Summary
The miR-200 microRNA family plays important tumor suppressive roles. The sole Drosophila miR-200 ortholog, miR-8 plays conserved roles in Wingless, Notch and Insulin signaling pathways linked to tumorigenesis, yet homozygous null animals are viable and often appear morphologically normal. We observed that wing tissues mosaic for miR-8 levels by genetic loss or gain of function exhibited patterns of cell death consistent with a role for miR-8 in modulating cell survival in vivo. Here we show that miR-8 levels impact several actin cytoskeletal regulators that can affect cell survival and epithelial organization. We show that loss of miR-8 can confer resistance to apoptosis independent of an epithelial to mesenchymal transition while the persistence of cells expressing high levels of miR-8 in the wing epithelium leads to increased JNK signaling, aberrant expression of extracellular matrix remodeling proteins and disruption of proper wing epithelial organization. Altogether our results suggest that very low as well as very high levels of miR-8 can contribute to hallmarks associated with cancer, suggesting approaches to increase miR-200 microRNAs in cancer treatment should be moderate.
Keywords: miRNA, actin cytoskeleton, Drosophila, Imaginal wing disc
Introduction
The miR-200 family plays a critical tumor suppressive role in several types of cancers (reviewed in Brabletz and Brabletz, 2010; Hill et al., 2013). In mammals the miR-200 family is made up of two genomic loci encoding microRNAs including miR-200a, miR-200b, miR-200c, miR-141, and miR-429. They are most strongly expressed in epithelial tissues, have similar seed regions with only one nucleotide difference generating two classes of target sites termed miR200a sites and miR200b sites, and can regulate overlapping targets involved in multiple signaling pathways (reviewed in Bracken et al., 2015). A key role for the miR-200 family in promoting the epithelial state has been shown, and high levels of miR-200 expression in mesenchymal cells can even promote a mesenchymal to epithelial transition (Gregory et al., 2008a). In this role, the Zeb family of transcription factors are important targets, as they transcriptionally repress E-cadherin expression (Comijn et al., 2001; Eger et al., 2005). There is also a miR-200/Zeb1/2 feedback loop, where Zeb1/2 represses miR-200 expression (Bracken et al., 2008). This creates a simple model for robustly establishing and maintaining the epithelial or mesenchymal state depending upon the levels of miR-200 vs. Zeb1/2. However additional studies have also suggested Zeb1/2-independent roles for miR-200 in promoting the epithelial state at least in part through targets that regulate the actin cytoskeleton such as moesin, the Formin Homology Domain Containing 1 protein (FHOD1) and WAVE3 a member of the WASP (Wiskott-Aldrich syndrome protein)/WAVE actin cytoskeleton remodeling family of proteins (Jurmeister et al., 2012; Li et al., 2014; Sossey-Alaoui et al., 2009). More recent work using an approach to identify miR-200 targets at the transcriptome-wide level, identified hundreds of potential miR-200a and miR-200b targets and revealed a predominant effect of miR-200 targets in widespread control of actin cytoskeletal regulators (Bracken et al., 2014). The current model for Zeb1/2-independent miR-200 functions in inhibition of the mesenchymal state posits that the down-regulation of miR-200 targets involved in modulating the actin cytoskeleton limits the formation of invadopodia and cell migration (Bracken et al., 2014). However this has largely been investigated in cell culture, and the effects of modulating miR-200 on the actin cytoskeleton in epithelial tissues in vivo needs to be further explored.
Importantly, the miR-200 family can also alter cell survival and metabolism (Belgardt et al., 2015; Guo et al., 2015; Howe et al., 2011; Jin et al., 2012; Jing et al., 2015), although it also remains largely unclear exactly how these roles intersect with the function of miR-200 in establishing and maintaining the epithelial state. Several studies have shown that reduced miR-200 can lead to chemotherapy and radiotherapy resistance in cancer, which can be rescued by re-introducing miR-200 expression (Adam et al., 2009; Cortez et al., 2014; Knezevic et al., 2015; Siebzehnrubl et al., 2013). A predominant model suggests that the sensitivity of cancer cells to cytotoxic treatments is somehow coupled with the transition to a mesenchymal state (Fischer et al., 2015), but whether miR-200 can reduce cancer cell survival independent of its roles in maintaining the epithelial state remains unknown.
The sole Drosophila miR-200 family homolog, miR-8 plays important roles in Insulin, Notch, and Wingless signaling as well as signaling via the fly steroid hormone ecdysone (Hyun et al., 2009; Jin et al., 2012; Kennell et al., 2008; Vallejo et al., 2011). More recently, miR-8 has been shown to also promote the epithelial state in the Drosophila intestine, where the transcription factors Escargot and the fly homolog of Zeb1, Zfh1 are critical targets (Antonello et al., 2015). Yet surprisingly, despite all of these conserved functions homozygous null miR-8 mutants are often properly patterned and viable, leaving unclear the full physiological significance of miR-8 function in these pathways during development (Hyun et al., 2009; Karres et al., 2007; Kennell et al., 2012). It also remains unclear whether there are Zfh1-independent roles for miR-8 in promoting the epithelial state in Drosophila. One target for miR-8 previously identified is the actin regulator Enabled (Ena), which is responsible for miR-8 phenotypes observed at the neuromuscular junction in Drosophila (Loya et al., 2009; Loya et al., 2014). The targeting of Ena provides a hint that miR-8 may also play a larger role in regulating the actin cytoskeleton in Drosophila, in a manner similar to that described for miR-200.
We previously identified miR-8 as a negative regulator of Wingless signaling in the Drosophila eye and wing (Kennell et al., 2008). We therefore examined whether mosaic analysis of miR-8 function in the wing epithelial tissue might reveal novel roles and targets for miR-8. Here we demonstrate that cell survival and the F-actin cytoskeleton is compromised by high levels of miR-8, in part through several novel direct targets of miR-8 that regulate actin dynamics via the SCAR/WAVE complex (Kunda et al., 2003) and F-actin meshwork formation (Isaji et al., 2011; Mavrakis et al., 2014). Conversely, we show that loss of miR-8 increases cell survival in the wing and resistance to DNA damage-induced apoptosis in a manner apparently unlinked to a mesenchymal transition, as epithelial organization and wing patterning remains intact in miR-8 mutants.
Results
miR-8 impacts cell survival in the wing
miR-8 and its mammalian homologs have been suggested to promote and maintain the epithelial state (Antonello et al., 2015; Brabletz and Brabletz, 2010; Mongroo and Rustgi, 2010). The developing Drosophila wing has been used extensively as a model to decipher mechanisms controlling epithelial organization and morphogenesis during development. We therefore examined the role of miR-8 in the wing epithelium in more detail. miR-8 was previously suggested to be expressed in the wing pouch and notum, based upon the expression of a Gal4 enhancer trap line (NP5247) (Karres et al., 2007; Vallejo et al., 2011). To ensure the enhancer trap reflects endogenous miR-8 expression and activity in wings, we examined the expression of a miR-8 EGFP sensor transgene in the wing (Kennell et al., 2012). The sensor transgene contains EGFP with two binding sites for miR-8 in its 3′UTR, driven by the α-tubulin promoter. Targeting by endogenous miR-8 leads to decreased expression of the EGFP sensor. Consistent with the expression pattern reported for the miR-8-Gal4 enhancer trap, the miR-8 sensor revealed higher endogenous miR-8 activity in the wing pouch and notum than in the hinge (Fig. 1A). The targeting of the sensor is completely lost in miR-8 null mutants (Fig. 1B, miR-8jk22/miR-8 jk22), confirming the specificity of the sensor for endogenous miR-8. Based upon these results, we focused on examining functions of miR-8 in the developing wing pouch.
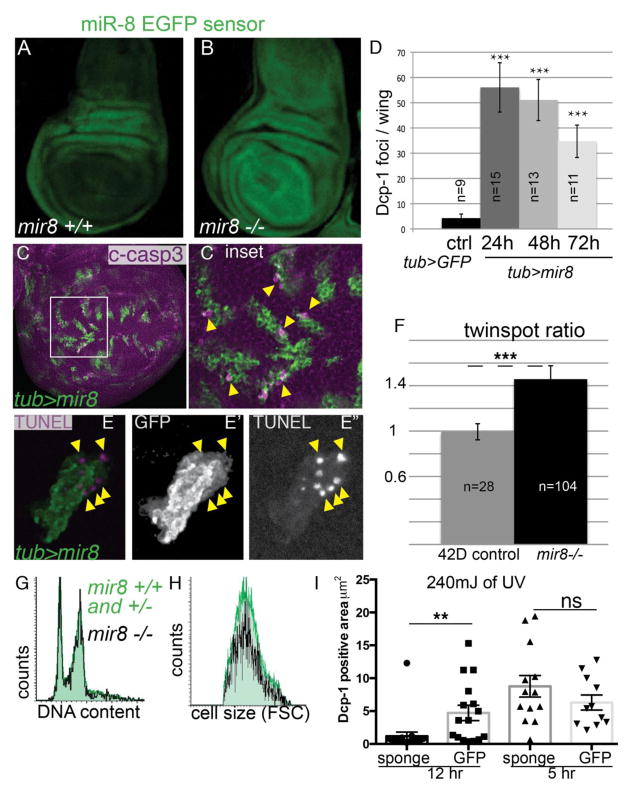
Fig. 1. miR-8 is expressed in the wing pouch where it impacts cell survival.
(A). In a wild type background, miR-8-sensor expression is decreased in the wing pouch and notum. (B). Expression of the miR-8-sensor is rescued in miR-8 mutant wings (miR-8JK22/miR-8JK22), suggesting that functional miR-8 is endogenously expressed in the wing pouch and notum. (C). GFP-labeled clones overexpressing miR-8 were generated using the tub>CD2>Gal4/UAS, Gal80TS system with heat-shock induced recombination from 38–80 hr of development and transgene induction at 28°C for 24–72hr prior to wing disc dissection at wandering third larval instar (L3). miR-8 overexpressing clones are positive for (C) cleaved-Caspase 3 (c-Casp3), (D) Dcp-1 (the full timecourse is shown in Supp. Fig. 1) and (E) TUNEL. (F) Negatively marked miR-8−/− clones with GFP-labeled twinspots were generated in larval wings using heat-shock induced FLP/FRT42D mitotic recombination and measured 75h post clone induction. miR-8 mutant tissue is overrepresented within the wing pouch compared to wild-type sibling clones (shown in additional data in Supp. Fig. 1) and FRT 42D control clones lacking the miR-8 allele. N indicates the total number of twinspots measured. (G) The DNA content and (H) cell size (via Forward scatter FSC) of miR-8 mutant clones is similar to wild-type GFP positive sibling clones. (I) Tissues were induced to express a miR-8 sponge using en-Gal4/UAS, Gal80TS with incubation at 28°C for 70hr prior to wing disc dissection. Wandering L3 larvae were exposed to 240mJ of UV and assayed 5–12hr later for cell death by Dcp-1 (staining shown in Supp. Fig. 1).
We previously demonstrated that miR-8 inhibits Wg signaling via multiple direct targets (Kennell et al., 2008). Wg signaling acts as an important cellular survival factor in the Drosophila wing (Giraldez and Cohen, 2003; Johnston and Sanders, 2003). Consistent with this, manipulating levels of miR-8 expression in the wing alters cell survival. We generated clones of cells overexpressing miR-8 via Gal4/UAS transgene induction (Duffy, 2002), in otherwise normal wing tissue. We found that miR-8 expressing clones were small, exhibited apoptotic morphologies and were rapidly eliminated from the wing epithelium by 72h after induction. To generate larger clones for more detailed analysis, we turned to the temperature-sensitive Gal80 repressor (Gal80TS) system (McGuire et al., 2004). With Gal80TS, we can induce clone formation early, allow clones to grow in the absence of transgene induction at low temperature where the Gal80 repressor is intact (18°C), and then switch to a non-permissive Gal80 temperature (29°C) to induce the Gal4/UAS driven transgene for a short time before analysis. Using this system, we induced clones to express miR-8 in the wing epithelium for 24–72h. We stained clones expressing miR-8 with cleaved-Caspase 3 (c-Casp3) antibody to detect apoptotic cells (Fan and Bergmann, 2010). Cells overexpressing miR-8 had high levels of c-Casp3 staining (Fig. 1C), indicating that miR-8 plays a role in cell survival. Similar results were also obtained with staining for a second Drosophila Caspase 1, Dcp-1 (Song et al., 1997), with Dcp-1 positivity increasing dramatically within 24h of miR-8 expression in the wing (Fig. 1D, Supplement to Fig. 1). Finally, TUNEL labeling confirmed DNA fragmentation consistent with late apoptosis in miR-8 expressing cells (Fig. 1E).
Since high miR-8 reduced cell survival, we next examined whether the loss of miR-8 could improve cell survival. Under normal rearing conditions, the amount of apoptosis in the larval wing pouch and hinge at any given point in time is very low (<10 TUNEL positive apoptotic cells/wing), and while we did observe a mild reduction in TUNEL labeling of miR-8−/− cells in fixed tissue samples, the numbers were too low to be conclusive. We therefore examined miR-8 genetic mosaic wing discs at the third larval instar stage using the miR-8 null allele JK22 (Kennell et al., 2012) to create mosaic discs via FLP/FRT mediated mitotic recombination (Xu and Rubin, 1993). With this technique, miR-8 GFP negative mutant clones and wild-type twin-spots (GFP-positive) are generated simultaneously by the same mitotic recombination event (examples of mosaic wings are shown in the Supplement to Fig. 1J). This allows for measurements of the relative clone area within the wing taken up by miR-8 mutant (no GFP) clones vs. wild-type sibling clones, providing a readout of the relative rates of cell growth, survival and proliferation. We restricted our measurements to the wing pouch as endogenous miR-8 is low or absent in the hinge, and we thus expect no mutant phenotype there.
