Abstract
Objective
To determine the association between altitude and obesity in a nationally representative sample of the Peruvian adult population.
Design and Methods
This is a cross-sectional analysis of publicly available data from the Food and Nutrition National Center (CENAN, Peru), period 2009-2010. Prevalence ratio of obesity and abdominal obesity was determined as a measure of association. Obesity and abdominal obesity were diagnosed based on direct anthropometric measurements.
Results
The final dataset consisted of 31,549 individuals ≥20 years old. The prevalence ratio of obesity was as follows: 1.00 between 0–499 m (reference category), 1.00 (95% confidence interval 0.87-1.16) between 500–1,499 m, 0.74 (0.63-0.86) between 1,500–2,999, and 0.54 (0.45-0.64) at ≥3,000 m, adjusting for age, sex, self-reported physical activity, out-migration rate, urbanization, poverty, education, and geographical latitude and longitude. In the same order, the adjusted prevalence ratio of abdominal obesity was 1.00, 1.01 (0.94-1.07), 0.93 (0.87-0.99), and 0.89 (0.82-0.95), respectively. We found an interaction between altitude and sex and between altitude and age (P<0.001, for both interactions) on the association with obesity and abdominal obesity.
Conclusions
Among Peruvian adult individuals, we found an inverse association between altitude and obesity, adjusting for multiple covariates. This adjusted association varied by sex and age.
Keywords: Abdominal obesity, altitude, BMI, obesity, prevalence ratio
Introduction
Obesity is a well established risk factor for type 2 diabetes, which is associated with severe chronic complications including blindness, renal failure, neuropathy, and amputations (1). Obesity is also a risk factor for cardiovascular diseases, hypertension, and several types of cancer including breast cancer, colorectal cancer, endometrial cancer, and kidney cancer (2). Thus, abnormal weight gain is a major issue for public health. The World Health Organization has estimated that ∼500 million adult individuals have obesity (3). The alarming increase in the prevalence of obesity is not only restricted to the United States (US), but it is a global hazard (4), affecting also countries with smaller income economies such as Peru (5).
In 2010, the Centers for Disease Control and Prevention (CDC) reported that the lowest prevalence of adult obesity in the US was in Colorado (6), one of the states with the highest mean elevation counties. Even more intriguing, an inverse association between altitude and self-reported body mass index (BMI) has been reported among US adult individuals, independent of risk factors and potential confounders (7,8). Whether this adjusted inverse association extends to other nationally representative populations when measured body mass index is used remains unknown.
The aim of the present study was to determine the association between altitude and obesity in a nationally representative sample of the Peruvian adult population. We utilized freely available online data from an on-site survey conducted in a nationally representative population of Peru, a country with different geographic (Figure S1), socio-economic, cultural, and ethnic features than the US. We estimated the prevalence ratio of obesity and abdominal obesity by altitude bands among adult individuals, 20 years or older, adjusting for age, sex, self-reported physical activity, and socio-demographic covariates.
Methods and Procedures
Characteristics of the population
The present study included a nationally representative sample of adult individuals (20 years or older) of Peru.
This study did not require approval or exemption from the Institutional Review Board at Cedars-Sinai Medical Center as it involved a cross-sectional analysis of publicly available, deidentified data.
Data from the National Household Survey (ENAHO)
Data from ENAHO for 2009–2010 was utilized to estimate the prevalence of overweight, obesity, and abdominal obesity among Peruvian adults and to estimate the association between altitude and these clinical conditions. ENAHO 2009–2010 is the largest on-site survey conducted by the Food and Nutrition National Center (CENAN) and the National Institute of Statistics and Informatics (INEI) to assess living conditions in Peru. ENAHO surveyed 21,680 homes, including 82,337 individuals, using a probabilistic, stratified, multi-stage design, independent for each region, including all ages starting as of 2 months (www.inei.gob.pe/web/enaho). The administrative division of Peru is organized in 25 regions, 196 provinces, and 1,850 districts. The survey questionnaire included, among other variables, information on age, sex, and self-reported physical activity. An important feature of this survey was the inclusion of direct anthropometric measurements such as height, body weight, and abdominal circumference, performed using standardized techniques and equipment. Abdominal circumference was measured at the level of the midway between the lowest rib and the top of the iliac crest. The survey did not ask for information on ethnicity, food intake, or smoking habits.
Data from the INEI
District-level data on altitude, latitude, longitude, poverty, and education, as well as province-level data on out-migration rate and urbanization were obtained from the INEI. Altitude of every province was estimated from the median of the altitudes of their corresponding districts (9). Poverty data represented the percentage of homes for which per capita income was below than the basic family needs (10). Data on education represented the percentage of the population who completed at least primary school (11). Data on out-migration rate and urbanization were available for the periods 2002–2007 and 2007, respectively (12). Out-migration data represented the rate of emigration, that is, those moving from one province to another different destination province within a 1-year period relative to the population registered in local databases (in a 5-year period estimate from 2002 to 2007). An urban area was defined as a conglomerate of 100 houses grouped contiguously. A town that is the capital of a district was also considered as an urban area (www.inei.gob.pe).
Prevalence estimates
Age-adjusted prevalence estimates of overweight and obesity were based on the relative age distribution for the Peruvian population reported by the INEI for 2010 (13). We excluded cases of self-reported pregnancy (n=464). We also excluded eight provinces with less than 10 subjects per age group (n=153 individuals).
Overweight was defined as a BMI (weight/height2) of 25–29.9. Obesity was defined as a BMI of 30 or higher, and classified as follows: class I (BMI 30.0–34.9), class II (BMI 35–39.9), and class III (BMI 40 or higher) (14). Abdominal obesity was diagnosed using criteria defined by the Adult Treatment Panel III, or ATP III (15) (if abdominal circumference was >102 cm for males or >88 cm for females) and the International Diabetes Federation (IDF) (16). We used the IDF criteria for South Asians (if abdominal circumference was ≥90 cm for males or ≥80 cm for females) as these criteria are currently recommended for the diagnosis of abdominal obesity among individuals of South American ethnicity (16).
