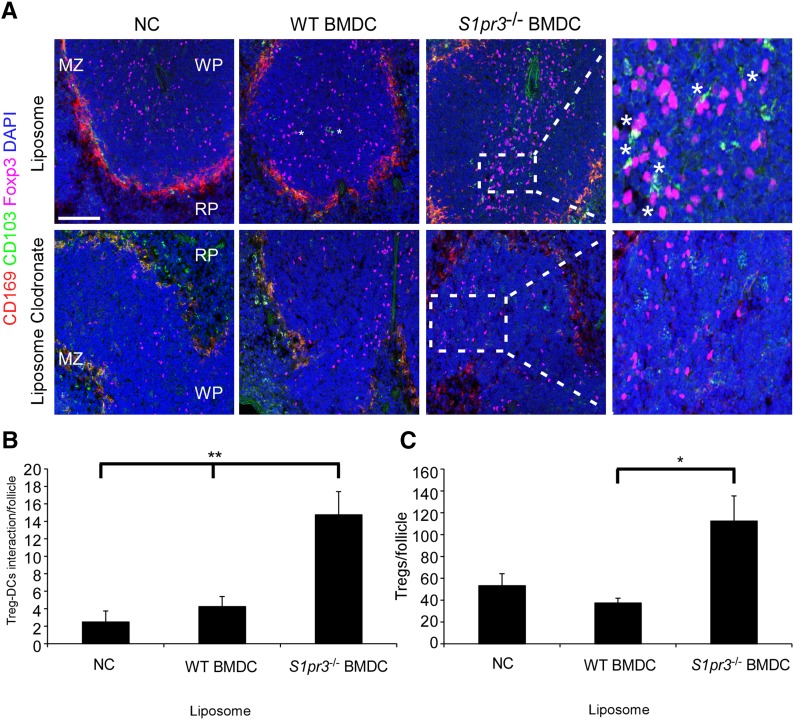
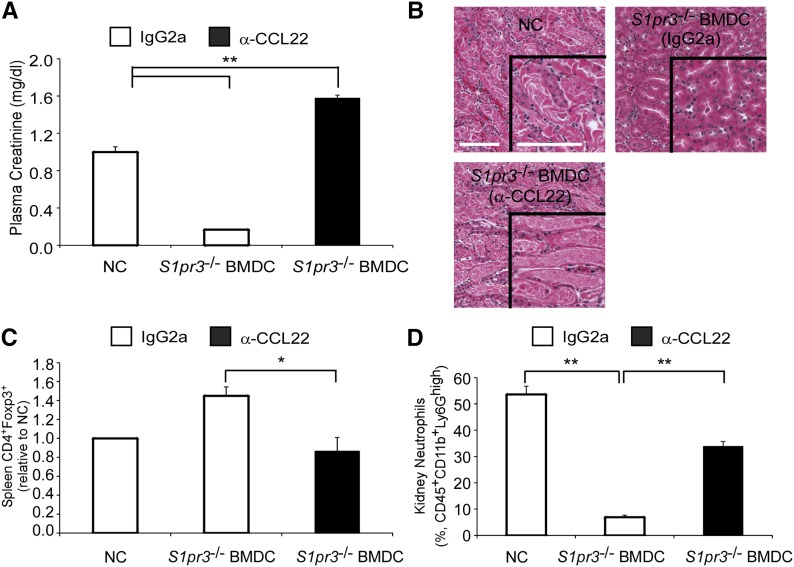
CD169 MZ macrophages are involved in the splenic accumulation of TREGs through the production of CCL22.31 We observed increased splenic DC (CD103+) and TREG (Foxp3+) interactions in the splenic white pulp of mice receiving S1pr3−/− BMDCs compared with mice receiving no cells or WT BMDCs (Figure 7). However, depletion of MZ CD169+ macrophages eliminated this interaction. To assess the importance of CCL22, we administered the CCL22 blocking antibody 4 hours before BMDC therapy. Blocking CCL22 before S1pr3−/− BMDC infusion eliminated the S1pr3−/− BMDC–dependent protection (Figure 8, A and B). Additionally, blocking CCL22 eliminated the S1pr3−/− BMDC–dependent increase in splenic TREG numbers (Figure 8C) and resulted in more kidney neutrophil infiltration (Figure 8D) after kidney IRI.
Figure 7.
Mice treated with S1pr3−/− BMDCs have higher numbers of TREGs and higher TREG-DC interactions. (A) Spleen sections of liposome- or LC-treated mice subsequently treated (as in Figure 6) with no cells (NCs), WT BMDCs, or S1pr3−/− BMDCs. Sections were labeled with antibodies for CD169 (red; macrophages), CD103 (green; DCs), or Foxp3 (magenta; TREGs) or with DAPI (blue; nuclei). Regions of the spleen are labeled: MZ, white pulp (WP), and red pulp (RP). *Interactions of CD103+ and Foxp3+ cells. (B and C) Quantification of (B) CD103+ DC and TREG (Foxp3+) interactions per white follicle and (C) TREGs per follicle in the WP of liposome-treated mice. Values are mean±SEM (n=3–4 mice). Scale bar, 100 μm. *P<0.05; **P<0.01.
Figure 8.
Neutralization of CCL22 abrogates the S1pr3−/− BMDC–dependent protection in kidney IRI. Mice were injected with α-CCL22 or control IgG2a (250 μg per mouse ip) 4 hours before S1pr3−/− BMDCs (0.5×106 iv), and IRI was performed 24 hours later. (A) PCr 24 hours after kidney IRI. (B) H&E staining of kidney sections from the same mice. Scale bar, 100 μm. (C) Total spleen TREG (CD4+Foxp3+) numbers in the same mice. (D) Kidney neutrophils as a percentage of total CD45+7AAD− cells. Values are mean±SEM (n=3–4 mice). NC, no cell. *P<0.01; **P<0.01. H&E, hematoxylin and eosin.
