Abstract
Rationale
Cognitive flexibility is a key component of executive function and is disrupted in major psychiatric disorders. Brain-derived neurotrophic factor (BDNF) exerts neuromodulatory effects on synaptic transmission and cognitive/affective behaviors. However the causal mechanisms linking BDNF hypofunction with executive deficits are not well understood.
Objectives
Here, we assessed the consequences of BDNF hemizygosity on cognitive flexibility in mice performing an operant conditioning task. As dopaminergic-glutamatergic interaction in the striatum is important for cognitive processing, and BDNF heterozygous (BDNF+/−) mice display a higher dopamine tone in the dorsal striatum, we also assessed the effects of partial striatal dopamine depletion on task performance and glutamate release.
Results
BDNF+/− mice acquired discrimination learning as well as new rule learning during set-shifting as efficiently as wild-type mice. However, partial removal of striatal dopaminergic inputs with 6-hydroxydopamine (6-OHDA) impaired these cognitive processes by impeding the maintenance of a new learning strategy in both genotypes. BDNF mutants exhibited performance impairments during reversal learning and these deficits were associated with increased perseveration to the previously acquired strategy. Partial dopamine depletion of the striatum reversed these cognitive impairments. Additionally, reduction in depolarization-evoked glutamate release noted in the dorsal striatum of BDNF+/− mice was not observed in 6-OHDA-infused BDNF mutants indicating normalization of glutamatergic transmission in these animals.
Conclusions
Our data illustrate that BDNF signaling regulates cognitive control processes presumably by maintaining striatal dopamine-glutamate balance. Moreover, aberrations in BDNF signaling may act as a common neurobiological substrate that accounts for executive dysfunction observed in multiple psychiatric conditions.
Keywords: BDNF, cognitive flexibility, dopamine, glutamate, mice
Introduction
Cognitive flexibility is the ability to update behavioral goals and maintain performance in the face of changing environmental demands. It is a key component of executive function, involving the adaptation of cognitive processing strategies and influencing judgement, problem-solving and decision-making abilities in new and unexpected contexts. The integrity of segregated frontostriatal circuits is critical for cognitive flexibility (Ragozzino 2007; Balleine and O'Doherty 2010) and disruption in these circuits may contribute to deficits in executive functions associated with various neuropsychiatric disorders such as schizophrenia (Floresco et al. 2009; Eisenberg and Berman 2010), depression (Mega and Cummings 1994), addiction (Kalivas and Volkow 2005) Huntington’s disease (Lawrence et al. 1996) and Parkinson’s disease (Dirnberger and Jahanshahi 2013). Therefore, delineation of neurobiological underpinnings of cognitive flexibility at the molecular and systems level may provide insights into the neurocognitive endophenotype common to the psychiatric manifestations of multiple brain disorders and the underlying mechanisms that subserve it.
Brain-derived neurotrophic factor (BDNF) is a growth-promoting protein that signals through tropomyosin-related kinase B (trkB) receptor, activates intracellular signaling pathways to protect and differentiate neurons, and produces plastic changes in the brain (Huang and Reichardt 2001; Chao 2003). This neurotrophin also exerts modulatory effects on synaptic transmission (Carvalho et al. 2008), and cognitive and affective/motivated behaviors such as spatial learning and memory (Mizuno et al. 2003), fear memory (Minichiello 2009), emotionality and mood (Lindholm and Castren 2014), and conditioned reward (Nestler and Carlezon 2006). BDNF is implicated in multiple psychiatric disorders. For example, lower BDNF levels are reported in the plasma and CSF of first-episode psychotic patients (Pillai et al. 2010). BDNF Val66met polymorphism produces memory impairments by disrupting activity-dependent secretion of mature BDNF (Egan et al. 2003; Kambeitz et al. 2012), and increases the risk to develop schizophrenia, anxiety-disorders and depression (Notaras et al. 2015). Long-term use of antidepressants increased BDNF expression in postmortem brains of individuals suffering from major depressive disorder (Duman and Monteggia 2006). Moreover, BDNF produced antidepressant-like effects in animal models of depression (Shirayama et al. 2002; Hu and Russek 2008), and BDNF heterozygous mice showed a depression-like phenotype when exposed to stress (Duman et al. 2007; Carola and Gross 2010). Additionally, dysregulation of BDNF signaling in the reward circuitry is hypothesized to underlie addiction-related behaviors (Bolanos and Nestler 2004; Kalivas and O'Brien 2008). Despite considerable evidence that suggests a possible association between deficient BDNF signaling and diverse behavioral symptoms of neuropsychiatric disorders (Autry and Monteggia 2012), the causal mechanisms linking BDNF and executive dysfunction, which constitutes a core cognitive symptom of these disorders, remain poorly understood.
There is substantial evidence that points towards aberrations in dopaminergic and glutamatergic transmission in major psychiatric disorders (Howes and Kapur 2009; Volkow et al. 2010; Moghaddam and Krystal 2012; Sanacora et al. 2012). The dorsal striatum receives converging glutamatergic inputs from the cortex and dopaminergic inputs from the midbrain regions (Haber et al. 2000). Moreover, a growing body of evidence suggests that the dorsal striatum may be critical for cognitive flexibility (Cools et al. 2006; van Schouwenburg et al. 2012). Activity-dependent release of dopamine was enhanced by exogenous BDNF administration in the striatum (Goggi et al. 2002). BDNF heterozygous mice that possess 50% reduced BDNF protein exhibited higher dopamine tone in the dorsal striatum (Bosse et al. 2012; Birbeck et al. 2014). BDNF is essential for long-term potentiation in the dorsal striatum (Jia et al. 2010). Moreover, we recently demonstrated that intracranial infusions of BDNF in the dorsal striatum facilitated strategy set-shifting in mice in an inverted-U manner and these effects were associated with parallel changes in striatal glutamatergic transmission (D'Amore et al. 2013). Therefore, modulation of corticostriatal dopaminergic-glutamatergic interactions by endogenous BDNF signaling may influence cognitive flexibility. In the present study, we assessed the consequences of BDNF hemizygosity on cognitive flexibility in mice performing an operant conditioning task. As optimal dopamine signaling is critical for cognitive flexibility (Floresco 2013), we also assessed the effects of partial striatal dopamine depletion on task performance in BDNF heterozygous mice. Lastly, the impact of genotype and dopamine manipulation was examined on striatal glutamatergic transmission.
Methods
Animals and genotyping
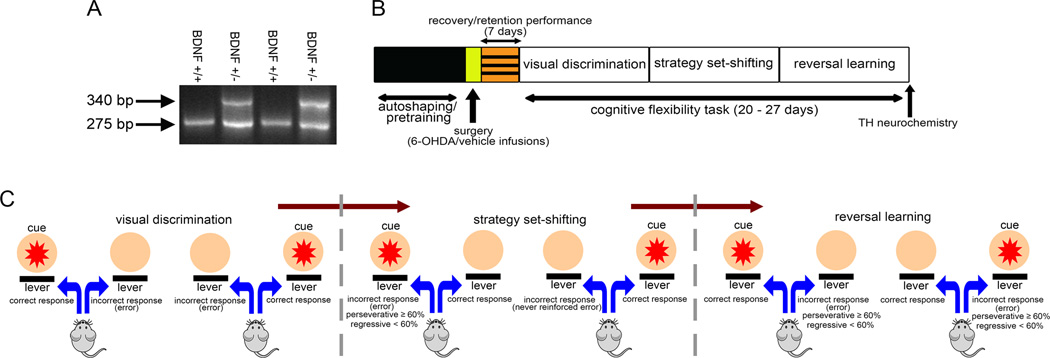
Breeding pairs of congenic mice with a heterozygous deletion of the BDNF gene and their wild-type siblings (Bdnf tm1Jae; Stock # 002266) were obtained from the Jackson laboratory (Bar Harbor, ME) and backcrossed for three generations in our facility. Prior to arrival at Temple, the transgenic mice were maintained on a C57BL/6J genetic background for six generations at Jackson. Genotyping was conducted from tail DNA with PCR using the following primers: 5’-ATG AAA GAA GTA AAC GTC CAC-3’ (common); 5’-CCA GCA GAA AGA GTA GAG GAG-3’ (wild-type reverse); and 5’-GGG AAC TTC CTG ACT AGG GG-3’ (reverse). The amplification products were 275 bp for the wild-type (BDNF+/+) and both 275 and 340 bp for the heterozygous (BDNF+/−) mice (Fig. 1A). Eight weeks old BDNF+/+ and BDNF+/− mice of either sex were housed individually in clear plastic cages under controlled conditions (12 h light/dark cycle; 25° C temperature) and progressively water-restricted to 5 min of water per day. Single housing was adopted so that each animal is restricted to the same amount of water intake per day. Since all mice used in the study were single housed prior to behavioral training and testing, any impact that single housing might have produced on the behavioral outcome was considered to remain similar to both genotypes. All behavioral training and testing took place 7 days/week between 9:00 and 16:00 h. At the completion of each behavioral session, mice received 5 min of water in addition to sweetened water received as a reward for each correct response (see below). Food (PMI LabDiet) was available ad libitum throughout the experiment. All experimental procedures were approved by the Institutional Care and Use Committee (IACUC) of Temple University and were in accordance with the National Institute of Health guidelines.
Figure 1.
