Abstract
Aim:
Argonaute2 (AGO2) protein is the active part of RNA-induced silencing complex, cleaving the target mRNA strand complementary to their bound siRNA. An increasing number of miRNAs has been identified as essential to angiogenesis of hepatocellular carcinoma (HCC). In this study we investigated how AGO2 affected HCC angiogenesis.
Methods:
Human HCC cell lines HepG2, Hep3B, Huh7, SMMC-7721, Bel-7404, MHCC97-H and LM-3, and human umbilical vein endothelial cells (HUVEC) were tested. The expression of AGO2 in HCC cells was knocked down with siRNA and restored using recombinant adenovirus expressing Ago2. The levels of relevant mRNAs and proteins were examined using RT-PCR, Western blot and EILSA. Nude mice were implanted with Huh7 or SMMC-7721 cells, and tumor volumes were measured. After the mice were euthanized, the xenograft tumors were used for immunohistological analysis.
Results:
In 6 HCC cell lines, AGO2 protein expression was significantly correlated with VEGF expression (r=+0.79), and with VEGF secretion (r=+0.852). Knockdown of Ago2 in Huh7 cells and SMMC-7721 cells substantially decreased VEGF expression, whereas the restoration of AGO2 reversed both VEGF expression and secretion. Furthermore, knockdown of Ago2 significantly up-regulated the expression of PTEN (a tumor suppressor involved in the inhibition of HCC angiogenesis), and vice versa. Moreover, the specific PTEN inhibitor bisperoxovanadate (7, 14, 28 nmol/L) dose-dependently restored the expression of VEGF and the capacity of HCC cells to induce HUVECs to form capillary tubule structures. In the xenograft nude mice, knockdown of Ago2 markedly suppressed the tumor growth and decreased PTEN expression and CD31-positive microvascular in the xenograft tumors.
Conclusion:
A direct relationship exists between the miRNA processing machinery AGO2 and HCC angiogenesis that is mediated by the AGO2/PTEN/VEGF signaling pathway. The results suggest the high value of Ago2 knockdown in anti-angiogenesis therapy for HCC.
Keywords: hepatocellular carcinoma, angiogenesis, Argonaute 2, microRNAs, VEGF, PTEN, bisperoxovanadate
Introduction
Hepatocellular carcinoma (HCC), the third most common cause for cancer mortality worldwide1,2, is characterized by neovascularization, which is closely associated with its metastasis, progression and therapy resistance3,4. Like other hypervascular solid tumors, angiogenesis of HCC is intricately regulated by vascular endothelial growth factor (VEGF), basic fibroblast growth factor(bFGF), platelet-derived growth factor
(PDGF), and other related factors5,6. However, the mechanisms for HCC angiogenesis have still not been fully elucidated.
AGO2 is one of the four members of Argonaute protein family, which is characterized by typical PAZ (Piwi-Argonaute-Zwille) and PIWI domains7. However, in humans, AGO2 is the only member with slicer activity, essential to microRNA (miRNA) maturation as well as the gene-silencing process guided by small RNAs8. Recent studies have revealed that AGO2 was frequently up-regulated in cancers and was linked to carcinogenesis, including head and neck squamous cell carcinoma9, colon cancer10, colorectal carcinomas11, ovarian carcinoma12, gastric carcinoma13, and urothelial carcinoma of the bladder14. In our previous study, we found that AGO2 was over-expressed in HCC cells and that Ago2-knockdown significantly impaired HCC growth in vivo, but barely affected HCC cell propagation in vitro15. This led us to speculate that AGO2 might promote HCC tumorigenesis through angiogenesis.
In this study, we investigated the involvement of AGO2 in HCC angiogenesis. Bi-directional manipulation of the Ago2 expression via RNAi-based knockdown and recombinant adenovirus-mediated expression restoration revealed that the expression of AGO2 protein was intensely associated with VEGF expression and secretion. Moreover, we found that PTEN was a mediator of AGO2 and VEGF interaction. These results provided new insight on the role of AGO2 in HCC angiogenesis via the PTEN/VEGF signaling pathway.
Materials and methods
Cell culture
The human HCC cell lines HepG2, Hep3B, Huh7, and Human Umbilical Vein Endothelial Cells (HUVEC) were purchased from the ATCC (Manassas, VA, USA), and human HCC cell lines SMMC-7721, Bel-7404, MHCC97-H, and LM-3 were obtained from the Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). All cell lines were cultured according to the instructions of the providers, that is, HepG2, Hep3B, Huh7, Bel7404, MHCC97-H, LM-3, HUVEC cells were cultured in high glucose DMEM with 10% FBS, and SMMC-7721 cells were cultured in RPMI-1640 medium with 10% FBS. All cells were incubated under 37 °C, 5% CO2 conditions.
