Abstract
The outcomes of flexor tendon repair are highly variable. As recent efforts to improve healing have demonstrated promise for growth factor- and cell-based therapies, the objective of the current study was to enhance repair via application of autologous adipose derived stromal cells (ASCs) and the tenogenic growth factor bone morphogenetic protein (BMP) 12. Controlled delivery of cells and growth factor was achieved in a clinically relevant canine model using a nanofiber/fibrin-based scaffold. Control groups consisted of repair-only (no scaffold) and acellular scaffold. Repairs were evaluated after 28 days of healing using biomechanical, biochemical, and proteomics analyses. Range of motion was reduced in the groups that received scaffolds compared to normal. There was no effect of ASC+BMP12 treatment for range of motion or tensile properties outcomes versus repair-only. Biochemical assays demonstrated increased DNA, glycosaminoglycans, and crosslink concentration in all repair groups compared to normal, but no effect of ASC+BMP12. Total collagen was significantly decreased in the acellular scaffold group compared to normal and significantly increased in the ASC+BMP12 group compared to the acellular scaffold group. Proteomics analysis comparing healing tendons to uninjured tendons revealed significant increases in proteins associated with inflammation, stress response, and matrix degradation. Treatment with ASC+BMP12 amplified these unfavorable changes. In summary, the treatment approach used in this study induced a negative inflammatory reaction at the repair site leading to poor healing. Future approaches should consider cell and growth factor delivery methods that do not incite negative local reactions.
Keywords: growth factor, stem cell, tendon, proteomics
Clinical outcomes following the operative repair and rehabilitation of transected intrasynovial tendons are highly variable.1–3 Prior reports have indicated that the two primary factors leading to poor results are adhesion formation within the digital sheath and repair-site elongation and rupture.2,4–8 Experimental studies have shown that as many as 48% of flexor tendon repairs result in repair-site elongation or catastrophic failure.2,7,9,10 These complications are attributed to a slow accrual of repair-site strength and stiffness and to an increase in gliding resistance within the digital sheath during the first few weeks following tendon suture.4–8,11–14 The healing of paucicellular, hypovascular intrasynovial tendon appears to be limited by the relatively low levels of collagen synthesis and remodeling during the early stages of healing.15,16 While attempts to improve repair by modifying rehabilitation variables have been partially successful in reducing adhesions and improving strength, these measures have not been fully effective in avoiding the complications associated with Zone 2 flexor tendon injury.2,17
A number of recent reports have indicated that biological approaches, such as the application of growth factors and mesenchymal stem cells (MSCs), have the potential to improve tendon and ligament repair.11,13,18–20 While the administration of selected exogenous growth factors has been an important strategy for improving the structural properties of repaired tendons and other tissues, the use of this approach for the repair of intrasynovial flexor tendons has been only modestly successful.11,13,15,21 Similarly, while multipotent MSCs from a variety of adult tissues have been shown to have an excellent capacity to regenerate and to provide potent immunosuppressive and anti-inflammatory effects in vitro and in vivo,22,23 MSCs alone have been ineffective in improving the strength and stiffness and in reducing adhesion formation following the repair of intrasynovial tendons in vivo.20
Recent in vitro experiments have indicated that MSCs, when used in combination with growth factors specifically expressed in the developing tendon, have considerable promise for accelerating tendon healing.18,19,24 The growth factors bone morphogenetic protein (BMP) 12, BMP13, and BMP14, which are expressed in developing tendons and ligaments, have been shown to have the greatest potential for improving tendon healing.18,25–27 In a recent in vitro study, it was demonstrated that interposition of a multilayered collagen patch seeded with muscle-derived MSCs and BMP-14 (a.k.a. GDF-5) into the repair site enhanced flexor tendon healing compared with a similar patch using cells alone.19 The purpose of the current in vivo experiment was to explore the application of a tenogenic growth factor, BMP12, and adipose-derived stromal cells (ASCs), for the repair of intrasynovial flexor tendons in a clinically relevant large animal model. We hypothesized that ASCs, prompted towards tenogenesis with BMP12, would enhance extracellular matrix synthesis at the repair site, leading to improved biomechanical properties.
METHODS
Study Design
To determine the effects of autologous ASCs and BMP12 on intrasynovial flexor tendon healing in canines, three experimental groups were created. In the first group, the flexor digitorum profundus tendon was transected and repaired in Zone 2 (Repair-only group). In the second group, the flexor tendon was transected and a heparin/fibrin-based delivery system (HBDS)/nanofiber scaffold28 was implanted between the tendon stumps prior to repair (Scaffold group). In the third group, the flexor tendon was transected and a scaffold loaded with 7.5 µg BMP12 and 1 × 106 autologous ASCs was implanted into pockets within the tendon stumps (ASC+BMP12 group) prior to repair. The scaffolds were alternated between the second and fifth digits of the right forelimbs in 17 adult mongrel dogs. Intrasynovial tendons of the opposite forelimbs were used for some comparisons (Normal group).
Heparin/Fibrin-Based Delivery System/Nanofiber Scaffold
All of the materials in the scaffold are approved by the FDA for medical use. The scaffold design consists of multiple alternating layers of the HBDS and an oriented, electrospun nanofiber polylactic co-glycolic acid (PLGA) mat (85:15 L:G ratio).28 The HBDS includes a bidomain peptide with a factor-XIIIa substrate derived from α2-plasmin inhibitor at the N-terminus, and a C-terminal heparin-binding domain derived from antithrombin III (ATIII).29,30 The peptide is covalently cross-linked to a fibrin matrix during coagulation by the transglutaminase activity of factor XIII. The peptide immobilizes heparin electrostatically to the matrix, which in turn immobilizes heparin-binding growth factors (e.g., PDGF, BMP12), preventing their diffusion from the matrix. Fibrin matrices (30 µl matrix volume) were made with the following final component amounts: 7.5 µg BMP12 (Pfizer, New York, NY), 10 µg/ml fibrinogen concentrate [preparation as previously described: 6.5 mM CaCl2, 13 units/ml thrombin (Sigma–Aldrich), 2.6 mM ATIII peptide (11) and 627 mM heparin (Sigma–Aldrich), in Trisbuffered saline solution (TBS; 137 mM NaCl, 2.7 mM KCl, 33 mM Tris–HCl, pH 7.4).11,21,30,31 Our prior in vitro studies in which this delivery system was used demonstrated that growth factors can be delivered in a controlled manner over a period of approximately 10 days and that BMP12 stimulates tenogenesis of ASCs.18,28,30,31
Adipose Derived Stromal Cells
Canine ASCs were isolated and cultured as described previously.18,28 In brief, subcutaneous fat tissues were minced into a fine slurry and digested with 0.2% collagenase A (Roche Diagnostics, GmbH, Mannheim, Germany) at 37 °C for up to 2 h. After washes and removing undigested tissues, the isolated cells were cultured in growth medium containing 10% FBS, 100 unit/ml penicillin, 100 µg/ml streptomycin, and 2.5 µg/ml amphotericin B in α-MEM in a T150 flask. Within the next 24–48 h, ASCs were selected by removing unattached cells with two PBS washes. The plastic adherent ASCs were then expanded in growth medium and passaged when they were 80–90% confluent at a density of 6,000 cells/cm2. One million cells at passage 2–3 and growth factor were incorporated into the fibrinogen solution of the nanofiber scaffolds prior to polymerization. The time from fat isolation to autologous cell implantation was 10–14 days. This timeframe accommodated surgical schedules, allowed animals to recover after fat isolation, and took into account cell isolation variations among animals.