Genotyping and experimental design. (A) PCR-based genotyping revealed two gene products in BDNF+/− mice (275 and 340 bp, respectively) as compared to the single product of 275 bp in WT mice. (B) Schematics of the experimental design. BDNF+/+ and BDNF+/− mice were initially autoshaped and then pretrained to press an activated lever within allotted time to receive reinforcement. Animals that reached pretraining criterion received bilateral infusions of either 6-OHDA or vehicle into the dorsal striatum (see Methods). Following recovery, mice were trained to retain pretraining criterion. Mice remained at pretraining phase for an additional 7 days following which they progressed to subsequent stages of the task, i.e. visual discrimination, strategy set-shifting and reversal learning. The total number of days required to complete behavioral testing for all three phases of the task varied between 20–27 days across all animals. After completion of behavioral testing, the brains of the animals were removed and examined for striatal tyrosine hydroxylase (TH) protein expression either via quantitative immunohistochemistry or immunoblotting. (C) Illustration depicting the task stages and error analysis. During visual discrimination phase, mice were required to press the lever associated with the stimulus light that illuminated above it. Incorrect responses on levers not paired with visual cue were scored as an error. Strategy set-shifting entailed a shift from visual cue- to egocentric spatial cue-based strategy. During this phase, the animals were required to press the lever always located at one side (e.g., right in this case) regardless of the position of the cue light. For set-shift to right, a left lever press was scored as an incorrect response (error). If the response was made while the cue light was illuminated above the incorrect lever, the errors were scored either as perseverative (≥60% incorrect responses/session) or regressive (<60% incorrect responses/session). An incorrect lever press in the presence of cue illuminated on the opposite side (left in this case) was scored as never-reinforced error. Both regressive and never-reinforced errors served as an index of animals’ ability to execute a new learning strategy and were categorized as learning errors. For reversal learning, the animals were required to always press the lever on the opposite side of the previously reinforced lever (left lever for set-shift to right assignment) to attain a reward. Incorrect responses on ≥60% trials/session were scored as perseverative errors. Regressive errors for reversal learning phase occurred when the mice made incorrect responses on <60% trials in a session and were categorized as learning errors.
Mouse Operant Cognitive Flexibility Task
Mouse modular operant conditioning chambers (MED Associates; St. Albans, VT, USA) equipped with a standard grid floor and house light (28V, 100mA), and a panel consisting of two large cue lights (2.5cm; 28V, 100mV), a central reward port attached to a fluid dipper, and two ultra-sensitive retractable levers were used. Control of all events, including light presentation, lever operations, and reward delivery, utilized a SmrtCtrl interface running MED-PC IV software on Dell PC (Optiplex 960).
Mice were trained in an automated operant cognitive flexibility task for mice as described previously in our studies (Cole et al. 2015; D'Amore et al. 2013; Ortega et al. 2013). This task places heavier emphasis on response conflicts and shares features similar to the operant conditioning tasks previously established to assess cognitive flexibility in rodents (Floresco et al. 2008; Haluk and Floresco 2009; Brigman et al. 2010; Scheggia et al. 2014) and the Wisconsin Card Sorting Task in humans (Monchi et al. 2001). Briefly, animals were autoshaped on a FR-1 schedule of reinforcement to acquire the lever press response and subsequent reward (10µl of .066% saccharin solution). After attaining at least 30 lever press responses with a 30-min session, animals were advanced to a pretraining phase where each trial consisted of a lever presentation (either left or right) for 10 s. Each lever press response was rewarded and terminated the lever. Trials were presented with an inter-trial interval (ITI) of 9±3 s. An omission was scored if no lever press response occurred within 10 s and the ITI was reinstated. To control for any novelty effect that might be associated with the visual stimulus during the subsequent stage of the task, the activated lever was randomly associated with an unpredictably occurring illumination of the panel light. After reaching criteria (30 rewards and ≤20% omissions for 3 consecutive days), animals underwent stereotaxic surgeries for striatal infusions of either vehicle or 6-hydroxydopamine (6-OHDA) and maintained on the pretraining phase following recovery for 1 week so that the neurotoxin could exert its maximal effect prior to the behavioral testing on all phases of the cognitive flexibility task (see Stereotaxic surgeries and experimental design).
Mice that retained criterion following recovery were progressed sequentially to all three phases of training and testing in the cognitive flexibility task; visual discrimination, strategy set-shifting, and reversal learning (see Fig 1B for schematic diagram of experiment). During the visual discrimination phase, mice were required to discern the lever with an activated cue light. The session began with the illumination of the house light and presentation of 30 trials with an ITI of 9 ± 3 s. All trials were started with the illumination of 7s visual cue (either from the left or right panel), followed by the presentation of both levers 2s later. Levers were present for 5s and both the stimulus light and levers were co-terminated. A lever press response on the cued lever was scored as a “correct response” and was followed by reward delivery. Responses on the incorrect lever were not rewarded and resulted in a “timeout” (punishment) phase characterized by a 10s extinguishing of the house light. Punishment on incorrect responses was introduced to discourage indiscriminate responding to levers. It is important to note that the ITI and punishment durations used for this study were selected to allow the animals to clearly distinguish between correct trials (i.e., reward + ITI) and incorrect trials (i.e., time out + ITI). The house light remained illuminated throughout the entire session except for the punishment phase. Animals trained to criterion (80% correct responses for 3 consecutive days) were advanced to the next phases of training.
During the set-shifting phase, experimental parameters remained identical to the previous phase except that the contingencies were altered in such a way that the animals were required to adopt an egocentric response strategy to achieve rewards. Animals were required to press the correct lever (either left or right; lever assignment was counterbalanced within a group) to earn a reward irrespective of cue presentation which remained pseudorandom. Animals that successfully attained criterion at this stage of training were moved to the reversal learning phase. During this phase of training, the proprioceptive rule was reversed; animals were required to press the lever opposite to the one assigned to them during the preceding phase (set-shifting) regardless of the position of the activated cue light. Daily training on 30-trial sessions continued until the animals’ attained criterion by exhibiting ≥80% correct responses for 3 consecutive days on both the strategy set-shifting and reversal learning phases of the task. It is important to note that the operant conditioning task to assess cognitive flexibility in mice is an incremental learning task that takes several days to attain criterion. Therefore, criterion performance for different phases could not be assessed based on a single session performance but rather stable performance over multiple sessions as reported previously for rodents (Brigman et al. 2008; Haluk and Floresco, 2009; Oualian and Gisquet-Verrier, 2010).
Behavioral Measures
The number of correct responses, errors, omissions, response latencies and reward retrieval latencies was obtained for each behavioral session. Response accuracies were calculated for each session using the formula: correct responses/(correct+incorrect responses)*100. The total number of performed trials to criterion, errors to criterion and omissions were obtained for each training phase using the above described criteria. A cartoon illustrating the rules of the cognitive flexibility task and different types of errors is presented in Fig. 1C. As the visual discrimination was the first stage of the task, there was no preceding phase at which the animals could perseverate. So, any incorrect response (pressing a lever not associated with a cue) was scored as an error. The strategy set-shifting and reversal learning performance was characterized by distinguishing whether an incorrect response occurred due to the perseverance of a previously learned strategy or failure to acquire/maintain a new strategy as described previously (D’Amore et al. 2013; Floresco et al. 2008; Ortega et al. 2013). For this analysis, errors were classified either as perseverative or learning errors. A perseverative error occurred if the animal responded to the incorrect lever when the visual cue was illuminated above it on ≥ 60% of trials within a session. For example, if the animal was set-shift to right lever and pressed the left lever in ≥ 9/15 of performed trials when the visual cue was presented from the left side, the error was scored as perseverative. Depending on the performance in the preceding session, an error was scored as a regressive error if the animal made < 60% incorrect responses by pressing the left lever paired with the cue in subsequent sessions. At this point, the animals were making fewer errors and considered to be inhibiting the previously learned strategy and acquiring the new strategy. Never-reinforced errors occurred when the animal responded on the incorrect lever while the visual cue was presented from the opposite side. While a regressive error might mean that the animals’ perseveration to the previous choice pattern is ceasing, a never-reinforced error reflect that the animals are trying to explore an alternate strategy that might be different from the visual discrimination as well as set-shifting phase. For strategy set-shifting, both regressive and never-reinforced errors were considered as learning errors as they reflected acquisition of a new learning strategy to optimize performance (Fig. 1C). Errors during the reversal learning phase occurred when the animal responded to the same lever that provided reward during the strategy set-shifting phase. Reversal errors were classified as perseverative if the animals made ≥ 60% presses (typically ≥18/30 of performed trials) on the incorrect lever. If the animals made < 60% incorrect responses, errors were scored as regressive in all subsequent sessions. Since the reversal learning phase followed the strategy set-shifting phase, the animals already learned to ignore the visual cue. Pressing the incorrect lever in the absence of the cue above it would not be considered never-reinforced in this situation because the animals knew that cue the did not have significance based on the previous stage of the task. Therefore, for reversal phase of the task, the learning errors consisted of regressive errors only. Learning errors for both the strategy set-shifting and reversal learning phases were estimated by subtracting the perseverative errors from the total errors for the respective phase of the task.
Stereotaxic surgeries and experimental design
Wild-type and BDNF mutant mice trained to criterion at the pretraining phase (above) were randomly assigned either to the vehicle or 6-OHDA injection group (N=7–10/genotype/manipulation; total N=36; 12 male and 7 female BDNF+/+; 10 male and 7 female BDNF+/−). All surgeries were conducted in isoflurane-anesthetized mice under aseptic conditions. 6-OHDA hydrobromide (Sigma Chemical Co., St. Louis, MO) solution was prepared in a vehicle consisting of 0.2% ascorbic acid in saline. BDNF+/+ (N=9) and BDNF+/− (N=7) mice received bilateral infusions of 6-OHDA (2 µg/µL; 4µL/hemisphere) into the dorsal striatum (A/P: +1.0, M/L: ±1.7, D/V: −3.0) to produce restricted loss of striatal dopaminergic fibers. Control animals of either genotype received vehicle (saline containing 0.2% ascorbic acid) infusions (BDNF+/+: N=10; BDNF+/−: N=10). All infusions were made at a rate of 1µL/min using a 10 µL Hamilton Syringe and the injection needle was left in place for 4 min prior to retraction to prevent reflux. Desipramine (20mg/Kg, i.p.; Sigma) was injected to all animals 30-min prior to surgeries to protect noradrenergic terminals from the toxic effects of 6-OHDA. For each manipulation, BDNF+/+ mice were littermate controls for BDNF+/− mice.
After a two-day recovery period, animals were placed back on the pretraining phase of the task to regain criterion performance. Mice remained at this phase for an additional 7 days for maximal lesion effect following which they progressed to subsequent stages of the task, i.e. visual discrimination, strategy set-shifting and reversal learning (see schematic in Fig. 1B). The total number of testing sessions varied between 20 and 27 sessions across animals. At the completion of behavioral testing, animals’ brains were examined for tyrosine hydroxylase (TH) protein expression either via quantitative immunohistochemistry or immunoblotting.
