Abstract
AKI remains a highly prevalent disease associated with poor short– and long–term outcomes and high costs. Although significant advances in our understanding of repair after AKI have been made over the last 5 years, this knowledge has not yet been translated into new AKI therapies. A consensus conference held by the Acute Dialysis Quality Initiative was convened in April of 2014 and reviewed new evidence on successful kidney repair to identify the most promising pathways that could be translated into new treatments. In this paper, we provide a summary of current knowledge regarding successful kidney repair and offer a framework for conceptualizing the therapeutic targeting that may facilitate this process. We outline gaps in knowledge and suggest a research agenda to more efficiently bring new discoveries regarding repair after AKI to the clinic.
Keywords: acute renal failure, cell biology and structure, cell survival, CKD, stem cell, interstitial fibrosis
AKI represents a serious complication in hospitalized patients. AKI is associated with increased intensive care unit (ICU) and hospital mortality as well as enhanced progression toward CKD. Indeed, some estimates hold that 20% of all patients on incident dialysis developed ESRD related to AKI.1 In patients >60 years of age, there is a 3- to 8-fold progressive and age–dependent increase in the frequency of AKI.2–4 The elderly also suffer increased rates of progression to CKD, ESRD, and death after an episode of AKI compared with younger patients.5 Because the number of United States citizens ages 65 years old or older will double over the next 20 years, the burden of AKI in this group will be substantial.
Ischemia-reperfusion injury, nephrotoxins, and sepsis are the main causes of AKI, sharing some pathogenic mechanisms of microvascular derangement and tubular epithelial cell dysfunction. The mechanisms of tissue repair after AKI are complex and involve epithelial, endothelial, stromal, and inflammatory cell types. This cellular complexity makes the task of measuring both injury and repair using biomarkers difficult. Despite this challenge, much has been learned concerning productive repair from AKI, and this knowledge can be used to guide therapeutic strategies aimed at accelerating this process. Here, we review very recent discoveries in this area and outline a framework for applying new basic discoveries for generation of novel AKI therapies.
Methods
The 13th Acute Dialysis Quality Initiative (ADQI) Consensus Conference on Therapeutic Targets of Human AKI held in Charlottesville, Virginia in April of 2014 (www.adqi.net) was attended by an international group of experts and focused on an objective scientific review of the current literature, developing a consensus of opinion, with evidence where possible, to distill current literature and articulate a research agenda to address important unanswered questions. Similar to other ADQI meetings, a modified Delphi approach was followed. Details of the methods can be found in the supplement of the introduction and summary by Okusa et al.6
Key Issues
How Can We Measure Kidney Repair in Human AKI?
Kidney repair is defined as restoration of kidney structure and recovery of renal function after AKI. Unfortunately, we do not have optimal biomarkers to evaluate kidney repair, except for changes in serum creatinine (Scr) that reflect alterations in GFR. However, Scr is considered a suboptimal GFR marker and an insensitive biomarker of AKI when both damage and functional changes occur together. Likewise, a decrease of Scr to normal range or baseline level does not necessarily reflect complete renal functional recovery or complete reconstitution of normal kidney structure.
The Goal: Is Complete Functional Repair Possible?
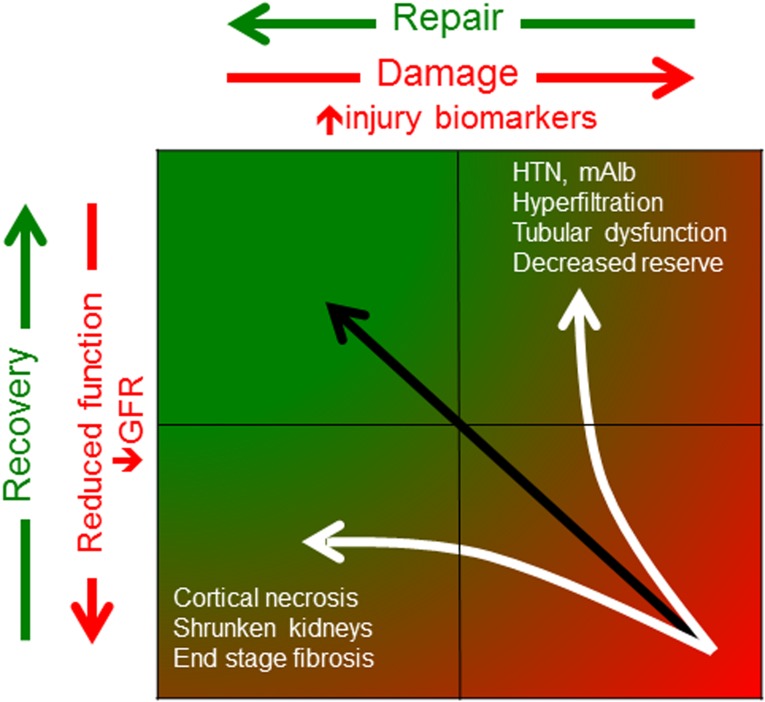
One way to summarize the goal after AKI is complete renal function repair (CFR), which incorporates restorations of renal perfusion, GFR, and tubular function. Whereas nearly all clinical studies now address only GFR, CFR encompasses a wider spectrum of renal functions, such as urinary concentrating ability, as well as histologic reconstitution, such as peritubular capillary density. Whether CFR actually occurs is not known at present. Even basic questions remain unanswered. What percentage of patients with AKI achieve CFR? Can patients benefit from CFR, and what are the consequences (e.g., increased morbidity and mortality) when repair is incomplete? Can therapeutic interventions improve CFR? To answer these questions, a panel of renal functional biomarkers is required to evaluate renal outcomes in clinical AKI studies. Figure 1 illustrates these concepts.
