Abstract
Wnt5a‐Ror2 signaling has been shown to play important roles in promoting aggressiveness of various cancer cells in a cell‐autonomous manner. However, little is known about its function in cancer‐associated stromal cells, including mesenchymal stem cells (MSCs). Thus, we examined the role of Wnt5a‐Ror2 signaling in bone marrow‐derived MSCs in regulating proliferation of undifferentiated gastric cancer cells. Coculture of a gastric cancer cell line, MKN45, with MSCs either directly or indirectly promotes proliferation of MKN45 cells, and suppressed expression of Ror2 in MSCs prior to coculture inhibits enhanced proliferation of MKN45 cells. In addition, conditioned media from MSCs, treated with control siRNA, but not siRNAs against Ror2, can enhance proliferation of MKN45 cells. Interestingly, it was found that expression of CXCL16 in MSCs is augmented by Wnt5a‐Ror2 signaling, and that recombinant chemokine (C‐X‐C motif) ligand (CXCL)16 protein can enhance proliferation of MKN45 cells in the absence of MSCs. In fact, suppressed expression of CXCL16 in MSCs or an addition of a neutralizing antibody against CXCL16 fails to promote proliferation of MKN45 cells in either direct or indirect coculture with MSCs. Importantly, we show that MKN45 cells express chemokine (C‐X‐C motif) receptor (CXCR)6, a receptor for CXCL16, and that suppressed expression of CXCR6 in MKN45 cells results in a failure of its enhanced proliferation in either direct or indirect coculture with MSCs. These findings indicate that Wnt5a‐Ror2 signaling enhances expression of CXCL16 in MSCs and, as a result, enhanced secretion of CXCL16 from MSCs might act on CXCR6 expressed on MKN45, leading to the promotion of its proliferation.
Keywords: CXCL16, CXCR6, gastric cancer cells, mesenchymal stem cells (MSCs), Wnt5a–Ror2 signaling
Ror2 receptor tyrosine kinase has been shown to act as a receptor for Wnt5a to mediate β‐catenin‐independent non‐canonical Wnt signaling.1, 2, 3 Ror2 is expressed highly in various types of cancer cells,4, 5 and both Ror2 and Wnt5a are also expressed at high levels in several cancer cells, including osteosarcomas and melanomas, resulting in constitutive activation of Wnt5a‐Ror2 signaling in a cell‐autonomous manner.6, 7, 8 In fact, it has been shown that constitutively activated Wnt5a‐Ror2 signaling results in the activation of Src‐family protein tyrosine kinases and of c‐Jun N‐terminal kinase, leading to expression of MMP‐13 and formation of invadopodia, which confer invasive properties on osteosarcoma cells.6, 9 It has been well appreciated that cancer progression is mediated not only by alterations in the cancer cells, but also by the cancer‐associated stroma.10 Among several cell types, constituting the cancer‐associated stroma, much attention has been paid to the role of bone marrow‐derived mesenchymal stem cells (MSCs), which show a strong tropism for cancer cells, in cancer progression.11, 12, 13 However, little is known about the role of Wnt5a‐Ror2 signaling in MSCs in regulating cancer cell behaviors.
Bone marrow‐derived MSCs represent a phenotypically heterogeneous population of cells, yet characterized by expression of cell surface molecules, including CD73, CD90, and CD105, their ability to proliferate ex vivo, and by their potential to differentiate into multilineage mesenchymal cells, including cancer‐associated fibroblasts (CAFs).11, 12, 13, 14, 15 The relationship between MSCs and cancer cells can be bidirectional. Mesenchymal stem cells express and produce a number of growth factors, cytokines, and chemokines, and also express receptors for various growth factors and inflammatory cytokines under different microenvironments or conditions.11, 12, 13 Thus, the effects of MSCs on cancer cells might be dependent on types or characteristics of the cancer cells.
In this study, we have examined the role of Wnt5a‐Ror2 signaling in bone marrow‐derived MSCs in regulating proliferation of an undifferentiated gastric cancer cell line, MKN45. It was found that MSCs express Wnt5a and Ror2 at relatively high levels, whereas MKN45 cells express Wnt5a and Ror2 at marginal levels, if at all.16 Coculture of MKN45 cells with MSCs either directly or indirectly promotes proliferation of MKN45 cells. We show that Wnt5a‐Ror2 signaling in MSCs plays a role in expression of chemokine (C‐X‐C motif) ligand (CXCL)16 in MSCs and its secretion from MSCs. Interestingly, MKN45 cells express a receptor for CXCL16, CXCR6, thereby they proliferate in response to CXCL16 produced by MSCs. Our findings show for the first time that Wnt5a‐Ror2 signaling in MSCs promotes proliferation of MKN45 cells by activating CXCR6 signaling in MKN45 cells through the binding of CXCL16, produced by MSCs. Therefore, it can be envisaged that Wnt5a‐Ror2 signaling in MSCs and/or the CXCL16–CXCR6 axis might be effective therapeutic targets for some types of gastric cancer cells.
Materials and Methods
Cell culture
MKN45‐Luc cells, which express luciferase stably, were obtained from JCRB cell bank (Osaka, Japan) and maintained in RPMI‐1640 medium (Nacalai Tesque, Tokyo, Japan) containing 10% FBS. Mesenchymal stem cells, primary human MSCs derived from bone marrow, were purchased from Lonza (Basel, Switzerland). The cells were maintained in MSCGM (Lonza) and used by passage 5. These cells were incubated at 37°C with 5% CO2 and 90% humidity. In some experiments, MKN45‐Luc cells were treated with soluble recombinant human CXCL16 (PeproTech, Oak Park, CA, USA) at a final concentration of 1.0 ng/mL.