Tyrosine Hydroxylase (TH) immunohistochemistry
Animals (N=4–6/genotype/manipulation)) were transcardially perfused with 4% paraformaldehyde and the brains were removed, postfixed and cryoprotected. Randomly sampled serial coronal sections (50 µM) from the rostral-caudal extent of the dorsal striatum (0.5–1.5 mm anterior to bregma) were incubated overnight with a monoclonal anti-TH antibody (1:1000; EMD Millipore, Billerica, MA). Slices were rinsed in 0.05 M TBS with 1% triton X-100 and incubated in biotinylated goat anti-mouse IgG (EMD Millipore) for 2 hrs followed by streptavidin-horseradish peroxidase for 1 hr. Staining was developed with 3,3’-diaminobenzidine in the presence of 0.01% nickle ammonium sulfate. All sections were visualized using a Leica microscope (Leica Microsystems Inc., Buffalo Grove, IL; Model DM4000B). Images were captured using the DFC 425C digital camera and Leica Application Suite image acquisition system. To analyze the extent of the 6-OHDA lesion, optical density (OD) measures were obtained from the striatum as described previously (Tripanichkul and Jaroensuppaperch, 2013). Briefly, images were captured at 2.5× magnification and OD measurements were conducted using NIH Image J. Images were converted to an 8-bit file and the pixel value was converted to an uncalibrated OD measure via the equation log10(255/pixel value). OD measurements were then calibrated against the cortex and corpus callosum by subtracting the background values. Average OD values were based on analyses from both hemispheres of three sections per animal.
Western blotting
Mice (N=3–6/genotype/manipulation) were decapitated and striatal tissues were collected and homogenized using 50mM HEPES NaOH buffer. Proteins were separated using 4–15% Tris–HCl polyacrylamide gels, transferred to PVDF membranes and probed with a mouse anti-TH antibody (1:2000 dilution; EMD Millipore). Blots were developed using a peroxidase-conjugated secondary antibody (GE Healthcare, Mickleton, NJ) and SuperSignal West Femto chemiluminescent substrate (Life Technologies, Grand Island, NY). Images were captured using Molecular Imager Chemidoc EQ system (Bio-Rad, Hercules, CA). β-tubulin served as a gel-loading control and detected using a mouse anti-β-tubulin antibody. Densitometric analysis was performed by calculating the integrated pixel densities using NIH Image J software. Blot densities were normalized to the levels of β-tubulin-immunoreactive bands for each sample assayed in duplicate.
In vivo amperometric recordings
A separate cohort of naïve mice were used for amperometric recordings of striatal glutamate transmission. Briefly, ceramic-based microelectrodes (Center for Microelectrode Technology, Lexington, KY), featuring an array of four (15 × 333 µm) platinum recording sites arranged in pairs (upper and lower) were coated with recombinant L-glutamate oxidase (EC 1.4.3.11; US Biologicals) and calibrated for glutamate sensitivity as reported earlier (D'Amore et al., 2013; Parikh et al. 2008; 2010; 2014). The enzyme was cross–linked with the BSA–glutaraldehyde mixture and immobilized onto the bottom pair of recording sites. The upper two recording sites were coated only with the BSA–glutaraldehyde solution and served to record background activity. Microelectrodes that displayed a sensitivity of ≥3pA/µM and limit of detection ≤500 nM for glutamate were subsequently used for in vivo recordings.
BDNF+/+ (N=4) and BDNF+/− (N=3) mice received unilateral infusions of 6-OHDA into either the right or left dorsal striatum 4 weeks prior to in vivo recordings. Infusion of vehicle into the contralateral hemisphere of the same animal served as within-animal control. Microelectrodes were implanted into the dorsal striatum of urethane-anesthetized mice. Recordings were made at a frequency of 2 Hz and data were digitized using a FAST-16 recording system (Quanteon, Nicholasville, KY). Experiments began after stabilization of the baseline current for 60 min. Currents were recorded for a period of 2-min for basal glutamate measurements. Depolarization-evoked glutamate release was induced by locally applying brief pulses of potassium (KCl 70mM; 50 nL) using a picospritzer (ALA Scientific Instruments, Farmingdale, NY, USA). Amperometric recordings were conducted in both hemispheres with side counterbalanced for 6-OHDA manipulation across animals within each genotype. Basal glutamate levels were measured by the self-referencing procedure that involves subtraction of the background current on sentinel channels from the currents recorded on glutamate-sensitive channels as described previously (Stephens et al., 2010). For this analysis, currents measured over 2-min period following the baseline stabilization period were averaged on all channels. Potassium-evoked glutamate signals were analyzed with respect to peak amplitudes, and signal decay rate (t80; time required for the signal to decline by 80% from peak amplitude). Self-referencing was adopted to eliminate any artifacts due to background noise levels or local drug application based on previous studies (D’Amore et al. 2013; Parikh et al. 2010). The averages of two responses per drug manipulation per animal were used for statistical analysis.
Statistics
Statistical analysis was performed with SPSS/PC+V21.0 (IBM SPSS Software, Armonk, NY). Data for trials to criterion, errors to criterion, error types, omissions, correct and incorrect response latencies, and neurochemical measures were analyzed using two-way ANOVA using genotype (two levels: BDNF+/+ and BDNF+/−) and manipulation (two levels: vehicle and 6-OHDA) as between-subject variables. As a priori analysis did not indicate a main effect of sex or sex × genotype interaction on any behavioral measure, data between sexes were aggregated across all groups to increase the statistical power. However, it must be noted that the chance of observing gender differences would be limited with small sample sizes by sex for each genotype, and therefore should not be entirely ruled out. Learning analyses were conducted by comparing response accuracies for the first 5 training sessions using mixed-factor repeated measures ANOVA. Post hoc tests were applied where necessary using Bonferroni corrections for multiple comparisons. For all statistical tests, p values <0.05 were considered significant.
Results
Striatal DA Depletion
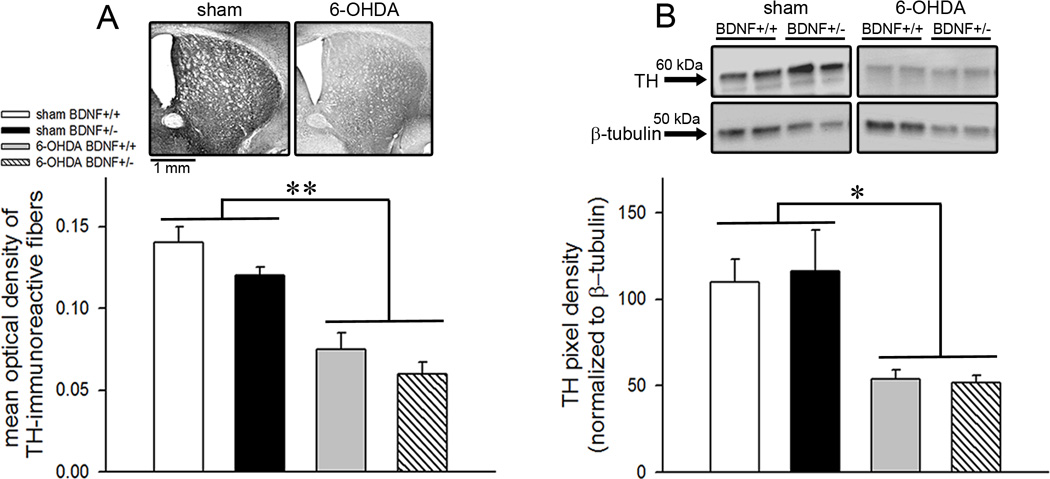
The impact of intrastriatal 6-OHDA infusions on striatal DA depletion was assessed by examining tyrosine hydroxylase expression in mice. Semiquantitative analysis of striatal TH immunoreactivity revealed a significant reduction in OD measures in the lesioned mice (main effect of manipulation: F(1,14)=38.97; p<0.001; Fig. 2A). Although OD values were pooled from both hemispheres, a separate analysis revealed no hemispheric differences in TH immunoreactivity (p=0.68) indicating that the effect of manipulations was similar in both hemispheres. Likewise, immunoblot analysis showed reduced TH protein densities in the 6-OHDA-infused mice (F(1,14)=8.91; p=0.01; Fig. 2B). On average, the loss of TH expression determined by immunohistochemistry was 46.93 ± 4.99% and estimated by immunoblot detection was 53.27 ± 3.09%, and TH reduction observed by the two measures did not differ (F(1,14)=0.84; p=0.38). These data illustrate that 6-OHDA concentration used in the present study produced partial DA depletion in the dorsal striatum in mice and are in line with previous studies (Branchi et al. 2010; Zurkovsky et al., 2013). As noted in TH-stained slices (Fig. 2A), it appears that some of the immunotoxin might have diffused into the ventral striatal regions such as nucleus accumbens. Therefore, partial denervation of dopaminergic terminals reported in our study is not restricted to only dorsal striatum but might have spanned the entire striatum. Genotypic differences in striatal TH expression were not observed either by immunohistochemistry (F(1,14)=2.84; p=0.11) or immunoblotting (F(1,14)=0.01; p=0.92). Moreover, the effects of 6-OHDA manipulation did not interact with the genotype (manipulation × genotype interaction: F(1,14)<0.05; p>0.77 for both measures).
Figure 2.
Tyrosine hydroxylase (TH) protein expression in the dorsal striatum. (A) Representative sections from the dorsal striatum show TH immunohistochemical staining from BDNF+/+ mice either infused with vehicle or 6-OHDA (top). Bar charts depicting optical density values show reduced TH-immunoreactivity in both BDNF+/+ and BDNF+/− mice (bottom). (B) Immunoblots depicting striatal TH expression from duplicate samples representative of all measured bands (top). TH-immunoreactive band (60 kDa) was detected with a monoclonal anti-TH antibody. Blot densities normalized to β-tubulin (gel-loading control) show ~53% decline in TH protein expression in both genotypes infused with 6-OHDA indicating partial dopamine depletion (bottom). TH expression did not differ by genotype. All data are Mean±SEM. (*, ** p<0.05, 0.001 main effect of 6-OHDA manipulation).