S1pr3−/− BMDCs Induce Protection in an Allogeneic Mouse Model in a TREG-Dependent Manner
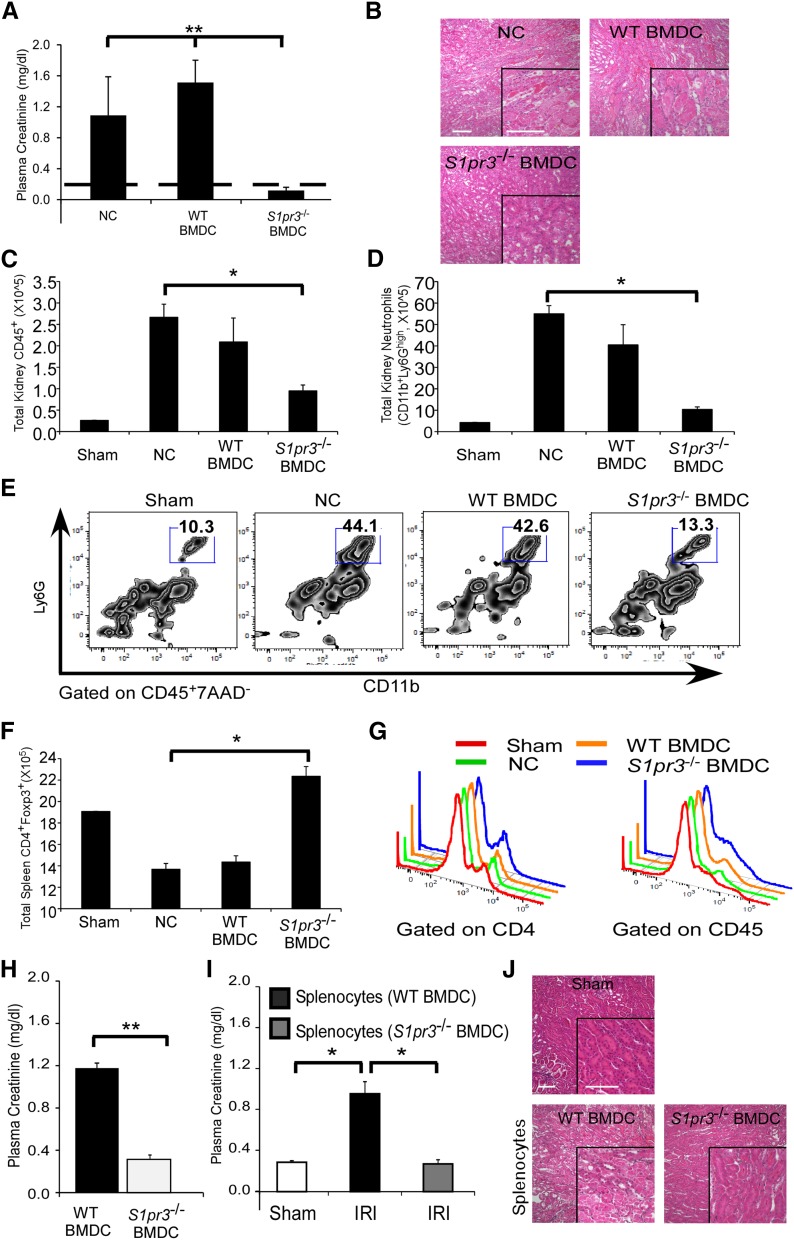
Given the anti-inflammatory nature of S1pr3−/− BMDCs and the potential involvement of TREGs, we next set out to determine the efficacy of S1pr3−/− BMDC therapy in allogeneic experiments (C57BL/6 BMDCs→Balb/c). Prior LPS activation of BMDCs is not required for allogeneic experiments given that the BMDC activation occurs in vivo because of stringent strain differences. Similar to the syngeneic studies, S1pr3−/− BMDC treatment (but not WT BMDCs) significantly protected Balb/c mice from IRI, and this effect lasted for 7 days (Figure 9, A, B, and H). Reduced AKI was associated with a significant reduction in the renal infiltration of total leukocytes and neutrophils (Figure 9, C and E). Similar to syngeneic studies, Balb/c mice treated with S1pr3−/− BMDCs had increased splenic TREG numbers (Figure 9F) and expression of Foxp3 (as indicated by elevated Foxp3 mean fluorescence intensity [MFI] when gating on CD4+ [sham: 1466±30.6, no cell: 1373±59.1, WT BMDC: 1323±28.6, and S1pr3−/− BMDC: 1585±24.5] or CD45+ cells) (Figure 9G).
Figure 9.
S1pr3−/− BMDCs or splenocytes induce tolerance in Balb/c mice and protect kidneys from ischemic injury. (A–F) Untreated C57BL/6 WT or S1pr3−/− BMDCs (0.5×106 iv) were adoptively transferred to naïve Balb/c mice 1 day before kidney IRI. (A) PCr (dashed line indicates mean value for sham-operated mice) and (B) H&E–stained kidney sections 24 hours after IRI. Total (C) CD45+ cells and (D) neutrophils (CD45+7AAD−CD11b+Ly6Ghigh) in kidney after IRI determined by flow cytometry. (E) Representative flow cytometry plots of kidney neutrophils. (F) Total spleen TREGs (CD4+Foxp3+) determined by flow cytometry. (G) Representative flow cytometry histograms of Foxp3+ cells derived from gating on CD4+ or CD45+ spleen cells. (H) PCr (24 hours after IRI) in Balb/c mice treated with C57BL/6 WT or S1pr3−/− BMDCs 7 days before kidney IRI. (I and J) Splenocytes were harvested from Balb/c mice 2 days after injection with WT (C57BL/6) or S1pr3−/− BMDCs and transferred (5.0×106 iv) to Balb/c recipient mice 1 day before IRI. (I) PCr was measured 24 hours after IRI. (J) H&E–stained kidney sections from the same mice. Values are mean±SEM (n=3–4 mice). NC, no cell. Scale bar, 100 μm. *P<0.05; **P<0.01. H&E, hematoxylin and eosin.
