Abstract
Nephrin belongs to a family of highly conserved proteins with a well characterized function as modulators of cell adhesion and guidance, and nephrin may have a role in metabolic pathways linked to podocyte and pancreatic β–cell survival. However, this role is incompletely characterized. In this study, we developed floxed nephrin mice for pancreatic β-cell–specific deletion of nephrin, which had no effect on islet size and glycemia. Nephrin deficiency, however, resulted in glucose intolerance in vivo and impaired glucose–stimulated insulin release ex vivo. Glucose intolerance was also observed in eight patients with nephrin mutations compared with three patients with other genetic forms of nephrotic syndrome or nine healthy controls. In vitro experiments were conducted to investigate if nephrin affects autocrine signaling through insulin receptor A (IRA) and B (IRB), which are both expressed in human podocytes and pancreatic islets. Coimmunoprecipitation of nephrin and IRB but not IRA was observed and required IR phosphorylation. Nephrin per se was sufficient to induce phosphorylation of p70S6K in an phosphatidylinositol 3-kinase–dependent but IR/Src-independent manner, which was not augmented by exogenous insulin. These results suggest a role for nephrin as an independent modulator of podocyte and pancreatic β–cell nutrient sensing in the fasting state and the potential of nephrin as a drug target in diabetes.
Keywords: nephrin, diabetes, metabolism, signaling
Nephrin (NP) and Neph1 (N1) are membrane proteins that were originally identified as components of an extracellular structure known as the slit diaphragm in podocytes.1–6 More recently, NP and several members of the Neph family were found to be expressed in pancreatic islets.7 Homophilic and heterophilic interactions between NP and N1 have been shown to affect the actin cytoskeleton and several signaling pathways.8,9 Among them, NP stimulates metabolic pathways linked to PI3K/Akt activity,10 a key pathway linked to podocyte survival11,12 as well as pancreatic β–cells survival.13–15 NP has several tyrosine residues in its cytosolic C–terminal domain that can be phosphorylated as a consequence of homophilic or heterophilic interactions16–18 and stimulate downstream signaling triggering endocytosis,19,20 actin remodeling,21–26 cell-cell adhesion,27 and calcium signaling.28 We have recently shown that glucose–induced NP phosphorylation of Y1176 and Y1193 tyrosine residues is essential to stimulate NP trafficking and insulin release in MIN6-C3 cells and human islets.29,30 Upon phosphorylation by Fyn,31 NP can form a complex with PI3K10 and Nck.21,23,24 Overexpression of NP in MIN6 cells results in increased total insulin content,29 but the mechanisms for such a phenomenon are yet to be uncovered. Interestingly, NP deficiency in podocytes leads to the impairment of insulin–stimulated glucose uptake,32 suggesting that NP may positively affect raft–dependent insulin signaling.33 In the pancreas, autocrine insulin signaling through insulin receptor A (IRA) leads to p70S6K–dependent insulin transcription, whereas insulin signaling through IRB augments Akt–dependent glucokinase transcription,34 thus facilitating pancreatic β–cell function in humans35 and rodents.36
NP expression has been described in both mouse and human pancreas,37,38 where NP regulates glucose–stimulated insulin release (GSIR) through Y1176 and Y1193 phosphorylation.30 Mutations in NP gene (NPHS1) cause congenital nephrotic syndrome.39 Although it does not result in diabetes, understanding its role in GSIR may lead to new drug discovery.
Here, we report that mice with a pancreatic-specific deletion of NP exhibit glucose intolerance and confirmed reduced GSIR in NP-deficient islets compared with the wild type. Additionally, patients with mutations in the NP gene are glucose intolerant compared with healthy and proteinuric controls. In vitro experiments showed that podocytes and pancreatic islets express both IRA and IRB and that NP interacts preferentially with the phosphorylated form of IRB. Although this interaction does not affect insulin–stimulated and –unstimulated IRB signaling, it negatively affects the ability of insulin to phosphorylate p70S6K. In the absence of insulin, NP upregulates p70S6K phosphorylation in an IR/Src-independent manner and a PI3K/Akt/mammalian target of rapamycin (mTOR) –dependent manner. NP deficiency does not affect GLUT2 abundance or pancreatic β–cell mass but is associated with decreased glucokinase expression. Therefore, therapeutic strategies that preserve NP expression in diabetes may protect both pancreatic β-cells and podocytes.
Results
Inborn NP Deficiency Does Not Affect Baseline Glucose Metabolism
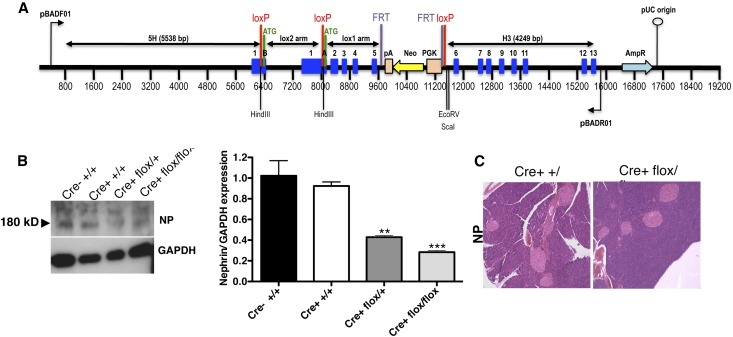
To determine the specific role of NP in the regulation of insulin secretion, we generated mice with floxed NP for the pancreatic-specific deletion of NP (Figure 1A). To verify that effective recombination of NP occurred, we used lysates from pancreatic islets of wild-type (Cre− +/+) mice, mice expressing Cre recombinase (Cre+ +/+) under the control of the rat insulin 2 promoter, and mice heterozygous and homozygous for NP deletion (Cre+ flox/+ and Cre+ flox/flox, respectively). A semiquantitative reduction to approximately 30% in NP expression was observed in NP (Cre+ flox/flox) mice compared with controls (Figure 1B). Pancreatic islet size and shape as examined on hematoxylin and eosin–stained tissue sections (Figure 1C) as well as body weight, random glycemia, and random plasma insulin levels were also not different across genotypes (Table 1). Deletion of NP did not affect islet size, number, and β-cell mass (Supplemental Figure 1) in NP Cre+ flox/flox mice compared with controls. NP expression remained unchanged in whole-brain lysates across genotypes (Supplemental Figure 1D). Given the fact that Cre+ +/+ mice have a metabolic defect per se,40 we used Cre+ +/+ as the appropriate control in subsequent experiments.
Figure 1.
NP conditional deletion strategy and efficacy. (A) Vector design for the conditional deletion of NP with loxP sites flanking exons 1B–5. (B) Representative WB analysis of NP expression and relative bar graph analysis of pancreatic islets purified and pooled from five different mice showing effective downregulation of NP. Data represent means±SEM (n=3 isolations from five mice each). **P<0.01; ***P<0.001 with ANOVA comparing Cre+ flox/flox with control. (C) Representative hematoxylin and eosin staining of sections from mouse pancreas showing normal architecture and islet size and shape in Cre+ flox/flox mice compared with Cre+ +/+ mice with the deletion of NP. Original magnification, ×20.
Table 1.
Metabolic profile of NP deficient mice
| NP | Cre− +/+ | Cre+ +/+ | Cre+ Flox/+ | Cre+ Flox/Flox |
|---|---|---|---|---|
| Body weight (g) | 23.0±0.0 | 19.0±0.0 | 20.0±1.4 | 25.0±2.1 |
| Glycemia (mg/dl) | 121±10.6 | 107.5±7.7 | 123±5.6 | 145.5±32 |
| Insulin (ng/ml) | 0.14±0.05 | 0.15±0.05 | 0.14±0.12 | 0.21±0.12 |
Body weight, random glycemia, and fasting plasma insulin in NP deficient 12-week-old littermates compared with their respective controls (n=5 per group).
