Abstract
Background
Cervical lymph node involvement in thyroid cancer is associated with locoregional recurrence and decreased disease-free survival. Preoperative lymph node mapping helps in planning surgery for neck dissection and improves patient outcomes. We sought to perform a qualitative and quantitative analysis of ultrasound mapping for thyroid cancer and evaluate the clinical importance of this exam in terms of identifying the group of patients who would benefit most from subsequent surgical dissection.
Methods
We retrospectively reviewed the cases of 263 patients who underwent thyroid surgery between 2009 and 2013. We calculated the positive predictive values (PPVs) of ultrasound mapping of both the lateral and central compartments together and the lateral or central compartment individually. A quantitative analysis was performed by comparing the number of positive lymph nodes at ultrasound imaging with histopathologic evaluation.
Results
A total of 136 cases of thyroid cancer in 120 patients met the inclusion criteria for ultrasound mapping analysis. The PPVs (and 95% confidence intervals) were 83.82 (0.76–0.89) for the lateral and central compartments, 85.39% (0.76–0.91) for the lateral compartment, and 80.48% (0.7–0.87) for the central compartment. When comparing the positive lymph nodes at ultrasound imaging with histopathologic evaluation, the result was χ2 = 10.33 (p = 0.006).
Conclusion
This single-institution study indicated that preoperative ultrasound mapping is an accurate imaging procedure for predicting lymphatic spread in differentiated and medullary thyroid cancer. Ultrasound mapping can be used as an efficient tool for surgical planning and prognosis determination, as well as for identifying the group of patients who would benefit most from subsequent surgical intervention.
Abstract
Contexte
Dans le contexte du cancer de la thyroïde, l’envahissement des nœuds lymphatiques cervicaux est associé à une récidive locorégionale et à une diminution de la survie sans récidive. Une cartographie préopératoire des nœuds lymphatiques facilite la planification de la dissection du cou et améliore les résultats pour les patients. Nous avons procédé à une analyse qualitative et quantitative de la cartographie par ultrasons dans le contexte du cancer de la thyroïde et avons évalué l’utilité de cette technique sur le plan clinique pour cibler le groupe de patients chez qui une dissection chirurgicale subséquente est indiquée.
Méthodes
Nous avons évalué rétrospectivement les dossiers de 263 patients ayant subi une thyroïdectomie entre 2009 et 2013. Nous avons calculé la valeur prédictive positive dans le contexte d’une cartographie par ultrasons du compartiment latéral et du compartiment central, conjointement et individuellement. Nous avons ensuite effectué une analyse quantitative en comparant le nombre de nœuds lymphatiques positifs détectés avec la cartographie par ultrasons et l’examen histopathologique.
Résultats
En tout, 136 cas de cancers de la thyroïde, ayant touché 120 patients, respectaient les critères d’inclusion de l’analyse. La valeur prédictive positive était de 83,82 % (0,76–0,89) pour la cartographie conjointe des compartiments latéral et central; de 85,39 % (0,76–0,91) pour le compartiment latéral; et de 80,48 % (0,7–0,87) pour le compartiment central (intervalle de confiance de 95 %). En comparant les résultats de l’imagerie avec ceux de l’examen histopathologique, nous avons obtenu un résultat de χ2 = 10,33 (p = 0,006).
Conclusion
Menée auprès de patients d’un seul établissement, cette étude a permis de conclure que la cartographie préopératoire par ultrasons est une technique d’imagerie médicale fiable qui permet de prévoir l’envahissement lymphatique chez les patients atteints d’un cancer médullaire ou différencié de la thyroïde. Cette technique peut être utilisée pour planifier une intervention chirurgicale ou établir un pronostic ainsi que pour cibler le groupe de patients chez qui une dissection chirurgicale subséquente serait indiquée.