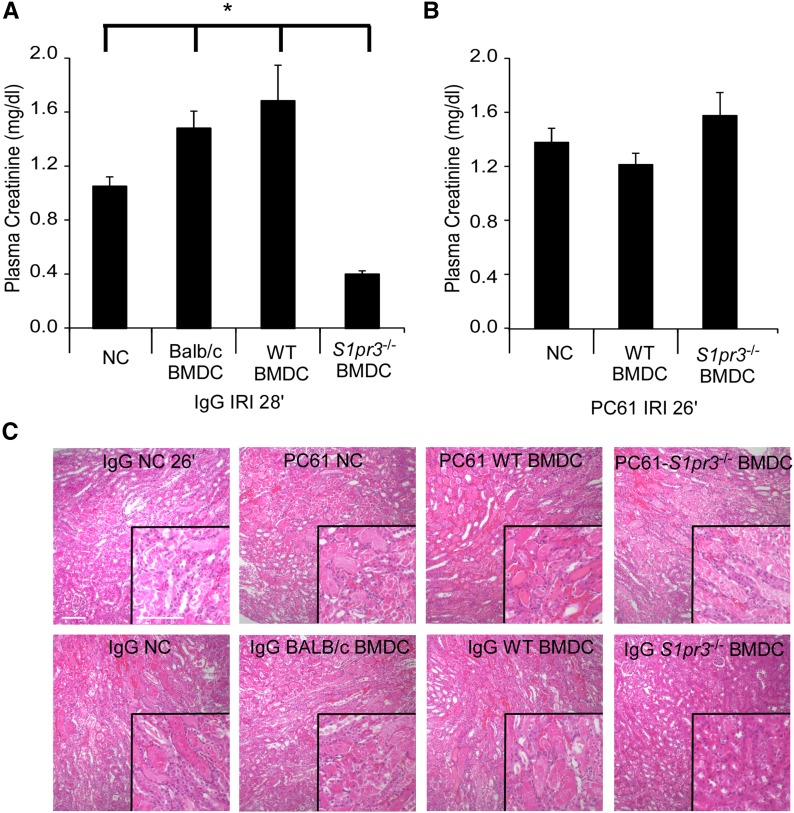
To support the specific involvement of the spleen in S1pr3−/− BMDC–mediated protection, splenocytes were isolated from Balb/c mice 2 days after receiving C57BL/6 WT or S1pr3−/− BMDCs. Splenocytes from S1pr3−/− BMDC–treated Balb/c mice had a higher percentage of CD4+Foxp3+ cells and a higher Foxp3+ MFI (gated on CD4+) compared with WT BMDC–treated mice (3.6% versus 2.8%, respectively; MFI was 2330 versus 1867, respectively). Adoptive transfer of splenocytes from S1pr3−/− BMDC–treated mice 1 day before IRI conferred protection from IRI in recipient naïve mice (Figure 9, I and J). We tested whether S1pr3−/− BMDCs were able to increase CD4+Foxp3+ TREG numbers in a mixed lymphocyte reaction using Balb/c T cells incubated for 7 days. Compared with B6 WT BMDCs (1:40; DC:T cell), incubation with B6 S1pr3−/− BMDCs resulted in a significantly higher percentage of CD4+Foxp3+ TREGs (4.3±0.69 versus 10.1±1.3, respectively; P<0.01). To determine the contribution of TREGs in this model, Balb/c mice were depleted of TREGs using PC61 antibody32 or received the IgG isotype control antibody; 5 days later, mice were injected with WT or S1pr3−/− BMDCs and challenged with IRI the following day. In agreement with our previously published observations,10 Balb/c mice treated with Balb/c BMDCs had greater kidney injury after IRI. In IgG–pretreated Balb/c mice, adoptive transfer of S1pr3−/− BMDCs protected kidneys from IRI (Figure 10, A and C). However, TREG depletion with PC61 eliminated the S1pr3−/− BMDC–induced protection from IRI (Figure 10, B and C, Table 4).
Figure 10.
Depletion of recipient TREGs (CD4+Foxp3+) prevents S1pr3−/− BMDC–dependent protection from kidney IRI. (A and B) Balb/c mice were (A) given control IgG or (B) depleted of TREGs by infusion of CD25 blocking antibody (PC61; 300 μg per mouse ip) 5 days before BMDC injection and subsequent kidney IRI. WT (C57BL/6) or S1pr3−/− BMDCs were injected 1 day before IRI. To achieve comparable levels of injury in no cell (NC) –treated mice, ischemic time was (A) 28 minutes in control mice and (B) 26 minutes in mice lacking TREGs. (C) H&E staining of kidney sections from the PC61- and IgG-treated mice. Values are mean±SEM (n=4–6 mice). Scale bar, 100 μm. *P<0.05. H&E, hematoxylin and eosin.
Table 4.
ATN scores in kidneys of PC61- and IgG-pretreated mice subsequently treated with no cells, Balb/c BMDCs, C57BL/6 WT BMDCs, and S1pr3−/− BMDCs
| PC61 | IgG | |||||
|---|---|---|---|---|---|---|
| NCs | WT BMDCs | S1pr3−/− BMDCs | NCs | Balb/c BMDCs | WT BMDCs | S1pr3−/− BMDCs |
| 3.0±0.35 | 2.75±0.17 | 3.12±0.13 | 3.0±0.70 | 3.1±0.54 | 3.5±0.35 | 1.3±0.27a |
Timing for IRI, PC61, IgG, and BMDC injections is the same as in Figure 10. Tissues were collected 24 hours after kidney IRI. Values are mean±SEM (n=3–4 in each group). NC, no cell.
P<0.05 compared with NCs, Balb/c BMDCs, and WT BMDCs in IgG-treated mice.
Discussion
In this study, we showed that immunosuppression and protection from kidney IRI induced by adoptive transfer of S1pr3−/− BMDCs are dependent on the spleen in recipient mice. Furthermore, the protective effects of S1pr3−/− BMDCs require splenic CD169+ macrophages along with recipient CD11c+ DCs and TREGs. The interactions of S1pr3−/− BMDCs with recipient CD169+ macrophages and DCs may be responsible for the increase in splenic TREGs. The increased numbers and recruitment of TREGs through the CCL22 chemokine are necessary for the protection observed by S1pr3−/− BMDCs.
AKI is a major health burden without major pharmacologic advances in its prevention or treatment.33 Additionally, current therapies for allograft rejection, cancer, or autoimmune diseases use nonspecific immunosuppressive drugs that are associated with adverse side effects and limited because of lack of antigen-specific tolerance. Cell-based therapy using regulatory immune cells (TREGs,34 myeloid cells,35 or DCs36,37) is a strategy that induces potential antigen–specific tolerance. Pharmacologic or biologic strategies induce regulatory or tolerogenic DCs,38 which are immature, maturation-resistant, or alternatively activated cells that express low levels of MHC and costimulatory molecules. Compared with mature DCs, immature DCs interact actively with T cells and direct them into a regulatory response. In kidney IRI, BMDCs tolerized with an A2AR agonist22 or DCs deficient of S1pr310 attenuate AKI.
Most of the biologic effects of S1P are mediated through the S1PR family, which includes the ubiquitously expressed S1P1, S1P2, and S1P3 subtypes.39 T cells express high levels of S1P1 followed by S1P4,24,25 and human DCs express S1P1, S1P2, S1P3, and S1P4.26 In DCs, S1P3 signaling is coupled to protease-activated receptor-1, leading to lethal outcomes in sepsis.40 Human and mouse DCs express S1P3, and after kidney IRI, S1P3 expression increases in kidney-resident and immune cells.10 After kidney IRI, S1pr3−/− mice have immature DCs compared with WT mice,10,20 and transfer of α-galactosyl-ceramide–loaded S1pr3−/− BMDCs before kidney IRI reduces AKI through a switch in T cell polarity from Th1 to Th2 without an increase in TREG numbers.10
Similarly, our previously published study showed that adoptive transfer of S1pr3−/− BMDCs loaded with α-galactosyl-ceramide induces an anti–inflammatory Th2 phenotype from natural killer T (NKT) cells. It is possible that TREG expansion in this study also induced an anti–inflammatory Th2–like response, possibly through production of IL-10. However, the prevailing paradigm that DCs with a regulatory phenotype produce high levels of IL-10 and low levels of IL-12p4038 is not consistent with our studies with S1pr3−/− BMDCs. S1pr3−/− BMDCs had lower IL-12 levels after LPS but failed to produce high levels of IL-10 compared with WT BMDCs. Nevertheless, therapeutic BMDCs require recipient IL-1022; TREGs modulate injury after kidney IRI through IL-10–mediated suppression of innate immunity, and use of IL-10−/− TREGs fails to protect kidneys from IRI.41 Transfer of immature S1pr3−/− BMDCs (lower MHCII, costimulatory molecules, and cytokines) in vivo could result in altered interaction with recipient splenocytes, leading to higher TREGs, possibly through IL-10.