Mice Deficient in NP Have Impaired GSIR
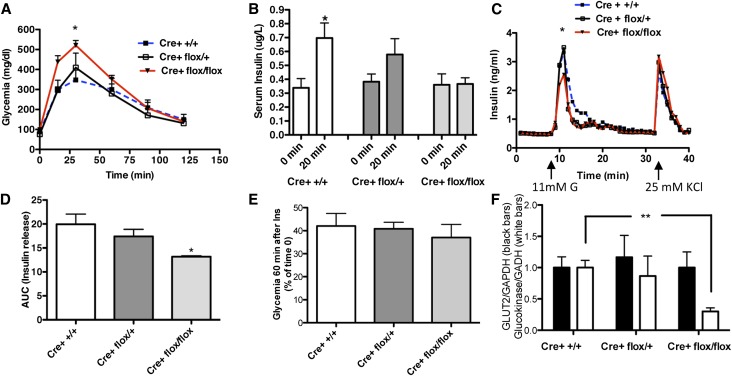
To determine if lower pancreatic expression levels of NP can affect GSIR, we performed an intraperitoneal glucose tolerance test (IPGTT) and showed that mice with pancreatic deletion of NP are glucose intolerant compared with Cre+ +/+ (Figure 2A). Glucose intolerance in NP-deficient mice was associated with an impairment of insulin release 20 minutes after glucose load (Figure 2B). Glucose intolerance in NP-deficient mice was not associated with altered insulin tolerance (Figure 2C). To further confirm that NP deficiency results in an impairment of GSIR, we performed perifusion experiments on islets isolated from mice with the different genotypes. After increases in glucose concentration from 3 to 11 mM, islets isolated from NP-deficient mice showed decreased insulin release compared with Cre+ flox/+ or Cre+ +/+ (Figure 2, D and E). However, the response to 25 mM KCl was similar in Cre+ flox/flox mice compared with Cre+ +/+ mice, suggesting that NP deficiency did not affect insulin content. Real–time PCR analysis of mRNA obtained from isolated islets showed an equal expression of GLUT2 accompanied by a reduced expression of glucokinase in NP-deficient islets versus control islets (Figure 2E), further suggesting that NP deficiency interferes with glucose sensing rather than insulin content. Together, these data indicate that, although pancreatic-specific deletion of NP is not lethal in mice and does not result in diabetes, NP-deficient islets do exhibit an inability to handle a glucose load compared with Cre+ +/+.
Figure 2.
β-Cell–specific deletion of NP results in glucose intolerance and lower GSIR. (A) Graphic representations of glycemia over time after intraperitoneal injection of 1.5 g/kg d-glucose in Cre+ +/+ (dotted blue line), Cre+ flox/+ (black line), and Cre+ flox/flox (red line) mice for the conditional deletion of NP. Experiments were repeated two times. *P<0.05 comparing Cre+ flox/flox with Cre+ +/+ (n=3–4 per group). (B) Bar graph analysis showing serum insulin levels before (t=0 minutes) and after (t=20 minutes) intraperitoneal d-glucose administration in Cre+ flox/flox compared with Cre+ flox/+ and Cre+ +/+ mice. Data represent means±SEM (n=3). *P<0.05 comparing 20 with 0 minutes in the same group. (C) Representative perifusion experiments and (D) relative bar graph analysis of AUC were performed in pooled islets isolated from Cre+ flox/flox; red line) compared with Cre+ flox/+ (black line) and Cre+ +/+ (dotted blue line) mice. *P<0.05 comparing Cre+ flox/flox with Cre+ +/+. (E) Insulin sensitivity was determined in Cre+ flox/flox compared with Cre+ flox/+ and Cre+ +/+ mice after intraperitoneal injection of short-acting insulin (2 milliunits per gram body weight); data are expressed as percentages of glycemia at time 60 minutes compared with time 0 minutes (n=5 across genotypes). (F) Bar graph analysis for GLUT2 and Glucokinase mRNA expression (normalized to GAPDH) in isolated islets from Cre+ flox/flox compared with Cre+ flox/+ and Cre+ +/+ mice (n=50). **P<0.01.
Pediatric Patients Carrying an NP Mutation Are Glucose Intolerant
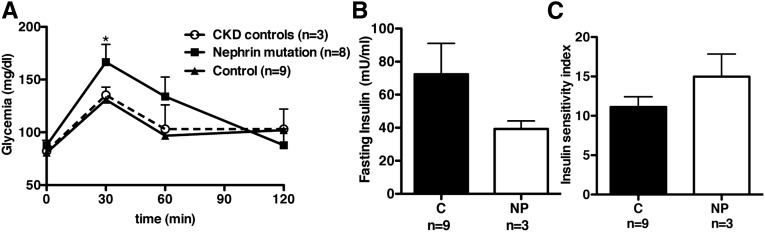
Mutations in the NP gene in humans lead to nephrotic syndrome of the Finnish Type (NPHS1).1 Oral glucose tolerance tests (OGTTs) performed in preparation of treatment with growth hormone were reviewed from eight patients born with mutations in the NP gene and compared with nine normal body mass index–matched patient controls. We also studied three patients with nephrotic syndrome of genetic origin caused, in two of three patients, by podocin mutation (NPHS2) as a proteinuric control with impaired GFR (CKD control). The specific mutations described in each of these patients are shown in Table 2, whereas patient characteristics are shown in Table 3. Similar to what we had observed in NP-deficient mice, patients with NP mutations are glucose intolerant compared with control patients or patients with podocin mutations (Figure 3A). This was accompanied with a trend to decreased fasting insulin levels in three of eight patients with NP mutations who had available fasting sera (Figure 3B) and unchanged insulin sensitivity assessed as described in Concise Methods (Figure 3C). Three years after the last patient was enrolled in this study, none of the patients had developed diabetes.
Table 2.
Genetic mutations in patients and CKD controls
| Patient ID | Age | Gender | Mutation Type |
|---|---|---|---|
| Patient 1 | 8.00 | Man | Compound heterozygote NP |
| Patient 2 | 6.00 | Woman | c2019C>A (h) N673K100 |
| Patient 3 | 15.00 | Woman | c.1019C>A (H) P340H |
| Patient 4 | 4.00 | Man | c.515_517del (H); p.T172del |
| Patient 5 | 2.00 | Woman | c.2339G>A (h);2928–3C>G (h), p.G796R; splicing of exon 22 |
| Patient 6 | 3.00 | Man | c.DelTCAinsCC2617 (H);2552C>T (H); p.L904x and A851V |
| Patient 7 | 3.00 | Woman | c.1699T>A (H); p.C567S |
| Patient 8 | 3.00 | Man | c.DelTCAinsCC2617 (H);2552C>T (H); p.L904x and A851V |
| CKD 1 | 6.00 | Woman | NPHS2: c.503G>A (H); p.503G>A (H) |
| CKD 2 | 4.00 | Man | No mutation in NPHS1/2 or WT1 |
| CKD 3 | 11.00 | Man | NPHS2: c.460_467insT (H); p.V165x |
Specific genetic mutations detected in each of eight patients carrying an NP mutation and patients with similar proteinuria and renal function impairment (CKD 1–3), two of which had podocin mutations, are shown.
Table 3.
Physiologic parameters evaluated in the patient cohorts
| Parameter | Control | NP Mutations | CKD Controls |
|---|---|---|---|
| n | 9 | 8 | 3 |
| Age (yr) | 11.88±3.79 | 11.75±3.65 | 17.33±7.37 |
| Sex (women/men) | 3/6 | 4/4 | 1/2 |
| Creatinine (mg/dl) | 0.44±0.10 | 2.89±2.25 | 6.25±2.98 |
| Weight (kg) | 41.65±15.40 | 15.66±7.06 | 18.1±7.29 |
| Height (cm) | 144.94±18.18 | 97.75±22.28 | 110.13±20.17 |
Clinical characteristics across the three different patient cohorts.
Figure 3.