Figure 1.
Cartoon illustrating that productive repair is restoration of both structure and recovery of function. The upper left reflects a normal, uninjured kidney, which is characterized by the absence of injury biomarkers (x axis) and unimpaired GFR (y axis). An episode of AKI moves the patient to the lower right, which characterized by elevated injury biomarkers and reduced GFR. Complete functional repair occurs along the black line, and this is the process that should be targeted therapeutically. However, after an AKI episode, some patients may experience a normalization in injury biomarkers without restoration of GFR (lower left). This would occur in cortical necrosis or end stage kidneys, where an absence of live parenchyma explains the reduction in injury biomarkers rather than an absence of injury. In other cases, after AKI, some patients may recover GFR but have residual injury, which reflected by ongoing injury biomarker levels (upper right). These patients have structural kidney damage that may manifest as hypertension, microalbuminuria, tubular dysfunction, or decreased reserve, despite the apparently normal GFR. It will be critical to target repair along the black line rather than either white line (www.adqi.net). HTN, hypertension; mAlb, microalbuminuria.
Restoration of renal perfusion relies on normal renal blood flow (RBF) and restoration of microvascular function. There are several techniques now available to evaluate functional biomarkers of RBF and microvascular tissue oxygenation. Contrast-enhanced ultrasonography using gas-filled microbubbles as contrast agents has been used extensively to assess myocardial perfusion during echocardiography with accepted safety.7 Contrast-enhanced ultrasonography has been used to determine renal reserve in healthy volunteers, diagnose renal artery stenosis, detect perfusion abnormalities in kidney transplantation, and identify subclinical AKI in ICU settings.6,8 Blood oxygenation level–dependent magnetic resonance imaging is a rapid, noninvasive method for assessing renal tissue oxygen bioavailability, relying on the paramagnetic properties of deoxyhemoglobin. There have been studies using blood oxygenation level–dependent magnetic resonance imaging in experimental ischemic AKI and clinical settings, including allograft rejection compared with normal functional transplants and transplants with acute tubular necrosis.9–11 Other noninvasive tools that are potential candidates for assessing RBF and tissue oxygenation include positron emission tomography and optical spectroscopy,12,13 both of which need validation in additional clinical studies.
The clearance of an ideal filtration marker, such as inulin, iothalamate, or iohexol, remains the gold standard for measuring GFR but is very cumbersome, with limited use in clinical practice. Recently, an experimental fluorescence–based measuring assay has been developed for bedside instantaneous measurement of GFR, with excellent agreement with the concurrent iohexol–based GFR measurement. This new technique has potential applicability in AKI, whereas clinical studies are needed to validate its efficacy and safety in humans.14 The renal tubular compartment is the most commonly injured target in various settings of AKI. There are multiple markers available to evaluated tubular dysfunction, such as albuminuria, small molecular weight proteinuria, renal glycosuria, impaired tubular acidification, and decreased urinary concentrating function. However, very few studies have implemented these assays in assessing renal functional recovery after AKI.
In all, the renal functional biomarker panel should reflect RBF, kidney tissue oxygenation, accurately assessed GFR, and renal tubular function. This will require a combination of imaging, biomarker, and functional studies. Prospective studies are needed to define the optimal components in the panel and evaluate its use in assessing renal functional repair after AKI. Such studies should include the elderly, because the incidences of both AKI and failed repair are highest in this group.
A Need for Kidney Repair Biomarkers
A plethora of urinary biomarkers for early detection of kidney structural injury are currently under evaluation in AKI. By contrast, biomarkers that reflect kidney tissue repair are few. When the kidney recovers after epithelial cells are lost, the surviving cells dedifferentiate, migrate along the basement membrane, proliferate to restore cell number, and then, differentiate, resulting in restoration of the integrity of the nephron. In this case, one example of a urinary repair biomarker might be a protein that reflects epithelial proliferation. Exfoliated mitotic renal tubular epithelial cells in urine sediment are a simple marker reflecting ongoing proliferation of tubular cells in the injured kidney. In a recent study of patients at high risk for AKI undergoing cardiac surgery with cardiopulmonary bypass, an increase in the product of urinary tissue inhibitor of metalloproteinase-2 (TIMP-2) and IGF-7 (TIMP-2 × IGFBP7) values served as a sensitive and specific urinary biomarker to predict AKI early, whereas the decline in the urinary concentrations thereafter was found to be a strong predictor for renal recovery, which was defined as an Scr value at hospital discharge equal to or lower than that at baseline.15 Because both TIMP-2 and IGFBP7 are inducers of G1 cell cycle arrest, which is a mechanism involved in the early injury phase of AKI, the decline of urinary (TIMP-2 × IGFBP7) concentrations may imply an initiation of normal proliferative repair in response to the injury.
Other possible biomarkers for kidney repair include proteins expressed by tubular brush border, such as Type 2a Na-phosphate cotransporter (NaPiT2a). The apical expression of NaPiT2a by the proximal tubule was decreased in kidneys from septic mice, and restored in a regenerating stage associated with the recovery from renal hypoxia.16 Thus, the recovery of the urinary NaPiT2a levels, if detectable, would reflect the restoration of tubular brush border. Recently, proteomic analysis of urinary exosomes, which contain proteins from cellular membranes, as well as cytoplasmic and nuclear compartments provides biomarker candidates for the diagnosis of AKI. For example, urinary exosomal Fetuin-A was found to be increased in experimental and human AKI and served as a biomarker for structural renal injury.17 It is not known whether a normalized urinary exosomal Fetuin-A would be observed during repair.