Animal Model
All procedures were approved by the institutional Animal Studies Committee. Flexor digitorum profundus tendons from the right forelimbs of dogs (Covance, Denver, Pennsylvania) were treated surgically and used as a repair-only group, an acellular scaffold group, or an ASC+BMP12-laden scaffold group. The tendons from the right second and fifth toes were transected sharply at the level of the proximal interphalangeal joint and repaired as described previously.11,21,28 Prior to completing the repair, longitudinally oriented horizontal slits were created in the center of each tendon stump using a beaver blade (5 mm depth, 2.5 mm width), as described previously.28 One of the two digits received the scaffold with cells and growth factor and the other digit received either repair only or the scaffold alone. The HBDS/nanofiber scaffold was secured within the repair-site by the core suture and maintained in that location by a 6–0 nylon running epitenon suture. Controlled passive motion exercise was applied to the digits postoperatively and animals were euthanized 28 days after repair. This animal model was identical to that used in prior experiments.11,21 Paws were frozen immediately after sacrifice and all tendons were mechanically tested within 24 h of thawing. Samples were then re-frozen prior to being processed and analyzed for biochemical assays. Detailed methods for the animal model can be found in the supplemental document.
Biomechanical Tests
Digital range-of-motion of the non-operated and the three experimental groups were assessed using a motion-analysis system (PC-Reflex; Qualisys, Glastonbury, CT).11,21,32 Using reflective markers, the joint positions were determined in flexed and extended positions. Proximal interphalangeal joint (PIP) rotation, distal interphalangeal joint (DIP) rotation, and linear tendon displacement were calculated.
The flexor tendons were then tested to failure in tension using a materials testing system (Instron 5,866, Canton, MA) as previously described.11,21,32 Each distal phalanx was held rigidly in a custom fixture and the proximal tendon stump was held in a soft tissue clamp. After application of a 1N preload and five preconditioning cycles in load control (triangle waveform, 1–5 N, 0.25 Hz), the specimens were pulled in tension to failure at a rate of 0.375 mm/s. From load-deformation plots, peak (ultimate) force, repair-site stiffness (slope of the linear portion of the force-elongation curve), and repair-site extension at 20N force were determined. From load-strain plots, repair-site rigidity (slope of the linear portion of the force-strain curve), resilience (the area under the curve until the yield point), and repair-site strain at 20N force were determined. Detailed biomechanical methods can be found in the supplemental document.
Biochemical Assays
Following biomechanical testing to failure, the proximal one-half centimeter and distal one-half centimeter of each tendon stump (total one centimeter) was divided longitudinally (Normal N = 12, Repair Only N = 8, Scaffold N = 6, ASC+BMP12 N = 12). Both wet weight and dry weight were determined for each segment. Samples were manually minced and dried overnight at 65°C. Dried samples (10 mg/ml) were further digested overnight at 65°C in a digestion buffer (pH 6.0) containing 2% papain (Sigma–Aldrich), 0.1 M sodium acetate, 0.01 M cysteine HCl, 0.05 M EDTA. Concentrated NaOH was subsequently added to the digestion solution to adjust pH to 7.0. Sulfated glycosaminoglycan (sGAG) concentrations were quantified with a Blyscan Assay according to manufacturer’s instructions (Biocolor, Carrickfergus, United Kingdom). dsDNA concentrations were determined using the Quant-iT Picogreen dsDNA assay (Invitrogen). Total collagen was determined using a modified hydroxyproline assay. Briefly, 200 µl of each sample was hydrolyzed with an equal volume of 4N NaOH at 121°C for 75 min, neutralized with an equal volume of 4N HCl, and then titrated to an approximate pH of 7.0. The resulting solution was combined with 1.2 ml chloramine-T (14.1 g/L) in buffer (50 g/L citric acid, 120 g/L sodium acetate trihydrate, 34 g/L sodium hydroxide, and 12.5 g/L acetic acid) and allowed to stand at room temperature for 30 min. The solution was then combined with 1.2 ml of 1.17 mM p-dimethylaminobenzaldehyde in perchloric acid and placed in a 65°C water bath for 20 min. An aliquot of 200 µl of each sample was added to a clear 96-well plate, in duplicate, and A550 values were read. PureCol bovine collagen (3.2 mg/ml) was diluted to provide a standard curve ranging from 0 to 1,000 µg/ml. Pyridinoline crosslinks were quantified using a commercially-available ELISA kit (Quidel Serum PYD EIA kit, San Diego, CA).
Proteomics
Proteomics evaluation was performed for the Normal, Repair-only, and ASC+BMP12 groups (N = 3 per group). Based on similar biochemistry and biomechanics outcomes in the Acellular scaffold and ASC+BMP12 study groups (see Results), we elected to forego proteomics evaluation in the Acellular scaffold group. 10 mm tendon fragments from the repair sites (5 mm distal and 5 mm proximal to the injury) and corresponding regions from non-operated tendons were isolated. Tendon fragments were diced into fine pieces and submitted to the Alvin J. Siteman Cancer Center Proteomics Shared Resource at Washington University School of Medicine for sample extraction (including Lys C and trypsin, high pressure digestion), time of flight liquid chromatography–tandem mass spectrometry (TOF LC-MS/MS) peptide analysis, and protein identification. All samples were analyzed using Mascot (Matrix Science, London, UK; version 2.4.1). Mascot was set up to search the Uni-Dog-Reference-20131009 database (26,438 entries) with trypsin as the digestion enzyme. Scaffold 4.1.1 (Proteome Software Inc., Portland, OR) was used to validate peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 90% probability by the Peptide Prophet algorithm33 with Scaffold delta-mass correction. Protein identifications were accepted if they could be established at greater than 95% probability and contained at least one identified peptide. Protein probabilities were assigned by the Protein Prophet algorithm.34 Proteins that contained similar peptides and could not be differentiated based on tandem mass spectroscopy analysis alone were grouped to satisfy the principles of parsimony.
Statistics
For biomechanics and biochemistry, comparisons between groups were made using an Analysis of Variance (ANOVA) followed by Tukey’s posthoc tests when appropriate. The significance level was set at p < 0.05. For proteomics, data analyses were performed on total spectrum count (TSC) of quantifiable proteins. Quantifiable proteins are defined as proteins, whose TSCs meet either of the two criteria: At least one group mean TSC ≥ 5; or one group mean TSC ≥ 4 and two individual TSCs within the group ≥ 5. If a protein’s group mean TSC is less than 1, the protein is deemed as absent from the group. Principle component analysis (PCA) was performed with TSCs of quantifiable proteins after z-score normalization using a customized code written in Matlab (The MathWorks, Inc., Natick, MA). Statistical analysis was performed with ANOVA after normalization using Scaffold_4.1.1 software. The significance was set to p < 0.05. Fold differences between groups were further validated by effect size analysis using Cohen’s D method. The effect size was set to large (> 0.8). Hierarchical clustering and heat map were generated with Genesis 1.7.6.35 Protein functional analysis was performed using the UniProt Knowledgebase (UniProtKB) at UniProt (http://www.uniprot.org).