Measures of association of altitude with overweight, obesity, and abdominal obesity
Prevalence ratio was used as a measure of association, adjusting for age, sex, self-reported physical activity, out-migration rate, urbanization, poverty, education, latitude, and longitude. The initial set of adult individuals (≥20 years old) comprised 48,394 (24,825 women). We excluded 464 cases of self-reported pregnancy, 3 subjects with a calculated BMI ≤10, 133 subjects with a BMI ≥60 (BMI cut-offs at which discordant measures between weight and height were observed), and 18 subjects with an abdominal circumference ≤40 cm. In fact, among those individuals with a BMI>60, 89% (n=119) had a height below 80 cm (range: 30.0-79.9 cm), which suggests error in data entry. There were 11,259 cases with missing data on body weight and/or height, 4,947 cases with missing information on physical activity, and 21 cases with missing information on city residence.
Based on the definition of high altitude (≥1,500 m) (17) and the frequency distribution of the number of individuals who reside within a given altitude band, altitude was grouped in 4 categories: 0–499 m, 500–1,499 m, 1,500–2,999 m, and ≥3,000 m. For the latter category, the median of the altitude was 3,397 m (interquartile range: 3,245.0–3,725.0 m). The district with the highest altitude surveyed by ENAHO was located at 4,660 m. Since moderate or vigorous physical activity for at least 150 min per week has been shown to result in a more sustained weight loss (18,19), we grouped the variable physical activity (total minutes per week of moderate and vigorous activity at work, home, and during recreation) in 4 categories: 0–149 min, 150–299 min, 300–449 min, and ≥450 min.
Because of the unique geographic location of Peru, near to the Equator, and the Central Andes crossing along the country (Figure S1), we included latitude and longitude as covariates in our model. Latitude was grouped in 2 categories: 0–9.9° S and 10–19.9° S. Longitude was also grouped in 2 categories: 65.0-75.9° W and 76.0-85.0° W. Poverty, education, out-migration rate, and urbanization were treated as continuous variables.
Statistical analysis
Bivariate associations were determined using Spearman rank order correlation. Since obesity is not a rare disease in Peru, we estimated the adjusted prevalence ratio and their 95% confidence intervals (95% CI), rather than prevalence odds ratio (20). Unadjusted prevalence ratios were estimated using Poisson regression. Fully adjusted prevalence ratios were determined using multilevel mixed-effects Poisson regression analysis (21), allowing us to account for nested data (region, province, and district level) and the random effects between subjects (22). Since obesity prevalence may vary with age and sex (23,24), we tested for interaction between altitude and sex and between altitude and age. These interactions terms were included in separate fully adjusted Poisson regression models, and the significance was assessed using the Wald test. Statistical analyses were performed using Statistica 7.0 (StatSoft Inc., Tulsa, OK) and STATA/SE 12.0 for Windows (StataCorp LP, College Station, TX).
Results
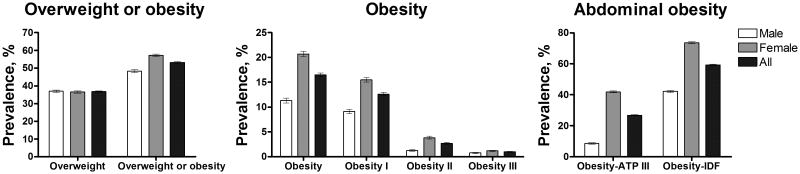
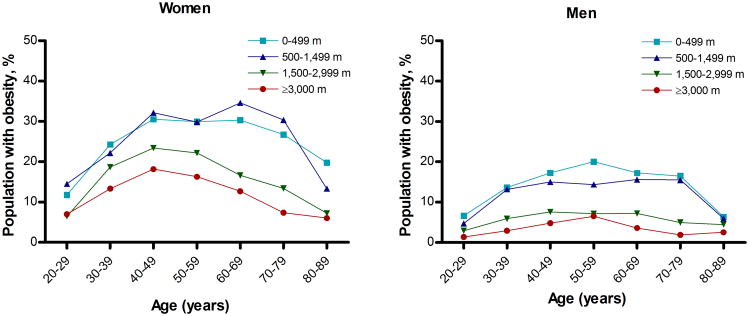
The final dataset for prevalence estimates included 36,540 individuals (20,123 women) from 185 provinces. The final dataset for regression analysis of overweight and obesity consisted of 31,549 individuals (17,537 women). Crude prevalences of overweight and obesity, respectively, were 37.0% (95% CI, 36.2–37.7%) and 11.3% (95% CI, 10.9–11.8%) among men and 36.5% (95% CI, 35.9–37.2%) and 20.7% (95% CI, 20.2–21.3%) among women (Figure 1). The estimated national age-adjusted adult median prevalences of overweight and obesity were 33.7% (interquartile range, 27.0–39.8%) and 10.3% (4.6–15.9%), respectively. In the same order, the mean age-adjusted prevalences of overweight and obesity were 33.0% (95% confidence interval, 31.7–34.4%) and 11.1% (95% CI, 10.0–12.2%). The age-specific percentage of obesity by altitude bands and sex is shown in Figure 2.
Figure 1. Crude prevalence of overweight and obesity in Peruvian adults ≥20 years old for 2009-2010.
Obesity and abdominal obesity is almost double among women as compared with men. Central panel shows the prevalences of obesity and obesity classes. In the right panel, obesity-ATP III and Obesity-IDF indicate the prevalences of abdominal obesity diagnosed using the criteria established by the Adult Treatment Panel III (ATP) and the International Diabetes Federation (IDF), respectively. Top ends of the bars indicate means. Error bars are 95% confidence intervals.