With a number of kidney repair biomarkers under investigation, it is important to keep in mind that it will be difficult to distinguish whether an AKI therapy accelerates repair or if it limits acute injury. It is expected that a maneuver that reduces initial injury will result in more complete repair, simply because fewer nephrons must undergo repair in the first place. This issue can be addressed in the preclinical realm, because the time of injury is known, and therefore, the acute fall in GFR (initial injury) can be compared with the rate of GFR recovery at later time points (repair) to distinguish between a therapy that targets one or the other. In the clinical realm, the time of injury is often not known or may be ongoing, complicating efforts to define a time when injury ends and repair begins. Despite this challenge, we believe that repair markers hold promise in the AKI biomarker field.
Mechanisms of Normal Repair
The kidney has a remarkable capacity for repair and regeneration, which is evidenced by apparently complete recovery of function after AKI. Previous studies using animal models of repair after acute ischemic injury have shown that tubule proliferation occurs in the straight segment of the proximal tubule, where most of the damage occurs.18 The origin of the tubular cells that replenish the epithelial population after such damage is now becoming increasingly clear. Using chimeric mice with the donor bone marrow–expressing enhanced EGFP gene, Duffield et al.19 concluded that bone marrow–derived cells do not make a significant contribution to the restoration of epithelial integrity after an ischemic insult. A subsequent study by Humphreys et al.20 used genetic fate mapping and transgenic mice to label tubular epithelial cells or tubular interstitium, concluding that regeneration by surviving epithelial cells was the predominant mechanism of repair after ischemic injury.
In contrast, other investigators suggested the existence of a specific tubular cell subpopulation with high regenerative potential and resistance to apoptosis also called scattered tubular cells or proximal tubule rare cells.21–24 These works hypothesized that these cells might represent a committed intratubular stem cell population.21–24 To address this controversy, Kusaba et al.25 and Berger et al.26 have used transgenic mice to genetically label this scattered tubular cell population. Both groups independently concluded that scattered tubular cells do not represent a fixed progenitor population but rather, a phenotype that can be adopted by almost any proximal tubular cell on injury. Tubular cells seem to switch to a common injury response program characterized by graded expression of specific markers (scattered tubular cell phenotype). Thus, the field is now reaching consensus that surviving epithelial cells repopulate the tubule in a process of dedifferentiation.
AKI is accompanied by a sterile inflammatory response that contributes to the tubular cell damage.27 Intracellular molecules released from dying tubular cells, also called damage–associated molecular patterns, elicit immunostimulatory effects. Damage–associated molecular patterns also can activate a set of pattern recognition receptors, such as Toll-like receptors (TLRs), on renal parenchymal as well as in the interstitium.28 A recent study29 examined the role of TLR4 and IL-22 secretion in renal repair. TLR4 blockade during the early phase suppressed IL-22 production, but also, TLR4 blockade during the healing phase suppressed IL-22 production and impaired kidney regeneration. These studies suggest that TLR4 signaling drives epithelial regeneration of a postischemic kidney through secretion of IL-22 from mononuclear phagocytes, which links renal inflammation and regeneration at the level of TLR4.
The role of macrophages in renal repair is of particular interest, because they can exhibit distinctly different functional phenotypes broadly characterized as proinflammatory (M1 or classically activated) and tissue reparative (M2 or alternatively activated) phenotypes.30 Zhang et al.31 examined the role of resident renal macrophages/dendritic cells in repair after AKI. Genetic or pharmacologic inhibition of macrophage colony–stimulating factor 1 signaling blocked macrophage/dendritic cell proliferation, decreased M2 polarization, and inhibited recovery. These findings showed an important role for colony-stimulating factor 1–mediated expansion and polarization of resident renal macrophages/dendritic cells in renal tubule epithelial regeneration.
Recent discoveries have advanced our understanding of the role of cell cycle control in AKI repair. After the first 24 hours of ischemic injury, tubular cells undergo apoptotic and necrotic cell death. In response, many of the surviving, normally quiescent proximal tubule epithelial cells proliferate and enter the cell cycle.32 Proliferating cells are acutely sensitive to genotoxic stresses in the postischemic inflammatory environment. To proliferate, cells enter the cell cycle, sequentially activate cyclin-dependent kinases (Cdks) Cdk4/6 and Cdk2, and begin to synthesize checkpoint proteins, including p21.33 This cell cycle reentry after injury has been viewed as a protective response. Previous studies suggest that expression of the Cdk inhibitor p21 ameliorates the injury34 and that overexpression of p21 or the use of other Cdk inhibitors can protect against cisplatin and ischemic cell death.35,36 Therefore, transient expression of Cdk2 or Cdk4/6 inhibitors represents a novel strategy to improve renal repair and could provide protection against early tubular cell death but still allow for normal repopulation of injured tubules to undergo subsequent proliferation.
What Aspects of Repair Can Be Targeted Therapeutically?