Partial dopaminergic deafferentation of the striatum and discrimination learning
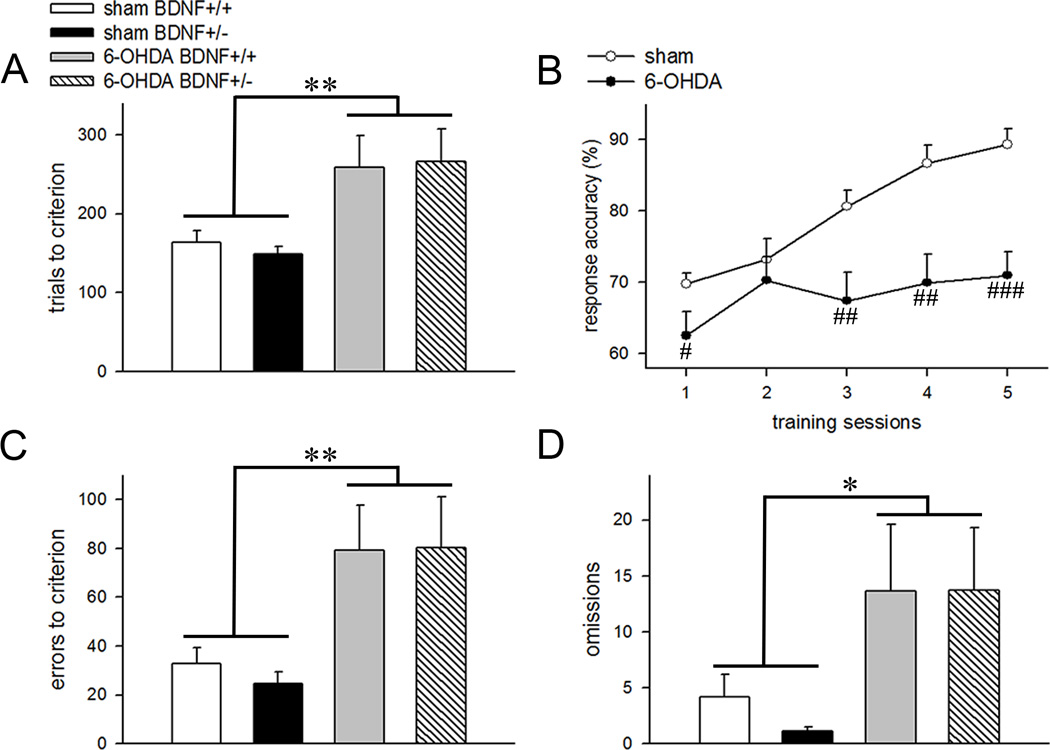
Mice with intrastriatal 6-OHDA infusions required higher number of trials to attain visual discrimination criterion as compared to vehicle-treated mice (main effect: F(1,28)=11.56; p=0.002; Fig. 3A). Trials to criterion remained similar between the BDNF+/+ and BDNF+/− mice (main effect of genotype: F(1,28)=0.01; p=0.94) and no interactions between genotype and 6-OHDA manipulation was observed (F(1,28)=0.27; p=0.61). Repeated-measure ANOVAs conducted on response accuracies for the first 5 behavioral sessions show a main effect of training illustrating effective discrimination learning (F(4,128)=15.42; p<0.001). Dopamine depletion significantly reduced response accuracies (F(1,32)=13.93; p=0.001; Fig. 3B) and this effect interacted with training sessions (manipulation × training interaction: F(4,128)=5.22; p=0.001). One way ANOVA shows that response accuracy was significantly lower on day 1, 3, 4 and 5 of training in the 6-OHDA-infused mice (all p<0.05; Fig. 3B). In general, dopamine-depleted mice committed higher errors (F(1,28)=14.63; p=0.001; Fig. 3C) which may explain diminished efficiency for discrimination learning in these animals. Response accuracies and errors to criterion remained similar between BDNF+/+ and BDNF+/− mice (main effect of genotype: both p>0.76) indicating that partial deafferentation of striatal dopaminergic terminals impaired visual discrimination learning irrespective of the genotype. Mice infused with 6-OHDA also omitted more trials as compared to the vehicle-infused animals (main effect of manipulation: F(1,28)=6.11; p=0.02; Fig. 3D) and this measure was not influenced by BDNF hemizygosity (main effect of genotype: F(1,28)=0.16; p=0.69). Average latencies for correct and incorrect responses did not differ between vehicle- and 6-OHDA-infused mice (vehicle: 0.72 ± 0.03 s correct, 0.93 ± 0.06 s incorrect; 6-OHDA: 0.85±0.03 s correct, 0.92 ± 0.04 s incorrect; both F(1,28)<1.25; p>0.27). Moreover, we neither found a main effect of genotype, nor a genotype × manipulation interaction for both latency measures (all main effects and interactions; F<0.72; p>0.41).
Figure 3.
Effects of partial dopamine depletion of the striatum on visual discrimination learning. (A) Number of trials required to reach visual discrimination criterion. (B) Response accuracies plotted for vehicle- and 6-OHDA-infused animals show learning impairments following partial dopamine depletion. 6-OHDA manipulation dramatically increased (C) errors to criterion and (D) total omissions in both BDNF+/+ and BDNF+/− mice. All data are Mean±SEM. (*, ** p<0.05, 0.01 main effect of 6-OHDA manipulation; #, ##, ### p<0.05, 0.01, 0.001 post hoc comparisons vs vehicle-infused mice for the corresponding testing day).
Effects of BDNF hemizygosity and striatal dopamine depletion on strategy shifting
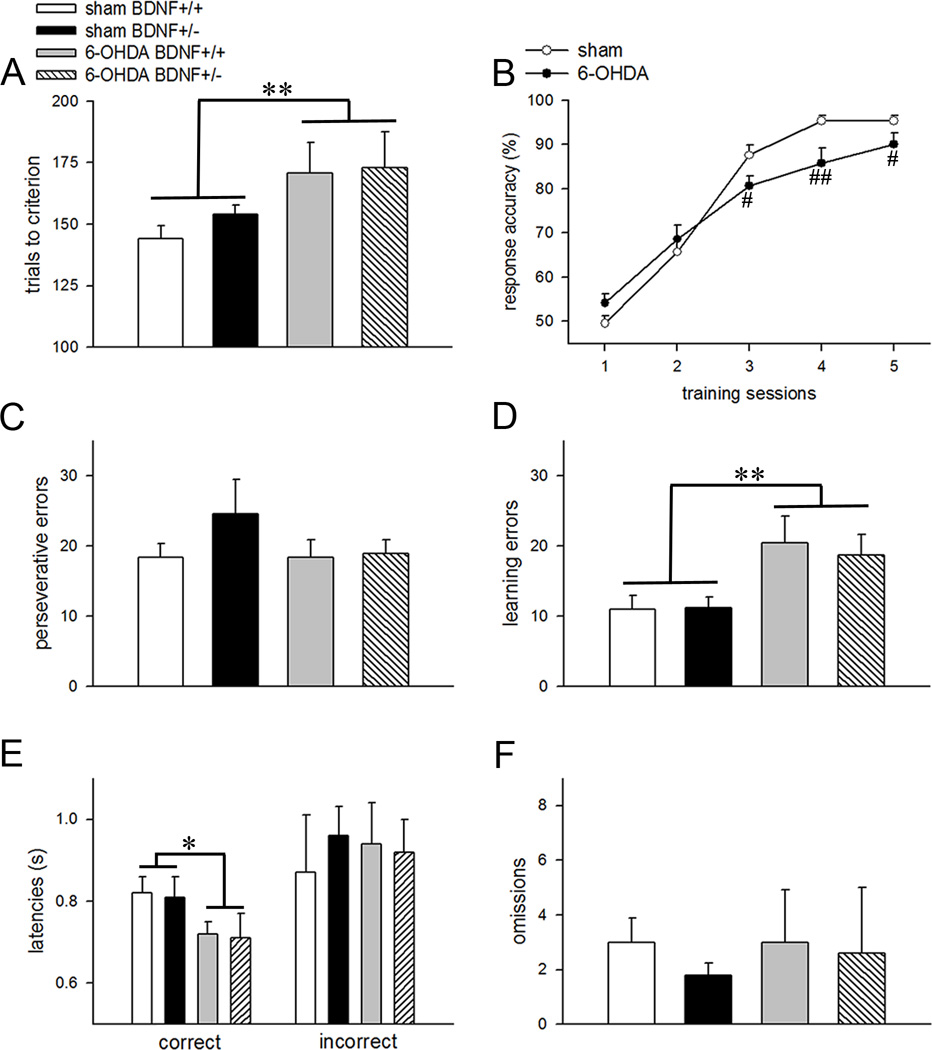
Trials to criterion on the set-shifting phase of the task were profoundly higher in animals that underwent 6-OHDA manipulation (main effect: F(1,28)=8.56; p=0.007; Fig. 4A). Partial deletion of the BDNF gene did not impact this measure (main effect: F(1,28)=3.67; p=0.07) and the effects of manipulation did not interact with the genotype (F(1,28)=0.57; p=0.46). Response accuracy analysis revealed session-dependent learning rates across all groups (F(4,128)=159.49; p<0.001). Learning rates remained unaffected by the genotype (main effect of genotype (F(1,32)=0.36; p=0.55; training × genotype interaction: F(4,128)=0.57; p=0.68) but were impacted by the manipulation; 6-OHDA-infused animals took longer to acquire the task (training × manipulation interaction: F(4,128)=5.02; p=0.001). One way-ANOVA showed that dopamine depleted mice exhibited reduced response accuracies during the later stages of the training (all p<0.03 for training days 3–5; Fig. 4B). Error analysis show that perseverative errors did not differ either by the manipulation (F(1,28)=0.41; p=0.53; Fig. 4C) or genotype (F(1,28)=1.24; p=0.28). However, intrastriatal 6-OHDA infusions markedly increased learning errors which are related to the inability to maintain the new learning strategy and are observed at the later stages of task acquisition (F(1,28)=9.05; p=0.005; Fig. 4D). Learning errors remained unaffected by the genotypes (main effect: F(1,28)=1.75; p=0.19; genotype × manipulation interaction: F(1,28)=1.55; p=0.22). Surprisingly, reaction time analysis showed reduced latencies for correct responses in both BDNF+/+ and BDNF+/− mice with partial loss of striatal dopaminergic inputs (main effect of manipulation: F(1,28)=4.61; p=0.04; Fig. 4E). It is possible that partial dopamine depletion of the striatum produced conditioning of the motor responses paired with the reward as reported previously (Jones and Robbins, 1992). However, the correct latencies for the 6-OHDA lesioned group during the set-shifting phase also remained significantly lower than correct latencies during the visual discrimination (p=0.004). Since the effects of partial dopamine depletion on reaction times remained inconsistent between the two phases, we could not rule out that the decrease in this behavioral measure noted during the strategy set-shifting phase occurred due to a chance. Incorrect response latencies and omissions remained unaffected by the genotype and manipulation (F(1,28)<0.44; p>0.50 for all main effects; Fig. 4E, 4F), and there was no interaction between the two factors (p>0.31 for both measures).