Coculture
For monoculture, MKN45‐Luc cells were plated in 12‐well plates at a density of 1 × 103 cells per well with 2 mL MSCGM. For direct coculture, MSCs and MKN45‐Luc cells were plated in the same well of 12‐well plates at a density of 1 × 103 cells per well for each cell type with 2 mL MSCGM. For indirect coculture, Transwells with 0.4‐μm pore membrane in 12‐well plates (Costar, Cambridge, MA, USA) were used to allow both types of cells to share media without making any direct contact. Unless otherwise indicated, MSCs (1 × 103 cells) were seeded in the upper chamber with 500 μL MSCGM, and MKN45‐Luc cells (1 × 103 cells) were seeded in the lower chamber with 1500 μL MSCGM. To neutralize CXCL16, anti‐human CXCL16 antibody (R&D Systems, Minneapolis, MN, USA) or control IgG (R&D Systems) was added to the media at a concentration of 0.25 μg/mL.
Conditioned media
Mesenchymal stem cells untreated or treated with the respective siRNAs were plated at 1 × 104 cells/mL in MSCGM and cultured for 6 days. The cell supernatants were collected as the conditioned media. To culture MKN45‐Luc cells with the conditioned media, cells were plated at 1 × 103 cells/mL in the well of 12‐well plates with 25% (v/v) of conditioned medium and 75% (v/v) of MSCGM.
Luciferase assay
Cells were lysed in Glo Lysis buffer (Promega, Madison, WI, USA). Aliquots of cell lysates were mixed with ONE Glo Luciferase Assay buffer (Promega), and the luciferase activities were measured by using the GloMax 96 microplate luminometer (Promega).
Enzyme‐linked immunosorbent assay
The culture supernatants from MSCs treated with si‐Ror2 or si‐CXCL16 siRNAs were collected. The CXCL16 concentration in the culture supernatants was determined using Quantikine ELISA kit (R&D Systems), according to the manufacturer's instructions.
Flow cytometric analysis
MKN45‐Luc cells treated with si‐CXCR6 for 5 days were collected and fixed with 10% (v/v) formalin in PBS. Then cells were washed with PBS and incubated with mouse anti‐human CXCR6 antibody (R&D Systems) at a final concentration of 25 μg/mL, followed by incubation with Alexa488‐conjugated anti‐mouse IgG (Life Technologies, Carlsbad, CA, USA). Flow cytometric analysis was performed using the BD FACSVerse (Becton Dickinson, Franklin Lakes, NJ, USA).
Human chemokine array
Conditioned media obtained from Ror2‐silenced MSCs were analyzed using the Proteome Profiler Human Chemokine Array Kit (ARY017; R&D Systems), according to the manufacturer's instructions. Briefly, array membranes, spotted with capture antibodies to specific target proteins, were incubated overnight at 4°C with the conditioned media pretreated with a cocktail of biotinylated detection antibodies. Then, membranes were washed and incubated with streptavidin–HRP for 30 min at room temperature. After washing the membranes, captured proteins on the membranes were visualized using chemiluminescent detection reagents. Supplementary materials and methods are described in Data S1.
Results
Promoted proliferation of MKN45 cells by direct and indirect coculture with MSCs
We first examined a possible role of human bone marrow‐derived MSCs on the proliferation of an undifferentiated gastric cancer cell line, MKN45 cells which express Wnt5a at marginal levels, if any.16 To this end, we used MKN45‐Luc cells, which express luciferase stably, to monitor proliferation of MKN45(‐Luc) cells. When MKN45‐Luc cells were cultured singly for 12 h at different densities, both total viable cell numbers and luciferase activities increased in proportion to increased cell densities (Fig. S1), indicating that measurement of luciferase activities can be a reliable readout of proliferation of MKN45‐Luc cells.
When MKN45‐Luc cells were either cultured singly (MSCs−) or cocultured directly with MSCs (MSCs+), proliferation of MKN45‐Luc cells was promoted remarkably at day 9 after coculture with MSCs (Fig. 1a). We next examined whether the effect of coculture with MSCs on promoted proliferation of MKN45‐Luc cells would be mediated by direct cell–cell interaction or by a soluble mediator(s) produced by MSCs. Thus, we carried out indirect cocultures of MKN45‐Luc cells and MSCs by using Transwells that prohibit any direct contact between cells in the lower and upper chambers. As shown in Figure 1(b,c), indirect coculture of MKN45‐Luc cells with MSCs promoted proliferation of MKN45‐Luc cells significantly at day 9 after coculture with MSCs, indicating that a soluble mediator(s) produced by MSCs might play a role in promoting proliferation of MKN45 cells.
Figure 1.