Patients with nephropathy of the Finnish Type are glucose intolerant. (A) Graphic representation of OGTT performed after 1.75 g/kg body wt oral glucodex. Glycemia was measured before and up to 120 minutes after glucose load in eight patients with NP (NPHS1) mutations (squares) compared with nine healthy controls (triangles) and three patients (NPHS2) with similar proteinuria and renal function impairment (CKD controls; circles). Data represent means±SEM. *P<0.05 compared with controls. (B and C) Bar graph analysis of (B) fasting plasma insulin and (C) insulin sensitivity index in patients with NP mutations (NP; n=3) compared with healthy controls (C; n=9).
NP Interacts with IRB
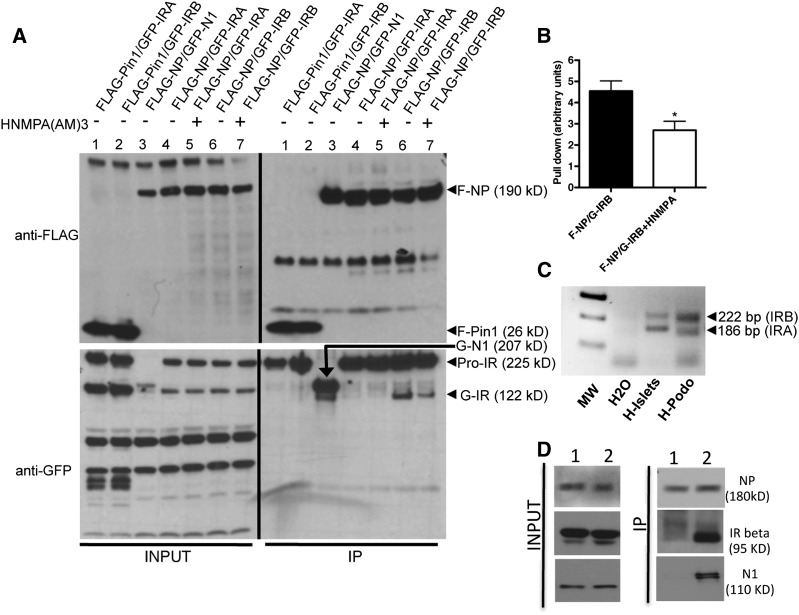
Given the observed metabolic effect of NP deficiency (Figures 2 and 3), the evidence that NP affects insulin signaling in podocytes,32 and the evidence that the two splicing variants of IRA and IRB have autocrine metabolic function in pancreatic β-cells,33,34,41,42 we investigated if NP interacts with IRA and/or IRB by immunoprecipitation (IP) experiments of exogenous proteins cotransfected in human embryonic kidney 293 (HEK293) cells. We showed that FLAG-NP (F-NP) can immunoprecipitate GFP-tagged IRB but not IRA (Figure 4A, lanes 4 and 6). F-NP and GFP-N1 cotransfection was used as a positive control (Figure 4A, lane 3), whereas FLAG-Pin1 (Figure 4A, lanes 1 and 2) cotransfected with IRA and IRB was used as a negative control. Although Pin1 immunoprecipitates the proform of IR as described43 (Figure 4A, lanes 1 and 2), NP immunoprecipitated the proform of IR but also, the mature 130- to 135-kD IR α-subunit (Figure 4A, lanes 6 and 7).44 Pretreatment with the IR phosphorylation inhibitor hydroxy-2-naphtalenylmethylphosphonic acid (HNMPA) partially inhibited the interaction between NP and IRB (Figure 4, A, lane 7 and B) without affecting NP phosphorylation at residues Y1176/1193 (Supplemental Figure 2). The NP-IR interaction did not require NP phosphorylation, because pretreatment with the Src inhibitor 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) did not affect the ability of NP to immunoprecipitate IR (Supplemental Figure 3A), although it effectively reduced Y1176/1193 NP phosphorylation (Supplemental Figure 3B). The interaction of NP and IRB may be relevant to both human islets and podocyte biology, because they both express IRA and IRB (Figure 4C). In fact, endogenous IP experiments in isolated mouse glomeruli showed that NP and IR interact in glomeruli (Figure 4D).
Figure 4.
NP immunoprecipitates IRB but not IRA. (A) Representative WB for FLAG and GFP from lysates (input) and IPs from HEK293 cells transfected with F-NP (lanes 3–7) and GFP-tagged IRA (G-IRA; lanes 4 and 5) or IRB (G-IRB; lanes 6 and 7). Negative (FLAG-Pin1; lanes 1 and 2) and positive (GFP-N1; lane 3) controls for IP were used. IP performed in the presence of an inhibitor of IR phosphorylation hydroxy-2-naphtalenylmethylphosphonic acid [HNMPA(AM)3], which was also used to preincubate cells for 60 minutes before lysate collection, is shown in lanes 5 and 7. (B) Bar graph analysis showing decreased ability of F-NP to pull down G-IRB when HNMPA(AM)3 was used. Data represent pull-down means±SEM (n=3). *P<0.05 comparing HNMPA(AM)3–treated with –untreated F-NP–transfected cells. (C) Representative PCR analysis of IRA (186 bp) and IRB (222 bp) expression in humans islets (H-Islets) and human podocytes (H-Podo). (D) Lysates were obtained from pooled isolated glomeruli from three mice, and WBs for NP, IR β-subunit, and N1 were performed (Input). Eluates from endogenous IP using (1) an irrelevant IgG or (2) an mAb against the IgG8-like domain of NP showed NP interaction with IR. Interaction with N1 was used as a positive control.
Figure 7.
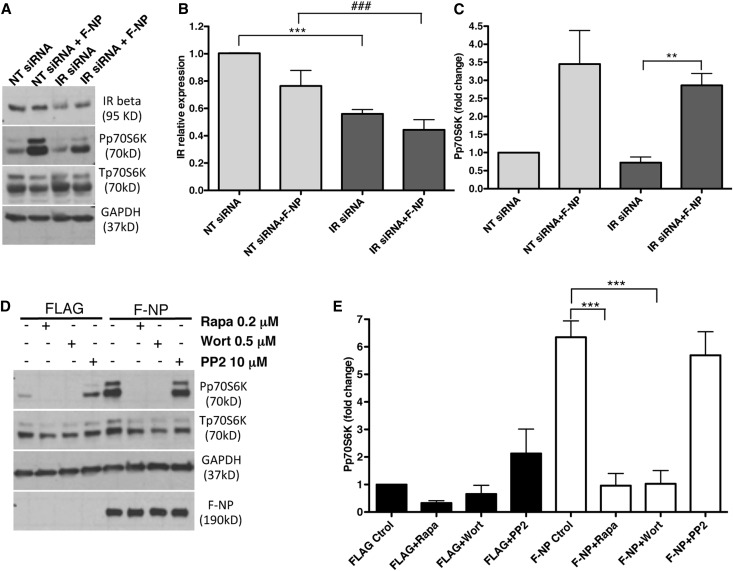
NP ability to induce p70S6K phosphorylation through the PI3K/Akt/mTOR pathway is independent of IR. (A) Representative WB for IR, p70S6K (phospho and total), and GAPDH expression in lysates obtained from F-NP–transfected HEK293 cells that were cotransfected with either control nontargeting (NT siRNA) siRNA or human IR–specific siRNA (IR siRNA). NT siRNA and IR siRNA alone served as controls. (B) Bar graph analysis of IR β-subunit expression levels in IR siRNA–transfected cells compared with NT siRNA–transfected control cells. Data represent means±SEM (n=5). ***P<0.001 comparing IR siRNA with NT siRNA; ###P<0.001 comparing IR siRNA with NT siRNA in F-NP–cotransfected cells. (C) Bar graph analysis of Pp70S6K levels in IR siRNA–transfected cells compared with NT siRNA–transfected control cells. Data represent means±SEM (n=5). Each value was corrected by the value of total protein (p70S6K) and GAPDH. **P<0.01 comparing IR siRNA with NT siRNA in F-NP–cotransfected cells. (D) Representative WB for p70S6K (phospho and total), GAPDH, and F-NP expression in lysates obtained from F-NP– or FLAG alone (FLAG-Ctrol)–transfected cells that were preincubated for 30 minutes with the mTOR inhibitor rapamycin (Rapa), the PI3K inhibitor wortmannin (Wort), or the Src inhibitor PP2. (E) Bar graph analysis of Pp70S6K levels comparing FLAG- and F-NP–transfected cells treated with the different inhibitors. Data represent means±SEM (n=3). Each value was corrected by the value of total protein (p70S6K) and GAPDH. ***P<0.001 comparing F-NP + Rapa and F-NP + Wort with control.