Figure 4.
Partial removal of striatal dopaminergic inputs impaired strategy shifting in both BDNF genotypes. (A) Mice that received striatal 6-OHDA infusions required a higher number of trials to attain criterion. (B) Learning curves depicting the main effect of manipulation. Response accuracies representing the proportion of correct responses were significantly lower during the latter part of training in dopamine-depleted mice. Error analysis showed no differences in perseverative errors (C) between the groups but higher learning errors (D) in the 6-OHDA-infused BDNF+/+ and BDNF+/− mice. Bar charts depicting response latencies (E) and omissions (F). All data are Mean±SEM. (*, ** p<0.05, 0.01 main effect; #, ## p<0.05, 0.01 post hoc comparisons vs vehicle-infused mice for the corresponding testing day).
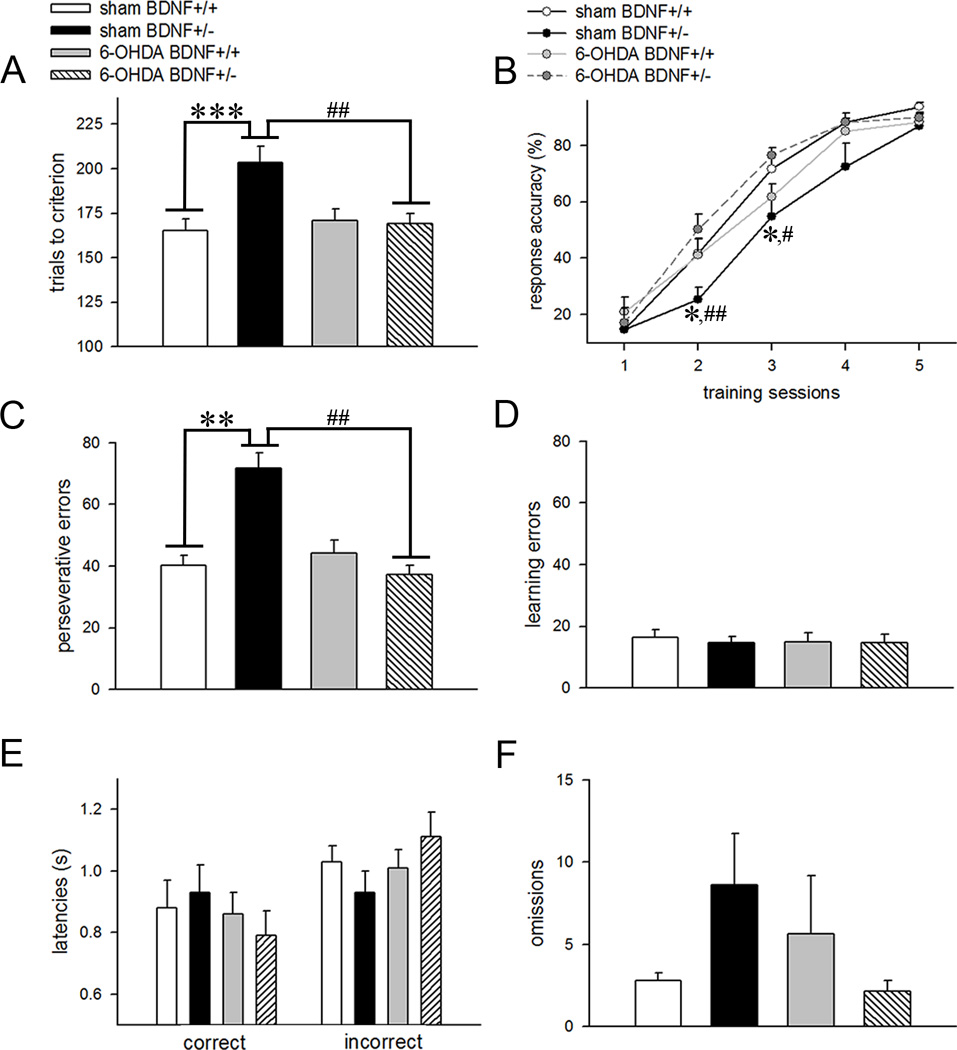
Partial dopamine depletion reduces reversal perseveration in BDNF heterozygous mice
Figure 5 summarizes the main results of reversal learning data. A two-way ANOVA applied on the number of trials performed during the reversal phase of the task to attain criterion yielded a main effect of genotype (F(1,28)=5.71; p=0.02), a main effect of manipulation (F(1,28)=4.53; p=0.04), and a significant interaction between the two factors (F(1,28)=6.15; p=0.02). Post hoc analysis shows that vehicle-infused BDNF+/− mice required a higher number of trials to attain criterion as compared to BDNF+/+ mice that received a similar manipulation (p<0.001; Fig 5A). Interestingly, trials to criterion remained significantly lower in 6-OHDA-infused BDNF mutants (p=0.009 vs. vehicle-infused BDNF+/− mice; Fig. 5A). However, this behavioral measure remained unaffected between the two genotypes following partial dopaminergic deafferentation (p=0.87). Reversal learning data analyzed using a repeated measures mixed factor ANOVA showed a main effect of training session (F(4,128)=291.71; p<0.001), no effect of genotype (F(1,32)=0.68; p=0.41) but a 3-way interaction (training × genotype × manipulation: F(4,128)=3.51; p=0.009; Fig. 5B). One-way ANOVA conducted to find the source of this interaction showed significant group differences during the second and third training sessions, respectively (both F(3,32)>3.15; p<0.05). Multiple comparisons showed reduced response accuracies in vehicle-infused BDNF mutants (p<0.03 vs. wild-types for both sessions; Fig 5B) indicating that these animals made more errors during the earlier stages of the reversal learning task. Error analysis further supported these results by showing that perseverative errors were significantly higher in BDNF+/− mice infused with vehicle as opposed to BDNF+/+ mice that received a similar treatment (genotype × manipulation interaction: F(4,128)=4.92; p=0.03; post hoc test: p=0.009; Fig. 5C). Higher response accuracies for earlier training sessions and reduced perseverative errors was also observed in 6-OHDA-infused BDNF+/− mice as compared to BDNF mutants infused with vehicle (all p<0.02; Fig. 5B and 5C). Learning errors remained similar between the genotypes (F(1,28)=0.01; p=0.92) and manipulations (F(1,28)=0.29; p=0.58). Collectively, these results indicate that reduction in striatal dopaminergic inputs may reverse the detrimental effect of partial BDNF gene deletion on reversal learning. Correct and incorrect response latencies (Fig. 5E) and omissions (Fig. 5F) during reversal learning were influenced neither by genotype (all p>0.54) nor manipulation (all p>0.14). Moreover, the two factors did not interact on these behavioral measures (all p>0.17). It is possible that sensorimotor and motivational functions during rule reversal are not influenced by BDNF hemizygosity and partial dopamine depletion of the striatum. However, this interpretation remains speculative and more specific behavioral tests are required to confirm it.
Figure 5.
Effects of BDNF hemizygosity and striatal dopaminergic deafferentation on reversal learning. (A) Control BDNF mutants required a significantly higher number of trials to attain reversal criterion as compared to BDNF+/+ mice. However, trials to criterion remained significantly lower in 6-OHDA-infused BDNF mutants. (B) Learning curves for 5 reversal sessions depict a gradual increase in response accuracy with training in all groups. Mixed factor ANOVA showed a session × genotype × manipulation interaction. Post hoc comparisons revealed lower response accuracies during the second and third training sessions in vehicle-infused BDNF+/− mice as compared to both vehicle-infused BDNF+/+ mice and dopamine-depleted BDNF+/− mice, respectively. (C) BDNF+/− mice with intact striatal dopaminergic afferents committed higher perseverative errors and this effect was not observed in 6-OHDA-infused animals. Bar charts for learning errors (D), response latencies (E), and omissions (F) illustrate no significant differences by genotype and manipulation, and no interaction between the two factors. All data are Mean±SEM. (*, **, *** p<0.05, 0.01, 0.001 vs vehicle-infused BDNF+/+ mice; #, ## p<0.05, 0.01 vs 6-OHDA-infused BDNF+/− mice; all post hoc comparisons).