RESULTS
Gross Observations and Repair-Site Gap Formation
At the time of dissection, there were no ruptures or gaps > 2.5 mm in the repair-only group. In contrast, ruptures or gaps > 2.5 mm were seen in 11% of the acellular scaffold group and 22% of the ASC+BMP12 group. Gap length, as determined at the time of dissection, was significantly higher in the acellular scaffold group compared to the repair-only group (Table 1). Based on a qualitative visual assessment, levels of adhesions (noted as none, slight, moderate, or severe) were similar among the three groups (moderate to severe adhesions were seen in 38% of repaironly samples, 44% of acellular scaffold samples, and 38% of ASC+BMP12 samples).
Table 1.
Range of Motion (PIP, DIP), Eextension and Strain at 20 N, and Gap for Normal, Repair-only, Scaffold, and ASC+BMP12 Groups
| Sample Size | PIP (degrees) | DIP (degrees) | Ext. @ 20N (mm) | Strain @ 20N | Gap (mm) | |
|---|---|---|---|---|---|---|
| Normal | 25 | 31.7±10.0a | 23.3±6.0a | |||
| Repair-only | 8 | 24.4±14.9 | 16.9±7.1 | 0.46±0.16 | 3.1±1.1 | 0.2±0.3a |
| Scaffold | 15 | 20.8±12.0a | 14.4±8.6a | 0.58±0.21 | 3.8±1.4 | 1.1±0.8a |
| ASC+BMP12 | 10 | 15.7±9.9a | 20.0±5.5 | 0.57±0.17 | 3.3±1.1 | 0.8±0.7 |
PIP, DIP: joint rotations under 1.5N of load. Ext./Strain @ 20N: extension/strain of tendon under 20N of tensile load. Gap: Distance between tendon stumps at the time of dissection.
Significantly different compared to each other (ANOVA followed by Tukey posthoc test).
Biomechanical Tests
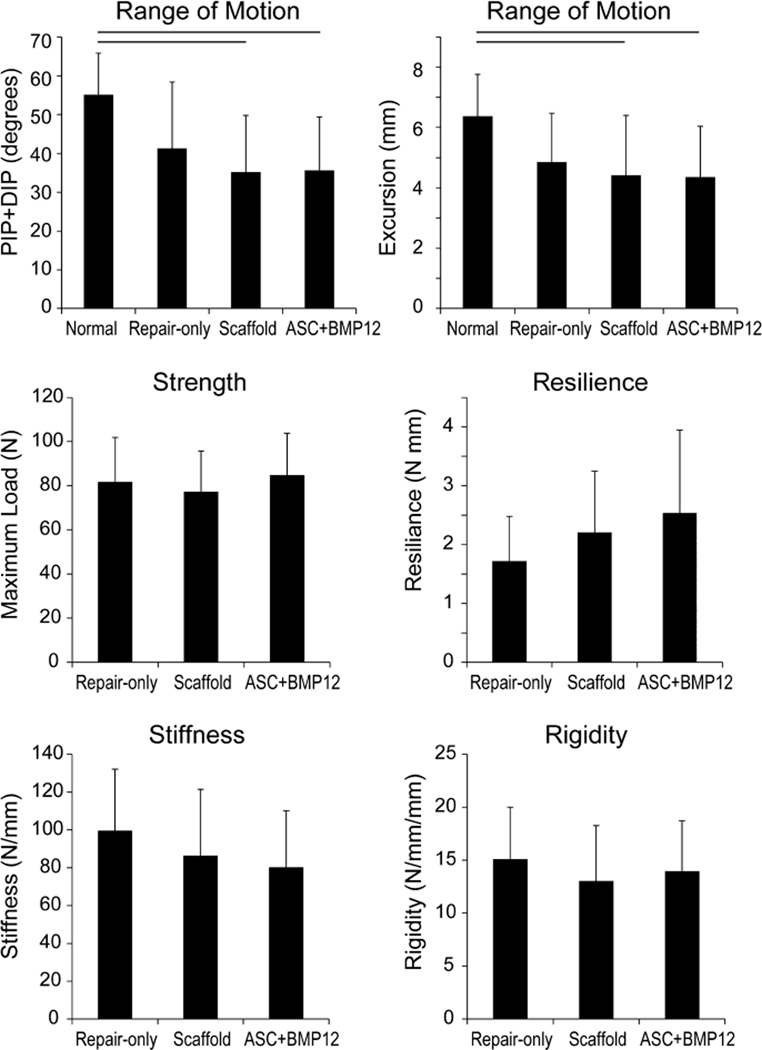
The primary measures of range of motion, PIP+DIP rotation and excursion, were significantly reduced in the groups containing the scaffold (i.e., Scaffold (acellular) and ASC+BMP12 groups compared to the Normal (uninjured) group; Fig. 1). In contrast, range of motion parameters were not different between the Repair-only group and the Normal group (Fig. 1, Table 1). There were no significant differences in any tensile properties when comparing Repair-only, Scaffold, and ASC+BMP12 groups (Fig. 1, Table 1).
Figure 1.
Functional (range of motion) and tensile properties for Repair-only, Acellular Scaffold, and ASC+BMP12 Scaffold groups at 28 d. Line above bars indicates p < 0.05 for that comparison.