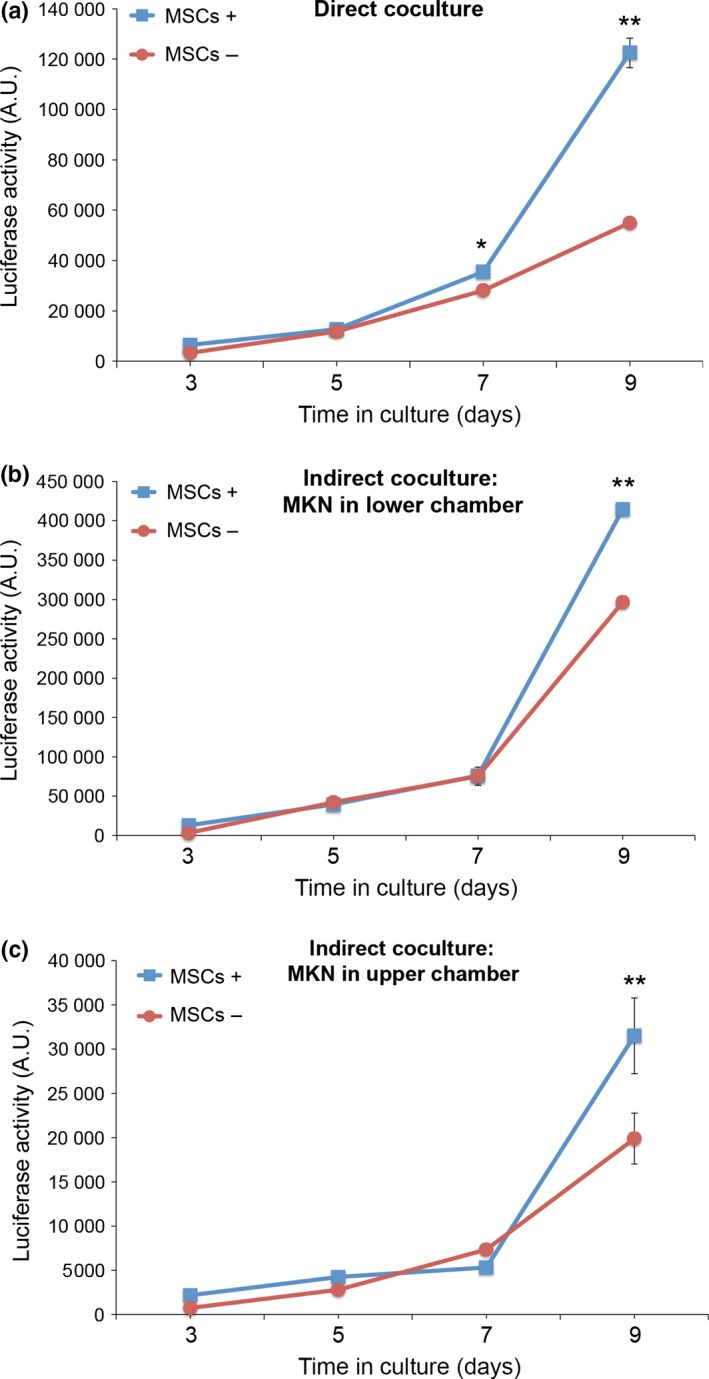
Coculture with mesenchymal stem cells (MSCs) promotes proliferation of MKN45‐Luc cells. (a) MKN45‐Luc cells were either cultured singly (MSCs−) or directly with MSCs (MSCs+). (b,c) MKN45‐Luc cells were either cultured singly (MSCs−) or indirectly with MSCs (MSCs+) in Transwell chambers in which MKN45‐Luc and MSCs were seeded in the lower and upper chambers, respectively (b) or in the upper and lower chambers, respectively (c). Luciferase activities were measured at the indicated time points. Data are expressed as mean ± SD (n = 3). *P < 0.005; **P < 0.001, t‐test.
Role of Wnt5a‐Ror2 signaling in MSCs in promoting proliferation of MKN45 cells
It was found that expression levels of both Ror2 and Wnt5a were relatively high in MSCs, but were marginal if any in MKN45‐Luc cells (Fig. S2). Thus, we then examined a possible role of Wnt5a‐Ror2 signaling in MSCs in promoting proliferation of MKN45‐Luc cells. Suppressed expression of Ror2 or Wnt5a in MSCs resulted in the inhibition of promoted proliferation of MKN45‐Luc cells by direct or indirect coculture with MSCs (Figs 2,S3). Furthermore, proliferation and viability of MSCs were unaffected by suppressed expression of Ror2 in the cells as assessed by WST assay (Fig. S4a). These results suggest that Wnt5a‐Ror2 signaling in MSCs might play a role in promoting proliferation of MKN45 cells.
Figure 2.