When we measured the ratio of GFP negative vs. positive sibling area of the wing pouch for a parental FRT chromosome lacking the miR-8 mutation, we observed a ratio close to 1, demonstrating that the FRT containing chromosome and GFP expression does not alter the growth, survival or proliferation of cells in the wing (Fig. 1F). By contrast, we observed a significant increase in the ratio of miR-8 mutant GFP-negative tissue compared to wild-type sibling clones (p<0.001, by two-tailed t-test Fig. 1F). This suggests that cells lacking miR-8 function either have increased cellular growth (size), an increased proliferation rate, or increased survival.
To examine whether miR-8 −/− cells had an altered cell cycle or grew larger than their wild-type siblings, we used flow cytometry (Flegel et al., 2013). We saw no alterations in cell cycle phase via DNA content, nor increased cell size in miR-8 −/− cells (Fig. 1G,H). Consistent with the flow cytometry results, miR-8 −/− wings (JK22/JK22) exhibited only a very mild phenotype of a slightly smaller size as previously reported, and a mild branching of the posterior crossvein also previously seen for the delta2 allele of miR-8 (Supplement to Fig. 1), (Hyun et al., 2009).
To test whether cells in the wing with reduced miR-8 exhibit increased survival, we performed a UV-challenge experiment in animals expressing a miR-8 “sponge”. The miR-8 sponge transgene has been shown to act as a competitive inhibitor for miR-8 binding to targets by expressing EGFP with 10 miR-8 target sites in the EGFP 3′UTR under the control of a UAS promoter (Loya et al., 2009). We expressed the miR-8 sponge in the posterior wing from L1 stage using engrailed-Gal4 (en-Gal4) with Gal80TS, and challenged animals with 240mJ of UV at 110–120 hours of development. We measured apoptosis in response to UV at two timepoints by Dcp-1 staining; 5 h post UV exposure which captures a p53-dependent phase of cell death and 12h which captures a secondary p53-independent phase of apoptosis (McNamee and Brodsky, 2009), (Supplement to Fig. 1). We found that expression of the miR-8 sponge did not inhibit the immediate response to UV, but protected against apoptosis during the secondary phase (Fig. 1I, p<0.0084 at 15h by two-tailed t-test, n=19 discs for the sponge, n=16 discs for the control). Altogether our data suggests that loss of miR-8 can increase cell survival.
Cells with high miR-8 in the wing activate JNK signaling and are basally extruded
We next tested whether miR-8 reduces expression of the Drosophila Death-associated inhibitor of apoptosis 1, Diap1. Using an antibody to the Diap1 protein that recapitulates the previously published expression pattern in fly wings (Ryoo et al., 2002), we found that expression of miR-8 reduced Diap1 levels within 24h, whether expressed in clones (using Gal80TS to limit expression) or with the patched-Gal4 (ptc-Gal4) driver combined with Gal80TS (Fig. 2A–D). The effects of miR-8 on Diap1 appear to be post-transcriptional, as miR-8 expression had no effect on the Diap1-LacZ transcriptional reporter (Ryoo et al., 2002), even when expressed from early stages using ptc-Gal4 without Gal80TS (Fig. 2E,F). Finally we used the Apterous-Gal4 driver (Ap-Gal4) combined with UAS-dIAP and Gal80TS to co-express miR-8 with Diap1 for 24h in the dorsal L3 wing to test whether providing exogenous Diap1 could prevent miR-8-induced apoptosis. miR-8 expression leads to apoptosis within 24h of expression even in the presence of exogenously restored Diap1 (Fig. 2G,H). This suggests the loss of Diap1 is most likely a secondary consequence of miR-8 induced apoptosis rather than a direct miR-8 target, as its inhibition is not required for miR-8 to induce apoptosis. Consistent with this, we found no strongly predicted miR-8 binding sites in the Diap1 3′UTR.
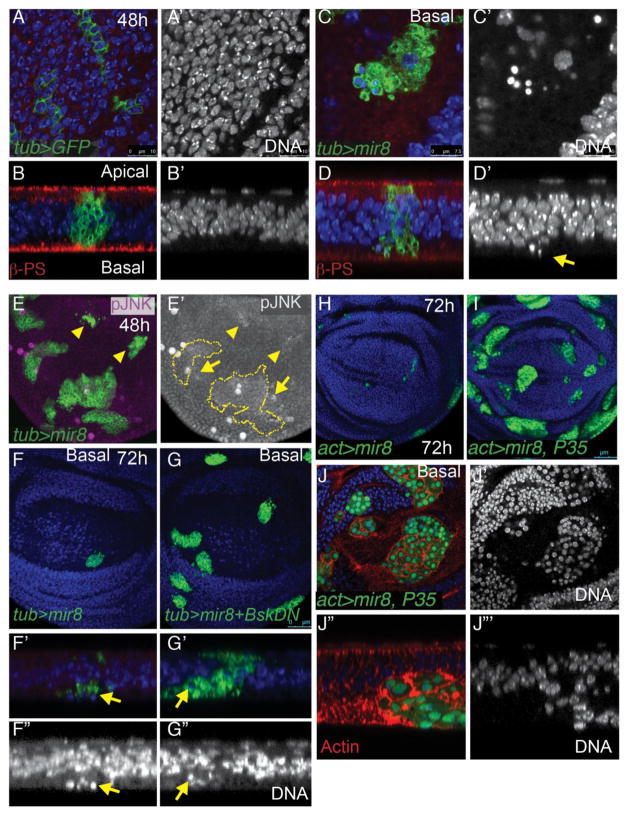
Fig. 2. miR-8 reduces Diap1 levels post-transcriptionally.
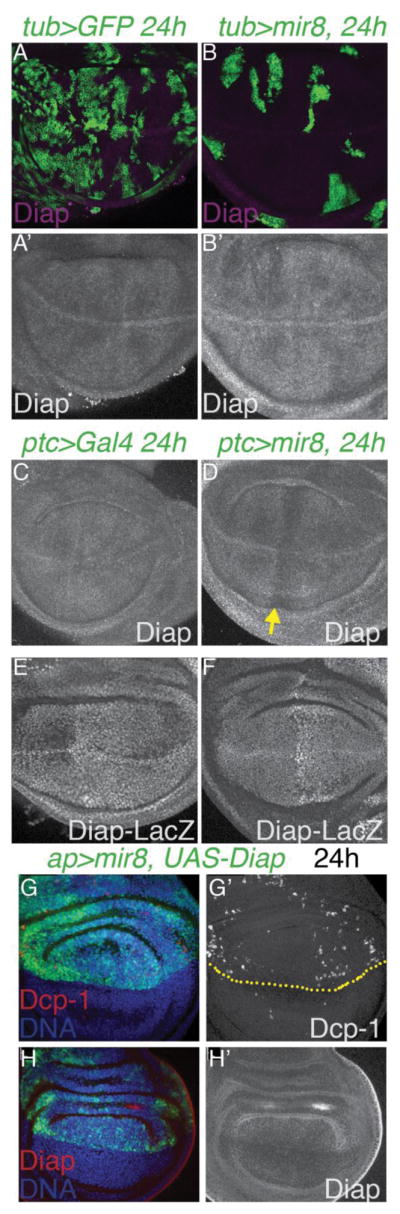
(A–B) miR-8 + GFP-expressing clones were generated using tub>CD2>Gal4/UAS, Gal80TS with heat-shock induced recombination at 38–48hr of development and transgene induction at 28°C for 24h prior to wing dissection at L3. miR-8 reduces Diap levels within 24hours (C–D) miR-8 expression driven by patched (ptc)-Gal4/UAS, Gal80TS for 24h reduces Diap. (E–F) miR-8 does not affect Diap-LacZ expression when overexpressed using the ptc-Gal4 driver. (G–H) Restoring Diap expression does not prevent apoptosis of high miR-8 expressing cells. Expression of miR-8 with UAS-Diap was driven in the dorsal wing using apterous (ap)-Gal4/UAS, Gal80TS for 24h. Apoptosis was assayed by Dcp-1 positivity and Diap expression confirmed using anti-Diap.
Cells undergoing apoptosis are often basally extruded from the wing epithelium and exhibit activation of c-Jun N-terminal Kinase (JNK) signaling (Dekanty et al., 2012; Shen and Dahmann, 2005). Cells expressing high miR-8 were also basally located (Fig. 3A–D) and exhibited an increase in phosphorylated JNK (pJNK, Fig. 3E). We attempted to examine a second readout of JNK signaling in miR-8 expressing clones, the expression of a puckered LacZ (puc-LacZ) transgene frequently used as a reporter of JNK signaling in the larval wing (Adachi-Yamada et al., 1999; Dekanty et al., 2012; Pastor-Pareja et al., 2004; Wu et al., 2010). However we could not recover any miR-8 expressing clones in backgrounds containing the puc-LacZ transgene. Puckered is a phosphatase that is both a target and a feedback inhibitor of JNK signaling (Martin-Blanco et al., 1998). Since the LacZ enhancer trap in puc is also a loss-of-function allele we speculate that a reduction of puc increases JNK signaling and hypersensitizes miR-8 expressing cells to rapid elimination from the wing epithelium.
Fig. 3. Cells with high miR-8 activate JNK signaling and are basally extruded.
(A.B) GFP-expressing clones or (C,D) miR-8 + GFP-expressing clones were generated using tub>CD2>Gal4/UAS, Gal80TS with heat-shock induced recombination at 38–48hr of development and transgene induction at 28°C for 48h prior to wing dissection at L3. Cells expressing GFP only in the wing pouch remain in the epithelium of disc proper. (B and D show representative x/z optical sections, all optical x/z sections are oriented with the peripodial epithelium marking the apical side of the disc at top). (C, D) Cells expressing high levels of miR-8 in the pouch are located basally and often have pyknotic nuclei. The arrow in D indicates basally located pyknotic nuclei. (E) miR-8 overexpression also causes increased phospho- JNK (pJNK) within miR-8 clones and in non miR-8-expressing cells bordering the clones (indicated by arrows, arrowheads indicate small miR-8 clones not outlined). Note that the large, round pJNK positive cells visible are located in the peripodial epithelia, which has been previously described (Tamori et al., 2010). This wing is from an L3 larva just prior to wandering to minimize the peripodial signal. (F) miR-8 overexpression using tub>CD2>Gal4 leads to elimination of most cells from the wing pouch by 72h and the few remaining cells are basally located (F′ x/z optical section of F). (G) Co-expression of miR-8 with a dominant negative form of Basket (BskDN) partially increases clone recovery (by 3.9-fold increase in clone area) at 72h. (G′) However miR-8+BskDN expressing cells are still basally located. (H– J) Co-expression of miR-8 with the apoptosis inhibitor P35 fully prevents clone elimination from the wing pouch (clone area is increased more than 10-fold comparing clones induced in parallel in H and I). (I, J) miR-8+P35 clones exhibit a rounded morphology, enlarged nuclei and are most often basally located.
To confirm whether JNK signaling is involved in elimination of miR-8 expressing cells from the wing epithelium, we compared basal extrusion and loss of miR-8 expressing clones with clones co-expressing miR-8 and a dominant negative form of the Drosophila JNK called Basket (BskDN). Co-expression of BskDN partially rescued the elimination of cells expressing miR-8 for 72 hours increasing average clone size 3.2-fold compared to miR-8 expressing clones induced in parallel (Fig. 3F,G, See also Supplement to Fig. 3). However, consistent with the reports of others, (Dekanty et al., 2012; Shen and Dahmann, 2005) inhibition of JNK signaling with BskDN did not prevent the basal extrusion of miR-8 expressing cells.
By contrast, co-expressing the apoptosis inhibitor P35 fully rescued miR-8 expressing clones from elimination in the wing epithelium (Fig. 3H–J). miR-8 expressing clones rescued by P35 co-expression exhibited smooth, round borders indicative of alterations in cell-cell adhesion (Prober and Edgar, 2002), an effect not observed in wing clones expressing P35 alone (Neufeld et al., 1998). These clones were also most often basally located (Fig. 3J) and in many cases persisted even after being fully extruded from the wing epithelium (Supplement to Fig. 3F). This demonstrates that the elimination of miR-8 expressing cells activates JNK signaling and proceeds normally via apoptosis, but that the events leading to basal extrusion of miR-8 expressing cells occurs independent or upstream of JNK and apoptosis. A p53/JNK-dependent feedback loop has been suggested to amplify apoptosis in response to stress in the Drosophila wing pouch (Shlevkov and Morata, 2012), but miR-8 induced cell death also occurs independent of p53, as co-expression of a dominant negative p53 sufficient to suppress apoptosis in response to gamma-irradiation in the wing (Supp. Table 1, Brodsky et al., 2000) completely failed to suppress apoptosis or basal extrusion of miR-8 expressing cells.
Loss or extrusion of miR-8 over-expressing cells leads to compensatory proliferation
Loss of cells via apoptosis in the fly wing epithelium can induce the compensatory proliferation of neighboring cells (Mollereau et al., 2013; Perez-Garijo et al., 2009; Poulton et al., 2014). To test whether dying cells expressing miR-8 triggered compensatory proliferation, we used the “tie-dye” method to create independently labeled non-miR-8 expressing neighbor clones in wings (Worley et al., 2013). With this method, Gal4/miR-8 expressing RFP-labeled clones are generated together with Gal4-negative GFP and Gal4-negative LacZ labeled clones. This provided us the opportunity to select wings which had GFP labeled clones near RFP-positive, miR-8 expressing (or control) clones. In addition, distant LacZ labeled clones, recovered at a lower frequency, can also serve as neutral controls to indicate the growth rate of the tissue. We measured clones for 10 wings expressing RFP alone, or miR-8 + RFP tie-dye clones (Fig. 4A,B). We consistently observed a severe loss of the miR-8 expressing cells with an expansion of GFP-labeled clones when neighbors (Fig. 4C). However more distant LacZ clones appeared similar between controls and miR-8 expressing wings, confirming that the loss of high miR-8 expressing cells in the wing epithelium induces local compensatory proliferation.