Figure 2. Age-specific percentage of Peruvian adult individuals with obesity by altitude bands, 2009–2010.
Profiles are shown for women (A) and for men (B).
Using the ATP III criteria, the crude prevalence of abdominal obesity was estimated in 8.6% (95% CI, 8.2–9.0%) and 41.9% (95% CI, 41.2–42.6%) in men and women, respectively. Since cut-off values established by the IDF are lower than those set by the ATP III, the prevalence of abdominal obesity was considerably higher when IDF criteria were applied: 42.2% (95% CI, 41.4–42.9%) and 73.5% (95% CI, 72.9–74.1%), men and women, respectively (Figure 1).
Prevalence of overweight and obesity in low- and high-altitude Peruvian provinces
High-altitude provinces (n=117) compared with those at lower altitudes (n=68) had lower age-adjusted adult prevalences of overweight [30.6% (95% CI, 28.9–32.3%) versus 37.2% (95% CI, 35.4–39.0%), respectively]. Likewise, age-adjusted obesity prevalence was lower in high-altitude provinces [8.0% (7.0–9.1%) versus 16.4% (14.7–18.2%)] (Table S1). Province median altitude was inversely correlated with adult prevalences of overweight and obesity (Figure S2).
Association of altitude with overweight, obesity, and abdominal obesity
The characteristics of the population included for regression analysis are shown in Table 1. Estimates for women and men combined revealed an inverse association between altitude and overweight that disappeared in the fully adjusted model. We found a significant interaction between altitude and sex (P<0.001). Men, but not women, had lower adjusted prevalence ratio of overweight at higher altitudes (Table 2).
Table 1.
Frequency distribution * of risk factors by BMI and altitude bands among Peruvian adults aged 20 years or older.
| Overweight (BMI 25-29) | Obesity (BMI ≥30) | Lean (BMI <25) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Altitude, m | 0–499 | 500–1,499 | 1,500–2,999 | ≥3,000 | 0–499 | 500–1,499 | 1,500–2,999 | ≥3,000 | 0–499 | 500–1,499 | 1,500–2,999 | ≥3,000 |
| n | 5,708 | 1,469 | 1,949 | 2,419 | 2,787 | 732 | 691 | 702 | 5,617 | 1,619 | 3,169 | 4,687 |
| Age, years | ||||||||||||
| 20–39 | 43.3 | 45.3 | 40.0 | 38.4 | 35.2 | 34.7 | 33.4 | 30.5 | 57.8 | 54.1 | 47.8 | 41.1 |
| 40–59 | 41.9 | 40.6 | 42.1 | 43.6 | 47.5 | 47.1 | 48.6 | 52.1 | 28.1 | 30.7 | 30.4 | 33.2 |
| 60–79 | 13.8 | 13.3 | 16.1 | 16.62 | 16.5 | 17.6 | 16.9 | 16.2 | 12.6 | 13.9 | 19.3 | 22.5 |
| ≥ 80 | 1.0 | 0.7 | 1.8 | 1.5 | 0.8 | 0.6 | 1.0 | 1.1 | 1.5 | 1.4 | 2.5 | 3.3 |
| Women | 53.5 | 53.4 | 57.9 | 62.3 | 68.1 | 70.8 | 78.0 | 83.1 | 50.8 | 46.3 | 48.1 | 51.1 |
| Physical activity, min/wk | ||||||||||||
| 0–149 | 32.9 | 24.8 | 23.2 | 19.4 | 36.6 | 29.9 | 27.7 | 23.7 | 30.9 | 20.8 | 18.7 | 16.2 |
| 150–299 | 23.9 | 27.1 | 24.7 | 19.3 | 24.8 | 29.1 | 28.5 | 20.7 | 24.1 | 22.5 | 21.9 | 16.9 |
| 300–449 | 23.7 | 26.5 | 23.0 | 22.5 | 23.1 | 24.0 | 19.5 | 24.6 | 24.9 | 25.2 | 22.3 | 24.2 |
| ≥450 | 19.5 | 21.6 | 29.1 | 38.8 | 15.5 | 17.0 | 24.3 | 31.0 | 20.2 | 31.5 | 37.2 | 42.7 |
| Latitude, °N | ||||||||||||
| 0–9.99 | 55.8 | 41.9 | 48.5 | 17.8 | 50.6 | 29.1 | 36.9 | 14.3 | 64.0 | 50.8 | 52.1 | 18.4 |
| 10–19.99 | 44.2 | 58.1 | 51.5 | 82.2 | 49.4 | 70.9 | 63.1 | 85.7 | 36.0 | 49.2 | 47.9 | 81.7 |
| Longitude, °W | ||||||||||||
| 65–75.99 | 30.5 | 57.5 | 48.2 | 71.2 | 31.8 | 69.9 | 58.8 | 73.8 | 32.8 | 50.8 | 44.0 | 71.8 |
| 76–85 | 69.5 | 42.5 | 51.8 | 28.8 | 68.2 | 30.1 | 41.2 | 26.2 | 67.2 | 49.2 | 56.0 | 28.2 |
| Poverty** | 24.4 | 30.4 | 45.2 | 52.3 | 22.3 | 25.9 | 40.0 | 46.9 | 30.2 | 35.2 | 52.5 | 61.1 |
| Completed primary school | 85.7 | 84.5 | 85.1 | 87.5 | 86.6 | 86.0 | 86.7 | 88.5 | 84.1 | 82.7 | 83.8 | 86.9 |
| Emigration*** | 16.0 | 23.4 | 22.9 | 23.1 | 15.5 | 21.8 | 23.1 | 24.1 | 17.0 | 24.4 | 23.1 | 22.4 |
| Urbanization | 83.4 | 65.6 | 56.0 | 56.2 | 85.7 | 71.7 | 62.0 | 62.9 | 79.1 | 59.7 | 49.7 | 49.8 |
Data are mean percentages. BMI, body mass index, calculated as weight/height2.