Depletion of DCs significantly protects mouse kidneys from IRI,6,10 and a dose-dependent increase in BMDC numbers exacerbates kidney injury,10 suggesting that DCs play a major role in inducing AKI. As our study shows, injected BMDCs accumulate in the spleen after systemic infusion42 and can persist for 2 weeks postinjection.43 In the spleen, injected BMDCs require host DCs for transferred BMDC–dependent tolerance.44,45 Transferred S1pr3−/− BMDCs require host CD11c+ DCs and CD169+ MZ macrophages to modulate protection, which was shown in this study. However, a major limitation of using CD11c-DTR mice is that diphtheria toxin depletes both outer MZ and CD169+ macrophages.46 We found similar degrees of injury after adoptive transfer of WT or S1pr3−/− BMDCs in mice depleted of MZ macrophages or CD11c+ cells. These data suggest that, in the absence of host CD11c+ cells or MZ macrophages to modulate the immunosuppressive effects of transferred immunosuppressive BMDCs, the addition of exogenous DCs worsens kidney injury after IRI, supporting our previously published study.10 Therapeutic use of DCs in transplantation also require recipient DCs to induce tolerance; depletion of recipient DCs abrogates the protection induced by injected DCs.44 Infusion of regulatory DCs in nonhuman primates prolongs allograft survival, justifying preclinical evaluation of DC therapy and its therapeutic potential in organ transplantation.47
Transfer of TREGs before kidney IRI significantly protects mice from kidney IRI,41 and depletion of TREGs with PC61 results in worsening of injury.32 The feasibility and safety of ex vivo expansion of TREGs with subsequent adoptive transfer to patients with transplants have been shown.48 Also, the results of several studies investigating adoptive transfer of freshly isolated TREGs before acute renal insults in mice have been encouraging.32,41 In our study, an increase in TREG numbers in the spleen may be responsible for the S1pr3−/− BMDC–induced protection from ischemic injury; TREG depletion or use of Rag-1−/− mice blocked protection. In models of transplantation, even long-term prevention by TREGs (ex vivo expanded) requires or involves host Foxp3+ T cells.49 Therefore, therapies that induce functionally better (higher IL-10 production) host TREGs are advantageous over studies using ex vivo–expanded TREG transfer. Similar to our study, mesenchymal stem cells attenuate kidney IRI by inducing TREGs through interactions that involve the recipient spleen.29 Mesenchymal cell therapy in animal models and pilot clinical trials shows encouraging results in conditions of renal IRI and renal transplantation.50
We found that the immunosuppressive capabilities of S1pr3−/− BMDCs require the recipient spleen. Strategic positioning allows the MZ to be involved in mounting innate and adaptive immune responses. Macrophages in the splenic MZ are essential for trapping blood-borne particulates. Using a low dose of LC that selectively eliminates phagocytic cells within the MZ,30,51 we showed that S1pr3−/− BMDCs require CD169+ cells to coordinate protection. It is possible that CD169+ macrophages respond to cell-associated antigens of injected BMDCs and transfer these antigens to host DCs in the spleen.52 CD169+ macrophages coordinate apoptotic cell–driven cellular recruitment and tolerance through induction of CCL22 and CCR4,53 resulting in accumulation and activation of TREGs and DCs.31 Similarly, in our studies, blocking CCL22 abrogated the protection induced by S1pr3−/− BMDCs and the associated increase in splenic TREGs. Conversely, a recent study by Karasawa et al.54 showed an anti-inflammatory phenotype for CD169+ macrophages, and depletion of CD169+ cells resulted in more kidney injury. It is possible that depletion of CD169+ cells and the associated increase in neutrophils in their study are caused by reduced TREG numbers. Depletion of DCs using CD11c-DTR mice could also deplete MZ macrophages, thus possibly reducing production of CCL22 and recruitment of TREGs. Therefore, as shown, depletion of DCs abrogates protection induced by S1pr3−/− BMDCs, perhaps leading to fewer TREG-DC aggregates and less TREG–dependent protection. Similar mechanisms of reduced aggregates could lead to the inability of S1pr3−/− BMDCs to provide protection in mice depleted of TREGs by PC61 or CD169+ cells by low-dose LC. The splenic MZ macrophages also coordinate the anti-inflammatory activity of intravenous (iv) Ig, and SPLX abrogates the anti-inflammatory activity of iv Ig.55 These studies help support our findings on the immunosuppressive effect and the potential therapeutic use of S1pr3−/− BMDCs. Injected S1pr3−/− BMDCs require splenic MZ macrophages that may coordinate the elaborate cross-talk between recipients CD11c+ DCs, ultimately resulting in increased numbers of TREGs that suppress inflammation.
In our studies, splenocytes isolated from mice that received S1pr3−/− BMDCs and were stimulated ex vivo with LPS contained CD11c+ cells with higher intracellular IFN-γ than those from mice receiving WT BMDCs. It is likely that IFN-γ production by DCs plays an important role in innate immunity and subsequent T cell responses. Propagation of tolerogenic BMDCs with GM-CSF and IL-4 also results in higher production of IFN-γ.56 IFN-γ production by DCs within an inflammatory environment has also been suggested to induce anti–inflammatory indoleamine 2,3-dioxygenase.57 In our preliminary studies, neutralization of IFN-γ abrogated the immunosuppressive BMDC–induced protection after kidney IRI (data not shown). Future studies will be done to better understand the functional immunosuppressive role of CD11c+-derived IFN-γ.
Previous studies from our laboratory have shown that NKT cells and IFN-γ are key mediators of kidney IRI.7 Recent studies have shown that NKT cells located in splenic MZ and red pulp58 also mediate lipid antigen processing and are rapidly activated by blood-borne antigens through interactions with the CD169 MZ macrophages.59 S1pr3−/− BMDCs loaded with α-galactosyl-ceramide fail to induce injury after kidney IRI, possibly because of the inability of recipient NKT cells to produce IFN-γ.10 Given our finding that depletion of CD169+ cells or use of Rag-1−/− (no NKT cells) mice abrogates the S1pr3−/− BMDC–dependent protection, it is plausible that similar overlapping mechanisms exist that could also involve CD169 MZ macrophages and NKT cells. Close proximity of CD169 MZ macrophages and NKT cells could result in higher MZ–NKT cell interactions, thus favoring fast initiation of anti–inflammatory innate immune responses.59 Future studies will be needed to better understand the involvement of the NKT–MZ–Treg axis. Current studies clearly show a functional change in splenocytes in response to S1pr3−/− BMDCs, and in conjunction with prior work, they also show a novel therapeutic anti–inflammatory use of S1pr3−/− BMDCs. This would be especially relevant in an allogeneic setting, which would be transferable to the area of transplantation.
