Abstract
The expression of estrogen receptor is the key in most breast cancers (BC) and binding of estrogen receptor to the genome correlates to Forkhead protein (FOXA1) expression. We herein assessed the correlation between the cancer stem cell (CSC) population and FOXA1 expression in luminal BC. We established luminal BC cells derived from metastatic pleural effusion and analyzed the potency of CSC and related factors with established luminal BC cell lines. We also confirmed that mammosphere cultures have an increased aldehyde dehydrogenase‐positive population, which is one of the CSC markers, compared with adherent culture cells. Using a quantitative PCR analysis, we found that mammosphere forming cells showed a higher expression of FOXA1 and stemness‐related genes compared with adherent culture cells. Furthermore, the growth activity and colony‐forming activity of 4‐hydroxytamoxifen‐treated BC cells were inhibited in a mammosphere assay. Interestingly, 4‐hydroxytamoxifen‐resistant cells had significantly increased FOXA1 gene expression levels. Finally, we established short hairpin RNA of FOXA1 (shFOXA1) MCF‐7 cells and investigated the relationship between self‐renewal potential and FOXA1 expression. As a result, we found no significant difference in the number of mammospheres but decreased colony formation in shFOXA1 MCF‐7 cells compared with control. These results suggest that the expression of FOXA1 appears to be involved in the proliferation of immature BC cells rather than the induction of stemness‐related genes and self‐renewal potency of CSCs.
Keywords: Breast cancer, cancer stem cells, FOXA1, mammosphere, tamoxifen resistance
Breast cancer (BC) treatment has progressed in recent decades. Many previous clinical studies clarified that pathological characteristics, such as estrogen receptor (ER), progesterone receptor (PgR), human epidermal growth factor receptor 2 (HER2), and Ki‐67 expression, correlate with the gene expression profile, such as PAM50,1, 2 prognosis, and treatment effects. Therefore, we can clinically divide BC into five types according to these pathological characteristics in consideration of the therapeutic plan: luminal A, luminal B, luminal‐HER2, HER2‐enrich, and triple negative type.3 For luminal A and B, which are categorized as ER‐positive BC, endocrine therapy can be used with chemotherapy treatment.
It is known that ER‐positive BC constitute approximately 70% of all BC, and it shows a good prognosis.4, 5, 6 However, it has been reported that ER‐positive and HER2‐negative BC patients with low Ki‐67 proliferation, so‐called luminal A type, can lead to late recurrence compared to other BC subtypes, possibly due to the presence of dormant tumor cells.7 The BC markers of late recurrence8, 9, 10 or commercialized prognostic prediction systems such as Oncotype DX11 and MammaPrint12 have been established. However, the BC cell mechanism of late recurrence is still poorly understood.
Cancer stem cells (CSCs) have been isolated and characterized to have high potential for tumor growth, self‐renewal, multidifferentiation, high metastatic ability, and drug resistance.13, 14, 15, 16, 17, 18 Interestingly, there are many similar biological characteristics between dormant cells and CSCs, especially in terms of therapy resistance and cell signals that regulate self‐renewal and the quiescent state of CSCs.19, 20, 21 Therefore, it is speculated that CSCs might contribute to late recurrence with dormant cells,22 and we hypothesized that some part of CSCs would be involved in the late recurrence of luminal type A in this study.
Al‐Hajj et al.13 reported that the CD44+/CD24−/low population of BC cells showed higher tumor initiating potential than other populations. In addition, Ginestier et al.23 reported that aldehyde dehydrogenase (ALDH) 1 is a good marker for both stem cells of normal mammary cells and BC cells. Moreover, it has been reported that the expression of these CSC markers correlate with a clinically poor outcome.23, 24, 25, 26 These markers were positively expressed in triple negative BC cells as well as in normal mammary stem/progenitor cells. Additionally, most of the previous reports showed that they were ER‐negative.27 Regarding CSCs of the luminal type of BC, Sun et al.28 reported that estrogen promotes stemness and invasiveness through Gli1 activation, however, the mechanisms that maintain CSCs in luminal BC remains obscure.
Forkhead protein (FOXA1) is the downstream target of GATA binding protein 3 and maintains ER sensitivity.29 FOXA1 directly binds to the estrogen receptor 1 promoter and activates ER mRNA expression.30 Moreover, it is known that FOXA1 binds to chromatin DNA and modulates ER activity.31, 32 Conversely, it has been reported that FOXA1 activates p27 transcription and works as a growth repressor.33 Until now, the correlation between FOXA1 and CSCs through ER has not yet been determined. According to tissue samples, Horimoto et al.34 clarified that those metastatic breast cancer patients whose tissue highly expressed FOXA1 took a long time to relapse compared to low FOXA1 patients, indicating that FOXA1 might be related to late recurrence. Taken together, the tendency for luminal A type BC and FOXA1‐enriched BC to cause late recurrence, and the similarity between CSCs and dormant cells, it may be hypothesized that FOXA1 expression, especially in CSCs, causes late recurrence and provides a poor prognosis for patients.
In this study, we investigated the mechanisms of initiation of luminal breast CSCs to understand how those CSCs influence BC late recurrence. First, we analyzed the potency of CSCs and related factors by a mammosphere assay compared to adherent culture BC cells. Under mammosphere culture, we found that CSC populations of luminal BC have increased FOXA1 expression compared with the control population. The FOXA1 knockdown study showed that FOXA1 is involved in the proliferation of immature BC cells rather than the induction of stemness‐related genes and self‐renewal potency of CSCs. Our findings may therefore help to establish a novel strategy to treat luminal BC and its late recurrence.
Materials and Methods
Cell lines
The MCF‐7 and MDA‐MB‐231 human breast cancer cell lines were purchased from RIKEN BioResource Center (Ibaraki, Japan). HCC1500 human breast cancer cells were purchased from ATCC (Manassas, VA, USA). All cells were cultured in DMEM high glucose medium (Gibco; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (Thermo Fisher Scientific), l‐glutamine, MEM–non‐essential amino acid solution, and penicillin/streptomycin. Cells were maintained in a humidified incubator at 37°C at an atmospheric pressure of 5% (v/v) CO2/air.