Biochemical Assays
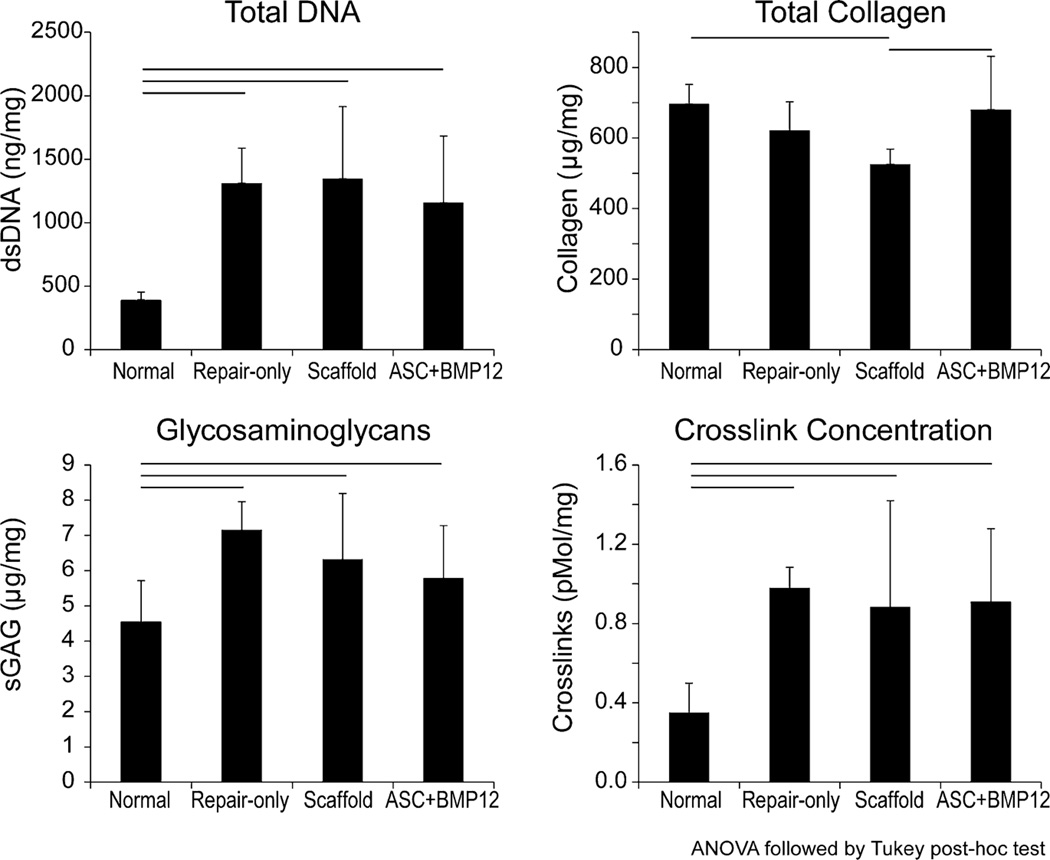
Total DNA, glycosaminoglycan concentration, and crosslink concentration were increased in all repair groups compared to normal (Fig. 2). There were no differences among the repair groups for these three outcome measures. Total collagen concentration was significantly reduced in the acellular scaffold group compared to normal and compared to the ASC+BMP12 group (Fig. 2).
Figure 2.
Total DNA and extracellular matrix biochemistry for Repair-only, Acellular Scaffold, and ASC+BMP12 Scaffold groups at 28 d. Line above bars indicates p < 0.05 for that comparison.
Proteomics
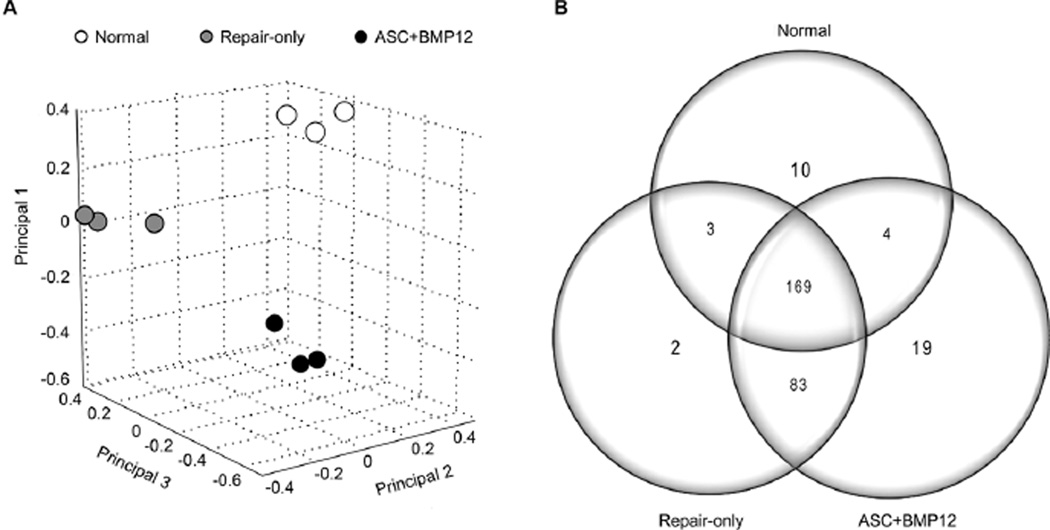
Proteomics analysis was performed to (i) identify the proteins and protein fragments in tendons and to (ii) compare the relative abundance of each protein between Normal, Repair-only, and ASC+BMP12 groups. A total of 1,900 proteins/protein fragments were identified (data will be made available on the PRIDE archive: http://www.ebi.ac.uk/pride/archive/). Based on principal components analysis, the samples from each of the three groups clustered in three distinct areas (Fig. 3A), indicating that the group differences were larger than those caused by individual and technical variations. Based on the criteria described in the methods, a total of 290 proteins were identified as statistically distinct from background noise (Supplemental Table S1). Over 50% of these proteins (169/290) were shared by tendons in all three groups (Fig. 3B). Eighty three proteins shared by the two repair groups were distinct from the Normal group, indicating a robust response to tendon injury and repair. Of note, although the two repair groups shared about 90% of their proteins, 19 proteins were unique to the ASC+BMP12 group and were absent from the Repair-only and Normal groups, supporting unique effects induced by ASC+BMP12 scaffold (Fig. 3B).
Figure 3.
Proteomic data analysis on tendon samples from Normal, Repair-only, and ASC+BMP12 groups. (A) Top three principals identified by principal component analysis based on total spectrum counts (TSCs) of quantifiable proteins after z-score normalization. There was clear separation between the three groups studied. (B) Distributions of differentially expressed proteins among groups. 169 proteins were found in all three groups. 83 proteins were distinct to the repair groups. 19 proteins were unique to the ASC+BMP12 group.
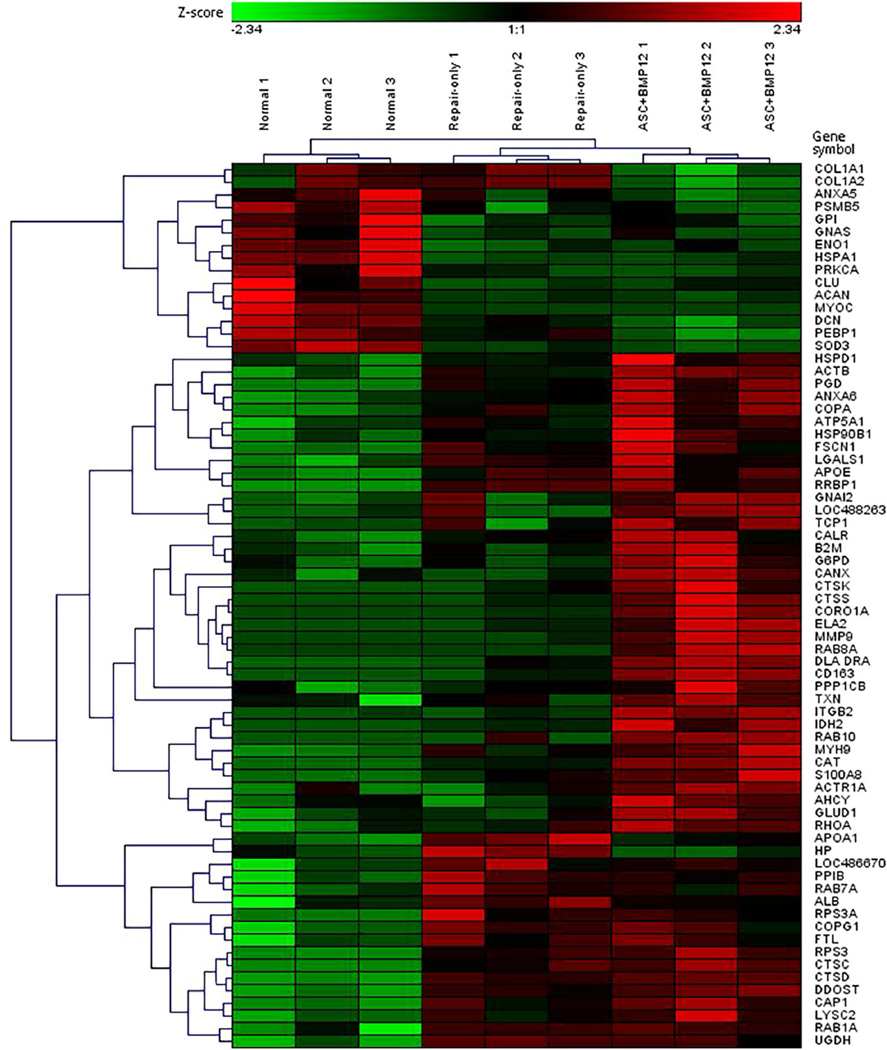
Only 70 of the 290 detected proteins have been characterized. These 70 proteins were the focus of further analyses and statistical comparison using hierarchical clustering and heat map approaches (Fig. 4). The vast majority of the proteins were significantly enriched in the repair groups compared to the normal group, and this difference was most apparent when comparing ASC+BMP12 to Normal.