Differentiated thyroid carcinomas are the most common types of thyroid malignancies. Papillary thyroid carcinoma (PTC) accounts for approximately 80% of all thyroid neoplasms1,2 and has shown a permanent increase in its incidence.3 The fastest increase has been in women,1 making PTC now the sixth most common cancer among women. There are several major risk factors for PTC, such as exposure to ionizing radiation and a family history of thyroid cancer. Papillary carcinoma is also associated with mutations of oncogenes, such as BRAF, RET/PTC, RAS and TRK.1
Despite a high survival rate (5- and 10-year overall survival of 90% and 95%, respectively4), about 20%–50% of patients require additional treatments for lymph node (LN) metastasis.4–6 In 80%–90% of patients, micrometastasis can be found with meticulous bilateral neck dissection.1,5,7 Although cervical LN metastasis has almost no impact on short-term survival, it greatly affects long-term survival, as it is a major risk factor for locoregional tumour recurrence.4 Lymph node metastasis is associated with several predisposing risk factors, such as male sex, age older than 45 years, tumour size greater than 1 cm and lymphovascular and extrathyroidal invasions.8 Moreover, PTC located in the upper neck has been shown to present a higher rate of lateral neck metastasis than PTC in the lower neck.8
Despite an increased rate of recurrence in patients with PTC and LN metastasis, there is no evidence that radical neck or routine central compartment dissection can improve disease-free survival, particularly in patients with papillary thyroid microcarcinomas measuring less than 10 mm.9 Central neck dissection is even associated with a higher rate of complications, such as hypocalcemia, recurrent laryngeal nerve palsy, hematoma, chyle leakage and spinal accessory nerve dysfunction.10,11
Lymph node involvement can be detected by physical examination, which has a sensitivity as low as 15%–30%,8,12 whereas neck imaging is highly efficient; high-resolution ultrasonography (US) in particular is able to detect nodules as small as 5 mm. In addition, computed tomography (CT) can be used for staging LNs in patients with thyroid carcinoma. Although most studies agree that US is the first choice,13–16 some investigators have reported comparable sensitivity with CT and that sensitivity was even slightly higher with CT than US for the central compartment.17,18 In these studies, it is interesting to observe that the sensitivity of US is lower (< 80%) than previously reported. Computed tomography necessitates the injection of a high quantity of iodine contrast, which could interfere in the follow-up investigation and treatment of the patient. After receiving iodine contrast, patients must wait 3–4 months before being able to receive radioactive iodine treatment or a thyroid iodine uptake scan. It is important to be aware of the patient therapy and coordinate CT with the medical team; this is clearly a disadvantage of this modality. Using US also gives the opportunity to practice fine needle aspiration (FNA) on LNs that are undetermined and influence the extent of the dissection.
A US-based preoperative evaluation of the primary tumour’s extent as well as LN involvement has become an essential procedure, which can modify the overall surgical approach in up to 40% of cases.9,19 This approach is recommended in the guidelines of the American Thyroid Association.20
The aim of this study is to evaluate the diagnostic reliability of preoperative US mapping of thyroid cancers in our institution by calculating the positive predictive value (PPV) of the test and trying to determine whether there is a quantitative association between US mapping and pathological analysis. The interpretation of such an association may help to target the group of patients who would benefit most from the subsequent surgical procedure and at the same time help avoid unproductive surgical dissection in patients with minimal risk of disease recurrence. To our knowledge, no study has been done to evaluate the existence of such a quantitative association between cervical LN mapping and pathological results.
Methods
The medical records of 263 patients who underwent surgery for thyroid cancer at the Centre hospitalier de l’Université de Montréal (CHUM) between 2009 and 2013 were retrospectively reviewed. The only exclusion criterion was the absence of US mapping. We used the electronic institutional database and Microsoft Access software for data collection. Surgeries were performed by 9 surgeons (1 endocrine surgeon and 8 otorhinolaryngologists). Ten radiologists were involved in the study, but more than 80% of cases were examined by 1 radiologist with advanced skills in US mapping of thyroid cancer. A US system equipped with a high-resolution (13 MHz) linear probe was used. Parameters including operative procedures, pathological analyses and mapping results according to neck compartments were collected.