Percentages were rounded to one decimal; thus, partial values within a column may not total 100.
Percentage of homes with a per-capita income below than the basic family needs.
Percentage of movers moving from one province to other different destination province within a 1-year period relative to the population registered in local databases (in a 5-year period estimate from 2002 to 2007).
Table 2.
Association of altitude with overweight, obesity, and abdominal obesity among Peruvian adults aged 20 years or older.
| All | Women* | Men* | ||||
|---|---|---|---|---|---|---|
| n | 31,549 | |||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |
| PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) | |
| Overweight (BMI 25-29.9) | ||||||
| 0–499 m | 1 [Reference] | 1 | 1 | 1 | 1 | 1 |
| 500–1,499 m | 0.95 (0.90–1.01) | 1.03 (0.96–1.11) | 0.98 (0.80–1.19) | 1.07 (0.86–1.32) | 0.92 (0.85–1.00) | 0.99 (0.90–1.09) |
| 1,500–2,999 m | 0.83 (0.79–0.87) | 0.99 (0.92–1.06) | 0.90 (0.75–1.09) | 1.08 (0.89–1.32) | 0.74 (0.69–0.80) | 0.88 (0.80–0.97) |
| ≥3,000 m | 0.77 (0.73–0.80) | 0.99 (0.92–1.08) | 0.86 (0.72–1.02) | 1.12 (0.92–1.36) | 0.65 (0.60–0.70) | 0.85 (0.77–0.93) |
| Obesity (BMI ≥30) | ||||||
| 0–499 m | 1 [Reference] | 1 | 1 | 1 | 1 | 1 |
| 500–1,499 m | 0.97 (0.89–1.05) | 1.00 (0.87–1.16) | 1.04 (0.75–1.44) | 1.07 (0.74–1.55) | 0.86 (0.74–1.00) | 0.87 (0.72–1.06) |
| 1,500–2,999 m | 0.60 (0.55–0.65) | 0.74 (0.63–0.86) | 0.69 (0.50–1.00) | 0.85 (0.56–1.28) | 0.41 (0.35–0.49) | 0.50 (0.41–0.62) |
| ≥3,000 m | 0.46 (0.42–0.49) | 0.54 (0.45–0.64) | 0.53 (0.36–0.80) | 0.65 (0.41–4.02) | 0.25 (0.21–0.31) | 0.31 (0.24–0.39) |
| Obesity class I (BMI 30-34.9) | ||||||
| 0–499 m | 1 [Reference] | 1 | 1 | 1 | 1 | 1 |
| 500–1,499 m | 0.98 (0.90–1.08) | 1.02 (0.88–1.18) | 1.08 (0.76–1.55) | 1.13 (0.76–1.68) | 0.82 (0.70–0.97) | 0.84 (0.68–1.02) |
| 1,500–2,999 m | 0.64 (0.58–0.70) | 0.77 (0.66–0.90) | 0.77 (0.51–1.14) | 0.93 (0.60–1.43) | 0.41 (0.34–0.50) | 0.50 (0.40–0.62) |
| ≥3,000 m | 0.47 (0.43–0.52) | 0.57 (0.48–0.67) | 0.58 (0.37–0.90) | 0.71 (0.44–1.15) | 0.25 (0.21–0.31) | 0.31 (0.24–0.39) |
| Obesity class II (BMI 35-39.9) | ||||||
| 0–499 m | 1 [Reference] | 1 | 1 | 1 | 1 | 1 |
| 500–1,499 m | 0.93 (0.76–1.13) | 0.94 (0.71–1.26) | 0.86 (0.37–2.03) | 0.86 (0.35–2.14) | 1.24 (0.83–1.84) | 1.25 (0.80–1.97) |
| 1,500–2,999 m | 0.50 (0.40–0.61) | 0.62 (0.45–0.84) | 0.49 (0.18–1.37) | 0.62 (0.21–1.80) | 0.51 (0.31–0.82) | 0.63 (0.37–1.07) |
| ≥3,000 m | 0.40 (0.33–0.49) | 0.48 (0.34–0.67) | 0.41 (0.13–1.28) | 0.50 (0.15–1.66) | 0.30 (0.17–0.52) | 0.37 (0.20–0.67) |
| Obesity class III (BMI ≥40)** | ||||||
| 0–499 m | 1 [Reference] | 1 | ||||
| 500–1,499 m | 0.94 (0.63–1.43) | 0.99 (0.63–1.56) | ||||
| 1,500–2,999 m | 0.37 (0.22–0.61) | 0.53 (0.29–0.96) | ||||
| ≥3,000 m | 0.32 (0.20–0.51) | 0.45 (0.23–0.85) | ||||
| Abdominal Obesity-ATP III*** | ||||||
| 0–499 m | 1 [Reference] | 1 | 1 | 1 | 1 | 1 |
| 500–1,499 m | 0.95 (0.89–1.02) | 1.04 (0.93–1.17) | 0.98 (0.69–1.40) | 1.06 (0.73–1.55) | 0.90 (0.76–1.07) | 0.96 (0.79–1.16) |
| 1,500–2,999 m | 0.75 (0.71–0.80) | 0.92 (0.81–1.03) | 0.81 (0.55–1.19) | 0.98 (0.65–1.48) | 0.48 (0.39–0.57) | 0.58 (0.47–0.71) |
| ≥3,000 m | 0.60 (0.56–0.63) | 0.73 (0.64–0.83) | 0.62 (0.42–0.91) | 0.78 (0.51–1.18) | 0.36 (0.30–0.44) | 0.45 (0.36–0.56) |
| Abdominal Obesity-IDF**** | ||||||
| 0–499 m | 1 [Reference] | 1 | 1 | 1 | 1 | 1 |
| 500–1,499 m | 0.96 (0.91–1.00) | 1.01 (0.94–1.07) | 0.99 (0.83–1.18) | 1.04 (0.86–1.25) | 0.92 (0.85–1.00) | 0.95 (0.87–1.04) |
| 1,500–2,999 m | 0.81 (0.78–0.84) | 0.93 (0.87–0.99) | 0.88 (0.75–1.05) | 1.01 (0.84–1.22) | 0.66 (0.61–0.71) | 0.75 (0.69–0.83) |
| ≥3,000 m | 0.73 (0.70–0.76) | 0.89 (0.82–0.95) | 0.80 (0.68–0.94) | 0.99 (0.82–1.19) | 0.56 (0.52–0.60) | 0.68 (0.62–0.75) |
PR (95% CI), prevalence ratio (95% confidence intervals), adjusted for age, sex, self-reported physical activity, out-migration rate, urbanization, poverty, education, and geographical latitude and longitude.