Patient sample and established cell lines
A surgical breast cancer tissue sample and metastatic pleural effusion from a breast cancer patient (79 years of age, ER [+], PgR [+], HER2 [0]), under aromatase inhibitor treatment, were harvested following the approval of the ethics committee at University of Tsukuba (Tsukuba, Japan). The pleural effusion was treated with RosetteSep Human CD45 Depletion Cocktail (Stemcell Technologies, Vancouver, BC, Canada) according to the manufacturer's instructions. The cells were then laid onto a density gradient buffer (HISTOPAQUE‐1083; Sigma‐Aldrich, St. Louis, MO, USA) and centrifuged at 460g for 20 min at room temperature. The mononuclear cells were collected and plated at the density of 2 × 105–2 × 106 cells/mL in a 25‐cm2 culture flask (Sumitomo Bakelite, Osaka, Japan) and maintained at 37°C in 5% CO2.
Establishment of shFOXA1 cells
We purchased OmicsLink shRNA Expression Clone targeted to FOXA1 and scrambled control gene (GeneCopoeia, Rockville, MD, USA). The shRNA clones were transfected into HEK293T cells using the Lenti‐Pac HIV Expression Packaging Kit (GeneCopoeia) and pseudo‐viral particles were harvested.
These particles were then transduced into target cells and selected with puromycin. The effect of shRNA of FOXA1 was assessed by an immunoblot analysis.
Flow cytometry
To characterize and analyze BC cells, flow cytometry was used (MoFlo XDP; Beckman Coulter, Tokyo, Japan) with CD44‐FITC antibody (BD Biosciences, San Jose, CA, USA) and CD24‐PE antibody (BioLegend, San Diego, CA, USA) as previously reported.31 For ALDH activity, an ALDEFLUOR kit (Stemcell Technologies) was used according to the manufacturer's instructions.
Mammosphere formation assay
Mammosphere culture was carried out using MammoCult medium (Stemcell Technologies), hydrocortisone, penicillin/streptomycin, and heparin according to the manufacturer's instructions. Approximately 1 × 104 cells were plated onto 35‐mm petri dishes (Sumitomo Bakelite), and the number of mammospheres over 100‐μm diameter was counted under a microscope. To analyze the effects of 4‐hydroxytamoxifen (4‐OHT; Sigma‐Aldrich, Tokyo, Japan) on the mammospheres derived from BC cells, a concentration of 1 μM 4‐OHT/dish was used.
Colony formation assay
The cells were seeded in triplicate at 100 cells/6‐well plate (Sumitomo Bakelite) in complete medium. After 2 weeks, the cells were fixed and stained with 0.5% w/v crystal violet in methanol. Visible colonies were scored by macroscopic observation.
Quantitative RT‐PCR
Total RNA was prepared from samples using extraction reagent (Sepasol‐RNA I Super G; Nacalai Tesque, Kyoto, Japan), and cDNA was synthesized by reverse transcription using the ReverTra Plus kit (Toyobo, Osaka, Japan). The expression levels of target genes were analyzed using a 7500 Fast Real‐Time PCR machine (Applied Biosystems, Foster City, CA, USA) with Thunderbird SYBR qPCR Mix (Toyobo). Experiments were carried out in triplicate and data were calculated using the ΔCt method. The sequences of the primers used for quantitative RT‐PCR are shown in Table 1.
Table 1.
Polymerase chain reaction primers used in this study
| Human Oct4 | Sense: | 5′‐CTGGGGGTTCTATTTGGGAAGGTA‐3′ |
| Antisense: | 5′‐CTGCAGGAACAGATTCTCCAGGTT‐3′ | |
| Human Nanog | Sense: | 5′‐ACAGAAATACCTCAGCCTCCAGCA‐3′ |
| Antisense: | 5′‐CTCCAGGTTGAATTGTTCCAGGTC‐3′ | |
| Human Sox2 | Sense: | 5′‐ GAGTGGAAACTTTTGTCGGAGACG‐3′ |
| Antisense: | 5′‐CCGGTATTTATAATCCGGGTGCTC‐3′ | |
| Human ESR1 | Sense: | 5′‐ATGTGTAGAGGGCATGGTGGAGAT‐3′ |
| Antisense: | 5′‐GACTTCAGGGTGCTGGACAGAAAT‐3′ | |
| Human FOXA1 | Sense: | 5′‐ACTCGTACATCTCGCTCATCACCA‐3′ |
| Antisense: | 5′‐CAAGTAGCAGCCGTTCTCGAACAT‐3′ | |
| Human β‐actin | Sense: | 5′‐CTGGCACCACACCTTCTACAATGA‐3′ |
| Antisense: | 5′‐TAGCACAGCCTGGATAGCAACGTA‐3′ |
Western blot analysis
Whole cell extract was prepared using RIPA buffer (50 mM Tris, 150 mM NaCl, 0.5% sodium deoxycholate, 1% NP‐40, and 0.1% SDS). Samples were electrophoresed on a 7.5% SDS–polyacrylamide gel and transferred onto PVDF membranes (Merck Millipore, Darmstadt, Germany). After blocking with 5% skim milk/TBST buffer, the membranes were incubated with anti‐FOXA1 antibody (clone2F83, dilution 1:1000; Abcam, Tokyo, Japan) or anti‐actin antibody as control (clone C‐11, dilution 1:2000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing, the membranes were incubated with HRP‐conjugated secondary antibodies, and positive signals were analyzed by a luminescence imager (Image Quant LAS4000; GE Healthcare, Little Chalfont, UK) using chemiluminescence reagents (Merck Millipore).
Immunohistochemical staining
The cell pellets were fixed in 10% formalin and 70% ethanol. After fixation, samples were embedded in paraffin blocks.
Immunohistochemical staining was carried out by the Translational Research and Resource Core at the University of Tsukuba. Nearly all staining methods, except for HER2, were based on the autostainer BenchMark ULTRA (Roche, Tokyo, Japan) protocols; HER2 was stained as recommended by the supplier. The following antibodies were used: mouse anti‐FOXA1 antibody (clone2F83, dilution 1:2000; Abcam); Ventana ultraView confirm ER (clone SP1; Roche); Ventana ultraView confirm PgR (clone 1E2; Roche); Histofine HER2 kit (Nichirei, Tokyo, Japan); cytokeratin (CK) 5/6 (clone D5/16 B4; Dako, Tokyo, Japan); and CK 8 (clone 35H11; Dako).
Growth inhibition curve analysis
To investigate the effects of 4‐OHT on cell growth, we modified the method reported previously.35 Both MCF‐7 and BC#1 cells were incubated at various concentrations of 4‐OHT for 72 h. Cells were harvested and viable cells were counted using the Trypan blue exclusion method.