Mesenchymal Stem Cells
Bone marrow–derived mesenchymal stem cells (MSCs) migrate to injured kidney in response to signals released from damaged tissues, including stromal-derived factor 1/CXCR and HGF/c-met axes,37,38 endothelium adhesion signals, such as VLA-4/VCAM-1,39 and matrix adhesion signals, such as CD44–dependent hyaluronic acid interaction.40 Several studies showed the engraftment of MSCs without a direct differentiation into mature tubular epithelial cells.40–42 MSCs can modulate the processes of dedifferentiation, migration, proliferation, and new redifferentiation of injured tubular cells to restore kidney homeostasis, mainly through paracrine mechanisms. The most compelling evidence for a paracrine mechanism for tissue repair comes from the demonstration that, in experimental models of AKI, intraperitoneal injection of conditioned medium from MSCs mimics the beneficial effects of intravenous injection of whole MSCs.43 MSCs release factors that promote tubular proliferation and trigger angiogenesis, such as bFGF, IL-6, HGF, TGF-β, EGF, stromal-derived factor 1, Angiopoietin-1, Epo, VEGF, and IGF. Knockdown of VEGF or IGF secretion decreases the protective effect of MSCs in experimental models of AKI.44,45 Other molecules released from MSCs (HLA-G5, PGE2, IL-10, NO, IDO, and HO-1) exert anti-inflammatory and immune-modulatory effects, such as inhibition of dendritic cell differentiation/activation, inhibition of CD4+/CD8+ T cell proliferation/cytokine release, reprogramming of macrophages toward an M2 phenotype,46 and stimulation of T regulatory cells.47,48
On the basis of these preclinical data, MSCs have been evaluated in clinical trials in patients with AKI with promising results (suprarenal aortic infusion in patients of cardiac surgery and induction therapy in patients of oncology with kidney transplantation subjected to cisplatin chemotherapy).48–50 Although no serious adverse effects have been reported in trials,51 a note of caution rose from experimental studies, in which the possibility of MSC maldifferentiation, tumorigenesis, and overimmunodepression has been reported.52–54 Three main aspects of MSC therapy for treating AKI should be better elucidated in the next years: (1) evaluation of possible long–term adverse effects, (2) comparison of regenerative effects of bone marrow–derived MSCs with MSCs isolated from other sources (adipose tissues, cord blood, etc.), and (3) identification of specific paracrine mediators released from MSCs involved in kidney regeneration. In this setting, recent studies suggested a possible role of MSC–derived extracellular vesicles, which are able to influence the biologic behavior of neighboring cells through the direct transfer of proteins and genetic material. MSC–derived extracellular vesicles are able to induce renal repair in experimental models of AKI through the horizontal transfer of mRNA and microRNA, synergizing with known growth factors.55–57
Other Therapeutic Targets
Tissue repair and limitation of damage after AKI are correlated with tubular cell proliferation and triggering of angiogenesis of endothelial cells. Recent studies have identified new potential therapeutic targets in ischemia-reperfusion and sepsis-associated AKI. It is clear that macrophages regulate repair after AKI as well. Macrophages rapidly accumulate in the postischemic kidney, and although they enhance inflammation early on, they also promote repair at later phases by phagocytosis of cell debris and through other mechanisms. For example, macrophages deliver reparative Wnt7b to tubular epithelia, enhancing proliferation and restoration of a functioning nephron.58,59 A summary of the most recent findings for other therapeutic targets is presented in Tables 1 and 2.
Table 1.
Novel repair targets in epithelial cells
| Target | Example | References |
|---|---|---|
| Mitochondrial dysfunction | SS-31, a mitochondria-targeted peptide inhibits permeability transition after AKI | 69 |
| Mitochondrial biogenesis | SIRT1 activator SRT1720 accelerates recovery from AKI through the enhanced expression of Peroxisome proliferator-activated receptor gamma coactivator-1-alpha (PGC-1alpha) | 70,71 |
| Resident macrophage function | Colony–stimulating factor signaling blocks macrophage/dendritic cell proliferation, decreases M2 polarization, and modulates tubular regeneration after AKI | 31 |
| Cellular senescence/cell cycle | Critical shortening leads to increased senescence and apoptosis, thereby limiting regenerative capacity in response to injury; Klotho is an antiaging molecule with pleiotropic actions, including regulation of mineral metabolism; AKI is associated with Klotho deficiency, and its restoration promotes recovery from ischemia-reperfusion (I/R)-associated AKI | 72,73 |
| Uremic toxins | The oral adsorbent AST-120 in association with low–protein intake diet may slow CKD progression by inhibiting p-cresyl sulfate and indoxyl sulfate levels, which increase NADPH oxidase activity and reactive oxygen species production | 74,75 |
| MicroRNAs | microRNA-127 protects tubular cells from I/R-associated AKI modulating its specific targets HIF-1α and kinesin family member 3B; antagoMirs allow targeting | 76,77 |
Sirt1, silent information regulator-1; PGC-1α peroxisome proliferator-activated receptor gamma coactivator-1α; I/R, ischemia-reperfusion; ROS, reactive oxygen species; HIF-1α, hypoxia-inducible factor.
Table 2.
Novel repair targets in endothelial cells
| Target | Example | References |
|---|---|---|
| Endothelin A receptor blockade | Endothelin A receptor antagonist to adult pigs undergoing cardiopulmonary bypass reverses endothelial dysfunction, regional hypoxia, and inflammation | 78 |
| Angiogenesis signaling | EphrinB2 reverse signaling protects against rarefaction of peritubular capillaries by regulating angiogenesis during kidney injury; cartilage oligomeric matrix protein–angiopoietin-1 is a possible endothelial cell–targeted therapy for preventing I/R- and endotoxemia-associated AKI | 79–81 |
| Sirtuin1 deficiency | SIRT1 depletion in vascular endothelial cells mediates endothelial dysfunction, premature senescence, and decreased angiogenesis in renal diseases | 82 |
| MicroRNAs | Hematopoietic microRNA-126 protects against kidney I/R injury by promoting vascular integrity | 83 |
SIRT1, silent information regulator-1; I/R, ischemia-reperfusion.
What Patient Factors and Diseases Affect Repair?
Current Knowledge
Factors that affect restoration after AKI can be divided into disease-specific factors and patient-specific factors.