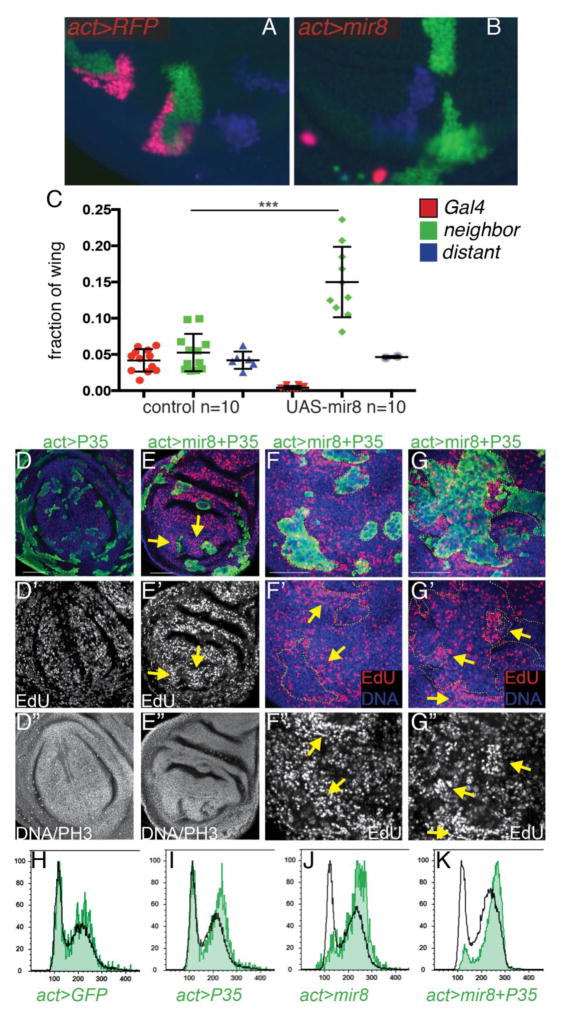
Fig. 4. Apoptotic signaling in miR-8 expressing cells leads to compensatory proliferation.
(A–C) Heat-shock induced clones expressing RFP alone (A) or RFP with miR-8 (B) were generated using the “Tie-Dye” method 72–80h hours before dissection of wandering L3 wings. We observed loss of the miR-8 expressing clones (red) and increased size of nearby GFP clones. We noted that “distant” LacZ clones (blue) were produced at a much lower rate than the other clones especially in the miR-8 expressing background, as previously described (Worley et al., 2013) therefore our distant measurements consist of fewer clones. Yellow bar = 50um. Error bars are std. dev. ***p<0.0001 by two-tailed Mann-Whitney test. (D) GFP-labeled clones expressing P35 alone or (E–G) GFP-labeled clones overexpressing miR-8 +P35 were generated using actin>CD2>Gal4/UAS system with heat-shock induced recombination at 30hr of development. Cells were labeled for S-phase with a 20 min EdU pulse and M-phase using Phospho-histone H3 Ser10 (PH3) positivity. DNA is labeled with Hoechst 33258. (F,G) Large clones expressing miR-8+P35 exhibit reduced EdU labeling while adjacent non-expressing tissue exhibits increased EdU positive cells. (H–K) Cell cycle analysis by flow cytometry was performed on larval wings containing GFP-labeled clones (H), expressing GFP+P35 (I), GFP+miR-8 (J) and miR-8+P35+GFP (K). The black trace shows the cell cycle profile of the non-clonal cells in the tissue. P35 expression alone shifts the cell cycle distribution in the wing slightly toward G2, while cells expressing miR-8 or miR-8+P35 show a dramatic shift toward late G2.
Defective, aneuploid or damaged cells normally destined to die that are rescued from elimination by co-expression of the caspase inhibitor P35, can trigger dramatic proliferation of neighboring cells. This phenomenon is termed Apoptosis Induced Proliferation (AIP), and occurs at least in part via JNK signaling (Dekanty et al., 2012; Mollereau et al., 2013; Poulton et al., 2014; Ryoo et al., 2004). Consistent with the phenomenon of AIP, miR-8 overexpressing clones that persist in the wing due to co-expression of P35 led to overgrowth and folding of the wing epithelium and an increase in cell proliferation of neighboring cells as measured by an EdU incorporation assay to label cells in S-phase (Fig 4D–G). We noted though that S-phase (and mitoses as indicated by phosphorylation of Ser10 on histone H3, PH3) was reduced within the miR-8 + P35 expressing clones, such that the overall level of EdU labeling in the miR-8 + P35 wings was not statistically significantly different from P35-only expressing controls (p=0.483). This led us to examine whether cells with high miR-8 expression exhibited cell cycle defects. We examined the cell cycle phasing of controls expressing GFP alone, GFP+P35, miR-8+GFP or miR-8+GFP+P35 using DNA content measurement by flow cytometry (Fig. 4H–K). The cell cycle profiles for GFP-positive and non-expressing GFP-negative larval wing cells are shown. We found that GFP has no effect on the relative cell cycle phasing in the wing, while P35 expression led to a mild increase in the proportion of cells in G2 (Fig. 4I). High levels of miR-8 with or without P35 led to a dramatic increase in the percentage of cells in G2-phase, suggestive of a defect in G2/M phase progression (Fig. 4J,K). However we did not observe any increase in aneuploidy even in the presence of P35. This may explain why the rescue of “undead” miR-8 expressing cells with P35 leads to tissue folding but not the more extreme phenotypes of tissue invasiveness or tumorigenesis as reported in P35-rescue contexts where cell cycle defects lead to aneuploidy (Dekanty et al., 2012). Indeed a prolonged G2 phase helps prevent aneuploidy and limits cell delamination in Drosophila wings (Dekanty et al., 2015), and recent work in C. elegans also confirms that blocking the cell cycle in G2 constrains cell migration (Matus et al., 2015). We suggest that the G2 elongation and arrest observed in miR-8 expressing cells may actually serve a protective function, in limiting cell invasiveness and migration.
High miR-8 expression disrupts wing epithelial organization and regulators of cell-extracellular matrix interactions
The basal extrusion of miR-8 expressing cells begins as early as 15h of miR-8 expression, occurs in the absence of apoptosis and when JNK signaling is inhibited (Fig. 3, Supplement to Fig. 3). This suggests high levels of miR-8 could affect proximal targets that impact epithelial organization. The miR-8 vertebrate homologs the miR-200 family, have well-described roles in epithelial cells where they inhibit epithelial-to-mesenchymal transitions in part by targeting transcriptional repressors of E-cadherin (E-cad) expression ZEB1 and ZEB2 (Brabletz and Brabletz, 2010; Hill et al., 2013). We therefore examined whether high levels of miR-8 in the fly wing affected E-cad levels. We found no clear, consistent differences in endogenous apical E-cad levels in miR-8 expressing cells, although we did observe loss of some E-cad staining in basal sections of the wing, consistent with holes in E-cad found in optical x/z sections due to the basal extrusion of dying cells (Fig. 5A,B). We also observed no loss of E-cad in miR-8 mutant clones in the wing, consistent with the largely normal development of the miR-8 mutant wing (Supplement to Fig. 1). We next examined whether miR-8 could reduce the expression of the Drosophila ZEB1/2 homolog Zfh1 in the wing. We found no loss of Zfh1 expression in clones expressing high levels of miR-8 for 48h. Rather Zfh1 appeared slightly increased, despite the presence of a predicted, conserved miR-8 binding site in the Zfh1 3′UTR (Supplement to Fig. 5).
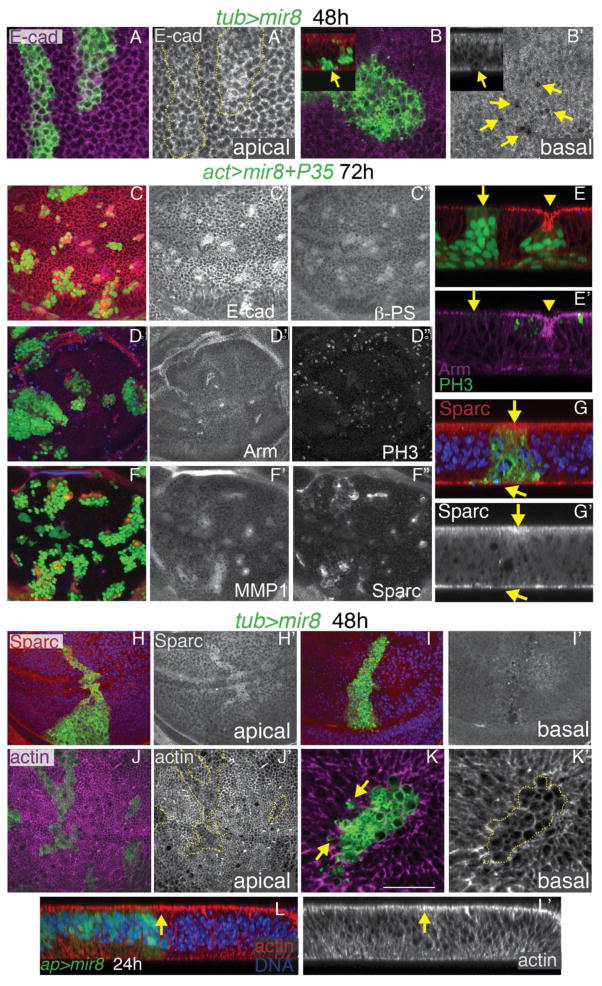
Fig. 5. High miR-8 remodels the wing epithelium and reduces apical f-actin.
GFP-labeled clones overexpressing miR-8 were generated by heat-shock induced recombination using tub>CD2>Gal4/UAS, Gal80TS with miR-8 expression induced at 28°C for 48h prior to dissection at wandering L3. (A) Apical x/y sections show little effect of miR-8 expression on E-cadherin (E-cad) intensity or localization. (B) Basal x/y sections show holes of E-cad (indicated by arrows) due to extrusion of miR-8-expressing cells from the wing epithelium. The inset shows an x/z section of this wing, confirming the loss of basal E-cad in a miR-8 clone undergoing basal extrusion. (C–G) GFP-labeled clones overexpressing miR-8 +P35 were generated using heat-shock induced recombination of actin>CD2>Gal4/UAS and dissected 72h post clone induction. (C) Persistent miR-8 + P35 leads to ectopic E-cad and integrin (β-PS). (D) miR-8 + P35 clones show concentrated foci of Arm staining in the center, (E) which in x/z optical sections correspond to locations of folded epithelium caused by basal extrusion of miR-8 cells. (F) Persistent miR-8 + P35 leads to Sparc and MMP1 upregulation, (G) with Sparc mis-localized apically (arrows). (H,I) Cells expressing miR-8 without P35 also show apical localization of Sparc. (J,K) Cells expressing miR-8 have reduced apical f-actin, (L) which can be seen by 24h of miR-8 expression in an optical x/z section. In this case miR-8 + GFP expression is driven by apterous (ap)-Gal4,Gal80TS with a shift to 28°C 24h prior to dissection. Ap-Gal4 drives in the dorsal wing, oriented to the left in this x/z section. The arrow indicates the dorso-ventral boundary.
However, when miR-8 over-expressing cells are allowed to persist in the wing epithelium, we observe severe disruptions of epithelial organization. In miR-8 over-expressing clones rescued by P35, we observed a strong up-regulation of apical E-Cad and ectopic Beta Integrin (β-PS) after 72h (Fig. 5C). To determine whether apico-basal polarity of the epithelium was lost under these conditions, we examined the apical localization of the Drosophila Beta-Catenin homolog Armadillo (Arm) and the positioning of mitoses in the wing epithelium (Fig. 5D). Arm is normally associated with the apical adherens junctions of the wing epithelium and mitoses in the wing normally occur apically (Supplement to Fig 5E). Even when a miR-8+P35 clone is being extruded basally, the apical localization of Arm is maintained (Fig. 5E arrowhead), suggesting high levels of miR-8 and extrusion does not primarily disrupt epithelial apico-basal polarity. However, we also observed some miR-8+P35 clones near those being extruded with reduced apical Arm levels (Fig. 5E, arrow). We previously examined whether high miR-8 can reduce Arm expression or protein stability, and found Arm protein levels are unchanged by miR-8 (Kennell et al., 2008). We therefore suggest the loss of apical Arm in these clones is due to defects in Arm localization rather than changes in Arm expression or Arm stability.
We also observed aberrant upregulation of the JNK signaling target Matrix Metalloproteinase 1 (MMP1) involved in basement membrane (BM) remodeling as well as the matricellular component Secreted protein acidic and rich in cysteine (Sparc) (Fig. 5F). We were particularly interested in these two proteins since they are involved in remodeling extracellular matrix (ECM) and modulating cell-ECM signaling. We reasoned that the elimination of high miR-8 cells from the wing epithelium by basal extrusion requires detachment of cell-ECM interactions and holes in the BM for cell elimination, similar to the type of JNK-dependent BM remodeling thought to occur in tumor invasion (Srivastava et al., 2007). MMP1 is a secreted protease with well-characterized roles in ECM degradation and remodeling as well as cell migration and invasion (Dekanty et al., 2012; Rudrapatna et al., 2013; Stevens and Page-McCaw, 2012; Uhlirova and Bohmann, 2006). Sparc has been shown to remodel ECM in Drosophila by promoting collagen solubility, but Sparc also plays additional roles in cell survival and epithelial cell competition (Isabella and Horne-Badovinac, 2015; Martinek et al., 2008; Portela et al., 2010). Sparc is normally high on the basal surface of epithelium, but we noticed that Sparc was apically localized in miR-8 expressing cells (Fig. 5G), and that this apical localization occurred even in the absence of cell rescue by P35 (Fig. 5H,J). Apical localization of Sparc has been reported before in vertebrate cells (Alpers et al., 2002; Hudson et al., 2005; Sodek et al., 2002) where it has been suggested to alter cell motility and ECM attachment, but it remains unclear exactly how. In mammals, Sparc directly binds Integrinβ1 and promotes integrin-linked kinase (ILK) activity intracellularly to increase cell survival and signaling to the actin cytoskeleton (Barker et al., 2005; Shi et al., 2007; Weaver et al., 2008). Apical re-localization of Sparc could limit this interaction, leading to reduced survival and reduced integrin-dependent signaling to the actin cytoskeleton.
miR-8 overexpression reduces apical f-actin
Our data suggests that supra-physiological levels of miR-8 in the wing epithelium leads to a cell-autonomous disruption of proper epithelial organization, which is exacerbated by the process of basal extrusion and prolonged JNK signaling when miR-8 expressing cells persist. The early events of basal extrusion require remodeling of the actin cytoskeleton (reviewed in Wu et al., 2015). When we examined clones in the wing expressing miR-8 for 48h, we noted that F-actin levels appeared reduced apically. More basal sections through these clones revealed cells with a rounded morphology and GFP-positive fragments indicative of cell elimination and apoptosis (Fig. 5J,K).