Regression models for each clinical condition were estimated for women and men combined including altitude-sex interaction term.
Inclusion of altitude-sex interaction term produced inconsistent values in the unadjusted model and did not produce outputs in the adjusted model.
Abdominal obesity defined as abdominal circumference >102 cm for males or >88 cm for females, according to the Adult Treatment Panel III (ATP III).
Abdominal obesity defined as abdominal circumference ≥90 cm for males or ≥80 cm for females, as recommended by the International Diabetes Federation (IDF) for individuals of South American ethnicity.
In the unadjusted model, women had lower prevalence ratio of obesity at higher altitudes. However, this association disappeared in the adjusted model (Table 2). We found a significant interaction between altitude and sex and between altitude and age (P<0.001, for both interactions). The association between altitude and obesity by age categories is shown in Table 3. Overall, the inverse association between obesity (and abdominal obesity) and altitude was stronger among individuals in the age category 60–79 years old. We found lower adjusted prevalence ratio of obesity in men who live at higher altitudes as compared with those who live below 500 m [prevalence ratio between 1,500–2,999 m: 0.50 (95% CI, 0.41–0.62); at ≥3,000 m: 0.31 (95% CI, 0.24–0.39)]. Among men, individuals who live below 500 m had two times higher adjusted prevalence ratio of obesity as compared with individuals who live between 1,500–2,999 m and 3.2 times higher adjusted prevalence ratio of obesity as compared with individuals who live at ≥3,000 m (Table 2). Also intriguing was the higher adjusted prevalence ratio of obesity for women as compared with men at different altitude bands: 1.65 (95% CI, 1.52–1.79) between 0–499 m, 2.03 (95% CI, 1.56–2.63) between 500–1,499 m, 2.80 (95% CI, 2.12–3.69) between 1,500–2,999 m, and 3.48 (95% CI, 2.59–4.67) at ≥3,000 m. Overall, women had two times higher adjusted prevalence ratio of obesity as compared with men, regardless the altitude of residence. Regression models for obesity classes also showed an inverse association between altitude and obesity, but only among men (Table 2).
Table 3.
Association of altitude with obesity and abdominal obesity by age categories among Peruvian adults.
| 20–39 years old* | 40–59 years old* | 60–79 years old* | ≥ 80 years old* | |||||
|---|---|---|---|---|---|---|---|---|
| n | 14,086 | 11,828 | 5,119 | 516 | ||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |
| PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) | PR (95% CI) | |
| Obesity | ||||||||
| 0–499 m | 1 [Reference] | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 500–1,499 m | 0.97 (0.84–1.11) | 0.98 (0.81–1.18) | 0.96 (0.70–1.32) | 0.96 (0.66–1.38) | 1.00 (0.68–1.45) | 1.10 (0.72–1.68) | 0.85 (0.25–2.87) | 0.95 (0.27–3.38) |
| 1,500–2,999 m | 0.63 (0.54–0.72) | 0.77 (0.64–0.94) | 0.63 (0.46–0.88) | 0.80 (0.55–1.17) | 0.48 (0.32–0.71) | 0.62 (0.40–0.96) | 0.44 (0.16–1.21) | 0.55 (0.19–1.58) |
| ≥3,000 m | 0.48 (0.41–0.55) | 0.55 (0.45–0.68) | 0.49 (0.35–0.69) | 0.61 (0.41–0.90) | 0.31 (0.21–0.46) | 0.40 (0.25–0.63) | 0.31 (0.12–0.83) | 0.40 (0.14–1.13) |
| Abdominal Obesity-ATP III | ||||||||
| 0–499 m | 1 [Reference] | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 500–1,499 m | 1.04 (0.93–1.15) | 1.11 (0.96–1.28) | 0.93 (0.72–1.20) | 0.98 (0.74–1.31) | 0.85 (0.63–1.14) | 1.03 (0.74–1.43) | 0.80 (0.35–1.83) | 0.97 (0.41–2.30) |
| 1,500–2,999 m | 0.81 (0.73–0.90) | 1.01 (0.87–1.16) | 0.74 (0.58–0.95) | 0.95 (0.71–1.26) | 0.61 (0.46–0.81) | 0.78 (0.56–1.07) | 0.93 (0.53–1.60) | 1.12 (0.62–2.02) |
| ≥3,000 m | 0.70 (0.63–0.77) | 0.84 (0.72–0.97) | 0.60 (0.47–0.75) | 0.76 (0.57–1.02) | 0.40 (0.31–0.53) | 0.54 (0.39–0.75) | 0.44 (0.24–0.79) | 0.61 (0.32–1.17) |
| Abdominal Obesity-IDF | ||||||||
| 0–499 m | 1 [Reference] | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 500–1,499 m | 0.97 (0.90–1.04) | 1.00 (0.92–1.09) | 0.96 (0.81–1.14) | 1.00 (0.83–1.20) | 0.91 (0.74–1.12) | 1.01 (0.81–1.26) | 0.81 (0.45–1.44) | 0.90 (0.50–1.63) |
| 1,500–2,999 m | 0.82 (0.77–0.88) | 0.95 (0.87–1.03) | 0.82 (0.70–0.96) | 0.96 (0.80–1.14) | 0.73 (0.61–0.88) | 0.86 (0.70–1.05) | 0.76 (0.51–1.14) | 0.87 (0.57–1.32) |
| ≥3,000 m | 0.80 (0.75–0.85) | 0.96 (0.88–1.05) | 0.73 (0.63–0.84) | 0.90 (0.76–1.08) | 0.59 (0.49–0.70) | 0.74 (0.61–0.90) | 0.63 (0.43–0.91) | 0.80 (0.54–1.19) |
PR (95% CI), prevalence ratio (95% confidence intervals), adjusted for age, sex, self-reported physical activity, out-migration rate, urbanization, poverty, education, and geographical latitude and longitude.