Disease-Specific Factors
Available clinical data suggest that the etiology of AKI plays an important role in the recovery of renal function. Using a Veterans Affairs database, Amdur et al.60 found that patients diagnosed with isolated acute tubular necrosis were significantly more likely to develop CKD stage 4 than patients with AKI without a specific etiology. After 7 years of follow-up, Schiffl and Fischer61 compared patients with single-cause AKI (e.g., ischemia) with those with multiple-cause AKI. Patients with multiple-cause AKI had a significantly higher incidence of CKD than those with single-cause AKI (38% versus 5%). Septic AKI is characterized by a higher burden of acute illness, longer hospitalization, and more frequent need for RRT compared with nonseptic AKI.62 Patients with septic AKI, however, have shown trends toward higher rates of recovery and independence from RRT.62 Although these findings are interesting, they also clearly show that additional studies are necessary to further substantiate these results and provide more detailed insight into the underlying mechanisms.
Patient-Specific Factors
Age and comorbidities represent the most important patient–specific factors that determine recovery from AKI. Aging itself is associated with changes that promote AKI and impair renal restoration, including oxidative stress, cellular senescence, increased susceptibility to apoptosis, decreased epithelial proliferation, and reduced stem cell function.63 Moreover, changes in the aging kidney are not uniform and vary between individuals (animal and clinical data).64 AKI in the elderly is most often multifactorial and depends on the setting (i.e., outpatient versus hospitalized).65,66 Sepsis, nephrotoxic drugs, hypoperfusion, and obstructive uropathy are among the most frequently observed contributing factors. The data as to whether age per se is a risk factor for nonrecovery remain somewhat controversial. Schmitt et al.66 found that, in a meta-analysis of patients with AKI receiving intermittent RRT, the risk of nonrecovery was 50% greater in elderly patients than younger patients. Contrary to that, Schiffl and Fischer61 showed that, in a cohort of 425 patients in the ICU with AKI, patients with complete or partial recovery did not differ significantly in their mean age. However, the good renal outcome in elderly patients in this study61 may be partially explained by the fact that patients with preexisting renal disease were excluded. Elderly patients more often have a higher burden of comorbidities, including CKD. It is not completely understood whether comorbidities, particularly CKD, or age per se increase the risk for nonrecovery.65,66
Congestive heart failure and complicated diabetes are the two most important nonrenal comorbidities that greatly affect recovery from AKI. Pannu et al.67 used an administrative database to conduct a population–based cohort study looking at various factors affecting recovery from AKI in >190,000 patients. Only preexisting congestive heart failure and complicated diabetes were significantly associated with nonrecovery from AKI. Not surprisingly, preexisting renal dysfunction was also associated with a higher rate of nonrecovery.
What are the Gaps in Knowledge, and What Are the Areas for Future Research to Bridge These Gaps?
The limited amount of conclusive data about factors modulating kidney restoration after AKI warrants both more in–depth research of already established fields and opening up new research avenues. For example, although the effects of age on the kidney and kidney function are rather well known, there is still no reliable biomarker to differentiate between normal aging and comorbidities mimicking renal aging.
Animal data to elucidate the mechanisms of kidney restoration have so far largely focused on ischemia-reperfusion–induced AKI. This work may not optimally model other forms of AKI that are prevalent, particularly in the elderly (e.g., sepsis, drug-induced, and even obstructive nephropathy). Even with ischemia-reperfusion injury, the vast majority of these studies are performed in young rodents, which do not reflect the clinical reality that AKI most often occurs in the elderly. Developing better animal models that mimic human disease,68 including AKI, must be a priority.
Available epidemiologic data are inconclusive regarding the role/effect of aging per se on recovery from AKI. Thus, future epidemiologic studies have to better differentiate between the effects of age per se and the effects of comorbidities that are more prevalent in the elderly. Whether targeting repair will reduce the risks of short-term morbidity, such as multiorgan failure, or the risks of long-term CKD, ESRD, and death is also unknown and requires investigation.
Conclusions
Our improved understanding of the cellular and molecular basis for repair after AKI has provided a better assessment of what constitutes complete repair. At the same time, there is a growing body of evidence suggesting that traditional measures of repair (i.e., GFR) fail to capture functional and structural changes that may accompany a cycle of acute injury followed by repair. New biomarkers are needed to target not simply injury but also, the normal repair process. Although MSCs represent one therapy that seems to target repair, several other pathways show promise as therapeutic targets to accelerate or promote complete repair. It will be important to conceive of repair as a targetable process in the future to maximize our chances of deriving effective new therapies for AKI.
Disclosures
No financial support. B.D.H., V.C., D.P., K.S., L.Y., M.H.R., and C.R. report no conflicts of interest. J.A.K. has received consulting fees from Abbott, Aethlon, Alere, Alung, AM Pharma, Astute Medical, Atox Bio, Baxter, Cytosorbents, venBio, Gambro, Grifols, Roche, Spectral Diagnostics, Sangart, and Siemens; has received research grants from Alere, Astute Medical, Atox Bio, Bard, Baxter, Cytosorbents, Gambro, Grifols, Kaneka, and Spectral Diagnostics; and has licensed technologies through the University of Pittsburgh to Astute Medical, Cytosorbents, and Spectral Diagnostics.