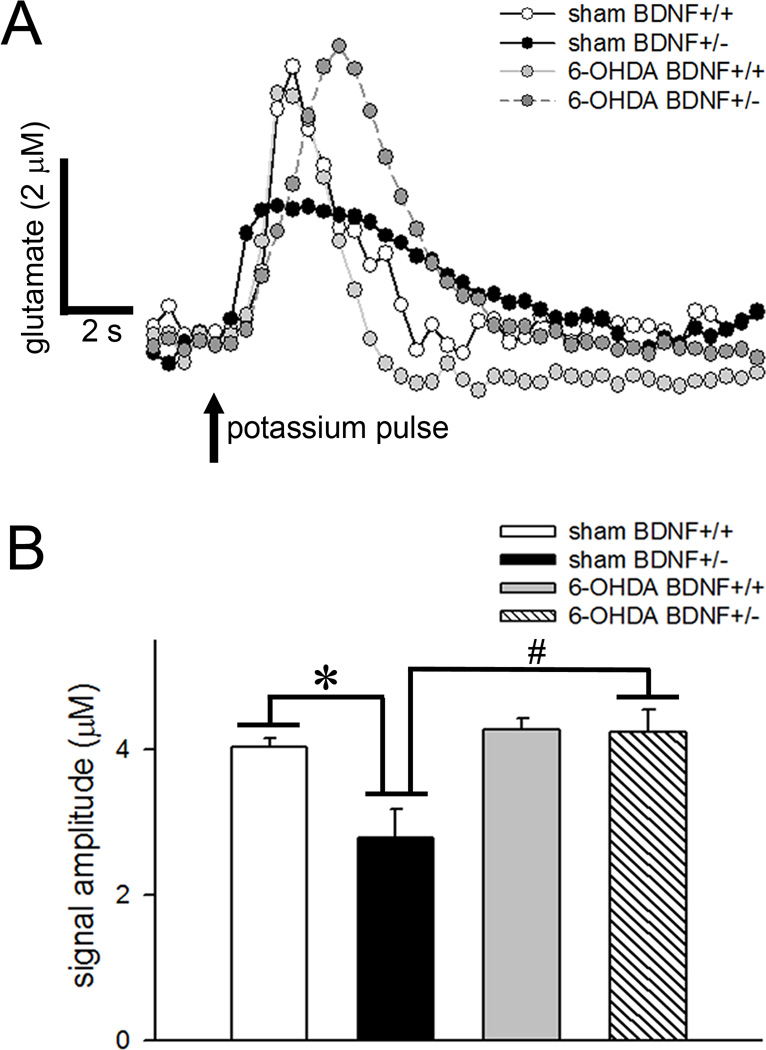
Basal and depolarization-evoked glutamate release
The extracellular levels of glutamate in the dorsal striatum under baseline conditions in sham wild-type mice and sham BDNF mutants were 3.27±0.49 µM and 3.47±0.32 µM, respectively. Resting glutamate levels remained unaffected by the genotype (F(1,10)=0.60; p=0.45) or manipulation (F(1,10)=2.80; p=0.12), and no interactions were observed between the two factors (F(1,10)=0.05; p=0.83). Local administration of potassium produced a robust increase in glutamate release that lasted 10–15 s (Fig. 6A). The amplitude of depolarization-evoked glutamate signals was significantly lower in BDNF+/− mice (main effect of genotype: F(1,10)=7.17; p=0.02). Potassium-elicited glutamate release was also reduced by 6-OHDA manipulation (main effect: F(1,10)=12.33; p=0.006) and the effects of genotype interacted with manipulation (F(1,10)=6.43; p=0.03; Fig. 6B). Post hoc comparisons show significant reductions in glutamate signal amplitudes in vehicle-infused BDNF+/− mice (p=0.008 vs. BDNF+/+). However, partial dopamine depletion of the striatum normalized depolarization-evoked glutamate release in these mutants (p=0.006 vs. vehicle-infused BDNF+/−; p=0.57 vs. vehicle-infused BDNF+/+). Interestingly, glutamate signals cleared slowly in BDNF+/− mice (t80: 8.16 ± 0.38 s vs. 5.68 ± 0.32 s in BDNF+/+; main effect: F(1,10)=24.49; p=0.001). As glutamate transporters eliminate glutamate spillover following depolarization from the extracellular space (Hascup et al., 2008), lower clearance of potassium-evoked glutamate signals observed in BDNF mutants may indicate alterations in glutamate transporters. It is unclear whether these biochemical changes represent a compensatory mechanism to restore glutamate signaling in these mice. 6-OHDA manipulation did not affect glutamate clearance (F(1,10)=2.11; p=0.18). Moreover, the effect of genotype on t50 did not interact with the manipulation (F(1,10)=1.46; p=0.26). Both resting and depolarization-evoked glutamate levels remained indifferent between the two hemispheres (F(1,13)<2.68; p>0.12).
Figure 6.
Amperometric measurements of depolarization-evoked glutamate release. (A) Representative traces depicting glutamate spikes following terminal depolarization by local application of potassium in the dorsal striatum of control and dopamine-depleted BDNF+/+ and BDNF+/− mice. (B) Bar charts show reduced glutamate signal amplitudes in BDNF mutants that previously received vehicle infusions. However, potassium-elicited glutamate release remained significantly higher in 6-OHDA-infused BDNF+/− mice as compared to vehicle-infused BDNF+/− mice indicating that partial depletion of dopamine normalized the effect of genotype on glutamatergic transmission. (* p<0.01 vs vehicle-infused BDNF+/+ mice; # 0.01 vs 6-OHDA-infused BDNF+/− mice; all post hoc comparisons).
Discussion
Visual discrimination requires selection of a goal-relevant stimulus that depends upon attentional processes (Muir, 1996). In the present study, the delayed acquisition of visual discrimination criterion in partial dopamine depleted mice may indicate that fundamental attentional mechanisms are disrupted in these animals. This interpretation is in line with previous studies that documented the involvement of striatal dopaminergic signaling mediated via D1 and D2 receptors in the regulation of attention (Agnoli et al. 2013; del Campo et al. 2013). However, we also observed higher omissions in these animals during the discrimination phase. Given the evidence that reduced dopamine transmission in the dorsal striatum impacts motivated behaviors (Palmiter 2008), we cannot rule out that higher omissions observed in dopamine-depleted mice occurred due to less motivation to perform the task. However, 6-OHDA-infused mice did not omit more trials during the subsequent phases of the task, which might indicate that impairments in visual discrimination learning performance reflect disruption of attentional rather than motivational mechanisms. Lack of any effect of BDNF genotype on discrimination learning reflects that BDNF haplo-insufficiency does not affect stimulus processing and attentional functions.
Strategy set-shifting like extradimensional shifting entails higher order cognitive processing when conditions demand learning about stimulus attributes (Ragozzino 2007). This form of cognitive flexibility is different from the perceptual attentional set-shifting and plays heavier emphasis on response conflicts as the same set of stimuli are presented during both visual discrimination and set-shifting. In our paradigm, mice were required to learn the relationship between different stimulus components in different dimensions: inhibit response to lever paired with a visual cue that previously predicted reward and use egocentric spatial location to respond to the lever to attain reinforcement. As the animals are still required to shift attention from one stimulus dimension (visual cue) to a different dimension (spatial cue; correct lever either left or right), the strategy set-shifting engages attentional set-shifting as a component process as reported previously in tasks with a similar set of rules (Floresco et al. 2006; Haluk and Floresco 2009; Ragozzino 2007). BDNF+/− mice switched to a new response strategy as efficiently as BDNF+/+ mice. It is noteworthy that although exogenous BDNF infusions into the dorsal striatum facilitated strategy set-shifting in our previous study, blockade of trkB receptors in this brain region per se did not affect this form of cognitive flexibility (D’Amore et al. 2013). Therefore, it is possible that endogenous BDNF signaling may not be critical for extradimensional shifting under baseline conditions. However, we could not rule out the possibility of the presence of compensatory processes which might have been triggered by partial knockout of BDNF gene and provided a rescue of cognitive deficits in BDNF+/− mice (discussed further below).
Striatal dopamine signaling is implicated in cognitive flexibility (Klanker et al. 2013). Restricting dopamine signaling to the dorsal striatum or moderate loss of dopaminergic projections to this brain region impaired acquisition of the strategy shifting in a cue-based water escape task in mice (Darvas and Palmiter 2011; Darvas et al. 2014). Systemic administration of sulpiride, a dopamine D2 receptor antagonist, impaired extradimensional set-shifting in human subjects (Mehta et al. 2004). Moreover, a human PET imaging study reported reduced D2 receptor binding in the dorsal striatum during the planning of set-shift in subjects performing the Montreal Card Sorting Task (Monchi et al. 2006). These studies are in strong support of our findings that partial elimination of dopaminergic inputs in the dorsal striatum impair strategy switching in our operant conditioning paradigm. However, we cannot conclude that the observed effects might have occurred merely due to the disruption of D2 receptor signaling as D1 receptors are also implicated in set-shifting (Agnoli et al. 2013; Nikiforuk 2012). Therefore, it is likely that aberrations in both D1 and D2 receptor signaling might have contributed to the detrimental performance effects in our study.
Reversal learning represents a lower-order rule learning process, as it involves a change in exemplar but not a category, and is used to measure the ability to suppress reward-related responding in the presence of conflicting response alternatives. BDNF+/− mice with intact striatal dopaminergic terminals displayed robust impairments in performance when the stimulus-reward contingencies were reversed. These impairments were associated with increased prepotent responding to the previously rewarded stimulus (higher perseverative errors) reflecting disruption in inhibitory control processes. It is important to note that BDNF hemizygosity per se did not affect discrimination learning and strategy switching. This may indicate that performance deficits observed in vehicle-infused BDNF+/− mice were specific for the reversal condition and not due to an overall impairment of the learning of task using the proprioceptive condition. The ability of striatal terminals to release glutamate following a depolarization stimulus was also found to be lower in these animals. Extensive evidence indicates that glutamate signaling is important for reversal learning. For example, mice either with a genetic deletion of the NMDA NR2A subunits or partial deletion of vesicular glutamate transporter exhibited deficits in reversal learning (Brigman et al. 2008; Granseth et al. 2015). Additionally, the administration of ADX47273, a metabotropic glutamate receptor 5 allosteric modulator, facilitated spatial reversal learning (Xu et al. 2013). Thus, reversal learning deficits observed in BDNF+/− mice may plausibly be linked to dysregulated glutamate transmission in the dorsal striatum.
However, we did not observe reversal learning deficits in the 6-OHDA-infused wild-type mice. Moreover, partial removal of striatal dopaminergic inputs reduced response perseveration to the previously acquire strategy in dopamine-depleted BDNF+/− mice as compared to BDNF mutants with intact dopaminergic inputs. Activation of dopamine D2 receptors increased perseverative responding in the reversal learning tasks (Boulougouris et al. 2008; Lee et al, 2007) illustrating that optimal activation of these receptors may be critical in preserving the ability to switch behavior in response to changes in reinforcement contingencies. As indicated earlier, BDNF hemizygosity elevated tonic dopamine levels in the dorsal striatum (Bosse et al. 2012). Moreover, D2 receptors have a high affinity for dopamine allowing them to be activated by tonic dopamine (Goto et al. 2007). Thus, it is conceivable that partial dopamine depletion in these mice would have restored reversal learning performance by normalizing tonic dopamine levels and D2 receptor activation. However, the involvement of D1 receptors in these behavioral changes could not be entirely dismissed as activation of these receptors is also known to produce reversal learning impairments (Izquierdo et al. 2006). The occupancy of D1 receptors is highly influenced by burst firing of dopaminergic neurons (Dreyer et al. 2010). Since BDNF+/− mice are shown to exhibit suppressed release of phasic dopamine in the dorsal striatum (Bosse et al. 2012), it is less likely that activation of D1 receptors in this brain region might have produced increased response perseveration during reversal in these animals.