Regression model for each clinical condition was estimated including altitude-age interaction term.
The adjusted prevalence ratio of abdominal obesity, regardless the diagnostic criteria, was lower at higher altitudes, but only among men (Table 2). Women had five times higher adjusted prevalence ratio of abdominal obesity as compared with men, regardless the altitude. We also found an interaction between altitude and sex and between altitude and age (P<0.001, for both interactions). The adjusted prevalence ratios of overweight and obesity among other covariates included in the full regression models are shown in Table S2.
The exclusion criteria applied to our data for regression analyses did not modify the statistical significance of the estimates of the prevalence ratios of overweight, obesity, obesity classes (Figure S3) or abdominal obesity (Figure S4).
Discussion
The present study conducted in a nationally representative sample of the population of Peru shows that adult individuals who live between 1,500–2,999 m had 26% less prevalence ratio of obesity than those who live between 0–500 m, adjusting for several covariates, including age, sex, and physical activity. A similar association has been reported among US adult individuals (7,8). The adjusted prevalence ratio of obesity among adults who live at ≥3,000 m was nearly 50% less compared with subjects who live below 500 m. Moreover, among men, individuals who live below 500 m had 3.2 times more adjusted prevalence ratio of obesity as compared with individuals who live at ≥3,000 m.
The inverse association between altitude and obesity, while adjusting for several covariates, was found among men but not women. The reason for this finding remains unclear, and should be further investigated. In fact, we found a higher crude prevalence of obesity and abdominal obesity among Peruvian women as compared with men (Figure 1). Although this sex-related difference in the prevalence of obesity has not been found in the US population (25), a higher obesity prevalence in women has been reported among countries with smaller income economies around the world (24). Interestingly, the prevalence of abdominal obesity among Peruvian adults is higher than that among US adults (59.3% versus 54.2%) (26), if the IDF criteria for South Asians is applied to Peruvians (i.e., if abdominal circumference was ≥90 cm for males or ≥80 cm for females).
Our findings of lower crude prevalence of obesity at higher altitudes confirm those from previous studies conducted in small or nationally non-representative samples of the adult populations of Peru (27,28) and Nepal (29), using the current diagnostic criteria. Our data are also consistent with the lower age-adjusted prevalence of obesity at higher altitudes in a nationally representative adult population of the United States (8).
We also found a lower age-adjusted prevalence ratio of abdominal obesity (IDF criteria) in individuals who reside at altitudes at ≥3,000 m as compared with individuals who reside closer to sea level. The age-adjusted prevalence ratio of abdominal obesity, an important predictor of type 2 diabetes (30) and overall mortality (31), was 25% and 32% less among men who live between 1500–2,999 m and ≥3,000 m, respectively, as compared with those who live below 500 m.
Our study has strengths. In contrast to the US, Peru has approximately one fourth of its population residing over 3,000 m, representing more than 6 million individuals (32). Thus, our regression analysis included a larger sample size of individuals (more than 7,000) who reside above 3,000 m, in contrast to those conducted in previous studies (7,8). It should also be noted that in the present study obesity and abdominal obesity were diagnosed based on direct anthropometric measurements, reducing data inaccuracy and preventing recall and response biases (33).
Our results should be interpreted according to the limitations of the study. First, this is a cross-sectional study. Thus, the inverse association between altitude and obesity does not prove causality. Since the ENAHO survey was designed to collect nation- and region-level data, there may well be some bias in the prevalence estimates at the province level. It was not possible to include final weights in our multilevel Poisson regression analysis to account for the ENAHO survey approach; thus, we cannot exclude a potential bias in our estimates. There is a possibility of residual confounding: 1) we adjusted for physical activity, but only self-reported information was available; 2) our regression model included district-level information on socio-economic status and education, and province-level information on out-migration rate and urbanization. Although unlikely, we cannot totally exclude a bias due to reverse causality; that is, individuals with obesity might tend to migrate to lower altitudes, explaining the lower prevalence of obesity at higher altitudes. However, we found no interaction between altitude and out-migration rate on the association with obesity (data not shown). Another limitation is that none of the diagnostic criteria used for abdominal obesity have been validated for the adult Peruvian population. Finally, information on food intake or ethnicity was not included in the survey and information on occupation was not systematically collected. In the US population, the inverse association between altitude and obesity remained while adjusting for ethnicity and fruit and vegetable consumption (7,8).
It can be argued that differences in height among ethnic groups could explain the lower obesity prevalence at higher altitudes. However, individuals who live at high altitudes had lower BMI despite having shorter height (mean height: 154.6 cm, 95% CI: 154.4-154.7 cm versus 156.9 cm, 95% CI: 156.8-157.1 cm; low- and high-altitude, respectively). Whether there are differences in adiposity remains unknown. It should be noted that Amerindian, mestizo (mixed Amerindian and white), and white individuals represent 45%, 37%, and 15% of the Peruvian population, respectively (34). The Caucasian admixture in high-altitude inhabitants appears to be less frequent, particularly in Aymara individuals (35), who are settled in Southeastern Peru. In addition, regional diet may vary among Peruvian places located at different altitudes (36). Thus, in the present study, the inverse association between altitude and obesity could be explained, at least in part, by possible differences in ethnic distribution and diet across altitude bands in the population studied.