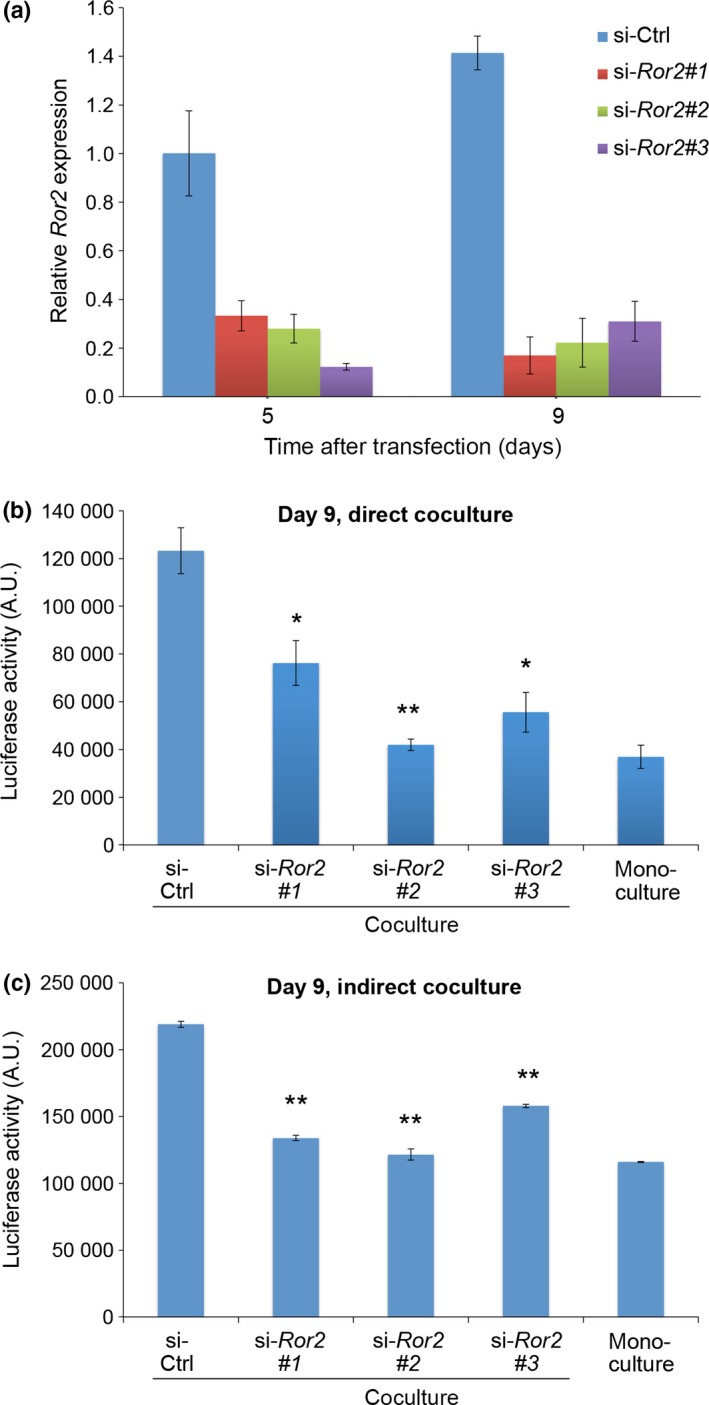
Expression of Ror2 in mesenchymal stem cells (MSCs) is required for the ability of MSCs to promote proliferation of MKN45‐Luc cells in coculture. (a) Suppressed expression of Ror2 in MSCs treated with siRNAs for Ror2. Cells were transfected with either control (ctrl) siRNA or three different siRNAs against Ror2 (#1, #2, #3) and cultured for 5 or 9 days. Expression levels of Ror2 mRNA were measured by quantitative RT‐PCR analyses. (b,c) MKN45‐Luc cells were cultured singly (monoculture) or cocultured with siRNA‐transfected MSCs either directly (b) or indirectly (c). After 9 days in culture, luciferase activities were measured. Data are expressed as mean ± SD (n = 3). *P < 0.005; **P < 0.001, t‐test.
Induced expression of CXCL16 by Wnt5a‐Ror2 signaling in MSCs
To assess further the findings that MSCs produce a soluble mediator(s) that promotes proliferation of MKN45 cells, we first tested the effect of conditioned media from MSCs on proliferation of MKN45‐Luc cells. As expected, conditioned media from MSCs promoted proliferation of MKN45‐Luc cells (Fig. 3a). Interestingly, suppressed expression of Ror2 in MSCs inhibited the effect of MSC‐conditioned media to promote proliferation of MKN45‐Luc cells (Fig. 3b), indicating that Wnt5a‐Ror2 signaling in MSCs might play a role in producing a soluble mediator(s) that promotes proliferation of MKN45 cells.
Figure 3.
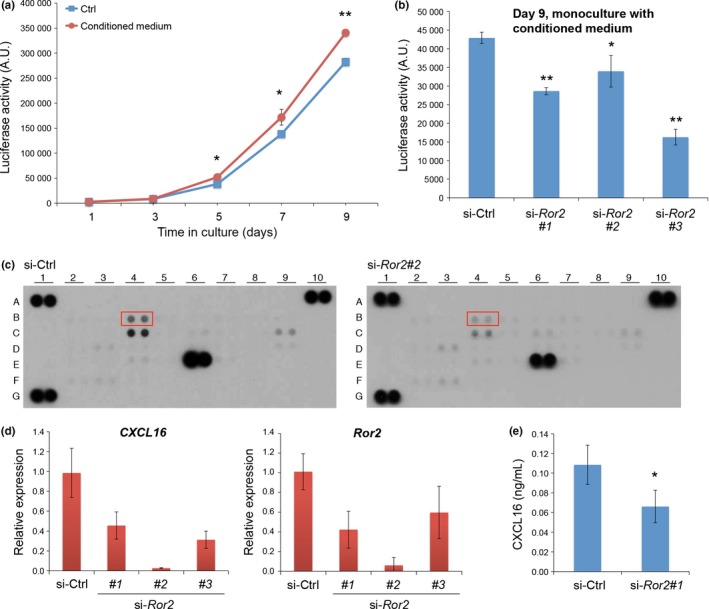
Conditioned media from mesenchymal stem cells (MSCs) promote proliferation of MKN45‐Luc cells in a manner dependent on Ror2 expression in MSCs. (a) Conditioned media from MSCs promotes proliferation of MKN45‐Luc cells. MKN45‐Luc cells were cultured in the presence or absence of MSC‐conditioned media. Luciferase activities were measured at the indicated time points. Data are expressed as mean ± SD (n = 3). *P < 0.05; **P < 0.001, t‐test. (b) MKN45‐Luc cells were cultured in the presence of conditioned media from MSCs pretreated with either ctrl siRNA or three different siRNAs against Ror2 (#1, #2, #3). After 9 days in culture, luciferase activities were measured. Data are expressed as mean ± SD (n = 3). *P < 0.05; **P < 0.001, t‐test. (c) MSCs were transfected with either ctrl or Ror2 (#2) siRNA. After 6 days in culture, conditioned media were collected and subjected to chemokine array analysis. Boxed spots (B4) indicate protein levels of CXCL16, which decreased significantly and reproducibly following suppressed expression of Ror2. Other spots with different intensities between si‐Ctrl and si‐Ror2 groups include C4 (interleukin [IL]‐8), C9 (CCL2), and E6 (CXCL12). A1, A10, and G1 represent reference spots. (d) Expression of CXCL16 is downregulated by suppressed expression of Ror2 in MSCs. Mesenchymal stem cells were transfected with either ctrl or Ror2 siRNAs. After 6 days in culture, mRNA levels of CXCL16 (left panel) and Ror2 (right panel) were measured by quantitative RT‐PCR analyses. (e) MSCs were transfected with either ctrl or Ror2 (#1) siRNA. After 6 days in culture, conditioned media were collected to measure relative amounts of CXCL16 protein by ELISA. Data are expressed as mean ± SD (n = 3). *P < 0.05, t‐test.