For the purpose of quantitative analysis, patients were divided into 2 groups based on the number of suspicious LNs at US mapping (group A: 1 or 2 suspicious LNs; group B: ≥ 3 suspicious LNs). Concerning the histopathological results, we created 3 groups according to the number of positive LNs (group 1: 0 positive LNs; group 2: 1 or 2 positive LNs; group 3: ≥ 3 positive LNs). The LN location in the neck was divided into central (CC) and lateral compartments (LC). The CC was defined as the space between the medial margins of bilateral carotid arteries, corresponding to level VI, whereas the LC extends from the carotid arteries to the medial border of the trapezius muscle and involves levels II, III, IV and V, according to the definitions of the American Head and Neck Society and the American Academy of Otolaryngology — Head and Neck Surgery.8,21,22
The imaging suspicion of LN involvement was based on various criteria, such as cystic changes, hyperechogenicity, loss of hilar echogenicity (fat centre), internal microcalcifications, poorly defined irregular borders, shape/dimension ratio, compressibility, vascularity, size > 5 mm in its shortest diameter and round shape (Fig. 1).1,6,19,23,24 In our study, we considered a node to be suspicious if at least 1 of these criteria was met. As for loss of hilar echogenicity on nodes smaller than 5 mm, an additional criterion was needed to reach the level of suspicious LN. The surgical exploration was guided by US mapping. Suspicious LNs were removed based on the compartment-oriented approach without further dissection. On histopathology, LNs with a diameter less than 5 mm were examined by 1-sliced specimen. We used 2-sliced specimen examination for the LNs with a diameter greater than 5 mm.
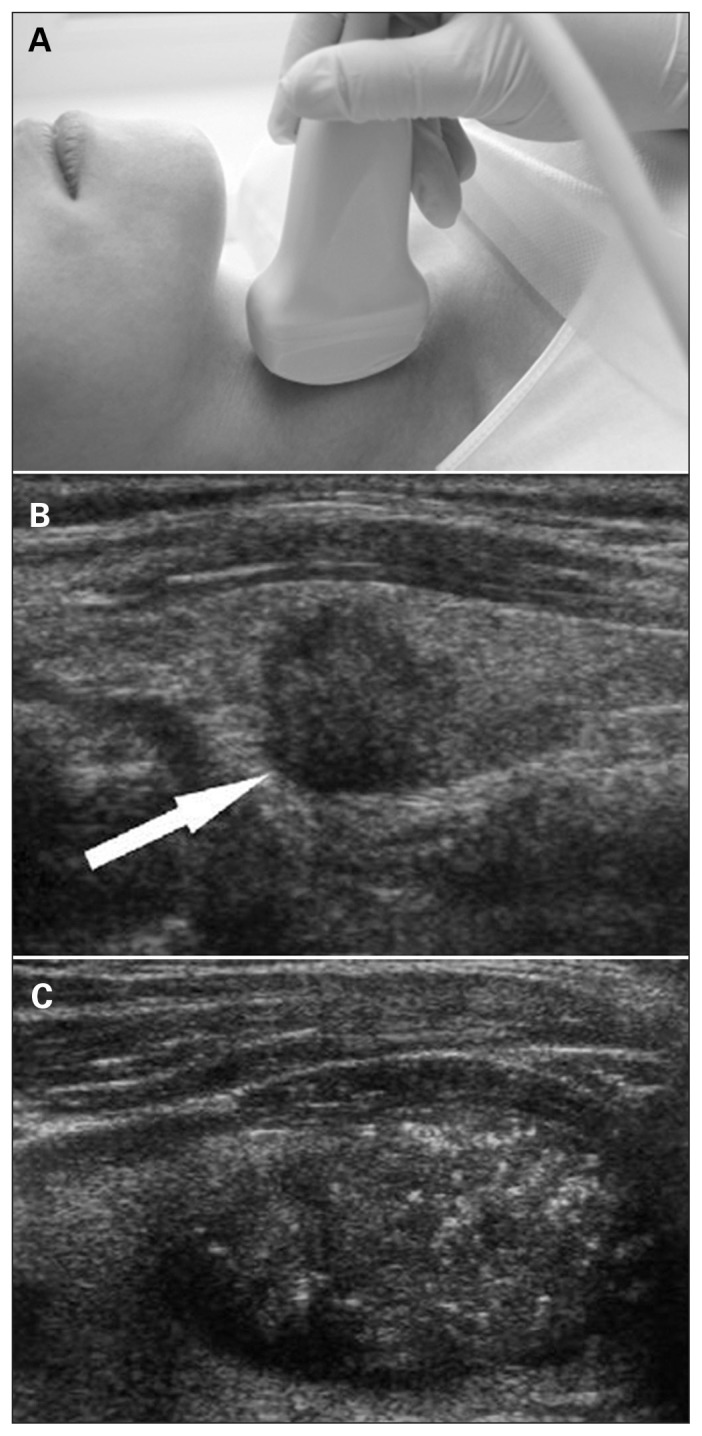
Fig. 1.