The biological mechanisms underlying the association between altitude and obesity are little understood (17). Although acute suppression of appetite and weight loss in lowlanders exposed to high altitudes is well documented (37,38), the direct effect of prolonged altitude exposure on appetite remains unknown. Basal metabolic rate and sympathetic activation does not appear to be higher among highlanders as compared with lowlanders, even if normalized to fat-free mass (17). Since there is an inverse relationship between elevation and ambient temperature (39), cold-induced increased thermogenesis could explain the lower prevalence of obesity, including abdominal obesity, at higher altitudes. Alternatively, non-exercise physical activity (e.g. sitting and standing, walking) (40), which was not accounted in our model, could also explain variations in body weight and abdominal circumference among populations residing at low- and high-altitude.
In conclusion, Peruvian individuals who live at higher altitudes have a lower prevalence ratio of obesity and abdominal obesity, adjusting for multiple covariates, as compared with individuals who live closer to sea level. This inverse association between altitude and obesity varied by sex and age. Our findings suggest that the adjusted inverse association between geographical elevation and obesity extends to different populations around the world. Future studies are needed to explore the source of this association and to determine whether simulated altitude conditions may have potential therapeutical applications for obesity and abdominal obesity.
Supplementary Material
What is already known about this subject?
An inverse association between altitude and self-reported body mass index has been described in a nationally representative sample of the adult population of the United States, adjusting for several risk factors and potential confounders.
What does this study add?
In a nationally representative sample of the adult population of Peru, individuals who live at higher altitudes have a lower adjusted prevalence ratio of obesity and abdominal obesity (based on direct anthropometric measurements) as compared with individuals who live closer to sea level.
The adjusted inverse association between altitude and obesity varies by sex and age.
Acknowledgments
We thank the National Institute of Statistics and Informatics (INEI) of Peru for providing publicly available access to data collected by the 2009-2010 ENAHO. This study was supported by the National Institutes of Health grants DK29867 and DK27619 (RNB), the Alexander von Humboldt Foundation, Bonn-Bad Godesberg, Germany (OAC), and the UCLA Clinical and Translational Science Institute grant UL1TR000124 (RME). The sponsors had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
OOW, CG, OC, and RNB designed the study. OOW, CG, and DS collected and assembled the data. OOW, CG, RME, DS, and RNB interpreted the data. OOW and CG performed the statistical analysis. OOW and RNB drafted the manuscript. OOW searched the literature and generated the figures. OOW, CG, OAC, RME, DS, and RNB critically revised the manuscript for important intellectual content and approved the final draft. OOW had full access to all the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis. OOW had full access to all of the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflicts of Interest Statement: All authors declare that they have no conflicts of interest.
References
- 1.American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2014;37:S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 2.Hjartaker A, Langseth H, Weiderpass E. Obesity and diabetes epidemics: cancer repercussions. Adv Exp Med Biol. 2008;630:72–93. doi: 10.1007/978-0-387-78818-0_6. [DOI] [PubMed] [Google Scholar]
- 3.WHO. [Accessed December 7, 2011];Media Centre: Obesity and overweight. 2011 Available at: http://www.who.int/mediacentre/factsheets/fs311/en/
- 4.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 5.Woolcott OO, Castillo OA, Bergman RN. Sobrepeso y obesidad en pobladores de la altura [Overweight and obesity in dwellers from highlands] Revista Peruana de Epidemiología. 2012;16:5. [Google Scholar]
- 6.Vital signs: state-specific obesity prevalence among adults --- United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:951–955. [PubMed] [Google Scholar]
- 7.Voss JD, Masuoka P, Webber BJ, Scher AI, Atkinson RL. Association of elevation, urbanization and ambient temperature with obesity prevalence in the United States. Int J Obes (Lond) 2013;37:1407–1412. doi: 10.1038/ijo.2013.5. [DOI] [PubMed] [Google Scholar]
- 8.Woolcott OO, Castillo OA, Gutierrez C, Elashoff RM, Stefanovski D, Bergman RN. Inverse association between diabetes and altitude: A cross-sectional study in the adult population of the United States. Obesity (Silver Spring) 2014;22:2080–2090. doi: 10.1002/oby.20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.INEI. Directorio nacional de municipalidades provinciales, distritales y de centros poblados [National directory of local councils at province, district, and centro-poblado level] Lima, Perú: Instituto Nacional de Estadística e Informática; 2012. [Google Scholar]
- 10.INEI. Mapa de pobreza provincial y distrital 2009: El enfoque de la pobreza monetaria [Map of poverty among provinces and districts 2009: Monetary poverty approach] Lima, Perú: Instituto Nacional de Estadística e Informática; 2009. [Google Scholar]
- 11.PNUD. Informe sobre Desarrollo Humano Perú 2009: Por una densidad del Estado al servicio de la gente Parte II: Una visión desde las cuencas [Peru Human Development Report 2009: For a density of the State at the service of the people Part II : A view from the basins] Lima: MIRZA Editores & Impresores SAC; 2010. [Google Scholar]
- 12.INEI. Perú: Migraciones internas 1993-2007 [Peru: Internal migration 1993-2007] Lima, Perú: Instituto Nacional de Estadística e Informática; 2009. [Google Scholar]