Virus construction
To conduct RNAi-based knockdown, two previously identified oligonucleotides encoding the Ago2-specific siRNA sequence and a loop sequence separating the complementary domains were synthesized and inserted into the lentiviral transfer vector pGC-LV (Genechem, Shanghai, China)15. A non-silencing-siRNA was used as a control. Then, the transfer vector with Ago2-specific shRNA was obtained and co-transfected with the lentiviral help plasmids pHelper 1.0 and pHelper 2.0 into HEK-293T cells using Lipofectamine 2000 reagent (Invitrogen) to package lentiviral particles. To transiently restore the expression of AGO2, a replication-defective recombinant adenovirus carrying the expression cassette of Ago2, Ad-Ago2, was constructed according to a previously described procedure16. The adenovirus was used to infect Ago2-knockdown HCC cells at five multiplicity of infection (MOI), and the adenovirus without transgene (ie, Ad-blank) served as the control.
Quantitative RT-PCR
Total RNA was isolated using TRIzol reagent (Invitrogen, Life Technology, USA) when cells covered approximately 80% of the bottom of 6-well plates. The reverse transcriptase reactions used the Quantscript RT Kit (TIANGEN, Beijing, China) and 1.0 μg RNA of each sample in a 20 μL reaction. Then, total cDNA was diluted to a volume of 100 μL, of which, 2 μL was used for target mRNA expression measurements by real-time PCR with SYBR Green Realtime PCR Master Mix (TOYOBO, Japan) according to the manufacturer's protocol and normalized to GAPDH. All reactions were performed in triplicate. However, for semi-quantitative reverse transcription and polymerase chain reaction, a volume of 5 μL was used, and analyzed by nucleic acid gel electrophoresis. The relative expression values of different samples were determined using Huh7 or SMMC-7721 cells as reference samples. The sequences of the primers used in these experiments are listed in Table 1.
Table 1. Sequences of primers for RT-PCR.
| Target | Sequence (5′–3′) |
|---|---|
| AGO2 | F: CGTGCCTGCTGGAATGTTTC |
| R: CCATCCGTGAGGCCTGTATC | |
| PTEN | F: CGGCAGCATCAAATGTTTCAG |
| R: AACTGGCAGGTAGAAGGCAACTC | |
| VEGF | F: CTACCTCCACCATGCCAAGT |
| R: AGCTGCGCTGATAGACATCC | |
| FGF1 | F: GTCAGTGCTGCCTGAATGCT |
| R: CTCCCGAAGGATTAAACGACG | |
| FGF2 | F: GGAGAAGAGCGACCCTCAC |
| R: AGCCAGGTAACGGTTAGCAC | |
| TGF-β | F: CCCTGGACACCAACTATTGC |
| R: AAGTTGGCATGGTAGCCCTT | |
| PDGFA | F: GGCACTTGACACTGCTCGT |
| R: GCAAGACCAGGACGGTCATTT | |
| PDGFB | F: GCGCTCTTCCTGTCTCTCTG |
| R: TCGAGTGGTCACTCAGCATC | |
| GAPDH | F: GGAGGAGTGGGTGTCGCTGT |
| R: GTGGACCTGACCTGCCGTCT |
Western blot
Protein lysates from cell lines were prepared in lysis buffer and centrifuged at 12 000×g at 4 °C. Western blotting was performed according to a previously described procedure15. The primary antibodies used were rabbit anti-AGO2 (CST, USA), rabbit anti-PTEN (CST, USA), mouse anti-VEGF (Beyotime, China), mouse anti-GAPDH (Beyotime, China). The secondary antibodies were HRP-labeled goat anti-rabbit or anti-mouse IgG (H+L) (Beyotime, China). The expression of each band was quantitatively analyzed using the Image LabTM Software (Bio-Rad, USA) and normalized to the expression of GAPDH in the same lane.
Enzyme-linked immunosorbent assay
Suspensions from Huh7, SMMC-7721, Ago2-knockdown Huh7 (ie Huh7-siAgo2), Ago2-knockdown SMMC-7721 (ie, 7721-siAgo2) cells were collected and diluted five-fold for VEGF secretion tests by Enzyme-linked Immunosorbent Assay using the Human VEGF Quantikine ELISA Kit (R&D, USA). Assays were carried out according to the manufacturer's instructions.
Capillary tube assay
The ability of Huh7, SMMC-7721, Huh7-siAgo2, and 7721-siAgo2 cells to induce endothelial cells to proliferate and organize into capillary-like sprouts was examined using HUVECs as previously described15 with a slight modification. In summary, 6.0×104 HUVEC cells were collected, re-suspended in conditioned medium, which was the suspension from Huh7, SMMC-7721, Huh7-siAgo2, 7721-siAgo2 cell culture for 48 h, and seeded in 24-well plates, each of which was coated with 50 μL growth factor-reduced matrigel (BD, USA). Photos were taken 24 h later.