Statistical analysis
All results are expressed as the means ± SD from three or more independent experiments. A statistical evaluation of the data was carried out using Student's t‐test. P‐values <0.05 were considered to be significantly different.
We used the GraphPad Prism 6 software program (GraphPad Software, San Diego, CA, USA) for all analyses.
Results
Characteristics of BC‐derived cells
To clarify the stemness of luminal BC, we first isolated BC cells from metastatic breast cancer pleural effusion and characterized them. Immunohistochemical staining of the surgical tissue is shown in Figure 1(a): ER‐positive, HER2‐negative, and FOXA1‐positive staining was evident in approximately 80–90% of cells. NANOG, OCT4, and SOX2 were not stained in the tissue sample (data not shown). In newly isolated cells, named BC#1 cells, ER was not expressed, whereas PgR was expressed in nearly 80% by immunohistochemical staining (Fig. 1b, Table 2). As the PgR expression in the tumor has been determined to be an indication of functional ER,36, 37 we analyzed the expression of ER in BC#1 cells. BC#1 cells were negative for ER according to immunohistochemical staining, whereas ER mRNA expression was higher in BC#1 than in MDA‐MB‐231 cells (Fig. 1c). The reason behind the decreased ER expression of BC#1 was surmised as follows. It has been reported that the hormone receptor discordance between primary and recurrent BC has occurred at the frequency of 10–40%, because of the change in tumor characteristics after treatment.38 In addition, it is known that long‐term estrogen deprivation in culture causes the instability of ER expression in vitro.39 These factors might cause the discrepancy of the ER expression in BC#1 cells isolated from the pleural effusion. According to epithelial markers, approximately 100% of BC#1 cells were strongly positive for CK 8 and partially positive for CK 5/6. Therefore, we confirmed that there is little contamination of the non‐epithelial population in BC#1 cells. MCF‐7 and HCC1500 cells were positive for CK 8 (Fig. 1b, Table 2). We next examined FOXA1 expression by immunohistochemical staining. FOXA1 was positively expressed in MCF‐7 and HCC1500 cells, but not in BC#1 cells (Fig. 1b).
Figure 1.
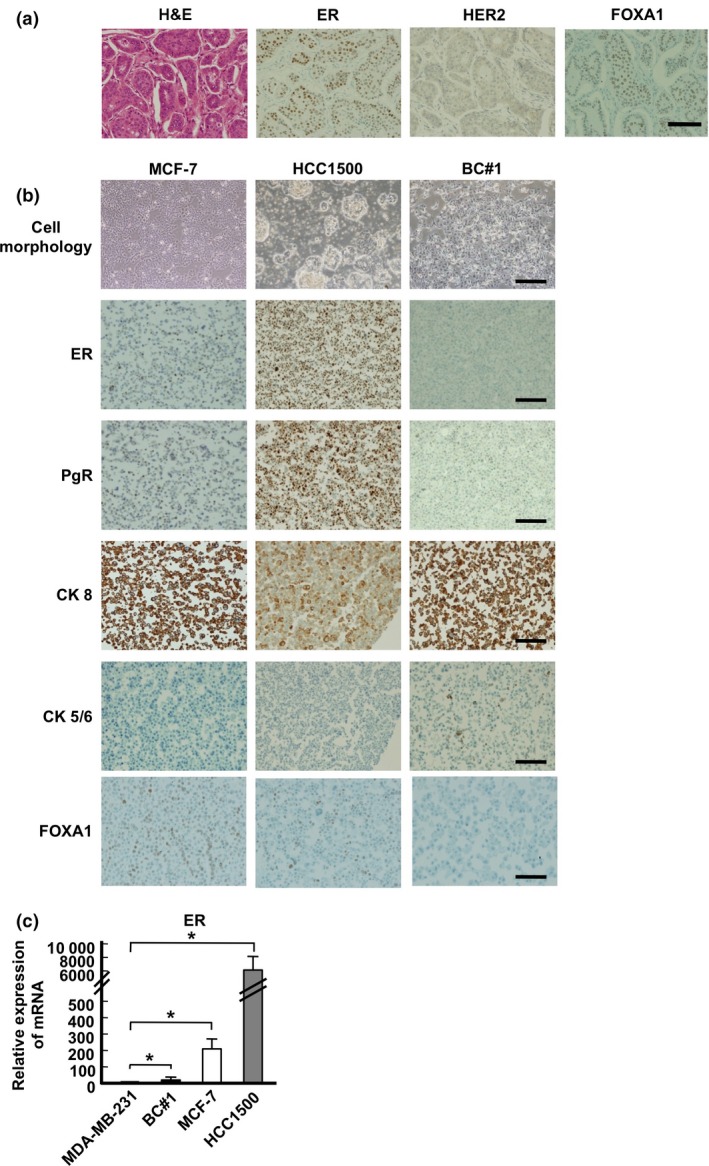
Characteristics of breast cancer cells. (a) Histopathological features of derived breast cancer tissue (magnification, 20×). (b) Cell morphology of culture cells (magnification, 4×) and immunocytochemical staining (magnification, 10×). (c) mRNA expression of estrogen receptor (ER) in MDA‐MB‐231, BC#1 (black bar), MCF‐7 (white bar), and HCC1500 (gray bar) cells. The data are presented as the means ± SD from independent measurements. P‐value compared between MDA‐MB‐231 (as a control) and other cell lines was calculated using Student's t‐test. *P < 0.05. Bar indicates 100 μm (a), 200 μm (b, except top column), 500 μm (b, top column). CK, cytokeratin; FOXA1, Forkhead protein; HER2, human epidermal growth factor receptor 2; PgR, progesterone receptor.
Table 2.
Characteristics of cell lines used in this study
| Cell line (ATCC name) | ER | PgR | HER2 | CK 8 | CK 5/6 |
|---|---|---|---|---|---|
| MCF7 (HTB‐22) | + | + | − | + | − |
| HCC1500 (CRL‐2329) | + | + | − | + | − |
| BC#1 | − | + | − | + | + |
+, Positively expressed; −, no expression. CK, cytokeratin; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PgR, progesterone receptor.
According to these findings, the newly isolated BC#1 cells were categorized into the luminal type of BC cells and were found to express different characteristics from commercially available BC cell lines.