IR Signaling through Akt and p70S6K Is Differentially Affected by NP
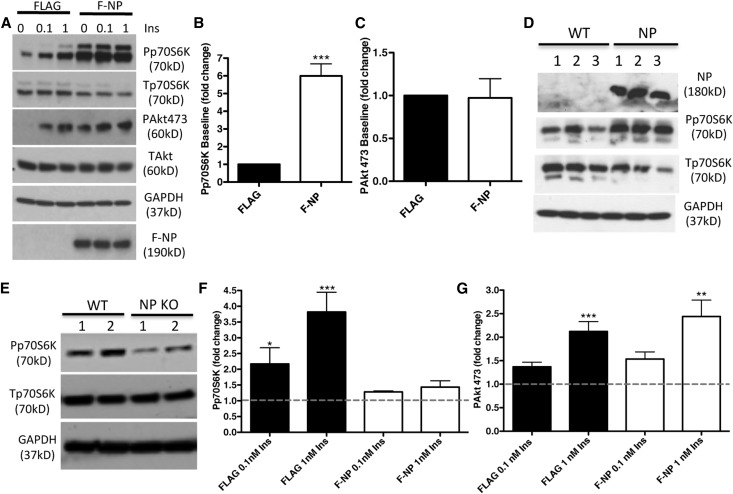
We next investigated whether overexpression of F-NP can affect phosphorylation of IR downstream targets Akt and p70S6K. Transfection of F-NP was sufficient to increase the phosphorylation of p70S6K in the absence of insulin compared with the FLAG alone control (Figure 5, A and B). F-NP did not affect the phosphorylation of Akt at S473 (Figure 5C). Consistent to what was shown in podocytes,10 NP–transfected MIN6 cells also showed increased phosphorylation of p70S6K (Figure 5D). In addition, islets isolated from NP-deficient mice showed decreased p70S6K (Figure 5E). To establish if transfection of F-NP further augments p70S6K and Akt phosphorylation in response to insulin, transfected cells were stimulated with 0, 0.1, and 1 nM insulin for 20 minutes. Although NP overexpression impaired the ability of insulin to further phosphorylate p70S6K (Figure 5F), the effect on Akt phosphorylation was preserved (Figure 5G).
Figure 5.
NP overexpression is sufficient to augment p70S6K phosphorylation. (A) Representative WB of lysates from F-NP– and FLAG–transfected HEK293 cells at baseline and after 20 minutes of insulin stimulation at indicated concentrations. Both phosphorylated (P) and total (T) Akt and p70S6K were studied. (B and C) Bar graph analyses of (B) Pp70S6K and (C) PAkt473 in F-NP–transfected cells compared with FLAG-transfected cells. (D) WB analysis of MIN6 cells transfected with an empty vector (wild-type [WT] clones 1–3) or NP (NP clones 1–3) showing increased phosphorylated p70S6K in NP cells compared with WT cells. (E) WB analysis of Pp70S6K and Tp70S6K performed in two different pools of islets (1 and 2; n=6 each) isolated from WT and NP knockout (KO) mice. (F and G) Bar graph analysis of (F) Pp70S6K and (G) PAkt473 in F-NP–transfected cells compared with FLAG-transfected cells exposed to exogenous insulin (0.1 and 1 nM Ins). Each value was corrected by the value of total protein (Akt or p70S6K) and GAPDH. Data represent means±SEM (n=3). Dotted lines represent baselines. *P<0.05; **P<0.01; ***P<0.001 compared insulin-treated with noninsulin controls.
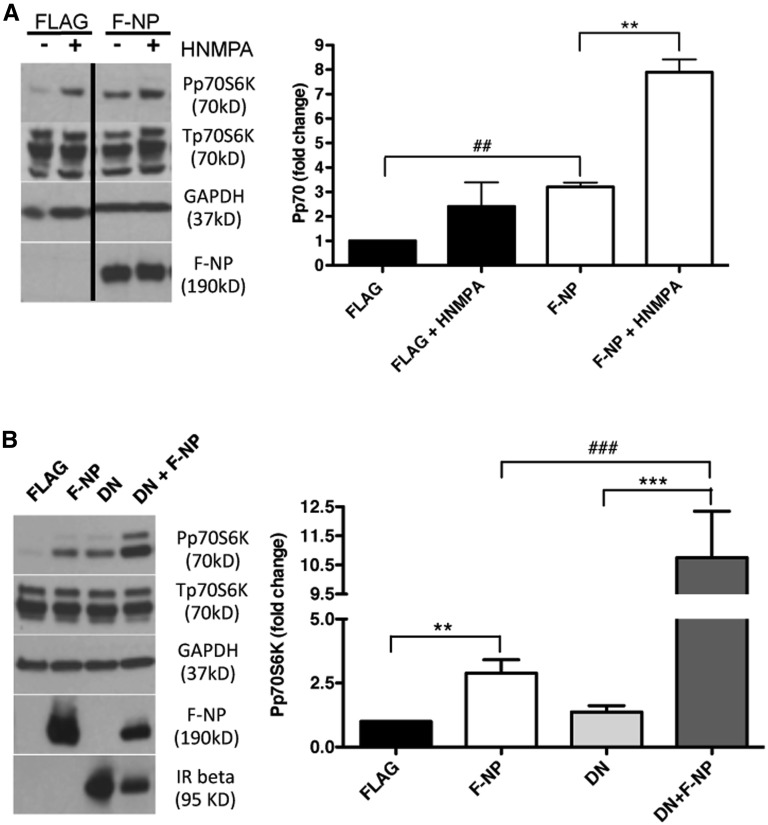
NP Induction of p70S6K Phosphorylation Is Augmented When IR Phosphorylation and Activity Are Inhibited
To determine if the phosphorylation and kinase activity of IR is required for NP to augment p70S6K phosphorylation, we overexpressed F-NP in HEK293 cells in the presence of the IR inhibitor HMNPA for 1 hour. Although the expected increase in p70S6K phosphorylation occurred in NP-transfected cells compared with FLAG-transfected cells, HMNPA treatment of F-NP–transfected cells further augmented p70S6K phosphorylation (Figure 6A), suggesting that the binding of IRB to NP may inhibit NP signaling. To support this hypothesis, we overexpressed a kinase inactive form of IR (dominant negative [DN] IR)45 in F-NP– or FLAG alone–transfected cells. Overexpression DN IR in NP-transfected cells resulted in an even stronger increase in p70S6K phosphorylation (Figure 6B), suggesting that phosphorylation–dependent IRB NP signaling may be inhibitory in nature. To further investigate whether the presence of IR per se influences NP induction of p70S6K phosphorylation, IR expression was downregulated with siRNA (Figure 7, A and B). Phosphorylation of p70S6K in F-NP–transfected cells was conserved, despite significant reduction of IR expression (Figure 7, A and C).
Figure 6.