Immunofluorescence assay
Cells were plated onto a confocal dish and fixed by paraformaldehyde for 20 min and solubilized with Triton X-100 for 10 min. The primary antibody rabbit anti-PTEN was used at a concentration of 1:100. Alexa Fluor 555-labeled donkey anti-rabbit IgG (H+L) (Beyotime, China) secondary antibody was used for the immunofluorescence assay. After immunolabeling, cells were washed, stained with DAPI, and then scanned with a confocal laser scanning microscope (Zeiss LSM700, Germany).
In vivo assay
All animal experiments were undertaken in accordance with the National Institute of Health guidelines for the care and use of laboratory animals, with the approval of the Scientific Investigation Board at the Second Military Medical University, Shanghai. Huh7, SMMC-7721 and Huh7-siAgo2, 7721-siAgo2 cells were injected subcutaneously into nude mice (1×107/mouse, 6 per group). Twelve days post injection, tumor volumes were measured every 4 d thereafter [volume=(W2×L)/2; W, width; L, length, in cubic millimeters]. The mice were euthanized four weeks post transplantation to weight the xenograft tumors, and the tumor tissues cells were separated for immunohistological analysis using the avidin biotin-peroxdase complex (ABC) method. The used primary antibodies were AGO2 (1:250, CST, USA), PTEN (1:250, CST, USA), and CD31 (1:200, Abcam, USA).
Statistical analysis
All data are presented as the mean±standard deviation. Independent Student's t-test was used to analyze the variation of two selected groups, and Pearson's chi-square test was used to analyze the correlation of two parameters. A P<0.05 was considered statistically significant and P<0.01 was considered highly statistically significant. All statistical analyses were performed with SPSS (Statistical Package for the Social Sciences) version 18.0 software.
Results
The expression and secretion of VEGF was significantly correlated with AGO2 protein in HCC cells
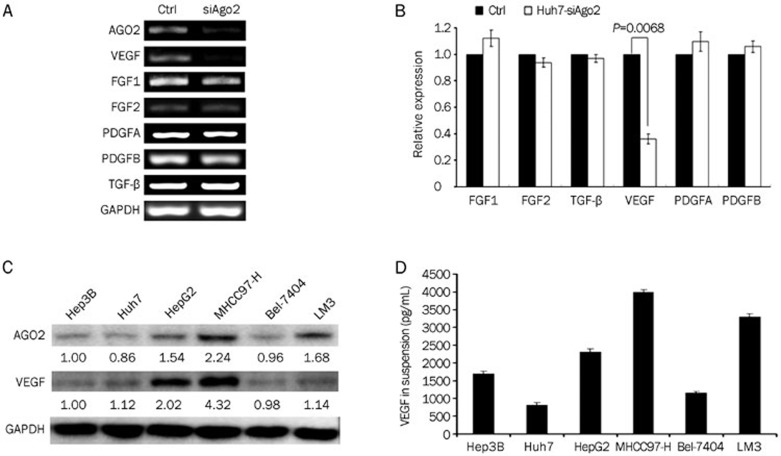
In our previous study, we had surprisingly noticed that Ago2-knockdown impaired the tumorigenesis of Huh7 cells in vivo, but barely affected the growth of Huh7 cells in vitro. These results indicate that AGO2 may be involved in HCC angiogenesis. To determine the validity of this hypothesis, we first examined the expression of the angiogenesis-related factors VEGF, TGF-β, PDGFA, PDGFB, FGF1, FGF2 in the Ago2-knockdown HCC cells. The results showed that VEGF expression was remarkably decreased (P=0.0068), while the expression of the other factors was almost unchanged under the condition when AGO2 was specifically reduced (Figure 1A, 1B). Thus, the correlation between AGO2 and VEGF was investigated by examining the expression of AGO2 and VEGF in a list of HCC cell lines (Figure 1C). From the results of quantitative Western blot analysis, we found that the expression of AGO2 and VEGF was significantly correlated at the protein level (r=+0.79, P=0.031). Furthermore, a more significant correlation was revealed when we analyzed AGO2 intracellular expression and VEGF secretion in HCC cell culture suspensions (r=+0.852, P=0.001).
Figure 1.
Expression of AGO2 and VEGF was significantly correlated in HCC cell lines. (A and B) RT-PCR was performed to detect the expressions of angiogenesis related factors in Huh7-ctrl and Huh7-siAgo2 cells; (C) Western blot was performed to detect VEGF and AGO2 expressions in HCC cell lines. (D) ELISA was performed to detect VEGF secretion in HCC cell lines suspensions.