Figure 4.
Hierarchical clustering of the 70 characterized proteins found in tendon samples from the three groups. Proteins are shown in rows and samples are shown in columns. The relative abundance of each protein is expressed as a z-score, with the color coding indicating high levels in red, low levels in green, and mean levels in black.
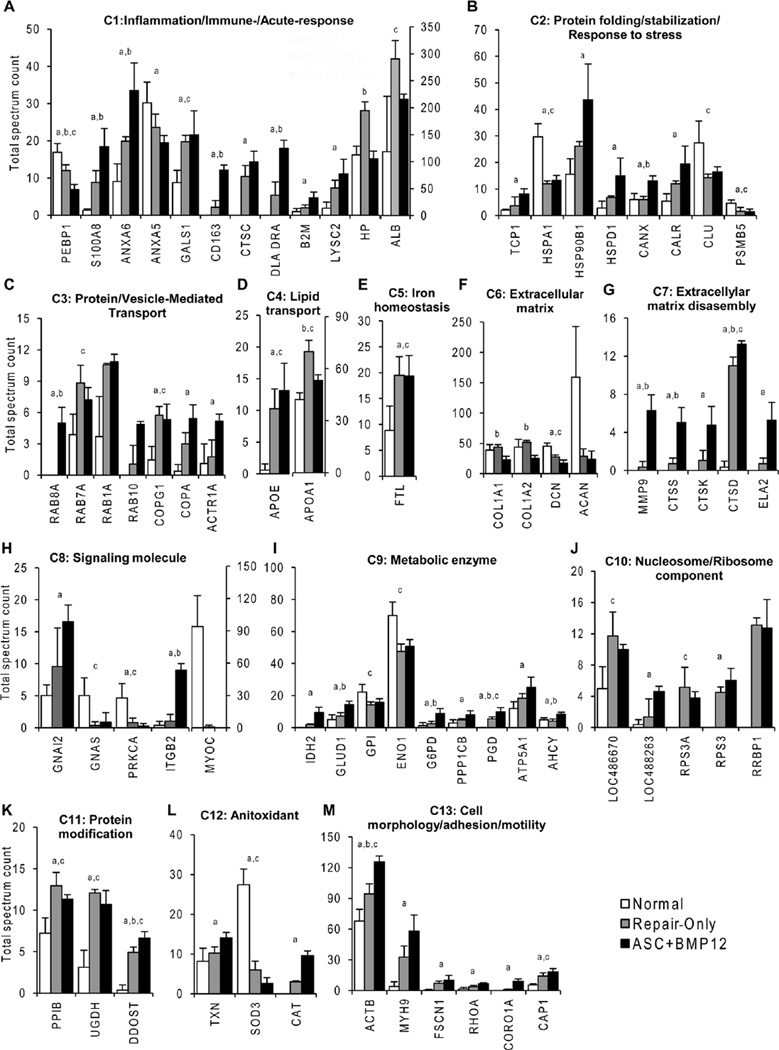
To better understand how the changes in tendon protein composition may affect tendon healing, we performed functional analysis on the 70 characterized proteins using UniProKB. According to this analysis, the proteins were classified into 13 categories based on their primary molecular functions, involvement in biological processes, and subcellular locations (Supplementary Fig. S1). The following protein categories (C) were identified: C1 Inflammation/Immune-/Acute-response, C2 Protein folding/stabilization/Response to stress, C3 Protein/Vesicle-mediated transport, C4 Lipid transport, C5 Iron homeostasis, C6 Extracellular matrix, C7 Extracellular matrix disassembly, C8 Signaling molecule, C9 Metabolic enzyme, C10 Nucleosome/Ribosome component, C11 Protein modification, C12 Antioxidant, and C13 Cell morphology/adhesion/motility.
As shown in Supplemental Figure S1 and Figure 5A, tendon injury and repair significantly affected proteins involved in inflammation, immune, and acute responses. Affected proteins included S100 calcium binding protein A8 (SA100A8), an important pro-inflammatory mediator, and its interacting protein Annexin 6 (ANXA6).36 The only protein that was significantly reduced in both repair groups was phosphatidylethanolamine-binding protein1 (PEBP1, also known as Raf kinase inhibitor protein, RKIP). This protein, an important inflammatory regulator involved in multiple signaling pathways, may either positively or negatively regulate inflammatory signaling.37 Many of these inflammation-related proteins were increased in the ASC+BMP12 group compared to the Repair-only group (Fig. 5A).
Figure 5.
Comparison of relative abundances of proteins differentially present in tendons from Normal, Repair-only, and ASC+BMP12 groups. All proteins are indicated with their gene symbols. The full descriptions of these proteins are listed in Supplemental Table S1. a, p < 0.05, ASC+BMP12 vs. Normal; b, p < 0.05, ASC+BMP12 vs. Repair-only; c, p < 0.05, Repair-only vs. Normal.
As expected, tendon injury and repair changed the composition of tendon matrix proteins (Fig. 5F) and their degrading enzymes (Fig. 5G). Specifically, tendon proteoglycan Decorin (DCN) was significantly down-regulated in both repair groups. The relative abundances of type I collagen α1 and α2 subunits (Col1A1 and α2 (Col1A2) in BMP12+ASC group were found to be lower than those in the Repair-only group. Consistently, collagenases were greatly accumulated in the ASC+BMP12 group, while they were barely detectable in the Normal group and rare in the Repair-only group (Fig. 5G).