(A) Ultrasonography of the neck. (B) Cancerous nodule detected on ultrasound (arrow). (C) Microcalcifications in the cancerous nodule.
Statistical analysis
We performed our statistical analyses using SPSS software, version 20. Continuous variables were analyzed as means ± standard deviations, and we used the χ2 test for categorical data.
Results
Among the 263 patients who underwent thyroid surgery, 261 had undergone US mapping, and suspicious LNs were found in 154 cases. Despite positive US mapping, 18 cases were excluded from the study owing to the absence of LNs in the surgical specimen (3 patients), the absence of pathological results (1 patient), the absence of neck compartment dissection (11 patients), and a change in surgical planning in favour of palliative tracheotomy (1 patient) and hemithyroidectomy (2 patients). Therefore, results were obtained after analyzing 136 cases of positive US mapping in 120 patients (Table 1).
Table 1.
Surgical and histopathological characteristics of patients who underwent ultrasound mapping followed by neck dissection
| Characteristic | No. (%)* |
|---|---|
| No. of patients/cases | 120/136 |
| Sex, F/M | 89 (74.1%)/31(25.9%) |
| Age, mean ± SD [range], yr | 49.9 ± 16.2 [16–90] |
| No. of primary/secondary surgeries | 87/49 |
| No. of postsurgical complications | 3 |
| Locoregional recurrence on US follow-up | 26 (21.6%) |
| Histopathology, no. patients | 110 papillary 8 medullary 1 follicular 1 Hurthle cell |
F = female; M = male; SD = standard deviation; US = ultrasound.
Unless indicated otherwise.
The patients’ mean age was 49.9 ± 16.2 (range 16–90) years. Of the 120 patients, 89 (74.1%) were women and 31 (25.9%) were men. Nine patients had been operated twice, 2 patients 3 times, and 1 patient 4 times. Among the 120 patients, histopathological analysis indicated 110 PTCs, 8 medullary cancers, 1 follicular cancer and 1 Hurthle cell tumour. In 87 cases, compartment-oriented surgical excision of suspected LNs along with thyroidectomy had been performed (primary surgeries) and 49 procedures had been carried out for recurrent disease (secondary surgeries). Three postsurgical complications had been recorded: 1 bilateral chylothorax, 1 hematoma in thyroid bed, and 1 case of bleeding from the innominate artery. During the data collection period (February to September 2014), radiological findings of locoregional recurrent disease were noted in 26 (21.6%) patients. On US mapping, there were 40 (29.4%) cases in group A and 96 (70.6%) cases in group B. On histopathology, there were 22 (16.2%) cases in group 1, 41 (31.1%) cases in group 2 and 73 (53.7%) cases in group 3 (Table 2). When comparing these groups using the χ2 test, the results were significant (χ2 = 10.33, p = 0.006).
Table 2.
Cross-tabulation of the results among the groups of US mapping and histopathology
| Mapping; group | Histopathology; group | |||
|---|---|---|---|---|
| 1 (0 PLN) | 2 (1 or 2 PLN) | 3 (≥ 3 PLN) | Total | |
| A (1 or 2 SLN) | 10 | 17 | 13 | 40 |
| B (≥ 3 SLN) | 12 | 24 | 60 | 96 |
| Total | 22 | 41 | 73 | |
PLN: positive lymph nodes; SLN = suspicious lymph nodes; US = ultrasound.
We calculated the PPVs of ultrasound mapping for LC alone, CC alone and both LC and CC. For LC, 76 of 89 cases were confirmed positive on pathological examination, resulting in a PPV of 85.39% (95% confidence interval [CI] 0.76–0.91). For CC, 66 of 82 cases were confirmed positive, resulting in a PPV of 80.48% (95% CI 0.7–0.87). Finally, for both LC and CC, 114 of 136 cases were confirmed positive, resulting in a PPV of 83.82 (95% CI 0.76–0.89). Positive results on histopathology were derived following the examination of 1766 and 659 LNs removed from the LC and CC, respectively (Table 3).
Table 3.