In summary, we have shown that tolerance induced by immature S1pr3−/− BMDCs is dependent on the spleen. In the spleen, S1pr3−/− BMDCs require the MZ CD169+ macrophages; interaction of CD169+ macrophages and S1pr3−/− BMDCs results in migration of DCs to the white pulp along with recruitment of TREGs, possibly through the CCL22–CCR4 pathway. In the white pulp, CD103+ DCs show increased interaction with T cells, which subsequently increase TREG numbers. We conclude that tolerogenic S1P3–deficient BMDCs may be useful in kidney IRI as well as other inflammatory states, such as transplantation and autoimmune disorders.
Concise Methods
All animals were handled and procedures were performed in adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the University of Virginia Institutional Animal Care and Use Committee. S1pr3−/− mice (gift from Richard Proia) and S1pr3+/+ WT progeny control male mice (C57BL/6 [B6] background) were used for all kidney IRI comparisons (Figures 2A and 3A) and bone marrow cell isolation. For all transfer studies, C57BL/6 and Balb/c mice were purchased from the National Cancer Institute (Frederick, MD). A Rag-1−/− (B6.129S7-Rag1tm1Mom/J) breeder pair was obtained from Jackson Laboratories (Bar Harbor, ME).
Immune Cell Depletion and Antibody Treatment In Vivo
Liposome PBS and LC were purchased from FormuMax Scientific (Palo Alto, CA). Mice were depleted of MZ macrophages with a low dose of LC (167 μg/kg) or injected with an equal volume of liposomes containing PBS 1 day before BMDC adoptive transfer studies.
The TIB-222 hybridoma (ATCC, Manassas, VA) was used by the Lymphocyte Culture Core Facility at the University of Virginia to produce the anti–mouse CD25 mAb (clone PC61), which was administered to mice (300 μg per mouse intraperitoneally [ip] 5 days before BMDC injection) to deplete Tregs.32 Control IgG ImmunoPure Rat IgG (300 μg per mouse ip) was purchased from Pierce (Rockford, IL).
The anti–CCL22 blocking antibody (clone 158132; R&D Systems, Minneapolis, MN) was administered to mice (250 μg per mouse ip 4 hours before BMDC injection). Control IgG2a (250 μg per mouse ip 4 hours before BMDC or PBS injection) was purchased from Bio X Cell (West Lebanon, NH).
Renal IRI and SPLX
Male mice (8–12 weeks old; C57BL/6 and Balb/c) were subjected to bilateral IRI (26 minutes of ischemia for C57BL/6 and Rag-1−/− mice and 28 minutes of ischemia for Balb/c mice followed by 20–24 hours of reperfusion) as previously described.3,7,60 Control sham–operated mice underwent a similar procedure; however, the renal pedicles were not clamped. For experiments that involved SPLX before IRI, mice were anesthetized with an ip injection of ketamine (120 mg/kg), xylazine (12 mg/kg), and atropine (0.324 mg/kg). The spleen was then removed through a small flank incision. Control sham–operated mice underwent the same procedure with the exception of splenic artery ligation and spleen removal. Buprenorphine (0.15 mg/kg) was administered as a postoperative analgesic for both IRI and splenectomized mice. Sham and splenectomized mice were allowed to recover for 7 days before BMDC transfer for IRI studies.
In Vivo Imaging
For in vivo imaging, adoptively transferred BMDCs (WT or S1pr3−/−) were labeled with VT-680 (Perkin Elmer Inc., Boston, MA) according to the manufacturer’s recommendations. BMDCs were labeled with 30 μg/ml VT-680 in 2 ml 1× PBS and washed three times; 5×106 labeled BMDCs were injected (iv) into mice. Two weeks before BMDC injection, standard chow for recipient mice was replaced with a purified chow (Teklad Global Rodent Diet, AIN-93G, alfalfa free; NFD:TD 94096; Harlan Laboratories, Madison, WI) to minimize tissue autofluorescence. Imaging was done with a Visen FMT 2500 Quantitative Fluorescence Tomography System (PerkinElmer Waltham, MA) 24, 44, and 72 hours after BMDC injection.
WT or S1pr3−/− BMDCs were labeled with 2 μM PKH26GL dye (Sigma-Aldrich, St. Louis, MO) according to the manufacturer’s recommendations, and then, they were washed and injected (2×106 labeled cells iv) into recipient mice. Spleens were harvested at 30 minutes or 24 hours after BMDC injection and used for immunofluorescence labeling.
Assessment of Kidney Function and Histology
PCr as a measure of kidney function was determined using a colorimetric assay according to the manufacturer’s protocol (Sigma-Aldrich). For histology, kidneys were fixed overnight in 0.2% sodium periodate, 1.4% DL-lysine, and 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 (4% PLP) and embedded in paraffin. Kidneys were prepared for hematoxylin and eosin staining as previously described3 and viewed by light microscopy (Carl Zeiss AxioSkop). Photographs were taken, and brightness/contrast adjustment was made with an SPOT RT Camera (software version 3.3; Diagnostic Instruments, Sterling Heights, MI). The histologic change in the outer medulla was expressed as ATN scored as previously described.10,61
Immunohistochemical Analyses
Kidneys and spleens were fixed in 1% PLP (as above except with 1% paraformaldehyde) overnight, incubated in 30% sucrose for 24 hours at 4°C, embedded, and frozen in Tissue-Tek OCT Compound (Ted Pella Inc., Redding, CA). Frozen sections (5–7 μm) were permeabilized with 0.3% Triton X-100, and nonspecific binding was blocked with 10% horse serum and rat anti–mouse CD16/32 (10 μg/ml; clone 2.4G2; BD Pharmingen, San Jose, CA). Sections were labeled by incubation for 1 hour with anti-mouse F4/80 (5 μg/ml; clone BM8; Molecular Probes, Fredrick, MD), anti-mouse CD169 (7 μg/ml; clone 3D6.112; BioLegend, San Diego, CA), anti-mouse CD169 (7 μg/ml; clone MOMA-1; AbD Serotec/BioRad, Raleigh, NC), FITC anti–mouse CD4 (7 μg/ml; clone RM4–5; BD Biosciences, San Jose, CA), FITC anti–mouse CD3 (7 μg/ml; clone 500A2; Caltag Invitrogen, Camarillo, CA), PE anti–mouse B220 (7 μg/ml; clone RA3–6B2; eBiosciences), A647 rat anti–Foxp3 (5 μg/ml; clone FJK-16s; eBiosciences), or FITC rat anti–CD103 (7 μg/ml; clone M290; BD Biosciences). All specimens were mounted with ProLong Gold Antifade Reagent with DAPI (Invitrogen, Carlsbad, CA) to label cell nuclei. Images were acquired using a Carl Zeiss Axiovert 200 Microscopy System with ApoTome Imaging and Axiovision 4.8 software (Carl Zeiss Microscopy, Thornwood, NY).