Expression of stemness markers and mammosphere forming capacity of different luminal BC cells
We evaluated the ALDH activity and frequency of CD44+/24− cells in our luminal BC cell lines (Figs 2a, S1). We found that BC#1 contains CSC populations at the highest frequency among the three BC cell lines investigated. The number of mammospheres was the highest in BC#1 compared to the other two BC cell lines (Fig. 2b and 2c). It has been previously reported that the changing of culture conditions from regular adherent culture to mammosphere culture led to the elevated expression of genes related to stemness.17 As shown in Figure 2(d,e), under a mammosphere culture, ALDH‐positive MCF‐7 cells increased compared to MCF‐7 cells cultured under adherent conditions.
Figure 2.
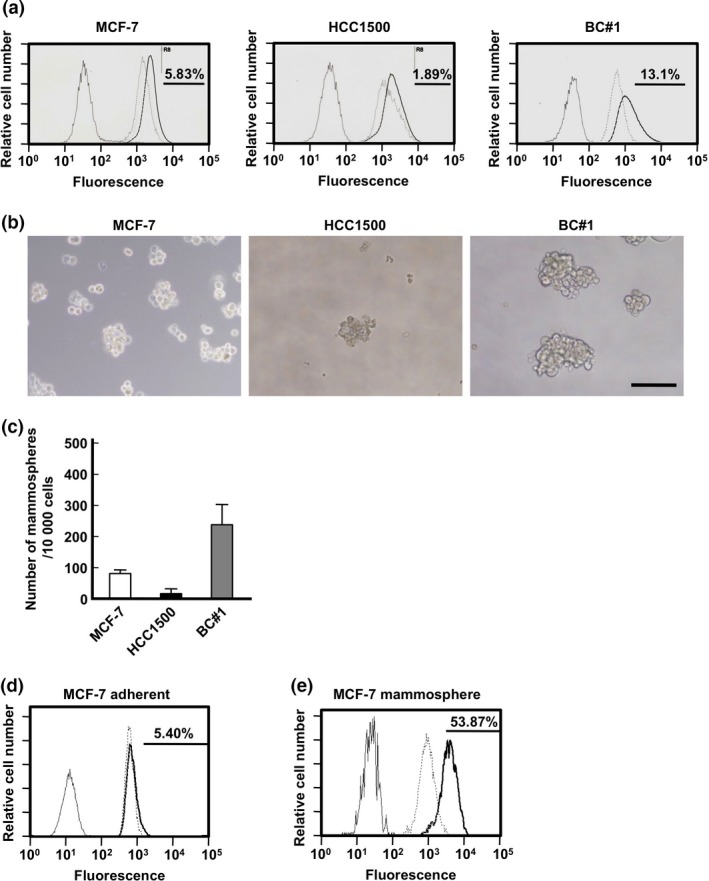
Assessment of the potential of cancer stemness. (a) Aldehyde dehydrogenase (ALDH) activity of MCF‐7 (left), HCC1500 (middle), and BC#1 (right) cell lines. Black line, unstained sample; broken line, sample treated with N,N‐diethylaminobenzaldehyde (inhibitor) and ALDH reagent; black bold line, sample treated with ALDH reagent. The black bar and rate shows the positive area. (b) Morphology of MCF‐7 (left), HCC1500 (middle), and BC#1 (right) cells in mammosphere culture. Bar indicates 200 μm. (c) The number of mammospheres greater than 100 μm in diameter in each cell line. Black bar, HCC1500; gray bar, BC#1; white bar, MCF‐7. (d, e) ALDH activity in adherent culture samples (d) and mammosphere culture samples (e) of MCF‐7 cells. Black line, unstained sample; broken line, sample treated with N,N‐diethylaminobenzaldehyde and ALDH reagent; black bold line, sample treated with ALDH reagent. The black bar and rate shows the positive area.
Mammosphere culture causes ectopic expression of FOXA1 and stemness‐related genes
First, the mRNA expression of ER, FOXA1, and stemness‐related genes NANOG, SOX2, and OCT4 were analyzed by quantitative PCR analyses.28, 40, 41, 42 There was no significant difference in the expression of ER between mammosphere and adhesion cultured cells. In contrast, the expression of FOXA1 under mammosphere culture was significantly higher than that under adherent culture (Fig. 3a). Similar to FOXA1 expression, stemness‐related genes were highly expressed in MCF‐7 and BC#1 cell lines under mammosphere culture compared to those under an adherent culture. Because lower mammosphere formation was observed in HCC1500 compared to that of MCF‐7 and BC#1 cell lines, we could not harvest sufficient samples for the analysis of gene expression. A Western blot analysis clearly indicated that FOXA1 protein expression was highly upregulated in both MCF‐7 and BC#1 cell lines under mammosphere culture compared to adherent culture (Fig. 3b).
Figure 3.
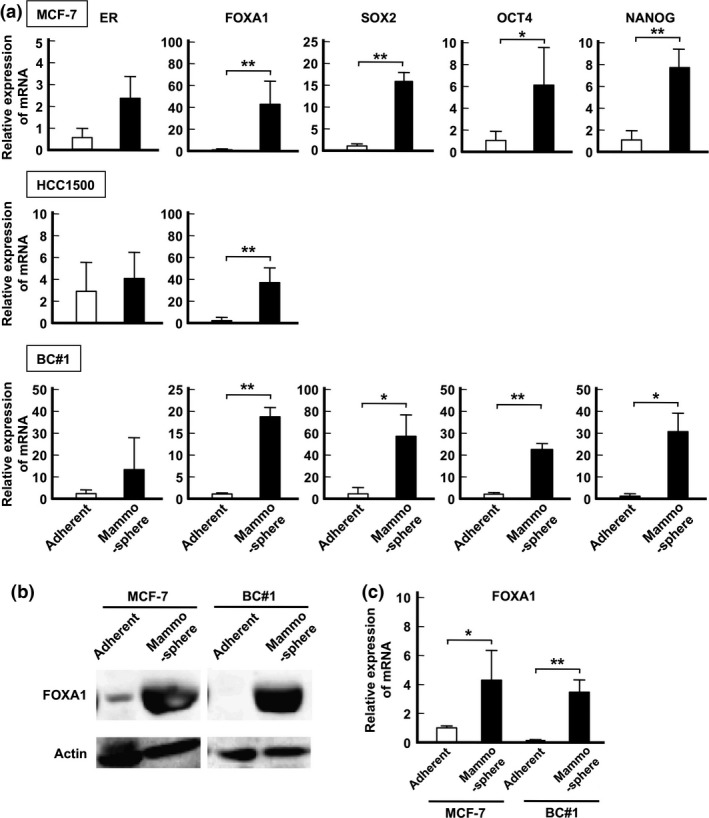
mRNA expression and Forkhead (FOXA1) levels compared between adherent culture samples and mammosphere culture samples. (a) mRNA expression levels of ER, FOXA1, SOX2, OCT4, and NANOG in each cell line are presented as the means ± SD from independent measurements. *P < 0.05, **P < 0.01, Student's t‐test. (b) Protein expression of FOXA1 in MCF‐7 (left) and BC#1 (right) cells compared between adherent culture and mammosphere culture by a Western blot analysis. (c) Relative expression of FOXA1 protein compared between cells grown in adherent culture and mammosphere culture. The expression levels were measured using Image J software and presented as the means ± SD from independent measurements. *P < 0.05, **P < 0.01, Student's t‐test.