To examine whether the reduction of apical F-actin in miR-8 expressing cells was a primary effect of miR-8 expression or a secondary consequence of basal extrusion and apoptosis, we examined an earlier timepoint in wings co-expressing miR-8 and Diap for 24h using the ap-Gal4 driver (Fig. 5H). At 24h of induction in this genetic background some apoptosis of miR-8 expressing cells is already observed as detected by Dcp-1 staining (Fig. 2H), but most cells maintain their proper position in the epithelium and very little basal extrusion or pyknotic nuclei are observed. Yet, after 24h of miR-8 expression, a reduction in apical actin throughout the dorsal wing pouch can be observed in optical x/z sections through the disc (Fig. 5L, Dorso-Ventral boundary indicated by arrow). This suggests a reduction in apical F-actin in miR-8 expressing cells may precede basal extrusion and apoptosis.
miR-8 targets cytoskeletal regulators in the Drosophila wing
miR-8 is known to target the actin regulator Enabled (Ena) in the larval muscles and larval wing (Loya et al., 2009), and inhibition of Ena is sufficient to rescue the post-synaptic miR-8 loss-of-function phenotypes observed at the neuromuscular junction (Loya et al., 2009). However adding back exogenous Ena does not rescue the effects of miR-8 overexpression on apical F-actin or cell survival in the wing. (Supplement to Fig.6). miR-8 also inhibits Wg signaling via multiple direct targets (Kennell et al., 2008) and Wg signaling acts as a cellular survival factor in the Drosophila wing (Giraldez and Cohen, 2003; Johnston and Sanders, 2003). However, providing exogenous TCF also fails to rescue cell death induced by miR-8 overexpression (Supplement to Fig.6).
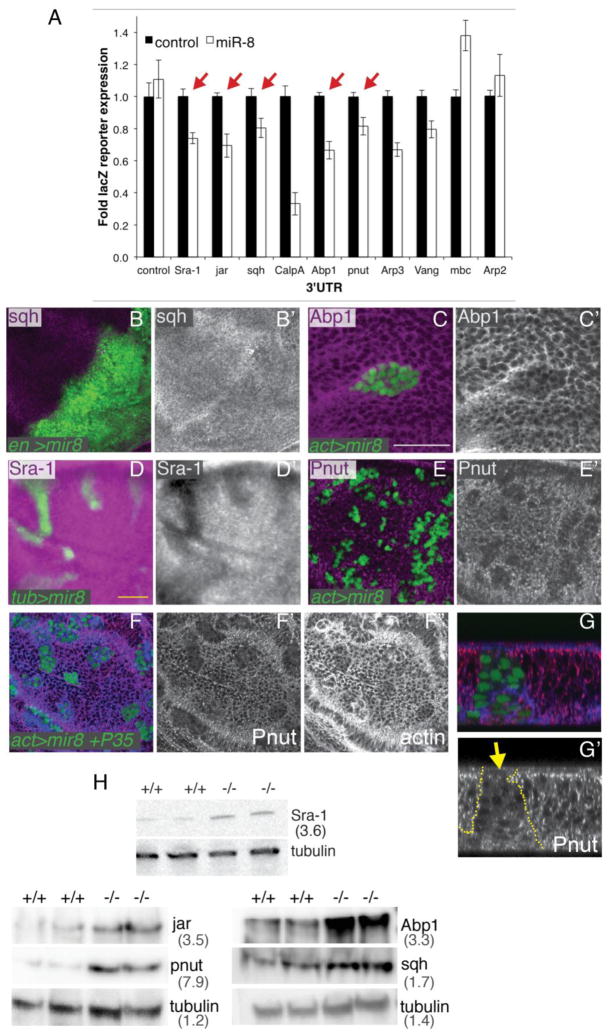
To find additional miR-8 targets that could be mediating the cytoskeletal and cell survival phenotypes we observe in the wing, we used publicly available microRNA target prediction programs (e.g. Targetscan, MiRanda and PicTar). Multiple programs predicted several cytoskeletal components and regulators to be miR-8 targets, which were conserved for the mammalian miR-200 family (Table 1) (Bracken et al., 2014). We examined several predicted miR-8 targets and found that the 3′UTRs for Specifically Rac1-associated protein 1 (Sra-1), jaguar (jar), spaghetti squash (sqh), Calpain A (CalpA), Actin binding protein 1 (Abp1), peanut (pnut), Actin-related protein 3 (Arp3), and Van Gogh (Vang), all involved in actin cytoskeletal regulation either directly or indirectly (Cooper and Kiehart, 1996; Emori and Saigo, 1994; Gautier et al., 2011; Jordan and Karess, 1997; Kellerman and Miller, 1992; Mavrakis et al., 2014; Zallen et al., 2002), can be targeted by miR-8 in in vitro reporter assays (Fig. 6A). For Sra-1 we confirmed that mutation of the predicted miR-8 target site in the 3′UTR abolished miR-8-dependent repression of the reporter (Supplement to Fig. 6E).
Table 1.
Potential targets of miR-8 tested in the 3′UTR reporter assay. Potential targets conserved with the miR-200 family in human cells were identified in Bracken et al., EMBO J, vol. 33 p 2040, 2014.
| Drosophila gene | function | Human homolog | Conserved target? |
|---|---|---|---|
| jaguar (jar) | Atypical myosinVI | MYO6 | Mir-200a coding |
| peanut (pnut) | septin | SEPT7 | - |
| Sra-1 | Arp2/3-dependent actin polymerization | CYFIP1/2 | - |
| enabled (ena) | Actin polymerization | ENAH/VASP | - |
| Actin binding protein 1 (Abp1) | Actin binding | DBNL/DBN1 | - |
| spaghetti squash (sqh) | Myosin regulatory light chain | MYL12A/B, MYL9 | MYL Kinase targeted by Mir-200c |
| Calpain-A (CalpA) | Ca2+-dependent cysteine endopeptidase | CAPN9/2/3/1 | Mir-200b 3′UTR |
| Actin-related protein (Arp3) | Arp2/3 complex, actin polymerization | ACTR3/3B/3C | Mir-200a 3′UTR |
| Van Gogh (Vang) | Planar polarity/Frizzled binding | VANGL1/2 | Mir-200b 3′UTR |
Fig. 6. Additional targets of miR-8 impact the actin cytoskeleton and epithelial organization.
(A) The 3′UTR of potential targets was cloned downstream of a LacZ coding region, and co-transfected into Kc167 cells with a miR-8 expression vector. The 3′UTRs of Sra-1, jar, sqh, CalpA, Abp1, pnut, Arp3, or Vang caused a significant decrease in LacZ expression in the presence of miR-8 (p<0.001 by two-tailed t-test). Arrows indicate targets further verified by immunofluorescence in vivo. (B) miR-8 overexpression in the posterior wing (using en-Gal4, Gal80TS) reduces levels of the myosin II regulatory light chain, Spaghetti Squash (Sqh) (C) miR-8 expressing GFP-positive clones display a decrease in Actin Binding Protein1 (Abp1) (D) Specifically Rac-associated protein 1 (Sra-1), (E) and the septin Peanut (Pnut). (F–G) Blocking apoptosis of miR-8 expressing cells does not prevent the decrease in miR-8 targets. GFP-positive clones co-expressing miR-8 and P35 show reduced f-actin as well as reduced Pnut levels. (G) shows an x/z optical section of a clone from F. (H) Loss of miR-8 leads to increased expression of novel miR-8 targets. miR-8JK22/miR-8Δ1 mutant larvae exhibit increased protein levels of the miR-8 targets Sra-1, Jar, Pnut, Abp1 and Sqh, ranging from 1.7-fold to 7.9-fold when normalized to tubulin levels.
We next confirmed that miR-8 overexpression in vivo reduces Sqh, Abp1, Sra-1, and Pnut protein levels by immunofluorescence (Fig. 6B–E). However, we did not see a clear decrease in Jar protein levels by immunofluorescence. We also did not see a decrease in Spitz protein levels upon overexpression of miR-8 in vivo by immunofluorescence, which has been shown to be a functionally relevant miR-8 target (Supplement to Fig. 6H), (Morante et al., 2013). miR-8 may therefore mildly reduce some targets at levels below that visible by immunofluorescent staining. Indeed, miR-8 has been suggested to mildly “tune” levels of certain targets (Karres et al., 2007). By contrast, Pnut is one of the most strongly affected miR-8 targets. We confirmed that the strong loss of Pnut staining was not due to cell death, as miR-8 overexpressing cells rescued by P35 exhibit a strong decrease in Pnut levels and loss of proper Pnut localization (Fig. 6F–G).
We next examined whether expression of the actin regulatory targets of miR-8 are increased in miR-8 mutants. miR-8 mutant larvae exhibited increased protein levels of the miR-8 targets Jar, Pnut, Abp1 and Sqh, ranging from 1.7-fold to 7.9-fold when normalized to tubulin levels (Fig. 6H) and miR-8 mutants also exhibit increased Ena (Loya et al., 2009). These proteins are involved in cortical F-actin bundling, F-actin polymerization and nucleation at adherens junctions and actomyosin contraction - molecular functions consistent with alterations in cell migration, cell-cell junction remodeling and cytokinesis, which may contribute to the G2 arrest of miR-8 expressing cells (Aldaz et al., 2013; Founounou et al., 2013; Geisbrecht and Montell, 2002; Koch et al., 2012; Lin et al., 2007; Mavrakis et al., 2014).
To examine whether reduction of miR-8 targets can recapitulate the phenotypes we observe upon miR-8 expression, we used RNAi to knockdown several miR-8 actin-regulatory targets (data confirming RNAi knockdown and the antibodies used in Figs. 6 and 7 is provided in the Supplement to Fig. 7). When we reduce miR-8 targets individually, we do not see a strong effect on actin staining (Supplement to Fig. 7), however when Sra-1 and ena are knocked down together by RNAi or Sra-1 is knocked down together with jar, we see reduced apical F-actin, similar to the effect observed with miR-8 overexpression (Fig. 7A,B). We also see increased apoptosis upon Sra-1 and ena knockdown for 48h, demonstrating that the reduction of these actin regulators is sufficient to compromise cell survival in the wing epithelium (Fig. 7C,D note that GFP expression is often reduced in DCP-1 positive cells).
Fig. 7. miR-8 targets involved in f-actin organization promote cell survival.
(A) Co-expression of Sra-1 and jar RNAi or (B) Sra-1 and ena RNAi in GFP-positive clones causes a decrease in apical f-actin. (C, D) Co-expression of Sra-1 and ena RNAi increases apoptosis as indicated by Dcp-1 staining. Note that GFP is often weaker but present in Dcp-1 positive cells as shown at higher magnification in D). (E) Knockdown of sqh by RNAi in GFP-positive clones also leads to reduced survival and basal localization similar to miR-8 expression (F) A summary of Drosophila miR-8 targets (dIAP may be an indirect target) that affect epithelial architecture, cell survival and their relationship to the known targets of miR-8 in WNT signaling.
Previous research has shown apically localized Sqh to be involved in apical constriction, invagination and MyoII-dependent regulation of tension in epithelia (Aldaz et al., 2013; Monier et al., 2015; Zimmerman et al., 2010). We therefore examined whether knocking down sqh via RNAi may reduce cell survival or promote basal extrusion. Reduction of Sqh had a very strong effect on cell survival (Supplement to Fig 7J) and nearly every sqh RNAi clone we recovered was basally located (Fig. 7E). Altogether our data suggests the reduction of a combination of miR-8 actin regulatory targets including Sra-1, Jar, Ena and Sqh can recapitulate the phenotypes of miR-8 overexpression in the wing.
Discussion
miR-8 and cell survival
The miR-200 family is highly conserved and its roles in promoting the epithelial state and inhibiting EMT have been extensively studied (reviewed in Brabletz and Brabletz, 2010; Bracken et al., 2015; Gregory et al., 2008b; Hill et al., 2013) and were recently shown to be conserved in Drosophila (Antonello et al., 2015). Here we examine roles for miR-8 in the epithelium of the Drosophila wing in vivo. Despite a number of conserved targets for miR-8/miR-200 that impact the actin cytoskeleton, cell adhesion and cell polarity, we find that wing epithelial architecture, patterning and differentiation are nearly completely normal in the absence of miR-8 (Supplement to Fig. 1). This may be due to the finding that a major role for the miR-200 family in maintaining the epithelial state by limiting Zeb1/2-dependent repression of E-Cad appears to be non-essential in the fly wing under normal conditions despite the capacity of miR-8 to inhibit Zfh1 in other contexts (Antonello et al., 2015).