Ago2-knockdown impaired angiogenesis by reducing VEGF expression in vitro
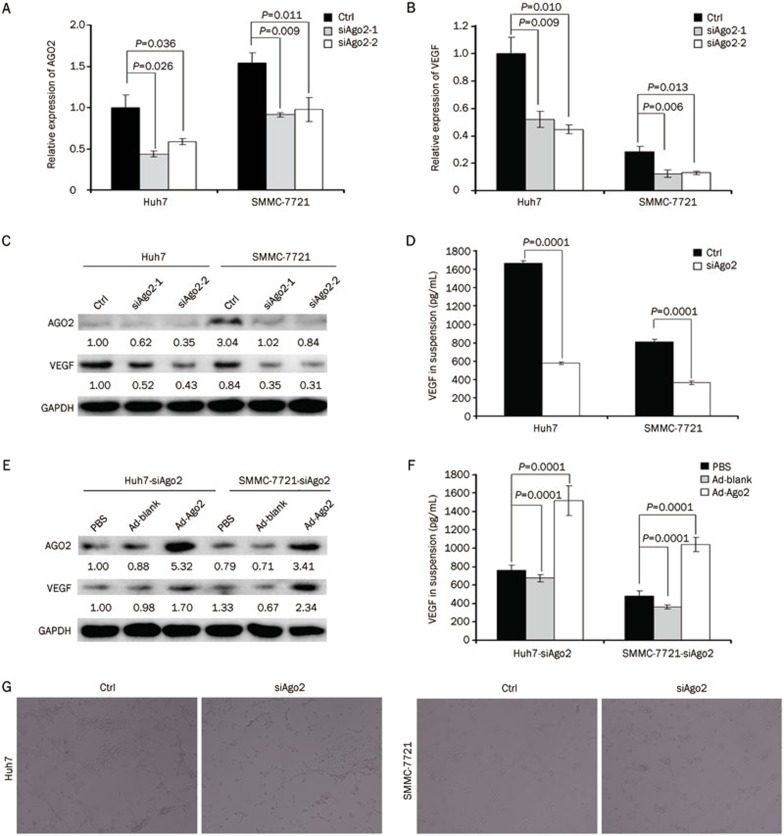
To confirm the correlation between AGO2 and VEGF, and due to their respective minimum and maximum up-regulation of AGO2 expression compared to normal liver cells, Huh7 and SMMC-7721 cells were chosen to conduct Ago2 knocking down with two individual siRNAs targeting different locations of the Ago2 gene (Figure 2A). The data showed that the expression of VEGF was decreased at the RNA level in all Ago2-knockdown HCC cells (ie, Huh7-siAgo2-1/Huh7-siAgo2-2 and 7721-siAgo2-1/7721-siAgo2-2) relative to the corresponding control cells expressing a non-silencing-siRNA (P<0.05, Figure 2B). These results were consistent with those from the Western blot and EILSA analyses, indicating that the expression of AGO2 and VEGF was synchronously down-regulated (P<0.001, Figure 2C and 2D). Interestingly, both the expression and secretion of VEGF were increased when we restored the expression of AGO2 by infection with the recombinant adenovirus expressing AGO2 (P<0.001, Figure 2E and 2F). These results suggested that AGO2 could promote HCC angiogenesis by the up-regulation of VEGF.
Figure 2.
Ago2-knockdown impaired HCC angiogenesis through reduction of VEGF. (A–D) Quantitative real-time RT-PCR, Western blot, and ELISA were performed to test the effect of Ago2-knockdown on VEGF expression. (E and F) Western blot and ELISA were performed to detect the effect of Ago2 restoration on VEGF expression. (G) 3-D matrigel system was used to test the ability of Ago2-knockdown HCC cells to induce capillary tubules formation from HUVECs (200×). Ad-Ago2 and Ad-blank indicated the recombinant adenovirus expressing Ago2 and blank adenovirus without transgene, respectively.
In vitro co-culture of cell culture suspensions with HUVECs in a three dimensional matrigel system has been ubiquitously applied for angiogenesis analysis6,17. Thus, we next co-cultured HCC cell culture suspensions with HUVECs in matrigel-coated 6-well plates to examine the influence of Ago2-knockdown on the capacity of the HCC cells to induce HUVECs to form capillary tubules. These results showed that the ability of HCC cells to induce HUVEC capillary tubule formation was greatly attenuated due to Ago2-knockdown in Huh7 and SMMC-7721 cells relative to the control (Figure 2G). These results indicated that AGO2 could promote HCC angiogenesis by up-regulating the expression of VEGF, illustrating that AGO2 could function in HCC angiogenesis through regulating the expression of VEGF.