The number of TREG (Foxp3+)–DC (CD103+) interactions in immunofluorescently labeled spleen sections was counted using a Carl Zeiss AxioImager Z2 Microscope with StereoInvestigator software (version 10.51; MBF Bioscience, Williston, VT). At least four to six white follicles/spleen were examined per liposome- or LC-treated mouse. Interaction between TREGs and DCs was considered contact if a cell with an Foxp3+ nucleus was adjacent to a CD103+ cell. If one Foxp3+ cell was surrounded by multiple CD103+ cells, TREG (Foxp3+)–DC (CD103+) interaction was only counted one time. Total number of TREGs was determined by counting Foxp3+ nuclei in white follicles.
Bone Marrow–Derived DC Culture and Adoptive Transfer
Eight-week-old C57BL/6 WT and S1pr3−/− male mice were used for generating highly pure DCs from whole bone marrow precursors.62 GM-CSF–rich supernatant was derived from J558L cells stably transfected with mouse GM-CSF. The cell line was a gift from Ira Mellman (Yale University). Briefly, freshly isolated bone marrow was cultured with 6 ng/ml recombinant mouse GM-CSF for 8–10 days with media replaced every 3 days; 80%–90% of resulting cells were CD11c+ DCs as determined by flow cytometry with CD11c antibody. Bone marrow-derived DCs (8- to 10-day-old cultures) were treated with 100 ng/ml LPS or vehicle (1× PBS) for 20 hours in culture medium for syngeneic studies (C57BL/6 BMDCs→C57BL/6 mice) and left untreated for allogenic studies (C57BL/6 BMDCs→Balb/c mice). Cells were washed, and 0.5×106 cells per mouse were introduced iv to naive mice 1 or in some studies, 7 days before kidney IRI.
Isolation and Adoptive Transfer of Splenocytes
Single-cell suspensions of splenocytes from mice treated with C57BL/6 WT, S1pr3−/−, or Balb/c BMDCs were obtained by passing through a 40-μm nylon filter and subjecting to hypotonic erythrocyte lysis; 200 μl freshly isolated splenocytes (5.0×106 per mouse) were transfused into the tail vein of Balb/c mice 1 day before kidney IRI.
Quantitative Real–time RT-PCR, Western Blot, and Multiplex Assay
Total RNA was extracted from kidneys with TriReagent according to the manufacturer’s protocol (Molecular Research Center Inc., Cincinnati, OH), and single-stranded cDNA was synthesized as previously described.62 Gene sequences were obtained from the GenBank Database. Primers were designed using IDT PrimerQuest (Integrated DNA Technologies; www.idtdna.com). Primer sequences were as previously published.11,61 RT-PCR was performed using the iScript 1-Step RT-PCR Kit with SYBR Green (Bio-Rad, Hercules, CA); samples were normalized to GAPDH. Melting curves were inspected to ensure specificity of product detection. The following PCR protocol was used: initial denaturation at 95°C for 3 minutes; denaturation, annealing, and elongation program repeated 35 times at 95°C for 45 seconds, 52°C for 60 seconds, and 72°C for 60 seconds; final elongation at 72°C for 7 minutes; and finally, a holding step at 4°C.
BMDC cultures from WT or S1pr3−/− mice were treated with LPS (100 ng/ml) for 1 hour, and total protein was isolated. Cell lysates were prepared by sonicating in RIPA Lysis Buffer (Thermo Fisher Scientific, Waltham, MA) enriched with 1% protease and phosphatase inhibitor cocktail (formulation: sodium fluoride, sodium orthovanadate, β-glycerophosphate, sodium pyrophosphate, aprotinin, bestatin, E64, leupeptin, and EDTA; Thermo Fisher Scientific). Homogenates were centrifuged (12,000 rpm for 15 minutes at 4°C), and the protein concentration in the supernatant was measured by using the BCA Protein Assay Kit (Pierce). Equal volumes of the remaining cell or tissue lysate supernatants were boiled with Leammli buffer and β-mercaptoethanol (10 minutes at 100°C); proteins were separated using 10% SDS-PAGE and transferred to polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA). The membranes were blocked with Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE) for 1 hour at room temperature and then incubated with primary antibody (mouse anti–β-tubulin; 1:5000; 3F3-G2; Santa Cruz Biotechnology, Dallas, TX and rabbit antiphospho-NFκB; 1:500; Cell Signaling Technology, Danvers, MA). The membrane was washed two times for 5 minutes each in 0.05% Tween and PBS, probed with goat anti–rabbit IRDye 680RD– and goat anti–mouse IRDye 800CW–labeled secondary antibody (1:20,000; LI-COR) in Odyssey Blocking Buffer, 0.1% Tween, and 0.01% SDS for 1 hour at room temperature, and washed two times for 5 minutes in Tween and PBS. Air-dried membranes were imaged by using an LI-COR Odyssey Infrared Imaging System with Odyssey 3.0 analytical software (LI-COR) for multiplex detection of the two different antigens.
A panel of cytokines and chemokines was assessed in supernatants from cultures of WT BMDCs and S1pr3−/− BMDCs (cells treated with PBS or LPS [100 ng/ml] for approximately 20 hours) using the Mouse Cytokine/Chemokine Magnetic Bead Multiplex Assay (EMD Millipore) as described by the manufacturer. Samples were analyzed as recommended by the manufacturer using a Luminex IS 100 System (UVA Flow Cytometry Core Facility). In the ex vivo stimulation studies, supernatant IL-6, TNF-α, and IL-10 were determined by ELISA following the manufacturer’s protocol (eBiosciences).
FACS Analyses
Flow cytometry was used to analyze kidney leukocyte content. In brief, kidneys were extracted, minced, and digested (1 mg/ml collagenase) as described.7 After blocking nonspecific Fc binding with anti-mouse CD16/32 (2.4G2), fresh kidney suspensions were incubated with fluorophore–tagged anti–mouse CD45 (30-F11) to determine total leukocyte cell numbers. CD45-labeled samples were further used for labeling with different combinations of fluorophore–tagged anti–mouse F4/80 (BM8), GR-1 (Ly6G), CD11b (M1/70), and CD11c (integrin-αX chain HL3) or IA (MHCII), CD4 (RM4–5), and Foxp3 (FJK-16s). 7-AAD (BD Biosciences) was added 15 minutes before analyzing the sample to separate live from dead cells. Appropriate fluorochrome–conjugated, isotype–matched, irrelevant mAbs were used as negative controls. Flow cytometry data acquisition was performed on an FACS Calibur (Becton Dickinson, San Jose, CA) with Cytek 8-Color Flow Cytometry Upgrade (Cytek Development, Inc., Fremont, CA). Data were analyzed by FlowJo software 9.0 (TreeStar, Inc., Ashland, OR). All antibodies (except as noted) were from eBiosciences and used at a concentration of 5 μg/ml.