Instead, we find that miR-8 reduces cell survival in the wing epithelium and that cells expressing supra-physiological levels of miR-8 exhibit cell rounding, detachment, basal extrusion, expression of basement membrane remodeling proteins and loss of proper epithelial organization - hallmarks often associated with an epithelial to mesenchymal transition (EMT) (Lamouille et al., 2013; Lamouille et al., 2014). However we do not think cells expressing supra-physiological levels of miR-8 are undergoing EMT. Rather we suggest levels of miR-8 must be maintained in an optimal range in epithelial tissues to avoid compromising cell survival and cell adhesion through miR-8 targets that influence the cytoskeleton. Here we identify the MyoII Regulatory Light Chain (Sqh), MyoVI (Jar), and the WAVE component Sra-1 as novel miR-8 targets in the fly wing. What these proteins have in common is that they play roles in generating proper epithelial tension at cell-cell junctions by their recruitment to adherens junctions and regulation of actin polymerization along with the previously identified miR-8 target Ena (Lecuit and Yap, 2015). In addition to these targets, we also identify the fly Septin, Peanut, as a strongly regulated miR-8 target. Septins play critical roles in bending and bundling of actin to generate curved actin filament networks (Mavrakis et al., 2014). The loss of Septins also alters tension in epithelia through promoting acto-myosin constriction during cytokinesis, which is required to remodel adherens junctions to maintain proper epithelial tension in proliferating tissues (Founounou et al., 2013). If tension is reduced in cells expressing high levels of miR-8, this could lead to defective junctions and basal extrusion culminating in JNK-dependent stress signaling (Fig. 3) and apoptosis.
In opposition to the effects of overexpressing miR-8 in the fly wing, we find that loss of miR-8 increases cell survival and promotes survival in the face of an apoptotic stimulus, in our case UV exposure (Fig. 1). This is consistent with studies showing that loss of miR-200 family members can reduce apoptosis in tissues (Belgardt et al., 2015) and can promote cancer cell survival in the face of chemotherapy (Feng et al., 2012; Pogribny et al., 2010). This is an important issue in light of recent work in lung cancer, which suggests that loss of miR-200 and subsequent EMT is not a driver of cancer metastasis, rather that the mesenchymal state and low miR-200 leads to survival in the face of chemotherapy. Importantly, the increased chemoresistance of mesenchymal cells is reversed when exogenous miR-200 was expressed (Fischer et al., 2015).
Whether the improved survival of miR-8 mutant cells impacts recovery from the UV damage in our experiment remains unclear, as the levels of UV damage disrupted pupariation for all the animals, including controls. However in a second experiment we compared miR-8 mutant animals to control animals reared on low doses of Paraquat (2mM PQ) sufficient to induce DNA damage but low enough to allow morphologically normal adults to develop. We found that 39% (7/18) of miR-8 null mutant animals (JK22/JK22) reared on low PQ exhibited wing and scutellar bristle defects while 0/8 control miR8+/+ animals grown on low PQ exhibited these defects (Supplement to Fig. 1). This suggests that a failure to eliminate defective cells by apoptosis in miR-8 mutants compromises recovery from developmentally induced damage and points to an important role for endogenous miR-8-induced apoptosis during development. A previous study in the Drosophila intestine has suggested damage induced by acute PQ treatment up-regulates endogenous miR-8 expression (Antonello et al., 2015), consistent with a role for endogenous miR-8 in the response to tissue damage. Based upon this observation and our results, we propose that the requirement for endogenous miR-8 becomes revealed upon a tissue challenge or damage, which induces high levels of miR-8 to assist in the elimination of defective cells from an epithelium.
How might our findings impact strategies aimed at using the miR-8 homologs as a potential target in cancer treatment for epithelial cancers? While our findings in Drosophila may not directly translate to miR-200 in human tissues, our results suggest that very low or no expression of the miR-200 family could make cells resistant to chemotherapy and apoptosis even in the absence of a mesenchymal transition. Conversely, very high levels of miR-200 expression and activity may induce JNK signaling and disrupt epithelial organization. This could explain the contradictory results obtained with using miR-200 expression levels as a prognostic marker in epithelial cancers, where in prostate and breast cancer higher expression levels are correlated with improved outcomes but in pancreatic and certain ovarian cancers the opposite is seen (Gregory et al., 2008a; Li et al., 2010; Mateescu et al., 2011; Nam et al., 2008; Park et al., 2008). It will therefore be critical to determine the factors that influence miR-8/miR-200 expression levels in epithelia outside of Zeb1/2, and the fly wing may be an optimal system to examine this.
Materials and Methods
Drosophila crosses
miR-8 overexpression clones: hs-flp; tub>CD2>gal4/+; UAS-miR-81F34/tub>gal80TS hs-flp; act>CD2>gal4/UAS-P35; UAS-miR-81F34/+hs-flp; act>CD2>gal4/+; UAS-miR-81F34/+
tie-dye assay: hs-flp; act>CD2>lacZ, ubi>CD2>GFPNLS/+; act>CD2>gal4,UAS-RFP/UAS-miR-81F34 Control: hs-flp; act>CD2>lacZ, ubi>CD2>GFPNLS/+; act>CD2>gal4,UAS-RFP/+
miR-8JK22 mitotic recombination clones: hs-flp; FRT 42D Ubi-GFP/FRT 42D miR-8jk22 Parental FRT42D control: hs-flp; FRT 42D Ubi-GFP/FRT 42D
MARCM miR-8 mutant for FACS: hs-flp, UAS-GFPNLS, tub-Gal4; FRT42D tub-Gal80/FRT 42D miR-8jk22
The following RNAi lines were crossed to w; tub>CD2>gal4, UAS-GFP; tub>gal80TS or w; en-gal4, UAS-GFP; tub-gal80TS: Sra-1RNAi (Trip RNAi BL#38294), JarRNAi (Trip RNAi BL#28064), EnaRNAi(Trip RNAi BL#31582), SqhRNAi(Trip RNAi BL#31542)
Sra-1RNAi + JarRNAi experiments: hsflp/+; tub>CD2>gal4, UAS-GFP/+; UAS-jarRNAi/Sra-1RNAi
Sra-1RNAi + EnaRNAi experiments: hsflp/+; tub>CD2>gal4, UAS-GFP/+; UAS-enaRNAi/Sra-1RNAi
miR-8 sensor: +/+; tubEGFP-2xmiR-8/+ and miR-8JK22/miR-8JK22; tubEGFP-2xmiR-8/+
miR-8 sponge experiment: w/+; miR-8-sponge/ap-Gal4,UAS-GFP; miR-8 sponge/tub-Gal80TS, UAS-DIAP
BskDN clone rescue: w/hs-flp; tub>CD2>gal4, UAS-GFP/+; UAS-BskDN/UAS-RFP (BL#9311)
P35 clone rescue: hs-flp/UAS-P35;+; act>CD2>gal4/UAS-miR-81F34
Experiments with Diap: w;apterous-Gal4,UAS-GFP/+; miR-81F34/UAS-DIAP, tub-gal80TS from (Buttitta et al., 2007), w;ptc-Gal4/UAS-miR-81F20; tub-gal80TS/Diap-LacZ Diap-LacZ on III (BL# 12093). All flystocks listed were generated with publicly available lines from the Bloomington Stock center, or are described in (Buttitta et al., 2007; Kennell et al., 2012; Kennell et al., 2008)
Immunofluorescence
Wing discs from wandering larvae at the third larval instar were fixed with 4% paraformaldehyde/1X PBS and processed as described (Buttitta et al., 2007), except for staining with anti-Sparc and TUNEL labeling, which required modifications described below. EdU incorporation was performed for 15 min in Ringer’s solution and detected using Click-iT EdU Alexa Fluor 555 Imaging Kit from Life Technologies. For UV treatment L3 larvae were placed in uncovered plastic 6cm dishes and exposed to 240mJ of UV using a Stratalinker 2400 with a UVC bulb as described (Kang and Bashirullah, 2014). Animals were allowed to recover for the indicated time and fixed and stained for DCP-1 immunofluorescence.
The antibodies and reagents used are as follows
Anti-Casp3: rabbit 1:100 (Cell Signaling)
TUNEL: see protocol Anti-Sparc: see protocol; mouse-anti Sparc 4 uL in 40 uL PAT + 0.3% Triton-X (gift of Eduardo Moreno, Bern, Switzerland) or rabbit anti-Sparc 1:100 (gift of M. Ringuette, Toronto, Canada).
Anti-pJNK: 1:100 rabbit (Promega)
F- actin staining: 1:100 in PBS rhodamine-labelled phalloidin (Invitrogen)
Anti-LacZ: 1:5,000 Rabbit (Cappel) or 1:500 mouse (Promega)
Anti-Sra-1: 1:1000 rabbit (provided by Alexis Gautreau, Laboratoire d’Enzymologie et Biochimie Structurales CNRS, FRANCE)
Anti-Jaguar: 1:20 mouse (3C7 provided by Kathryn Miller, St. Louis, MO, USA)
Anti-Enabled: 1:50 mouse (Developmental Studies Hybridoma Bank, DSHB, USA)
Anti-Sqh: 1:300 mouse (provided by Robert Edwin Ward IV, University of Kansas, USA)
Anti-Dcp1: 1:100 rabbit (Cell signaling) Anti-Pnut 1:100 mouse (DSHB, USA)
Anti-Abp1: 1:250 Rabbit (provided by Michael Kessels Jena University Hospital, Germany)
Anti-Diap 1:100 goat (Santa Cruz Biotechnology)
Anti-Arm 1:100 mouse (DSHB, USA)
Anti-DE-Cad 1:20 rat (DSHB, USA)
Anti-βPS1 (Integrin) 1:100 mouse (DSHB, USA)
Anti-MMP1 1:100 mouse (DSHB, USA)
Anti-PH3 1:2,000 Rabbit (Upstate Biotechnology)
Clone size measurements
Crosses to compare miR-8 expressing clone sizes with and without P35 were performed with a UAS-P35 on the X, such that only females expressed P35 while males served as a non-P35 control from the exact same vial. Similarly, crosses to compare miR-8 expressing clone sizes with and without UAS-BskDN were performed with males carrying UAS-BskDN on III over a UAS-RFP transgene, such that only RFP negative animals expressed BskDN while RFP positive animals served as non- BskDN control from the exact same vials. This was done to ensure that the level of heat-shock, efficiency of clone formation and rearing conditions were identical between the two genotypes compared in Fig. 3. Crosses to collect animals to make miR-8 clones and control FRT42D clones were performed in parallel. Animals were roughly synchronized by collecting eggs for 12 h on grape-agar plates. Hatched larvae were transferred to vials uncrowded vials (50/vial) containing cornmeal-agar food and reared at 25°C for 24h. Clones were induced by 18 min. heat-shock in a 37°C water bath and animals were reared for 75h at 25°C prior to dissection and fixation at the wandering L3 stage. For clone size measurements, images were analyzed using Nikon NIS Elements D software. The “polygon area” tool was used to outline the wing pouch as defined by the location of the hinge wrinkles. Next, individual clones were outlined with the “polygon area tool” labeling each one for area measurement export to an Excel spreadsheet. The area of all clones of the same genotype: all GFP+ and all GFP- were summed. This was used to calculate the percentage of the total wing pouch area taken up by GFP+ and GFP- clones respectively. The average percentage of the wing pouch taken up by each clone type was then averaged and the standard deviation of the percentages, and the p-value were calculated using an unpaired, two-tailed t-test.
In vitro 3′UTR reporter assays
All 3′UTR reporter genes were generated by cloning a stop codon, followed by the entire 3′UTR of the predicted miR-8 target, downstream of lacZ in pAclacZ (Invitrogen). Kc167 Drosophila cells were transiently transfected with the indicated 3′UTR reporter gene along with pAc control or pAc-miR-8, and pAc-luciferase, to control for transfection efficiency. β-galactosidase and luciferase activities were measured as described previously (Blauwkamp et al., 2008).
TUNEL labeling detailed protocol
For Rhodamine-TUNEL labeling the Apoptag Detection Kit was used (Millipore) with the following modifications. Tissues were fixed with 4% paraformaldehyde (PFA)/PBS. Staining for clonal markers (ie. GFP) was performed via standard procedures before TUNEL labeling. Before TUNEL reaction, tissues were fixed in 100% methanol for 6 min. followed by washes with PBS + 0.1% Triton-X. Tissues were then exposed to Equilibration Buffer for 2 min. prior to addition of the TdT enzyme. Tissues were incubated with TdT enzyme for 1hr at 37C and stopped by addition of diluted Stop/Wash Buffer Digoxygenin (DIG)-labeled dT was detected with anti-DIG-Rhodamine antibody and samples were mounted on slides using standard procedures.
Sparc labeling detailed protocol
For anti-Sparc staining, tissues were fixed for 1 hour in 4% PFA/PBS and blocked in PBS + 1% BSA + 0.3% Triton-X overnight. Clone 30A/B4 anti-Sparc antibody (provided by Dr. E Moreno) or rabbit anti-dSparc (provided by Dr. M. Ringuette) was used at 4 uL in 40 uL of blocking solution or 1:100 respectively and incubated with tissues for 1–2 days at room temperature. Tissues were washed and secondary antibody labeling was performed overnight as previously described.
Microscopy
All images were obtained using a Zeiss LSM 510 confocal or a Leica SP5 confocal except for Fig. 6D and Supplement to Fig. 5G, which was obtained using a Leica DMI6000 epi-fluorescence system with de-convolution (ImageQuant). All images were cropped, rotated and processed using Adobe Photoshop. For brightness/contrast the Auto Contrast function was used. All brightness/contrast adjustments were applied equally on the entire image. All x/z optical sections were obtained on a Leica SP5 confocal. Images for x/z sections are maximum projections of 1–3 y-sections of 0.5 micron intervals or less. All image quantifications were performed using Image J.
Flow cytometry
Flow cytometry on larval wings was performed as described (Flegel et al., 2013).
Western blots
Wandering third instar wild type (w; +/+) and miR-8 mutant (w; miR-8JK22/miR-8JK22 or w; miR-8JK22/miR-8Δ1 (Karres et al., 2007)) larvae were lysed in RIPA buffer or for Sra-1 detection directly in Laemmli buffer. The primary antibodies used were mouse anti-Sqh (1:5000), mouse anti-Jar (1:20), rabbit anti-Abp1 (1:1000), rabbit anti-Sra-1 (1:1,000), mouse anti-Pnut (1:500, 4C9H4 concentrate from DSHB) and mouse anti-tubulin (1:500 for E7 or 1:1,000 for 12A10, DSHB). HRP-conjugated secondary antibodies (Jackson ImmunoResearch) were visualized using ECL kits and digitally imaged to avoid band saturation. Bands were analyzed using NIH ImageJ. Due to band interference, the tubulin control for the blots of Abp1 and Sqh are from a separate blot which was loaded identically and processed in parallel.