Signaling molecules were altered after tendon repair (Fig. 5H). Meanwhile, the relative abundances of many metabolic enzymes were also changed in the repaired tendons (Fig. 5I). These proteins are critical for multiple metabolic pathways, including glycolysis (Glucose-6-phosphate isomerase, GPI; Enolase, ENO1), tricarboxylic acid cycle (Isocitrate dehydrogenase, IDH2; Glutamate dehydrogenase, GLUD1), pentose-phosphate shunt (Glucose-6-phosphate 1-dehydrogenase, G6PD; 6-phosphoglucnate dehydrogenase, decarboxylating, PGD), and ATP synthesis (ATP synthase subunit α, ATP5A1). Of note, the relative abundances of many of these enzymes were comparable in the Normal and Repair-only groups but significantly elevated in the ASC+BMP12 group (Fig. 5I).
Nucleosome/ribosome components participating in transcriptional and translational processes (Fig. 5J) as well as those involved in protein posttranslational modification (Fig. 5K) were increased in the repair groups. Of note, Peptidyl-prolyl cis-trans insomerase (PPIB, also known as Cyclophilin B, CypB) is an enzyme necessary for collagen type I posttranslational modification and crosslinking38 and interacts with two molecular chaperones, Calnexin (CANX) and Calreticulin (CALR),39 identified in this study (Fig. 5B). UDP-glucose 6-dehydrogenase (UGDH) is involved in glycosaminoglycan (GAG) biosynthesis.
DISCUSSION
Flexor tendon healing depends on cell migration from the tendon’s surface and digital sheath to the repair site, cell proliferation between the aligned tendon stumps, and extracellular matrix (ECM) synthesis by tendon fibroblasts.6,40,41 These processes are inherently slow in the hypocellular environment of intrasynovial flexor tendons. Partially due to the paucity of tendon fibroblasts in the region of repair, growth factor application in isolation has been insufficient to stimulate an improvement in tensile mechanical properties following tendon suture, although some improvements have been achieved in digital range of motion.11,13,21 In an effort to improve the biological response to tendon injury and repair, we used a clinically relevant suture technique, a controlled method of ASC and BMP12 administration, and early passive motion in a canine model. The canine model was chosen because of its clinical relevance42 and for ease of comparison with prior studies.6,11,13,17,21,40,41 The results of the current study indicate that the application of MSCs and the tenogenic growth factor BMP12 at the time of intrasynovial tendon suture does not enhance repair and that the scaffold used for delivery and may be deleterious to the healing process. When examining the biomechanics data and the protein content of healing tendons, our findings demonstrated that repaired tendons remain far from normal 28 days after surgical repair. Our biochemical studies indicate that total DNA (a measure of tendon cellularity), GAG content (a measure of ECM formation), and collagen crosslinks (a measure of repair site remodeling and maturation) are significantly increased compared to normal. Consistent with these findings, proteomics analysis showed that UGDH and PPIB, involved in GAG biosynthesis and collagen crosslinking, respectively, are increased in both repair groups. The results are also consistent with prior reports indicating a significant up-regulation of mRNA levels for proteoglycans following tendon injury.43
Proteomics assessment of healing tendons compared to uninjured tendons demonstrated increases in proteins associated with inflammation and immunologic response, stress response, matrix degradation, metabolism, protein modification, and transcriptional and translational processes. These results indicate that injury and repair leads to extensive changes in tendon protein composition, and that treatment with ASC+BMP12 amplifies those changes. Of the subset of previously characterized and differentially expressed proteins (i.e., the 70 proteins categorized and explicitly compared), almost all were enriched in the repair groups. There were two striking exceptions to this trend. MYOC, a secreted glycoprotein involved in Wnt/integrin signaling44 and SOD3, an antioxidant enzyme, had significantly reduced concentrations in repaired tendons compared to normal. High levels of MYOC were found in normal tendon, whereas the protein’s levels were nearly undetectable in the repair groups. MYOC was recently reported to be enriched in male tendon/ligament compared to female tendon/ligament45 and may be involved in regulating tendon size and strength. Similarly, SOD3 was abundant in normal tendons but scarce in repaired tendons. Taken together, these findings indicate that intrasynovial tendon repair is associated with dramatic upregulation of numerous cell processes, and that the delivery of ASCs and BMP12 with a PLGA/fibrin-based scaffold amplified these processes. Neither of the repair responses, however, came close to returning the tendon to its native properties by 28 days of healing. The healing response, even in the presence of stem cells and a tenogenic growth factor, appeared to be driven by inflammation rather than by regeneration. Although there are no definitive markers for tendon, genes such as Scx, Mkx, Dcn, Col1, Col3, Tnc-C, and others, are characteristic of tenogenesis. Proteomics analysis, however, was limited by the available databases defining proteins and protein fragments, and these data are incomplete for the canine. In our analysis, there were a number of proteins and protein fragments that were identified but could not be assigned to known proteins (Supplemental Table S1).
The treatment used in the current study, autologous ASCs and BMP12 delivered with a PLGA/fibrin-based scaffold, was ineffective for improving tendon healing. To the contrary, biomechanical and biochemical results implied a potentially detrimental effect of the treatment. The growth factor BMP12 was chosen in the current study based on experiments indicating that this factor is capable of dose- and time-dependent induction of tenogenic differentiation of ASCs.18 In a side-by-side comparison between BMP12 and BMP14, it was noted that BMP12 was more effective than BMP14 as a tenogenic factor. We elected to use a PLGA/fibrin-based scaffold to administer the ASCs and BMP12 based on recent histological and gene expression findings showing that both cells and growth factors can be delivered simultaneously in this manner without any apparent adverse reaction.28 In a prior large animal study, we demonstrated that these scaffolds were well-tolerated, that sustained delivery of growth factors could be achieved, and that cell viability could be maintained, as demonstrated by intravital staining for up to 9 days post tendon repair.28 However, the proteomics analysis reported here demonstrated that inflammation was amplified in this treatment group compared to the repair-only group through 28 days, potentially impairing the healing process. High levels of inflammation-related proteins were seen in the ASC+BMP12 scaffold group compared to Repair-only and to Normal. These findings indicate a chronic level of inflammation induced by a component of the treatment or delivery system. Furthermore, range of motion was reduced in the groups that contained scaffolds, and total collagen content and gap formation were reduced in the scaffold-only group compared to the Normal and ASC+BMP12 groups. These results suggest a negative effect of the scaffold, perhaps due to fragments of degraded scaffold inciting a low-grade inflammatory reaction and/or negative effects associated with the pocket that was created to secure the scaffold (Supplemental Table S2). The positive findings noted in prior in vitro and in vivo studies notwithstanding, our results indicate that the intrasynovial tendon repair process is not substantially enhanced, either structurally or functionally, by the application of an ASC+BMP12 scaffold compared to tendon repair alone.