To identify a candidate soluble mediator(s) produced by MSCs through Wnt5a‐Ror2 signaling, we carried out a human chemokine array analysis using conditioned media from either MSCs treated with control (ctrl) siRNA or siRNAs against Ror2. As a result, among several candidate chemokines identified, CXCL16 was the most prominent one whose expression was inhibited significantly and reproducibly by suppressed expression of Ror2 (Fig. 3c). In fact, expression of CXCL16 was inhibited by suppressed expression of Ror2 in MSCs as assessed by quantitative RT‐PCR (Fig. 3d). As CXCL16 can exist as a transmembrane protein and a soluble protein by cleavage with the ADAM family of proteases,17, 18, 19, 20, 21 we measured amounts of soluble CXCL16 secreted from MSCs following treatment with either ctrl siRNA or siRNAs against Ror2 by ELISA. As shown in Figure 3(e), secretion of soluble CXCL16 from MSCs was inhibited significantly by suppressed expression of Ror2. In addition, expression of CXCL16 was inhibited by suppressed expression of Wnt5a (Fig. S4b). These results indicate that Wnt5a‐Ror2 signaling in MSCs plays an important role in promoting expression and secretion of CXCL16.
Critical role of soluble CXCL16 in promoting proliferation of MKN45 cells
We next examined the effect of soluble recombinant CXCL16 (rCXCL16) on proliferation of MKN45 cells. Importantly, proliferation of MKN45‐Luc cells was promoted by the addition of rCXCL16 after day 7 (Fig. 4a). To further assess the role of soluble CXCL16 secreted from MSCs in the promotion of MKN45 cell proliferation, MKN45‐Luc cells were cocultured directly or indirectly with MSCs pretreated with either ctrl siRNA or siRNA against CXCL16. As shown in Figure 4(b,c), expression of CXCL16 mRNA and secretion of soluble CXCL16 were inhibited drastically by pretreatment of MSCs with siRNA against CXCL16 and subsequent culture for 6 days. Interestingly, suppressed expression of CXCL16 in MSCs resulted in the inhibition of promoted proliferation of MKN45‐Luc cells by direct or indirect coculture with MSCs (Fig. 4d,e), indicating that soluble CXCL16, expressed in and secreted from MSCs through Wnt5a‐Ror2 signaling, plays an important role in promoting proliferation of MKN45 cells. To confirm further the role of soluble CXCL16 from MSCs in promoting proliferation of MKN45 cells, we examined the effect of a neutralizing antibody against CXCL16 on proliferation of MKN45‐Luc cells cocultured directly or indirectly with MSCs. As expected, promoted proliferation of MKN45‐Luc cells by direct or indirect coculture with MSCs was inhibited significantly by an addition of the neutralizing anti‐CXCL16 antibody (Fig. 4f,g), confirming the critical role of soluble CXCL16 in promoting proliferation of MKN45.
Figure 4.