Descriptive statistics of the removed LNs according to compartments
| No. LNs | LC, n = 91 | CC, n = 93 | ||
|---|---|---|---|---|
|
|
|
|||
| Positive LNs | Total LNs | Positive LNs | Total LNs | |
| Mean ± SD | 4.21 ± 0.66 | 19.41± 1.6 | 3.24± 0.42 | 7.09 ±0.61 |
|
| ||||
| Median [range] | 2 [0–49] | 17 [1–72] | 2 [0–24] | 5 [1–34] |
|
| ||||
| Sum | 383 | 1766 | 301 | 659 |
CC = central compartnemtn; LC = lateral compartment; LN = lymph node; SD = standard deviation.
Discussion
This study examined the qualitative and quantitative reliability of US mapping of thyroid cancer in a single institution. Our results confirmed that US mapping is a reliable tool for detecting affected LNs. With an PPV of 83.82% for the LC and CC together with advantages, such as simplicity, ease in terms of performance, wide availability, comparatively low price, noninvasiveness and lack of radiation,12 US mapping is an excellent tool for surgery guidance. The relatively low rate of detection of metastatic LNs in the CC might be explained by anatomic limitations in that area of the neck, such as the clavicle, sternum and tracheal air shadow.3,20
Micrometastases (< 2 mm) in patients undergoing prophylactic LN dissection were found in up to 80% of individuals.12,25 Nevertheless, such micrometastases are not clincially relevant, as palpable lymphadenopathies do not develop in most patients with PTC. On the contrary, patients presenting with macrometastases (> 2 mm) have 5%–40% persistent/recurrent (P/R) disease after surgery,26,27 which has a tremendous impact on quality of life, and despite the additional therapeutic interventions, 10% of patients die from the disease.28 Reoperation of P/R disease involves many difficulties because the extensive scarring from previous surgery can obscure normal anatomy, which in turn contributes to longer surgeries and increased morbidity.29
Ultrasound mapping guides the surgeon for precise neck dissection, resulting in decreased locoregional tumour recurrence and lower risk of postsurgical complications due to reoperation. To analyze this test more thoroughly, we decided to go a step further in our study by evaluating not only PPV, but also the quantitative reliability of the test, which, to our knowledge, had not been done before. Such an approach seemed highly valuable as patients with multiple positive LNs have been shown to be at greater risk of recurrence.30 Based on the quantitative results of US mapping, patients can be stratified according to the level of risk of recurrent disease, which in turn will help to better select surgical candidates. The use of US mapping to plan compartment-oriented LN dissection could therefore benefit patients undergoing thyroid cancer surgery, as recognized by the American Thyroid Association.30
Limitations
Our results showed a statistically significant association between US mapping analysis and LN pathological examination, indicating that U/S mapping is an effective tool in the armamentarium of quantitative prediction for lymphatic spread in thyroid cancer. Another notable observation was derived by analyzing the false-positive cases on US mapping. In 10 of 22 cases (45.4%), patients had Hashimoto’s thyroiditis, which was mimicking affected LNs in thyroid cancer. The retrosternal localization and small size (< 5 mm) of the affected LNs made it extremely difficult to distinguish them from metastatic LNs. Consequently, we believe that Hashimoto’s thyroiditis might be considered a limiting factor for US mapping in patients with suspicion of thyroid cancer.
Another major subject of discussion is the test’s specificity and sensitivity. In fact, it is impossible to calculate either sensitivity or specificity because negative findings on US cannot be considered true negatives owing to the lack of supporting “gold standard” results. In the literature, these 2 values have a very wide range (29%–100%) depending on the study design and chosen “gold standard” methods, as shown in the meta-analysis by Wu and colleagues.31 We therefore believe that the best way to solve this contradiction is to conduct a prospective study with long-term follow-up of negative cases on US mapping.
Conclusion
Ultrasound mapping is a reliable tool for guiding surgical dissection of the neck, both for a primary tumour and for P/R disease. It has a sufficiently high PPV and strong quantitative association with histopathological analysis, which makes it possible to focus on patients with higher risk of recurrent disease. Meanwhile, there may be some limitations to the test in cases such as thyroiditis, and further research needs to be conducted to obtain more reliable values for both sensitivity and specificity.
Footnotes
Competing interests: None declared.