Corticostriatal synapses constitute the major pool of BDNF in the dorsal striatum (Altar et al. 1992). Moreover, BDNF exerts neuromodulatory effects on striatal dopaminergic (Goggi et al. 2002) and glutamatergic transmission (Jia et al. 2010; D'Amore et al. 2013), and a functional interaction between these neurotransmitter systems in the striatum is considered critical for the processing of cognitive and affective information (David et al. 2005). As the tonic dopamine D2 receptor has been shown to reduce the activity of striatal output neurons by suppressing glutamatergic transmission (West et al. 2003), performance deficits in this form of cognitive flexibility might have occurred as a consequence of striatal dopamine/glutamate imbalance. This view is supported by our amperometry data that show reduced depolarization-evoked glutamate release in the dorsal striatum of BDNF+/− mice and complete restoration of glutamatergic transmission following partial dopaminergic deafferentation in these animals. We did not find any changes in the basal glutamate levels between the genotypes which might have also been influenced by higher dopamine tone. There could be two potential explanations for this finding. First, it is possible that striatal dopamine negatively regulates glutamate release from corticostriatal terminals only in an impulse-dependent fashion as noted previously (Bamford et al. 2004). Since, we did not observe any effect of partial dopaminergic deafferentation on depolarization-evoked glutamate release in the wild-type mice, this association is plausibly related to higher tonic dopamine and overstimulation of D2 receptors present on glutamatergic afferents as noted in BDNF+/− mice. Second, as the glial cells constitute a major source of extracellular glutamate under resting conditions (Moussawi et al. 2011), compensatory glial activation in BDNF+/− mice might have possibly accounted for the lack of genotypic differences in basal glutamate levels.
It is interesting to note that despite glutamate aberrations in the dorsal striatum of vehicle-infused BDNF+/− mice, strategy shifting performance in these mice remained unaffected. There could be multiple interpretations for this observation. 1) Elevated dopamine tone observed in the dorsal striatum of BDNF+/− mice reported earlier (Bosse et al. 2012) may be sufficient to maintain set-shifting performance despite disruptions in glutamate signaling in this brain region. 2) Glutamate signaling in the dorsal striatum may provide only limited support for strategy shifting. 3) Dopamine-glutamate interactions in the ventral striatum may be more important to maintaining higher forms of cognitive flexibility as opposed to the dorsal striatum. In this context, a recent study showed that infusions of AP5, a NMDA receptor antagonist, into the ventral striatum impaired strategy shifting (Ding et al. 2014). A similar observation was reported by Haluk and Floresco (2009) when a D1 receptor antagonist was infused into the nucleus accumbens of rats. More importantly, set shifting deficits observed in these studies were related to impediments in the execution of new learning strategies which were also observed in partially dopaminergic deafferented mice in the present experiment. It must be noted that some of the 6-OHDA did diffuse into the nucleus accumbens in our study (see Results). As postsynaptic D1 receptors potentiate extrasynaptic NMDA receptor function in the striatum (Flores-Hernandez et al. 2002), it is possible that the association between dopaminergic and glutamatergic mechanisms in the ventral striatum is imperative for the learning of new stimulus-reward associations during set-shifting and these interactions are maintained in BDNF+/− mice.
To conclude, our data illustrate that endogenous BDNF signaling modulates reversal learning presumably by maintaining striatal dopamine-glutamate balance. Moreover, normalization of striatal dopamine-glutamate imbalance that occurs as a consequence of BDNF hemizygosity restores reversal learning performance. Frontostriatal circuits involving discrete regions of the striatum regulate cognitive control and decision processes (Balleine et al. 2007) and disruption in these cognitive processes is present in major psychiatric disorders (Mega and Cummings 1994; Kalivas and Volkow 2005; Floresco et al. 2009). Our findings add a critical piece of evidence that perturbations in striatal dopaminergic-glutamatergic interactions produced by disrupted BDNF signaling may serve as a common molecular mechanism that links the homogenous cluster of behavioral/cognitive symptoms across these disorders. Further studies are required to determine the causality of these relationships by targeting BDNF in specific frontostriatal circuits in animals that model fundamental characteristics of psychiatric disorders.
Acknowledgments
This work was supported by grants from the Brain and Behavioral Research Foundation, Pennsylvania Department of Health (# 4100050909) and the National Institute of Health (NIH DA 037421) to V.P. MARC Undergraduate Student Training in Academic Research (NIH 5T34 GM 087239) provided research training support to D.G. We thank Brittany Tracy for the assistance with the genotyping.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Agnoli L, Mainolfi P, Invernizzi RW, Carli M. Dopamine D1-like and D2-like receptors in the dorsal striatum control different aspects of attentional performance in the five-choice serial reaction time task under a condition of increased activity of corticostriatal inputs. Neuropsychopharmacol. 2013;38:701–714. doi: 10.1038/npp.2012.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Boylan CB, Jackson C, Hershenson S, Miller J, Wiegand SJ, Lindsay RM, Hyman C. Brain-derived neurotrophic factor augments rotational behavior and nigrostriatal dopamine turnover in vivo. Proc Natl Acad Sci USA. 1992;89:11347–11351. doi: 10.1073/pnas.89.23.11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacological reviews. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, O'Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacol. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK. Dopamine modulates release from corticostriatal terminals. J Neurosci. 2004;24:9541–9552. doi: 10.1523/JNEUROSCI.2891-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbeck JA, Khalid M, Mathews TA. Potentiated striatal dopamine release leads to hyperdopaminergia in female brain-derived neurotrophic factor heterozygous mice. ACS Chem Neurosci. 2014;5:275–281. doi: 10.1021/cn400157b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. Neuromol Med. 2004;5:69–83. doi: 10.1385/NMM:5:1:069. [DOI] [PubMed] [Google Scholar]
- Bosse KE, Maina FK, Birbeck JA, France MM, Roberts JJ, Colombo ML, Mathews TA. Aberrant striatal dopamine transmitter dynamics in brain-derived neurotrophic factor-deficient mice. J Neurochem. 2012;120:385–395. doi: 10.1111/j.1471-4159.2011.07531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulougouris V, Castane A, Robbins TW. Dopamine D2/D3 receptor agonist quinpirole impairs spatial reversal learning in rats: investigation of D3 receptor involvement in persistent behavior. Psychopharmacol. 2008;202:611–620. doi: 10.1007/s00213-008-1341-2. [DOI] [PubMed] [Google Scholar]
- Branchi I, D'Andrea I, Armida M, Carnevale D, Ajmone-Cat MA, Pezzola A, Potenza RL, Morgese MG, Cassano T, Minghetti L, Popoli P, Alleva E. Striatal 6-OHDA lesion in mice: Investigating early neurochemical changes underlying Parkinson's disease. Behav Brain Res. 2010;208:137–143. doi: 10.1016/j.bbr.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Feyder M, Saksida LM, Bussey TJ, Mishina M, Holmes A. Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit. Learn Mem. 2008;15:50–54. doi: 10.1101/lm.777308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Graybeal C, Holmes A. Predictably irrational: assaying cognitive inflexibility in mouse models of schizophrenia. Front Neurosci. 2010;4:19–28. doi: 10.3389/neuro.01.013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carola V, Gross C. BDNF moderates early environmental risk factors for anxiety in mouse. Genes Brain Behv. 2010;9:379–389. doi: 10.1111/j.1601-183X.2010.00572.x. [DOI] [PubMed] [Google Scholar]
- Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. British journal of pharmacology. 2008;153(Suppl 1):S310–S324. doi: 10.1038/sj.bjp.0707509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Cole RD, Poole RL, Guzman DM, Gould TJ, Parikh V. Contributions of beta2 subunit-containing nAChRs to chronic nicotine-induced alterations in cognitive flexibility in mice. Psychopharmacol. 2015;232:1207–1217. doi: 10.1007/s00213-014-3754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Ivry RB, D'Esposito M. The human striatum is necessary for responding to changes in stimulus relevance. J Cog Neurosci. 2006;18:1973–1983. doi: 10.1162/jocn.2006.18.12.1973. [DOI] [PubMed] [Google Scholar]
- D'Amore DE, Tracy BA, Parikh V. Exogenous BDNF facilitates strategy set-shifting by modulating glutamate dynamics in the dorsal striatum. Neuropharmacol. 2013;75:312–323. doi: 10.1016/j.neuropharm.2013.07.033. [DOI] [PubMed] [Google Scholar]
- Darvas M, Henschen CW, Palmiter RD. Contributions of signaling by dopamine neurons in dorsal striatum to cognitive behaviors corresponding to those observed in Parkinson's disease. Neurobiol Dis. 2014;65:112–123. doi: 10.1016/j.nbd.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas M, Palmiter RD. Contributions of striatal dopamine signaling to the modulation of cognitive flexibility. Biol Psychiatr. 2011;69:704–707. doi: 10.1016/j.biopsych.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David HN, Ansseau M, Abraini JH. Dopamine-glutamate reciprocal modulation of release and motor responses in the rat caudate-putamen and nucleus accumbens of "intact" animals. Brain research Brain Res Rev. 2005;50:336–360. doi: 10.1016/j.brainresrev.2005.09.002. [DOI] [PubMed] [Google Scholar]
- del Campo N, Fryer TD, Hong YT, Smith R, Brichard L, Acosta-Cabronero J, Chamberlain SR, Tait R, Izquierdo D, Regenthal R, Dowson J, Suckling J, Baron JC, Aigbirhio FI, Robbins TW, Sahakian BJ, Muller U. A positron emission tomography study of nigro-striatal dopaminergic mechanisms underlying attention: implications for ADHD and its treatment. Brain. 2013;136:3252–3270. doi: 10.1093/brain/awt263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Qiao Y, Piao C, Zheng X, Liu Z, Liang J. N-methyl-D-aspartate receptor-mediated glutamate transmission in nucleus accumbens plays a more important role than that in dorsal striatum in cognitive flexibility. Front Behav Neurosci. 2014;8:304. doi: 10.3389/fnbeh.2014.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnberger G, Jahanshahi M. Executive dysfunction in Parkinson’s disease: a review. J Neuropsychol. 2013;7:193–224. doi: 10.1111/jnp.12028. [DOI] [PubMed] [Google Scholar]
- Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD. Influence of phasic and tonic dopamine release on receptor activation. J Neurosci. 2010;30:14273–14283. doi: 10.1523/JNEUROSCI.1894-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatr. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatr. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Eisenberg DP, Berman KF. Executive function, neural circuitry, and genetic mechanisms in schizophrenia. Neuropsychopharmacol. 2010;35:258–277. doi: 10.1038/npp.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Hernandez J, Cepeda C, Hernandez-Echeagaray E, Calvert CR, Jokel ES, Fienberg AA, Greengard P, Levine MS. Dopamine enhancement of NMDA currents in dissociated medium-sized striatal neurons: role of D1 receptors and DARPP-32. J Neurophysiol. 2002;88:3010–3020. doi: 10.1152/jn.00361.2002. [DOI] [PubMed] [Google Scholar]
- Floresco SB. Prefrontal dopamine and behavioral flexibility: shifting from an "inverted-U" toward a family of functions. Front Neurosci. 2013;7:62. doi: 10.3389/fnins.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MT. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behav Brain Res. 2008;190:85–96. doi: 10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MT. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacol. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behav Brain Res. 2009;204:396–409. doi: 10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Goggi J, Pullar IA, Carney SL, Bradford HF. Modulation of neurotransmitter release induced by brain-derived neurotrophic factor in rat brain striatal slices in vitro. Brain Res. 2002;941:34–42. doi: 10.1016/s0006-8993(02)02505-2. [DOI] [PubMed] [Google Scholar]
- Goto Y, Otani S, Grace AA. The Yin and Yang of dopamine release: a new perspective. Neuropharmacol. 2007;53:583–587. doi: 10.1016/j.neuropharm.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granseth B, Andersson FK, Lindström SH. The initial stage of reversal learning is impaired in mice hemizygous for the vesicular glutamate transporter (VGluT1) Genes, Brain Behav. 2015;14:477–485. doi: 10.1111/gbb.12230. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluk DM, Floresco SB. Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacol. 2009;34:2041–2052. doi: 10.1038/npp.2009.21. [DOI] [PubMed] [Google Scholar]
- Hascup KN, Hascup ER, Pomerleau F, Huettl P, Gerhardt GA. Second-by-second measures of L-glutamate in the prefrontal cortex and striatum of freely moving mice. J Pharmacol Expt Ther. 2008;324:725–731. doi: 10.1124/jpet.107.131698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Russek SJ. BDNF and the diseased nervous system: a delicate balance between adaptive and pathological processes of gene regulation. J Neurochem. 2008;105:1–17. doi: 10.1111/j.1471-4159.2008.05237.x. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Ann Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wiedholz LM, Millstein RA, Yang RJ, Bussey TJ, Saksida LM, Holmes A. Genetic and dopaminergic modulation of reversal learning in a touchscreen-based operant procedure for mice. Behav Brain Res. 2006;171:181–188. doi: 10.1016/j.bbr.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Jia Y, Gall CM, Lynch G. Presynaptic BDNF promotes postsynaptic long-term potentiation in the dorsal striatum. J Neurosci. 2010;30:14440–14445. doi: 10.1523/JNEUROSCI.3310-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GH, Robbins TW. Differential effects of mesocortical, mesolimbic, and mesostriatal dopamine depletion on spontaneous, conditioned, and drug-induced locomotor activity. Pharmacol Biochem Behav. 1992;43:887–895. doi: 10.1016/0091-3057(92)90422-c. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacol. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatr. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kambeitz JP, Bhattacharyya S, Kambeitz-Ilankovic LM, Valli I, Collier DA, McGuire P. Effect of BDNF val(66)met polymorphism on declarative memory and its neural substrate: a meta-analysis. Neurosci Biobehav Rev. 2012;36:2165–2177. doi: 10.1016/j.neubiorev.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Klanker M, Feenstra M, Denys D. Dopaminergic control of cognitive flexibility in humans and animals. Front Neurosci. 2013;7:201. doi: 10.3389/fnins.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AD, Sahakian BJ, Hodges JR, Rosser AE, Lange KW, Robbins TW. Executive and mnemonic functions in early Huntington’s disease. Brain. 1996;119:1633–1645. doi: 10.1093/brain/119.5.1633. [DOI] [PubMed] [Google Scholar]
- Lee B, Groman S, London ED, Jentsch JD. Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacol. 2007;32:2125–2134. doi: 10.1038/sj.npp.1301337. [DOI] [PubMed] [Google Scholar]
- Lindholm JS, Castren E. Mice with altered BDNF signaling as models for mood disorders and antidepressant effects. Front Behav Neurosci. 2014;8:143. doi: 10.3389/fnbeh.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mega MS, Cummings JL. Frontal-subcortical circuits and neuropsychiatric disorders. J Neuropsychiatr Clin Neurosci. 1994;6:358–370. doi: 10.1176/jnp.6.4.358. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Manes FF, Magnolfi G, Sahakian BJ, Robbins TW. Impaired set-shifting and dissociable effects on tests of spatial working memory following the dopamine D2 receptor antagonist sulpiride in human volunteers. Psychopharmacol. 2004;176:331–342. doi: 10.1007/s00213-004-1899-2. [DOI] [PubMed] [Google Scholar]
- Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci. 2009;10:850–860. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, He J, Nakajima A, Nabeshima T. Involvement of BDNF receptor TrkB in spatial memory formation. Learn Mem. 2003;10:108–115. doi: 10.1101/lm.56003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Krystal JH. Capturing the angel in "angel dust": twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophr Bull. 2012;38:942–949. doi: 10.1093/schbul/sbs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Ko JH, Strafella AP. Striatal dopamine release during performance of executive functions: A [(11)C] raclopride PET study. NeuroImage. 2006;33:907–912. doi: 10.1016/j.neuroimage.2006.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Riegel A, Nair S, Kalivas PW. Extracellular glutamate: functional compartments operate in different concentration ranges. Front Syst Neurosci. 2011;5:94. doi: 10.3389/fnsys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir JL. Attention and stimulus processing in the rat. Brain Res Cog Brain Res. 1996;3:215–225. doi: 10.1016/0926-6410(96)00008-0. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatr. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A. Dopamine D1 receptor modulation of set shifting: the role of stress exposure. Behav Pharmacol. 2012;23:434–438. doi: 10.1097/FBP.0b013e328356522f. [DOI] [PubMed] [Google Scholar]
- Notaras M, Hill R, van den Buuse M. The BDNF gene Val66Met polymorphism as a modifier of psychiatric disorder susceptibility: progress and controversy. Mol Psychiatr. 2015;20:916–930. doi: 10.1038/mp.2015.27. [DOI] [PubMed] [Google Scholar]
- Ortega LA, Tracy BA, Gould TJ, Parikh V. Effects of chronic low- and high-dose nicotine on cognitive flexibility in C57BL/6J mice. Behavioural brain research. 2013;238:134–145. doi: 10.1016/j.bbr.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oualian C, Gisquet-Verrier P. The differential involvement of the prelimbic and infralimbic cortices in response conflict affects behavioral flexibility in rats trained in a new automated strategy-switching task. Learn Mem. 2010;17:654–668. doi: 10.1101/lm.1858010. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann NY Acad Sci. 2008;1129:35–46. doi: 10.1196/annals.1417.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Ji J, Decker MW, Sarter M. Prefrontal beta2 subunit-containing and alpha7 nicotinic acetylcholine receptors differentially control glutamatergic and cholinergic signaling. J Neurosci. 2010;30:3518–3530. doi: 10.1523/JNEUROSCI.5712-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Man K, Decker MW, Sarter M. Glutamatergic contributions to nicotinic acetylcholine receptor agonist-evoked cholinergic transients in the prefrontal cortex. J Neurosci. 2008;28:3769–3780. doi: 10.1523/JNEUROSCI.5251-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Naughton SX, Shi X, Kelley LK, Yegla B, Tallarida CS, Rawls SM, Unterwald EM. Cocaine-induced neuroadaptations in the dorsal striatum: glutamate dynamics and behavioral sensitization. Neurochem Int. 2014;75:54–65. doi: 10.1016/j.neuint.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Pillai A, Kale A, Joshi S, Naphade N, Raju MS, Nasrallah H, Mahadik SP. Decreased BDNF levels in CSF of drug-naive first-episode psychotic subjects: correlation with plasma BDNF and psychopathology. Int J Neuropsychopharmacol. 2010;13:535–539. doi: 10.1017/S1461145709991015. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann NY Acad Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacol. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheggia D, Bebensee A, Weinberger DR, Papaleo F. The ultimate intra-/extra-dimensional attentional set-shifting task for mice. Biol Psychiatr. 2014;75:660–670. doi: 10.1016/j.biopsych.2013.05.021. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens ML, Pomerleau F, Huettl P, Gerhardt GA, Zhang Z. Real-time glutamate measurements in the putamen of awake rhesus monkeys using an enzyme-based human microelectrode array prototype. J Neurosci Methods. 2010;185:264–272. doi: 10.1016/j.jneumeth.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripanichkul W, Jaroensuppaperch EO. Ameliorating effects of curcumin on 6-OHDA-induced dopaminergic denervation, glial response, and SOD1 reduction in the striatum of hemiparkinsonian mice. Eur Rev Med Pharmacol Sci. 2013;17:1360–1368. [PubMed] [Google Scholar]
- van Schouwenburg MR, O'Shea J, Mars RB, Rushworth MF, Cools R. Controlling human striatal cognitive function via the frontal cortex. J Neurosci. 2012;32:5631–5637. doi: 10.1523/JNEUROSCI.6428-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain's control circuit. BioEssays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AR, Floresco SB, Charara A, Rosenkranz JA, Grace AA. Electrophysiological interactions between striatal glutamatergic and dopaminergic systems. Ann NY Acad Sci. 2003;1003:53–74. doi: 10.1196/annals.1300.004. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Kraniotis S, He Q, Marshall JJ, Nomura T, Stauffer SR, Lindsley CW, Conn PJ, Contractor A. Potentiating mGluR5 function with a positive allosteric modulator enhances adaptive learning. Learn Mem. 2013;20:438–445. doi: 10.1101/lm.031666.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Bychkov E, Tsakem EL, Siedlecki C, Blakely RD, Gurevich EV. Cognitive effects of dopamine depletion in the context of diminished acetylcholine signaling capacity in mice. Dis Model Mech. 2013;6:171–183. doi: 10.1242/dmm.010363. [DOI] [PMC free article] [PubMed] [Google Scholar]