Supplementary Material
Supplement to Figure 1. (A–F) GFP-labeled clones expressing GFP alone or GFP with miR-8 were generated using the tub>CD2>Gal4/UAS, Gal80TS system with heat-shock induced recombination from 38–80 hr of development and transgene induction at 28°C for 24–72hr prior to wing disc dissection at wandering third larval instar (L3). F-actin is shown by phalloidin staining in blue and anti-Dcp-1 positivity is shown in red. miR-8 overexpressing clones are positive for Dcp-1 after 24h of miR-8 expression. We observed no increases in Dcp-1 positivity in control GFP expressing wings, even after 72h at 28°C. (G) GFP-labeled miR-8-expressing clones were generated using tub>CD2>Gal4/UAS with heat-shock induced recombination 48h prior to pupariation. Adult wings expressing miR-8 mosaically show defects including notching at the wing margin and disrupted planar polarity. (H) Wings mosaic for the FRT42D, miR-8JK22 allele or a control FRT42D chromosome were generated using heat-shock induced FLP/FRT mitotic recombination to generate miR-8 mutant (GFP-negative) and wild-type (GFP-positive) sibling clones and examined at 75h post induction at 25°C. Sibling clone sizes are compared in the wing pouch and represented as the percent of wing pouch area for over 100 clones, with the control FRT42D clones induced, cultured and measured in parallel. (I) The clone size distribution for miR-8 mutant clones and control FRT42D clones. miR-8 mutant clones show a greater variability in size and an increase in larger clones within the wing pouch. (J) Examples of wings used for sibling clone twinspot analysis are shown, DNA is labeled in blue by Hoechst 33258. (K) Tissues were induced to express a GFP alone (top) or GFP with a miR-8 sponge using en-Gal4/UAS, Gal80TS induced at 28°C for 70hr prior to the UV challenge. Wandering L3 larvae were exposed to 240mJ of UV and assayed 12hr later for cell death by Dcp-1 staining (red). Dcp-1 staining is significantly reduced in animals expressing the miR-8 sponge, although this is not restricted to the posterior wing only, suggesting some effects of reducing miR-8 may be non-compartment autonomous. (L) miR-8 homozygous mutant adult wings show only mild defects, such as the mild branching observed near the posterior cross-vein (arrow in M). (N) miR-8 mutant animals or parental miR8+/+ controls were reared on food with low doses of Paraquat (2mM PQ). For miR-8 null animals (JK22/JK22) 39% (7/18) exhibited wing and scutellar bristle defects while 0/8 control miR8+/+ animals grown on low PQ exhibited these defects.
Supplement to Fig. 3 (A) GFP-labeled miR8-expressing clones or (B) miR-8 + BskDN-expressing clones were generated in parallel using tub>CD2>Gal4/UAS with heat-shock induced recombination 72h prior to wing dissection at L3. Cells expressing miR-8 are almost completely eliminated from the wing pouch epithelium by 72h, while cell expressing miR-8 + BskDN are partially rescued from elimination in the pouch, but exhibit basal localization. (C–D) GFP-expressing clones or miR-8 + GFP-expressing clones were generated using tub>CD2>Gal4/UAS, Gal80TS with heat-shock induced recombination at 30hr of development and transgene induction at 28°C for 15h prior to wing dissection at L3. By 15h of miR-8 expression basally located pyknotic nuclei (arrow, D) can be observed in an optical x/z section. DNA is labeled by Hoechst 33258 and a sqh-RFP transgene provides counterstaining of the epithelial tissue boundaries (the peripodial cells on the apical side is oriented at top). (E,F) GFP-labeled clones expressing the apoptosis inhibitor P35 or miR+P35 were generated using heat-shock induced recombination of the act>CD2>Gal4/UAS system 72h prior to tissue dissection. (E) P35 has no effect on the apico-basal location of GFP expressing control clones, (F) while P35 co-expression fully prevents elimination of miR-8 expressing clones from the wing pouch but these clones exhibit full basal extrusion from the wing pouch epithelium by 72h. Optical x/z sections are shown with apical to top. DNA (blue) is labeled with Hoechst 33258 and f-actin is labeled with phalloidin (red).
Supplement to Fig. 5 Control genotypes for Figure 5 are shown. (A) GFP-labeled clones expressing P35 or (B) miR-8 without P35 were generated by heat-shock induced recombination using act>CD2>Gal4/UAS 72h prior to dissection at wandering L3. (A) Control clones expressing P35 show no alterations in E-cad or integrin (βPS1). (B) Clones expressing miR-8 show no changes to apical E-Cad or βPS1 but in x/z optical sections show a basal location and holes of E-Cad (indicated by arrows), suggesting basal extrusion. (C) Control clones expressing P35 only show no alterations in Sparc or MMP1. (D) Clones expressing miR-8 without P35 exhibit increased apical Sparc, even in clones containing pyknotic nuclei and undergoing basal extrusion. (E) Control clones expressing GFP + P35 show normal apical localization of Armadillo (Arm) and apical mitoses indicated by PH3. (F) Expression of miR-8 driven in the posterior wing during pupal stages (for 30h) disrupts proper formation of actin rich wing hairs. (G) Clones expressing miR-8 for 48h in the larval wing show no decrease in Zfh1 protein levels at wandering L3, rather a moderate increase is observed.
Supplement to Fig. 6 GFP-labeled clones overexpressing miR-8 plus the indicated transgenes were generated by heat-shock induced recombination using tub>CD2>Gal4/UAS, Gal80TS with miR-8 expression induced at 28°C for 72h prior to dissection at wandering L3. (A) miR-8 expression reduces apical f-actin at the dorso-ventral wing margin. (B) Co-expressing the known miR-8 target Enabled (ena) does not rescue the reduced f-actin at the margin (C) or miR-8 induced apoptosis as indicated by Dcp-1 staining. (D) Ena expression was confirmed in miR-8 co-expressing clones by anti-Ena staining. (E) High levels of TCF expression alone does not induce apoptosis. (F) Co-expression of miR-8 with the known target TCF does not rescue the apoptosis of miR-8 expressing cells, (G) nor the basal location of many dying miR-8 expressing cells. (H) Clones expressing miR-8 for 48h in the larval wing show no decrease in Spitz protein levels. (I) Co-transfection of Kc167 cells with an Ac-miR-8 vector causes decreased LacZ expression of a LacZ reporter containing the 3′UTR of Sra-1 cloned downstream of the LacZ coding sequence. Mutation of the putative miR-8 binding site in the Sra-1 3′UTR reporter prevents the decrease caused by miR-8 co-transfection. LacZ activity is reported as fold activation relative to 3′UTR reporter activity with the control pAc empty vector alone, error bars indicate standard deviation.
Supplement to Fig. 7 (A) Clones overexpressing miR-8 for 48h (generated using tub>CD2>Gal4/UAS, Gal80TS) do not display reduced Jar. (B) Clones overexpressing jarRNAi, (C) enaRNAi (D) Sra-1RNAi and (F) sqhRNAi effectively knock-down their targets, and this also serves to validate the antibodies. (E) Overexpression of enaRNAi in the posterior wing for 48h using en-Gal4, Gal80TS reduces f-actin as shown by phalloidin staining. (G) jarRNAi (H), Sra-1RNAi (I) or sqhRNAi alone had no obvious effect on f-actin levels, however, sqhRNAi led to a basal location of cells and a rounded morphology similar to that of dying and extruded miR-8 expressing cells. (J) Apoptosis of cells expressing sqhRNAi in the posterior wing for 48h (using en-Gal4, Gal80TS) was confirmed by Dcp-1 staining.
Highlights.
miR-8 impacts cell survival and targets cytoskeletal regulators in Drosophila
miR-8 alters apoptosis resistance in Drosophila independent of an epithelial-to-mesenchymal transition
Persistence of cells with supra-physiological miR-8 disrupts epithelial organization.
Acknowledgments
We thank Dr. A. Miller, Dr. K. Cadigan, the Buttitta and Kennell lab members for helpful discussions. We thank Dr. E. Moreno, Dr. M. Ringuette, Dr. A. Gautreau, Dr. M. Kesells, Dr. R. Ward, IV and Dr. K. Miller for kindly providing antibodies. Additional stocks and antibodies were obtained from the Bloomington Drosophila Stock Center, the Vienna Drosophila RNAi Center and the Developmental Studies Hybridoma Bank (DSHB). Work in the Buttitta Lab was supported by NIH R00 GM086517 and startup funding from the University of Michigan, J.K. was supported by NIH F32GM074465 and R15 GM101598.
Footnotes
Author contributions:
K.B. and L.B. conceived of the project. K.B., N.R., K.M., K.P., J.K. and L.B. performed the experiments. L.B. and J.K wrote the manuscript with assistance from N.R. and K.B. The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi-Yamada T, Fujimura-Kamada K, Nishida Y, Matsumoto K. Distortion of proximodistal information causes JNK-dependent apoptosis in Drosophila wing. Nature. 1999;400:166–169. doi: 10.1038/22112. [DOI] [PubMed] [Google Scholar]
- Adam L, Zhong M, Choi W, Qi W, Nicoloso M, Arora A, Calin G, Wang H, Siefker-Radtke A, McConkey D, Bar-Eli M, Dinney C. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin Cancer Res. 2009;15:5060–5072. doi: 10.1158/1078-0432.CCR-08-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldaz S, Escudero LM, Freeman M. Dual role of myosin II during Drosophila imaginal disc metamorphosis. Nat Commun. 2013;4:1761. doi: 10.1038/ncomms2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpers CE, Hudkins KL, Segerer S, Sage EH, Pichler R, Couser WG, Johnson RJ, Bassuk JA. Localization of SPARC in developing, mature, and chronically injured human allograft kidneys. Kidney Int. 2002;62:2073–2086. doi: 10.1046/j.1523-1755.2002.00680.x. [DOI] [PubMed] [Google Scholar]
- Antonello ZA, Reiff T, Ballesta-Illan E, Dominguez M. Robust intestinal homeostasis relies on cellular plasticity in enteroblasts mediated by miR-8-Escargot switch. EMBO J. 2015;34:2025–2041. doi: 10.15252/embj.201591517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker TH, Baneyx G, Cardo-Vila M, Workman GA, Weaver M, Menon PM, Dedhar S, Rempel SA, Arap W, Pasqualini R, Vogel V, Sage EH. SPARC regulates extracellular matrix organization through its modulation of integrin-linked kinase activity. J Biol Chem. 2005;280:36483–36493. doi: 10.1074/jbc.M504663200. [DOI] [PubMed] [Google Scholar]
- Belgardt BF, Ahmed K, Spranger M, Latreille M, Denzler R, Kondratiuk N, von Meyenn F, Villena FN, Herrmanns K, Bosco D, Kerr-Conte J, Pattou F, Rulicke T, Stoffel M. The microRNA-200 family regulates pancreatic beta cell survival in type 2 diabetes. Nat Med. 2015;21:619–627. doi: 10.1038/nm.3862. [DOI] [PubMed] [Google Scholar]
- Blauwkamp TA, Chang MV, Cadigan KM. Novel TCF-binding sites specify transcriptional repression by Wnt signalling. EMBO J. 2008;27:1436–1446. doi: 10.1038/emboj.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz S, Brabletz T. The ZEB/miR-200 feedback loop--a motor of cellular plasticity in development and cancer? EMBO Rep. 2010;11:670–677. doi: 10.1038/embor.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, Goodall GJ. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 2008;68:7846–7854. doi: 10.1158/0008-5472.CAN-08-1942. [DOI] [PubMed] [Google Scholar]
- Bracken CP, Khew-Goodall Y, Goodall GJ. Network-Based Approaches to Understand the Roles of miR-200 and Other microRNAs in Cancer. Cancer Res. 2015;75:2594–2599. doi: 10.1158/0008-5472.CAN-15-0287. [DOI] [PubMed] [Google Scholar]
- Bracken CP, Li X, Wright JA, Lawrence DM, Pillman KA, Salmanidis M, Anderson MA, Dredge BK, Gregory PA, Tsykin A, Neilsen C, Thomson DW, Bert AG, Leerberg JM, Yap AS, Jensen KB, Khew-Goodall Y, Goodall GJ. Genome-wide identification of miR-200 targets reveals a regulatory network controlling cell invasion. EMBO J. 2014;33:2040–2056. doi: 10.15252/embj.201488641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky MH, Nordstrom W, Tsang G, Kwan E, Rubin GM, Abrams JM. Drosophila p53 binds a damage response element at the reaper locus. Cell. 2000;101:103–113. doi: 10.1016/S0092-8674(00)80627-3. [DOI] [PubMed] [Google Scholar]
- Buttitta LA, Katzaroff AJ, Perez CL, de la Cruz A, Edgar BA. A double-assurance mechanism controls cell cycle exit upon terminal differentiation in Drosophila. Dev Cell. 2007;12:631–643. doi: 10.1016/j.devcel.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Kiehart DP. Septins may form a ubiquitous family of cytoskeletal filaments. J Cell Biol. 1996;134:1345–1348. doi: 10.1083/jcb.134.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez MA, Valdecanas D, Zhang X, Zhan Y, Bhardwaj V, Calin GA, Komaki R, Giri DK, Quini CC, Wolfe T, Peltier HJ, Bader AG, Heymach JV, Meyn RE, Welsh JW. Therapeutic delivery of miR-200c enhances radiosensitivity in lung cancer. Mol Ther. 2014;22:1494–1503. doi: 10.1038/mt.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekanty A, Barrio L, Milan M. Contributions of DNA repair, cell cycle checkpoints and cell death to suppressing the DNA damage-induced tumorigenic behavior of Drosophila epithelial cells. Oncogene. 2015;34:978–985. doi: 10.1038/onc.2014.42. [DOI] [PubMed] [Google Scholar]