Contributors: D. Kocharyan and E. Nassif designed the study, acquired and analyzed the data, which F. Schwenter and M. Bélair also analyzed. D. Kocharyan and E. Nassif wrote the article, which all authors reviewed and approved for publication.
References
- 1.Cisco RM, Shen WT, Gosnell JE. Extent of surgery for papillary thyroid cancer: preoperative imaging and role of prophylactic and therapeutic neck dissection. Curr Treat Options Oncol. 2012;13:1–10. doi: 10.1007/s11864-011-0175-z. [DOI] [PubMed] [Google Scholar]
- 2.Li QS, Chen S, Xiong X, et al. Papillary thyroid carcinoma on sonography. Clin Imaging. 2010;34:121–6. doi: 10.1016/j.clinimag.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Iyer NG, Shaha AR. Management of thyroid nodules and surgery for differentiated thyroid cancer. Clin Oncol (R Coll Radiol) 2010;22:405–12. doi: 10.1016/j.clon.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conzo G, Docimo G, Pasquali D, et al. Predictive value of nodal metastases on local recurrence in the management of differentiated thyroid cancer. Retrospective clinical study. BMC Surg. 2013;13(Suppl 2):S3. doi: 10.1186/1471-2482-13-S2-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang HS, Orloff LA. Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer. Laryngoscope. 2011;121:487–91. doi: 10.1002/lary.21227. [DOI] [PubMed] [Google Scholar]
- 6.Lee YS, Lim YS, Lee JC, et al. Ultrasonographic findings relating to lymph node metastasis in single micropapillary thyroid cancer. World J Surg Oncol. 2014;28:273. doi: 10.1186/1477-7819-12-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giacomini CP, Jeffrey RB, Shin LK. Ultrasonographic evaluation of malignant and normal cervical lymph nodes. Semin Ultrasound CT MR. 2013;34:236–47. doi: 10.1053/j.sult.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Choi YJ, Yun JS, Kook SH, et al. Clinical and imaging assessment of cervical lymph node metastasis in papillary thyroid carcinomas. World J Surg. 2010;34:1494–9. doi: 10.1007/s00268-010-0541-1. [DOI] [PubMed] [Google Scholar]
- 9.Kim KE, Kim EK, Yoon JH, et al. Preoperative prediction of central lymph node metastasis in thyroid papillary microcarcinoma using clinicopathologic and sonographic features. World J Surg. 2013;37:385–91. doi: 10.1007/s00268-012-1826-3. [DOI] [PubMed] [Google Scholar]
- 10.Conzo G, Mauriello C, Docimo G, et al. Clinicopathological pattern of lymph node recurrence of papillary thyroid cancer. Implications for surgery. Int J Surg. 2014;12(Suppl 1):S194–7. doi: 10.1016/j.ijsu.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Lee DW, Ji YB, Sung ES, et al. Roles of ultrasonography and computed tomography in the surgical management of cervical lymph node metastases in papillary thyroid carcinoma. Eur J Surg Oncol. 2013;39:191–6. doi: 10.1016/j.ejso.2012.07.119. [DOI] [PubMed] [Google Scholar]
- 12.Sakorafas GH, Christodoulou S, Lappas C, et al. Preoperative detection of cervical lymph node metastasis in papillary thyroid cancer: a surgical prospective. Onkologie. 2009;32:762–6. doi: 10.1159/000255337. [DOI] [PubMed] [Google Scholar]
- 13.King AD. Imaging for staging and management of thyroid cancer. Cancer Imaging. 2008;8:57–69. doi: 10.1102/1470-7330.2008.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosário PW, de Faria S, Bicalho L, et al. Ultrasonographic differentiation between metastatic and benign lymph nodes in patients with papillary thyroid carcinoma. J Ultrasound Med. 2005;24:1385–9. doi: 10.7863/jum.2005.24.10.1385. [DOI] [PubMed] [Google Scholar]
- 15.Langer JE, Mandel SJ. Sonographic imaging of cervical lymph nodes in patients with thyroid cancer. Neuroimaging Clin N Am. 2008;18:479–89. doi: 10.1016/j.nic.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Stulak JM, Grant CS, Farley DR, et al. Value of preoperative ultrasonography in the surgical management of initial and reoperative papillary thyroid cancer. Arch Surg. 2006;141:489–96. doi: 10.1001/archsurg.141.5.489. [DOI] [PubMed] [Google Scholar]