Data and Statistical Analyses
GraphPad Instat 3 (GraphPad Inc.), SigmaPlot 11.0 (Systat Software Inc.), and Canvas X (ACD Systems of America Inc.) were used to analyze and present the data. Data were analyzed after transformation, if needed, to generate a normal distribution by two–tailed t test or two- or one-way ANOVA with post hoc analysis as appropriate. P<0.05 was used to indicate significance.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the University of Virginia Research Histology Core Facility and all members of the laboratory of M.D.O. We also thank our students Bansi Patel and Nisha Christian for assistance and support with experiments.
Research reported in this work was supported by National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NIH) Awards K01-DK091444 (to A.B.), R01-DK085259 (to M.D.O.), and R01-DK062324 (to M.D.O.); a National Kidney Foundation Fellowship (to A.B.); and American Heart Association Career Development Grant 11S-DG7000007 (to A.B.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015010095/-/DCSupplemental.
References
- 1.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grenz A, Bauerle JD, Dalton JH, Ridyard D, Badulak A, Tak E, McNamee EN, Clambey E, Moldovan R, Reyes G, Klawitter J, Ambler K, Magee K, Christians U, Brodsky KS, Ravid K, Choi DS, Wen J, Lukashev D, Blackburn MR, Osswald H, Coe IR, Nürnberg B, Haase VH, Xia Y, Sitkovsky M, Eltzschig HK: Equilibrative nucleoside transporter 1 (ENT1) regulates postischemic blood flow during acute kidney injury in mice. J Clin Invest 122: 693–710, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Li L, Huang L, Sung SS, Vergis AL, Rosin DL, Rose CE, Jr., Lobo PI, Okusa MD: The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int 74: 1526–1537, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson PJ: Renal ischemia-reperfusion injury: Renal dendritic cells loudly sound the alarm. Kidney Int 71: 604–605, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Soos TJ, Sims TN, Barisoni L, Lin K, Littman DR, Dustin ML, Nelson PJ: CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int 70: 591–596, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Li L, Okusa MD: Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Semin Nephrol 30: 268–277, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Huang L, Sung SS, Lobo PI, Brown MG, Gregg RK, Engelhard VH, Okusa MD: NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J Immunol 178: 5899–5911, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Huang Y, Rabb H, Womer KL: Ischemia-reperfusion and immediate T cell responses. Cell Immunol 248: 4–11, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burne MJ, Daniels F, El Ghandour A, Mauiyyedi S, Colvin RB, O’Donnell MP, Rabb H: Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest 108: 1283–1290, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bajwa A, Huang L, Ye H, Dondeti K, Song S, Rosin DL, Lynch KR, Lobo PI, Li L, Okusa MD: Dendritic cell sphingosine 1-phosphate receptor-3 regulates Th1-Th2 polarity in kidney ischemia-reperfusion injury. J Immunol 189: 2584–2596, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bajwa A, Jo SK, Ye H, Huang L, Dondeti KR, Rosin DL, Haase VH, Macdonald TL, Lynch KR, Okusa MD: Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J Am Soc Nephrol 21: 955–965, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park SW, Kim M, Brown KM, D’Agati VD, Lee HT: Inhibition of sphingosine 1-phosphate receptor 2 protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 23: 266–280, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park SW, Kim M, Kim M, D’Agati VD, Lee HT: Sphingosine kinase 1 protects against renal ischemia-reperfusion injury in mice by sphingosine-1-phosphate1 receptor activation. Kidney Int 80: 1315–1327, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Spiegel S, Milstien S: Functions of a new family of sphingosine-1-phosphate receptors. Biochim Biophys Acta 1484: 107–116, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Ancellin N, Colmont C, Su J, Li Q, Mittereder N, Chae SS, Stefansson S, Liau G, Hla T: Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem 277: 6667–6675, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Spiegel S, Milstien S: Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem 277: 25851–25854, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Jo SK, Bajwa A, Awad AS, Lynch KR, Okusa MD: Sphingosine-1-phosphate receptors: Biology and therapeutic potential in kidney disease. Kidney Int 73: 1220–1230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Means CK, Xiao CY, Li Z, Zhang T, Omens JH, Ishii I, Chun J, Brown JH: Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 292: H2944–H2951, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Theilmeier G, Schmidt C, Herrmann J, Keul P, Schäfers M, Herrgott I, Mersmann J, Larmann J, Hermann S, Stypmann J, Schober O, Hildebrand R, Schulz R, Heusch G, Haude M, von Wnuck Lipinski K, Herzog C, Schmitz M, Erbel R, Chun J, Levkau B: High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation 114: 1403–1409, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Jo SK, Bajwa A, Ye H, Vergis AL, Awad AS, Kharel Y, Lynch KR, Okusa MD: Divergent roles of sphingosine kinases in kidney ischemia-reperfusion injury. Kidney Int 75: 167–175, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K: Immunobiology of dendritic cells. Annu Rev Immunol 18: 767–811, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Li L, Huang L, Ye H, Song SP, Bajwa A, Lee SJ, Moser EK, Jaworska K, Kinsey GR, Day YJ, Linden J, Lobo PI, Rosin DL, Okusa MD: Dendritic cells tolerized with adenosine A₂AR agonist attenuate acute kidney injury. J Clin Invest 122: 3931–3942, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinsey GR, Huang L, Jaworska K, Khutsishvili K, Becker DA, Ye H, Lobo PI, Okusa MD: Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J Am Soc Nephrol 23: 1528–1537, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allende ML, Dreier JL, Mandala S, Proia RL: Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem 279: 15396–15401, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Rosen H, Goetzl EJ: Sphingosine 1-phosphate and its receptors: An autocrine and paracrine network. Nat Rev Immunol 5: 560–570, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Idzko M, Panther E, Corinti S, Morelli A, Ferrari D, Herouy Y, Dichmann S, Mockenhaupt M, Gebicke-Haerter P, Di Virgilio F, Girolomoni G, Norgauer J: Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. FASEB J 16: 625–627, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Lee DW, Faubel S, Edelstein CL: Cytokines in acute kidney injury (AKI). Clin Nephrol 76: 165–173, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Gigliotti JC, Huang L, Ye H, Bajwa A, Chattrabhuti K, Lee S, Klibanov AL, Kalantari K, Rosin DL, Okusa MD: Ultrasound prevents renal ischemia-reperfusion injury by stimulating the splenic cholinergic anti-inflammatory pathway. J Am Soc Nephrol 24: 1451–1460, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu J, Zhang L, Wang N, Ding R, Cui S, Zhu F, Xie Y, Sun X, Wu D, Hong Q, Li Q, Shi S, Liu X, Chen X: Mesenchymal stem cells attenuate ischemic acute kidney injury by inducing regulatory T cells through splenocyte interactions. Kidney Int 84: 521–531, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGaha TL, Chen Y, Ravishankar B, van Rooijen N, Karlsson MC: Marginal zone macrophages suppress innate and adaptive immunity to apoptotic cells in the spleen. Blood 117: 5403–5412, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Ravishankar B, Shinde R, Liu H, Chaudhary K, Bradley J, Lemos HP, Chandler P, Tanaka M, Munn DH, Mellor AL, McGaha TL: Marginal zone CD169+ macrophages coordinate apoptotic cell-driven cellular recruitment and tolerance. Proc Natl Acad Sci U S A 111: 4215–4220, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinsey GR, Huang L, Vergis AL, Li L, Okusa MD: Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney. Kidney Int 77: 771–780, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okusa MD, Molitoris BA, Palevsky PM, Chinchilli VM, Liu KD, Cheung AK, Weisbord SD, Faubel S, Kellum JA, Wald R, Chertow GM, Levin A, Waikar SS, Murray PT, Parikh CR, Shaw AD, Go AS, Chawla LS, Kaufman JS, Devarajan P, Toto RM, Hsu CY, Greene TH, Mehta RL, Stokes JB, Thompson AM, Thompson BT, Westenfelder CS, Tumlin JA, Warnock DG, Shah SV, Xie Y, Duggan EG, Kimmel PL, Star RA: Design of clinical trials in acute kidney injury: A report from an NIDDK workshop–prevention trials. Clin J Am Soc Nephrol 7: 851–855, 2012 [DOI] [PubMed] [Google Scholar]