A limitation of this experiment is that the comparison groups did not include all possible combinations of ASCs and growth factor administration. Specifically, we did not explore the isolated applications of ASCs and BMP12 with our biodegradable scaffold. Another shortcoming is the exclusion of the acellular scaffold group from the proteomics assessment. This choice was made to keep the scope of the proteomics analysis at a manageable level, as the findings on the biomechanics studies indicated that there were no significant differences between the two control groups (i.e., repair-only vs. acellular scaffold groups). A third limitation is that only a single time-point, 28 days, was evaluated. Although this is a relatively early time-point when considering tendon healing, we note that that the early period after flexor tendon repair dictates the long-term success of the repair, as most complications occur in the first month after repair. Nevertheless, future studies at longer time-points could provide important additional information about the healing process, particularly for extracellular matrix formation and repair site mechanical properties. A fourth limitation is the lack of histologic assessment, which prevented the local assessment of inflammation around the scaffold. Despite the lack of this qualitative outcome measure, the quantitative protein expression and biomechanical data support the hypothesis that inflammation, in response to the scaffold, was deleterious to healing. As the cells were autologous, fibrin was well received in our previous studies, and BMP12 has been used in numerous animal models without negative effects, it is likely that the negative reaction was due to the PLGA used in the scaffold. It was beyond the scope of this large animal study to mechanistically determine the cause of increased inflammation in the scaffold-containing groups.
The hypothesis of the current study was that ASCs, prompted towards tenogenesis with BMP12, would enhance extracellular matrix synthesis at the repair site, leading to improved biomechanical properties. This approach was supported by data from prior studies that combined administration of cells and growth factors for improving both the biological response and the structural properties of the repair.11,13,20,46,47 Pilot studies using the growth factor PDGF and higher doses of BMP12 did not show promise with the approach presented in the current study (data not shown). Furthermore, according to the proteomics analysis, the treatment approach induced a negative long-term inflammatory reaction at the repair site. Future studies should consider delivery techniques that are minimally invasive (i.e., without the creation of a slit within the tendon), alternative scaffolding approaches, and/or different choices of growth factors/stem cells for flexor tendon cell and growth therapies.
Supplementary Material
Acknowledgments
The authors thank Dr. Wenying Liu for preparing nanofiber mats, Ryan Potter and Dr. Cionne Manning for organizational efforts, and Stephen Linderman and Shivam Shah for isolation of adipose derived stromal cells. The study was funded by the National Institutes of Health (NIH) R01 AR06294. Biomechanics was performed with support from the Washington University Musculoskeletal Research Center ASCs/BMP12 (NIH P30 AR057235). Proteomics was performed at the Alvin J. Siteman Cancer Center (Washington University School of Medicine) Proteomics Shared Resource, supported in part by NIH P30 CA91842 with help from Drs. James Malone and Reid Townsend.
Conflicts of interest: SSE is an inventor on patents covering the heparin-binding delivery system technology. Kuros Therapeutics has licensed these patents. SSE may receive royalties if additional licensing agreements occur as a result of this publication. Kuros did not fund this research and SSE does not have any role as a consultant or board member at Kuros. ST, SSE, MJS, RHG, and YX are inventors on a patent covering the layered HBDS/PLGA nanofiber mat used in this study. This patent has not been licensed and no royalties or other funds have been derived from this patent.
Footnotes
AUTHORS’ CONTRIBUTIONS
RHG, ST, MJS, and SSE developed the study design; RHG and IK performed the surgeries; HS prepared the cells and scaffolds; BR, GY, and RT performed the biochemical assays; YX, SSE, and ST developed the scaffold; all authors edited/approved article.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
REFERENCES
- 1.Boyer MI, Strickland JW, Engles D, et al. Flexor tendon repair and rehabilitation: state of the art in 2002. Instr Course Lect. 2003;52:137–161. [PubMed] [Google Scholar]
- 2.Boyer MI, Goldfarb CA, Gelberman RH. Recent progress in flexor tendon healing. The modulation of tendon healing with rehabilitation variables. J Hand Ther. 2005;18:80–85. doi: 10.1197/j.jht.2005.02.009. quiz 86. [DOI] [PubMed] [Google Scholar]
- 3.Khanna A, Friel M, Gougoulias N, et al. Prevention of adhesions in surgery of the flexor tendons of the hand: what is the evidence? Br Med Bull. 2009;90:85–109. doi: 10.1093/bmb/ldp013. [DOI] [PubMed] [Google Scholar]
- 4.Khan U, Kakar S, Akali A, et al. Modulation of the formation of adhesions during the healing of injured tendons. J Bone Joint Surg Br Vol. 2000;82:1054–1058. doi: 10.1302/0301-620x.82b7.9892. [DOI] [PubMed] [Google Scholar]
- 5.Matthews P, Richards H. Factors in the adherence of flexor tendon after repair: an experimental study in the rabbit. J Bone Joint Surg Br Vol. 1976;58:230–236. doi: 10.1302/0301-620X.58B2.777010. [DOI] [PubMed] [Google Scholar]
- 6.Gelberman RH, Vandeberg JS, Manske PR, et al. The early stages of flexor tendon healing: a morphologic study of the first fourteen days. J Hand Surg Am. 1985;10:776–784. doi: 10.1016/s0363-5023(85)80151-9. [DOI] [PubMed] [Google Scholar]
- 7.Gelberman RH, Boyer MI, Brodt MD, et al. The effect of gap formation at the repair site on the strength and excursion of intrasynovial flexor tendons. An experimental study on the early stages of tendon-healing in dogs. J Bone Joint Surg Am. 1999;81:975–982. doi: 10.2106/00004623-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Zhao C, Amadio PC, Paillard P, et al. Digital resistance and tendon strength during the first week after flexor digitorum profundus tendon repair in a canine model in vivo. J Bone Joint Surg Am. 2004;86-A:320–327. doi: 10.2106/00004623-200402000-00015. [DOI] [PubMed] [Google Scholar]
- 9.May EJ, Silfverskiold KL. Rate of recovery after flexor tendon repair in zone II. A prospective longitudinal study of 145 digits. Scand J Plast Reconstr Surg Hand Surg. 1993;27:89–94. doi: 10.3109/02844319309079789. [DOI] [PubMed] [Google Scholar]
- 10.Silfverskiold KL, May EJ. Gap formation after flexor tendon repair in zone II. Results with a new controlled motion programme. Scand J Plast Reconstr Surg Hand Surg. 1993;27:263–268. [PubMed] [Google Scholar]
- 11.Thomopoulos S, Das R, Silva MJ, et al. Enhanced flexor tendon healing through controlled delivery of PDGF-BB. J Orthop Res. 2009;27:1209–1215. doi: 10.1002/jor.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beredjiklian PK, Favata M, Cartmell JS, et al. Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng. 2003;31:1143–1152. doi: 10.1114/1.1616931. [DOI] [PubMed] [Google Scholar]