Acknowledgments
Members of the Acute Dialysis Quality Initiative XIII Workgroup are chairs: Mark Okusa (University of Virginia Health System), M.H.R., J.A.K., and C.R.; and members: Anupam Agarwal (University of Alabama at Birmingham and Birmingham Veterans Administration Medical Center), David P. Basile (Indiana University), Roland Blantz (University of California, San Diego), Joseph V. Bonventre (Harvard Medical School), Lakhmir S. Chawla (Veterans Affairs Medical Center), Zheng Dong (Georgia Regents University), Matthew Griffin (National University of Ireland), Raymond Harris (Vanderbilt University School of Medicine), Can Ince (Erasmus MC University Hospital Rotterdam), Martin Matejovic (Charles University in Prague), Dianne McKay (University of California, San Diego), Ravindra Mehta (University of California, San Diego), Bruce Molitoris (Indiana University School of Medicine and the Rouderbush Veterans Affairs Medical Center), Patrick Murray (University College Dublin), Masaomi Nangaku (University of Tokyo Graduate School of Medicine), Peter Pickkers (Radboud University Medical Center), Hamid Rabb (Johns Hopkins University), Sundararaman Swaminathan (University of Virginia Health System), Robert Unwin (University College London), B.D.H., V.C., D.P., K.S., and L.Y.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Coca SG: Long-term outcomes of acute kidney injury. Curr Opin Nephrol Hypertens 19: 266–272, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Feest TG, Round A, Hamad S: Incidence of severe acute renal failure in adults: Results of a community based study. BMJ 306: 481–483, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groeneveld AB, Tran DD, van der Meulen J, Nauta JJ, Thijs LG: Acute renal failure in the medical intensive care unit: Predisposing, complicating factors and outcome. Nephron 59: 602–610, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Macías-Núñez JF, López-Novoa JM, Martínez-Maldonado M: Acute renal failure in the aged. Semin Nephrol 16: 330–338, 1996 [PubMed] [Google Scholar]
- 5.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ: Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okusa MD, Jaber BL, Doran P, Duranteau J, Yang L, Murray PT, Mehta RL, Ince C: Physiological biomarkers of acute kidney injury: A conceptual approach to improving outcomes. Contrib Nephrol 182: 65–81, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang RM, Mor-Avi V: Clinical utility of contrast-enhanced echocardiography. Clin Cardiol 29[Suppl 1]: I15–I25, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Göcze I, Wohlgemuth WA, Schlitt HJ, Jung EM: Contrast-enhanced ultrasonography for bedside imaging in subclinical acute kidney injury. Intensive Care Med 40: 431, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Juillard L, Lerman LO, Kruger DG, Haas JA, Rucker BC, Polzin JA, Riederer SJ, Romero JC: Blood oxygen level-dependent measurement of acute intra-renal ischemia. Kidney Int 65: 944–950, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Sadowski EA, Fain SB, Alford SK, Korosec FR, Fine J, Muehrer R, Djamali A, Hofmann RM, Becker BN, Grist TM: Assessment of acute renal transplant rejection with blood oxygen level-dependent MR imaging: Initial experience. Radiology 236: 911–919, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Han F, Xiao W, Xu Y, Wu J, Wang Q, Wang H, Zhang M, Chen J: The significance of BOLD MRI in differentiation between renal transplant rejection and acute tubular necrosis. Nephrol Dial Transplant 23: 2666–2672, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Juillard L, Janier MF, Fouque D, Lionnet M, Le Bars D, Cinotti L, Barthez P, Gharib C, Laville M: Renal blood flow measurement by positron emission tomography using 15O-labeled water. Kidney Int 57: 2511–2518, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Scheeren TW, Martin K, Maruschke M, Hakenberg OW: Prognostic value of intraoperative renal tissue oxygenation measurement on early renal transplant function. Transpl Int 24: 687–696, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Wang E, Meier DJ, Sandoval RM, Von Hendy-Willson VE, Pressler BM, Bunch RM, Alloosh M, Sturek MS, Schwartz GJ, Molitoris BA: A portable fiberoptic ratiometric fluorescence analyzer provides rapid point-of-care determination of glomerular filtration rate in large animals. Kidney Int 81: 112–117, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meersch M, Schmidt C, Van Aken H, Martens S, Rossaint J, Singbartl K, Görlich D, Kellum JA, Zarbock A: Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS ONE 9: e93460, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamimoto M, Mizuno S, Ohnishi H, Mizuno-Horikawa Y: Type 2a sodium-phosphate co-transporter serves as a histological predictor of renal dysfunction and tubular apical damage in the kidneys of septic mice. Biomed Res 30: 251–258, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Zhou H, Pisitkun T, Aponte A, Yuen PS, Hoffert JD, Yasuda H, Hu X, Chawla L, Shen RF, Knepper MA, Star RA: Exosomal Fetuin-A identified by proteomics: A novel urinary biomarker for detecting acute kidney injury. Kidney Int 70: 1847–1857, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witzgall R, Brown D, Schwarz C, Bonventre JV: Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest 93: 2175–2188, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duffield JS, Park KM, Hsiao L-L, Kelley VR, Scadden DT, Ichimura T, Bonventre JV: Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 115: 1743–1755, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV: Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Lindgren D, Boström A-K, Nilsson K, Hansson J, Sjölund J, Möller C, Jirström K, Nilsson E, Landberg G, Axelson H, Johansson ME: Isolation and characterization of progenitor-like cells from human renal proximal tubules. Am J Pathol 178: 828–837, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angelotti ML, Ronconi E, Ballerini L, Peired A, Mazzinghi B, Sagrinati C, Parente E, Gacci M, Carini M, Rotondi M, Fogo AB, Lazzeri E, Lasagni L, Romagnani P: Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells 30: 1714–1725, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Smeets B, Boor P, Dijkman H, Sharma SV, Jirak P, Mooren F, Berger K, Bornemann J, Gelman IH, Floege J, van der Vlag J, Wetzels JFM, Moeller MJ: Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J Pathol 229: 