Ago2-knockdown impaired HCC angiogenesis in vivo
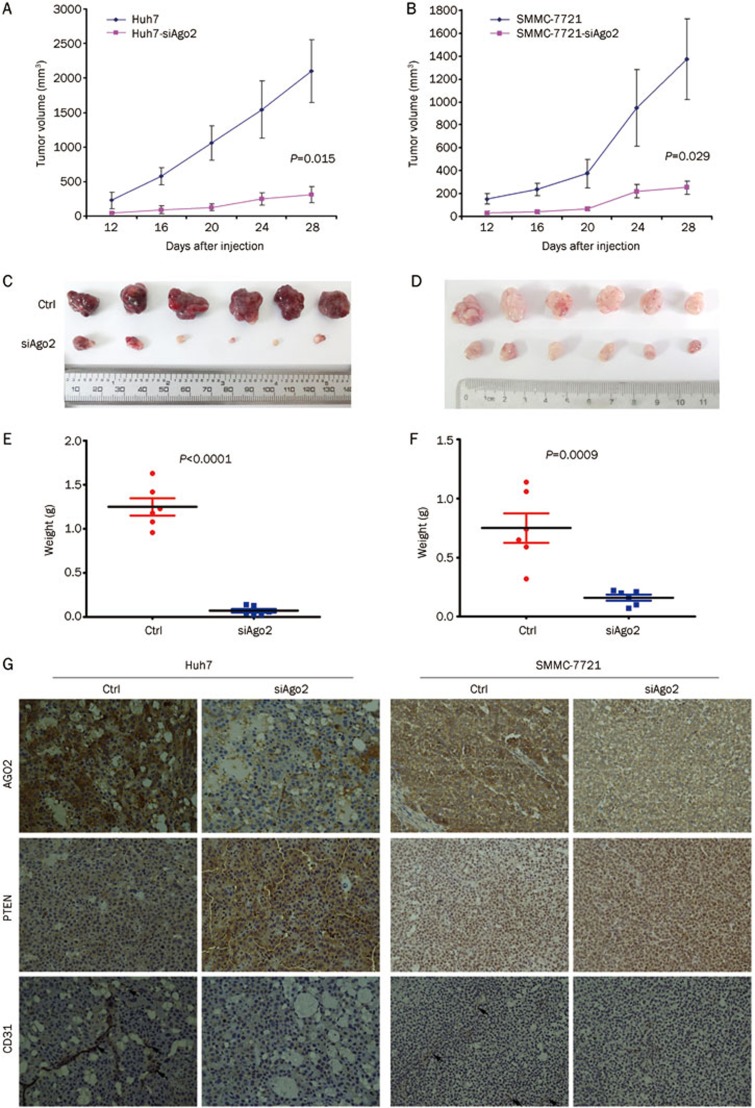
To examine the function of AGO2 on HCC angiogenesis in vivo, Huh7-siAgo2/Huh7-ctrl and 7721-siAgo2/7721-ctrl cells were subcutaneously transplanted into nude mice. Twelve days post transplantation, the solid tumors could be detected in all mice. However, the tumor growth of the test group (ie, Huh7-siAgo2 and 7721-siAgo2) was significantly slower than those of the control group (ie, Huh7-ctrl and 7721-ctrl) during the following half month (P<0.05, Figure 3A and 3B). On the day of euthanasia, the sizes of xenograft from Huh7-siAgo2 and 7721-siAgo2 group were remarkably smaller than those from Huh7-ctrl and 7721-ctrl, respectively (Figure 3C and 3D). The xenograft tumor weights between the test group and control group also exhibited a significant difference (P<0.0001, Figure 3E and 3F).
Figure 3.
Ago2-knockdown impaired HCC angiogenesis and tumor growth in vivo. (A–G) The growth curve, morphology, and weight of xenograft were measured. (H) Immunochemistry was conducted by using antibody for AGO2, PTEN, and CD31 (200×).
Immunohistochemistry analysis was then performed to examine the variance of microvascular richness between the xenografts from the test and control groups. As a result, we found that the CD31-postive microvascular within the xenograft tissues was greatly decreased with the knockdown of Ago2 (Figure 3G) in two HCC transplantation models. These results indicated that Ago2-knockdown could effectively impair HCC angiogenesis and thereby inhibit tumor growth in vivo, suggesting a potent utilization of Ago2-knockdown in antiangiogenic therapy for HCC.