Baseline p70S6K phosphorylation is independent of IR activity in NP–transfected HEK293 cells. (A) Representative WB of FLAG– or F-NP–transfected HEK293 cells preincubated 60 minutes with 10 μM IR Tyrosine Kinase inhibitor hydroxy-2-naphtalenylmethylphosphonic acid [HNMPA(AM)3] at baseline (left panel). Bar graph analysis of Pp70S6K expression comparing FLAG- and F-NP–transfected cells preincubated with HNMPA(AM)3 with their respective controls (right panel). Each value was corrected by the value of total protein (Tp70S6K) and GAPDH. Data represent means±SEM (n=3). **P<0.01 comparing F-NP + HNMPA with F-NP; #P<0.01 comparing F-NP with FLAG alone. (B) Representative WB from HEK293 cells transfected with human DN IR and control FLAG plasmids at baseline (left panel). DN transfection efficiency was determined by WB for IR β-subunit. Bar graph analysis of Pp70S6K levels at baseline comparing F-NP– and DN-cotransfected cells with their respective controls (right panel). Each value was corrected by the value of total protein (p70S6K) and GAPDH. Data represent means±SEM (n=3). **P<0.01 comparing F-NP with FLAG; ***P<0.001 comparing DN + F-NP with DN; ###P<0.001 comparing DN + F-NP with F-NP.
NP Phosphorylation Is Not Required for p70S6K Phosphorylation
Because NP can activate PI3K,10 an upstream modulator of mTOR and p70S6K signaling,46 we investigated if the ability of NP to augment p70S6K phosphorylation was mediated by PI3K and involved mTOR and Src kinases. Although the PI3K inhibitor wortmannin and the mTORC1 inhibitor rapamycin abolished the ability of NP to increase p70S6K phosphorylation, inhibition of Src kinase activity with PP2 had no effect on p70S6K (Figure 7, D and E) and IRB binding (Supplemental Figure 3A), despite successful reduction in NP phosphorylation (Supplemental Figure 3B).22,31 To further support the fact that the effect of NP on p70S6K is NP phosphorylation independent, we compared p70S6K phosphorylation between F-NP– and triple mutant (Y1176F/1193F/Y1217F) F-NP–transfected cells and showed that p70S6K phosphorylation is augmented when NP is unphosphorylated (Supplemental Figure 4). Use of a different PI3K inhibitor (LY294002) led to a similar reduction in NP–induced p70S6K phosphorylation (Supplemental Figure 5). However, use of a specific mTORC2 inhibitor 2-(4-amino-1-isopropyl-1H-pyrazolo[3,4-d]pyrimidin-3-yl)-1H-indol-5-ol (PP 242) resulted in only partial suppression of NP–induced p70S6K phosphorylation (Supplemental Figure 5), suggesting that mTORC1 is primarily involved in NP-dependent signaling.
Discussion
The study is innovative for three reasons. First, we showed that newly generated mice with the conditional deletion of NP in pancreatic β-cells have impaired glucose–stimulated insulin secretion. Second, we studied a small cohort of pediatric patients with nephropathy of the Finnish type (NPHS1) and showed that clinical NP deficiency is associated with glucose intolerance and normal insulin sensitivity. Third, we performed experimental in vitro studies to show that NP is not only a master regulator of cell-cell adhesion and guidance in podocytes but also, affects insulin signaling, even in the absence of IR.
More recently, a metabolic function of NP has emerged. In podocytes, NP can signal through PI3K and Akt,10 which could eventually lead to p70S6K phosphorylation. Furthermore, NP is required for insulin–stimulated glucose uptake in podocytes through regulation of GLUT4 trafficking,32 a function that is disposable in pancreatic β-cells. Because we have previously shown that NP augments insulin synthesis and glucose–stimulated insulin secretion by pancreatic β-cells29,30 and that the two isoforms of the IR (IRA and IRB) are differentially involved in autocrine insulin signaling,34,36 we investigated if NP may predispose to diabetes and differentially affect IRA- and IRB-dependent signaling. Despite a >70% decreased expression of NP in pancreatic β-cells from NP-deficient mice (Figure 1), NP-deficient mice had normal islet architecture, distribution size, and β-cell mass (Supplemental Figure 1), and fasting insulin and glucose were identical to control mice (Table 1). After glucose stimulation, however, NP-deficient mice consistently showed a mild glucose–intolerant phenotype (Figure 2, A and B), which was proven to occur centrally, because insulin secretion was suppressed in NP-deficient mice 20 minutes after glucose load and insulin sensitivity was unchanged (Figure 2, C and D). Perifusion experiments in isolated islets confirmed impaired GSIR in NP-deficient islets with conserved secretion in response to KCl, suggesting that NP deficiency affects glucose sensitivity rather than insulin production. (Figure 2E). The reduced glucose sensitivity observed in NP-deficient islets is unrelated to the amount of GLUT2 transporters expressed in NP-deficient islets but may result from the suppression of glucokinase expression (Figure 2F). We also studied eight pediatric patients carrying different NP mutations and confirmed that NP deficiency can also lead to glucose intolerance in humans (Figure 3A) without affecting peripheral insulin sensitivity (Figure 3, B and C) compared with nine healthy controls. Glucose intolerance was specific to patients who were NP deficient and was not related to CKD, because control patients with CKD were not glucose intolerant (Figure 3A).
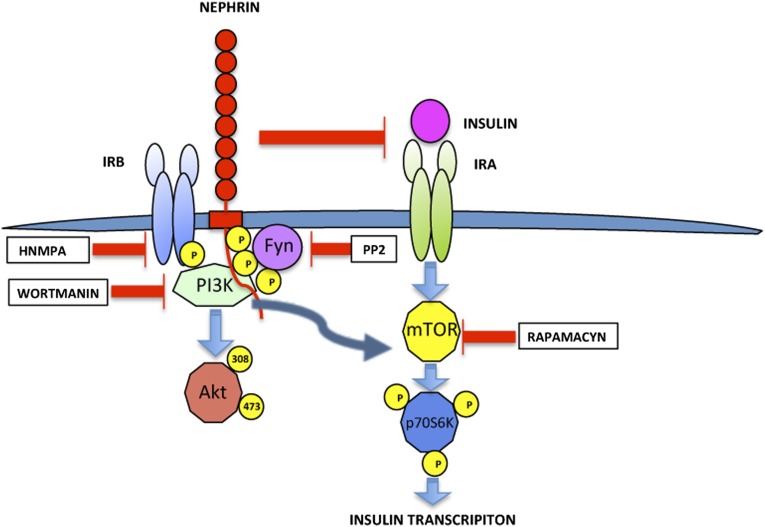
The mild phenotype of pancreatic β-cells–specific NP deficiency in mice and humans does not exclude the possibility suggested by our prior studies that increased NP expression and/or stimulation of NP signaling may lead to improved cell function and survival. In fact, although we have shown increased insulin content in NP-overexpressing cells,29 NP deficiency did not alter β-cell mass (Supplemental Figure 1) and resulted in decreased glucokinase expression in isolated islets (Figure 2F). We, therefore, investigated how NP may affect metabolic pathways that have been shown to be important for insulin and glucokinase gene transcription.34 The observation that NP stimulates the phosphorylation of p70S6K in both HEK- and MIN6-transfected cells (Figure 5) and the fact that this occurs independently of IR function and activity (Figure 6) are intriguing and raise the question as to why NP interacts with IRB when IRB is phosphorylated (Figure 4). One possibility is that IRB binding to NP may be a mechanism to modulate NP signaling through p70S6K. This is suggested by the fact that inhibitors of IR phosphorylation and transfection of DN IR augment the ability of NP to signal (Figure 6). It has been established that NP is phosphorylated by Fyn when activated by high glucose or protamine sulfate.16,47,48 In our model, Src inhibition by PP2 could partially inhibit NP tyrosine phosphorylation at Y1176/1193 without affecting the ability of NP to bind IRB (Supplemental Figure 3A) and cause p70S6K phosphorylation (Figure 7), suggesting that the phosphorylation status of NP does not interfere with IRB binding. Although we have shown that glucose–induced Y1176 and Y1193 phosphorylation leads to increased GSIR and insulin vesicle trafficking,29,30 the new observation that transfection of a triple mutant (Y1176F/1193F/Y1217F) F-NP resulted in increased p70S6K phosphorylation (Supplemental Figure 4) suggests that the pathways by which NP regulates GSIR and p70S6K may not always be linked. We propose a model where, in the fasting state, NP stimulates p70S6K signaling in an NP phosphorylation– and IR–independent manner, leading to increased insulin transcription in preparation of the next round of glucose stimulation. IRB phosphorylation by a glucose load will cause NP and IRB interaction, suppress NP signaling, and allow physiologic insulin signaling. NP interaction with phosphorylated IRB after glucose stimulation results in suppression of NP signaling without affecting insulin’s ability to signal through IRB and IRA (Figure 8).