Ectopic FOXA1 expression in mammospheres is correlated to 4‐OHT resistance
The correlation between drug therapy resistance and FOXA1 has also been reported previously.34, 43, 44 To determine the concentration of 4‐OHT for BC cell resistance, we examined the proliferative activity of MCF‐7 and BC#1 cell lines in the presence of various concentrations of 4‐OHT. As shown in Figure 4(a), both MCF‐7 and BC#1 cells tended to maintain growth activity over 50% when 4‐OHT was added at a concentration less than 1 μM. No significant morphologic changes were observed in MCF‐7 or BC#1 cell lines (data not shown). Therefore, we used 4‐OHT at the concentration of 1 μM in this study. Next, we examined the effect of 4‐OHT on mammosphere culture. The number of mammospheres in MCF‐7 and BC#1 cell lines drastically decreased in the presence of 4‐OHT (Fig. 4b,c). The expression of ER mRNA and stemness‐related genes in mammospheres from MCF‐7 and BC#1 cells did not differ according to the presence or absence of 4‐OHT. In contrast, FOXA1 mRNA expression of 4‐OHT‐treated mammospheres was significantly higher than that of the control (MCF‐7, P = 0.0210; BC#1, P = 0.0353; Fig. 4d).
Figure 4.
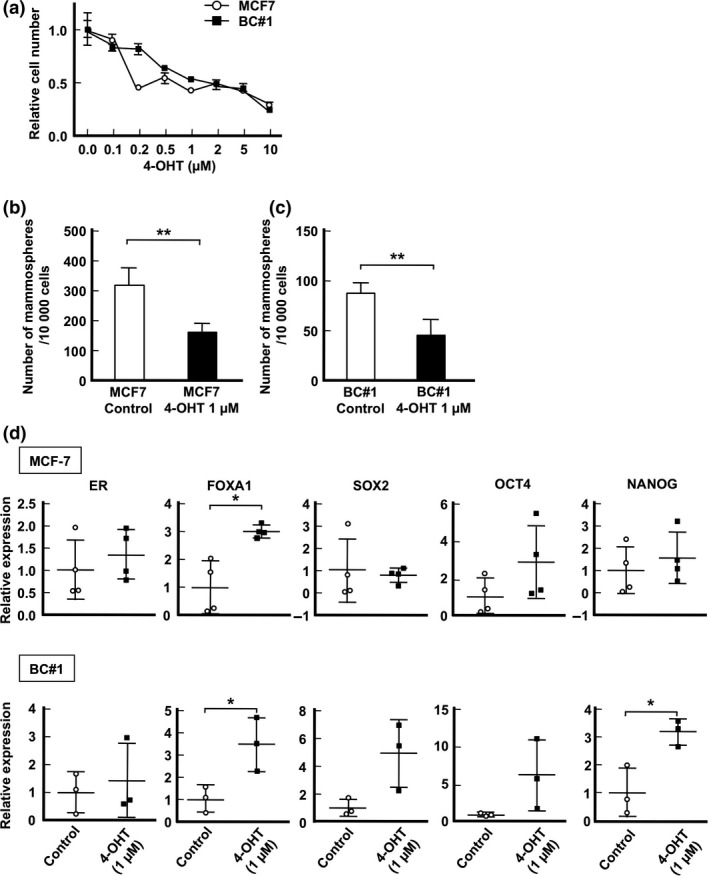
Effect of 4‐hydroxytamoxifen (4‐OHT) treatment on mammosphere assay. (a) Cell growth inhibition curves with 4‐OHT treatment in MCF‐7 (circle) and BC#1 (square) cell lines are presented as the means ± SD. (b, c) Mammosphere numbers greater than 100 μm in diameter in MCF‐7 (b) and BC#1 (c) cells. (d) mRNA expression levels of mammospheres from MCF‐7 and BC#1 cells in control and 4‐OHT‐treated samples. Data are presented as the means ± SD from independent measurements. *P < 0.05, **P < 0.01, Student's t‐test.
Knockdown expression of FOXA1 does not correlate with expression of stemness‐related genes
To investigate the effect of FOXA1 on the expression of stemness‐related genes or mammosphere forming cells, we established FOXA1 knockdown MCF‐7 cells (shFOXA1 MCF‐7 cells, Fig. 5a). These cells showed downregulated ER and AGR2 expression, which have been reported to be downstream of FOXA1.45 However, the expression of stemness‐related genes, NANOG and OCT4, was maintained at a high level compared to the control (Fig. 5b). No significant difference in the number of mammospheres between shFOXA1 and control cells was observed (Fig. 5c), suggesting that FOXA1 may not affect the generation of CSCs. The colony formation assay is widely used to evaluate the proliferative ability of immature cells.28, 46 As shown in Figure 5(d,e), the number of colonies derived from shFOXA1 MCF‐7 cells significantly decreased compared to control cells.
Figure 5.