645–659, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansson J, Hultenby K, Cramnert C, Pontén F, Jansson H, Lindgren D, Axelson H, Johansson ME: Evidence for a morphologically distinct and functionally robust cell type in the proximal tubules of human kidney. Hum Pathol 45: 382–393, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD: Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci U S A 111: 1527–1532, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berger K, Bangen J-M, Hammerich L, Liedtke C, Floege J, Smeets B, Moeller MJ: Origin of regenerating tubular cells after acute kidney injury. Proc Natl Acad Sci U S A 111: 1533–1538, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anders H-J, Schaefer L: Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J Am Soc Nephrol 25: 1387–1400, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulkarni OP, Hartter I, Mulay SR, Hagemann J, Darisipudi MN, Kumar Vr, Sr., Romoli S, Thomasova D, Ryu M, Kobold S, Anders HJ: Toll-like receptor 4-induced IL-22 accelerates kidney regeneration. J Am Soc Nephrol 25: 978–989, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rees AJ: Monocyte and macrophage biology: An overview. Semin Nephrol 30: 216–233, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Zhang M-Z, Yao B, Yang S, Jiang L, Wang S, Fan X, Yin H, Wong K, Miyazawa T, Chen J, Chang I, Singh A, Harris RC: CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest 122: 4519–4532, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price PM, Safirstein RL, Megyesi J: The cell cycle and acute kidney injury. Kidney Int 76: 604–613, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgan DO: The Cell Cycle: Principles of Control, London, New Science Press, 2007 [Google Scholar]
- 34.Megyesi J, Safirstein RL, Price PM: Induction of p21WAF1/CIP1/SDI1 in kidney tubule cells affects the course of cisplatin-induced acute renal failure. J Clin Invest 101: 777–782, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price PM, Yu F, Kaldis P, Aleem E, Nowak G, Safirstein RL, Megyesi J: Dependence of cisplatin-induced cell death in vitro and in vivo on cyclin-dependent kinase 2. J Am Soc Nephrol 17: 2434–2442, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DiRocco DP, Bisi J, Roberts P, Strum J, Wong K-K, Sharpless N, Humphreys BD: CDK4/6 inhibition induces epithelial cell cycle arrest and ameliorates acute kidney injury. Am J Physiol Renal Physiol 306: F379–F388, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neuss S, Becher E, Wöltje M, Tietze L, Jahnen-Dechent W: Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells 22: 405–414, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A: Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells 24: 1254–1264, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Rüster B, Göttig S, Ludwig RJ, Bistrian R, Müller S, Seifried E, Gille J, Henschler R: Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood 108: 3938–3944, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Herrera MB, Bussolati B, Bruno S, Morando L, Mauriello-Romanazzi G, Sanavio F, Stamenkovic I, Biancone L, Camussi G: Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int 72: 430–441, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C: Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int 68: 1613–1617, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Tögel F, Yang Y, Zhang P, Hu Z, Westenfelder C: Bioluminescence imaging to monitor the in vivo distribution of administered mesenchymal stem cells in acute kidney injury. Am J Physiol Renal Physiol 295: F315–F321, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG: Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol 18: 2486–2496, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Tögel F, Zhang P, Hu Z, Westenfelder C: VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J Cell Mol Med 13[8B]: 2109–2114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imberti B, Morigi M, Tomasoni S, Rota C, Corna D, Longaretti L, Rottoli D, Valsecchi F, Benigni A, Wang J, Abbate M, Zoja C, Remuzzi G: Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol 18: 2921–2928, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, Kim YS, Ahn Y: Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med 46: e70, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan Z, Zhuansun Y, Chen R, Li J, Ran P: Immunomodulation of mesenchymal stromal cells on regulatory T cells and its possible mechanism. Exp Cell Res 324: 65–74, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Cantaluppi V, Biancone L, Quercia A, Deregibus MC, Segoloni G, Camussi G: Rationale of mesenchymal stem cell therapy in kidney injury. Am J Kidney Dis 61: 300–309, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Tögel FE, Westenfelder C: Kidney protection and regeneration following acute injury: Progress through stem cell therapy. Am J Kidney Dis 60: 1012–1022, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Perico N, Casiraghi F, Gotti E, Introna M, Todeschini M, Cavinato RA, Capelli C, Rambaldi A, Cassis P, Rizzo P, Cortinovis M, Noris M, Remuzzi G: Mesenchymal stromal cells and kidney transplantation: Pretransplant infusion protects from graft dysfunction while fostering immunoregulation. Transpl Int 26: 867–878, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ, Canadian Critical Care Trials Group : Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of clinical trials. PLoS ONE 7: e47559, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, Fries JW, Tiemann K, Bohlen H, Hescheler J, Welz A, Bloch W, Jacobsen SE, Fleischmann BK: Potential risks of bone marrow cell transplantation into infarcted hearts. Blood 110: 1362–1369, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Kunter U, Rong S, Boor P, Eitner F, Müller-Newen G, Djuric Z, van Roeyen CR, Konieczny A, Ostendorf T, Villa L, Milovanceva-Popovska M, Kerjaschki D, Floege J: Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol 18: 1754–1764, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, Noël D, Jorgensen C: Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood 102: 3837–3844, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Tomasoni S, Longaretti L, Rota C, Morigi M, Conti S, Gotti E, Capelli C, Introna M, Remuzzi G, Benigni A: Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev 22: 772–780, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, Camussi G: Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant 26: 1474–1483, 2011 [DOI] [PubMed] [Google Scholar]