Figure 8.
Proposed model for NP signaling. Schematic representation of the proposed model for NP signaling. NP interacts with IRB. This interaction does not require NP phosphorylation but is partially dependent on IR phosphorylation. NP interaction with IRB does not interfere with the ability of insulin to stimulate Akt phosphorylation and does not affect the degree of Akt phosphorylation at baseline. On the contrary, NP prevents insulin ability to stimulate phosphorylation of p70S6K and in the absence of insulin, acts itself as a strong inducer of p70S6K phosphorylation through the PI3K/Akt/mTOR pathway in a way that is totally independent of IR phosphorylation, activity, and protein expression. Decreased IR phosphorylation and kinase activity augment NP signaling, suggesting that binding of IRB to NP may inhibit NP signaling. Decreased NP phosphorylation further augments NP signaling through p70S6K. It is, therefore, possible that, during starvation, NP independently contributes to stimulate p70S6K–dependent insulin transcription in preparation of the next round of glucose exposure.
Our study has several limitations, including the fact that a limited number of patients were studied and that most of the in vitro mechanistic studies were done in transfected HEK293 cells. We conclude that, although NP deficiency may not result in diabetes, strategies that augment NP expression and/or signaling may lead to a combined protection of pancreatic β-cells and podocytes.
Concise Methods
Experimental Design
In vivo experiments in newly developed genetically modified mice were conducted to determine how pancreatic β-cell–specific NP deficiency affects islet cell function. A small subset of patients with NP deficiency was also studied to suggest a role of NP in the modulation of β-cell function in humans. Finally, in vitro experiments were performed to study the influence of NP overexpression on cell signaling as well as the ability of NP to immunoprecipitate key elements of the insulin signaling cascade.
Strategy to Generate Floxed NP for the Pancreatic-Specific Deletion of NP
All animal studies were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. We have generated an NP floxed mouse with loxP sites flanking exons 1B–5 with Ozgene (Bentley, Western Australia, Australia). Targeting vector included three loxP sites: one in the 5′-UTR of exon 1B, one in the 5′-UTR of exon 1A, and one flanking the PGK–neo selection cassette downstream of exon 5 (JAX Stock No. 027824). The complete targeting vector was then screened by sequencing and restriction enzymes digestion. NP-floxed/ΔNEO mice were bred with homozygous B6.Cg-Tg(Ins2-Cre)25Mgn/J, which expresses CRE recombinase under the control of the rat insulin 2 promoter to generate pancreatic β-cell–specific NP–deficient mice. To test the effective homologous recombination, Western blots (WBs) of lysates from purified islets were performed. NP and N1 protein expression levels were quantified in Cre+ flox/flox mice, Cre+ +/+ mice, and Cre− +/+ mice. WBs of lysates from purified islets pooled from five mice each and obtained from three different sets of mice were performed. Mouse pancreas sections were stained with hematoxylin and eosin to evaluate islet size and morphology in Cre+ flox/flox mice compared with Cre+ +/+ NP mice. The number of islet equivalents yields from each isolation was also compared among groups, and islet size distribution was quantified by light microscopy on purified islets. β-Cell mass was determined by point counting morphometry as previously described.49 Ninety points per section were analyzed. The β–cell relative volume was calculated by dividing the number of points over β-cells by the number of points over the total pancreatic tissue. β-Cell mass was determined by multiplying the relative volume by the total weight of the pancreas.
IPGTT, Insulin Tolerance, and Perifusions Assays
Mice bred in our facility were used for in vivo and ex vivo assays. Blood samples were taken from the tail vein for the determination of fasting serum insulin content after 12 hours of fasting and during the IPGTT. For the IPGTT, a sterile glucose solution (1.5 g/kg=15% solution) was injected, and glycemia was measured by a glucometer (ONETOUCH Ultra). For insulin sensitivity determination, glycemia was evaluated before and after intraperitoneal injection of short-acting insulin (2 milliunits per 1 g body weight) as we have described.50 For perifusion assays, 50 isolated islets cultured overnight in CMRL-1066 (Invitrogen) were loaded on columns connected to an inflow port and an outflow port of a customized perifusion system (Biorep, Miami, FL). Islets were perifused with media of defined composition (3 mmol/L glucose, 11 mmol/L glucose, 3 mmol/L glucose, and 25 mmol/L KCl), and samples were collected every 1 minute for insulin determination, which was performed using the Mouse Insulin ELISA Kit (Mercodia). For perifusion assay normalization, DNA from isolated islets was extracted and quantified. Briefly, islets/beads from the columns after perifusion assays were collected in T-PER tissue protein extraction reagent (Thermo Fisher Scientific). Samples were vortexed and centrifuged. Supernatants were used for DNA measurement and compared with DNA standards using the Pico-Green Kit (Invitrogen) with fluorescence quantified at 480 nm excitation and 520 nm emission.
Real-Time PCR
mRNA was isolated from 500 islet equivalents isolated from NP Cre+ flox/flox mice, Cre+ +/+ mice, and Cre− +/+ mice with the RNAeasy Extraction Kit (Qiagen, Valencia, CA); 1 μg RNA was reverse transcribed to obtain cDNA, and PCR assays were performed to measure GLUT2, glucokinase, and GAPDH using the ABI Prism 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA). Data are expressed as fold change expression compared with Cre+ +/+ controls. Amplification of IRA and IRB subtypes from human islets and podocytes was performed using a forward primer in exon 10 (TGAGGATTACCTGCACAACG) and a reverse primer in exon 12 (CGACTCCTTGTTCACCACCT), yielding a 222-bp product for IRB and a 186-variant product for IRA.
Patient Data Collection
We studied eight children ages 2 months old to 15 years old with documented positive mutational analysis for NPHS1 (National Center for Biotechnology Information accession no. NG_013356.1) undergoing a routine OGTT performed before initiation of treatment with recombinant growth hormone as a standard of care. Nine body mass index–matched controls as well as three patients with proteinuria and altered GFR carrying mutations other than NPHS1 were used as controls (ages 4 months old to 18 years old). All patients were treated at the University Children’s Hospitals of Essen, Berlin, and Hamburg in Germany. All 20 patients underwent an OGTT. OGTT was carried out with 1.75 g/kg body wt glucodex given, and insulin and glucose were measured from blood drawn at 0, 30, 60, 90, and 120 minutes. The quantitative insulin sensitivity check index was derived using the inverse of the sum of the logarithms of the fasting insulin and fasting glucose as described: 1/(log(fasting insulin in microunits per milliliter) + log(fasting glucose in milligrams per deciliter)).51 All studies were conducted in accordance with the Declaration of Helsinki.
Transfections and Cell Culture
A full–length human NP FLAG-pcDNA3 vector, NP FLAG mutant (Y1176F/1193F/Y1217F), N1-GFP-pcDNA6, N1-FLAG-pcDNA6, Pin1-pFLAGCMV5a, and Human IRA or B-GFP-pRC/CMV were used for overexpression in HEK293 cell line (ATCC) after transfection with FuGENE-6 (Promega). HIR2-pRT3 DN plasmid45 was purchased from Addgene. FLAG-pCDNA3 vector was used as a negative control. NP–transfected MIN6 cells were previously described.29 For IR knockdown, ON-TARGETplus SMARTpool siRNA and nontargeting siRNA were used (Thermo Fisher Scientific Inc.). HEK293 cells were grown until 60% confluence, transfected, and incubated in DMEM (10% FBS and 1% P/S; Gibco) for 24 or 48 hours. For insulin stimulation and IP assays, HEK293 cells were grown until 80% confluence and starved for 24 hours in DMEM (0.1% FBS and 1% P/S) before preincubation with different inhibitors and/or insulin stimulation for 20 minutes.