- Dekanty A, Barrio L, Muzzopappa M, Auer H, Milan M. Aneuploidy-induced delaminating cells drive tumorigenesis in Drosophila epithelia. Proc Natl Acad Sci U S A. 2012;109:20549–20554. doi: 10.1073/pnas.1206675109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JB. GAL4 system in Drosophila: a fly geneticist’s Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, Berx G, Cano A, Beug H, Foisner R. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24:2375–2385. doi: 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- Emori Y, Saigo K. Calpain localization changes in coordination with actin-related cytoskeletal changes during early embryonic development of Drosophila. J Biol Chem. 1994;269:25137–25142. [PubMed] [Google Scholar]
- Fan Y, Bergmann A. The cleaved-Caspase-3 antibody is a marker of Caspase-9-like DRONC activity in Drosophila. Cell Death Differ. 2010;17:534–539. doi: 10.1038/cdd.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Wang R, Song HZ, Chen LB. MicroRNA-200b reverses chemoresistance of docetaxel-resistant human lung adenocarcinoma cells by targeting E2F3. Cancer. 2012;118:3365–3376. doi: 10.1002/cncr.26560. [DOI] [PubMed] [Google Scholar]
- Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, Schwabe RF, Vahdat LT, Altorki NK, Mittal V, Gao D. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegel K, Sun D, Grushko O, Ma Y, Buttitta L. Live Cell Cycle Analysis of Drosophila Tissues using the Attune Acoustic Focusing Cytometer and Vybrant DyeCycle Violet DNA Stain. J Vis Exp. 2013 doi: 10.3791/50239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Founounou N, Loyer N, Le Borgne R. Septins regulate the contractility of the actomyosin ring to enable adherens junction remodeling during cytokinesis of epithelial cells. Dev Cell. 2013;24:242–255. doi: 10.1016/j.devcel.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Gautier JJ, Lomakina ME, Bouslama-Oueghlani L, Derivery E, Beilinson H, Faigle W, Loew D, Louvard D, Echard A, Alexandrova AY, Baum B, Gautreau A. Clathrin is required for Scar/Wave-mediated lamellipodium formation. J Cell Sci. 2011;124:3414–3427. doi: 10.1242/jcs.081083. [DOI] [PubMed] [Google Scholar]
- Geisbrecht ER, Montell DJ. Myosin VI is required for E-cadherin-mediated border cell migration. Nat Cell Biol. 2002;4:616–620. doi: 10.1038/ncb830. [DOI] [PubMed] [Google Scholar]
- Giraldez AJ, Cohen SM. Wingless and Notch signaling provide cell survival cues and control cell proliferation during wing development. Development. 2003;130:6533–6543. doi: 10.1242/dev.00904. [DOI] [PubMed] [Google Scholar]
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008a;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- Gregory PA, Bracken CP, Bert AG, Goodall GJ. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle. 2008b;7:3112–3118. doi: 10.4161/cc.7.20.6851. [DOI] [PubMed] [Google Scholar]
- Guo L, Wang J, Yang P, Lu Q, Zhang T, Yang Y. MicroRNA-200 promotes lung cancer cell growth through FOG2-independent AKT activation. IUBMB Life. 2015;67:720–725. doi: 10.1002/iub.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill L, Browne G, Tulchinsky E. ZEB/miR-200 feedback loop: at the crossroads of signal transduction in cancer. Int J Cancer. 2013;132:745–754. doi: 10.1002/ijc.27708. [DOI] [PubMed] [Google Scholar]
- Howe EN, Cochrane DR, Richer JK. Targets of miR-200c mediate suppression of cell motility and anoikis resistance. Breast Cancer Res. 2011;13:R45. doi: 10.1186/bcr2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AE, Feng WC, Delostrinos CF, Carmean N, Bassuk JA. Spreading of embryologically distinct urothelial cells is inhibited by SPARC. J Cell Physiol. 2005;202:453–463. doi: 10.1002/jcp.20140. [DOI] [PubMed] [Google Scholar]
- Hyun S, Lee JH, Jin H, Nam J, Namkoong B, Lee G, Chung J, Kim VN. Conserved MicroRNA miR-8/miR-200 and its target USH/FOG2 control growth by regulating PI3K. Cell. 2009;139:1096–1108. doi: 10.1016/j.cell.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Isabella AJ, Horne-Badovinac S. Dynamic regulation of basement membrane protein levels promotes egg chamber elongation in Drosophila. Dev Biol. 2015;406:212–221. doi: 10.1016/j.ydbio.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaji M, Lenartowska M, Noguchi T, Frank DJ, Miller KG. Myosin VI regulates actin structure specialization through conserved cargo-binding domain sites. PLoS One. 2011;6:e22755. doi: 10.1371/journal.pone.0022755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Kim VN, Hyun S. Conserved microRNA miR-8 controls body size in response to steroid signaling in Drosophila. Genes Dev. 2012;26:1427–1432. doi: 10.1101/gad.192872.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Xiong S, Li Z, Wu J, Zhou L, Gui JF, Mei J. A feedback regulatory loop involving p53/miR-200 and growth hormone endocrine axis controls embryo size of zebrafish. Sci Rep. 2015;5:15906. doi: 10.1038/srep15906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LA, Sanders AL. Wingless promotes cell survival but constrains growth during Drosophila wing development. Nat Cell Biol. 2003;5:827–833. doi: 10.1038/ncb1041. [DOI] [PubMed] [Google Scholar]
- Jordan P, Karess R. Myosin light chain-activating phosphorylation sites are required for oogenesis in Drosophila. J Cell Biol. 1997;139:1805–1819. doi: 10.1083/jcb.139.7.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurmeister S, Baumann M, Balwierz A, Keklikoglou I, Ward A, Uhlmann S, Zhang JD, Wiemann S, Sahin O. MicroRNA-200c represses migration and invasion of breast cancer cells by targeting actin-regulatory proteins FHOD1 and PPM1F. Mol Cell Biol. 2012;32:633–651. doi: 10.1128/MCB.06212-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Bashirullah A. A steroid-controlled global switch in sensitivity to apoptosis during Drosophila development. Dev Biol. 2014;386:34–41. doi: 10.1016/j.ydbio.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karres JS, Hilgers V, Carrera I, Treisman J, Cohen SM. The conserved microRNA miR-8 tunes atrophin levels to prevent neurodegeneration in Drosophila. Cell. 2007;131:136–145. doi: 10.1016/j.cell.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Kellerman KA, Miller KG. An unconventional myosin heavy chain gene from Drosophila melanogaster. J Cell Biol. 1992;119:823–834. doi: 10.1083/jcb.119.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell JA, Cadigan KM, Shakhmantsir I, Waldron EJ. The microRNA miR-8 is a positive regulator of pigmentation and eclosion in Drosophila. Dev Dyn. 2012;241:161–168. doi: 10.1002/dvdy.23705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell JA, Gerin I, MacDougald OA, Cadigan KM. The microRNA miR-8 is a conserved negative regulator of Wnt signaling. Proc Natl Acad Sci U S A. 2008;105:15417–15422. doi: 10.1073/pnas.0807763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knezevic J, Pfefferle AD, Petrovic I, Greene SB, Perou CM, Rosen JM. Expression of miR-200c in claudin-low breast cancer alters stem cell functionality, enhances chemosensitivity and reduces metastatic potential. Oncogene. 2015;34:5997–6006. doi: 10.1038/onc.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch N, Dharmalingam E, Westermann M, Qualmann B, Thomas U, Kessels MM. Abp1 utilizes the Arp2/3 complex activator Scar/WAVE in bristle development. J Cell Sci. 2012;125:3578–3589. doi: 10.1242/jcs.101451. [DOI] [PubMed] [Google Scholar]
- Kunda P, Craig G, Dominguez V, Baum B. Abi, Sra1, and Kette control the stability and localization of SCAR/WAVE to regulate the formation of actin-based protrusions. Curr Biol. 2003;13:1867–1875. doi: 10.1016/j.cub.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Lamouille S, Subramanyam D, Blelloch R, Derynck R. Regulation of epithelial-mesenchymal and mesenchymal-epithelial transitions by microRNAs. Curr Opin Cell Biol. 2013;25:200–207. doi: 10.1016/j.ceb.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit T, Yap AS. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat Cell Biol. 2015;17:533–539. doi: 10.1038/ncb3136. [DOI] [PubMed] [Google Scholar]
- Li A, Omura N, Hong SM, Vincent A, Walter K, Griffith M, Borges M, Goggins M. Pancreatic cancers epigenetically silence SIP1 and hypomethylate and overexpress miR-200a/200b in association with elevated circulating miR-200a and miR-200b levels. Cancer Res. 2010;70:5226–5237. doi: 10.1158/0008-5472.CAN-09-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Roslan S, Johnstone CN, Wright JA, Bracken CP, Anderson M, Bert AG, Selth LA, Anderson RL, Goodall GJ, Gregory PA, Khew-Goodall Y. MiR-200 can repress breast cancer metastasis through ZEB1-independent but moesin-dependent pathways. Oncogene. 2014;33:4077–4088. doi: 10.1038/onc.2013.370. [DOI] [PubMed] [Google Scholar]
- Lin HP, Chen HM, Wei SY, Chen LY, Chang LH, Sun YJ, Huang SY, Hsu JC. Cell adhesion molecule Echinoid associates with unconventional myosin VI/Jaguar motor to regulate cell morphology during dorsal closure in Drosophila. Dev Biol. 2007;311:423–433. doi: 10.1016/j.ydbio.2007.08.043. [DOI] [PubMed] [Google Scholar]
- Loya CM, Lu CS, Van Vactor D, Fulga TA. Transgenic microRNA inhibition with spatiotemporal specificity in intact organisms. Nat Methods. 2009;6:897–903. doi: 10.1038/nmeth.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loya CM, McNeill EM, Bao H, Zhang B, Van Vactor D. miR-8 controls synapse structure by repression of the actin regulator enabled. Development. 2014;141:1864–1874. doi: 10.1242/dev.105791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Blanco E, Gampel A, Ring J, Virdee K, Kirov N, Tolkovsky AM, Martinez-Arias A. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 1998;12:557–570. doi: 10.1101/gad.12.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinek N, Shahab J, Saathoff M, Ringuette M. Haemocyte-derived SPARC is required for collagen-IV-dependent stability of basal laminae in Drosophila embryos. J Cell Sci. 2008;121:1671–1680. doi: 10.1242/jcs.021931. [DOI] [PubMed] [Google Scholar]
- Mateescu B, Batista L, Cardon M, Gruosso T, de Feraudy Y, Mariani O, Nicolas A, Meyniel JP, Cottu P, Sastre-Garau X, Mechta-Grigoriou F. miR-141 and miR-200a act on ovarian tumorigenesis by controlling oxidative stress response. Nat Med. 2011;17:1627–1635. doi: 10.1038/nm.2512. [DOI] [PubMed] [Google Scholar]
- Matus DQ, Lohmer LL, Kelley LC, Schindler AJ, Kohrman AQ, Barkoulas M, Zhang W, Chi Q, Sherwood DR. Invasive Cell Fate Requires G1 Cell-Cycle Arrest and Histone Deacetylase-Mediated Changes in Gene Expression. Dev Cell. 2015;35:162–174. doi: 10.1016/j.devcel.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrakis M, Azou-Gros Y, Tsai FC, Alvarado J, Bertin A, Iv F, Kress A, Brasselet S, Koenderink GH, Lecuit T. Septins promote F-actin ring formation by crosslinking actin filaments into curved bundles. Nat Cell Biol. 2014;16:322–334. doi: 10.1038/ncb2921. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004;2004:pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- McNamee LM, Brodsky MH. p53-independent apoptosis limits DNA damage-induced aneuploidy. Genetics. 2009;182:423–435. doi: 10.1534/genetics.109.102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollereau B, Perez-Garijo A, Bergmann A, Miura M, Gerlitz O, Ryoo HD, Steller H, Morata G. Compensatory proliferation and apoptosis-induced proliferation: a need for clarification. Cell Death Differ. 2013;20:181. doi: 10.1038/cdd.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongroo PS, Rustgi AK. The role of the miR-200 family in epithelial-mesenchymal transition. Cancer Biol Ther. 2010;10:219–222. doi: 10.4161/cbt.10.6312548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier B, Gettings M, Gay G, Mangeat T, Schott S, Guarner A, Suzanne M. Apico-basal forces exerted by apoptotic cells drive epithelium folding. Nature. 2015;518:245–248. doi: 10.1038/nature14152. [DOI] [PubMed] [Google Scholar]
- Morante J, Vallejo DM, Desplan C, Dominguez M. Conserved miR-8/miR-200 defines a glial niche that controls neuroepithelial expansion and neuroblast transition. Dev Cell. 2013;27:174–187. doi: 10.1016/j.devcel.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim JH, Kim JW, Kim S. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res. 2008;14:2690–2695. doi: 10.1158/1078-0432.CCR-07-1731. [DOI] [PubMed] [Google Scholar]
- Neufeld TP, de la Cruz AF, Johnston LA, Edgar BA. Coordination of growth and cell division in the Drosophila wing. Cell. 1998;93:1183–1193. doi: 10.1016/s0092-8674(00)81462-2. [DOI] [PubMed] [Google Scholar]
- Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Pareja JC, Grawe F, Martin-Blanco E, Garcia-Bellido A. Invasive cell behavior during Drosophila imaginal disc eversion is mediated by the JNK signaling cascade. Dev Cell. 2004;7:387–399. doi: 10.1016/j.devcel.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Perez-Garijo A, Shlevkov E, Morata G. The role of Dpp and Wg in compensatory proliferation and in the formation of hyperplastic overgrowths caused by apoptotic cells in the Drosophila wing disc. Development. 2009;136:1169–1177. doi: 10.1242/dev.034017. [DOI] [PubMed] [Google Scholar]
- Pogribny IP, Filkowski JN, Tryndyak VP, Golubov A, Shpyleva SI, Kovalchuk O. Alterations of microRNAs and their targets are associated with acquired resistance of MCF-7 breast cancer cells to cisplatin. Int J Cancer. 2010;127:1785–1794. doi: 10.1002/ijc.25191. [DOI] [PubMed] [Google Scholar]