Ago2-knockdown impaired HCC angiogenesis through the up-regulation of PTEN
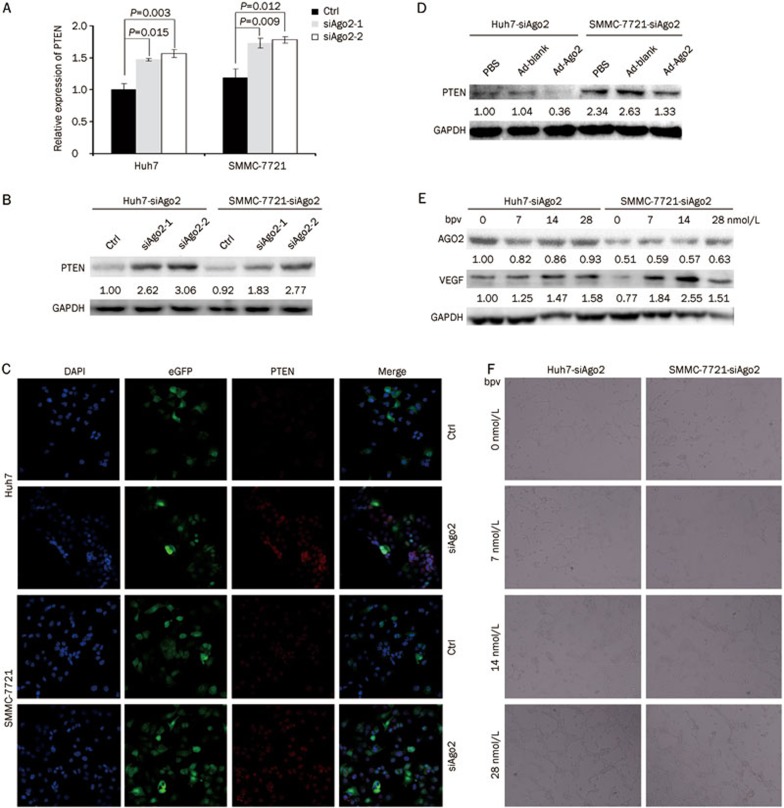
We further investigated the underlying mechanism through which AGO2 regulates HCC angiogenesis. In our previous study15, Ago2-knockdown was revealed to elicit an up-regulation of PTEN, a tumor suppressor which has been identified as a typical inhibitor of angiogenesis and VEGF expression18,19,20,21,22. Therefore, it was reasonable that PTEN might serve as a mediator of the association between AGO2 and VEGF. In accordance with our previous finding, the expression of PTEN was significantly increased in either Huh7 or SMMC-7721 cells when Ago2 was knocked down both in vitro and in vivo (P<0.05, Figure 4A, 4B, and 3G). The results were further confirmed by immunofluorescence analysis, in which PTEN signal was stronger in the HCC cells expressing Ago2-specific siRNA (eGFP was co-expressed with the siRNA) relative to the control (Figure 4C). Conversely, the expression of PTEN could be reduced by the restoration of AGO2 (Figure 4D).
Figure 4.
Ago2-Knockdown impaired HCC angiogenesis by up-regulation of PTEN. (A–C) Quantitative real-time RT-PCR, Western blot, and immunofluorescence assay (200×) was used to test the effect of Ago2-knockdown on PTEN expression, respectively. (D) Western blot was performed to test the effect of Ago2 restoration on PTEN expression. (E) Western blot was performed to test the impact of PTEN inhibitor bisperoxovanadate (bpv) at four different concentrations on AGO2 and VEGF expression. (F) 3-D matrigel system was used to test Ago2-knockdown HCC cells' ability to induce capillary tubules formation from HUVECs after utilizing PTEN inhibitor bpv (200×).
To further investigate the role of PTEN in mediating the capacity of AGO2 to regulate VEGF, the function of PTEN was blocked with the PTEN inhibitor bisperoxovanadate (bpv). In agreement with our expectations, we found that VEGF was increased when bpv was utilized at four different concentrations (ie, 0, 7, 14, and 28 nmol/L), while the expression of AGO2 was barely affected (Figure 4E). Subsequently, capillary tube assays were also conducted with HUVECs. The results showed that the capacity of Huh7-siAgo2 and 7721-siAgo2 cell culture suspensions to induce capillary tubule formation with HUVECs could be restored when the function of PTEN was inhibited (Figure 4F). Taken together, these results indicated that AGO2 might regulate HCC angiogenesis through the deregulation of PTEN and the consequent up-regulation of VEGF.
Discussion
In the previous study, we had demonstrated that up-regulation of AGO2, an executor of miRNA-guided gene silencing, could regulate HCC growth by specifically enhancing the capacity of oncogenic miRNAs (eg, miR-21a) to repress their target tumor suppressor genes (eg, PTEN), as expression profiling showed that oncogenic miRNAs such as miR-21a15 and miR-22123 are ectopically over-expressed while tumor suppressive miRNAs such as miR-199a24 and let-7a25 are dysfunctionally restricted or even lose expression. In this study, we have further documented the role of AGO2 and its role in the regulation of HCC angiogenesis. It was revealed that the expression and secretion of VEGF are significantly correlated with the expression of AGO2 protein among several HCC cell lines, whereas Ago2-knockdown can induce down-regulation of VEGF and consequently lead into the suppression of HCC angiogenesis in vitro and in vivo. Interestingly, this suppression can be reversed through either restoration of AGO2 or functional blockade of PTEN, which is frequently mutated or down-regulated in HCC cells (Supplementary Figure 1A). Thus, the results presented here have confirmed the regulatory relationship between AGO2 and PTEN, and then further extended it to VEGF. The results have broadened our understanding of HCC angiogenesis by providing the novel signaling pathway of AGO2/PTEN/VEGF and offering a reasonable explanation for the irrelevant impact of Ago2-knockdown in vitro and in vivo.
It has been well documented that specific miRNAs play important roles in the regulation of cancer angiogenesis26. In HCC, miRNAs such as miR-21a and miR-195, among others, have been identified to regulate HCC angiogenesis27. Recently, Dicer, a miRNA processing machinery that has universal function in miRNA maturation, has been demonstrated to regulate cancer angiogenesis28,29. Data reported by Tomohiro et al have also indicated that AGO2 participated in controlling the growth of endothelial cells, thereby directly regulating angiogenesis30. In this study, another member of miRNA processing machinery-Ago2, has been revealed to indirectly regulate HCC angiogenesis through its regulatory effect on VEGF but not HIF1α (Supplementary Figure 1B). It might be of great interest to investigate the role of AGO2 in angiogenesis in other types of malignancy because its ectopic expression is present in various cancers9,10,11,12,13,14.