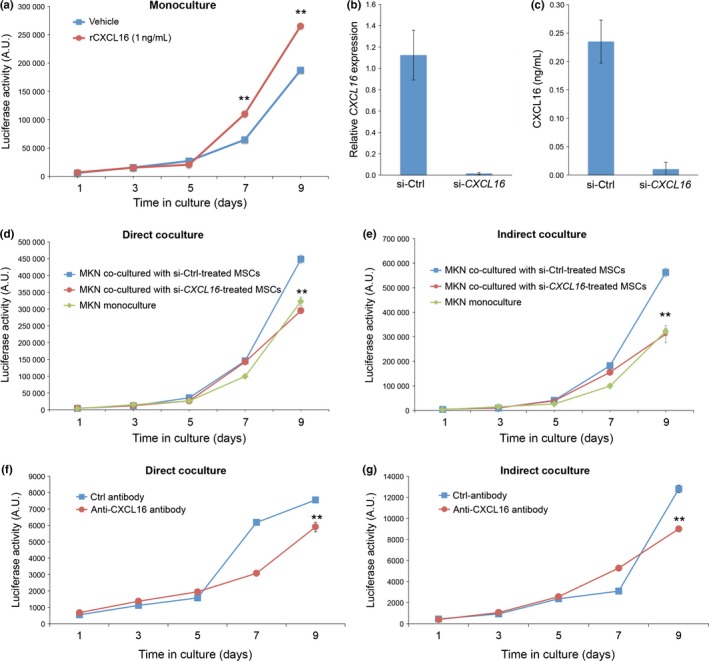
Expression of CXCL16 in mesenchymal stem cells (MSCs) is required for the ability of MSCs to promote proliferation of MKN45‐Luc cells in coculture. (a) Recombinant CXCL16 (rCXCL16) promotes proliferation of MKN45‐Luc cells. MKN45‐Luc cells were cultured in the absence (vehicle) or presence of 1 ng/mL rCXCL16, and luciferase activities were measured at the indicated time points. Data are expressed as mean ± SD (n = 3). **P < 0.001, t‐test. (b,c) Suppressed expression of CXCL16 in MSCs. MSCs were transfected with either ctrl or CXCL16 siRNA. After 6 days in culture, mRNA levels of CXCL16 were measured by quantitative RT‐PCR analyses (b). Conditioned media from the siRNA‐treated MSCs were collected to measure relative amounts of CXCL16 protein by ELISA (c). (d,e) MKN45‐Luc cells were cultured singly or cocultured with siRNA‐treated MSCs either directly (d) or indirectly (e). Luciferase activities were measured at the indicated time points. Data are expressed as mean ± SD (n = 3). **P < 0.001, t‐test. (f,g) Effect of neutralizing antibody against CXCL16 on proliferation of MKN45‐Luc cells cocultured with MSCs. MKN45‐Luc cells were cocultured with MSCs either directly (f) or indirectly (g) in the presence of anti‐CXCL16 neutralizing antibody or control IgG. Luciferase activities were measured at the indicated time points. Data are expressed as mean ± SD (n = 3). **P < 0.001, t‐test.
Critical role of CXCL16–CXCR6 signaling in promoted proliferation of MKN45 cells
It has been shown that CXCR6 acts as a receptor for soluble and transmembranous forms of CXCL16.18, 19, 22 Therefore, it can be envisaged that CXCR6 might be expressed on MKN45 cells to mediate CXCL16‐induced proliferation signaling. In fact, quantitative RT‐PCR analysis revealed remarkably high levels of CXCR6 mRNA in MKN45‐Luc cells compared with those in MSCs (Fig. S5). Treatment of MKN45‐Luc cells with siRNA against CXCR6 inhibited expression of CXCR6 (Fig. 5a). Flow cytometric analysis also showed expression of cell surface CXCR6 proteins on MKN45‐Luc cells and its inhibition by the siRNA treatment (Fig. 5b). It was found that suppressed expression of CXCR6 in MKN45‐Luc cells by itself had marginal effect, if any, on proliferation of MKN45‐Luc cells (monoculture) (Fig. S6). However, suppressed expression of CXCR6 in MKN45‐Luc cells significantly inhibited the promotion of MKN45‐Luc cell proliferation by direct or indirect coculture with MSCs (Figs 5c,d,S6), indicating that CXCR6 expressed on MKN45 cells plays a critical role in the promotion of MKN45 cell proliferation by CXCL16 secreted from MSCs.
Figure 5.
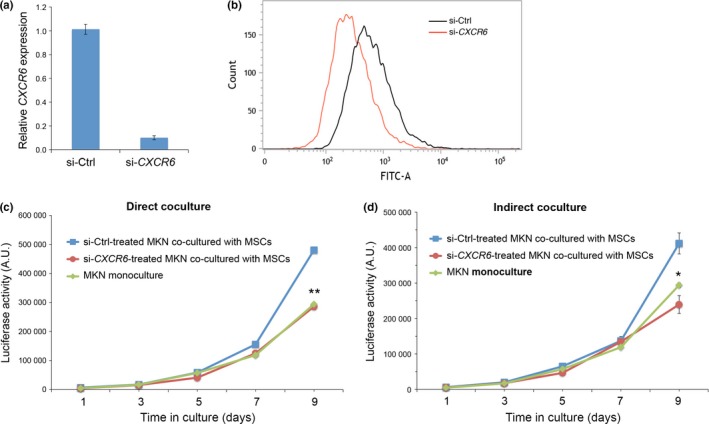
Expression of CXCR6 in MKN45‐Luc cells is required for the ability of mesenchymal stem cells (MSCs) to promote proliferation of MKN45‐Luc cells in coculture. (a,b) Suppressed expression of CXCR6 in MKN45‐Luc cells. MKN45‐Luc cells were transfected with either ctrl or CXCR6 siRNA. After 6 days in culture, mRNA levels of CXCR6 were measured by quantitative RT‐PCR analyses (a). Cell surface expression of CXCR6 protein was measured by flow cytometry (b). (c,d) MKN45‐Luc cells transfected with either ctrl or CXCR6 siRNA were cocultured with MSCs either directly (c) or indirectly (d). Untreated MKN45‐Luc cells were also cultured singly (monoculture). Luciferase activities were measured at the indicated time points. Data are expressed as mean ± SD (n = 3). *P < 0.005; **P < 0.001 between si‐Ctrl and si‐CXCR6 groups, t‐test.
Discussion
Growing evidence indicates the important roles of Wnt5a‐Ror2 signaling in the progression of various types of cancer cells.4, 5, 6, 7, 8 Here we show that Wnt5a‐Ror2 signaling plays an important role in bone marrow‐derived MSCs to produce soluble CXCL16, which in turn, acting on CXCR6, a receptor for CXCL16 expressed on MKN45 undifferentiated gastric cancer cells, to mediate the promotion of its proliferation. To our knowledge, this is for the first time the role of Wnt5a‐Ror2 signaling has been revealed by an external cue, in this case MSCs, to promote cancer cell progression.