- Portela M, Casas-Tinto S, Rhiner C, Lopez-Gay JM, Dominguez O, Soldini D, Moreno E. Drosophila SPARC is a self-protective signal expressed by loser cells during cell competition. Dev Cell. 2010;19:562–573. doi: 10.1016/j.devcel.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Poulton JS, Cuningham JC, Peifer M. Acentrosomal Drosophila epithelial cells exhibit abnormal cell division, leading to cell death and compensatory proliferation. Dev Cell. 2014;30:731–745. doi: 10.1016/j.devcel.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prober DA, Edgar BA. Interactions between Ras1, dMyc, and dPI3K signaling in the developing Drosophila wing. Genes Dev. 2002;16:2286–2299. doi: 10.1101/gad.991102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudrapatna VA, Bangi E, Cagan RL. Caspase signalling in the absence of apoptosis drives Jnk-dependent invasion. EMBO Rep. 2013;14:172–177. doi: 10.1038/embor.2012.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo HD, Bergmann A, Gonen H, Ciechanover A, Steller H. Regulation of Drosophila IAP1 degradation and apoptosis by reaper and ubcD1. Nat Cell Biol. 2002;4:432–438. doi: 10.1038/ncb795. [DOI] [PubMed] [Google Scholar]
- Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell. 2004;7:491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Shen J, Dahmann C. Extrusion of cells with inappropriate Dpp signaling from Drosophila wing disc epithelia. Science. 2005;307:1789–1790. doi: 10.1126/science.1104784. [DOI] [PubMed] [Google Scholar]
- Shi Q, Bao S, Song L, Wu Q, Bigner DD, Hjelmeland AB, Rich JN. Targeting SPARC expression decreases glioma cellular survival and invasion associated with reduced activities of FAK and ILK kinases. Oncogene. 2007;26:4084–4094. doi: 10.1038/sj.onc.1210181. [DOI] [PubMed] [Google Scholar]
- Shlevkov E, Morata G. A dp53/JNK-dependant feedback amplification loop is essential for the apoptotic response to stress in Drosophila. Cell Death Differ. 2012;19:451–460. doi: 10.1038/cdd.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebzehnrubl FA, Silver DJ, Tugertimur B, Deleyrolle LP, Siebzehnrubl D, Sarkisian MR, Devers KG, Yachnis AT, Kupper MD, Neal D, Nabilsi NH, Kladde MP, Suslov O, Brabletz S, Brabletz T, Reynolds BA, Steindler DA. The ZEB1 pathway links glioblastoma initiation, invasion and chemoresistance. EMBO Mol Med. 2013;5:1196–1212. doi: 10.1002/emmm.201302827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodek J, Zhu B, Huynh MH, Brown TJ, Ringuette M. Novel functions of the matricellular proteins osteopontin and osteonectin/SPARC. Connect Tissue Res. 2002;43:308–319. doi: 10.1080/03008200290001050. [DOI] [PubMed] [Google Scholar]
- Song Z, McCall K, Steller H. DCP-1, a Drosophila cell death protease essential for development. Science. 1997;275:536–540. doi: 10.1126/science.275.5299.536. [DOI] [PubMed] [Google Scholar]
- Sossey-Alaoui K, Bialkowska K, Plow EF. The miR200 family of microRNAs regulates WAVE3-dependent cancer cell invasion. J Biol Chem. 2009;284:33019–33029. doi: 10.1074/jbc.M109.034553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Pastor-Pareja JC, Igaki T, Pagliarini R, Xu T. Basement membrane remodeling is essential for Drosophila disc eversion and tumor invasion. Proc Natl Acad Sci U S A. 2007;104:2721–2726. doi: 10.1073/pnas.0611666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens LJ, Page-McCaw A. A secreted MMP is required for reepithelialization during wound healing. Mol Biol Cell. 2012;23:1068–1079. doi: 10.1091/mbc.E11-09-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamori Y, Bialucha CU, Tian AG, Kajita M, Huang YC, Norman M, Harrison N, Poulton J, Ivanovitch K, Disch L, Liu T, Deng WM, Fujita Y. Involvement of Lgl and Mahjong/VprBP in cell competition. PLoS Biol. 2010;8:e1000422. doi: 10.1371/journal.pbio.1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlirova M, Bohmann D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 2006;25:5294–5304. doi: 10.1038/sj.emboj.7601401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo DM, Caparros E, Dominguez M. Targeting Notch signalling by the conserved miR-8/200 microRNA family in development and cancer cells. EMBO J. 2011;30:756–769. doi: 10.1038/emboj.2010.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver MS, Workman G, Sage EH. The copper binding domain of SPARC mediates cell survival in vitro via interaction with integrin beta1 and activation of integrin-linked kinase. J Biol Chem. 2008;283:22826–22837. doi: 10.1074/jbc.M706563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley MI, Setiawan L, Hariharan IK. TIE-DYE: a combinatorial marking system to visualize and genetically manipulate clones during development in Drosophila melanogaster. Development. 2013;140:3275–3284. doi: 10.1242/dev.096057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Pastor-Pareja JC, Xu T. Interaction between Ras(V12) and scribbled clones induces tumour growth and invasion. Nature. 2010;463:545–548. doi: 10.1038/nature08702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SK, Lagendijk AK, Hogan BM, Gomez GA, Yap AS. Active contractility at E-cadherin junctions and its implications for cell extrusion in cancer. Cell Cycle. 2015;14:315–322. doi: 10.4161/15384101.2014.989127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Zallen JA, Cohen Y, Hudson AM, Cooley L, Wieschaus E, Schejter ED. SCAR is a primary regulator of Arp2/3-dependent morphological events in Drosophila. J Cell Biol. 2002;156:689–701. doi: 10.1083/jcb.200109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman SG, Thorpe LM, Medrano VR, Mallozzi CA, McCartney BM. Apical constriction and invagination downstream of the canonical Wnt signaling pathway require Rho1 and Myosin II. Dev Biol. 2010;340:54–66. doi: 10.1016/j.ydbio.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement to Figure 1. (A–F) GFP-labeled clones expressing GFP alone or GFP with miR-8 were generated using the tub>CD2>Gal4/UAS, Gal80TS system with heat-shock induced recombination from 38–80 hr of development and transgene induction at 28°C for 24–72hr prior to wing disc dissection at wandering third larval instar (L3). F-actin is shown by phalloidin staining in blue and anti-Dcp-1 positivity is shown in red. miR-8 overexpressing clones are positive for Dcp-1 after 24h of miR-8 expression. We observed no increases in Dcp-1 positivity in control GFP expressing wings, even after 72h at 28°C. (G) GFP-labeled miR-8-expressing clones were generated using tub>CD2>Gal4/UAS with heat-shock induced recombination 48h prior to pupariation. Adult wings expressing miR-8 mosaically show defects including notching at the wing margin and disrupted planar polarity. (H) Wings mosaic for the FRT42D, miR-8JK22 allele or a control FRT42D chromosome were generated using heat-shock induced FLP/FRT mitotic recombination to generate miR-8 mutant (GFP-negative) and wild-type (GFP-positive) sibling clones and examined at 75h post induction at 25°C. Sibling clone sizes are compared in the wing pouch and represented as the percent of wing pouch area for over 100 clones, with the control FRT42D clones induced, cultured and measured in parallel. (I) The clone size distribution for miR-8 mutant clones and control FRT42D clones. miR-8 mutant clones show a greater variability in size and an increase in larger clones within the wing pouch. (J) Examples of wings used for sibling clone twinspot analysis are shown, DNA is labeled in blue by Hoechst 33258. (K) Tissues were induced to express a GFP alone (top) or GFP with a miR-8 sponge using en-Gal4/UAS, Gal80TS induced at 28°C for 70hr prior to the UV challenge. Wandering L3 larvae were exposed to 240mJ of UV and assayed 12hr later for cell death by Dcp-1 staining (red). Dcp-1 staining is significantly reduced in animals expressing the miR-8 sponge, although this is not restricted to the posterior wing only, suggesting some effects of reducing miR-8 may be non-compartment autonomous. (L) miR-8 homozygous mutant adult wings show only mild defects, such as the mild branching observed near the posterior cross-vein (arrow in M). (N) miR-8 mutant animals or parental miR8+/+ controls were reared on food with low doses of Paraquat (2mM PQ). For miR-8 null animals (JK22/JK22) 39% (7/18) exhibited wing and scutellar bristle defects while 0/8 control miR8+/+ animals grown on low PQ exhibited these defects.
Supplement to Fig. 3 (A) GFP-labeled miR8-expressing clones or (B) miR-8 + BskDN-expressing clones were generated in parallel using tub>CD2>Gal4/UAS with heat-shock induced recombination 72h prior to wing dissection at L3. Cells expressing miR-8 are almost completely eliminated from the wing pouch epithelium by 72h, while cell expressing miR-8 + BskDN are partially rescued from elimination in the pouch, but exhibit basal localization. (C–D) GFP-expressing clones or miR-8 + GFP-expressing clones were generated using tub>CD2>Gal4/UAS, Gal80TS with heat-shock induced recombination at 30hr of development and transgene induction at 28°C for 15h prior to wing dissection at L3. By 15h of miR-8 expression basally located pyknotic nuclei (arrow, D) can be observed in an optical x/z section. DNA is labeled by Hoechst 33258 and a sqh-RFP transgene provides counterstaining of the epithelial tissue boundaries (the peripodial cells on the apical side is oriented at top). (E,F) GFP-labeled clones expressing the apoptosis inhibitor P35 or miR+P35 were generated using heat-shock induced recombination of the act>CD2>Gal4/UAS system 72h prior to tissue dissection. (E) P35 has no effect on the apico-basal location of GFP expressing control clones, (F) while P35 co-expression fully prevents elimination of miR-8 expressing clones from the wing pouch but these clones exhibit full basal extrusion from the wing pouch epithelium by 72h. Optical x/z sections are shown with apical to top. DNA (blue) is labeled with Hoechst 33258 and f-actin is labeled with phalloidin (red).
Supplement to Fig. 5 Control genotypes for Figure 5 are shown. (A) GFP-labeled clones expressing P35 or (B) miR-8 without P35 were generated by heat-shock induced recombination using act>CD2>Gal4/UAS 72h prior to dissection at wandering L3. (A) Control clones expressing P35 show no alterations in E-cad or integrin (βPS1). (B) Clones expressing miR-8 show no changes to apical E-Cad or βPS1 but in x/z optical sections show a basal location and holes of E-Cad (indicated by arrows), suggesting basal extrusion. (C) Control clones expressing P35 only show no alterations in Sparc or MMP1. (D) Clones expressing miR-8 without P35 exhibit increased apical Sparc, even in clones containing pyknotic nuclei and undergoing basal extrusion. (E) Control clones expressing GFP + P35 show normal apical localization of Armadillo (Arm) and apical mitoses indicated by PH3. (F) Expression of miR-8 driven in the posterior wing during pupal stages (for 30h) disrupts proper formation of actin rich wing hairs. (G) Clones expressing miR-8 for 48h in the larval wing show no decrease in Zfh1 protein levels at wandering L3, rather a moderate increase is observed.
Supplement to Fig. 6 GFP-labeled clones overexpressing miR-8 plus the indicated transgenes were generated by heat-shock induced recombination using tub>CD2>Gal4/UAS, Gal80TS with miR-8 expression induced at 28°C for 72h prior to dissection at wandering L3. (A) miR-8 expression reduces apical f-actin at the dorso-ventral wing margin. (B) Co-expressing the known miR-8 target Enabled (ena) does not rescue the reduced f-actin at the margin (C) or miR-8 induced apoptosis as indicated by Dcp-1 staining. (D) Ena expression was confirmed in miR-8 co-expressing clones by anti-Ena staining. (E) High levels of TCF expression alone does not induce apoptosis. (F) Co-expression of miR-8 with the known target TCF does not rescue the apoptosis of miR-8 expressing cells, (G) nor the basal location of many dying miR-8 expressing cells. (H) Clones expressing miR-8 for 48h in the larval wing show no decrease in Spitz protein levels. (I) Co-transfection of Kc167 cells with an Ac-miR-8 vector causes decreased LacZ expression of a LacZ reporter containing the 3′UTR of Sra-1 cloned downstream of the LacZ coding sequence. Mutation of the putative miR-8 binding site in the Sra-1 3′UTR reporter prevents the decrease caused by miR-8 co-transfection. LacZ activity is reported as fold activation relative to 3′UTR reporter activity with the control pAc empty vector alone, error bars indicate standard deviation.
Supplement to Fig. 7 (A) Clones overexpressing miR-8 for 48h (generated using tub>CD2>Gal4/UAS, Gal80TS) do not display reduced Jar. (B) Clones overexpressing jarRNAi, (C) enaRNAi (D) Sra-1RNAi and (F) sqhRNAi effectively knock-down their targets, and this also serves to validate the antibodies. (E) Overexpression of enaRNAi in the posterior wing for 48h using en-Gal4, Gal80TS reduces f-actin as shown by phalloidin staining. (G) jarRNAi (H), Sra-1RNAi (I) or sqhRNAi alone had no obvious effect on f-actin levels, however, sqhRNAi led to a basal location of cells and a rounded morphology similar to that of dying and extruded miR-8 expressing cells. (J) Apoptosis of cells expressing sqhRNAi in the posterior wing for 48h (using en-Gal4, Gal80TS) was confirmed by Dcp-1 staining.