As an indispensable mediator of miRNA-related regulatory pathways, AGO2 also has been shown to directly interact with common cancer associated genes, such as EGFR31, FAK32, AKT33, MAPK34, P4H35, etc. Among them, several genes have already been proven to be involved in regulating angiogenic-related factors (ie, VEGF). It is noted that EGFR and P4H are effectors of hypoxia, which is a major factor in angiogenesis promotion. Under hypoxic conditions, EGFR can phosphorylate AGO2 and inhibit its capacity to regulate the maturation of tumor suppressive miRNAs36,37, and P4H can also regulate the stability of HIF1α and AGO238. Therefore, it is possible that AGO2 may exert its regulation of angiogenesis through different signaling pathways besides AGO2/PTEN/VEGF. Nevertheless, the data showed here that the expression of VEGF is significantly correlated with AGO2 and Ago2-knockdown in two different HCC cells (ie, Huh7 and SMMC-7721) can remarkably impair HCC angiogenesis in vitro and in vivo. This finding clearly supports the regulatory role of AGO2 in HCC angiogenesis and suggests that AGO2 might serve as a potential target for anti-angiogenic therapy against malignant tumors.
Author contribution
Qi-jun QIAN, Hua-jun JIN and Zhen-long YE designed research and revised the manuscript; Zhen-long YE and Yao HUANG performed research and wrote the manuscript; Lin-fang LI contributed new reagents; Hai-li ZHU assited with animal experiment; Hai-xia GAO assisted with Immunofluorescence experiment ; Hui LIU, Jing-lei ZHANG and Sai-qun LV assisted with ELISA, Zeng-hui XU, Luo-ning ZHENG and Tao LIU assisted with the confocal laser scanning microscope operation.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(No 81001013) and Creative Research Groups (No 81221061), the Chinese Key Project for Infectious Diseases (No 2012ZX0002-014-005, 2013ZX10002-010-007), the Military Youth Project (No 13QNP101)and the State Project for Essential Drug Research and Development (2013ZX09102-060).
Footnotes
Supplementary information is available at website of Acta Pharmacologica Sinica.
Supplementary Information
(A) Western Blot was performed to detect PTEN expression in HCC cell lines, and showed no correlation with the interaction between AGO2 and VEGF.
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29. [DOI] [PubMed] [Google Scholar]
- Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology 2015; 61: 191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AX, Duda DG, Sahani DV, Jain RK. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol 2011; 8: 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D, Vacca A, Nico B, Sansonno D, Dammacco F. Angiogenesis and anti-angiogenesis in hepatocellular carcinoma. Cancer Treat Rev 2006; 32: 437–44. [DOI] [PubMed] [Google Scholar]
- Italiano JE Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood 2008; 111: 1227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel RS. Tumor angiogenesis. N Engl J Med 2008; 358: 2039–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirle NT, MacRae IJ. The crystal structure of human Argonaute2. Science 2012; 336: 1037–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock J, Meister G. The Argonaute protein family. Genome Biol 2008; 9: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SS, Smith I, Glazer C, Hennessey P, Califano JA. EIF2C is overexpressed and amplified in head and neck squamous cell carcinoma. ORL J Otorhinolaryngol Relat Spec 2010; 72: 337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yu C, Gao H, Li Y. Argonaute proteins: potential biomarkers for human colon cancer. BMC Cancer 2010; 10: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papachristou DJ, Korpetinou A, Giannopoulou E, Antonacopoulou AG, Papadaki H, Grivas P, et al. Expression of the ribonucleases Drosha, Dicer, and Ago2 in colorectal carcinomas. Virchows Arch 2011; 459: 431–40. [DOI] [PubMed] [Google Scholar]
- Vaksman O, Hetland TE, Trope CG, Reich R, Davidson B. Argonaute, Dicer, and Drosha are up-regulated along tumor progression in serous ovarian carcinoma. Hum Pathol 2012; 43: 2062–9. [DOI] [PubMed] [Google Scholar]
- Zhang J, Fan XS, Wang CX, Liu B, Li Q, Zhou XJ. Up-regulation of Ago2 expression in gastric carcinoma. Med Oncol 2013; 30: 628. [DOI] [PubMed] [Google Scholar]