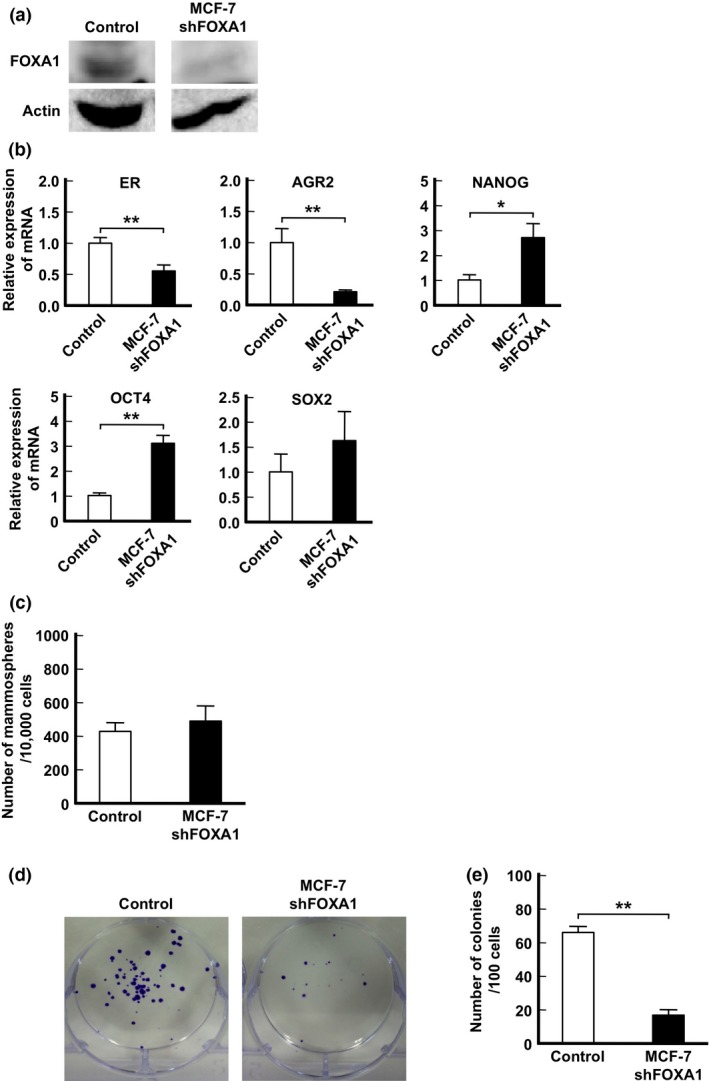
Colony formation of Forkhead (FOXA1) knockdown MCF‐7 cells. (a) Protein expression of FOXA1 compared between control and FOXA1 knockdown cells by a Western blot analysis. (b) mRNA expression of MCF‐7 compared between control and FOXA1 knockdown cells. Data are presented as the means ± SD from independent measurements. *P < 0.05, **P < 0.01, Student's t‐test. (c) Number of mammospheres greater than 100 μm in diameter in MCF‐7 shControl and shFOXA1 cells. Data are presented as the means ± SD from independent measurements. (d) Representative photographs of colony formation. The colonies were stained purple. (e) Comparison of number of colonies formed between control and FOXA1 knockdown cells. Data are presented as the means ± SD from independent measurements. **P < 0.01, Student's t‐test.
Taken together, these findings indicate that FOXA1 appears to work as a factor in the proliferation rather than maintenance of stemness‐related genes or the activation of CSCs.
Discussion
Understanding the characteristics and properties of CSCs is crucial for clinical application. Metastases and recurrence are known to correlate with CSCs, and chemotherapeutic resistance and endocrine therapy‐resistance of BC are generally known to correlate with CSC populations.47, 48, 49, 50 Therefore, CSCs are the major challenge in BC treatment difficulties.
One of the methods to assess the potency of CSCs is the mammosphere assay. Mammosphere culture was first reported by Dontu et al.17 to evaluate the potency of progenitor/stem cells. A correlation has also been shown between mammospheres and epithelial–mesenchymal transition51, 52, 53 and drug resistance.53 Thus, we speculated that analyses of BC colonies formed under the mammosphere assay would elucidate the mechanism by which CSCs in luminal BC arise. In order to enrich BC cells for CSC populations, mammosphere culture was used and investigated in this study. Harrison et al.54 previously showed that the CD44+/CD24− population generates more mammospheres than other cell populations. Furthermore, it has been reported that an assessment of mammospheres could be useful to predict prognosis.55 In this study, we determined that both established BC cell lines and commercially established BC cell lines had CSC marker positive populations and could form mammospheres. Each mammosphere showed high expression of stemness‐related genes, such as NANOG, SOX2, and OCT4. Interestingly, mammosphere‐forming cells showed high expression of FOXA1 mRNA and protein compared to adherent culture samples. By contrast, BC#1 cells in adherent culture did not express FOXA1 according to immunohistochemical staining and Western blotting analyses. These findings suggest that CSCs cultured under specific culture atmospheres increase the CSC population and induce stemness‐related genes as well as FOXA1 expression.
Because BC is composed of various subtypes, the FOXA1 expression determined by immunohistochemical staining was observed in the majority of the luminal subtype. Therefore, we hypothesized that the prognosis of the luminal subtype regarding FOXA1 expression would be superior to other types. Indeed, a tissue analysis by immunohistochemical staining showed that FOXA1 is a good prognosis marker of formalin‐fixed paraffin‐embedded tissue staining.33, 44, 56 It has also been reported that late recurrence also tends to occur in luminal BC,7 not in other subtypes. Some studies also show that FOXA1 expression correlates with metastatic lesions of luminal BC.34, 57 Furthermore, Ross‐Innes et al.57 showed that there are specific and reoccurring cis‐regulatory elements that are occupied by ER in BC; however, these vary according to good and poor outcome samples, and FOXA1 mediates ER reprogramming. The ectopic expression of FOXA1 observed in our study may correlate with this mechanism.
Drug resistance is a significant problem that influences the CSC ability. Raffo et al.47 compared the mammosphere formation capacity and in vivo tumorigenicity between parental MCF‐7 and 4‐OHT‐resistant MCF‐7 cells and showed that 4‐OHT‐resistant MCF‐7 cells had an increased mammosphere number and tumorigenicity compared to parent MCF‐7 cells. Moreover, Calcagno et al.48 reported that doxorubicin‐resistant MCF‐7 cells showed high stemness potential using a mammosphere assay, CSC markers, and tumorigenicity. Lin et al.58 described that tamoxifen‐resistant BC cells possibly emerge from CSC populations by validating the DNA methylation and SOX2 gene expression. In our study, the colony‐forming activity of 4‐OHT‐treated BC cells was inhibited in the mammosphere assay. Interestingly, 4‐OHT‐resistant cells had significantly increased FOXA1. Moreover, we established shFOXA1 MCF‐7 cells and investigated the relationship between their self‐renewal potential and FOXA1 expression. As a result, the number of mammospheres was not significantly different between shFOXA1 MCF‐7 cells and control cells. We also observed decreased colony formation in shFOXA1 MCF‐7 cells than control cells, indicating the contribution of specific culture conditions in the mammosphere assay to activate CSCs formation.