In this study we used undifferentiated MKN45 gastric cancer cells to exclude the possibility of cancer cells proliferating in a cell‐autonomous manner through Wnt5a‐Ror2 signaling. MKN45 cells express both Wnt5a and Ror2 marginally, if at all, whereas MSCs express them at relatively high levels (Fig. S2). Although the extent of the luciferase activities detected was varied under different experimental settings, proliferation of MKN45‐Luc cells was promoted remarkably at day 9 after direct or indirect coculture with MSCs through Wnt5a‐Ror2 signaling (Figs 1, 2,S3). We further showed that CXCL16, expressed in and secreted from MSCs by Wnt5a‐Ror2 signaling, acts on CXCR6 expressed on MKN45‐Luc cells, thereby promoting proliferation of MKN45 cells (Figs 3, 4, 5,S4b). In this respect, it should be noted that the CXCL16–CXCR6 axis has been shown to play an important role in promoting progression of prostate cancer cells by activating the AKT–mTOR pathways.23, 24, 25, 26
As shown, the promoted effects of coculture with MSCs, and the addition of conditioned media from MSCs and rCXCL16 on proliferation of MKN45 cells can be detected only after day 7 or day 9 in culture (Figs 1, 2, 3, 4, 5,S3). It is conceivable that the CXCL16–CXCR6 axis might activate further signaling to promote the eventual proliferation of MKN45 cells. Further study will be required to clarify this issue. It has also been reported that in vitro culture of MSCs alone or with cancer‐derived medium can induce its conversion into CAFs.11, 27 Although expression levels of Ror2 and Wnt5a increased and decreased, respectively, during in vitro culture of MSCs alone (Fig. S2c), suppressed expression of either Ror2 or Wnt5a failed to affect expression patterns and levels of CAF marker genes, including α‐SMA, SDF‐1, and FAP (data not shown), suggesting that Wnt5a‐Ror2 signaling in MSCs might not affect its conversion into CAFs and vice versa.
Collectively, our present findings reveal that soluble CXCL16, produced by MSCs through Wnt5a‐Ror2 signaling, can promote proliferation of MKN45 cells expressing CXCR6. Thus, it can be assumed that CXCL16, produced by MSCs, might also contribute to the promoted proliferation of other cancer cells that express CXCR6. Therefore, Wnt5a‐Ror2 signaling in MSCs and/or the CXCL16–CXCR6 axis between MSCs and cancer cells might be effective therapeutic targets to prevent cancer progression. Future study with in vivo xenograft analyses will be required to test this possibility.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. Cell densities in culture do not affect proliferation rates of MKN45‐Luc cells.
Fig. S2. Expression levels of Ror2 and Wnt5a in MKN45‐Luc cells and mesenchymal stem cells.
Fig. S3. Expression of Wnt5a in mesenchymal stem cells is required for the ability of mesenchymal stem cells to promote proliferation of MKN45‐Luc cells in culture.
Fig. S4. Effect of suppressed expression of Ror2 or Wnt5a on viability of mesenchymal stem cells and expression of CXCL16 in mesenchymal stem cells, respectively.
Fig. S5. Expression levels of CXCR6 in MKN45‐Luc cells and mesenchymal stem cells.
Fig. S6. Effects of suppressed expression of CXCR6 on proliferation of MKN45‐Luc cells cultured singly or cocultured with mesenchymal stem cells.
Data S1. Supplementary materials and methods.
Acknowledgments
This study was supported in part by a grant‐in‐aid for Scientific Research on Innovative Area from the Ministry of Education, Culture, Sports, Science and Technology of Japan (23112007 to Y.M.).