- Yang FQ, Huang JH, Liu M, Yang FP, Li W, Wang GC, et al. Argonaute 2 is up-regulated in tissues of urothelial carcinoma of bladder. Int J Clin Exp Pathol 2014; 7: 340–7. [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Jin H, Liu H, Lv S, Wang B, Wang R, et al. MiRNA-99a directly regulates AGO2 through translational repression in hepatocellular carcinoma. Oncogenesis 2014; 3: e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Lv S, Yang J, Wang X, Hu H, Su C, et al. Use of microRNA Let-7 to control the replication specificity of oncolytic adenovirus in hepatocellular carcinoma cells. PLoS One 2011; 6: e 21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Htay A, Dos Santos W, Gillies GT, Fillmore HL, Sholley MM, et al. In vitro angiogenesis by human umbilical vein endothelial cells (HUVEC) induced by three-dimensional co-culture with glioblastoma cells. J Neurooncol 2009; 92: 121–8. [DOI] [PubMed] [Google Scholar]
- Kim KR, Moon HE, Kim KW. Hypoxia-induced angiogenesis in human hepatocellular carcinoma. J Mol Med (Berl) 2002; 80: 703–14. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 2007; 6: 273–86. [DOI] [PubMed] [Google Scholar]
- Tian T, Nan KJ, Wang SH, Liang X, Lu CX, Guo H, et al. PTEN regulates angiogenesis and VEGF expression through phosphatase-dependent and -independent mechanisms in HepG2 cells. Carcinogenesis 2010; 31: 1211–9. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Liu LZ. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv Cancer Res 2009; 102: 19–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keniry M, Parsons R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene 2008; 27: 5477–85. [DOI] [PubMed] [Google Scholar]
- Rong M, Chen G, Dang Y. Increased miR-221 expression in hepatocellular carcinoma tissues and its role in enhancing cell growth and inhibiting apoptosis in vitro. BMC Cancer 2013; 13: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Liu X, Lin L, Hou J, Li N, Wang C, et al. MicroRNA-99a inhibits hepatocellular carcinoma growth and correlates with prognosis of patients with hepatocellular carcinoma. J Biol Chem 2011; 286: 36677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YM, Xia Y, Dai W, Han HY, Dong YX, Cai J, et al. Cholesterol-conjugated let-7a mimics: antitumor efficacy on hepatocellular carcinoma in vitro and in a preclinical orthotopic xenograft model of systemic therapy. BMC Cancer 2014; 14: 889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong Q, et al. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell 2011; 19: 232–43. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhao N, Li S, Fang JH, Chen MX, Yang J, et al. MicroRNA-195 suppresses angiogenesis and metastasis of hepatocellular carcinoma by inhibiting the expression of VEGF, VAV2, and CDC42. Hepatology 2013; 58: 642–53. [DOI] [PubMed] [Google Scholar]
- Chen S, Xue Y, Wu X, Le C, Bhutkar A, Bell EL, et al. Global microRNA depletion suppresses tumor angiogenesis. Genes Dev 2014; 28: 1054–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res 2007; 101: 59–68. [DOI] [PubMed] [Google Scholar]
- Asai T, Suzuki Y, Matsushita S, Yonezawa S, Yokota J, Katanasaka Y, et al. Disappearance of the angiogenic potential of endothelial cells caused by Argonaute2 knockdown. Biochem Biophys Res Commun 2008; 368: 243–8. [DOI] [PubMed] [Google Scholar]
- Shen J, Xia W, Khotskaya YB, Huo L, Nakanishi K, Lim SO, et al. EGFR modulates microRNA maturation in response to hypoxia through phosphorylation of AGO2. Nature 2013; 497: 383–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N, Li Y, Han ZG. Argonaute2 promotes tumor metastasis by way of up-regulating focal adhesion kinase expression in hepatocellular carcinoma. Hepatology 2013; 57: 1906–18. [DOI] [PubMed] [Google Scholar]
- Horman SR, Janas MM, Litterst C, Wang B, MacRae IJ, Sever MJ, et al. Akt-mediated phosphorylation of argonaute 2 downregulates cleavage and upregulates translational repression of MicroRNA targets. Mol Cell 2013; 50: 356–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams BD, Claffey KP, White BA. Argonaute-2 expression is regulated by epidermal growth factor receptor and mitogen-activated protein kinase signaling and correlates with a transformed phenotype in breast cancer cells. Endocrinology 2009; 150: 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi HH, Ongusaha PP, Myllyharju J, Cheng D, Pakkanen O, Shi Y, et al. Prolyl 4-hydroxylation regulates Argonaute 2 stability. Nature 2008; 455: 421–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida N, Mimori K, Mori M, Calin GA. EGFR gets in the way of microRNA biogenesis. Cell Res 2013; 23: 1157–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann J, Meister G. Argonaute regulation: two roads to the same destination. Dev Cell 2013; 25: 553–4. [DOI] [PubMed] [Google Scholar]
- Myllyharju J. Prolyl 4-hydroxylases, master regulators of the hypoxia response. Acta Physiol (Oxf) 2013; 208: 148–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Western Blot was performed to detect PTEN expression in HCC cell lines, and showed no correlation with the interaction between AGO2 and VEGF.