- 13.Gelberman RH, Thomopoulos S, Sakiyama-Elbert SE, et al. The early effects of sustained platelet-derived growth factor administration on the functional and structural properties of repaired intrasynovial flexor tendons: an in vivo biomechanic study at 3 weeks in canines. J Hand Surg [Am] 2007;32:373–379. doi: 10.1016/j.jhsa.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Miller A. In: Mineral phases in biology. Miller A, Phillips D, Williams RJP, editors. London: Royal Society; 1984. pp. 45–68. [Google Scholar]
- 15.Thomopoulos S, Zaegel M, Das R, et al. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J Orthop Res. 2007;25:1358–1368. doi: 10.1002/jor.20444. [DOI] [PubMed] [Google Scholar]
- 16.Gelberman RH, Amiel D, Gonsalves M, et al. The influence of protected passive mobilization on the healing of flexor tendons: a biochemical and microangiographic study. Hand. 1981;13:120–128. doi: 10.1016/s0072-968x(81)80051-4. [DOI] [PubMed] [Google Scholar]
- 17.Woo SL, Gelberman RH, Cobb NG, et al. The importance of controlled passive mobilization on flexor tendon healing. A biomechanical study. Acta Orthop Scand. 1981;52:615–622. doi: 10.3109/17453678108992156. [DOI] [PubMed] [Google Scholar]
- 18.Shen H, Gelberman RH, Silva MJ, et al. BMP12 induces tenogenic differentiation of adipose-derived stromal cells. PLoS ONE. 2013;8:e77613. doi: 10.1371/journal.pone.0077613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi M, Zhao C, An KN, et al. The effects of growth and differentiation factor 5 on bone marrow stromal cell transplants in an in vitro tendon healing model. J Hand Surg Eur. 2011;36:271–279. doi: 10.1177/1753193410394521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao C, Ozasa Y, Reisdorf RL, et al. CORR(R) ORS Richard A. Brand Award for Outstanding Orthopaedic Research: engineering flexor tendon repair with lubricant, cells, and cytokines in a canine model. Clin Orthop Relat Res. 2014;472:2569–2578. doi: 10.1007/s11999-014-3690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomopoulos S, Kim HM, Das R, et al. The effects of exogenous basic fibroblast growth factor on intrasynovial flexor tendon healing in a canine model. J Bone Joint Surg Am. 2010;92:2285–2293. doi: 10.2106/JBJS.I.01601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanez R, Lamana ML, Garcia-Castro J, et al. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582–2591. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 23.Cui L, Yin S, Liu W, et al. Expanded adipose-derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Eng. 2007;13:1185–1195. doi: 10.1089/ten.2006.0315. [DOI] [PubMed] [Google Scholar]
- 24.Park A, Hogan MV, Kesturu GS, et al. Adipose-derived mesenchymal stem cells treated with growth differentiation factor-5 express tendon-specific markers. Tissue Eng Part A. 2010;16:2941–2951. doi: 10.1089/ten.tea.2009.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfman NM, Hattersley G, Cox K, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321–330. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tashiro T, Hiraoka H, Ikeda Y, et al. Effect of GDF-5 on ligament healing. J Orthop Res. 2006;24:71–79. doi: 10.1002/jor.20002. [DOI] [PubMed] [Google Scholar]
- 27.Lou J, Tu Y, Burns M, et al. BMP-12 gene transfer augmentation of lacerated tendon repair. J Orthop Res. 2001;19:1199–1202. doi: 10.1016/S0736-0266(01)00042-0. [DOI] [PubMed] [Google Scholar]
- 28.Manning CN, Schwartz AG, Liu W, et al. Controlled delivery of mesenchymal stem cells and growth factors using a nanofiber scaffold for tendon repair. Acta Biomater. 2013;9:6905–6914. doi: 10.1016/j.actbio.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J Controlled Release. 2000;69:149–158. doi: 10.1016/s0168-3659(00)00296-0. [DOI] [PubMed] [Google Scholar]
- 30.Sakiyama-Elbert SE, Das R, Gelberman RH, et al. Controlled-release kinetics and biologic activity of platelet-derived growth factor-BB for use in flexor tendon repair. J Hand Surg [Am] 2008;33:1548–1557. doi: 10.1016/j.jhsa.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomopoulos S, Das R, Sakiyama-Elbert S, et al. BFGF and PDGF-BB for tendon repair: controlled release and biologic activity by tendon fibroblasts in vitro. Ann Biomed Eng. 2010;38:225–234. doi: 10.1007/s10439-009-9844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson GN, Potter R, Ntouvali E, et al. Intrasynovial flexor tendon repair: a biomechanical study of variations in suture application in human cadavera. J Orthop Res. 2012;30:1652–1659. doi: 10.1002/jor.22108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller A, Nesvizhskii AI, Kolker E, et al. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 34.Nesvizhskii AI, Keller A, Kolker E, et al. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 35.Sturn A, Quackenbush J, Trajanoski Z. Genesis: cluster analysis of microarray data. Bioinformatics. 2002;18:207–208. doi: 10.1093/bioinformatics/18.1.207. [DOI] [PubMed] [Google Scholar]
- 36.Bode G, Luken A, Kerkhoff C, et al. Interaction between S100A8/A9 and annexin A6 is involved in the calcium-induced cell surface exposition of S100A8/ A9. J Biol Chem. 2008;283:31776–31784. doi: 10.1074/jbc.M803908200. [DOI] [PubMed] [Google Scholar]
- 37.Zhao J, Wenzel S. Interactions of RKIP with inflammatory signaling pathways. Crit Rev Oncog. 2014;19:497–504. doi: 10.1615/critrevoncog.2014011950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cabral WA, Perdivara I, Weis M, et al. Abnormal type I collagen post-translational modification and crosslinking in a cyclophilin B KO mouse model of recessive osteogenesis imperfecta. PLoS Genet. 2014;10:e1004465. doi: 10.1371/journal.pgen.1004465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kozlov G, Bastos-Aristizabal S, Maattanen P, et al. Structural basis of cyclophilin B binding by the calnexin/calreticulin P-domain. J Biol Chem. 2010;285:35551–35557. doi: 10.1074/jbc.M110.160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gelberman RH, Vande Berg JS, Lundborg GN, et al. Flexor tendon healing and restoration of the gliding surface. An ultrastructural study in dogs. J Bone Joint Surg Am. 1983;65:70–80. [PubMed] [Google Scholar]
- 41.Gelberman RH, Amiel D, Harwood F. Genetic expression for type I procollagen in the early stages of flexor tendon healing. J Hand Surg Am Vol. 1992;17:551–558. doi: 10.1016/0363-5023(92)90370-5. [DOI] [PubMed] [Google Scholar]
- 42.Potenza AD. Tendon healing within the flexor digital sheath in the dog. J Bone Joint Surg Am. 1962;44-A:49–64. [PubMed] [Google Scholar]
- 43.Berglund M, Reno C, Hart DA, et al. Patterns of mRNA expression for matrix molecules and growth factors in flexor tendon injury: differences in the regulation between tendon and tendon sheath. J Hand Surg Am. 2006;31:1279–1287. doi: 10.1016/j.jhsa.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Kwon HS, Tomarev SI. Myocilin, a glaucoma-associated protein, promotes cell migration through activation of integrin-focal adhesion kinase-serine/threonine kinase signaling pathway. J Cell Physiol. 2011;226:3392–3402. doi: 10.1002/jcp.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Little D, Thompson JW, Dubois LG, et al. Proteomic differences between male and female anterior cruciate ligament and patellar tendon. PLoS ONE. 2014;9:e96526. doi: 10.1371/journal.pone.0096526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young RG, Butler DL, Weber W, et al. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res. 1998;16:406–413. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- 47.Awad HA, Boivin GP, Dressler MR, et al. Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res. 2003;21:420–431. doi: 10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.