WB and IPs
For WB, a polyclonal guinea pig anti–NP antibody (C terminus; Fitzgerald Laboratories), monoclonal rabbit anti–Phospho-NP (Y1176/Y1193) antibody (Epitomics), polyclonal rabbit anti–Phospho-Akt (Ser473) or total Akt, polyclonal rabbit anti–Phospho-p70S6K (Thr389) or total p70S6K, polyclonal rabbit anti–IR β-subunit (Cell Signaling Technology, Beverly, MA), polyclonal rabbit anti–N1 developed against a peptide corresponding to amino acids 767–788, and monoclonal mouse anti–GAPDH (Calbiochem, Nottingham, UK) were used. IP was performed in HEK293 cells transfected with F-NP. FLAG-Pin1 served as the negative control. Cotransfections of F-NP with GFP-N1 were used as positive controls. Lysates collected in Triton buffer (50 mM Tris, pH 7.5, 150 mM NaCl, and 1% Triton) were immunoprecipitated with anti-FLAG M2 Affinity Gel (Sigma-Aldrich, St. Louis, MO); after a wash, the pellets were analyzed by standard SDS gel electrophoresis, and WB detection of FLAG (Sigma-Aldrich) and GFP (Clontech, Mountain View, CA) was performed. Bands were analyzed with ImageJ software. IR inhibitor hydroxy-2-naphtalenylmethylphosphonic acid was purchased from Santa Cruz Biotechnology (Dallas, TX), LY204002 wortmanin and 2-(4-amino-1-isopropyl-1H-pyrazolo[3,4-d]pyrimidin-3-yl)-1H-indol-5-ol were purchased from Sigma-Aldrich, PP2 was purchased from Calbiochem (San Diego, CA), and rapamycin was purchased from InvivoGen (San Diego, CA). NP endogenous IP was performed on isolated glomeruli as described52 using an mAb against the IgG8-like domain of NP (50A9; gift of Karl Tryggvason, Karolinska Instittute, Stocholm, Sweden). Immunoprecipitates were stained for NP and IR as well as N1. N1 was detected using a newly developed rabbit anti–mouse antibody developed as described in Supplemental Material that was proven to be specific to N1.
Statistical Analyses
Data are expressed as means±SEM. A number of experiments (ranging between three and six) were used as specified for each experiment. When one-way ANOVA showed statistical significance, results were compared using t test after Tukey’s correction for multiple comparisons (Graph Pad Prism software). Statistical significance was set at P<0.05.
Special Considerations
All animal procedures were conducted under protocols approved by the Institutional Animal Care and Use Committee. The retrospective clinical data presented in Figure 3 and Tables 2 and 3 were obtained in accordance with the regulations from the institute where the data were generated and the German local ethical committees’ regulations. Written informed consent was received from participant’s families.
Disclosures
S.M., G.W.B., and A.F. are inventors on pending or issued patents aimed to diagnose or treat proteinuric renal diseases, and they stand to gain royalties from their future commercialization. A.F. is a consultant for Hoffman-La Roche, Genentech, Mesoblast, Abbvie, Boehringher Ingelheim, and Alexion on subject matters that are unrelated to this publication.
Supplementary Material
Acknowledgments
We thank Dr. Damaris Molano, Dr. Antonello Pileggi, Yalena Gadea, Elsie Zahr-Akrawi, and Maite Lopez from the Preclinical Cell Processing and Translational Models Program at the Diabetes Research Institute for their excellent technical assistance.
R.V. is supported by American Heart Association Grant 12POST11640007. S.M. and A.F. are supported by National Institutes of Health and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK090316, DK104753, and U24-DK076169. A.F. is also supported by National Institutes of Health and National Institute of Diabetes and Digestive and Kidney Diseases Grants U54-DK083912, UL1-TR000460, and UM1-DK100846; National Center for Advancing Translational Sciences Grant 1UL1-TR000460; the Diabetes Research Institute Foundation, and the Peggy and Harold Katz Family Foundation.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Nephrin Trafficking beyond the Kidney—Role in Glucose–Stimulated Insulin Secretion in β Cells,” on pages 965–968.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015020210/-/DCSupplemental.
References
- 1.Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestilä M, Jalanko H, Holmberg C, Tryggvason K: Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci U S A 96: 7962–7967, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, Mathur BN, Turner CA, Geske R, Montgomery CA, Starbuck M, Brandt M, Gupta A, Ramirez-Solis R, Zambrowicz BP, Powell DR: Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol 21: 4829–4836, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wartiovaara J, Ofverstedt LG, Khoshnoodi J, Zhang J, Mäkelä E, Sandin S, Ruotsalainen V, Cheng RH, Jalanko H, Skoglund U, Tryggvason K: Nephrin strands contribute to a porous slit diaphragm scaffold as revealed by electron tomography. J Clin Invest 114: 1475–1483, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Grahammer F, Schell C, Huber TB: The podocyte slit diaphragm--from a thin grey line to a complex signalling hub. Nat Rev Nephrol 9: 587–598, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Rinta-Valkama J, Palmén T, Lassila M, Holthöfer H: Podocyte-associated proteins FAT, alpha-actinin-4 and filtrin are expressed in Langerhans islets of the pancreas. Mol Cell Biochem 294: 117–125, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Patrakka J, Tryggvason K: Nephrin--a unique structural and signaling protein of the kidney filter. Trends Mol Med 13: 396–403, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P: Actin up: Regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 17: 428–437, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Huber TB, Hartleben B, Kim J, Schmidts M, Schermer B, Keil A, Egger L, Lecha RL, Borner C, Pavenstädt H, Shaw AS, Walz G, Benzing T: Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol 23: 4917–4928, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canaud G, Bienaimé F, Viau A, Treins C, Baron W, Nguyen C, Burtin M, Berissi S, Giannakakis K, Muda AO, Zschiedrich S, Huber TB, Friedlander G, Legendre C, Pontoglio M, Pende M, Terzi F: AKT2 is essential to maintain podocyte viability and function during chronic kidney disease. Nat Med 19: 1288–1296, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Tejada T, Catanuto P, Ijaz A, Santos JV, Xia X, Sanchez P, Sanabria N, Lenz O, Elliot SJ, Fornoni A: Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int 73: 1385–1393, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Aikin R, Rosenberg L, Maysinger D: Phosphatidylinositol 3-kinase signaling to Akt mediates survival in isolated canine islets of Langerhans. Biochem Biophys Res Commun 277: 455–461, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA: Islet beta cell expression of constitutively active Akt1/PKB alpha induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Invest 108: 1631–1638, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuttle RL, Gill NS, Pugh W, Lee JP, Koeberlein B, Furth EE, Polonsky KS, Naji A, Birnbaum MJ: Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat Med 7: 1133–1137, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Barletta GM, Kovari IA, Verma RK, Kerjaschki D, Holzman LB: Nephrin and Neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers. J Biol Chem 278: 19266–19271, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Heikkilä E, Ristola M, Havana M, Jones N, Holthöfer H, Lehtonen S: Trans-interaction of nephrin and Neph1/Neph3 induces cell adhesion that associates with decreased tyrosine phosphorylation of nephrin. Biochem J 435: 619–628, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Gerke P, Huber TB, Sellin L, Benzing T, Walz G: Homodimerization and heterodimerization of the glomerular podocyte proteins nephrin and NEPH1. J Am Soc Nephrol 14: 918–926, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Qin XS, Tsukaguchi H, Shono A, Yamamoto A, Kurihara H, Doi T: Phosphorylation of nephrin triggers its internalization by raft-mediated endocytosis. J Am Soc Nephrol 20: 2534–2545, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quack I, Rump LC, Gerke P, Walther I, Vinke T, Vonend O, Grunwald T, Sellin L: beta-Arrestin2 mediates nephrin endocytosis and impairs slit diaphragm integrity. Proc Natl Acad Sci U S A 103: 14110–14115, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, Huang H, Larose L, Li SS, Takano T, Quaggin SE, Pawson T: Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440: 818–823, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Li H, Lemay S, Aoudjit L, Kawachi H, Takano T: SRC-family kinase Fyn phosphorylates the cytoplasmic domain of nephrin and modulates its interaction with podocin. J Am Soc Nephrol 15: 3006–3015, 2004 [DOI] [PubMed] [Google Scholar]