- 57.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L: Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int 78: 838–848, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, Pollard JW, McMahon AP, Lang RA, Duffield JS: Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A 107: 4194–4199, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kinsey GR: Macrophage dynamics in AKI to CKD progression. J Am Soc Nephrol 25: 209–211, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE: Outcomes following diagnosis of acute renal failure in U.S. veterans: Focus on acute tubular necrosis. Kidney Int 76: 1089–1097, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Schiffl H, Fischer R: Clinical cause of presumed acute tubular necrosis requiring renal replacement therapy and outcome of critically ill patients: Post hoc analysis of a prospective 7-year cohort study. Int Urol Nephrol 44: 1779–1789, 2012 [DOI] [PubMed] [Google Scholar]
- 62.Bagshaw SM, Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Oudemans-van Straaten HM, Ronco C, Kellum JA, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Septic acute kidney injury in critically ill patients: Clinical characteristics and outcomes. Clin J Am Soc Nephrol 2: 431–439, 2007 [DOI] [PubMed] [Google Scholar]
- 63.Schmitt R, Cantley LG: The impact of aging on kidney repair. Am J Physiol Renal Physiol 294: F1265–F1272, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Karam Z, Tuazon J: Anatomic and physiologic changes of the aging kidney. Clin Geriatr Med 29: 555–564, 2013 [DOI] [PubMed] [Google Scholar]
- 65.Chronopoulos A, Rosner MH, Cruz DN, Ronco C: Acute kidney injury in elderly intensive care patients: A review. Intensive Care Med 36: 1454–1464, 2010 [DOI] [PubMed] [Google Scholar]
- 66.Schmitt R, Coca S, Kanbay M, Tinetti ME, Cantley LG, Parikh CR: Recovery of kidney function after acute kidney injury in the elderly: A systematic review and meta-analysis. Am J Kidney Dis 52: 262–271, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Pannu N, James M, Hemmelgarn B, Klarenbach S, Alberta Kidney Disease Network : Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol 8: 194–202, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perrin S: Preclinical research: Make mouse studies work. Nature 507: 423–425, 2014 [DOI] [PubMed] [Google Scholar]
- 69.Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, Zhao Z, Ganger M, Tow CY, Seshan SV: Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol 22: 1041–1052, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Funk JA, Schnellmann RG: Accelerated recovery of renal mitochondrial and tubule homeostasis with SIRT1/PGC-1α activation following ischemia-reperfusion injury. Toxicol Appl Pharmacol 273: 345–354, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, Zsengeller ZK, Akhavan-Sharif MR, Khankin EV, Saintgeniez M, David S, Burstein D, Karumanchi SA, Stillman IE, Arany Z, Parikh SM: PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest 121: 4003–4014, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Westhoff JH, Schildhorn C, Jacobi C, Hömme M, Hartner A, Braun H, Kryzer C, Wang C, von Zglinicki T, Kränzlin B, Gretz N, Melk A: Telomere shortening reduces regenerative capacity after acute kidney injury. J Am Soc Nephrol 21: 327–336, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu MC, Shi M, Zhang J, Quiñones H, Kuro-o M, Moe OW: Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int 78: 1240–1251, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Owada A, Nakao M, Koike J, Ujiie K, Tomita K, Shiigai T: Effects of oral adsorbent AST-120 on the progression of chronic renal failure: A randomized controlled study. Kidney Int Suppl 63: S188–S190, 1997 [PubMed] [Google Scholar]
- 75.Anders HJ, Andersen K, Stecher B: The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int 83: 1010–1016, 2013 [DOI] [PubMed] [Google Scholar]
- 76.Lan YF, Chen HH, Lai PF, Cheng CF, Huang YT, Lee YC, Chen TW, Lin H: MicroRNA-494 reduces ATF3 expression and promotes AKI. J Am Soc Nephrol 23: 2012–2023, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aguado-Fraile E, Ramos E, Sáenz-Morales D, Conde E, Blanco-Sánchez I, Stamatakis K, del Peso L, Cuppen E, Brüne B, Bermejo ML: miR-127 protects proximal tubule cells against ischemia/reperfusion: Identification of kinesin family member 3B as miR-127 target. PLoS ONE 7: e44305, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patel NN, Toth T, Jones C, Lin H, Ray P, George SJ, Welsh G, Satchell SC, Sleeman P, Angelini GD, Murphy GJ: Prevention of post-cardiopulmonary bypass acute kidney injury by endothelin A receptor blockade. Crit Care Med 39: 793–802, 2011 [DOI] [PubMed] [Google Scholar]
- 79.Kida Y, Ieronimakis N, Schrimpf C, Reyes M, Duffield JS: EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury. J Am Soc Nephrol 24: 559–572, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim DH, Jung YJ, Lee AS, Lee S, Kang KP, Lee TH, Lee SY, Jang KY, Moon WS, Choi KS, Yoon KH, Sung MJ, Park SK, Kim W: COMP-angiopoietin-1 decreases lipopolysaccharide-induced acute kidney injury. Kidney Int 76: 1180–1191, 2009 [DOI] [PubMed] [Google Scholar]
- 81.Jung YJ, Kim DH, Lee AS, Lee S, Kang KP, Lee SY, Jang KY, Sung MJ, Park SK, Kim W: Peritubular capillary preservation with COMP-angiopoietin-1 decreases ischemia-reperfusion-induced acute kidney injury. Am J Physiol Renal Physiol 297: F952–F960, 2009 [DOI] [PubMed] [Google Scholar]
- 82.Vasko R, Xavier S, Chen J, Lin CH, Ratliff B, Rabadi M, Maizel J, Tanokuchi R, Zhang F, Cao J, Goligorsky MS: Endothelial sirtuin 1 deficiency perpetrates nephrosclerosis through downregulation of matrix metalloproteinase-14: Relevance to fibrosis of vascular senescence. J Am Soc Nephrol 25: 276–291, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bijkerk R, van Solingen C, de Boer HC, van der Pol P, Khairoun M, de Bruin RG, van Oeveren-Rietdijk AM, Lievers E, Schlagwein N, van Gijlswijk DJ, Roeten MK, Neshati Z, de Vries AA, Rodijk M, Pike-Overzet K, van den Berg YW, van der Veer EP, Versteeg HH, Reinders ME, Staal FJ, van Kooten C, Rabelink TJ, van Zonneveld AJ: Hematopoietic microRNA-126 protects against renal ischemia/reperfusion injury by promoting vascular integrity. J Am Soc Nephrol 25: 1710–1722, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]