In conclusion, our findings showed for the first time that the CSC population in luminal BC induces ectopic expression of FOXA1. The expression of FOXA1 appears to be involved in the proliferation of immature BC cells rather than the induction of stemness‐related genes and self‐renewal potency of CSCs.
Further studies are necessary to investigate the process of ectopic FOXA1 expression in BC development and its biological influence. These findings are thus expected to help establish a novel strategy to treat luminal BC and its late recurrence.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. CD44 and CD24 expression in luminal breast cancer cells. The horizontal axis shows the intensity of CD44 staining and vertical axis shows the intensity of CD24 staining. R5 fracture shows the CD44+/CD24− population. Left column, MCF‐7 cells; middle column, HCC1500 cells; right column, BC#1 cells.
Acknowledgments
This work was supported by a Grant‐in Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Cancer Sci 107 (2016) 281–289
Funding Information
Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. Perou CM, Sorlie T, Eisen MB et al Molecular portraits of human breast tumours. Nature 2000; 406: 747–52. [DOI] [PubMed] [Google Scholar]
- 2. Parker JS, Mullins M, Cheang MC et al Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 2009; 27: 1160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coates AS, Winer EP, Goldhirsch A et al Tailoring therapies‐improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015; 26: 1533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sorlie T, Perou CM, Tibshirani R et al Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001; 98: 10869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamashita H, Iwase H, Toyama T et al Estrogen receptor‐positive breast cancer in Japanese women: trends in incidence, characteristics, and prognosis. Ann Oncol 2011; 22: 1318–25. [DOI] [PubMed] [Google Scholar]
- 6. Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res 2007; 9: R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nishimura R, Osako T, Nishiyama Y et al Evaluation of factors related to late recurrence – later than 10 years after the initial treatment – in primary breast cancer. Oncology 2013; 85: 100–10. [DOI] [PubMed] [Google Scholar]
- 8. Dubsky P, Brase JC, Jakesz R et al The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2− breast cancer patients. Br J Cancer 2013; 109: 2959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sgroi DC, Sestak I, Cuzick J et al Prediction of late distant recurrence in patients with oestrogen‐receptor‐positive breast cancer: a prospective comparison of the breast‐cancer index (BCI) assay, 21‐gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol 2013; 14: 1067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahn SG, Lee HM, Cho SH et al The difference in prognostic factors between early recurrence and late recurrence in estrogen receptor‐positive breast cancer: nodal stage differently impacts early and late recurrence. PLoS ONE 2013; 8: e63510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paik S, Shak S, Tang G et al A multigene assay to predict recurrence of tamoxifen‐treated, node‐negative breast cancer. N Engl J Med 2004; 351: 2817–26. [DOI] [PubMed] [Google Scholar]
- 12. van't Veer LJ, Dai H, van de Vijver MJ et al Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002; 415: 530–6. [DOI] [PubMed] [Google Scholar]
- 13. Al‐Hajj M, Wicha MS, Benito‐Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003; 100: 3983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abdullah LN, Chow EK. Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med 2013; 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dylla SJ, Beviglia L, Park IK et al Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS ONE 2008; 3: e2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guler G, Balci S, Costinean S et al Stem cell‐related markers in primary breast cancers and associated metastatic lesions. Mod Pathol 2012; 25: 949–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dontu G, Abdallah WM, Foley JM et al In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 2003; 17: 1253–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Connor ML, Xiang D, Shigdar S et al Cancer stem cells: a contentious hypothesis now moving forward. Cancer Lett 2014; 344: 180–7. [DOI] [PubMed] [Google Scholar]
- 19. Jo M, Eastman BM, Webb DL, Stoletov K, Klemke R, Gonias SL. Cell signaling by urokinase‐type plasminogen activator receptor induces stem cell‐like properties in breast cancer cells. Cancer Res 2010; 70: 8948–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tobar N, Villar V, Santibanez JF. ROS‐NFkappaB mediates TGF‐beta1‐induced expression of urokinase‐type plasminogen activator, matrix metalloproteinase‐9 and cell invasion. Mol Cell Biochem 2010; 340: 195–202. [DOI] [PubMed] [Google Scholar]
- 21. Kusumbe AP, Bapat SA. Cancer stem cells and aneuploid populations within developing tumors are the major determinants of tumor dormancy. Cancer Res 2009; 69: 9245–53. [DOI] [PubMed] [Google Scholar]
- 22. Kleffel S, Schatton T. Tumor dormancy and cancer stem cells: two sides of the same coin? Adv Exp Med Biol 2013; 734: 145–79. [DOI] [PubMed] [Google Scholar]
- 23. Ginestier C, Hur MH, Charafe‐Jauffret E et al ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007; 1: 555–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsang JY, Huang YH, Luo MH et al Cancer stem cell markers are associated with adverse biomarker profiles and molecular subtypes of breast cancer. Breast Cancer Res Treat 2012; 136: 407–17. [DOI] [PubMed] [Google Scholar]
- 25. Yoshioka T, Umekita Y, Ohi Y et al Aldehyde dehydrogenase 1 expression is a predictor of poor prognosis in node‐positive breast cancers: a long‐term follow‐up study. Histopathology 2011; 58: 608–16. [DOI] [PubMed] [Google Scholar]