- 23.New LA, Keyvani Chahi A, Jones N: Direct regulation of nephrin tyrosine phosphorylation by Nck adaptor proteins. J Biol Chem 288: 1500–1510, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB: Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest 116: 1346–1359, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sellin L, Huber TB, Gerke P, Quack I, Pavenstädt H, Walz G: NEPH1 defines a novel family of podocin interacting proteins. FASEB J 17: 115–117, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Garg P, Verma R, Nihalani D, Johnstone DB, Holzman LB: Neph1 cooperates with nephrin to transduce a signal that induces actin polymerization. Mol Cell Biol 27: 8698–8712, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khoshnoodi J, Sigmundsson K, Ofverstedt LG, Skoglund U, Obrink B, Wartiovaara J, Tryggvason K: Nephrin promotes cell-cell adhesion through homophilic interactions. Am J Pathol 163: 2337–2346, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harita Y, Kurihara H, Kosako H, Tezuka T, Sekine T, Igarashi T, Ohsawa I, Ohta S, Hattori S: Phosphorylation of nephrin triggers Ca2+ signaling by recruitment and activation of phospholipase C-gamma1. J Biol Chem 284: 8951–8962, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fornoni A, Jeon J, Varona Santos J, Cobianchi L, Jauregui A, Inverardi L, Mandic SA, Bark C, Johnson K, McNamara G, Pileggi A, Molano RD, Reiser J, Tryggvason K, Kerjaschki D, Berggren PO, Mundel P, Ricordi C: Nephrin is expressed on the surface of insulin vesicles and facilitates glucose-stimulated insulin release. Diabetes 59: 190–199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeon J, Leibiger I, Moede T, Walter B, Faul C, Maiguel D, Villarreal R, Guzman J, Berggren PO, Mundel P, Ricordi C, Merscher-Gomez S, Fornoni A: Dynamin-mediated Nephrin phosphorylation regulates glucose-stimulated insulin release in pancreatic beta cells. J Biol Chem 287: 28932–28942, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verma R, Wharram B, Kovari I, Kunkel R, Nihalani D, Wary KK, Wiggins RC, Killen P, Holzman LB: Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J Biol Chem 278: 20716–20723, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Coward RJ, Welsh GI, Koziell A, Hussain S, Lennon R, Ni L, Tavaré JM, Mathieson PW, Saleem MA: Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes 56: 1127–1135, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Uhles S, Moede T, Leibiger B, Berggren PO, Leibiger IB: Isoform-specific insulin receptor signaling involves different plasma membrane domains. J Cell Biol 163: 1327–1337, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leibiger B, Leibiger IB, Moede T, Kemper S, Kulkarni RN, Kahn CR, de Vargas LM, Berggren PO: Selective insulin signaling through A and B insulin receptors regulates transcription of insulin and glucokinase genes in pancreatic beta cells. Mol Cell 7: 559–570, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Bouche C, Lopez X, Fleischman A, Cypess AM, O’Shea S, Stefanovski D, Bergman RN, Rogatsky E, Stein DT, Kahn CR, Kulkarni RN, Goldfine AB: Insulin enhances glucose-stimulated insulin secretion in healthy humans. Proc Natl Acad Sci U S A 107: 4770–4775, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leibiger IB, Leibiger B, Berggren PO: Insulin feedback action on pancreatic beta-cell function. FEBS Lett 532: 1–6, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Putaala H, Soininen R, Kilpeläinen P, Wartiovaara J, Tryggvason K: The murine nephrin gene is specifically expressed in kidney, brain and pancreas: Inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet 10: 1–8, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Palmén T, Ahola H, Palgi J, Aaltonen P, Luimula P, Wang S, Jaakkola I, Knip M, Otonkoski T, Holthöfer H: Nephrin is expressed in the pancreatic beta cells. Diabetologia 44: 1274–1280, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Rapola J: Congenital nephrotic syndrome. Pediatr Nephrol 1: 441–446, 1987 [DOI] [PubMed] [Google Scholar]
- 40.Lee JY, Ristow M, Lin X, White MF, Magnuson MA, Hennighausen L: RIP-Cre revisited, evidence for impairments of pancreatic beta-cell function. J Biol Chem 281: 2649–2653, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Seino S, Bell GI: Alternative splicing of human insulin receptor messenger RNA. Biochem Biophys Res Commun 159: 312–316, 1989 [DOI] [PubMed] [Google Scholar]
- 42.Seino S, Seino M, Nishi S, Bell GI: Structure of the human insulin receptor gene and characterization of its promoter. Proc Natl Acad Sci U S A 86: 114–118, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakatsu Y, Sakoda H, Kushiyama A, Zhang J, Ono H, Fujishiro M, Kikuchi T, Fukushima T, Yoneda M, Ohno H, Horike N, Kanna M, Tsuchiya Y, Kamata H, Nishimura F, Isobe T, Ogihara T, Katagiri H, Oka Y, Takahashi S, Kurihara H, Uchida T, Asano T: Peptidyl-prolyl cis/trans isomerase NIMA-interacting 1 associates with insulin receptor substrate-1 and enhances insulin actions and adipogenesis. J Biol Chem 286: 20812–20822, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siegel TW, Ganguly S, Jacobs S, Rosen OM, Rubin CS: Purification and properties of the human placental insulin receptor. J Biol Chem 256: 9266–9273, 1981 [PubMed] [Google Scholar]
- 45.Jacob KK, Whittaker J, Stanley FM: Insulin receptor tyrosine kinase activity and phosphorylation of tyrosines 1162 and 1163 are required for insulin-increased prolactin gene expression. Mol Cell Endocrinol 186: 7–16, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Dalle Pezze P, Sonntag AG, Thien A, Prentzell MT, Gödel M, Fischer S, Neumann-Haefelin E, Huber TB, Baumeister R, Shanley DP, Thedieck K: A dynamic network model of mTOR signaling reveals TSC-independent mTORC2 regulation. Sci Signal 5: ra25, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Agarwal R: Effects of statins on renal function. Am J Cardiol 97: 748–755, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Dor Y, Brown J, Martinez OI, Melton DA: Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429: 41–46, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Xu G, Stoffers DA, Habener JF, Bonner-Weir S: Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes 48: 2270–2276, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Ijaz A, Tejada T, Catanuto P, Xia X, Elliot SJ, Lenz O, Jauregui A, Saenz MO, Molano RD, Pileggi A, Ricordi C, Fornoni A: Inhibition of C-jun N-terminal kinase improves insulin sensitivity but worsens albuminuria in experimental diabetes. Kidney Int 75: 381–388, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ: Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85: 2402–2410, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Yoo TH, Pedigo CE, Guzman J, Correa-Medina M, Wei C, Villarreal R, Mitrofanova A, Leclercq F, Faul C, Li J, Kretzler M, Nelson RG, Lehto M, Forsblom C, Groop PH, Reiser J, Burke GW, Fornoni A, Merscher S: Sphingomyelinase-like phosphodiesterase 3b expression levels determine podocyte injury phenotypes in glomerular disease. J Am Soc Nephrol 26: 133–147, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.