- 34.McMurchy AN, Bushell A, Levings MK, Wood KJ: Moving to tolerance: Clinical application of T regulatory cells. Semin Immunol 23: 304–313, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosborough BR, Raïch-Regué D, Turnquist HR, Thomson AW: Regulatory myeloid cells in transplantation. Transplantation 97: 367–379, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gross CC, Wiendl H: Dendritic cell vaccination in autoimmune disease. Curr Opin Rheumatol 25: 268–274, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Hilkens CM, Isaacs JD, Thomson AW: Development of dendritic cell-based immunotherapy for autoimmunity. Int Rev Immunol 29: 156–183, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Raïch-Regué D, Glancy M, Thomson AW: Regulatory dendritic cell therapy: From rodents to clinical application. Immunol Lett 161: 216–221, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takuwa Y, Takuwa N, Sugimoto N: The Edg family G protein-coupled receptors for lysophospholipids: Their signaling properties and biological activities. J Biochem 131: 767–771, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Niessen F, Schaffner F, Furlan-Freguia C, Pawlinski R, Bhattacharjee G, Chun J, Derian CK, Andrade-Gordon P, Rosen H, Ruf W: Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature 452: 654–658, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, Ju ST, Okusa MD: Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol 20: 1744–1753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eggert AA, Schreurs MW, Boerman OC, Oyen WJ, de Boer AJ, Punt CJ, Figdor CG, Adema GJ: Biodistribution and vaccine efficiency of murine dendritic cells are dependent on the route of administration. Cancer Res 59: 3340–3345, 1999 [PubMed] [Google Scholar]
- 43.Pêche H, Trinité B, Martinet B, Cuturi MC: Prolongation of heart allograft survival by immature dendritic cells generated from recipient type bone marrow progenitors. Am J Transplant 5: 255–267, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Divito SJ, Shufesky WJ, Sumpter T, Wang H, Tkacheva OA, Wang W, Liu C, Larregina AT, Morelli AE: Dendritic cell therapies in transplantation revisited: Deletion of recipient DCs deters the effect of therapeutic DCs. Am J Transplant 12: 1398–1408, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Divito SJ, Wang Z, Shufesky WJ, Liu Q, Tkacheva OA, Montecalvo A, Erdos G, Larregina AT, Morelli AE: Endogenous dendritic cells mediate the effects of intravenously injected therapeutic immunosuppressive dendritic cells in transplantation. Blood 116: 2694–2705, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Probst HC, Tschannen K, Odermatt B, Schwendener R, Zinkernagel RM, Van Den Broek M: Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells. Clin Exp Immunol 141: 398–404, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ezzelarab MB, Zahorchak AF, Lu L, Morelli AE, Chalasani G, Demetris AJ, Lakkis FG, Wijkstrom M, Murase N, Humar A, Shapiro R, Cooper DK, Thomson AW: Regulatory dendritic cell infusion prolongs kidney allograft survival in nonhuman primates. Am J Transplant 13: 1989–2005, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, Defor T, Levine BL, June CH, Rubinstein P, McGlave PB, Blazar BR, Wagner JE: Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: Safety profile and detection kinetics. Blood 117: 1061–1070, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pasquet L, Douet JY, Sparwasser T, Romagnoli P, van Meerwijk JP: Long-term prevention of chronic allograft rejection by regulatory T-cell immunotherapy involves host Foxp3-expressing T cells. Blood 121: 4303–4310, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Erpicum P, Detry O, Weekers L, Bonvoisin C, Lechanteur C, Briquet A, Beguin Y, Krzesinski JM, Jouret F: Mesenchymal stromal cell therapy in conditions of renal ischaemia/reperfusion. Nephrol Dial Transplant 29: 1487–1493, 2014 [DOI] [PubMed] [Google Scholar]
- 51.Aichele P, Zinke J, Grode L, Schwendener RA, Kaufmann SH, Seiler P: Macrophages of the splenic marginal zone are essential for trapping of blood-borne particulate antigen but dispensable for induction of specific T cell responses. J Immunol 171: 1148–1155, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Martinez-Pomares L, Gordon S: CD169+ macrophages at the crossroads of antigen presentation. Trends Immunol 33: 66–70, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW: Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med 201: 1037–1044, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karasawa K, Asano K, Moriyama S, Ushiki M, Monya M, Iida M, Kuboki E, Yagita H, Uchida K, Nitta K, Tanaka M: Vascular-resident CD169-positive monocytes and macrophages control neutrophil accumulation in the kidney with ischemia-reperfusion injury. J Am Soc Nephrol 26: 896–906, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV: Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci U S A 105: 19571–19578, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fukao T, Matsuda S, Koyasu S: Synergistic effects of IL-4 and IL-18 on IL-12-dependent IFN-gamma production by dendritic cells. J Immunol 164: 64–71, 2000 [DOI] [PubMed] [Google Scholar]
- 57.Williams CA, Harry RA, McLeod JD: Apoptotic cells induce dendritic cell-mediated suppression via interferon-gamma-induced IDO. Immunology 124: 89–101, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barral P, Sánchez-Niño MD, van Rooijen N, Cerundolo V, Batista FD: The location of splenic NKT cells favours their rapid activation by blood-borne antigen. EMBO J 31: 2378–2390, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barral P, Polzella P, Bruckbauer A, van Rooijen N, Besra GS, Cerundolo V, Batista FD: CD169(+) macrophages present lipid antigens to mediate early activation of iNKT cells in lymph nodes. Nat Immunol 11: 303–312, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD: IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest 120: 331–342, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Awad AS, Ye H, Huang L, Li L, Foss FW, Jr., Macdonald TL, Lynch KR, Okusa MD: Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol 290: F1516–F1524, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Lutz MB, Kukutsch N, Ogilvie AL, Rössner S, Koch F, Romani N, Schuler G: An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods 223: 77–92, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.