- 17.Na DK, Choi YJ, Choi SH, et al. Evaluation of cervical lymph node metastasis in thyroid cancer patients using real-time CT-navigated ultrasonography: preliminary study. Ultrasonography. 2015;34:39–44. doi: 10.14366/usg.14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahn JE, Lee JH, Shong YK, et al. Diagnostic accuracy of CT and ultrasonography for evaluating metastatic cervical lymph nodes in patients with thyroid cancer. World J Surg. 2008;32:1552–8. doi: 10.1007/s00268-008-9588-7. [DOI] [PubMed] [Google Scholar]
- 19.Lew JI, Rodgers SE, Solorzano CC. Developments in the use of ultrasound for thyroid cancer. Curr Opin Oncol. 2010;22:11–6. doi: 10.1097/CCO.0b013e3283337f16. [DOI] [PubMed] [Google Scholar]
- 20.Choi JS, Chung WY, Kwak JY, et al. Staging of papillary thyroid carcinoma with ultrasonography: performance in a large series. Ann Surg Oncol. 2011;18:3572–8. doi: 10.1245/s10434-011-1783-3. [DOI] [PubMed] [Google Scholar]
- 21.Machens A, Hauptmann S, Dralle H. Lymph node dissection in the lateral neck for completion in central node-positive papillary thyroid cancer. Surgery. 2009;145:176–81. doi: 10.1016/j.surg.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Sugitani I, Fujimoto Y, Yamada K, et al. Prospective outcomes of selective lymph node dissection for papillary thyroid carcinoma based on preoperative ultrasonography. World J Surg. 2008;32:2494–502. doi: 10.1007/s00268-008-9711-9. [DOI] [PubMed] [Google Scholar]
- 23.Lew JI, Solorzano CC. Use of ultrasound in the management of thyroid cancer. Oncologist. 2010;15:253–8. doi: 10.1634/theoncologist.2009-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shuzhen C. Comparison analysis between conventional ultrasonography and ultrasound elastography of thyroid nodules. Eur J Radiol. 2012;81:1806–11. doi: 10.1016/j.ejrad.2011.02.070. [DOI] [PubMed] [Google Scholar]
- 25.Shin LK, Olcott EW, Jeffrey RB, et al. Sonographic evaluation of cervical lymph nodes in papillary thyroid cancer. Ultrasound Q. 2013;29:25–32. doi: 10.1097/RUQ.0b013e31827c7a9e. [DOI] [PubMed] [Google Scholar]
- 26.Binyousef HM, Alzahrani AS, Al-Sobhi SS, et al. Preoperative neck ultrasonographic mapping for persistent/recurrent papillary thyroid cancer. World J Surg. 2004;28:1110–4. doi: 10.1007/s00268-004-7636-5. [DOI] [PubMed] [Google Scholar]
- 27.Lim YS, Lee JC, Lee YS, et al. Lateral cervical lymph node metastases from papillary thyroid carcinoma: predictive factors of nodal metastasis. Surgery. 2011;150:116–21. doi: 10.1016/j.surg.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Sakorafas GH, Sampanis D, Safioleas M. Cervical lymph node dissection in papillary thyroid cancer: current trends, persisting controversies, and unclarified uncertainties. Surg Oncol. 2010;19:e57–70. doi: 10.1016/j.suronc.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 29.McCoy KL, Yim JH, Tublin ME, et al. Same-day ultrasound guidance in reoperation for locally recurrent papillary thyroid cancer. Surgery. 2007;142:965–72. doi: 10.1016/j.surg.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 30.Nixon IJ, Wang LY, Palmer FL, et al. The impact of nodal status on outcome in older patients with papillary thyroid cancer. Surgery. 2014;156:137–46. doi: 10.1016/j.surg.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 31.Wu LM, Gu HY, Qu XH, et al. The accuracy of ultrasonography in the preoperative diagnosis of cervical lymph node metastasis in patients with papillary thyroid carcinoma: a meta-analysis. Eur J Radiol. 2012;81:1798–805. doi: 10.1016/j.ejrad.2011.04.028. [DOI] [PubMed] [Google Scholar]