- 26. Park SY, Lee HE, Li H, Shipitsin M, Gelman R, Polyak K. Heterogeneity for stem cell‐related markers according to tumor subtype and histologic stage in breast cancer. Clin Cancer Res 2010; 16: 876–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Honeth G, Lombardi S, Ginestier C et al Aldehyde dehydrogenase and estrogen receptor define a hierarchy of cellular differentiation in the normal human mammary epithelium. Breast Cancer Res 2014; 16: R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun Y, Wang Y, Fan C et al Estrogen promotes stemness and invasiveness of ER‐positive breast cancer cells through Gli1 activation. Mol Cancer 2014; 13: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kouros‐Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA‐3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell 2006; 127: 1041–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bernardo GM, Lozada KL, Miedler JD et al FOXA1 is an essential determinant of ERalpha expression and mammary ductal morphogenesis. Development 2010; 137: 2045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hurtado A, Holmes KA, Ross‐Innes CS, Schmidt D, Carroll JS. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet 2011; 43: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bernardo GM, Keri RA. FOXA1: a transcription factor with parallel functions in development and cancer. Biosci Rep 2012; 32: 113–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wolf I, Bose S, Williamson EA, Miller CW, Karlan BY, Koeffler HP. FOXA1: growth inhibitor and a favorable prognostic factor in human breast cancer. Int J Cancer 2007; 120: 1013–22. [DOI] [PubMed] [Google Scholar]
- 34. Horimoto Y, Arakawa A, Harada‐Shoji N et al Low FOXA1 expression predicts good response to neo‐adjuvant chemotherapy resulting in good outcomes for luminal HER2‐negative breast cancer cases. Br J Cancer 2015; 112: 345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnson N, Bentley J, Wang LZ et al TAM GI50: pre‐clinical evaluation of cyclin‐dependent kinase 2 and 1 inhibition in anti‐estrogen‐sensitive and resistant breast cancer cells. Br J Cancer 2010; 102: 342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jensen EV. Steroid hormones, receptors, and antagonists. Ann N Y Acad Sci 1996; 784: 1–17. [DOI] [PubMed] [Google Scholar]
- 37. Freifeld ML, Feil PD, Bardin CW. The in vivo regulation of the progesterone “receptor” in guinea pig uterus: dependence on estrogen and progesterone. Steroids 1974; 23: 93–103. [DOI] [PubMed] [Google Scholar]
- 38. Matsumoto A, Jinno H, Murata T et al Prognostic implications of receptor discordance between primary and recurrent breast cancer. Int J Clin Oncol 2015; 20: 701–8. [DOI] [PubMed] [Google Scholar]
- 39. Milosevic J, Klinge J, Borg AL, Foukakis T, Bergh J, Tobin NP. Clinical instability of breast cancer markers is reflected in long‐term in vitro estrogen deprivation studies. BMC Cancer 2013; 13: 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lengerke C, Fehm T, Kurth R et al Expression of the embryonic stem cell marker SOX2 in early‐stage breast carcinoma. BMC Cancer 2011; 11: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ezeh UI, Turek PJ, Reijo RA, Clark AT. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer 2005; 104: 2255–65. [DOI] [PubMed] [Google Scholar]
- 42. Ponti D, Costa A, Zaffaroni N et al Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res 2005; 65: 5506–11. [DOI] [PubMed] [Google Scholar]
- 43. Xu C, Wei Q, Guo J et al FOXA1 expression significantly predict response to chemotherapy in estrogen receptor‐positive breast cancer patients. Ann Surg Oncol 2015; 22: 2034–9. [DOI] [PubMed] [Google Scholar]
- 44. Kawase M, Toyama T, Takahashi S et al FOXA1 expression after neoadjuvant chemotherapy is a prognostic marker in estrogen receptor‐positive breast cancer. Breast Cancer 2015; 22: 308–16. [DOI] [PubMed] [Google Scholar]
- 45. Wright TM, Wardell SE, Jasper JS et al Delineation of a FOXA1/ERalpha/AGR2 regulatory loop that is dysregulated in endocrine therapy‐resistant breast cancer. Mol Cancer Res 2014; 12: 1829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ma SR, Wang WM, Huang CF, Zhang WF, Sun ZJ. Anterior gradient protein 2 expression in high grade head and neck squamous cell carcinoma correlated with cancer stem cell and epithelial mesenchymal transition. Oncotarget 2015; 6: 8807–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Raffo D, Berardi DE, Pontiggia O, Todaro L, de Kier Joffe EB, Simian M. Tamoxifen selects for breast cancer cells with mammosphere forming capacity and increased growth rate. Breast Cancer Res Treat 2013; 142: 537–48. [DOI] [PubMed] [Google Scholar]
- 48. Calcagno AM, Salcido CD, Gillet JP et al Prolonged drug selection of breast cancer cells and enrichment of cancer stem cell characteristics. J Natl Cancer Inst 2010; 102: 1637–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu H, Zhang HW, Sun XF et al Tamoxifen‐resistant breast cancer cells possess cancer stem‐like cell properties. Chin Med J (Engl) 2013; 126: 3030–4. [PubMed] [Google Scholar]
- 50. Yenigun VB, Ozpolat B, Kose GT. Response of CD44+/CD24−/low breast cancer stem/progenitor cells to tamoxifen and doxorubicininduced autophagy. Int J Mol Med 2013; 31: 1477–83. [DOI] [PubMed] [Google Scholar]
- 51. Guttilla IK, Phoenix KN, Hong X, Tirnauer JS, Claffey KP, White BA. Prolonged mammosphere culture of MCF‐7 cells induces an EMT and repression of the estrogen receptor by microRNAs. Breast Cancer Res Treat 2012; 132: 75–85. [DOI] [PubMed] [Google Scholar]
- 52. Ziegler E, Hansen MT, Haase M, Emons G, Grundker C. Generation of MCF‐7 cells with aggressive metastatic potential in vitro and in vivo. Breast Cancer Res Treat 2014; 148: 269–77. [DOI] [PubMed] [Google Scholar]
- 53. Qian X, Anzovino A, Kim S et al N‐cadherin/FGFR promotes metastasis through epithelial‐to‐mesenchymal transition and stem/progenitor cell‐like properties. Oncogene 2014; 33: 3411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Harrison H, Farnie G, Howell SJ et al Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res 2010; 70: 709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ito T, Sato N, Yamaguchi Y et al Differences in stemness properties associated with the heterogeneity of luminal‐type breast cancer. Clin Breast Cancer 2015; 15: e93–103. [DOI] [PubMed] [Google Scholar]
- 56. Habashy HO, Powe DG, Rakha EA et al Forkhead‐box A1 (FOXA1) expression in breast cancer and its prognostic significance. Eur J Cancer 2008; 44: 1541–51. [DOI] [PubMed] [Google Scholar]
- 57. Ross‐Innes CS, Stark R, Teschendorff AE et al Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 2012; 481: 389–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lin X, Li J, Yin G et al Integrative analyses of gene expression and DNA methylation profiles in breast cancer cell line models of tamoxifen‐resistance indicate a potential role of cells with stem‐like properties. Breast Cancer Res 2013; 15: R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. CD44 and CD24 expression in luminal breast cancer cells. The horizontal axis shows the intensity of CD44 staining and vertical axis shows the intensity of CD24 staining. R5 fracture shows the CD44+/CD24− population. Left column, MCF‐7 cells; middle column, HCC1500 cells; right column, BC#1 cells.