Cancer Sci 107 (2016) 290–297
Funding Information
Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. Oishi I, Suzuki H, Onishi N et al The receptor tyrosine kinase Ror2 is involved in non‐canonical Wnt5a/JNK signaling pathway. Genes Cells 2003; 8: 645–54. [DOI] [PubMed] [Google Scholar]
- 2. Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits β‐catenin‐TCF signaling depending on receptor context. PLoS Biol 2006; 4: e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nishita M, Yoo SK, Nomachi A et al Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a‐induced cell migration. J Cell Biol 2006; 175: 555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nishita M, Enomoto M, Yamagata K, Minami Y. Cell/tissue‐tropic functions of Wnt5a signaling in normal and cancer cells. Trends Cell Biol 2010; 20: 346–54. [DOI] [PubMed] [Google Scholar]
- 5. Endo M, Nishita M, Fujii M, Minami Y. Insight into the role of Wnt5a‐induced signaling in normal and cancer cells. Int Rev Cell Mol Biol 2015; 314: 117–48. [DOI] [PubMed] [Google Scholar]
- 6. Enomoto M, Hayakawa S, Itsukushima S et al Autonomous regulation of osteosarcoma cell invasiveness by Wnt5a/Ror2 signaling. Oncogene 2009; 28: 3197–208. [DOI] [PubMed] [Google Scholar]
- 7. O'Connell MP, Flori JL, Xu M et al The orphan tyrosine kinase receptor, ROR2, mediates Wnt5a signaling in metastatic melanoma. Oncogene 2010; 29: 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ren D, Minami Y, Nishita M. Critical role of Wnt5a‐Ror2 signaling in motility and invasiveness of epidermoid carcinoma cells following Snail‐mediated epithelial‐mesenchymal transition. Genes Cells 2011; 16: 304–15. [DOI] [PubMed] [Google Scholar]
- 9. Yamagata K, Li X, Ikegaki S et al Dissection of Wnt5a‐Ror2 signaling leading to matrix metalloproteinase (MMP‐13) expression. J Biol Chem 2012; 287: 1588–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–74. [DOI] [PubMed] [Google Scholar]
- 11. Bergfeld SA, DeClerck YA. Bone marrow‐derived mesenchymal stem cells and the tumor microenvironment. Cancer Metastasis Rev 2010; 29: 249–61. [DOI] [PubMed] [Google Scholar]
- 12. Barcellos‐de‐Souza P, Gori V, Bambi F, Chiarugi P. Tumor microenvironment: bone marrow‐mesenchymal stem cells as key players. Biochim Biophys Acta 2013; 1836: 321–35. [DOI] [PubMed] [Google Scholar]
- 13. Sun Z, Wang S, Zhao RC. The roles of mesenchymal stem cells in tumor inflammatory microenvironment. J Hematol Oncol 2014; 7: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mishra PJ, Mishra PJ, Glod JW, Banerjee D. Mesenchymal stem cells: flip side of the coin. Cancer Res 2009; 69: 1255–8. [DOI] [PubMed] [Google Scholar]
- 15. Quante M, Tu SP, Tomita H et al Bone marrow‐derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 2011; 19: 257–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kurayoshi M, Oue N, Yamamoto H et al Expression of Wnt‐5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res 2008; 68: 10439–48. [DOI] [PubMed] [Google Scholar]
- 17. Shimaoka T, Kume N, Minami M et al Molecular cloning of a novel scavenger receptor for oxidized low density lipoprotein, SR‐PSOX, on macrophages. J Biol Chem 2000; 275: 40663–6. [DOI] [PubMed] [Google Scholar]
- 18. Matloubian M, David A, Engel S, Ryan JE, Cyster JG. A transmembrane CXC chemokine is a ligand for HIV‐coreceptor Bonzo. Nat Immunol 2000; 4: 298–304. [DOI] [PubMed] [Google Scholar]
- 19. Wilbanks A, Zondlo SC, Murphy K et al Expression cloning of the STRL33/BONZO/TYMSTRligand reveals elements of CC, CXC, and CX3C chemokines. J Immunol 2001; 166: 5145–54. [DOI] [PubMed] [Google Scholar]
- 20. Gough PJ, Garton KJ, Wille PT et al A disintegrin and metalloproteinase 10‐mediated cleavage and shedding regulates the cell surface expression of CXC chemokine ligand 16. J Immunol 2004; 172: 3678–85. [DOI] [PubMed] [Google Scholar]
- 21. Abel S, Hundhausen C, Mentlein R et al The transmembrane CXC‐chemokine ligand 16 is induced by IFN‐γ and TNF‐α and shed by the activity of the disintegrin‐like metalloproteinase ADAM10. J Immunol 2004; 172: 6362–72. [DOI] [PubMed] [Google Scholar]
- 22. Liao F, Alkhatib G, Peden KW, Sharma G, Berger EA, Farber JM. STRL33, a novel chemokine receptor‐like protein, functions as a fusion cofactor for both macrophage‐tropic and T cell line‐tropic HIV. J Exp Med 1997; 185: 2015–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu W, Zhen X, Xiong B, Wang B, Zhang W, Zhou W. CXCR6 is expressed in human prostate cancer in vivo and is involved in the in vitro invasion of PC3 and LNCap cells. Cancer Sci 2008; 99: 1362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang J, Lu Y, Wang J, Koch AE, Zhang J, Taichman RS. CXCR6 induces prostate cancer progression by the AKT/mammalian target if rapamycin signaling pathway. Cancer Res 2008; 68: 10367–76. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25. Darash‐Yahana M, Gillespie JW, Hewitt SM et al The chemokine CXCL16 and its receptor, CXCR6, as markers and promoters of inflammation‐associated cancers. PLoS ONE 2009; 4: e6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deng L, Chen N, Li Y, Zheng H, Lei Q. CXCR6/CXCL16 functions as a regulator in metastasis and progression of cancer. Biochim Biophys Acta 2010; 1806: 42–9. [DOI] [PubMed] [Google Scholar]
- 27. Kidd S, Spaeth E, Watson K et al Origins of the tumor microenvironment: quantitative assessment of adipose‐derived and bone marrow‐derived stroma. PLoS ONE 2012; 7: e30563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Cell densities in culture do not affect proliferation rates of MKN45‐Luc cells.
Fig. S2. Expression levels of Ror2 and Wnt5a in MKN45‐Luc cells and mesenchymal stem cells.
Fig. S3. Expression of Wnt5a in mesenchymal stem cells is required for the ability of mesenchymal stem cells to promote proliferation of MKN45‐Luc cells in culture.
Fig. S4. Effect of suppressed expression of Ror2 or Wnt5a on viability of mesenchymal stem cells and expression of CXCL16 in mesenchymal stem cells, respectively.
Fig. S5. Expression levels of CXCR6 in MKN45‐Luc cells and mesenchymal stem cells.
Fig. S6. Effects of suppressed expression of CXCR6 on proliferation of MKN45‐Luc cells cultured singly or cocultured with mesenchymal stem cells.
Data S1. Supplementary materials and methods.