- 13.INEI. Perú: Estimaciones y proyecciones de población departamental, por años calendario y edades simples 1995-2025 [Peru: Estimations and projections of department-level population, by calendar years and ages1995-2025] Lima, Perú: Instituto Nacional de Estadística e Informática; 2010. [Google Scholar]
- 14.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am J Clin Nutr. 1998;68:899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 15.Expert Panel on Detection E, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 16.Alberti KG, Zimmet P, Shaw J. International Diabetes Federation: a consensus on Type 2 diabetes prevention. Diabet Med. 2007;24:451–463. doi: 10.1111/j.1464-5491.2007.02157.x. [DOI] [PubMed] [Google Scholar]
- 17.Woolcott OO, Ader M, Bergman RN. Glucose homeostasis during short-term and prolonged exposure to high altitudes. Endocr Rev. 2015;36:149–173. doi: 10.1210/er.2014-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaMonte MJ, Blair SN, Church TS. Physical activity and diabetes prevention. J Appl Physiol. 2005;99:1205–1213. doi: 10.1152/japplphysiol.00193.2005. [DOI] [PubMed] [Google Scholar]
- 19.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1433–1438. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- 20.Tajeu GS, Sen B, Allison DB, Menachemi N. Misuse of odds ratios in obesity literature: an empirical analysis of published studies. Obesity (Silver Spring) 2012;20:1726–1731. doi: 10.1038/oby.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cummings P. Methods for estimating adjusted risk ratios. Stata Journal. 2009;9:175–196. [Google Scholar]
- 22.Hamilton LC. Statistics with STATA: Updated for version 10. Belmont: Brooks/Cole; 2008. Multilevel and mixed-effects modeling; pp. 387–421. [Google Scholar]
- 23.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief. 2012;82:1–8. [PubMed] [Google Scholar]
- 24.Martorell R, Khan LK, Hughes ML, Grummer-Strawn LM. Obesity in women from developing countries. Eur J Clin Nutr. 2000;54:247–252. doi: 10.1038/sj.ejcn.1600931. [DOI] [PubMed] [Google Scholar]
- 25.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 26.Ford ES, Maynard LM, Li C. Trends in mean waist circumference and abdominal obesity among US adults, 1999-2012. Jama. 2014;312:1151–1153. doi: 10.1001/jama.2014.8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pajuelo-Ramírez J, Sánchez-Abanto J, Arbañil-Huamán H. Las enfermedades crónicas no transmisibles en el Perú y su relación con la altitud [Non-transmissible chronic diseases in Peru and their relationship with altitude] Revista de la Sociedad Peruana de Medicina Interna. 2010;23:45–52. [Google Scholar]
- 28.Segura-Vega L, Agusti R, Parodi-Ramirez J. Factores de riesgo de las enfermedades cardiovasculares en el Perú (Estudio TORNASOL) [Risk factors of cardiovascular diseases in Peru (TORNASOL study)] Revista Peruana de Cardiología. 2006;XXXII:82–128. [Google Scholar]
- 29.Sherpa LY, Deji, Stigum H, Chongsuvivatwong V, Thelle DS, Bjertness E. Obesity in Tibetans aged 30-70 living at different altitudes under the north and south faces of Mt. Everest. Int J Environ Res Public Health. 2010;7:1670–1680. doi: 10.3390/ijerph7041670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kodama S, Horikawa C, Fujihara K, Heianza Y, Hirasawa R, Yachi Y, et al. Comparisons of the strength of associations with future type 2 diabetes risk among anthropometric obesity indicators, including waist-to-height ratio: a meta-analysis. Am J Epidemiol. 2012;176:959–969. doi: 10.1093/aje/kws172. [DOI] [PubMed] [Google Scholar]
- 31.Cerhan JR, Moore SC, Jacobs EJ, Kitahara CM, Rosenberg PS, Adami HO, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89:335–345. doi: 10.1016/j.mayocp.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Center for International Earth Science Information Network (CIESIN), Columbia University. National Aggregates of Geospatial Data: Population, Landscape and Climate Estimates (PLACE) Palisades, NY: CIESIN, Columbia University; 2002. [Accessed on April 27, 2012]. Available at: http://sedac.ciesin.columbia.edu/plue/nagd/place. [Google Scholar]
- 33.Le A, Judd SE, Allison DB, Oza-Frank R, Affuso O, Safford M, et al. The geographic distribution of obesity in the US and the potential regional differences in misreporting of obesity. Obesity. 2013 doi: 10.1002/oby.20451. n/a http://dx.doi.org/10.1002/oby.20451. [DOI] [PMC free article] [PubMed]
- 34.Central Intelligence Agency. The World Factbook. South America, Peru: [Accessed April 20, 2015]. Available at: http://www.cia.gov/library/publications/the-world-factbook/geos/pe.html. [Google Scholar]
- 35.Rupert JL, Hochachka PW. Genetic approaches to understanding human adaptation to altitude in the Andes. J Exp Biol. 2001;204:3151–3160. doi: 10.1242/jeb.204.18.3151. [DOI] [PubMed] [Google Scholar]
- 36.Picon-Reategui E. The food and nutrition of high-altitude populations. In: Baker PT, editor. The biology of high-altitude peoples. Cambridge: Cambridge University Press; 1978. pp. 219–249. [Google Scholar]
- 37.Stock MJ, Norgan NG, Ferro-Luzzi A, Evans E. Effect of altitude on dietary-induced thermogenesis at rest and during light exercise in man. J Appl Physiol. 1978;45:345–349. doi: 10.1152/jappl.1978.45.3.345. [DOI] [PubMed] [Google Scholar]
- 38.Hannon JP, Sudman DM. Basal metabolic and cardiovascular function of women during altitude acclimatization. J Appl Physiol. 1973;34:471–477. doi: 10.1152/jappl.1973.34.4.471. [DOI] [PubMed] [Google Scholar]
- 39.Montgomery K. Variation in temperature with altitude and latitude. Journal of Geography. 2006;105:133–135. [Google Scholar]
- 40.Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, et al. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307:584–586. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.